Abstract
Colorectal carcinoma (CRC) ranks as the third most common malignancy. Long non-coding RNA DLGAP1-AS1 was reported to be dysregulated and to play a pivotal role in hepatocellular carcinoma (HCC). This work aims to analyze the functions and molecular basis of DLGAP1-AS1 in CRC progression and 5-fluorouracil resistance. Cell Counting Kit-8 (CCK-8) assay, Transwell assay, flow cytometry, and western blot were utilized to measure the CRC cell activity, invasiveness, and apoptosis. RNA immunoprecipitation (RIP) and dual-luciferase reporter gene assay were adopted to verify the direct mutual action between DLGAP1-AS1 and miR-149-5p. The effect of DLGAP1-AS1 knockdown on tumor growth and chemosensitivity of 5-fluorouracil (5-FU) were investigated in the mouse CRC xenograft models. Functional assays showed that silencing DLGAP1-AS1 expression remarkably inhibited cell proliferation and aggressiveness ability and enhanced apoptosis rate and cell chemosensitivity to 5-FU. In addition, miR-149-5p was identified as a tumor suppressor and a direct downstream target of DLGAP1-AS1 in CRC. Furthermore, miR-149-5p was confirmed to directly bind to TGFB2 and DLGAP1-AS1 could regulate the expression of TGFB2 signaling pathway via miR-149-5p in CRC. These new findings indicate that DLGAP1-AS1 knockdown inhibited the progression of CRC and enhanced the 5-FU sensitivity of CRC cells through miR-149-5p/TGFB2 regulatory axis, suggesting that DLGAP1-AS1 may be a promising therapeutic target for CRC.
Keywords: colorectal cancer, lncRNA, DLGAP1-AS1, miR-149-5p, TGFB2, chemoresistance
Graphical Abstract

This research is the first to explore the effect of the interactions among DLGAP1-AS1, miR-149-5p, and TGFB2 in CRC. The authors found that DLGAP1-AS1 knockdown repressed progression and chemoresistance of CRC by modulating the miR-149-5p/TGFB2/Smad2 signaling pathway, providing a promising therapeutic target for CRC.
Introduction
Colorectal carcinoma (CRC) is the third most common cancer disease and the second leading cause of cancer-associated deaths globally.1 According to cancer statistics in 2019, there were an estimated 1.8 million newly diagnosed cases and 700,000 deaths from CRC worldwide.2 Recently, an epidemiological research suggested a continuous increase of morbidity rate in CRC patients under the age of 50 in the past few decades.3 Both environmental and genetic factors are believed to be involved in the development of CRC.4 Despite the advances made in the treatment of CRC in recent years, such as targeted therapies and immunotherapy, the cure rates and long-term survival of CRC is still far from satisfactory.5 Therefore, it is still imperative to further understand the molecular pathogenesis of CRC in order to identify novel cancer biomarker and therapeutic targets for CRC.
Long non-coding RNAs (lncRNAs) were a class of non-coding transcripts longer than 200 nucleotides with limited protein-coding potential.6 lncRNAs have been demonstrated as critical mediators in the processes of tumorigenesis by influencing microRNA (miRNA)-mediated gene regulation, mRNA stability, chromatin structure, and RNA splicing.7 Moreover, plenty of evidence suggested that aberrant lncRNA expression was also involved in chemoresistance of multiple tumors through various signaling pathways and regulation mechanisms.8 As to CRC, a large number of lncRNAs are dys-regulated.9, 10, 11 NEAT1 was identified as a novel diagnostic biomarker and demonstrated to be associated with various clinical prognosis in CRC.12 LINC01567 was verified to participate in the development and progression of CRC through sponging miR-93.13 Yu et al.14 validated that the overexpression of a new lncRNA u50535 was closely related to adverse prognosis in CRC patients. Located at chromosome 18:3,593,730–3,598,350, lncRNA DLGAP1 antisense 1 (DLGAP1-AS1) is a lncRNA with few annotations, whose involvement in CRC remains uncharacterized. Recently, some studies showed that DLGAP1-AS1 was able to promote epithelial-mesenchymal transition and tumorigenesis in hepatocellular carcinoma (HCC) via various regulation mechanisms and signaling pathways, but the functions of DLGAP1-AS1, particularly its role in the progression and chemoresistance of CRC, is still unknown.
miRNAs are defined as a class of small non-coding transcripts of 20–22 nucleotides in length that exert their functions through directly binding to the 3′ untranslated region (3′ UTR) of mRNA at complementary sequences, which results in mRNA cleavage or translation inhibition.15 Abundant studies indicated that miRNAs participated in the regulation of tumorigenesis and drug resistance in various tumors,16 including CRC.17 Recently, miR-149-5p has been identified as a tumor suppressor in the progression and chemoresistance of a variety of tumors including CRC.18 However, the role of miR-149-5p on tumorigenesis and drug resistance in CRC is still not very clear to this day. It is worth noting that more and more studies have shown that lncRNAs can function as a molecular sponge or competing endogenous RNA (ceRNA) to suppress the expressions and functions of miRNAs, hence leading to derepression of its target mRNA.19 Interestingly, DLGAP1-AS1 was predicted to directly interact with miR-149-5p. Hence, this study was aimed to examine whether DLGAP1-AS1 could participate in the modulation of tumorigenesis and drug resistance by interacting with miR-149-5p in CRC.
In this study, we first validated the upregulation of DLGAP1-AS1 in CRC tissues and that the expression level of hsa-miR-149-5p was negatively related with DLGAP1-AS1 expression as well as clinicopathologic characteristics in CRC. We further explored the functions and underlying molecular mechanisms of DLGAP1-AS1 in the proliferation, apoptosis, invasion, and drug resistance of CRC cells.
Results
lncRNA DLGAP1-AS1 was highly expressed in colorectal cancer and indicated a poor prognosis
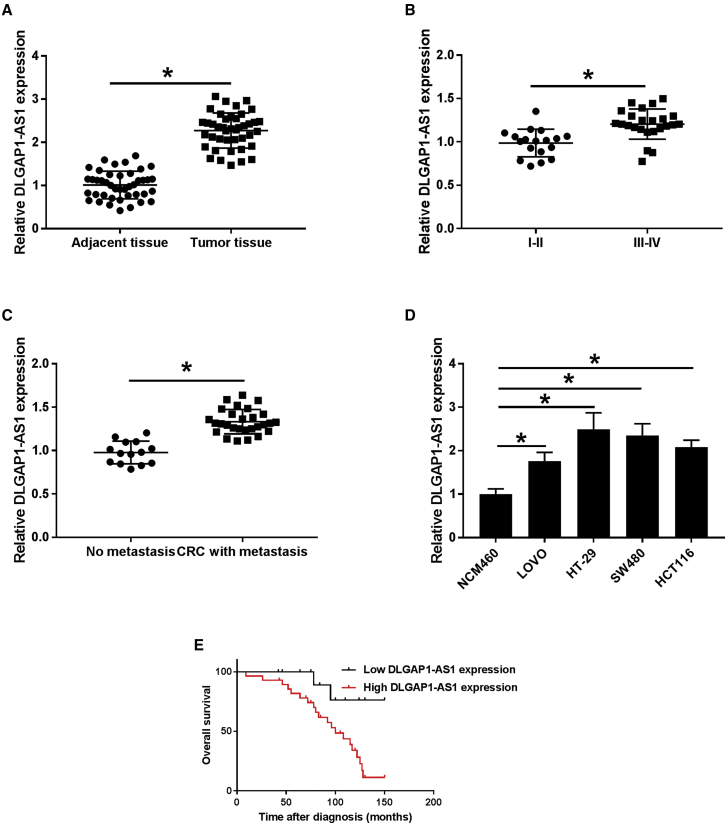
DLGAP1-AS1 was confirmed to be associated with the tumorigenesis and progression of HCC, but the relationship between DLGAP1-AS1 and CRC has not been reported. lncRNA DLGAP1-AS1 expression in colorectal cancer tissues and cell lines was first determined using qRT-PCR. The results suggested that DLGAP1-AS1 was significantly upregulated in CRC tissues compared with matched para-carcinoma tissues (Figure 1A). Additionally, the DLGAP1-AS1 expression level in patients with advanced clinical stage (phase III–IV) was found to be significantly upregulated compared with the patients in early clinical stage (phase I–II) (Figure 1B). Moreover, the patients with distant metastases showed higher DLGAP1-AS1 expression compared to those without distant metastases (Figure 1C). As shown in Table S1, the high expression level of DLGAP1-AS1 was positively associated with distant metastasis (p = 0.019), pathological stage (p < 0.001), and tumor size (p = 0.003) but had no correlation with age, gender, and tumor localization of the patients (each p > 0.05). These results indicated that DLGAP1-AS1 might be involved in the regulation of CRC tumorigenesis and progression. DLGAP1-AS1 expression in 4 CRC cell lines was also markedly elevated compared with that in normal colorectal epithelial cell line NCM460 (Figure 1D). The correlation between overall survival rate and DLGAP1-AS1 expression was evaluated through Kaplan-Meier analysis. As shown in Figure 1E, high DLGAP1-AS1 expression level indicates a poorer prognosis than those with lower level in CRC patients.
Figure 1.
The expression level of DLGAP1-AS1 was verified in CRC samples and CRC cell lines, and the correlation between DLGAP1-AS1 expression and clinical manifestations and prognosis was analyzed
(A) The expression level of DLGAP1-AS1 was detected by qRT-PCR in cancer and adjacent tissues of CRC patients. (B) The expression of DLGAP1-AS1 was detected by qRT-PCR in different tumor stages. (C) The expression level of DLGAP1-AS1 was compared between metastatic and non-metastatic CRC patients. (D) The expression levels of DLGAP1-AS1 in LoVo, HT-29, SW480, HCT116 CRC cell lines, and NCM460 normal colon epithelial cells were detected by qRT-PCR. (E) Kaplan-Meier overall survival (OS) curves were illustrated on the basis of DLGAP1-AS1 level. Data are the means ± SD of triplicate determinants (∗p < 0.05).
lncRNA DLGAP1-AS1 promoted CRC cell proliferation and reduced cell apoptosis
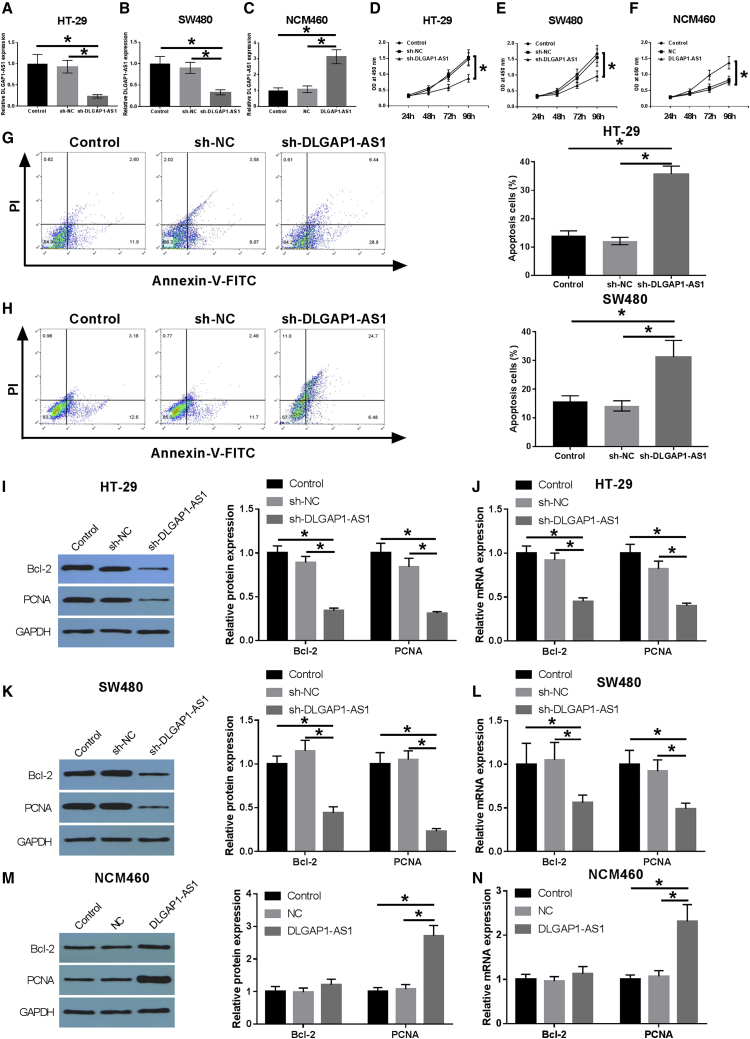
To explore the biological function of DLGAP1-AS1 in CRC cells, DLGAP1-AS1 expressions in HT-29 and SW480 cells were inhibited by transfection with sh-DLGAP1-AS1, and its expression in NCM460 was upregulated by transfection with plasmid encoding DLGAP1-AS1. As shown in Figures 2A and 2B, sh-DLGAP1-AS1 markedly downregulates the expression of DLGAP1-AS1 in HT-29 and SW480 cells (p < 0.05), pointing out a high transfection efficiency. The expression of DLGAP1-AS1 was significantly increased in NCM460 cells with the transfection of DLGAP1-AS1 (Figure 2C). Proliferation of the treated cells was evaluated using a Cell Counting Kit-8 (CCK-8) assay. Data showed that HT-29 and SW480 cells proliferation were significantly inhibited by DLGAP1-AS1 silencing (Figures 2D and 2E), and NCM460 cells proliferation was markedly promoted by DLGAP1-AS1 overexpression (Figure 2F). The apoptosis rate of transfected HT-29 and SW480 cells were detected by flow cytometry. Our results indicated that downregulation of DLGAP1-AS1 remarkably augmented the apoptosis rate in CRC cells (Figures 2G and 2H). In addition, western blot and qRT-PCR assay revealed that the silencing or overexpression of DLGAP1-AS1 significantly influenced cell proliferation levels and apoptosis-related proteins in HT-29, SW480, and NCM460 cells, including Bcl-2 and proliferating cell nuclear antigen (PCNA) (all p < 0.05; Figures 2I–2N). These results revealed that lncRNA DLGAP1-AS1 may promote the development of CRC cells as an oncogene.
Figure 2.
DLGAP1-AS1 can promote the proliferation and inhibit apoptosis of HT-29 and SW480 cells
(A and B) The HT-29 and SW480 cells were divided into control group, shNC group, and sh-DLGAP1-AS1 group. The expression level of DLGAP1-AS1 mRNA was detected by qRT-PCR. (C) The NCM460 cells were divided into control group, NC group, and DLGAP1-AS1 group. The expression level of DLGAP1-AS1 mRNA was detected by qRT-PCR. (D–F) The CCK-8 method was used to detect the cell viability of HT-29, SW480, and NCM460 cells. (G and H) Apoptosis was detected by flow cytometry in HT-29 and SW480 cells. (I) Expression levels of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) were detected by western blot (WB) in HT-29 cells. (J) qRT-PCR was used to detect the expression level of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) in HT-29 cells. (K) Expression levels of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) were detected by WB in SW480 cells. (L) qRT-PCR was used to detect the expression level of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) in SW480 cells. (M) Expression levels of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) were detected by WB in NCM460 cells. (N) qRT-PCR was used to detect the expression level of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) in NCM460 cells. Data are the means ± SD of triplicate determinants (∗p < 0.05).
lncRNA DLGAP1-AS1 promoted CRC cell migration and invasion
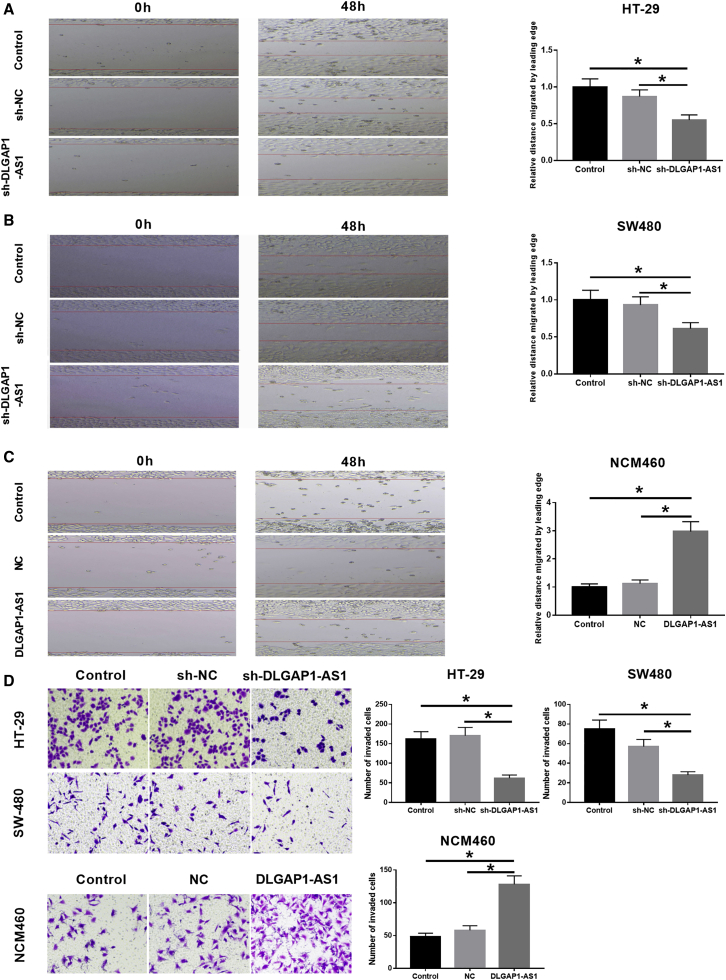
The data of the wound-healing assay suggested that silencing of DLGAP1-AS1 obviously repressed the migration abilities of HT-29 and SW480 cells based on the distance migrated by the leading edge of cells (Figures 3A and 3B). On the contrary, overexpression of DLGAP1-AS1 significantly increased the migration abilities of NCM460 cells (Figure 3C). Additionally, we conducted a Matrigel Transwell invasion assay to observe the effects of DLGAP1-AS1 on CRC cell invasion. The results suggested that the invasive abilities were markedly decreased in DLGAP1-AS1-silenced HT-29 or SW480 cells and increased in DLGAP1-AS1-overexpressed NCM460 cells (Figure 3D).
Figure 3.
DLGAP1-AS1 promotes the migration and invasion of CRC cells
(A–C) Scratch healing tests were used to detect the mobility of cells in HT-29, SW480, and NCM460 cells. (D) Matrigel experiments were used to detect cell invasion in HT-29, SW480, and NCM460 cells. Data are the means ± SD of triplicate determinants (∗p < 0.05).
DLGAP1-AS1 acted as a sponge for miR-149-5p in CRC cells
Previous evidence indicated that lncRNAs always function as ceRNAs for miRNAs to regulate the downstream target genes. Thus, we investigated whether lncRNA DLGAP1-AS1 functions as a sponge for a certain miRNA in CRC cells. First, the bioinformatics prediction software StarBase predicted that miR-149-5p contains the potential binding site for DLGAP1-AS1 (Figure 4A). To verify the direct interaction between miR-149-5p and DLGAP1-AS1, a luciferase reporter assay was conducted with wild-type or mutant DLGAP1-AS1 3′ UTR luciferase vector (DLGAP1-AS1-WT or DLGAP1-AS1-Mut) in HT-29 and SW480 cells. It was found that miR-149-5p overexpression remarkably reduced the luciferase activity of WT DLGAP1-AS1 (DLGAP1-AS1-WT) rather than that of Mut DLGAP1-AS1 (DLGAP1-AS1-Mut) in HT-29 cells (Figures 4B and 4C). Furthermore, miR-149-5p expression level was markedly increased following DLGAP1-AS1 knockdown in HT-29 and SW480 cells (Figures 4D and 4E). In addition, the results showed that miR-149-5p expression was markedly repressed in CRC tissues (Figure 4F) as well as cell lines (Figure 4H) compared with control. The negative correlation was found between DLGAP1-AS1 and miR-149-5p expression (Figure 4G). As shown in Figures 4I–4L, DLGAP1-AS1 and miR-149-5p were highly enriched in the Ago2 complex compared with the immunoprecipitates in negative immunoglobulin G (IgG) control. The combination of DLGAP1-AS1 and miR-149-5p was corroborated by the results of an RNA immunoprecipitation (RIP) assay, which validated the interaction between DLGAP1-AS1 and miR-149-5p in CRC cell lines. These data showed that DLGAP1-AS1 acted as a sponge for miR-149-5p in CRC cells.
Figure 4.
There is a targeted regulation relationship between DLGAP1-AS1 and hsa-miR-149-5p
(A) The binding site of hsa-miR-149-5p on DLGAP1-AS1 was predicted by the bioinformatics method. (B and C) The target regulation of DLGAP1-AS1 and hsa-miR-149-5p was confirmed by double luciferase reporter assay in HT-29 and SW480 cells, respectively. (D and E) After interfering with the expression level of DLGAP1-AS1, the expression level of hsa-miR-149-5p was detected by qRT-PCR in HT-29 and SW480 cells, which suggested that DLGAP1-AS1 was negatively correlated with the expression level of hsa-miR-149-5p. (F) qRT-PCR was used to detect the expression of hsa-miR-149-5p in CRC tissues and adjacent tissues. (G) A negative correlation between hsa-miR-149-5p and DLGAP1-AS1 was determined by Spearman’s correlation analysis. (H) The expression levels of hsa-miR-149-5p in LoVo, HT-29, SW480, HCT116 CRC cell lines, and NCM460 normal colon epithelial cells were detected by qRT-PCR. (I–L) The co-precipitated RNA were detected by an RNA immunoprecipitation experiment and qRT-PCR in HT-29 and SW480 cells. DLGAP1-AS1 and hsa-miR-149-5p were presented as fold enrichment in Ago2 relative to IgG immunoprecipitate. Data are the means ± SD of triplicate determinants (∗p < 0.05).
Ectopic expression of miR-149-5p suppressed proliferation and enhanced apoptosis in CRC cells
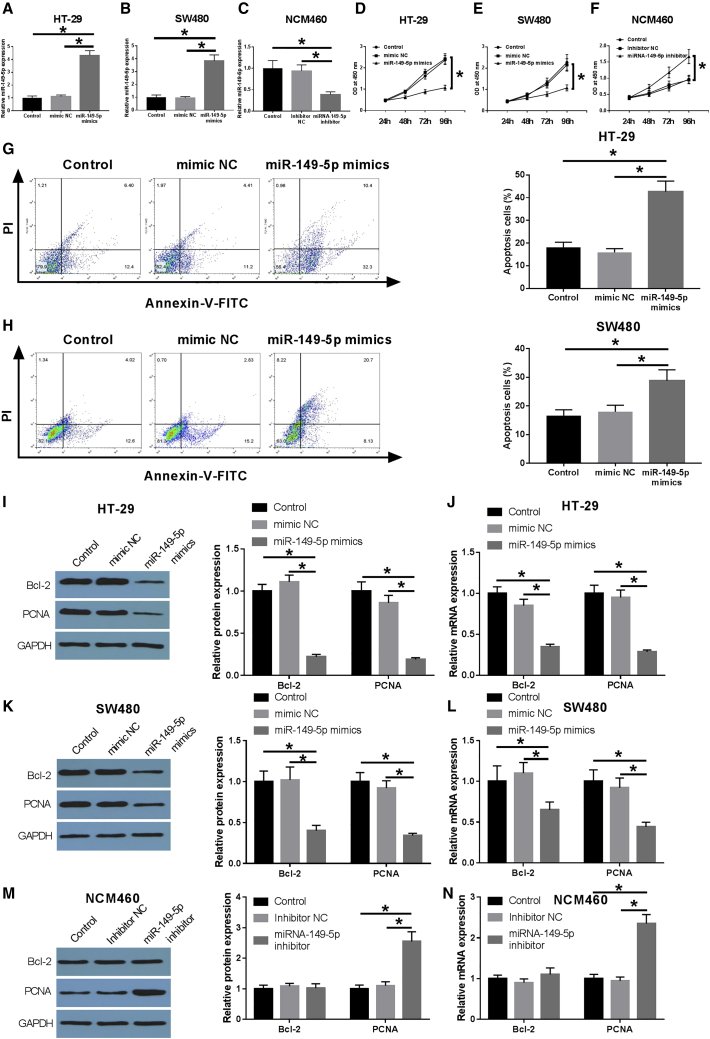
To investigate the biological functions of miR-149-5p on CRC cells, we transfected miR-149-5p mimics into HT-29 and SW480 cells to increase the expression level of miR-149-5p (Figures 5A and 5B). miR-149-5p inhibitor was transfected into NCM460 cells to block the expression of miR-149-5p (Figure 5C). Data of CCK-8 assay indicated that miR-149-5p overexpression could notably suppress the proliferation of HT-29 and SW480 cells (Figures 5D and 5E), and miR-149-5p inhibitor could markedly promote the proliferation of NCM460 cells (Figure 5F). Cell apoptosis analysis demonstrated that the apoptosis rates in HT-29 and SW480 cells were markedly increased after miR-149-5p overexpression (Figures 5G and 5H). Western blot and qRT-PCR assay showed that the overexpression or inhibition of miR-149-5p remarkably influences the levels of cell proliferation and apoptosis-related proteins in HT-29, SW480, and NCM460 cells, including Bcl-2 and PCNA (Figures 5I–5N). These above results suggested that miR-149-5p might function as a tumor suppressor in CRC development.
Figure 5.
Hsa-miR-149-5p inhibited the proliferation of CRC cells and promoted apoptosis
(A and B) The HT-29 and SW480 cells were divided into control group, mimic NC group, and miR-149-5p mimic group. The expression level of miR-149-5p was detected by qRT-PCR. (C) The NCM460 cells were divided into control group, inhibitor NC group, and miR-149-5p inhibitor group. The expression level of miR-149-5p was detected by qRT-PCR. (D–F) The CCK-8 method was used to detect the cell viability of HT-29, SW480, and NCM460 cells. (G and H) Apoptosis was detected by flow cytometry in HT-29 and SW480 cells. (I) Expression levels of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) were detected by WB in HT-29 cells. (J) qRT-PCR was used to detect the expression level of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) in HT-29 cells. (K) Expression levels of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) were detected by WB in SW480 cells. (L) qRT-PCR was used to detect the expression level of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) in SW480 cells. (M) Expression levels of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) were detected by WB in NCM460 cells. (N) qRT-PCR was used to detect the expression levels of apoptosis- and proliferation-related indexes (Bcl-2, PCNA) in NCM460 cells. Data are the means ± SD of triplicate determinants (∗p < 0.05).
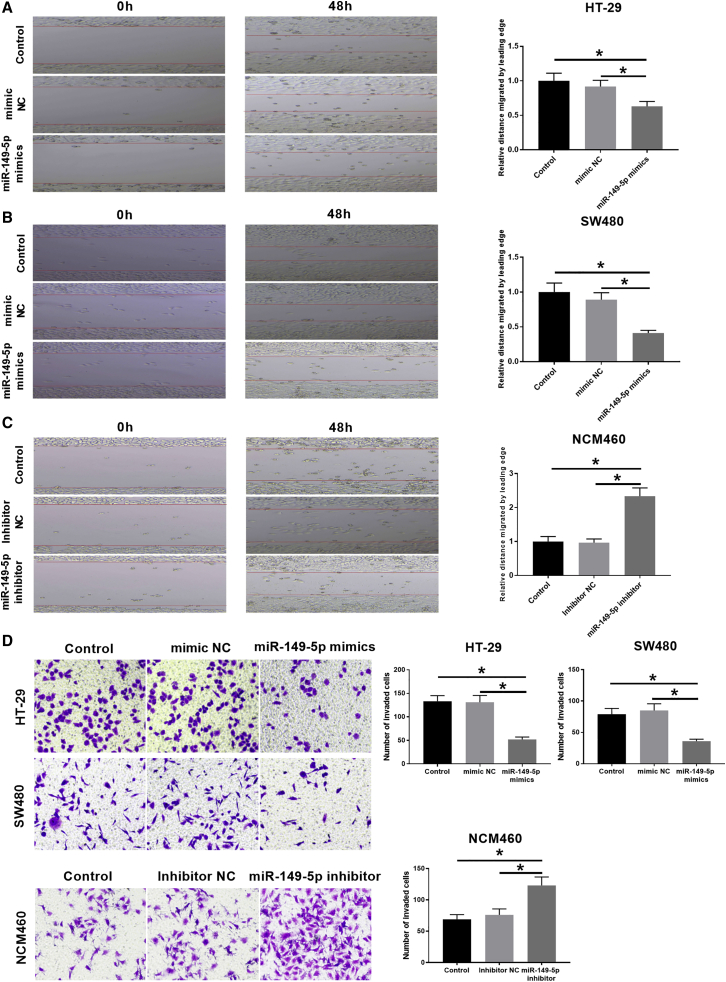
Ectopic expression of miR-149-5p suppressed CRC cell migration and invasion
The data of the wound-healing assay suggested that miR-149-5p overexpression could obviously suppress the migration abilities of HT-29 and SW480 cells based on the distance migrated by the leading edge of cells (Figures 6A and 6B). On the contrary, the miR-149-5p inhibitor significantly increased the migration abilities of NCM460 cells (Figure 6C). Additionally, we conducted a Matrigel Transwell invasion assay to observe the effects of miR-149-5p overexpression on CRC cell invasion. The results suggested that the invasive abilities were markedly decreased in miR-149-5p overexpressed HT-29 or SW480 cells and increased in miR-149-5p inhibited NCM460 cells (Figure 6D).
Figure 6.
Hsa-miR-149-5p inhibited the migration and invasion of CRC cells
(A–C) Scratch healing tests were used to detect the mobility of cells in HT-29, SW480, and NCM460 cells. (D) Matrigel experiments were used to detect cell invasion in HT-29, SW480, and NCM460 cells. Data are the means ± SD of triplicate determinants (∗p < 0.05).
TGFB2 was a direct target of miR-149-5p in colorectal cancer
Our results show that miR-149-5p was downregulated in CRC and acted as a tumor suppressor. Here, we further explored the possible target gene of miR-149-5p in CRC. Using the TargetScan tool, TGFB2 was found to be a potential target of miR-149-5p (Figure 7A). Then, a luciferase reporter assay was conducted to verify the prediction. Our data indicated that the luciferase activities were notably reduced in HT-29 and SW480 cells when co-transfected with WT TGFB2 vectors (TGFB2-WT) and miR-149-5p mimics but no change when co-transfected with Mut TGFB2 vectors (TGFB2-Mut) and miR-149-5p mimics (Figures 7B and 7C). In addition, we detected the mRNA level and protein expression of TGFB2 in HT-29. SW480 and NCM460 cells after miR-149-5p overexpression or inhibition. Data showed that miR-149-5p overexpression remarkably repressed the protein and mRNA levels of TGFB2 in CRC cells (Figures 7D–7G), and miR-149-5p inhibition significantly increased the protein and mRNA levels of TGFB2 in NCM460 cells (Figures 7H and 7I). In addition, TGFB2 expression was found to be significantly upregulated in CRC tissues (Figure 7J) and cell lines (Figure 7K) compared with control. The negative correlation was found between TGFB2 and miR-149-5p expression (Figure 7L). As shown in Figures 7M–7P, TGFB2 and miR-149-5p were highly enriched in Ago2 complex compared with the immunoprecipitates in negative IgG control. These above results indicated that TGFB2 was directly targeted by miR-149-5p in CRC cells.
Figure 7.
There is a targeted regulation relationship between TGFB2 and hsa-miR-149-5p
(A) The binding site of hsa-miR-149-5p on TGFB2 was predicted by the bioinformatics method. (B and C) The binding relationship of TGFB2 and hsa-miR-149-5p was confirmed by double luciferase reporter assay in HT-29 and SW480 cells, respectively. (D–I) After increasing or inhibiting the expression level of hsa-miR-149-5p, the expression levels of TGFB2 were detected by WB and qRT-PCR in HT-29, SW480, and NCM460 cells, respectively. (J) qRT-PCR was used to detect the expression of TGFB2 in CRC and adjacent tissues. (K) The expression levels of TGFB2 in LoVo, HT-29, SW480, HCT116 CRC cell lines, and NCM460 normal colon epithelial cells were detected by qRT-PCR. (L) A negative correlation between hsa-miR-149-5p and TGFB2 was determined by Spearman’s correlation analysis. (M–P) The co-precipitated RNA were detected by RNA immunoprecipitation experiment and qRT-PCR in HT-29 and SW480 cells. TGFB2 and hsa-miR-149-5p were presented as fold enrichment in Ago2 relative to IgG immunoprecipitate. Data are the means ± SD of triplicate determinants (∗p < 0.05).
DLGAP1-AS1 promoted CRC cell proliferation and reduced cell apoptosis via miR-149-5p/TGFB2 axis
Based on the results above, we hypothesized that lncRNA DLGAP1-AS1 may promote CRC cell proliferation and reduced cell apoptosis via the miR-149-5p/TGFB2 axis. Thus, we transfected miR-149-5p inhibitors into HT-29 and SW480 cells to downregulate miR-149-5p expression after DLGAP1-AS1 knockdown (Figures 8A and 8B). Then, a CCK-8 assay indicated that miR-149-5p downregulation partly reversed the suppressive effect of DLGAP1-AS1 knockdown on proliferation in HT-29 and SW480 cells (Figures 8C and 8D). The apoptosis assay suggested that miR-149-5p downregulation partly reversed the stimulative effect of DLGAP1-AS1 knockdown on HT-29 and SW480 cells apoptosis (Figures 8E and 8F). In addition, we examined the mRNA and protein levels of the TGFB2/Smad2 signaling pathway and proliferation-related genes in HT-29 cells when transfected with DLGAP1-AS1 siRNA or/and co-transfected with miR-149-5p inhibitors. Data showed that the activity of the TGFB2/Smad2 signaling pathway was dramatically decreased after DLGAP1-AS1 small interfering RNA (siRNA) transfection, while these changes were partly abolished by co-transfecting with miR-149-5p inhibitors (Figures 8G–8I). These data revealed that DLGAP1-AS1 could promote cell proliferation and reduce cell apoptosis via the miR-149-5p/TGFB2/Smad2 axis in CRC cells.
Figure 8.
DLGAP1-AS1 directly bound hsa-miR-149-5p and further affects the proliferation and apoptosis of CRC cells by regulating the TGFB2 signaling pathway
(A and B) CRC cells were divided into four groups: control, shRNA NC, sh-DLGAP1-AS1, and sh-DLGAP1-AS1+hsa-miR-149-5p inhibitor. The mRNA expression level of DLGAP1-AS1 was detected by qRT-PCR in HT-29 and SW480 cells. (C and D) CCK-8 assay was used to detect the cell viability of HT-29 and SW480 cells, respectively. (E and F) Flow cytometry was adopted to detect the level of apoptosis in HT-29 and SW480 cells. (G and H) The expression level of TGFB2 and its downstream signal pathway molecules p-Smad2, Smad2, and proliferation-related index (PCNA) was detected by WB. (I) The expression level of TGFB2 and its downstream signal pathway molecules p-Smad2, Smad2, and proliferation-related index (PCNA) was detected by qRT-PCR. Data are the means ± SD of triplicate determinants (∗p < 0.05).
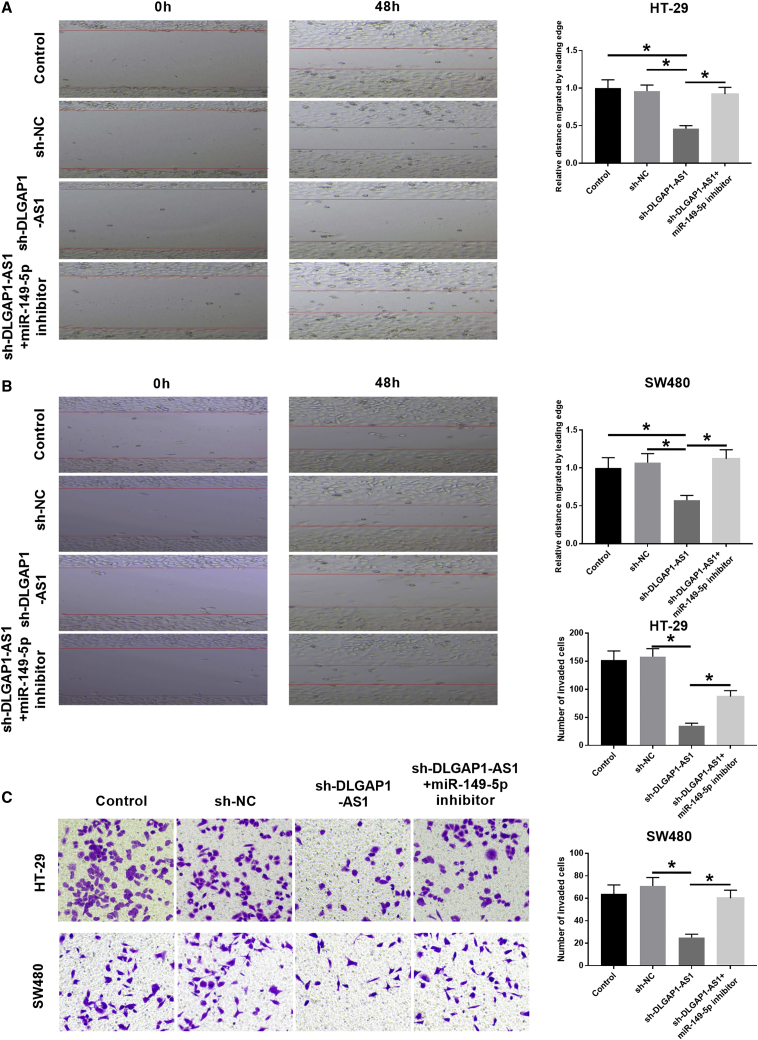
DLGAP1-AS1 promoted CRC cell migration and invasion via the miR-149-5p/TGFB2 axis
The data of the wound-healing assay indicated that miR-149-5p downregulation partly reversed the repressive influence of DLGAP1-AS1 knockdown on HT-29 and SW480 cell migration abilities (Figures 9A and 9B). Additionally, the Matrigel Transwell invasion assay showed that the suppressive effect of DLGAP1-AS1 silencing on HT-29 and SW480 cell invasion could be partly reversed by miR-149-5p downregulation (Figure 9C).
Figure 9.
DLGAP1-AS1 directly bound hsa-miR-149-5p and further affects the migration and invasion of CRC cells by regulating the TGFB2 signaling pathway
(A and B) Scratch healing test was used to detect the movement ability of HT-29 and SW480 cells. (C) Matrigel experiments were used to detect cell invasion in HT-29 and SW480 cells. Data are the means ± SD of triplicate determinants (∗p < 0.05).
DLGAP1-AS1 promotes the chemoresistance of CRC cells through targeting the miR-149-5p-TGFB2 signaling pathway
To analyze whether DLGAP1-AS1 and miR-149-5p could modulate the drug resistance of CRC cells, the expression levels of DLGAP1-AS1, miR-149-5p, and TGFB2 were compared between HT-29 cells and 5-FU-resistant HT-29 (HT-29/5FU) cells (Figure 10A). The results showed that DLGAP1-AS1 and TGFB2 were upregulated in HT-29/5-FU cells, while hsa-miR-149-5p was the opposite. CRC cells were treated with 5-FU after DLGAP1-AS1 knockdown and/or miR-149-5p inhibition. The results indicated that the use of 5-FU could reduce the expression level of DLGAP1-AS1, and the inhibition of hsa-miR-149-5p could partially restore the expression level of DLGAP1-AS1 (Figure 10B). The results of the CCK-8 (Figure 10C) and apoptosis assays (Figures 10D an 10E77) showed that DLGAP1-AS1 knockout could enhance the sensitivity of HT-29 cells to 5-FU, but this influence was also partially reversed by silencing of miR-149-5p. Data also indicated that the protein and mRNA levels of TGFB2 were dramatically reduced after DLGAP1-AS1 siRNA transfection, while these changes were partly abolished by co-transfecting with miR-149-5p inhibitors (Figures 10F–10H). Therefore, these data suggested that miR-149-5p upregulation was crucial for increasing the sensitivity of CRC cells to 5-FU induced by DLGAP1-AS1 silencing.
Figure 10.
DLGAP1-AS1 contributed to the chemosensitivity of 5-FU in HT-29 cells
(A) The expression levels of DLGAP1-AS1, hsa-miR-149-5p, and TGFB2 in HT-29 and 5-FU-resistant HT-29 (HT-29/5FU) cells were detected by qRT-PCR. HT-29 cells were treated with 5-FU after DLGAP1-AS1 knockdown and/or miR-149-5p inhibition. (B) The expression level of DLGAP1-AS1 was detected by qRT-PCR. (C) The cell viability of each group was detected by CCK-8. (D and E) Apoptosis was detected by flow cytometry. (F and G) The expression level of TGFB2 was detected by WB. (H) The expression level of TGFB2 was detected by qRT-PCR. Data are the means ± SD of triplicate determinants (∗p < 0.05).
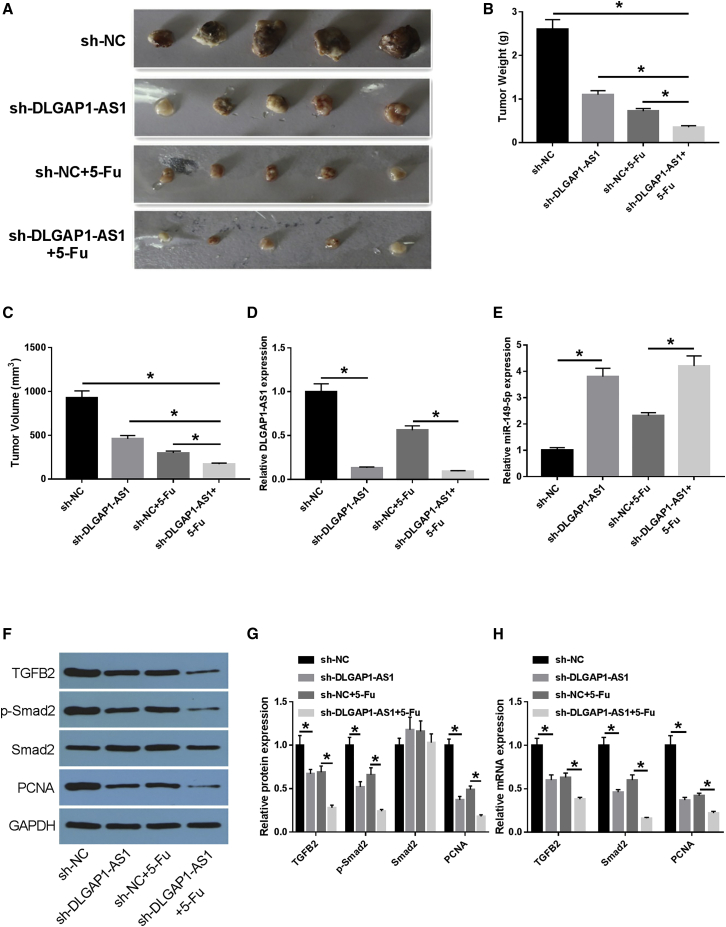
DLGAP1-AS1 knockdown inhibited tumor growth and enhanced 5-FU sensitivity in CRC in vivo
To further explore the effect of DLGAP1-AS1 silencing on CRC development and 5-FU sensitivity, the mouse xenograft models of CRC were established, and results indicated that 5-FU administration or DLGAP1-AS1 silencing suppressed tumor growth, and DLGAP1-AS1 knockdown increased the 5-FU-induced tumor-suppressive effect in vivo (Figures 11A–11C). Moreover, we further demonstrated that DLGAP1-AS1 and TGFB2/Smad2 signaling pathway activities were decreased (Figures 11D and 11F–11H), while miR-149-5p expression was enhanced (Figure 11E) in tumor tissues originating from HT-29 cells transfected with sh-DLGAP1-AS1. Finally, on the basis of the above findings, we concluded that DLGAP1-AS1 silencing could repress tumor development and enhance 5-FU sensitivity, possibly by regulating the miR-149-5p/TGFB2/Smad2 axis in CRC.
Figure 11.
DLGAP1-AS1 can inhibit the growth of xenograft tumor model of CRC in nude mice and promote the sensitivity of tumor tissue to 5-FU
(A) The subcutaneous transplanted tumor (drug-resistant cell line) model of CRC in nude mice was established. At 21 days upon cell implantation, tumors were excised and imaged. (B) Tumor weight of xenografts. (C) Tumor volume of xenografts. (D) The expression level of DLGAP1-AS1 was detected by qRT-PCR. (E) The expression of hsa-miR-149-5p was detected by qRT-PCR. (F and G) The expression levels of TGFB2 and its downstream signal pathway molecules p-Smad2, Smad2, and proliferation-related index (PCNA) were detected by WB. (H) The expression levels of TGFB2 and its downstream signal pathway molecule Smad2 and proliferation-related index (PCNA) were detected by qRT-PCR. Data are the means ± SD of triplicate determinants (∗p < 0.05).
Discussion
Recently, accumulative evidence has manifested that lncRNAs participate in the oncogenesis of various human cancers including CRC, indicating that lncRNAs could be used as biomarkers for prognosis, diagnosis, and treatment of CRC.20,21 For example, the lncRNA prostate cancer-associated ncRNA transcript 6 (PCAT6) was demonstrated to be upregulated and promote cell proliferation by regulating anti-apoptotic protein in CRC.22 Ma et al.23 reported that the CRC development was suppressed and chemosensitivity of CRC cells to adriamycin (ADR) was enhanced with BRAF-activated noncoding RNA (BANCR) downregulation possibly by modulating the miR-203/CSE1L axis, suggesting BANCR as a potential target for CRC therapy. Sun et al.24 showed that lncRNA SNHG15 promotes the CRC progression via acting as a sponging RNA through the miR-141/SIRT1/Wnt/β-catenin axis. These studies indicate that lncRNAs may play pivotal roles in a variety of biological processes of CRC. Therefore, identifying potential novel lncRNAs that are involved in the tumorigenesis and chemoresistance of CRC is valuable to establishing new therapeutic strategies and improving the prognosis for patients with CRC.
Herein, DLGAP1-AS1 was found to be upregulated in CRC tissues and cells and associated with clinical stage and distant metastases. Deng et al.25 reported that DLGAP1-AS1 promotes the aggressive behavior of gastric cancer by acting as a ceRNA for miRNA-628-5p and raising astrocyte-elevated gene 1 expression. Functional analyses showed that proliferation and invasion potency were decreased while apoptosis rate was increased in HT-29 and SW480 cells after DLGAP1-AS1 expression was silenced. To exclude the influence of apoptosis on the detection of cell invasion, the apoptotic inhibitor (Q-VD-OPH) was applied in HT-29, SW480 and NCM460 cells. The results indicates the same tendency of cells without treatment of Q-VD-OPH (Figure S1). In addition, data also suggested that DLGAP1-AS1 silencing enhanced 5-FU sensitivity in HT-29 cells. Also, our data suggested that DLGAP1-AS1 act as miRNA sponges to miR-149-5p and miR-149-5p inhibitor remarkably reverses the effects of decreased DLGAP1-AS1 on tumor progression and chemoresistance. Furthermore, we identified TGFB2 as a direct functional target gene of miR-149-5p in CRC cells.
Consistent with previous studies reported by Lin et al.,26 DLGAP1-AS1 promotes epithelial-mesenchymal transition and tumor development in HCC through the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and the Wnt/β-catenin pathway. Our results indicated that DLGAP1-AS1 expression was increased in CRC tissues and cell lines. Moreover, DLGAP1-AS1 silencing notably repressed the proliferation, migration, and invasion, while increasing apoptosis rate of CRC cells. Therefore, we deduce that DLGAP1-AS1 might play a pivotal role in carcinogenesis and development of CRC.
It is well established that lncRNAs can serve as a ceRNA of miRNAs to modulate the downstream target genes of miRNAs.27 Hence, an online prediction website was employed to screen for miRNAs that have potential binding sites in the 3′ UTR of DLGAP1-AS1. Among various candidate miRNAs, we selected miR-149-5p for the following research, considering its anti-tumor functions in a variety of cancers including HCC,28 oral squamous cell carcinoma,29 cervical cancer,30 and colorectal cancer.18 Results of the luciferase reporter assay indicated that DLGAP1-AS1 was a direct target gene of miR-149-5p. In addition, expression of miR-149-5p was dramatically decreased in CRC tissues and cell lines, and miR-149-5p overexpression could repress proliferation, migration, and invasion, while enhancing apoptosis rate in CRC cells. Moreover, TGFB2 was identified as a direct target of miR-149-5p in CRC. By using rescue assays, we found that DLGAP1-AS1 can promote cell proliferation and 5-FU chemoresistance and regulate the expression level of TGFB2 by acting as a ceRNA of miR-149-5p in CRC cells. These results suggest that lncRNA DLGAP1-AS1 promoted tumor progression via regulating the miR-149-5p/TGFB2 axis in CRC.
Next, in vivo assays further revealed that 5-FU administration or DLGAP1-AS1 silencing restrained tumor growth, and DLGAP1-AS1 knockdown could enhance 5-FU-induced anti-tumor function in the mouse xenograft model of CRC. Furthermore, an upregulation of miR-149-5p expression and a downregulation of TGFB2 were triggered by DLGAP1-AS1 knockdown, indicating that DLGAP1-AS1 reduction inhibited tumor growth and augmented 5-FU sensitivity through modulation of miR-149-5p/TGFB2/Smad2 pathway in vivo. Nevertheless, experiments around the relationship of DLGAP1-AS1 with the downstream signaling pathway of TGFB2 were not conducted in the current study. Further research is still needed to confirm our conclusions.
In conclusion, our study elucidated that DLGAP1-AS1 knockdown repressed CRC development and promoted 5-FU sensitivity by modulating the miR-149-5p/TGFB2/Smad2 signaling pathway in vitro and in vivo. DLGAP1-AS1-miR-149-5p-TGFB2 axis might provide a promising strategy for CRC diagnosis and treatment.
Materials and methods
Clinical specimens
This study gained the approval of the Ethics Committee of the First Hospital of Jilin University. A total of 42 pairs of CRC tumor tissues and the matched para-carcinoma tissues were collected in the surgery process from patients. The pathological diagnosis of CRC was confirmed by two pathologists independently. None of the participants had received any chemotherapy or radiotherapy treatment before surgery. The pathological features of CRC patients were shown in Table S1. Resected tumor tissues were immediately frozen and maintained in liquid nitrogen. Every patient signed an informed consent.
Bioinformatics analysis
ENCORI (http://starbase.sysu.edu.cn/) was employed to search and predict the potential interactive miRNAs of DLGAP1-AS1 as well as the potential targets of miR-149-5p. Pearson product-moment correlation coefficient was applied to perform correlation analysis.
Cell culture and treatment
Four kinds of human CRC cell lines (LoVo, HT-29, SW480, and HCT116) and the normal colon mucosal epithelial cell line NCM460, which were gained from the Cell Bank of Type Culture Collection (Chinese Academy of Sciences, Shanghai, China), were chosen for this experiment. DMEM mixed with 10% fetal bovine serum (FBS; Sangon Biotech, Shanghai, China) and 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) were used to cultivate cells at 37°C containing 5% CO2. siRNA targeting lncRNA DLGAP1-AS1, control siRNA, miR-149-5p mimics, and miR-149-5p inhibitors were ordered from Sangon Biotech (China). Lipofectamine 2000 reagent (Invitrogen) was employed to perform the cell transient transfection in accordance with the manufacturer’s protocol. Cells were treated with 5-FU (Sigma, USA) at the concentration of 2 mM.
qRT-PCR assay
We extracted the total RNA from CRC tissues and cultured cells using TRIZOL reagent (Invitrogen) following the manufacturer’s protocol. The cDNA was then synthesized by a PrimeScript RT reagent kit (Takara, Dalian, China). The qRT-PCR assay was performed using a SYBR green PCR kit (Takara, Dalian, China) according to the manufacturer’s guidelines. glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6 were chosen as the internal controls for RNAs and miRNAs, respectively. The 2−ΔΔCt method was used to calculate the relative gene expression levels. The experiments were performed in triplicate.
Western blot
The isolated protein samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose filter membranes (Millipore, Bedford, MA, USA). Afterward, the membranes were then incubated with primary antibodies for a whole night, including Bcl-2, PCNA, TGFB2, Smad2, p-Smad2, and glyceraldehydes 3-phosphate dehydrogenase (1:1,000; Cell Signaling Technology, Beverly, MA, USA), followed by probing with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (1:3,000, Abcam) for 1 h at 37°C. Protein bands were detected by enhanced chemiluminescence (ECL) plus western blotting detection reagents (GE Healthcare Life Sciences, Piscataway, NJ, USA) and analyzed by Quantity One analysis software (Bio-Rad, San Francisco, CA, USA).
Luciferase activity assay
WT DLGAP1-AS1 or TGFB2 containing a potential miR-149-5p binding site and DLGAP1-AS1 or TGFB2-Mut containing a variant site were synthesized and fused to the dual-luciferase reporter vector pMIR-Report (Promega, Madison, WI, USA). The above luciferase reporter plasmids and miR-149-5p mimic or control miRNA were co-transfected into HT-29 or SW480 cells, and the luciferase activity in each group was determined using a dual-luciferase reporter assay system (Promega, Madison, WI, USA).
RNA binding protein immunoprecipitation assay
The RIP assay was conducted by using the Magna RIPTM RNA binding protein immunoprecipitation kit (Millipore, USA). The collected cells were lysed in RNA lysis buffer. The cell lysis was treated with the RIP immunoprecipitation buffer containing magnetic beads conjugated with human anti-Ago2 antibody or normal mouse IgG (Millipore). After a whole night of incubation at 4°C, the immunoprecipitated RNA was obtained by digesting protein-RNA complex with proteinase K. Afterward, the qRT-PCR assay was performed to validate the RNA concentration.
Cell proliferation assay
Cell proliferation patterns and cell survival rates were analyzed by using a CCK-8 (Meilunbio, Dalian, China) in accordance with the manufacturer’s protocol. The CCK-8 assay was conducted at different time points (0, 24, 48, and 72 h), respectively, after cell transfection to detect the patterns of cell proliferation. We treated the untransfected or transfected cells with 5-FU at the concentration of 2 mM for 48 h. Then the CCK-8 assay was performed to determine the cell survival rate. At each time point (0, 24, 48, and 72 h), 20 μL of CCK-8 solution was applied into each well, and the plates were maintained for 1 h at 37°C. Afterward, a microplate reader (Bio-Rad, Hercules, CA, USA) was employed to measure the absorbance at 450 nm.
Cell apoptosis assay
Cell apoptosis analysis was performed by using flow cytometry. The annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Pharmingen, CA, USA) was used to detect the cell apoptosis rate following the manufacturer’s instruction. Generally, the cultured cells were collected and stained with annexin V-FITC and propidium iodide (PI) at room temperature for 15 min after being resuspended in 200 μL of binding buffer. Then, flow cytometry (FACScan; BD Biosciences, San Jose, CA, USA) was used to determine the apoptotic rates of cells. Three independent assays were conducted.
Cell invasion assay
The Matrigel Transwell assay was chosen to assess the cell invasion ability. Transwell chambers with 8 μm pore size were purchased from Corning (Corning, NY, USA). Matrigel matrix (1 mg/mL) was added to the upper Transwell chamber and precoated at 37°C for 30 min. Cells with different treatments were collected and resuspended in serum-free DMEM with a concentration of 1 × 106 cells in 100 μL. Afterward, the upward chambers in the 24-well plates were inoculated with cells, while the lower chambers were filled with medium containing 10% FBS. Cells were incubated for 24 h. Finally, a cotton swab was used to remove the cells on the upward surface of the membranes, while cells that migrated into the lower surface of the membranes were fixed with methanol and stained using crystal violet solution (0.1%; Sigma-Aldrich, St. Louis, MO, USA). The number of invaded cells was counted at least in 5 visual fields of 400× magnification, which was randomly selected by using a light microscope (BX41, Olympus, Japan).
Wound-healing assay
In order to perform the wound-healing assay, the cells with different treatments were inoculated into a 24-well cell plate and cultured in DMEM. A sterile pipette tip was used to create a 1-mm-wide scratch. We photographed the plates at 0 and 48 h, respectively, and counted the distance migrated by the leading edge of the cells.
In vivo assay
All experimental procedures were performed in accordance with the internal biosafety and bioethics guidelines of the First Hospital of Jilin University. The 6-week-old female nude mice were obtained from the Chinese Academy of Medical Sciences (Beijing, China). Afterward, mice were randomly divided into 4 groups (sh-NCl, sh-NC+5-FU, sh-DLGAP1-AS1, sh-DLGAP1-AS1+5-FU, n = 6/group) and subcutaneously injected into the left flank, with HT-29 cells (5 × 106) stably transfected with sh-NC or sh-DLGAP1-AS1. 6 days later, 5-FU (1 mg/kg) was administrated in mice of sh-NC+5-FU and sh-DLGAP1-AS1+5-FU groups every 3 days. Tumor volume was measured every 3 days with a caliper and calculated by the formula volume = (length × width)2/2. Finally, at 21 days upon cell inoculation, tumors were dissected, photographed, and weighted. Moreover, qRT-PCR assays were conducted to detect the expressions of DLGAP1-AS1 and miR-149-5p in xenograft tumors.
Statistical analysis
Data were exhibited as mean ± standard deviation (SD). SPSS 18.0 software (Chicago, IL, USA) and GraphPad Prism 6.0 (San Diego, CA, USA) were used to perform all statistical analysis. Two-tailed Student’s t test or one-way ANOVA with post hoc contrasts by Student-Newman-Keuls test was applied to determine the difference between groups. p <0.05 was considered statistically significant.
Acknowledgments
The study was supported by Science and Technology Department of Jilin Province under grant no. 20170520011JH and the Youth Mainstay Program of the First Hospital of Jilin University. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
L.Q. and Y.C. gave substantial contributions to the conception and the design of the manuscript and L.Q. and F.Z. to acquisition and analysis and interpretation of the data. All authors participated in drafting the manuscript, and L.H. revised it critically. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2021.01.003.
Contributor Information
Linlin Qu, Email: qull@jlu.edu.cn.
Liang He, Email: he_liang@jlu.edu.cn.
Supplemental information
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Zhong X., Fang Y.J., Pan Z.Z., Lu M.S., Zheng M.C., Chen Y.M., Zhang C.X. Dietary fiber and fiber fraction intakes and colorectal cancer risk in Chinese adults. Nutr. Cancer. 2014;66:351–361. doi: 10.1080/01635581.2013.877496. [DOI] [PubMed] [Google Scholar]
- 4.Torre L.A., Siegel R.L., Ward E.M., Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 5.Sun L., Jiang C., Xu C., Xue H., Zhou H., Gu L., Liu Y., Xu Q. Down-regulation of long non-coding RNA RP11-708H21.4 is associated with poor prognosis for colorectal cancer and promotes tumorigenesis through regulating AKT/mTOR pathway. Oncotarget. 2017;8:27929–27942. doi: 10.18632/oncotarget.15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponting C.P., Oliver P.L., Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Kondo Y., Shinjo K., Katsushima K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017;108:1927–1933. doi: 10.1111/cas.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia H., Hui K.M. Mechanism of cancer drug resistance and the involvement of noncoding RNAs. Curr. Med. Chem. 2014;21:3029–3041. doi: 10.2174/0929867321666140414101939. [DOI] [PubMed] [Google Scholar]
- 9.Fang C., Qiu S., Sun F., Li W., Wang Z., Yue B., Wu X., Yan D. Long non-coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53 feedback loop promotes the metastatic progression of colon cancer by functioning as a competing endogenous RNA. Cancer Lett. 2017;410:50–62. doi: 10.1016/j.canlet.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Kong J., Sun W., Li C., Wan L., Wang S., Wu Y., Xu E., Zhang H., Lai M. Long non-coding RNA LINC01133 inhibits epithelial-mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett. 2016;380:476–484. doi: 10.1016/j.canlet.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Han P., Li J.W., Zhang B.M., Lv J.C., Li Y.M., Gu X.Y., Yu Z.W., Jia Y.H., Bai X.F., Li L. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol. Cancer. 2017;16:9. doi: 10.1186/s12943-017-0583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y., Yang L., Zhao J., Li C., Nie J., Liu F., Zhuo C., Zheng Y., Li B., Wang Z., Xu Y. Nuclear-enriched abundant transcript 1 as a diagnostic and prognostic biomarker in colorectal cancer. Mol. Cancer. 2015;14:191. doi: 10.1186/s12943-015-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y., Li L., Zheng Z., Chen S., Chen E., Hu Y. Long non-coding RNA linc00261 suppresses gastric cancer progression via promoting Slug degradation. J. Cell. Mol. Med. 2017;21:955–967. doi: 10.1111/jcmm.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X., Yuan Z., Yang Z., Chen D., Kim T., Cui Y., Luo Q., Liu Z., Yang Z., Fan X. The novel long noncoding RNA u50535 promotes colorectal cancer growth and metastasis by regulating CCL20. Cell Death Dis. 2018;9:751. doi: 10.1038/s41419-018-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 16.Magee P., Shi L., Garofalo M. Role of microRNAs in chemoresistance. Ann. Transl. Med. 2015;3:332. doi: 10.3978/j.issn.2305-5839.2015.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju J. Implications of miRNAs in Colorectal Cancer Chemoresistance. Int. Drug Discov. 2011;2011:2063. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang A.H., Fan W.J., Fu L., Wang X.T. LncRNA PCAT-1 regulated cell proliferation, invasion, migration and apoptosis in colorectal cancer through targeting miR-149-5p. Eur. Rev. Med. Pharmacol. Sci. 2019;23:8310–8320. doi: 10.26355/eurrev_201910_19142. [DOI] [PubMed] [Google Scholar]
- 19.Yoon J.H., Abdelmohsen K., Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han D., Wang M., Ma N., Xu Y., Jiang Y., Gao X. Long noncoding RNAs: novel players in colorectal cancer. Cancer Lett. 2015;361:13–21. doi: 10.1016/j.canlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Xie X., Tang B., Xiao Y.F., Xie R., Li B.S., Dong H., Zhou J.Y., Yang S.M. Long non-coding RNAs in colorectal cancer. Oncotarget. 2016;7:5226–5239. doi: 10.18632/oncotarget.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W., Su G., Huang X., Zou A., Wu J., Yang Y., Zhu Y., Liang S., Li D., Ma F., Guo L. Long noncoding RNA PCAT6 inhibits colon cancer cell apoptosis by regulating anti-apoptotic protein ARC expression via EZH2. Cell Cycle. 2019;18:69–83. doi: 10.1080/15384101.2018.1558872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma S., Yang D., Liu Y., Wang Y., Lin T., Li Y., Yang S., Zhang W., Zhang R. LncRNA BANCR promotes tumorigenesis and enhances adriamycin resistance in colorectal cancer. Aging (Albany NY) 2018;10:2062–2078. doi: 10.18632/aging.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X., Bai Y., Yang C., Hu S., Hou Z., Wang G. Long noncoding RNA SNHG15 enhances the development of colorectal carcinoma via functioning as a ceRNA through miR-141/SIRT1/Wnt/β-catenin axis. Artif. Cells Nanomed. Biotechnol. 2019;47:2536–2544. doi: 10.1080/21691401.2019.1621328. [DOI] [PubMed] [Google Scholar]
- 25.Deng J., Zhang Q., Lu L., Fan C. Long Noncoding RNA DLGAP1-AS1 Promotes the Aggressive Behavior of Gastric Cancer by Acting as a ceRNA for microRNA-628-5p and Raising Astrocyte. Elevated Gene 1 Expression. 2020;12:2947–2960. doi: 10.2147/CMAR.S246166. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Lin Y., Jian Z., Jin H., Wei X., Zou X., Guan R., Huang J. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and Wnt/β-catenin pathway. Cell Death Dis. 2020;11:34. doi: 10.1038/s41419-019-2188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi X., Zhang D.H., Wu N., Xiao J.H., Wang X., Ma W. ceRNA in cancer: possible functions and clinical implications. J. Med. Genet. 2015;52:710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 28.Liu G., Yin L., Ouyang X., Zeng K., Xiao Y., Li Y. M2 Macrophages Promote HCC Cells Invasion and Migration via miR-149-5p/MMP9 Signaling. J. Cancer. 2020;11:1277–1287. doi: 10.7150/jca.35444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo K., He J., Yu D., Açil Y. MiR-149-5p regulates cisplatin chemosensitivity, cell growth, and metastasis of oral squamous cell carcinoma cells by targeting TGFβ2. Int. J. Clin. Exp. Pathol. 2019;12:3728–3739. [PMC free article] [PubMed] [Google Scholar]
- 30.Shao S., Wang C., Wang S., Zhang H., Zhang Y. Hsa_circ_0075341 is up-regulated and exerts oncogenic properties by sponging miR-149-5p in cervical cancer. Biomed. Pharmacother. 2020;121:109582. doi: 10.1016/j.biopha.2019.109582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.