Abstract
BACKGROUND
Since coronavirus disease 2019 vaccines have been distributed, a debate has raised on whether pregnant women should get the vaccine. No available data exist so far regarding the safety, efficacy, and toxicology of these vaccines when administered during pregnancy. Most of the Obstetrics and Gynecology societies suggested that pregnant could agree to be vaccinated, after a thorough counseling of risks and benefits with their gynecologists, thus leading to an autonomous decision.
OBJECTIVE
This study aimed to evaluate the attitude to coronavirus disease 2019 vaccination in pregnant and breastfeeding women in Italy.
STUDY DESIGN
A survey was made at the University of Naples Federico II and the Ospedale Cristo Re, Tor Vergata University of Rome, on pregnant and breastfeeding women asking their perspectives on the available vaccines after reading the recommendations issued by our national Obstetrics, Gynecology, and Neonatology societies. The questionnaire included 12 items finalized to evaluate general features of the women and 6 items specifically correlated to their attitudes toward the severe acute respiratory syndrome coronavirus 2 vaccination. Chi-square or Fisher's exact tests were used to compare group differences of categorical variables and Wilcoxon signed rank or Mann-Whitney U test for continuous variables. The study was approved by the institutional review boards of the University of Naples Federico II (ref. no. 409/2020) and the Ospedale Cristo Re, Tor Vergata University of Rome (ref. #Ost4-2020).
RESULTS
Most of the included women did not agree to eventually receive severe acute respiratory syndrome coronavirus 2 vaccine during pregnancy (40 [28.2%] vs 102 [71.8%]). Being pregnant was considered a determinant factor to refuse the vaccine prophylaxis (99 [69.7%] vs 43 [30.3%]; chi-square test=24.187; P<.001), even if a very large percentage declared to be generally in favor of vaccines (128 [90.1%] vs 14 [9.9%]; chi-square test=6.091; P=.014) and most of them confirmed they received or would receive other recommended vaccines during pregnancy (75 [52.8%] vs 67 [47.2%]; chi-square test=10.996; P=.001).
CONCLUSION
Urgent data are needed on the safety, efficacy, and toxicology of severe acute respiratory syndrome coronavirus 2 vaccines during pregnancy to modify this trend and to help obstetricians during the counseling. Furthermore, pregnant women should be included in future vaccine development trials to not incur again in such uncertainty.
Key words: COVID-19; SARS-COV-2, survey; vaccine during pregnancy
AJOG MFM at a Glance.
Why was this study conducted?
This study aimed to know the current perspectives of Italian pregnant women, during the first phase of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine distribution, regarding its uptake.
Key findings
Italian pregnant women are still largely suspicious toward the SARS-CoV-2 vaccination program, independently from social, cultural, and pregnancy-related features.
What does this add to what is known?
These results are the first report from a patient's point of view that reinforce the need for urgent data from vaccine trials, in which women should be included since now on, to avoid the current uncertainty and denial. Counseling without data lacks power.
Introduction
Coronavirus disease 2019 (COVID-19) pandemic has determined an incredible burden on national healthcare systems worldwide. In Obstetrics and Gynecology practice, all nonurgent clinical and surgical activities have been postponed during the most critical phases, also raising the debate on which conditions should be considered as urgent and how to reorganize obstetrical, gynecologic, and reproductive medicine units.1, 2, 3, 4, 5, 6, 7, 8
Large multicenter cohort studies have found that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy was associated with a 0.8% rate of maternal mortality and 11.1% rate of intensive care unit admissions, with increased rates of preterm deliveries (both spontaneous and iatrogenic) and cesarean deliveries.9 , 10 The earlier the infection, the more the increase of the risk of adverse fetal outcomes,11 even though the risk of vertical transmission seems to be negligible.9 , 10 Actually, no general consensus exists on the optimal management for pregnant women with SARS-CoV-2 infection12, 13, 14, 15; moreover, there is a huge heterogeneity among hospitals, and regarding therapy, combinations of azithromycin or other antibiotic agents, hydroxychloroquine, low-molecular-weight heparin, and a large variety of antiviral agents have been used, without any substantial difference among therapeutic regimes.9 , 16
Less than a year after the recognition of this new infection, vaccines against SARS-CoV-2 virus have been developed and open to worldwide distribution, to counteract the pandemic. It has been widely proposed to include pregnant women into COVID-19 vaccine trials,17 , 18 because they could be considered at higher risk, and the International Federation of Gynecology and Obstetrics endorsed this recommendation.19
On December 27, 2020, the vaccine against SARS-CoV-2 infection started to be administered in Italy and across Europe. Thereafter, the most important Italian Obstetrics and Gynecology societies released a position paper ad interim on COVID-19 vaccine and pregnancy, stating that it is not recommended but not contraindicated to receive SARS-CoV-2 vaccine during pregnancy and breastfeeding and in women with reproductive desire and that each woman should evaluate with her gynecologist risks and benefits of its administration.20
Therefore, the aim of the present survey is to understand which are the perspectives of pregnant and breastfeeding women regarding the possibility to receive COVID-19 vaccine during pregnancy.
Materials and Methods
Study design and participants
This was a multicenter cross-sectional cohort study involving 2 centers in Italy (University of Naples Federico II and Ospedale Cristo Re, Tor Vergata University of Rome), conducted in January 2021.
Our study included pregnant women attending the 2 centers for outpatient visits and early postpartum inpatient women who were asked to participate to a survey on the possible uptake of the SARS-CoV-2 vaccine during pregnancy and puerperium. The exclusion criteria were inability to comprehend the text and to sign the informed consent.
After signing an informed consent and reading the position paper ad interim on “Pregnancy and COVID-19 vaccine,”20 participants were given a questionnaire to fill. An anonymous online semistructured questionnaire was developed using google forms (https://docs.google.com/forms. Google Mountain View, CA).
Outcome measures
The questionnaire was structured in 2 sections: Part A was finalized to acquire data on maternal characteristics (sociocultural and demographic variables, past and current obstetrical history, and maternal age and gestational age at the receipt of the questionnaire); Part B was structured to test women's knowledge and concerns about vaccines (Supplemental Material).
We defined education as of a medium-low level in case of primary school or early secondary school and of a medium-high level in case of late secondary school or degree and more. Any work was considered to fill in the worker subgroup compared with women who were housewives or unemployed.
Women were specifically asked whether they were in favor or against the SARS-CoV-2 vaccine during pregnancy. Furthermore, questions were asked regarding general acceptance of vaccines, whether the acceptance of SARS-CoV-2 vaccine was dependent on the pregnant/breastfeeding status, and whether they would receive other vaccine recommended during pregnancy (referring to the trivalent—diphtheria, tetanus, and acellular pertussis [DTaP]—and the influenza vaccines).
Patients were then grouped according to their response to the survey (acceptance or decline of the SARS-CoV-2 vaccine during pregnancy or breastfeeding).
Statistical analysis
Descriptive statistics were calculated for the variables considered, and data were expressed as number and percentage for categorical variables and median and interquartile range for continuous variables. Chi-square (χ2) or Fisher exact tests were used to compare group differences of categorical variables and Wilcoxon signed rank or Mann-Whitney U test for continuous variables. Pearson correlation analysis was used to calculate the univariate associations variables. All hypotheses were tested at a significance level of P=.05. Statistical analysis was performed using SPSS Statistic 21.0 (IBM Corp, Armonk, NY).
Ethical approval
The study was approved by the institutional review boards of the University of Naples Federico II (ref. no. 409/2020) and the Ospedale Cristo Re, Tor Vergata University of Rome (ref. #Ost4-2020).
Results
A total of 168 women were asked to participate in the study, 26 of whom (15.5%) refused the invitation; therefore, 142 women (84.5%) were enrolled in the survey.
Of 142 women, 119 (83.8%) were pregnant and 23 (16.2%) were in the early postpartum period. Maternal and obstetrical characteristics of included women are described in Table 1 .
Table 1.
General characteristics of the participants to the survey (Questionnaire Part A)
| Features | Options | Value |
|---|---|---|
| Age | 34 [31–37.25] | |
| Nationality | Italian European Non-European |
137 (96.5) 1 (0.7) 4 (2.8) |
| Marital status | Unmarried Married Divorced No answer |
11 (7.7) 71 (50) 2 (1.4) 58 (40.8) |
| Education | Medium-low Medium-high |
121 (85.2) 21 (14.8) |
| Employment | Worker Housewife/unemployed |
90 (63.4) 52 (36.6) |
| Smoke | Yes No |
12 (8.5) 130 (81.5) |
| Preexisting diseases | Yes No |
108 (76.1) 34 (23.9) |
| Previous pregnancy | Yes No |
91 (64.1) 51 (35.9) |
| Previous children | Yes No No answer |
58 (40.8) 65 (45.8) 19 (13.4) |
| Previous miscarriage | Yes No No answer |
30 (21.1) 93 (65.5) 19 (13.4) |
| Conception | Spontaneous IVF |
120 (84.5) 22 (15.5) |
| Gestational age during the survey | 29 [19–35] | |
| Trimester during the survey | 1 2 3 Postpartum |
18 (12.7) 33 (23.2) 68 (47.9) 23 (16.2) |
| Still pregnant during the survey | Yes No |
119 (83.8) 23 (16.2) |
| Disease during pregnancy | Yes No |
31 (21.8) 111 (78.2) |
Data are presented as median [interquartile range] or number (percentage).
IVF, in vitro fertilization.
Carbone. SARS-CoV-2 vaccine and pregnancy. Am J Obstet Gynecol MFM 2021.
The median age of the women was 34 years [31–37.25]. For pregnant women, the median gestational age during the survey was 29 weeks [19–35]. Most of the included patients were Italian (96.5%), 1 (0.7%) was from a European country, and 4 (2.8%) were non-European. In our cohort, 58 pregnant women (40.8%) were >35 years old, and 31 (21.8%) had pathologic conditions during pregnancy (pregnancy-induced hypertension, gestational diabetes, threatened miscarriage, and threatened preterm birth) (Table 1).
Most of the included women did not express their agreement to eventually receive SARS-CoV-2 vaccine during pregnancy (40 [28.2%] vs 102 [71.8%]). Interestingly, no patient affirmed to be totally sure about the safety of SARS-CoV-2 vaccine, only 39 patients (27.5%) were quite secure, 69 patients (48.6%) were quite insecure, and 34 patients (23.9%) were totally insecure about vaccine safety (Table 2 ). Furthermore, they affirmed that being pregnant was a determinant factor guiding the eventual choice to accept the vaccine prophylaxis (99 [69.7%] vs 43 [30.3%]; χ2=24.187; P<.001), even if a very large percentage declared to be generally in favor of vaccines (128 [90.1%] vs 14 [9.9%]; χ2=6.091; P=.014) and most of them confirmed they received or would receive other recommended vaccines during pregnancy (75 [52.8%] vs 67 [47.2%]; χ2=10.996, P=.001) (Table 3 ). When we compared maternal characteristics and survey answers according to women's agreement to receive SARS-CoV-2 vaccine in pregnancy, no statistically significant differences were found in relation to nationality, marital status, education, employment, smoke, preexisting diseases, type of conception, pregnancy trimester during the survey, and pregnancy complications during current pregnancy, between women who would undergo SARS-CoV-2 vaccine in pregnancy and women who would not (Table 3). Indeed, women who had a previous pregnancy (irrespective of defining it as a livebirth or a miscarriage) (91 [64.1%] vs 51 [35.9%]; χ2=4.354, P=.037) and women who were still pregnant during the survey (119 [83.8%] vs 23 [16.2%]; χ2=10.904, P=.001) would preferably decline the SARS-CoV-2 vaccine in a statistically significant manner (Table 3).
Table 2.
Perspectives on SARS-CoV-2 vaccine of the included women (Questionnaire Part B)
| Questions | Answers | n (%) |
|---|---|---|
| Agreement to be vaccinated against SARS-CoV-2 | Yes No |
40 (28.2) 102 (71.8) |
| Feeling safe about SARS-CoV-2 vaccine | Totally insecure Rather insecure Safe enough Totally safe |
34 (23.9) 69 (48.6) 39 (27.5) 0 |
| Being pregnant influence the choice | Yes No |
99 (69.7) 43 (30.3) |
| Usually in favor of vaccines | Yes No |
128 (90.1) 14 (9.9) |
| Received or would receive other vaccine during pregnancy (DTaP and/or influenza) | Yes No |
75 (52.8) 67 (47.2) |
Data are presented as number (percentage).
DTaP, diphtheria, tetanus, and acellular pertussis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Carbone. SARS-CoV-2 vaccine and pregnancy. Am J Obstet Gynecol MFM 2021.
Table 3.
Comparison of women characteristics according to their attitude to coronavirus disease 2019 vaccine
| Items | Would get the SARS-CoV-2 vaccine |
Chi-square test | P value | |
|---|---|---|---|---|
| Yes=40 | No=102 | |||
| Nationality - Italian - European - Non-European |
38 (95) 0 2 (5) |
99 (97.2) 1 (0.9) 2 (1.9) |
189.347 | .51 |
| Marital status - Unmarried - Married - Divorced - Separated - No answer |
2 (5) 20 (50) 0 0 18 (45) |
9 (8.9) 51 (50) 1 (0.9) 1 (0.9) 40 (39.3) |
1.562 | .81 |
| Education - Medium-low - Medium-high |
37 (92.5) 3 (7.5) |
84 (82.3) 18 (17.7) |
2.348 | .125 |
| Employment - Worker - Housewife/unemployed |
29 (72.5) 11 (27.5) |
61 (59.8) 41 (40.2) |
1.995 | .158 |
| Smoke - Yes - No |
2 (5) 38 (95) |
10 (9.8) 92 (90.2) |
0.857 | .355 |
| Preexisting diseases - Yes - No |
9 (22.5) 31 (77.5) |
25 (24.5) 77 (75.5) |
0.064 | .801 |
| Previous pregnancy - Yes - No |
31 (77.5) 9 (22.5) |
60 (58.8) 42 (41.2) |
4.354 | .037a |
| Previous children - Yes - No - No answer |
22 (55) 15 (37.5) 3 (7.5) |
36 (35.3) 50 (49) 16 (15.7) |
3.215 |
.073 |
| Previous miscarriage - Yes - No - No answer |
10 (25) 27 (67.5) 3 (7.5) |
20 (19.6) 66 (64.7) 16 (15.7) |
0.2 | .655 |
| Conception - Spontaneous - IVF |
34 (85) 6 (15) |
86 (84.3) 16 (15.7) |
0.01 | .919 |
| Trimester during the survey - 1 - 2 - 3 - Postpartum |
7 (17.5) 9 (22.5) 11 (27.5) 13 (32.5) |
11 (10.8) 24 (23.5) 57 (55.9) 10 (9.8) |
4.732 | .094 |
| Still pregnant during the survey - Yes - No |
27 (67.5) 13 (32.5) |
92 (90.2) 10 (9.8) |
10.904 | .001a |
| Disease during pregnancy - Yes - No |
6 (15) 34 (85) |
25 (24.5) 77 (75.5) |
1.523 | .217 |
| Age of >35 y - Yes - No |
20 (50) 20 (50) |
46 (45) 56 (55) |
0.278 | .598 |
| Preexisting diseases at the age of >35 y - Yes - No |
4 (10) 16 (40) |
12 (11.7) 34 (33.3) |
0.2812 | .59 |
| Preexisting diseases in still pregnant >35-y-olds - Yes - No |
3 (7.5) 12 (30) |
12 (11.7) 31 (30.4) |
0.3626 | .547 |
| Disease during pregnancy at the age of >35 y still pregnant during the survey - Yes - No |
2 (5) 13 (32.5) |
12 (11.7) 31 (30.4) |
1.2899 | .256 |
| Being pregnant influence the choice - Yes - No |
40 (100) 0 |
59 (57.8) 43 (42.1) |
24.187 | .000a |
| Usually in favor of vaccines - Yes - No |
40 (100) 0 |
88 (86.3) 14 (13.7) |
6.091 | .014a |
| Received or would receive other vaccine during pregnancy (DTaP and/or influenza) - Yes - No |
30 (75) 10 (25) |
45 (44.1) 57 (55.9) |
10.996 | .001a |
Data are presented as number (percentage).
DTaP, diphtheria, tetanus, and acellular pertussis; IVF, in vitro fertilization; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Carbone. SARS-CoV-2 vaccine and pregnancy. Am J Obstet Gynecol MFM 2021.
Statistically significant for P<.05.
Performing a subgroup analysis, we evaluated women who were >35 years old who had previous diseases or had complications during pregnancy and were still pregnant during the survey, but there were no statistically significant differences in the eventual SARS-CoV-2 vaccine uptake for these subgroups (Table 3).
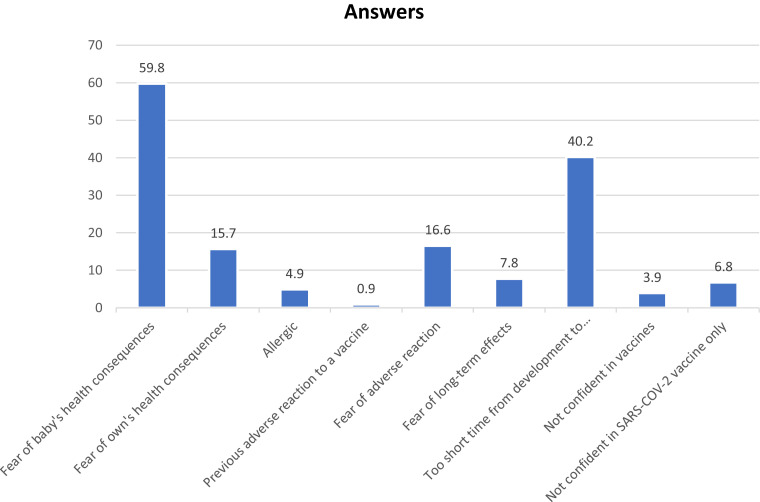
Finally, we asked women who did not want to receive the SARS-CoV-2 vaccine during pregnancy about the reasons for such refusal, and the 2 most frequent answers were “fear of baby's health consequences” in 61 of cases (59.8%) and “too short time from development to commercialization” in 41 of cases (40.2%) (Figure ).
Figure.
Main reason reported by the women to decline SARS-CoV-2 vaccine
Participants could indicate more than 1 reason. Data are presented as percentage.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Carbone. SARS-CoV-2 vaccine and pregnancy. Am J Obstet Gynecol MFM 2021.
Discussion
Main findings
This survey analyzed the grade of acceptance of women during pregnancy and breastfeeding to eventually receive SARS-CoV-2 vaccine during pregnancy after being informed on the basis of actual evidence and recommendations from the main Italian Obstetrics, Gynecology, and Neonatology societies. Our data show that most women were not in favor to receive SARS-CoV-2 vaccine in pregnancy, being overall insecure about the safety of this vaccine, even if most of them were usually in favor to receive vaccines and almost half of them received or would receive other vaccines recommended during pregnancy. Indeed, the state of pregnancy itself has been largely considered as a determinant factor to refuse the SARS-CoV-2 vaccine, combined with the current lack of certainty.
Results in the context of what is known
Although the absolute risk of SARS-CoV-2 severe infection is low, the Centers for Disease Control and Prevention (CDC) has included pregnancy as a risk factor for severe COVID-19 illness.21 Unfortunately, clinical trials for the available vaccines excluded pregnant and lactating women. Therefore, because the safety and efficacy of the vaccines for pregnant women, the fetus, and the newborn remain unknown, there is no general consensus on whether pregnant women should be vaccinated against SARS-CoV-2. The last recommendations of the Royal College of Obstetricians and Gynaecologists in the United Kingdom on SARS-CoV-2 vaccine in pregnancy stated that although the available data do not indicate any safety concern or harm to pregnancy, there is insufficient evidence to recommend the routine use of COVID-19 vaccines during pregnancy, and pregnant women should undergo an individualized and autonomous evaluation with their obstetrician on whether they should receive the vaccine.22
Clinical and research implications
The last recommendations of the United States CDC on SARS-CoV-2 vaccine in pregnancy, issued on February 12, 2021, stated that SARS-CoV-2 vaccine is a messenger RNA (mRNA)–based vaccine that, first, does not contain the live virus that causes COVID-19 and, therefore, cannot transmit COVID-19 to someone and, second, mRNA vaccines do not interact with a person's DNA because the mRNA does not enter the nucleus of the cell.23 Nevertheless, the CDC stated that getting vaccinated is a personal choice for people who are pregnant.23 In January 2021, the European Medicines Agency authorized the use of COVID-19 vaccines and issued a report24 stating that no harmful effects with respect to pregnancy and lactation were observed during animal studies. However, it also declared that the decision to receive the vaccine during pregnancy and lactation should be based on a discussion with the healthcare provider and on a case-by-case basis, taking into consideration benefits and possible risks. Because currently there are no available data on the safety or possible established risks for SARS-CoV-2 vaccination in pregnancy, it seems to be difficult for women and for their doctors to perform and individualized counseling on the possible benefits and risks of the vaccination. The lack of strong recommendations based on evidence for clear safety seems to explain the results of our survey, where 71.8% of women felt unsure on whether eventually undergo SARS-CoV-2 vaccination, 59.8% of women who did not agree for SARS-CoV-2 vaccine were afraid of possible baby's health consequences, and 40.2% of women were afraid of vaccine safety considering the short amount of time from development to commercialization compared with other vaccines.
Authors have pointed out how pregnancy is considered an emotional phase in women life and how it can impact women's mental health even in uneventful pregnancies. Recently, the concept of maternal anxiety has been described, distinct from general anxiety or depression.25 Maternal anxiety is characterized by the fear of real or anticipated threat to pregnancy or its outcomes and low perceived control. Silva et al26 found that 26.8% women with low-risk pregnancies scored a high grade of anxiety, with a greater incidence in the third trimester (42.9%). These results are in line with findings from Giardinelli et al,27 who found that 21.9% of pregnant women in the study group had a high score at Edinburgh Postnatal Depression Scale.
SARS-CoV-2 pandemic, national lockdown, severe restrictions, and safety measures into hospitals and labor wards caused a great impact on pregnant women's emotional balance. Moyer et al28 analyzed 2740 pregnant women and found that 93% of them reported increased stress about getting infected with COVID-19, and Saccone et al29 reported that 68% of pregnant women included in a survey performed a high score of State-Trait Anxiety Inventory scale during the first months of the COVID-19 pandemic; Mappa et al30 observed that 47% had fear of structural anomalies and 51% of preterm birth. These data are consistent with our findings of increased perception of risk of pregnancy or baby outcomes associated with SARS-CoV-2 vaccine in pregnancy, mostly because of the lack of adequate safety data and of strong clear recommendations from the main scientific societies and agencies. In this view, it is of outmost importance the evidence that, even if most women in the survey would not get vaccinated against SARS-CoV-2, 90.1% of them are generally in favor of vaccines and 52.8% of them received or would receive other vaccines strongly and clearly recommended in pregnancy (DTaP or influenza vaccine). Klein et al31 affirmed that it is not ethical to ask pregnant women or their providers to decide whether to get the COVID-19 vaccine or not, given the such limited evidence so far; in addition, they acknowledge the need to include them in phase III trials, especially if preclinical data on safety and toxicology seem encouraging.
Other authors urged to include pregnant and lactating women in future clinical trials on SARS-CoV-2 vaccines in the development and deployment of COVID-19 vaccines and early investment in this field.32 The inclusion of these women will ensure that pregnant women and their infants could benefit from vaccine candidates that prove successful and help ensure that they will ultimately be protected against COVID-19.33 Furthermore, as soon as rigorously designed studies with proactive data collection, recording both vaccine-related symptoms and obstetrical outcomes, will provide evidence-based recommendations regarding mRNA vaccination to reduce harms from COVID-19, expert opinion will be replaced.34 As Heath et al32 stated, to enable the inclusion of pregnant and lactating women in the development of COVID-19 vaccines, it is important to understand whether pregnant women wish to be vaccinated against COVID-19 and also participate in trials for vaccine development.
Strength and limitations
This survey reports women's attitudes toward the vaccination against COVID-19, in the context of lack of definitive data regarding its safety during pregnancy, demonstrating with its results that counseling is fundamental, but data are a very strong added value to reinforce it. A limitation of our analysis depends on the small sample size, which in part is caused by the short amount of time we decided to apply for the distribution of the survey. Another limitation is given by the survey method itself, because we gave the questionnaire after the administration of the abovementioned document produced by our national societies,20 which could eventually be considered too technical by some women, resulting in a higher rate of negative answers.
Conclusions
This survey describes current perspectives in pregnant and breastfeeding women about SARS-CoV-2 vaccine, of which the majority would opt to not get the vaccine, highlighting the need of evidence-based recommendations to guide pregnant women decision to get vaccinated. According to our results, first safety reports on pregnant and lactating women who got vaccinated during these months and more clear indications on SARS-CoV-2 vaccine in pregnancy from scientific societies will increase women adherence rate to the vaccine during pregnancy and lactation, hence reducing maternal morbidity owing to SARS-CoV-2 infection.
Acknowledgments
We sincerely thank all the women who took part in the survey.
Footnotes
G.R. and G.M.M. share last authorship.
The authors report no conflict of interest.
Cite this article as: Carbone L, Mappa I, Sirico A, et al. Pregnant women's perspectives on severe acute respiratory syndrome coronavirus 2 vaccine. Am J Obstet Gynecol MFM 2021;XX;x.ex–x.ex.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ajogmf.2021.100352.
Appendix. Supplementary materials
References
- 1.Chervenak FA, McCullough LB, Grünebaum A, et al. Professionally responsible advocacy for women and children first during the COVID-19 pandemic: guidance from World Association of Perinatal Medicine and International Academy of Perinatal Medicine. J Perinat Med. 2020;48:867–873. doi: 10.1515/jpm-2020-0329. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone IF, Conforti A, Farina A, Alviggi C. A practical approach for the management of obstetric and infertile women during the phase two of the novel coronavirus disease 2019 (COVID -19) pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;251:266–267. doi: 10.1016/j.ejogrb.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alviggi C, Esteves SC, Orvieto R, et al. COVID-19 and assisted reproductive technology services: repercussions for patients and proposal for individualized clinical management. Reprod Biol Endocrinol. 2020;18:45. doi: 10.1186/s12958-020-00605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaiarelli A, Bulletti C, Cimadomo D, et al. COVID-19 and ART: the view of the Italian Society of Fertility and Sterility and Reproductive Medicine. Reprod Biomed Online. 2020;40:755–759. doi: 10.1016/j.rbmo.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picarelli S, Conforti A, Buonfantino C, et al. IVF during coronavirus pandemic: who comes first? The poseidon viewpoint. Int J Gynaecol Obstet. 2020;32:223–228. [Google Scholar]
- 7.Alviggi C, Borini A, Costa M, et al. Sterility Special Interest Group position paper on ART treatments and COVID-19 pandemic. Int J Gynaecol Obstet. 2020;32:154–162. [Google Scholar]
- 8.Harvey S, Zalud I. Obstetric hospital preparedness for a pandemic: an obstetric critical care perspective in response to COVID-19. J Perinat Med. 2020;48:874–882. doi: 10.1515/jpm-2020-0281. [DOI] [PubMed] [Google Scholar]
- 9.WAPM (World Association of Perinatal Medicine) Working Group on COVID-19. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol. 2021;57:232–241. doi: 10.1002/uog.23107. [DOI] [PubMed] [Google Scholar]
- 10.Huntley BJF, Huntley ES, Di Mascio D, Chen T, Berghella V, Chauhan SP. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic Review. Obstet Gynecol. 2020;136:303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 11.Di Mascio D, Sen C, Saccone G, et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by coronavirus disease 2019 (COVID-19): a secondary analysis of the WAPM study on COVID-19. J Perinat Med. 2020;48:950–958. doi: 10.1515/jpm-2020-0355. [DOI] [PubMed] [Google Scholar]
- 12.Carbone L, Esposito R, Raffone A, Verrazzo P, Carbone IF, Saccone G. Proposal for radiologic diagnosis and follow-up of COVID-19 in pregnant women. J Matern Fetal Neonatal Med. 2020:1–2. doi: 10.1080/14767058.2020.1793325. [DOI] [PubMed] [Google Scholar]
- 13.Api O, Sen C, Debska M, et al. Clinical management of coronavirus disease 2019 (COVID-19) in pregnancy: recommendations of WAPM-World Association of Perinatal Medicine. J Perinat Med. 2020;48:857–866. doi: 10.1515/jpm-2020-0265. [DOI] [PubMed] [Google Scholar]
- 14.Afshar Y, Silverman NS, Han CS, Platt LD. Clinical guidance and perinatal care in the era of coronavirus disease 2019 (COVID-19) J Perinat Med. 2020;48:925–930. doi: 10.1515/jpm-2020-0400. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo G, Mappa I, Maqina P, et al. Effect of SARS-CoV-2 infection during the second half of pregnancy on fetal growth and hemodynamics: a prospective study. Acta Obstet Gynecol Scand. 2021 doi: 10.1111/aogs.14130. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giampreti A, Eleftheriou G, Gallo M, et al. Medications prescriptions in COVID-19 pregnant and lactating women: the Bergamo Teratology Information Service experience during COVID-19 outbreak in Italy. J Perinat Med. 2020;48:1001–1007. doi: 10.1515/jpm-2020-0339. [DOI] [PubMed] [Google Scholar]
- 17.Dashraath P, Nielsen-Saines K, Madhi SA, Baud D. COVID-19 vaccines and neglected pregnancy. Lancet. 2020;396:e22. doi: 10.1016/S0140-6736(20)31822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehead CL, Walker SP. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet. 2020;395:e92. doi: 10.1016/S0140-6736(20)31029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nassar AH, Theron G. COVID-19 vaccines and neglected pregnancy: FIGO SMNH Committee Letter of Support. 2020. Available at:https://www.figo.org/sites/default/files/2020-10/covid-19%20vaccines%20and%20pregnancy.pdf. Accessed February 16, 2021.
- 20.Società Italiana di Ginecologia e Ostetricia. Position Paper ad interim VACCINAZIONE ANTI-COVID19 e GRAVIDANZA 2 gennaio 2021. Available at: https://www.sigo.it/wp-content/uploads/2021/01/VaccinoCovid19eGravidanza-SIGO-AOGOI-AGUI-AGITE-SIN_02-01-2021.pdf. Accessed January 3, 2021.
- 21.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royal College of Obstetricians and Gynaecologists . 2020. Updated advice on COVID-19 vaccination in pregnancy and women who are breastfeeding.https://www.rcog.org.uk/en/news/updated-advice-on-covid-19-vaccination-in-pregnancy-and-women-who-are-breastfeeding/ Available at: Accessed February 21, 2021. [Google Scholar]
- 23.Centers for Disease Control and Prevention. Information about COVID-19 vaccines for people who are pregnant or breastfeeding. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html. Accessed February 21, 2021.
- 24.European Medicines Agency . 2020. COVID-19 vaccine moderna (COVID-19 mRNA vaccine [nucleoside modified])https://www.ema.europa.eu/en/documents/overview/covid-19-vaccine-moderna-epar-medicine-overview_en.pdf Available at: Accessed February 21, 2021. [Google Scholar]
- 25.Bayrampour H, Ali E, McNeil DA, Benzies K, MacQueen G, Tough S. Pregnancy-related anxiety: a concept analysis. Int J Nurs Stud. 2016;55:115–130. doi: 10.1016/j.ijnurstu.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Silva MMJ, Nogueira DA, Clapis MJ, Leite EPRC. Anxiety in pregnancy: prevalence and associated factors. Rev Esc Enferm USP. 2017;51:e03253. doi: 10.1590/S1980-220X2016048003253. [DOI] [PubMed] [Google Scholar]
- 27.Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21–30. doi: 10.1007/s00737-011-0249-8. [DOI] [PubMed] [Google Scholar]
- 28.Moyer CA, Compton SD, Kaselitz E, Muzik M. Pregnancy-related anxiety during COVID-19: a nationwide survey of 2740 pregnant women. Arch Womens Ment Health. 2020;23:757–765. doi: 10.1007/s00737-020-01073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saccone G, Florio A, Aiello F, et al. Psychological impact of coronavirus disease 2019 in pregnant women. Am J Obstet Gynecol. 2020;223:293–295. doi: 10.1016/j.ajog.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mappa I, Distefano FA, Rizzo G. Effects of coronavirus 19 pandemic on maternal anxiety during pregnancy: a prospectic observational study. J Perinat Med. 2020;48:545–550. doi: 10.1515/jpm-2020-0182. [DOI] [PubMed] [Google Scholar]
- 31.Klein SL, Creisher PS, Burd I. COVID-19 vaccine testing in pregnant females is necessary. J Clin Invest. 2021;131 doi: 10.1172/JCI147553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heath PT, Le Doare K, Khalil A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect Dis. 2020;20:1007–1008. doi: 10.1016/S1473-3099(20)30638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianchi DW, Kaeser L, Cernich AN. Involving pregnant individuals in clinical research on COVID-19 vaccines. JAMA. 2021;325:1041–1042. doi: 10.1001/jama.2021.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhikari EH, Spong CY. COVID-19 vaccination in pregnant and lactating women. JAMA. 2021;325:1039–1040. doi: 10.1001/jama.2021.1658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.