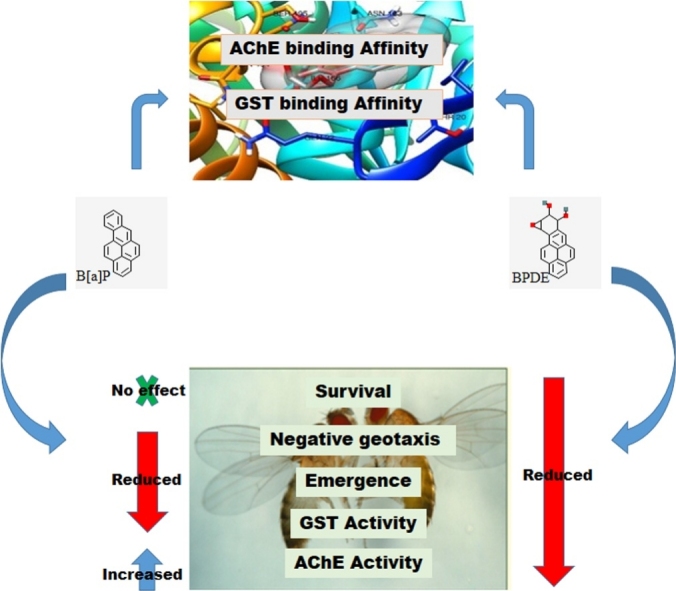
Graphical abstract
Keywords: Drosophila melanogaster; Canton-S; Benzo[a]pyrene; Benzo[a]pyrene- 7, 8-dihydrodiol-9,10-epoxide; Glutathione-S-transferase; Acetylcholinesterase; Molecular docking
Highlights
-
•
Canton-S strain of Drosophila melanogaster was investigated as a possible experimental model of Benzo[a]pyrene (B[a]P) toxicity.
-
•
B[a]P and its metabolite, BPDE, caused significant changes in negative geotaxis, fecundity and biochemical parameters of toxicity of the flies.
-
•
The compounds interacted with Drosophila glutathione-S-transferase (GST) and acetylcholinesterase (AChE) in a molecular docking analysis.
-
•
BPDE induced a high rate of mortality in this strain of flies possibly through AChE inhibition.
-
•
Canton-S strain of Drosophila melanogaster could be an alternative model to study B[a]P toxicity.
Abstract
Benzo[a]pyrene (B[a]P) is a polycyclic aromatic hydrocarbon (PAH) commonly found in cigarette smoke, automobile exhaust fumes, grilled meat, and smoked food among others. Exposure to B[a]P is associated with a range of toxic effects including developmental, neurological, oxidative, inflammatory, mutagenic, carcinogenic and mortal. Efficient and more affordable experimental models like Drosophila melanogaster could provide more insight into the mechanism of PAH toxicity and help develop new strategies for prevention, diagnosis and treatment of PAH-related conditions. In this study, we examined the induction of some biochemical changes along with mortality and functional senescence by B[a]P and its metabolite, benzo[a]pyrene- 7,8-dihydrodiol-910-epoxide (BPDE) in the Canton-S strain of Drosophila melanogaster, with the aim to establish an alternative assay medium for B[a]P toxicity in flies. Flies were exposed to 2–200 μM of B[a]P and 1–10 μM of BPDE through diet for a seven-day survival assay followed by a four-day treatment to determine the effects of the compounds on negative geotaxis, fecundity and some biochemical parameters of oxidative damage. BPDE significantly reduced the survival rate of flies along the 7 days of exposure whereas B[a]P did not cause any significant change in the survival rate of flies. B[a]P and BPDE significantly reduced the climbing ability of flies after 4 days of exposure. Rate of emergence of flies significantly reduced at 10–200 μM of B[a]P and 5–10 μM of BPDE. Both compounds caused various levels of alterations in the values of reduced glutathione (GSH), total thiol (T—SH), glutathione-S-transferase (GST), catalase (CAT), hydrogen peroxide (H2O2), nitric oxide (NO) and acetylcholinesterase (AChE) of the flies. The compounds also exhibited high binding affinities and molecular interactions with the active site amino acid residues of Drosophila GST and the inhibitor binding site of Drosophila AChE in an in silico molecular docking analysis, with BPDE forming stable hydrogen bonds with AChE. Hence, the Canton-S strain of Drosophila melanogaster could offer a simple and affordable assay medium to study B[a]P toxicity.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are toxic environmental pollutants produced by partial or incomplete combustion of organic matter and are thus found in exhaust fumes, cigarette smoke and in charcoal-broiled and smoked food [1,2]. These highly abundant, semi-volatile organic compounds represent a potential health hazard worldwide. They are known to cause several toxic effects, including developmental, neurological, oxidative, inflammatory, mutagenic, carcinogenic and lethal [1,3,4].
Benzo[a]pyrene (B[a]P) is a PAH known to be highly toxic to humans and animals. Upon ingestion, this pro-carcinogen undergoes biotransformation reactions catalyzed by cytochrome P450 family members (CYP1A1 and CYP1B1) and epoxide hydrolase, producing highly reactive intermediates and ultimately Benzo[a]pyrene- 7,8-dihydrodiol-910-epoxide (BPDE) [5,6]. The reaction is also characterized by the generation of reactive oxygen species (ROS) and consequent oxidative damage leading to several toxicological outcomes [1]. Furthermore, BPDE as a reactive electrophile binds covalently to critical macromolecules (proteins, lipids, and nucleic acids) to produce BPDE adducts, thereby interfering with key biological processes [7]. Hence, exposure to this ubiquitous chemical compound either accidentally or intentionally, through inhalation, the oral or dermal route, is a serious health concern. Studies aimed to develop strategies for prevention, diagnosis and treatment of PAH related conditions are very essential, and this requires efficient experimental models.
Drosophila melanogaster is an effective and affordable experimental model that is currently been used as an alternative to rodents in toxicological studies [8]. However, some strains of Drosophila melanogaster are reported to possess zero or weak susceptibility to PAH due to evolutionary adaptive responses. Insects have developed several adaptive strategies and modifications of their detoxification mechanisms in response to several classes of toxicants. [9]. The sensitivity of Drosophila melanogaster to B[a]P toxicity has been reported by several studies, some presenting Drosophila as an inefficient assay medium due to little or no susceptibility [10,11]. Analysis of metabolism of B[a]P by Hallstrom and Grafstrom showed different spectra of metabolites in Drosophila and rat liver, with Drosophila exhibiting a much lower production of 7,8-dihydrodiol epoxide and high production of B[a]P quinones that effectively inhibit B[a]P oxidation and the metabolism of B[a]P-7,8-dihydrodiol to the ultimate carcinogen, BDPE [10,12] thereby limiting its toxicity. The dissimilarity in metabolic profile following B[a]P exposure is attributable to differential expression of multiple P450s resulting in variations in susceptibility of mammals and flies to PAH toxicity [13].
Successful toxicity testing of B[a]P in Drosophila melanogaster have however depended on genetically modified strains and most of these studies are mainly for mutagenicity and genotoxicity testing. Lee et al. successfully used a repair-deficient strain for B[a]P-induced mutagenicity study [14], Fahmy and Fahmy used the 3-methyl and 6-hydroxymethyl derivatives of B[a]P in the Oregon-K strain [15], Frölich and Würgler created a tester strain by introducing chromosomes 1 and 2 from a wild-type strain with high CYP450-dependent metabolism linked to a gene on chromosome 2 for genotoxicity testing [16], Fujikawa et al. also achieved genotoxicity testing of B[a]P using D. melanogaster stock comprising of meiotic recombination deficient double mutant males and females [17]. These studies are based on either the sex-linked recessive lethal test for mutagenesis, DNA repair assay or the somatic mutation and recombination tests (SMART) for genotoxicity testing. It is, therefore, necessary to have a simpler fly model to study the several toxicological outcomes and biochemical changes associated with B[a]P toxicity.
Some wild-type strains of Drosophila melanogaster like the Canton-S regarded as insecticide-sensitive have been reported to under-express the cyp6 genes implicated in the resistance of insects to xenobiotics [18,19] thereby making them more susceptible to these compounds. In this study, we investigated the inducibility of mortality, functional senescence and some toxic biochemical changes by B[a]P as well as its toxic metabolite (BPDE) in the Canton-S strain of Drosophila melanogaster with the aim to establish an alternate assay medium for B[a]P toxicity in flies.
2. Materials and methods
2.1. Test compounds
Benzo [a]pyrene and benzo[a]pyrene- 7,8-dihydrodiol-910-epoxide were purchased from Toronto Research Chemicals, North York, Ontario, Canada.
2.2. Drosophila melanogaster stock and culture
Canton-S strain of Drosophila melanogaster firstly gotten from the National Species Stock Center, Bowling Green, Oklahoma in the USA by the Federal University of Santa Maria, Brazil were obtained and cultured at the Drosophila Research Laboratory, Department of Biochemistry, University of Ibadan, Ibadan, Nigeria. One to three days old flies of both genders were reared on a cornmeal diet supplemented with 2% w/v sucrose, 1% w/v brewer's yeast, 1% w/v agar and 0.08 % v/w methylparaben, under 12 h dark/light cycle, at constant temperature (22–24 °C) and humidity (60–70 %).
2.3. Survival assay
Flies were divided into 9 experimental groups with each group separated into 5 vials of 30 flies each. The flies were exposed to different concentrations of B[a]P (2, 10, 100 and 200 μM in 3 g diet) dissolved in 2% v/v ethyl acetate and BPDE (1, 5, 10 μM in 3 g diet) dissolved in 1% v/v DMSO for 7 days. Control flies were exposed to 2% ethyl acetate and 1% DMSO respectively. The flies were monitored daily and the total number of live/dead flies in each vial was recorded [20]. The result was analyzed and a survival curve was generated using the Kaplan Meier survival test. The data from the survival assay informed the period of exposure to varying concentrations of the B[a]P and BPDE for negative geotaxis, fecundity and biochemical assay.
2.4. Negative geotaxis (climbing) and Fecundity (emergent) assays
The climbing assay was performed as previously described by Abolaji et al. [20]. Briefly, 10 flies (1–3 days old) per vial in five replicates were exposed to 10–200 μM of B[a]P and 1–10 μM of BPDE as described above for 4 days. The number of flies that reach the 6 cm line of graded treatment vial in 6 s was recorded. For the emergent test, newly emerged male and female flies of equal numbers were used to ascertain the onset of offspring emergence after treatment with the B[a]P and BPDE.
2.5. Determination of biochemical parameters of oxidative damage
Flies exposed to 10–200 μM of B[a]P and 1–10 μM of BPDE for 4 days were prepared and used for biochemical assays following the protocols previously described by Abolaji et al. [21]. According to these protocols, the method of Lowry et al. [22] was adopted for the determination of protein concentration. Total thiol content was determined as described by Ellman [23]. Briefly, the assay medium containing 510 μL of 0.1 M phosphate buffer (pH 7.4), 35 μL of 1 mM DTNB, 35 μL of distilled water and 20 μL of sample, was incubated for 30 min at room temperature and the absorbance was read at 412 nm. The Habig and Jakoby method [17] was used for the assay of GST activity. Solution A was prepared using 0.25 M potassium phosphate buffer (20 μL), 2.5 mM EDTA, 10.5 μL of distilled water and 0.1 M GSH (500 μL) at pH 7.0 and 25 °C. 20 μL of the sample (1:5 dilution) was added to 270 μL of solution A and 10 μL of 25 mM CDNB. Absorbance was read at 340 nm for 5 min (10-seconds interval) using a spectrophotometer.
The method of Aebi was used for the determination of catalase activity [24]. The sample (1:50 dilution) was mixed with 50 mM potassium phosphate buffer (pH 7.0) and 300 mM H2O2. Change in absorbance was read at 240 nm for 2 min and the activity of catalase was estimated as μmol of H2O2 consumed per min/mg of protein. The method of Ellman et al. [25] was used for the assay of AChE activity. 0.1 M potassium phosphate buffer (pH 7.4) was mixed with 1 mM of DTNB followed by addition of 0.8 mM acetylthiocholine to initiate the reaction. The absorbance was measured every 30 s for 2 min at 412 nm. AChE activity was calculated as μmol of acetylthiocholine hydrolyzed/min/mg protein.
Hydrogen peroxide level was measured as described by Wolff [26]. The sample was incubated in FOX 1 reagent for 30 min at room temperature followed by measurement of absorbance at 560 nm. H2O2 values estimated using a standard curve were expressed in μmole/mg protein. The Griess reaction assay [21] was used for the determination of Nitric oxide level. 250 μL of sample was incubated in 250 μL of Griess reagent for 20 min at room temperature. The absorbance was measured spectrophotometrically at 550 nm and the nitrite concentration was estimated by comparing the OD of the sample with that of a standard solution of known nitrite concentration.
2.6. Molecular modelling of biological interactions
Molecular docking analysis was conducted to determine the inhibitory potential and mode of biological interactions of B[a]P and BPDE against GST and AChE.
2.6.1. Ligands and protein collection
The structure data file (SDF) format of B[a]P, BPDE, GST substrate (glutathione), GST standard inhibitor (6-[(7-nitro-2,1,3-benzoxadiazol-4-yl)sulfanyl]hexan-1-ol or NBDHEX), AChE substrate (acetylcholine) and AChE standard inhibitor (galantamine) were obtained from PubChem database. The crystal structures of Drosophila melanogaster glutathione-S-transferase (PDB ID: 1 M0U) and acetylcholinesterase (PDB ID: 6XYS) were obtained from the RCSB protein data bank (PDB).
2.6.2. Ligand preparation
A library containing the SDF format of the compounds were uploaded to PyRx software and converted to PDBQT format using the OpenBabel plugin. The output files were minimized to obtain minimum energy for the ligand docking.
2.6.3. Protein preparation
Each of the crystal structure of the proteins retrieved from the RCSB directory was uploaded to Chimera 1.14 workspace. The non-standard residues including ions, water and bounded ligands were removed. The proteins were structurally minimized at 200 steepest descent steps, 0.02 steepest descent steps size (Ᾰ), 10 conjugate gradient steps, 0.02 conjugate gradient steps size (Ᾰ), and 10 update intervals, using the structure editing wizard. Solvents were removed, hydrogen bonds were added, charges were assigned using Gasteiger force field, histidine was set for the protonation state. Every available selenomethione (MSE) were changed to methionine (MET), bromo-UMP (5BU) to UMP (U), methylselenyl-dUMP (UMS) to UMP (U) and methylselenyl-dCMP (CSL) to CMP (C). The output files were converted to PDBQT using the PyRx software.
2.6.4. Molecular docking
The prepared ligands and proteins were uploaded to the PyRx workspace, the grid box was set based on the protein structural dimensions docking, each ligand was set to have eight exhaustive ligand conformation protein receptor binding, using autodock vina of PyRx software. The output files were uploaded to Chimera 1.14 workspace for post docking analysis and visualization followed by Discovery Studio 2020 for 2D ligand-protein interaction generation.
2.7. Statistical analysis
Estimation of significant differences (p < 0.05) on GraphPad Prism5.0 software was through One-way Analysis of Variance (ANOVA) and Dunett’s posthoc test. Data are represented as the Mean ± SEM. The Kaplan-Meier test was used for analyzing the survival rate and the log-rank test was used to make comparisons between groups.
3. Results
3.1. Survival assay
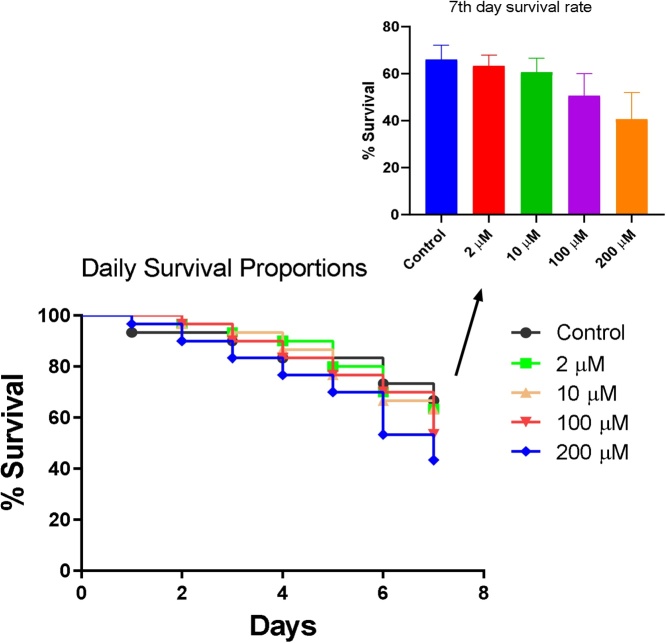
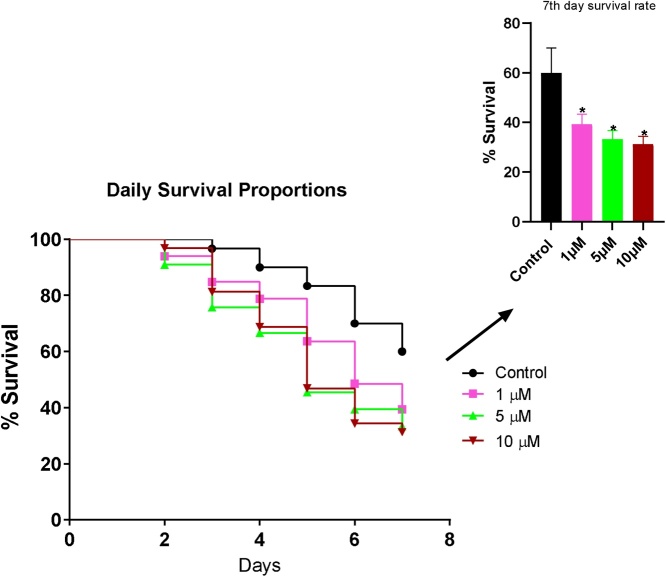
B[a]P did not cause any significant change in the survival rate of flies throughout the exposure period compared with the control (Fig. 1), whereas BPDE significantly reduced the survival rate of flies along the 7 days of exposure (Fig. 2).
Fig. 1.
Percentage Survival of flies exposed to various concentrations of B[a]P for 7 days.
Fig. 2.
Percentage Survival of flies exposed to various concentrations of BPDE for 7 days. * Significantly lower (p < 0.05) compared with the control.
3.2. Negative geotaxis and fecundity assays
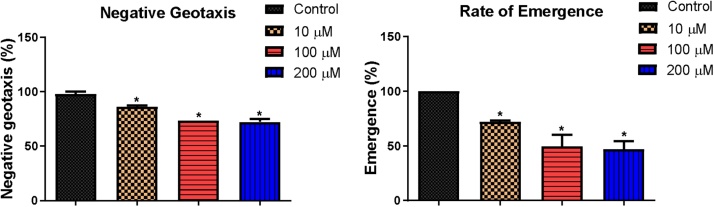
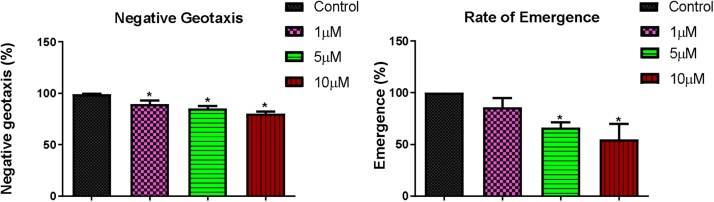
At the end of the 4-day exposure, 10, 100 and 200 μM of B[a]P significantly reduced the climbing ability of flies from 97.89 % to 86.32, 73.32 and 72.37 % respectively; and 1, 5 and 10 μM of BPDE significantly reduced the climbing rate from 99.17%–89.58, 85.42 and 80.21 % respectively. Rate of emergence of flies significantly reduced from 100 % to 72.22, 49.57 and 47.01 % at 10, 100 and 200 μM of B[a]P respectively; and 85.79, 66.44 and 54.79 % at 1, 5 and 10 μM of BPDE respectively (Fig. 3, Fig. 4).
Fig. 3.
Percentage negative geotaxis and rate of emergence of flies exposed to B[a]P for 4 days. * Significantly lower (p < 0.05) compared with the control.
Fig. 4.
Percentage negative geotaxis and rate of emergence of flies exposed to BPDE for 4 days. * Significantly lower (p < 0.05) compared with the control.
3.3. Biochemical parameters
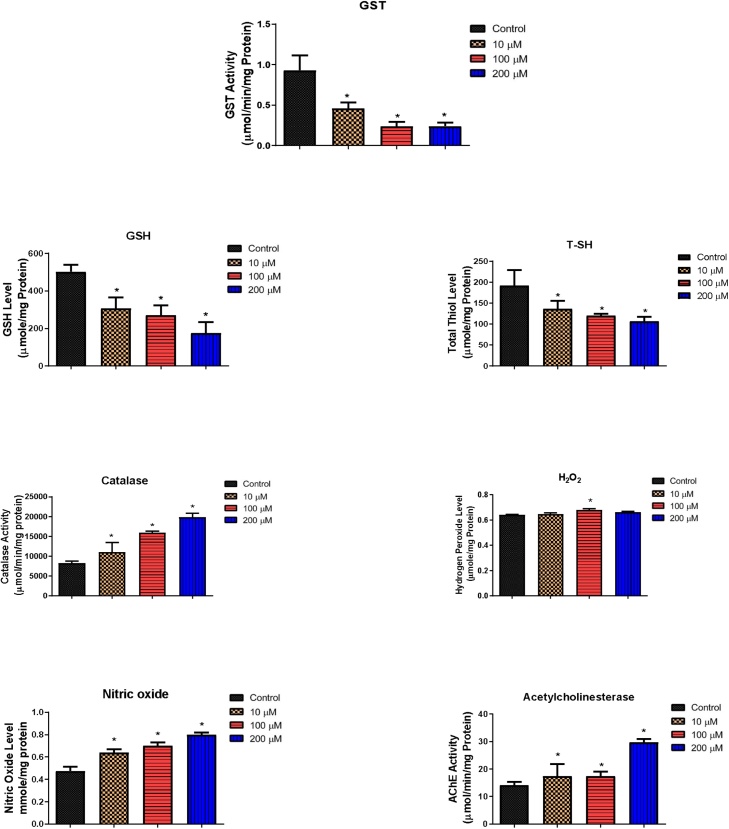
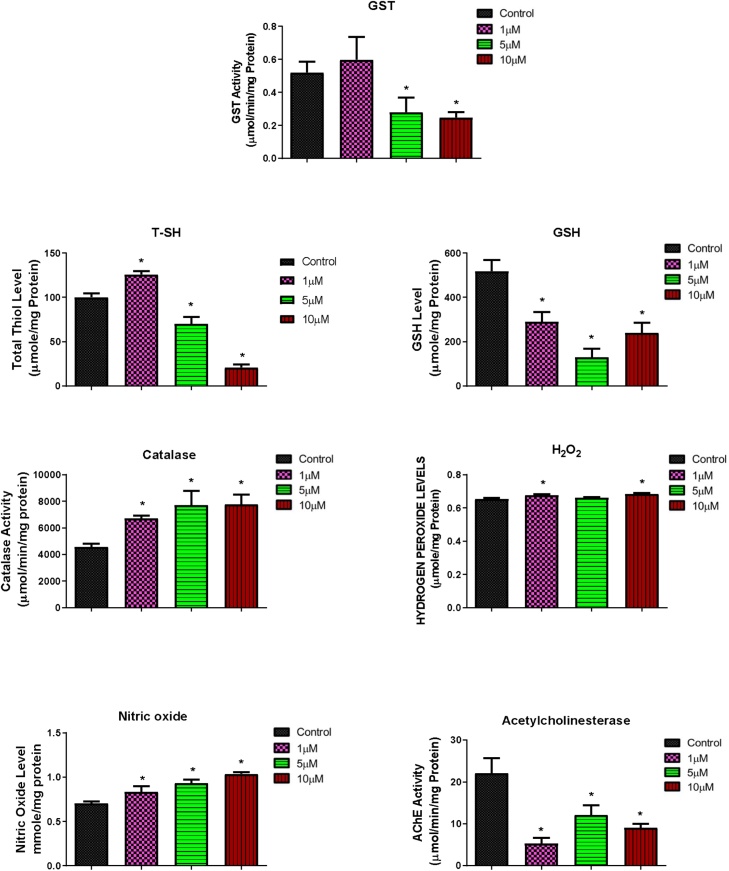
Both B[a]P and BPDE altered the levels of biochemical parameters as shown in Fig. 5, Fig. 6. At 10–200 μM of B[a]P, GST, GSH and T—SH values of flies were significantly reduced compared with the control, NO levels and activities of catalase and AChE were significantly increased, while H2O2 levels only significantly increased at 100 μM (Fig. 5). For the BPDE exposed groups, GST activity and T—SH levels were significantly increased at 1 μM and significantly decreased at 5 and 10 μM. GSH level and AChE activity significantly decreased and catalase activity, as well as NO level significantly increased at 1, 5 and 10 μM. Furthermore, H2O2 levels significantly increased at 1 and 10 μM (Fig. 6).
Fig. 5.
Changes in the levels of biochemical parameters (Glutathione-S-Transferase, catalase and acetylcholinesterase activities; and reduced glutathione, total thiol, hydrogen peroxide and nitric oxide concentrations) after 4 days exposure of D. melanogaster to B[a]P. * Significantly different (p < 0.05) compared with the control.
Fig. 6.
Changes in the levels of biochemical parameters (Glutathione-S-Transferase, catalase and acetylcholinesterase activities; and reduced glutathione, total thiol, hydrogen peroxide and nitric oxide concentrations) after 4 days exposure of D. melanogaster to BPDE. * Significantly lower (p < 0.05) compared with the control.
3.4. In silico molecular modelling of biological interactions
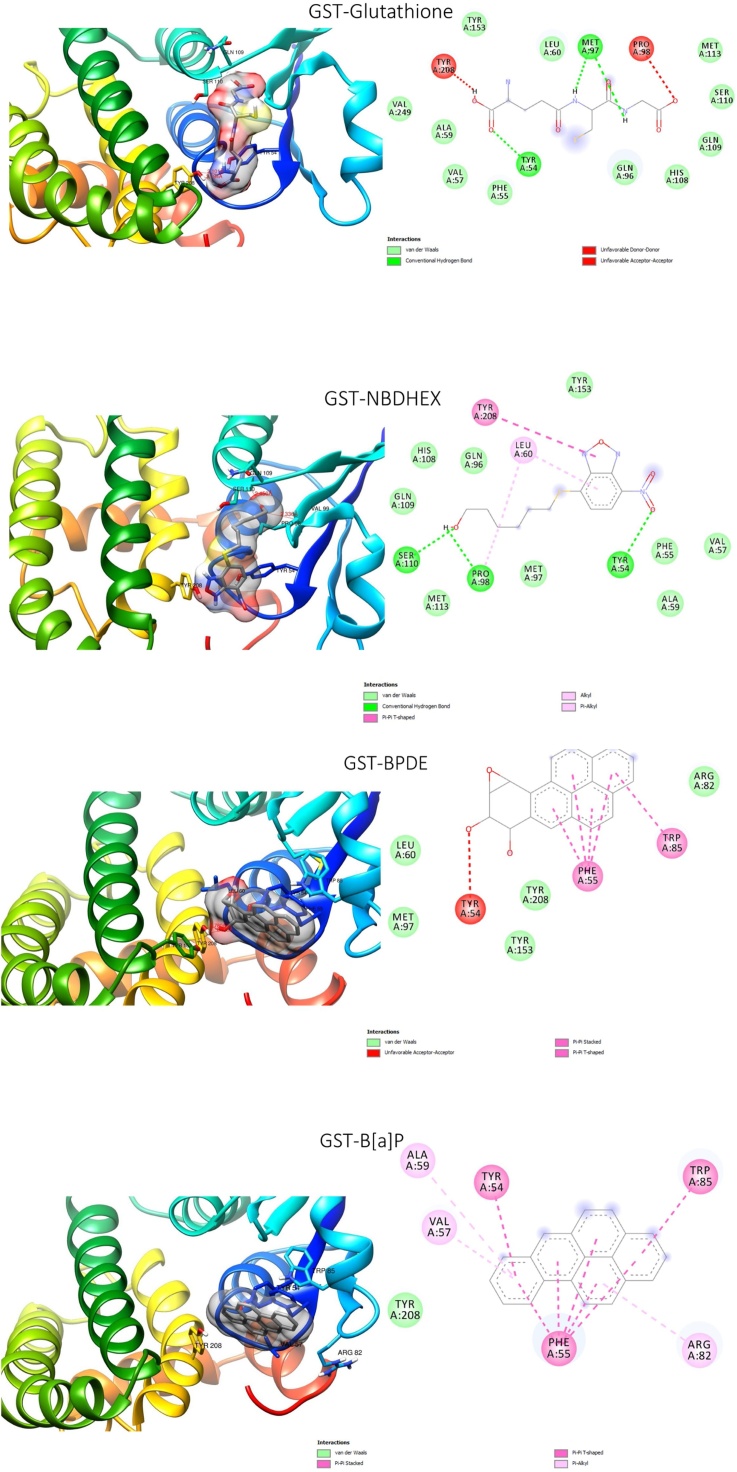
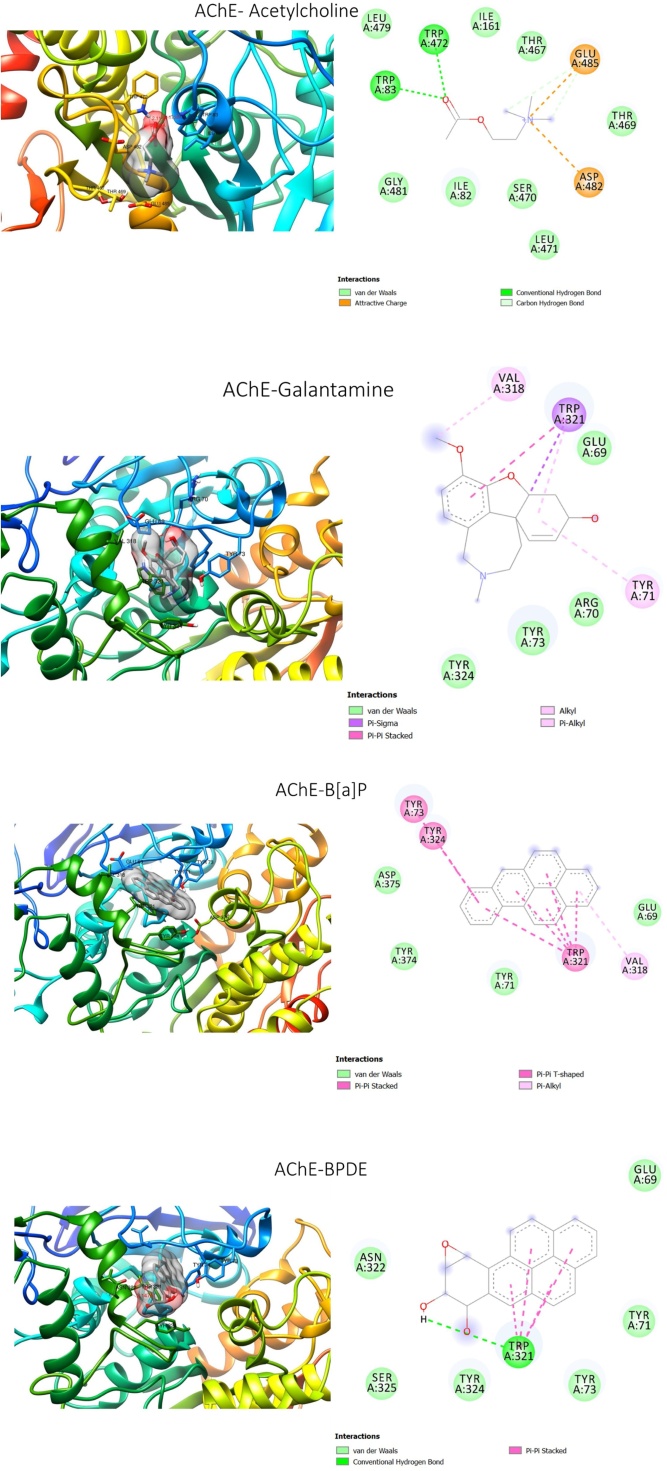
B[a]P and BPDE exhibited binding affinities for GST and AChE higher than those exhibited by the substrates and the standard inhibitors (Table 1). Both compounds occupied the binding site of the substrate (glutathione) and standard inhibitor (NBDHEX) (Fig. 7) of GST. The four compounds interacted with TYR A:54, TRY A:208, and PHE A:55, at the glutathione binding site of the enzyme. Additionally, BPDE together with glutathione and NBDHEX interacted with TYR A:153, LEU A: 60, MET A: 97, PRO A:98. For AChE, B[a]P and BPDE occupied the same binding site as the standard inhibitor (galantamine) which is different from the acetylcholine binding site. The three compounds commonly interacted with TRP A: 321, TRP A: 324, TRP A: 73, TRP A: 71 and GLU A: 69. Besides, a hydrogen bond interaction was detected between BPDE and TRP A: 321 in addition to other types of bonds (Fig. 8).
Table 1.
Binding affinities of B[a]P, BPDE, substrates and standard inhibitors for GST and AChE.
| Compounds | PubChem CID | ΔG Energy (Kcal/mol) |
|
|---|---|---|---|
| GST | AChE | ||
| Substrates | |||
| Glutathione | 124,886 | −5 | |
| Acetylcholine | 187 | −4.2 | |
| Standard inhibitors | |||
| NBDHEX | 9,817,686 | −5.3 | |
| Galantamine | 9651 | −6.4 | |
| B[a]P | 2336 | −7.9 | −9.2 |
| BPDE | 41,322 | −7.5 | −9.3 |
Fig. 7.
3D (left) and 2D (right) views of the molecular interactions of amino-acid residues of glutathione – S - transferase with the substrate (glutathione), the standard inhibitor (NBDHEX), B[a]P and BPDE.
Fig. 8.
3D (left) and 2D (right) views of the molecular interactions of amino-acid residues of acetylcholinesterase with the substrate (acetylcholine), the standard inhibitor (galantamine), B[a]P and BPDE.
4. Discussion
The Canton-S strain of Drosophila melanogaster showed susceptibility for B[a]P and BPDE toxicity in this study. Both chemicals caused significant changes in negative geotaxis, fecundity and some biochemical parameters of toxicity, with BPDE significantly reducing the survival rate of the experimental flies at 1–10 μM per 3 g diet. BPDE is the carcinogenic metabolite of B[a]P produced through cytochrome P450 and epoxide hydrolase catalyzed reactions. This highly electrophilic compound is known to be the most toxic metabolite of B[a]P, with high mutagenic and tumorigenic potencies [5,6]. During B[a]P metabolism in flies, series of defence mechanisms and detoxification processes are elicited that tend to limit the toxic effects of the compound [27] and this might be the reason for the little or no effect of B[a]P on the survival rate of flies even at concentrations as high as 200μM per 3 g diet. BPDE, on the other hand, does not require further metabolism and its immediate availability and direct electrophilic attack on critical proteins, lipids, and nucleic acids [7] makes the animal more susceptible to its toxic effects. It is on this basis that 1–10 μM BPDE and 10–200 μM B[a]P were used for negative geotaxis, fecundity and biochemical assays.
Negative geotaxis and fecundity assays are some of the parameters used to determine the functional senescence of flies [28]. Senescence involves the gradual deterioration of the function of a cell or the whole organism due to ageing or diseased conditions and it can be induced by exposure to toxic compounds and/or their reactive metabolites/bi-products [28]. This condition is characterized by several functional changes including behavioural, neurological, and reproductive [28]. Negative geotaxis is a behavioural and neurological parameter that measures the climbing rate (locomotor performance) of flies demonstrated by the number of flies that climbed beyond a specified distance within a set time or the length of time it takes to climb a set distance [29]. Negative geotactic ability has been shown to be affected by oxidative damage resulting from exposure to toxic chemicals [20]. This is in line with the results obtained in this study as exposure to B[a]P and BPDE caused a significant decline in the percentage negative geotaxis of flies.
The two compounds also demonstrated the potential capacity to induce a deteriorating effect on the reproductive ability of Canton-S Drosophila melanogaster as demonstrated by the significant decline in the rate of emergence of B[a]P and BPDE exposed flies. Exposure to toxic environmental chemicals have been reported to cause reproductive adversities in humans [30]. Biotransformation of B[a]P and other PAHs produce some reactive intermediates, the most notable of which are diol epoxides and quinones, coupled with reactive oxygen species (ROS); and these are found to be directly toxic to the gonads, thus resulting into declining fertility in males and females [31].
The significant decrease in negative geotaxis and fecundity of flies exposed to B[a]P and BPDE occurred with a corresponding reduction in the values of GSH, T—SH and GST. These are protective biomolecules whose reduction predisposes the organism to oxidative damage. Significant reduction in GSH value due to B[a]P exposure has been previously demonstrated in mice. Deng et al. reported a significant decrease in GSH concentration following 72 h of B[a]P exposure in mice with a corresponding increase in the concentration of its oxidized form - glutathione disulfide (GSSG). Excessive GSH oxidation to GSSG by B[a]P-generated ROS is said to overwhelm the rate at which GSSG is reduced back to GSH leading to a drastic reduction in GSH level, hence the animal is exposed to the devastating effects of the toxic compound [32,33]. This is further compounded by a reduction in the level of total thiols (T—SH). Thiols are a group of organic compounds that - like glutathione - contain a sulfhydryl group (-SH), and they contribute significantly to the defence against oxidative damage caused by ROS and electrophilic intermediates. Natural thiols include: glutathione (GSH), homocysteine (HcySH), cysteinylglycine (CysGlySH) and cysteine (CysSH) [34]. These nucleophilic compounds are highly susceptible to electrophilic and oxidative attack, and decreased T—SH concentration as observed with B[a]P and BPDE exposed flies in this study predisposes the cell to the toxic effects of ROS and electrophiles. GSTs are a superfamily of enzymes that play significant metabolic roles especially during phase II reactions of xenobiotics metabolism. They catalyze the conjugation of GSH with electrophilic compounds of exogenous and endogenous origins [35], thereby protecting the cells against xenobiotic-induced toxicity [36]. This mechanism, which also prevents lipid peroxidation and oxidative stress is a significant pathway for the detoxification of B[a]P and other PAHs. Hence, inhibition of GST activity coupled with the reduced GSH and T—SH levels of the flies, represents a decline in these protective biochemical mechanisms, thus exposing the animals to oxidative damage.
Catalase, an oxidoreductase is another enzyme responsible for protecting the cell from oxidative damage. It catalyzes the breakdown of two H2O2 molecules into one molecule of oxygen and two molecules of water, thereby preventing the damaging effect of H2O2 generated from the dismutation of the toxic free radical - superoxide anion (O2−) [37]. The significant increase in the catalase activity of experimental flies in this study disagrees with previous reports obtained from experiments with rat models. Kim and Lee reported a decreased catalase activity in the liver and kidneys of rats following B[a]P treatment [38] and Adedara et al. reported a significant decrease in the activity of this enzyme in the kidneys of B[a]P exposed rats [39]. However, there seem to be no available reports on the effect of B[a]P and BPDE on catalase activity in Drosophila melanogaster. The observed increase in catalase activity in this study represent a defensive response to regulate the devastating effects of the chemicals on the flies and this is reflected in the levels of H2O2 observed in the experimental flies. The H2O2 levels of exposed flies were close to the control values even though statistical analysis showed significant differences at 100μM of B[a]P and 1 and 10 μM of BPDE. Nonetheless, this beneficial response seems to have been counteracted by the reduced GSH, T-SH and GST levels of the flies (Fig. 5, Fig. 6).
Nitric oxide (NO) is a free radical synthesized endogenously from l-arginine by nitric oxide synthases (NOS) and it plays a key role in a variety of biological processes [40]. This signalling molecule can exert both cytoprotective and cytotoxic effects depending on some factors [41]. Increased production of NO by inducible NOS (iNOS) could be an initial adaptive response to a chemical or pathogen-induced insult but may develop into a promoter of tissue damage when in excess [41,42]. Increased NO production upon B[a]P exposure have been demonstrated in rat models and has been corroborated in vitro with a time and dose-dependent rise in NO production in microglial and hepatic cells exposed to B[a]P [[42], [43], [44]]. In this present study, both B[a]P and BPDE caused a dose-dependent increase in NO production which may have contributed to the toxic responses observed. B[a]P induced NO production has been recognized as a survival signal leading to inhibition of B[a]P-induced apoptosis, thereby promoting carcinogenesis [42].
AChE is an enzyme found mainly at neuromuscular junctions and in chemical synapses of the cholinergic type and they catalyze the hydrolysis of acetylcholine (ACh) and some other choline esters that function as neurotransmitters. Hence, they are responsible for the termination of synaptic transmission [45]. Alterations in AChE activity has been recognized as one of the toxicological consequences of exposure to PAHs [46]. Niu et al. reported a significant decrease in AChE activity in the blood samples of coke oven workers occupationally exposed to B[a]P [47]. Kang and Fang showed a dose-dependent competitive inhibition in the activity of AChE purified from electric eel as a result of exposure to PAHs [46]. In this study, however, B[a]P caused a significant increase, while BPDE caused a significant decrease in the AChE activity of D. melanogaster. The reduced AChE activity in the BPDE-exposed flies could have contributed to the increased mortality observed in this group of flies compared with the B[a]P-exposed flies. Inhibition of AChE activity is reported to be associated with the insecticidal activity of some compounds and its over-expression has been suggested to contribute to the resistance of some insects to the toxic effects of theses insecticides [48].
To further understand some of the mechanisms of the toxicological action of B[a]P and BPDE in the flies, the compounds were subjected to molecular docking analysis against GST and AChE. These enzymes were selected due to the observed significant reduction in their activities on exposure to either or both of the compounds. The reducing effect of B[a]P and BPDE on the GST activity of exposed flies corresponded with the result of the molecular docking analysis. Both compounds demonstrated higher binding affinities for GST than the substrate (glutathione) and the standard inhibitor ((NBDHEX) (Table 1) and they interacted with some important amino acid residues at the binding site of the substrate and the standard inhibitor. These include TRY A: 208 and TYR A:153 suggested to be key residues in the polar-binding region of Drosophila GST substrate binding site [49]. Both compounds interacted with TRY A: 208 while BPDE interacted with both TRY A: 208 and TYR A: 153 along with other amino acid residues. The potential ability of B[a]P and BPDE to interact with the active site amino acid residues of this enzyme, therefore, suggests the involvement of competitive inhibition of GST in the toxicological actions of these compounds.
B[a]P and BPDE also showed AChE inhibitory potential as both compounds exhibited higher binding affinities for the enzyme than the substrate and standard inhibitor. Both compounds interacted with some amino acids at the binding site of the standard inhibitor, with BPDE possessing a hydrogen bond interaction with TRP A: 321 of the enzyme. The hydrogen bond interaction could confer a more stable binding with the enzyme, suggesting BPDE as a stronger AChE inhibitor than the parent compound. Moreover, since B[a]P is immediately metabolized and detoxified in vivo on exposure, it might not be readily available to inhibit the enzyme. This may be the reason for the discrepancy between the molecular docking result and the in vivo effect of B[a]P on AChE. The molecular docking result showed possible AChE inhibitory activity while the in vivo study showed an increase in AChE activity by B[a]P.
5. Conclusion
In this study, exposure to B[a]P and BPDE caused a significant decline in the rate of emergence and negative geotactic ability of Canton-S Drosophila melanogaster. The compounds also altered some biochemical parameters of oxidative damage in these flies. Furthermore BPDE significantly reduced the survival rate of the experimental flies possibly through AChE inhibition.
Credit Authorship Contribution Statement
Titilayo O. Johnson: Conceptualization, data analysis, writing - original draft, funding acquisition, visualization. Amos O. Abolaji: Investigation, data analysis, writing- review & editing. Simeon Omale: Conceptualization, writing- review & editing. Ishaya Y. Longdet: Conceptualization, validation, funding acquisition, writing - review & editing. Richard Joseph Kutshik: Conceptualization, validation, funding acquisition, writing - review & editing. Bolaji O. Oyetayo: Investigation, data analysis, writing - original draft. Abayomi Emmanuel Adegboyega: Investigation, software, data analysis, writing - review & editing. Atiene Sagay: Validation, supervision, funding acquisition, writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research reported in this publication was supported by Fogarty International Centre (FIC) of the US National Institutes of Health (NIH) and Support of Training and Mentoring in Nigeria for Academics (STAMINA), University of Jos, Nigeria under the award number 5D43TW010130-03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Edited by Dr. A.M Tsatsaka
References
- 1.Lin S., Ren A., Wang L., Huang Y., Wang Y., Wang C. Free radical biology and medicine oxidative stress and apoptosis in Benzo [a] pyrene-induced neural tube defects. Free Radic. Biol. Med. 2018;116:149–158. doi: 10.1016/j.freeradbiomed.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zedeck M.S. Polycyclic aromatic hydrocarbons: a review. J. Environ. Pathol. Toxicol. 1980;3:537–567. doi: 10.1080/23311843.2017.1339841. [DOI] [PubMed] [Google Scholar]
- 3.Dreij K., Rhrissorrakrai K., Gunsalus K.C., Geacintov N.E., Scicchitano D.A. Benzo[a]pyrene diol epoxide stimulates an inflammatory response in normal human lung fibroblasts through a p53 and JNK mediated pathway. Carcinogenesis. 2010;31:1149–1157. doi: 10.1093/carcin/bgq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geier M.C., Chlebowski A.C., Truong L., Massey Simonich S.L., Anderson K.A., Tanguay R.L. Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Arch. Toxicol. 2018;92:571–586. doi: 10.1007/s00204-017-2068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penning T.M., Burczynski M.E., Hung C.F., McCoull K.D., Palackal N.T., Tsuruda L.S. Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: Generation of reactive and redox active o-quinones. Chem. Res. Toxicol. 1999;12:1–18. doi: 10.1021/tx980143n. [DOI] [PubMed] [Google Scholar]
- 6.Gelboin H.V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 7.Hang B. Formation and repair of tobacco carcinogen-derived bulky DNA adducts. J. Nucleic Acids. 2010;2010 doi: 10.4061/2010/709521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abolaji A., Kamdem J.P., Farombi O., Batista J., Da Rocha T. vol. 1. 2013. pp. 33–38. (Drosophila Melanogaster as a Promising Model Organism in Toxicological Studies: A Mini Review Neurotoxicity of Methylmercury in Insects View Project Science Education View Project). [Google Scholar]
- 9.Misra J.R., Horner M.A., Lam G., Thummel C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011;25:1796–1806. doi: 10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallstrom I., Grafstrom R. The metabolism of drugs and carcinogens in isolated subcellular fractions of. Chem. Biol. Interact. 1981;34:145–159. doi: 10.1016/0009-2797(81)90127-7. [DOI] [PubMed] [Google Scholar]
- 11.Zijlstra J.A., Vogel E.W. vol. 125. 1984. pp. 243–261. (Mutagens in Drosophila Melanogaster). [DOI] [PubMed] [Google Scholar]
- 12.Muhammed A. 2012. Basic and Applied Mutagenesis: With Special Reference to Agricultural Chemicals in Developing Countries. [PubMed] [Google Scholar]
- 13.Mohammed B.R., Mailafia S., Simon M.K., Agbede R.I.S. Cytochrome P450s in Anopheles gambiae (Diptera : culicidae) and insecticide resistance in Africa : a mini review. Entomol. Ornithol. Herpetol. Curr. Res. 2017;6:200. doi: 10.4172/2161-0983.1000200. [DOI] [Google Scholar]
- 14.Lee W.R., Abrahamson S., Valencia R., von Halle E.S., E WF, S Z The sex-linked recessive lethal test for mutagenesis in Drosophila melanogaster. Mutat. Res. 1983;123:189–279. doi: 10.1016/0165-1110(83)90025-8. [DOI] [PubMed] [Google Scholar]
- 15.Fahmy O.G., Fahmy M.J. Mutagenic Properties of Benzo(a)pyrene and Its Methylated Derivatives in Relation to the Molecular Mechanisms of Hydrocarbon Carcinogenesis1. Cancer Res. 1973;33:302–309. [PubMed] [Google Scholar]
- 16.Frolich A., Wurgler F.E. Drosophila wing-spot test : improved detectability of genotoxicity of polycyclic aromatic hydrocarbons. Mutat. Res. 1990;234:71–80. doi: 10.1016/0165-1161(90)90033-k. [DOI] [PubMed] [Google Scholar]
- 17.Fujikawa K., Fort F.L., Samejima K., Sakamoto Y. Genotoxic potency in Drosophila melanogaster of selected aromatic amines and polycyclic aromatic hydrocarbons as assayed in the DNA repair test. Mutat. Res. 1993;290:175–182. doi: 10.1016/0027-5107(93)90157-b. [DOI] [PubMed] [Google Scholar]
- 18.Seong K.M., Coates B.S., Berenbaum M.R., Clark J.M., Pittendrigh B.R. Comparative CYP-omic analysis between the DDT-susceptible and -resistant Drosophila melanogaster strains 91-C and 91-R. Pest Manag. Sci. 2018;74:2530–2543. doi: 10.1002/ps.4936. [DOI] [PubMed] [Google Scholar]
- 19.Brun A., Cuany A., Mouel T.L.E., Berge J., Amichot M. Inducibility of the Drosophila melanogaster phenobarbital in insecticide susceptible or resistant strains. Insect Biochem. Mol. Biol. 1996;26:697–703. doi: 10.1016/s0965-1748(96)00036-7. [DOI] [PubMed] [Google Scholar]
- 20.Abolaji A.O., Kamdem J.P., Lugokenski T.H., Nascimento T.K., Waczuk E.P., Farombi E.O. Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic. Biol. Med. 2014;71:99–108. doi: 10.1016/j.freeradbiomed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Abolaji A.O., Fasae K.D., Iwezor C.E., Aschner M., Farombi E.O. Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Toxicol Reports. 2020;7:261–268. doi: 10.1016/j.toxrep.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 26.Wolff S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. doi: 10.1016/S0076-6879(94)33021-2. [DOI] [Google Scholar]
- 27.Mohammed B.R., Malang S.M., Kawe S., Agbede R.I.S., Finn R.D. Cytochrome P450s in Anopheles gambiae (Diptera: Culicidae) and Insecticide Resistance in Africa: A Mini Review. Entomol. Ornithol. Herpetol. Curr. Res. 2017;06 doi: 10.4172/2161-0983.1000200. [DOI] [Google Scholar]
- 28.Grotewiel M.S., Martin I., Bhandari P., Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Alexander E.M., Aguiyi J.C., Mdekera I.W., Ogwu O.S., Imoleayo O.O., Ugokwe C.V. The climbing performance, neuromuscular transmitter (ACHE) activity, reproductive performance and survival of Drosophila melanogaster fed diet with Mangifera indica cold aqueous leaf extract. J. Appl. Life Sci. Int. 2019:1–11. doi: 10.9734/jalsi/2019/v22i230120. [DOI] [Google Scholar]
- 30.Ramesh A., Harris K.J., Archibong A.E. Reproductive toxicity of polycyclic aromatic hydrocarbons. Reprod. Dev. Toxicol. 2017:745–763. doi: 10.1016/B978-0-12-804239-7.00040-8. [DOI] [Google Scholar]
- 31.Madeen E.P., Williams D.E. vol. 32. 2017. pp. 73–81. (Environmental PAH Exposure and Male Idiopathic Infertility: A Review on Early Life Exposures and Adult Diagnosis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng C., Dang F., Gao J., Zhao H., Qi S., Gao M. Acute benzo[a]pyrene treatment causes different antioxidant response and DNA damage in liver, lung, brain, stomach and kidney. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shelly C.L.M.D. Regulation of glutathione synthesis. Mol. Aspects Med. 2009;30:42–59. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash M., Shetty M.S., Tilak P., Anwar N. Total Thiols: Biomedical importance and their alteration in various disorders. Online J. Health Allied Sci. 2009;8 [Google Scholar]
- 35.Cummins I., Dixon D.P., Freitag-Pohl S., Skipsey M., Edwards R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab. Rev. 2011;43:266–280. doi: 10.3109/03602532.2011.552910. [DOI] [PubMed] [Google Scholar]
- 36.Deavall D.G., Martin E.A., Horner J.M., Roberts R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012;2012 doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nandi A., Yan L.J., Jana C.K., Das N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H.S., Kwack S.J., Lee B.M. Lipid peroxidation, antioxidant enzymes, and benzo[a]pyrene-quinones in the blood of rats treated with benzo[a]pyrene. Chem. Biol. Interact. 2000;127:139–150. doi: 10.1016/S0009-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 39.Adedara I.A., Daramola Y.M., Dagunduro J.O., Aiyegbusi M.A., Farombi E.O. Renoprotection of Kolaviron against benzo (A) pyrene-induced renal toxicity in rats. Ren. Fail. 2015;37:497–504. doi: 10.3109/0886022X.2015.1006085. [DOI] [PubMed] [Google Scholar]
- 40.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Habib S., Ali A. Biochemistry of nitric oxide. Indian J. Clin. Biochem. 2011;26:3–17. doi: 10.1007/s12291-011-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardonnière K., Huc L., Podechard N., Fernier M., Tekpli X., Gallais I. Benzo[a]pyrene-induced nitric oxide production acts as a survival signal targeting mitochondrial membrane potential. Toxicol. In Vitro. 2015;29:1597–1608. doi: 10.1016/j.tiv.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A., Patel S., Gupta Y.K., Singh M.P. Involvement of endogenous nitric oxide in myeloperoxidase mediated benzo(a)pyrene induced polymorphonuclear leukocytes injury. Mol. Cell. Biochem. 2006;286:43–51. doi: 10.1007/s11010-005-9083-5. [DOI] [PubMed] [Google Scholar]
- 44.Dutta K., Ghosh D., Nazmi A., lal Kumawat K., Basu A. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colovic M.B., Krstic D.Z., Lazarevic-Pasti T.D., Bondzic A.M., Vasic V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159x11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang J.J., Fang H.W. Polycyclic aromatic hydrocarbons inhibit the activity of acetylcholinesterase purified from electric eel. Biochem. Biophys. Res. Commun. 1997;238:367–369. doi: 10.1006/bbrc.1997.7293. [DOI] [PubMed] [Google Scholar]
- 47.Niu Q., Zhang H., Li X., Li M. Benzo[a]pyrene-induced neurobehavioral function and neurotransmitter alterations in coke oven workers. Occup. Environ. Med. 2010;67:444–448. doi: 10.1136/oem.2009.047969. [DOI] [PubMed] [Google Scholar]
- 48.Menozzi P., Shi M.A., Lougarre A., Tang Z.H., Fournier D. Mutations of acetylcholinesterase which confer insecticide resistance in Drosophila melanogaster populations. BMC Evol. Biol. 2004;4:1–7. doi: 10.1186/1471-2148-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agianian B., Tucker P.A., Schouten A., Leonard K., Bullard B., Gros P. Structure of a Drosophila sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidation products. J. Mol. Biol. 2003;326:151–165. doi: 10.1016/S0022-2836(02)01327-X. [DOI] [PubMed] [Google Scholar]