Highlights
-
•
Amygdala & striatal neural activity may underlie Social Anxiety Disorder (SAD).
-
•
80 individuals with SAD completed an emotion processing task during fMRI.
-
•
Dorsal striatal & amygdala response to angry > happy related to illness severity.
-
•
Activity in these regions may contribute to individual differences in SAD.
Keywords: Anxiety, Emotion processing, Neuroimaging, fMRI, Reward, Threat
Abstract
Social anxiety disorder (SAD) is a common heterogeneous disorder characterized by excessive fear and deficient positive experiences. Case-control emotion processing studies indicate that altered amygdala and striatum function may underlie SAD; however, links between these regions and symptomatology have yet to be established. Therefore, in the current study, 80 individuals diagnosed with SAD completed a validated emotion processing task during functional magnetic resonance imaging. Anatomy-based regions of interest were amygdala, caudate, putamen, and nucleus accumbens. Neural activity in response to angry > happy faces and fearful > happy faces in these regions were submitted to multiple linear regression analysis with bootstrapping. Additionally, multiple linear regression analysis was performed to explore clinical features of SAD. Results showed greater putamen activity and less amygdala activity in response to angry > happy faces were related to greater social anxiety severity. In the model consisting of caudate and amygdala activity in response to angry > happy faces, results were marginally related to social anxiety severity and the pattern of activity was similar to the regression model comprising putamen and amygdala. Nucleus accumbens activity was not related to social anxiety severity. There was no correspondence between brain activity in response to fearful > happy faces and social anxiety severity. Clinical variables revealed greater levels of anhedonia and general anxiety were related to social anxiety severity, however, neural activity was not related to these features of SAD. Neuroimaging findings suggest that variance in dorsal striatal and amygdala activity in response to certain social signals of threat contrasted with an approach/rewarding social signal may contribute to individual differences in SAD. Clinical findings indicate variance in anhedonia and general anxiety symptoms may contribute to individual differences in social anxiety severity.
1. Introduction
Social anxiety disorder (SAD) is a prevalent disorder affecting>15 million adults in the United States (Kessler et al., 2005) and>155 million adults worldwide in the past year alone (Stein et al., 2017). Therefore, SAD is a major public health concern (Hambrick et al., 2003, Safren et al., 1996, Schneier et al., 1994). SAD has an early age of onset (i.e., 80% of individuals develop SAD by 20 years old; Stein and Stein, 2008), follows a chronic course (Bandelow et al., 2015), is more common in women than men (Asher et al., 2017), and often precedes and is a risk factor for other psychiatric illnesses such as major depressive disorder (Beesdo et al., 2007, Stein et al., 2001). Hallmarks of SAD are excessive fear and avoidance behaviors (or endurance of distress if not engaging in avoidance) in various situations that involve potential negative evaluation by others (American Psychiatric Association, 2013). More recently, however, it is also recognized that individuals with SAD have less positive emotional experiences compared to individuals with other anxiety disorders (Richey et al., 2019). There is also evidence of links between SAD and anhedonia, not explained by depression, indicating that motivation and reward functioning may be disrupted in SAD (Kashdan, 2007).
Due to fears of embarrassment, disapproval, or rejection, threatening faces are especially motivationally relevant in SAD (Staugaard and Rosenberg, 2011). Individuals with SAD may also perceive positive socio-emotional cues (i.e., happy faces) as less approachable (Campbell et al., 2009) or more negative (e.g., mocking, untrustworthy) (Gutiérrez-García and Calvo, 2016, Hunter et al., 2009) than individuals without social anxiety. Findings are consistent with longstanding cognitive models of anxiety that propose threat bias plays a key role in the development and maintenance of excessive anxiety (Beck and Clark, 1997). Yet, despite the emphasis on threat bias, anxious individuals vary considerably in their response to threatening stimuli (Bar-Haim et al., 2007) pointing to heterogeneity in threat processing.
In light of social fears, neuroimaging work aimed at elucidating the brain pathophysiology of SAD has frequently examined amygdala response to threatening facial expressions. Accumulating case-control findings indicate an amplified ‘fear circuit’ involving amygdala underlies SAD (Brühl et al., 2014, Etkin and Wager, 2007). However, individual differences in amygdala reactivity to socio-emotional signals of threat relative to social signals of reward in SAD is less clear, as studies have largely focused on case-control characterization.
Associations between amygdala activitation to the processing of threatening faces and the severity of social anxiety symptoms have been mixed. Some studies show a positive correlation (Ball et al., 2012, Phan et al., 2006), while others show no correlation (Klumpp et al., 2010, Ziv et al., 2013). Inconsistent findings may be due in part to methodological differences across studies, including neuroimaging tasks and sample characteristics (e.g., students, patients, comorbidity). Therefore, further study is warranted. Moreover, the amygdala is part of an emotion processing network (Fusar-Poli et al., 2009, Skelly and Decety, 2012) that includes the striatum. The striatum is a component of the basal ganglia circuit that encompasses the caudate, putamen, and nucleus accumbens (‘NAcc’) (Marchand, 2010) and is involved in emotion recognition (Fusar-Poli et al., 2009), reward/approach responses (Ernst and Spear, 2009), and avoidance behavior (Darvas et al., 2011). Until recently, the striatum has not been thought of as an important brain region underlying anxiety (Lago et al., 2017). Yet, resting-state data shows a positive functional relationship between the amygdala and striatum (Roy et al., 2009).
Neuroimaging studies of emotion processing suggest the caudate and putamen are altered in SAD. For example, individuals with SAD exhibit greater putamen activation in response to threat-relevant images (e.g., job interview, giving a speech, etc.) (Heitmann et al., 2016) compared to healthy controls. Also, we observed increased caudate activity in response to threatening > happy faces (Klumpp et al., 2012) and greater putamen activity in response to threatening faces of moderate intensity in SAD relative to healthy controls (Klumpp et al., 2010). In a study by Brühl et al. (2011), patients with SAD showed exaggerated caudate activation in anticipation of negative > neutral images compared to healthy controls. In a similar study, patients with SAD or obsessive–compulsive disorder exhibited more caudate activity in response to negative > neutral images and more caudate activity in anticipation of viewing positive or negative emotional > neutral images compared to healthy participants (Weidt et al., 2016). Furthermore, since neural function maps on to brain structure to some extent (Greicius et al., 2009, Honey et al., 2010), it is notable that a recent volumetric mega-analysis of brain structure found larger gray matter volume in the putamen in individuals with SAD relative to healthy controls and among participants with SAD, social anxiety severity positively correlated with putamen volume (Bas-Hoogendam et al., 2017). However, in contrast to hypotheses, no significant amygdala effects were detected.
Therefore, the primary objective of the current study was to extend the literature by using multivariate regression analysis to examine individual differences in striatal and amygdala response to emotional facial expressions in SAD. Based on the literature, we hypothesized greater caudate, putamen, and amygdala activity in response to threatening faces would be related to greater symptom severity in SAD. Although prior emotion processing findings do not suggest ventral striatal (i.e., NAcc) activity in response to threatening faces > happy faces is disrupted in SAD (Brühl et al., 2011, Klumpp et al., 2010, Klumpp et al., 2012, Weidt et al., 2016), we included the NAcc for comprehensiveness. A secondary goal was to elucidate symptoms (e.g., general anxiety, depression) that may correspond to social anxiety severity and explore whether such symptoms also corresponded to striatal and amygdala activity in response to emotional faces. We expected higher levels of general anxiety and depression would correspond with greater social anxiety severity. Additionally, in light of links between social anxiety and anhedonia (Kashdan, 2007), we hypothesized higher levels of anhedonia would be related to greater social anxiety severity. For comprehensiveness, we also evaluated melancholic symptoms, although we did not expect such symptoms would correspond to severity of SAD symptoms due to lack of evidence.
2. Materials and methods
2.1. Participants
The study consisted of 80 un-medicated treatment-seeking adults with SAD. Participants were recruited from the Mood and Anxiety Clinic at the University of Illinois at Chicago (UIC) via referrals and in the community through flyers and internet advertisements. Interested participants completed a phone screen followed by a psychiatric evaluation during which time they reviewed the consent form as approved by the local Institutional Review Board at UIC.
After attaining consent, all participants completed the Structured Clinical Interview (SCID-5; American Psychiatric Association, 2015) conducted by a trained master’s level clinician in addition to clinician-administered measures, which included the Hamilton Depression Rating Scale (HAMD; Hamilton, 1960), Hamilton Anxiety Rating Scale (HAMA; Hamilton, 1959) and Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987). Overall social anxiety severity indexed with the LSAS total score was in the moderate-to-severe range (81.1 ± 17.0) (Liebowitz, 1987), depression level was in the mild range as assessed with HAMD (9.1 ± 5.0) (Hamilton, 1960), and anxiety level was in the mild range as assessed with HAMA (14.63 ± 7.61) (Hamilton, 1959). Self-report measures included the Beck Depression Inventory (BDI-II; Beck and Steer, 1987), which indicated depression was in the mild-to-moderate range (22.2 ± 9.6). Consistent with previous studies (e.g., Pizzagalli et al., 2005), BDI-II items were used to examine anhedonic and melancholic symptoms. Specifically, anhedonia comprised the sum of the following items: loss of pleasure (Item #4), loss of interest (Item #12), loss of energy (Item #15), and loss of interest in sex (Item #21) (Pizzagalli et al., 2005). Regarding melancholic symptoms, the following items were summed: loss of pleasure (item #4), guilty feelings (item #5), agitation (item #11), loss of interest (item #12), early morning awakening (item #16b), and loss of interest in sex (item #21) (Pizzagalli et al., 2005).
Inclusion criteria for all participants were as follows: adults ages 18 to 65 years, inclusive, and free of major medical or neurologic illness as confirmed by a Board-Certified physician. Exclusion criteria for all participants were as follows: inability to provide consent; current or history of psychotic symptoms, bipolar disorder, traumatic brain injury, mental retardation, pervasive developmental disorder, or dementia; active suicide ideation; current alcohol or substance dependence (within the 6 months of study); treatment (e.g., pharmacotherapy, psychotherapy); and contraindications to magnetic resonance imaging (e.g., pregnancy, non-removable ferrous objects, claustophobia). None of the participants tested positive on a urine toxicology screen before scans.
Diagnosis was determined by SCID and a Best-Estimate/Consensus Panel of study staff members (e.g., clinical psychologist, psychiatrist, clinical assessor). Comorbidity was permitted. See Table 1 for clinical and demographic characteristics and comorbid diagnoses. A portion of participants were previously reported (Gorka et al., 2019, Klumpp et al., 2013, MacNamara et al., 2017, Phan et al., 2013), however, the research topics differed from the current study.
Table 1.
Clinical and demographic descriptives.
| Clinical Measures | M | SD |
|---|---|---|
| LSAS | 81.08 | 17.03 |
| HAMA | 14.63 | 7.61 |
| HAMD | 9.11 | 4.96 |
| Anhedonia | 3.84 | 2.25 |
| Melancholic | 6.13 | 5.94 |
| Demographics | M | SD |
| Age (range 18–46 yrs) | 24.76 | 10.02 |
| N | % | |
| Gender | ||
| Female | 57 | 71.3 |
| Male | 23 | 28.8 |
| Race | ||
| Caucasian | 51 | 63.8 |
| Asian | 15 | 18.8 |
| African American | 6 | 7.5 |
| More than one race | 5 | 6.3 |
| Other/Unknown | 2 | 2.5 |
| American Indian/Alaskan Native | 1 | 1.3 |
| Ethnicity | ||
| Hispanic/Latino | 21 | 26.3 |
| Medication History | 32 | 40.0 |
| Comorbidity | N | % |
| Generalized anxiety disorder | 33 | 50 |
| Major depressive disorder | 31 | 47.0 |
| Panic disorder | 16 | 24.2 |
| Specific phobia | 16 | 24.2 |
| Persistent depressive disorder | 11 | 16.7 |
| Post-traumatic stress disorder | 6 | 9.1 |
| Eating disorder | 5 | 7.6 |
| Alcohol abuse | 3 | 4.6 |
| Adjustment disorder | 1 | 1.5 |
| Attention deficit hyperactivity disorder | 1 | 1.5 |
| Obsessive compulsive disorder | 1 | 1.5 |
LSAS = Liebowitz Social Anxiety Scale; HAMA = Hamilton Anxiety Rating Scale; HAMD = Hamilton Depression Rating Scale; Anhedonia = BDI-II sub-score; Melancholic = BDI-II sub-score.
All participants were compensated for their time and all procedures complied with the Helsinki Declaration.
All analyses were two-tailed with an alpha level of 0.05 and performed in the Statistical Package for the Social Sciences (Chicago, IL; Version 24).
2.2. fMRI task
All participants performed an Emotional Face Matching Task shown to engage regions involved in emotion processing (Gorka et al., 2019, Klumpp et al., 2013, MacNamara et al., 2017, Phan et al., 2013). Participants viewed a trio of faces (one target at the top, two probes on the bottom) consisting of photographs from a validated set of face stimuli (Gur et al., 2002) presented in a block design. The target congruent probe faces displayed one of three expressions (happy, fearful, angry),1 while the other (incongruent) probe face always displayed a neutral expression. The task was one run with 18 blocks in total, consisting of 9 blocks of matching emotional faces (3 blocks for each target angry, fear, or happy facial expression) interleaved with 9 blocks of matching shapes (triangles, circles, squares) as a sensorimotor control “baseline” condition. There was no rest period between blocks; immediately after one block ended, the next block began. Prior to performing the task in the scanner, participants completed practice trials consisting of one matching faces block and one matching shapes block. Faces used in the practice session were not used in the experimental session. During the scan, participants viewed a blank black screen for 8 s at the beginning of the task. Each block lasted 20 s and comprised four back-to-back 5-second trials with no inter-trial interval.
The primary contrast of interest was threatening > happy faces. Since the emotional content conveyed by a facial expression may influence neural response (Gorka et al., 2019, Klumpp et al., 2013, MacNamara et al., 2017, Phan et al., 2013), primary contrasts of interest were angry > happy faces and fear > happy faces.
2.3. fMRI data acquisition
Functional imaging was performed with blood-oxygen-level-dependent (BOLD) sensitive whole-brain fMRI on a 3.0 Tesla GE Discovery System (General Electric Healthcare; Waukesha, WI) with an 8-channel head coil. Functional data were acquired using gradient-echo echo planar imaging sequence with the following parameters: TR = 2 s, TE = 0.0222 s, flip angle = 90°, FOV = 22 × 22 cm2, acquisition matrix 64 × 64, 3-mm slice thickness, 44 axial slices, and 180 volumes per run.
2.4. fMRI data preprocessing
Data from all participants were required to meet quality control parameters (i.e., <3 mm in any direction or 3 degrees of displacement across the functional run). Before the data was preprocessed, the first 4 volumes from were discarded to allow for T1 equilibration effects. Conventional preprocessing steps were used in Statistical Parametric Mapping (SPM8) software package (Wellcome Trust Centre for Neuroimaging, London www.fil.ion.ucl.ac.uk/spm). The scans were preprocessed using the following conventional steps: 1) temporally/slice-time, motion corrected; 2) warped (non-linear) based on the parameters of warping the mean T2* volume to the EPI template, a canonical brain in Montreal Neurologic Institute (MNI) space; 3) resampled to 2 mm3 voxels; and 4) smoothed with an 8 mm isotropic Gaussian kernel.
2.5. Statistical analysis of fMRI data
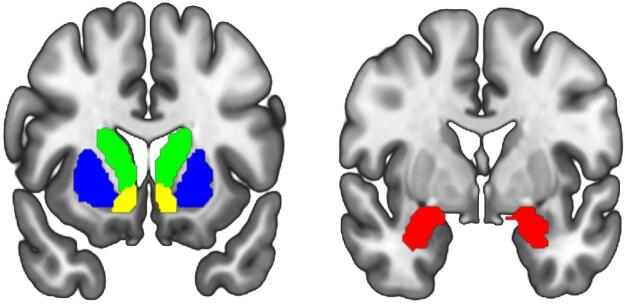
Regarding first-level analysis, we modelled task conditions as a box-car model convolved with the HRF as regressors and had the six motion parameters, outputted from the motion correction step, as covariates to account for motion artifacts. The time series was processed with a 128-second high pass filter and corrected for serial correlation using an autoregressive AR(1) model during Classical (ReML) parameter estimation. Contrasts of interest (angry > happy faces; fear > happy faces) were generated for each participant. The Automatic Anatomical Labeling Atlas 3 (Rolls et al., 2020) was used to select anatomically-based regions of interest (ROIs), which were bilateral caudate (volume = 15,672 mm3), bilateral putamen (volume = 16,584 mm3), bilateral NAcc (volume = 8,576 mm3), and bilateral amygdala (volume = 3,744 mm3) (Fig. 1). The mean ROI-extracted contrast estimates of activation (β weights, arbitrary units [a.u.]) were subsequently submitted to multiple linear regression analysis.
Fig. 1.
Illustration of anatomy-based regions of interest; green = caudate, blue = putamen, yellow nucleus accumbens, red = amygdala. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.6. Social anxiety measure
The Liebowitz Social Anxiety Scale (Liebowitz, 1987) is a 24-item measure that assesses social and performance fears and related avoidance behaviors. Therefore, the total score is based on 4 sub-scales: Social Fear, Performance Fear, Social Avoidance, Performance Avoidance such that higher scores represent more fear and avoidance (Liebowitz, 1987). The LSAS is one of the most commonly used clinician-administered measures to evaluate SAD and has been shown to have acceptable psychometric properties (Heimberg et al., 1999). However, the discriminability between LSAS fear and LSAS avoidance is generally weak calling into question the utility of these sub-scores (Heimberg et al., 1999). Therefore, regression analysis was based on the LSAS total score as the dependent variable though all relationships between independent variables and sub-scores are reported.
2.7. Data analysis
To verify participants followed task instructions, we collapsed across emotional faces and report average accuracy and standard deviation. To test for potential differences between emotional faces, accuracy and reaction time when matching for angry, fear, and happy faces were submitted to an Analysis of Variance (ANOVA) with repeated measures.
Multiple linear regression analysis (simultaneous entry) with bootstrapping (based on 1000 samples) was conducted to examine the extent to which variance in a priori ROI-extracted contrast estimates of activation (β weights, arbitrary units [a.u.]) related to social anxiety severity (neural model). The same analysis was conducted to examine the extent to which variance in general anxiety and depression symptoms related to social anxiety severity (clinical model). Post-hoc multiple linear regression analysis was performed to evaluate whether clinical variables (e.g., general anxiety, depression) that were significantly associated with social anxiety severity related with ROI-extracted contrast estimates of activation (secondary model).
To reduce collinearity among neural variables, one model consisted of striatal and amygdala activity in response to angry > happy faces and a separate model for fear > happy faces. All independent variables were standardized (z-score). To evaluate whether collinearity was acceptable, tolerance was required to be > 2.0 (Allison, 1999, Weisburd and Britt, 2013).
3. Results
3.1. Task performance
Average accuracy across emotional faces was high (94% ± 6%). When exploring potential emotional face effects, ANOVA results revealed a significant effect of emotional face type (angry, fear, happy) [F(2,158) = 50.57, p < 0.005]. Follow-up paired t-tests showed participants were more accurate when matching happy faces (98% ± 6%) than angry faces (87% ± 10%) [t(79) = 9.11, p < 0.005] and fear faces (96% ± 8%) [t(79) = 2.17, p = 0.033]. Moreover, participants were more accurate when matching fear faces relative to angry faces [t(79) = 6.75, p < 0.005].
When examining reaction time (RT) in milliseconds (ms) for accurate trials, ANOVA results revealed a significant effect of emotional face type (angry, fear, happy) [F(2,158) = 20.53, p < 0.005]. Follow-up paired t-tests showed participants were faster when matching happy faces (1300.37 ± 299.19 ms) than angry faces (1442.29 ± 418.31 ms) [t(79) = 4.27, p < 0.005] and fear faces (1505.07 ± 393.25 ms). RT between angry and fear faces was not significant [t(79) = 1.73, p = 0.09].
3.2. Multiple linear regression
3.2.1. Angry > happy faces
With LSAS total score as the dependent variable (DV) and bilateral caudate, putamen, NAcc, and amygdala activation as independent variables (IVs), the omnibus result was significant [R2 = 0.135, F(4,75) = 2.932, p = 0.026]. However, tolerance for caudate and putamen was < 2.0, therefore, collinearity was a concern. Pearson’s correlation revealed a significant positive association between caudate and putamen activation (r = 0.863, p < 0.005). Thus, subsequent analyses were performed separately for caudate and putamen.
When the same analysis was performed with bilateral caudate, NAcc, and amygdala ROIs, the model was significant [R2 = 0.104, F(3,76) = 2.943, p = 0.038] (tolerance was > 2.0). Results showed caudate (B = 7.049, p = 0.026) and amygdala activation (B = -5.698, p = 0.017), but not NAcc activation (B = -1.741, p = 0.574), were related to social anxiety severity. When the same analysis was repeated with bilateral putamen, NAcc, and amygdala activation, the model was significant [R2 = 0.132, F(3,76) = 3.856, p = 0.013] (tolerance was > 2.0). Putamen (B = 8.966, p = 0.007) and amygdala activation (B = -8.801, p = 0.002), but not NAcc activation (B = -0.817, p = 0.755), were related to social anxiety severity.
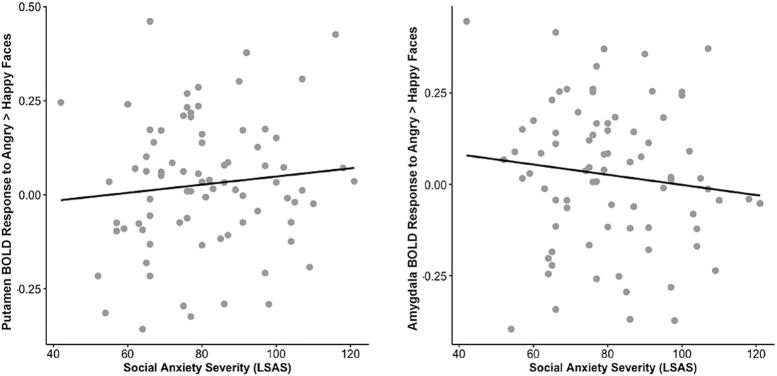
The scatterplot comprising significant variables (putamen, caudate, amygdala) from the regression model suggested a potential outlier. When removed, results remained significant [R2 = 0.082, F(2,76) = 3.401, p = 0.038] (tolerance > 2.0) as putamen (B = 7.752, p = 0.012) and amygdala activation (B = -8.387, p = 0.014) were related to social anxiety severity (Fig. 2). However, when the model comprised caudate and amygdala activation, the omnibus result was not significant [R2 = 0.050, F(2,76) = 2.006, p = 0.142] (tolerance > 2.0) as caudate (B = 4.731, p = 0.052) and amygdala activation (B = -5.484, p = 0.054) were marginally related to social anxiety severity.
Fig. 2.
Scatter plots depicting significant relationship between putamen and amygdala response to angry > happy facial expressions and social anxiety severity measured with the Liebowitz Social Anxiety Scale with a putamen response on the left and amygdala response on the right.
Table 2 shows Pearson’s correlation coefficients for all dependent and independent variables for all LSAS sub-scores (uncorrected for multiple comparisons).
Table 2.
Correlation coefficients.
| Contrast of Interest Regions of Interest | Social Fear | Performance Fear | Social Avoidance | Performance Avoidance | Anhedonia | HAMA |
|---|---|---|---|---|---|---|
| Angry (vs. Happy) | ||||||
| L Caudate | 0.245* | 0.066 | 0.100 | −0.025 | 0.152 | 0.063 |
| R Caudate | 0.257* | 0.138 | 0.102 | 0.027 | 0.133 | 0.129 |
| L Putamen | 0.279* | 0.040 | 0.127 | −0.093 | 0.049 | 0.068 |
| R Putamen | 0.252* | 0.093 | 0.109 | −0.073 | 0.118 | 0.168 |
| L Nucleus Accumbens | 0.137 | −0.077 | 0.059 | −0.107 | 0.162 | 0.057 |
| R Nucleus Accumbens | 0.156 | 0.018 | 0.062 | −0.014 | 0.136 | 0.072 |
| L Amygdala | 0.027 | −0.180 | −0.094 | −0.325** | 0.134 | 0.165 |
| R Amygdala | 0.086 | −0.107 | −0.008 | −0.255* | 0.142 | 0.202 |
| Fear (vs. Happy) | ||||||
| L Caudate | 0.118 | −0.031 | 0.038 | −0.058 | 0.086 | 0.031 |
| R Caudate | 0.121 | 0.034 | 0.017 | −0.020 | 0.055 | 0.034 |
| L Putamen | 0.088 | 0.063 | 0.019 | −0.082 | −0.026 | −0.103 |
| R Putamen | 0.062 | −0.018 | −0.026 | −0.052 | −0.020 | −0.038 |
| L Nucleus Accumbens | 0.096 | −0.088 | −0.004 | −0.092 | 0.052 | −0.015 |
| R Nucleus Accumbens | 0.067 | −0.058 | −0.039 | −0.036 | 0.073 | −0.010 |
| L Amygdala | −0.053 | −0.166 | −0.096 | −0.168 | −0.040 | −0.107 |
| R Amygdala | 0.053 | −0.067 | −0.014 | −0.056 | 0.060 | −0.054 |
| Measures of Interest | Social Fear | Performance Fear | Social Avoidance | Performance Avoidance |
|---|---|---|---|---|
| HAMA | 0.307** | 0.245* | 0.328** | 0.262* |
| HAMD | 0.200 | 0.184 | 0.265* | 0.247* |
| Anhedonia | 0.221* | 0.322** | 0.297** | 0.277* |
| Melancholic | 0.181 | 0.239** | 0.227* | 0.204 |
| Age | −0.114 | 0.100 | −0.061 | 0.202 |
| Gender | 0.001 | −0.080 | −0.038 | −0.138 |
L = left hemisphere; R = right hemisphere.
HAMA = Hamilton Anxiety Rating Scale; HAMD = Hamilton Depression Rating Scale; Anhedonia = BDI-II sub-score; Melancholic = BDI-II sub-score; Social Fear = Liebowitz Social Anxiety Scale sub-score.
Performance Fear = Liebowitz Social Anxiety Scale sub-score; Social Avoidance = Liebowitz Social Anxiety Scale sub-score; Performance Avoidance = Liebowitz Social Anxiety Scale sub-score.
*p < 0.05.
**p < 0.01.
3.2.2. Fear > happy faces
With LSAS total score as the DV and bilateral caudate, NAcc, and amygdala activation as IVs, the omnibus result was not significant [R2 = 0.028, F(3,76) = 0.726, p = 0.539] (tolerance > 2.0) and none of the IVs were significant (lowest p = 0.294). When the model consisted of bilateral putamen, NAcc, and amygdala activation, the model was not significant [R2 = 0.025, F(3,76) = 0.650, p = 0.585] (tolerance > 2.0) and none of the ROIs were related to social anxiety severity (lowest p = 0.175).
Table 2 shows Pearson’s correlation coefficients for all dependent and independent variables for all LSAS sub-scores (uncorrected for multiple comparisons).
3.2.3. Clinical variables
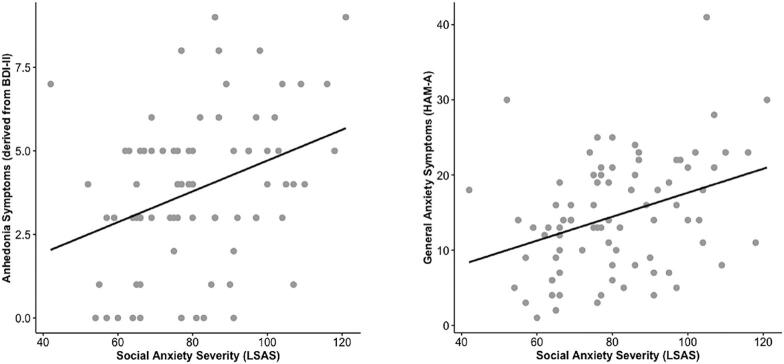
With LSAS total score as the DV and general anxiety (HAMA), general depression (HAMD), anhedonia symptoms (derived from BDI-II), melancholic symptoms (derived from BDI-II) as IVs showed the omnibus result was significant [R2 = 0.190, F(4,75) = 4.404, p = 0.003] (tolerance > 2.0). Anhedonia (B = 7.450, p = 0.015) and general anxiety (B = 5.178, p = 0.031) but not melancholic symptoms (p = 0.230) or general depression (HAMD) (p = 0.937) were related to social anxiety severity (see Fig. 3).
Fig. 3.
Scatter plot illustrating that anhedonia symptoms, assessed with certain items in the Beck Depression Inventory (on the left), and general anxiety indexed with the Hamilton Anxiety Rating Scale (on the right) were significantly related to social anxiety severity measured with the Liebowitz Social Anxiety Scale.
3.2.4. Post-hoc analysis
Since anhedonia (derived from BDI-II) and general anxiety (HAMA) were significantly related to social anxiety severity, multiple linear regression analysis was performed to evaluate the extent to which neural activity corresponded with anhedonia and general anxiety symptoms. Specifically, in one model anhedonia was the DV and in another model general anxiety was the DV. In both models bilateral caudate, NAcc, and amygdala activity in response to angry > happy faces were the IVs.
When anhedonia was the DV, the omnibus result was not significant [R2 = 0.028, F(3,76) = 0.741, p = 0.531] (tolerance was > 2.0) nor were any of the IVs (lowest p = 0.606). When the same analysis was performed with bilateral putamen (but not caudate) activation, the model was not significant [R2 = 0.036, F(3,76) = 0.948, p = 0.422] (tolerance was > 2.0) nor were any of the IVs (lowest p = 0.294). Similarly, omnibus results were not significant for striatal and amygdala activation in response to fear > happy faces (lowest p = 0.530) and neural activity was not related to anhedonia in any model (lowest p = 0.172).
When general anxiety was the DV and bilateral caudate, NAcc, and amygdala activity in response to angry > happy faces were IVs, the omnibus result was not significant [R2 = 0.032, F(3,76) = 0.831, p = 0.481] (tolerance was > 2.0) nor were any of the IVs (lowest p = 0.387). When the same analysis was performed with bilateral putamen (but not caudate) activation, the model was not significant [R2 = 0.029, F(3,76) = 0.766, p = 0.156] (tolerance was > 2.0) nor were any of the IVs (lowest p = 0.484). Also, omnibus results for striatal and amygdala activity in response to fear > happy faces was not related to general anxiety (lowest p = 0.367) nor did activation in any region correspond with general anxiety (lowest p = 0.074).
Table 2 shows Pearson’s correlation coefficients for all dependent and independent variables for all LSAS sub-scores (uncorrected for multiple comparisons).
4. Discussion
The primary objective of the present study was to examine the extent to which striatal and amygdala activity in response to social signals of threat, compared to social signals of approach/reward, were related to social anxiety severity in un-medicated patients with SAD. Results revealed bilateral putamen, caudate, and amygdala activation corresponded with social anxiety severity such that greater putamen and caudate activity in response to angry > happy faces was related to higher levels of social anxiety. Unexpectedly, less amygdala activation in response to angry > happy faces corresponded with higher levels of social anxiety when taking striatal activation into consideration. Individual differences in NAcc activation in response to angry > happy faces was not related to social anxiety severity. Our secondary goal was to explicate clinical features of SAD and explore whether striatum and amygdala activation were related to symptoms outside of social anxiety. We observed higher levels of anhedonia and general anxiety, but not melancholy or general depression, corresponded with social anxiety severity. Nonetheless, variance in striatal and amygdala activity in response to angry > happy faces did not significantly explain anhedonia or general anxiety. Overall task performance accuracy was high indicating participants followed task instructions.
Our hypothesis that greater caudate, putamen, and amygdala activity in response to threatening faces would correspond with greater symptom severity in SAD was partially supported. We found that caudate and putamen activation were highly correlated, therefore, the contribution of these regions on social anxiety severity were evaluated separately, namely, one model comprised caudate and amygdala activation and another model consisted of putamen and amygdala activation. Our results indicate that more caudate and putamen activation in response to angry > happy faces and less amygdala activation in response to angry > happy faces corresponded with greater social anxiety severity. However, when removing a potential outlier, caudate and amygdala activity in response to angry > happy faces was only marginally related to social anxiety severity.
Our dorsal striatal findings extend previous case-control studies that have shown individuals with SAD demonstrate greater putamen activity in response to threat-related images (Heitmann et al., 2016), greater caudate and putamen activity to threatening faces (Klumpp et al., 2010, Klumpp et al., 2012), and more caudate activation in anticipation of negative > neutral images compared to healthy controls (Brühl et al., 2011, Weidt et al., 2016). Outside of SAD, negative images > positive images or negative images > neutral images are reported to engage the caudate in healthy participants (Carretié et al., 2009) and the putamen has been implicated in the sensory features of pain (Starr et al., 2011). Taken together, results provide support that the striatum is involved in aversive processing in addition to appetitive or rewarding processing (Levita et al., 2009, Roiser et al., 2008). Relevant to the current study, the dorsal striatum, which encompasses the caudate and putamen (Marchand, 2010) is involved in social and emotional processes (Balleine et al., 2007, Marchand, 2010, Shohamy, 2011, Stathis et al., 2007). Thus, findings suggest individuals with greater levels of social anxiety severity may be more sensitive to negative socio-emotional signals when contrasted with positive socio-emotional cues, suggesting heterogeneity of SAD may be due in part to differential variance in dorsal striatum engagement.
Although symptom severity was partly explained by amygdala activity in response to angry > happy faces, the direction was not expected. Specifically, we found that more amygdala activation in response to happy > angry faces was related to greater social anxiety severity. While this finding was unexpected based on prior studies (Brühl et al., 2014), individuals with SAD have demonstrated increased activation in response to happy faces compared to healthy controls (Straube et al., 2005). It is possible that our finding pertains to the salience of happy faces, which were easier to match than angry faces, as evidenced by higher accuracy and faster response times. Salient faces, regardless of emotional valence, engage the amygdala (Santos et al., 2011). Thus, happy faces may have been relatively more striking than angry faces for some patients in our cohort due to the inherent conflict happy faces represent given socially anxious individuals both want and fear social interaction (Stein and Stein, 2008). Higher levels of social anxiety have been shown to be associated with lower approachability ratings for happy faces among individuals with SAD (Campbell et al., 2009). However, since our study did not collect such ratings or ratings concerning other factors that may have contributed to results (e.g., ratings of arousal or valence of faces), our interpretation is speculative.
Importantly, results were significant when happy faces were contrasted with angry faces, but not with fearful faces. Angry faces convey direct interpersonal aggression whereas the threat signal for fearful faces is more ambiguous (Biehl et al., 1997, Ekman, 1994). In addition to differences in socio-emotional content, angry faces are seen more often than fearful faces (Bond and Siddle, 1996, Whalen, 1998). Prior exposure to angry faces coupled with a block design, which does not control for habituation effects, may also have contributed to results. Indeed, individuals differ in their rate of habituation to stimuli (Blackford et al., 2013) and data show individual differences in emotion-dependent amygdala habituation are reliable (Plichta et al., 2014). Thus, it is also possible that results reflect variance in differential habituation between angry and happy faces. Our study was not designed to test this, therefore, it will be important for future investigations to examine possible habituation effects.
Clinically, higher levels of anhedonia and general anxiety were related to greater social anxiety severity. Comorbidity was permitted and many of the participants were diagnosed with a concurrent major depressive disorder and/or another anxiety disorder. Consequently, it is not surprising that depression and general anxiety symptoms were associated with social anxiety symptoms. As anticipated, higher levels of anhedonia, the reduction of the ability to feel pleasure, but not melancholy was related to social anxiety severity. Social anxiety is associated with diminished positive experiences, not explained by depression, which may be due to an amplified negative valence system and corresponding expenditure of regulatory resources to manage fear or a more general impairment in reward-related processes (Kashdan, 2007). Our results provide further support that anhedonia is a feature of SAD. Even so, neither anhedonia nor general anxiety were related to striatal or amygdala activity suggesting individual differences in brain activity in response to threat > happy faces may be specific to severity of social anxiety symptoms. The lack of a significant relationship between depression symptoms assessed with HAMD and social anxiety severity (LSAS) may be due to restricted range, as HAMD signified depression severity was in the mild range.
Findings should be considered in the context of important limitations. Certain comorbidity was allowed; therefore, results may not replicate in a sample of participants who differ in comorbidity or other characteristics (e.g., age, gender frequency). We focused on striatum and amygdala activation as potential neural variables related to social anxiety symptoms, however, neural activity in other brain structures may correspond to social anxiety symptoms. Anhedonia and melancholy symptoms were estimated from BDI-II items; therefore, results may not generalize to measures designed to assess anhedonia and melancholy. The Emotional Faces Matching Task was designed to engage regions central to emotion processing and may not be adequate in engaging the NAcc. Personality traits are an important source of heterogeneity in SAD (Costache et al., 2020), however, we did not assess personality traits, which may provide better measurements of anhedonia or (low) social motivation than our measures. Future studies should include personality measures and examine striatal functioning in SAD with other reward-related paradigms since happy faces may not be rewarding in SAD. Concerning neuroimaging, no field maps or phase-encoded EPIs were collected during acquisition; therefore, we were unable to correct for any potential field distortions.
5. Conclusions
In summary, differential response between threatening and happy faces in the dorsal striatum, particularly the putamen, along with amygdala, were related to social anxiety severity. Variance in anhedonia and general anxiety symptoms also corresponded with social anxiety severity, however, such symptoms were not linked with neural activity in response to emotional faces. Social anxiety disorder is a heterogeneous disorder and findings suggest variance in striatum and amygdala activation may contribute to social anxiety severity.
CRediT authorship contribution statement
NAC and HK made substantial contributions to the analysis and writing of the manuscript. HK led the project administration and conceptualization for the manuscript and provided supervision for the team. FC and KLK contributed to the data curation and the review and editing of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by NIH/NIMH K23 MH093679, R01 MH112705, R01 MH101497, T32 MH067631 (NAC, KLK), and the Center for Clinical and Translational Research (CCTS) UL1RR029879.
Footnotes
A sub-sample of participants (n=59) also completed a modified version of the task that included sad faces. However, the primary focus of the study was response to threat, as opposed to loss. Additionally, restricting analysis to participants who completed the version with angry, fearful, happy, and sad faces would reduce the sample size. Therefore, we elected to perform analysis on participants who completed the original task comprising angry, fear, and happy faces.
References
- Allison P.D. Pine Forge Press; 1999. Multiple Regression: A Primer. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, 5th edition (5 edition). American Psychiatric Publishing.
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2015. Structured clinical interview for DSM-5 (SCID-5) [Google Scholar]
- Asher M., Asnaani A., Aderka I.M. Gender differences in social anxiety disorder: A review. Clin. Psychol. Rev. 2017;56:1–12. doi: 10.1016/j.cpr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Ball T.M., Sullivan S., Flagan T., Hitchcock C.A., Simmons A., Paulus M.P., Stein M.B. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. NeuroImage. 2012;59(2):1879–1887. doi: 10.1016/j.neuroimage.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Balleine B.W., Delgado M.R., Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B., Reitt M., Röver C., Michaelis S., Görlich Y., Wedekind D. Efficacy of treatments for anxiety disorders: a meta-analysis. Int. Clin. Psychopharmacol. 2015;30(4):183–192. doi: 10.1097/YIC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bas-Hoogendam, J.M., van Steenbergen, H., Nienke Pannekoek, J., Fouche, J.-P., Lochner, C., Hattingh, C. J., Cremers, H. R., Furmark, T., Månsson, K. N. T., Frick, A., Engman, J., Boraxbekk, C.-J., Carlbring, P., Andersson, G., Fredrikson, M., Straube, T., Peterburs, J., Klumpp, H., Phan, K. L., … van der Wee, N.J.A., 2017. Voxel-based morphometry multi-center mega-analysis of brain structure in social anxiety disorder. NeuroImage: Clin., 16, 678–688. 10.1016/j.nicl.2017.08.001. [DOI] [PMC free article] [PubMed]
- Beck A.T., Clark D.A. An information processing model of anxiety: Automatic and strategic processes. Behav. Res. Ther. 1997;35(1):49–58. doi: 10.1016/s0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Beck, Aaron T., Steer, R.A., 1987. Manual for the revised Beck depression inventory.
- Beesdo K., Bittner A., Pine D.S., Stein M.B., Höfler M., Lieb R., Wittchen H.-U. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Biehl M., Matsumoto D., Ekman P. Matsumoto and Ekman’s Japanese and Caucasian facial expressions of emotion (JACFEE): Reliability data and cross-national differences. J. Nonverbal Behav. 1997;21:3–21. [Google Scholar]
- Blackford J.U., Allen A.H., Cowan R.L., Avery S.N. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc. Cogn. Affect. Neurosci. 2013;8(2):143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond N., Siddle D. The preparedness account of social phobia: Some data and alternative explanations. In: Rapee R.M., editor. Current controversies in the anxiety disorders. Guilford Press; 1996. pp. 291–316. [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Brühl A.B., Rufer M., Delsignore A., Kaffenberger T., Jäncke L., Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Campbell, D.W., Sareen, J., Stein, M.B., Kravetsky, L. B., Paulus, M. P., Hassard, S.T., Reiss, J.P., 2009. Happy but not so approachable: The social judgments of individuals with generalized social phobia. Depress. Anxiety, 26(5), 419–424. 10.1002/da.20474. [DOI] [PubMed]
- Carretié L., Albert J., López-Martín S., Tapia M. Negative brain: An integrative review on the neural processes activated by unpleasant stimuli. Int. J. Psychophysiol. 2009;71(1):57–63. doi: 10.1016/j.ijpsycho.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Costache M.E., Frick A., Månsson K., Engman J., Faria V., Hjorth O., Hoppe J.M., Gingnell M., Frans Ö., Björkstrand J., Rosén J., Alaie I., Åhs F., Linnman C., Wahlstedt K., Tillfors M., Marteinsdottir I., Fredrikson M., Furmark T., Sudzina F. Higher- and lower-order personality traits and cluster subtypes in social anxiety disorder. PLoS ONE. 2020;15(4):e0232187. doi: 10.1371/journal.pone.0232187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M., Fadok J.P., Palmiter R.D. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn. Memory. 2011;18(3):136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Strong evidence for universals in facial expressions: a reply to Russell’s mistaken critique. Psychol. Bull. 1994;115(2):268–287. doi: 10.1037/0033-2909.115.2.268. [DOI] [PubMed] [Google Scholar]
- Ernst M., Spear L.P. Reward systems. In: de Haan M., Gunnar M., editors. Handbook of Developmental Social Neuroscience. The Guilford Press; 2009. pp. 324–341. [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., Benedetti F., Abbamonte M., Gasparotti R., Barale F., Perez J., McGuire P., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry & Neurosci.: JPN. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gorka S.M., Young C.B., Klumpp H., Kennedy A.E., Francis J., Ajilore O., Langenecker S.A., Shankman S.A., Craske M.G., Stein M.B., Phan K.L. Emotion-based brain mechanisms and predictors for SSRI and CBT treatment of anxiety and depression: a randomized trial. Neuropsychopharmacology. 2019;44(9):1639–1648. doi: 10.1038/s41386-019-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Sara R., Hagendoorn M., Marom O., Hughett P., Macy L., Turner T., Bajcsy R., Posner A., Gur R.E. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods. 2002;115(2):137–143. doi: 10.1016/S0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-García A., Calvo M.G. Social anxiety and perception of (un)trustworthiness in smiling faces. Psychiatry Res. 2016;244:28–36. doi: 10.1016/j.psychres.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Hambrick J.P., Turk C.L., Heimberg R.G., Schneier F.R., Liebowitz M.R. The experience of disability and quality of life in social anxiety disorder. Depress. Anxiety. 2003;18(1):46–50. doi: 10.1002/da.v18:110.1002/da.10110. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg R.G., Horner K.J., Juster H.R., Safren S.A., Brown E.J., Schneier F.R., Liebowitz M.R. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol. Med. 1999;29(1):199–212. doi: 10.1017/S0033291798007879. [DOI] [PubMed] [Google Scholar]
- Heitmann C.Y., Feldker K., Neumeister P., Zepp B.M., Peterburs J., Zwitserlood P., Straube T. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder: processing of disorder-related scenes in SAD. Hum. Brain Mapp. 2016;37(4):1559–1572. doi: 10.1002/hbm.v37.410.1002/hbm.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C.J., Thivierge J.-P., Sporns O. Can structure predict function in the human brain? NeuroImage. 2010;52(3):766–776. doi: 10.1016/j.neuroimage.2010.01.071. [DOI] [PubMed] [Google Scholar]
- Hunter L.R., Buckner J.D., Schmidt N.B. Interpreting facial expressions: the influence of social anxiety, emotional valence, and race. J. Anxiety Disord. 2009;23(4):482–488. doi: 10.1016/j.janxdis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan T.B. Social anxiety spectrum and diminished positive experiences: Theoretical synthesis and meta-analysis. Clin. Psychol. Rev. 2007;27(3):348–365. doi: 10.1016/j.cpr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Angstadt M., Nathan P.J., Phan K.L. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Res.: Neuroimag. 2010;183(2):167–169. doi: 10.1016/j.pscychresns.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Angstadt M., Phan K.L. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol. Psychol. 2012;89(1):273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Fitzgerald D.A., Phan K.L. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;45:83–91. doi: 10.1016/j.pnpbp.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago T., Davis A., Grillon C., Ernst M. Striatum on the anxiety map: small detours into adolescence. Brain Res. 2017;1654:177–184. doi: 10.1016/j.brainres.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L., Hare T.A., Voss H.U., Glover G., Ballon D.J., Casey B.J. The bivalent side of the nucleus accumbens. NeuroImage. 2009;44(3):1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz M.R. Social phobia. Mod. Probl. Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- MacNamara, A., Klumpp, H., Kennedy, A. E., Langenecker, S. A., & Phan, K. L. (2017). Transdiagnostic neural correlates of affective face processing in anxiety and depression: MACNAMARA et al. Depress. Anxiety, 34(7), 621–631. 10.1002/da.22631. [DOI] [PMC free article] [PubMed]
- Marchand W.R. Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct. Funct. 2010;215(2):73–96. doi: 10.1007/s00429-010-0280-y. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Coccaro E.F., Angstadt M., Kreger K.J., Mayberg H.S., Liberzon I., Stein M.B. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biol. Psychiatry. 2013;73(4):329–336. doi: 10.1016/j.biopsych.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol. Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Jahn A.L., O’Shea J.P. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol. Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Grimm O., Morgen K., Mier D., Sauer C., Haddad L., Tost H., Esslinger C., Kirsch P., Schwarz A.J., Meyer-Lindenberg A. Amygdala habituation: a reliable fMRI phenotype. NeuroImage. 2014;103:383–390. doi: 10.1016/j.neuroimage.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Richey J.A., Brewer J.A., Sullivan-Toole H., Strege M.V., Kim-Spoon J., White S.W., Ollendick T.H. Sensitivity shift theory: a developmental model of positive affect and motivational deficits in social anxiety disorder. Clin. Psychol. Rev. 2019;72:101756. doi: 10.1016/j.cpr.2019.101756. [DOI] [PubMed] [Google Scholar]
- Roiser J.P., Levy J., Fromm S.J., Wang H., Hasler G., Sahakian B.J., Drevets W.C. The Effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008;33(8):1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls, E. T., Huang, C.-C., Lin, C.-P., Feng, J., & Joliot, M. (in press). Automated anatomical labelling atlas 3. NeuroImage, 116189. 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren, S.A., Heimberg, R.G., Brown, E.J., Holle, C., 1996. Quality of life in social phobia. Depress. Anxiety, 4(3), 126–133. 10.1002/(SICI)1520-6394(1996)4:3<126::AID-DA5>3.0.CO;2-E. [DOI] [PubMed]
- Santos A., Mier D., Kirsch P., Meyer-Lindenberg A. Evidence for a general face salience signal in human amygdala. NeuroImage. 2011;54(4):3111–3116. doi: 10.1016/j.neuroimage.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Heckelman L.R., Garfinkel R., Campeas R., Fallon B.A., Gitow A., Street L., Del Bene D., Liebowitz M.R. Functional impairment in social phobia. J Clin. Psychiatry. 1994;55(8):322–331. [PubMed] [Google Scholar]
- Shohamy D. Learning and motivation in the human striatum. Curr. Opin. Neurobiol. 2011;21(3):408–414. doi: 10.1016/j.conb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Skelly, L.R., Decety, J., 2012. Passive and motivated perception of emotional faces: qualitative and quantitative changes in the face processing network. PLoS ONE, 7(6), e40371. 10.1371/journal.pone.0040371. [DOI] [PMC free article] [PubMed]
- Starr C.J., Sawaki L., Wittenberg G.F., Burdette J.H., Oshiro Y., Quevedo A.S., McHaffie J.G., Coghill R.C. The contribution of the putamen to sensory aspects of pain: Insights from structural connectivity and brain lesions. Brain. 2011;134(7):1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathis, P., Panourias, I., Themistocleous, M., & Sakas, D. E. (2007). Connections of the basal ganglia with the limbic system: Implications for neuromodulation therapies of anxiety and affective disorders. In Operative Neuromodulation (pp. 575–586). Springer. [DOI] [PubMed]
- Staugaard, S.R., Rosenberg, N.K., 2011. Processing of emotional faces in social phobia. Mental Illness, 3(1), 5. 10.4081/mi.2011.e5. [DOI] [PMC free article] [PubMed]
- Stein, D.J., Lim, C.C.W., Roest, A.M., de Jonge, P., Aguilar-Gaxiola, S., Al-Hamzawi, A., Alonso, J., Benjet, C., Bromet, E. J., Bruffaerts, R., de Girolamo, G., Florescu, S., Gureje, O., Haro, J. M., Harris, M.G., He, Y., Hinkov, H., Horiguchi, I., Hu, C., … WHO World Mental Health Survey Collaborators. (2017). The cross-national epidemiology of social anxiety disorder: Data from the World Mental Health Survey Initiative. BMC Medicine, 15(1), 143. 10.1186/s12916-017-0889-2. [DOI] [PMC free article] [PubMed]
- Stein M.B., Fuetsch M., Müller N., Höfler M., Lieb R., Wittchen H.-U. Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Arch. Gen. Psychiatry. 2001;58(3):251. doi: 10.1001/archpsyc.58.3.251. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Stein D.J. Social anxiety disorder. Lancet. 2008;371(9618):1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Weidt S., Lutz J., Rufer M., Delsignore A., Jakob N.J., Herwig U., Bruehl A.B. Common and differential alterations of general emotion processing in obsessive-compulsive and social anxiety disorder. Psychol. Med. 2016;46(7):1427–1436. doi: 10.1017/S0033291715002998. [DOI] [PubMed] [Google Scholar]
- Weisburd D., Britt C. Springer Science & Business Media; 2013. Statistics in Criminal Justice. [Google Scholar]
- Whalen P.J. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr. Direct. Psychol. Sci. 1998;7(6):177–188. doi: 10.1111/cdir.1998.7.issue-610.1111/1467-8721.ep10836912. [DOI] [Google Scholar]
- Ziv M., Goldin P., Jazaieri H., Hahn K.S., Gross J.J. Is there less to social anxiety than meets the eye? behavioral and neural responses to three socio-emotional tasks. Biol. Mood Anxiety Dis. 2013;3(1):5. doi: 10.1186/2045-5380-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]