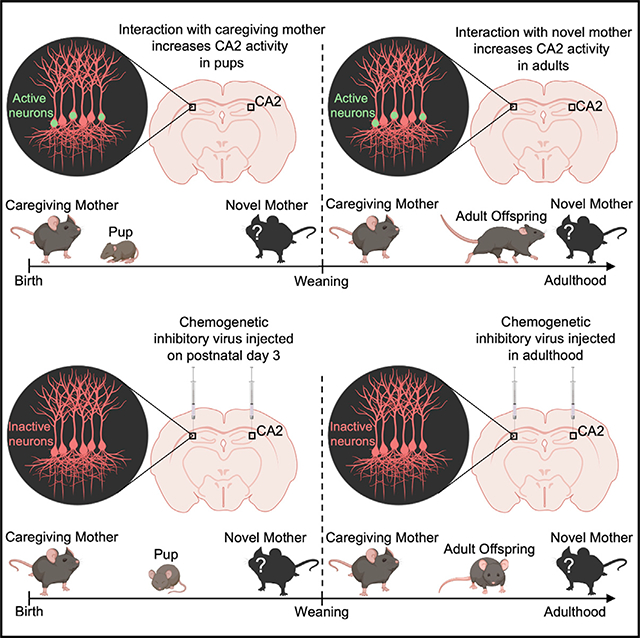
SUMMARY
Some of the most enduring social connections begin when infants first recognize their caregivers, memories that form the basis of many family relationships. It remains unknown whether these early social memories persist into adulthood in mice and, if so, which brain regions support them. Here we show that mice form memories of their mother within days after birth and that these memories persist into adulthood. Pups display greater interest in the mother than in an unfamiliar dam before weaning, after which this preference reverses. Inhibition of CA2 neurons in the pup temporarily blocks the ability to discriminate between the mother and an unfamiliar dam, whereas doing so in adulthood prevents the formation of short-term memories about conspecifics, as well as social discrimination related to long-term memories of the mother. These results suggest that the CA2 supports memories of the mother during infancy and adulthood with a developmental switch in social preference.
In Brief
Laham et al. report that mice recognize their caregiving mothers and distinguish them from novel mothers with a postweaning reversal in social preference. In response to mother stimuli, immediate-early gene expression in the CA2 parallels social preference. CA2 inhibition impairs memory for the caregiving mother in pups and adults.
Graphical Abstract
INTRODUCTION
The earliest social memory, which sets the stage for the dynamic social interactions experienced later in life, is the memory an infant has for its primary caregiver, typically the mother. Across the animal kingdom, evidence indicates that memory for the mother is driven by prenatal and postnatal experience, with some studies suggesting that genetics play a role (Schaal et al., 2020; Todrank et al., 2005). Differences exist in the degree to which species rely on inborn versus learned cues for early maternal recognition (Blank and Yang, 2017; El-Showk et al., 2010; Logan et al., 2012; Porter and Winberg, 1999; Raineki et al., 2015; Sreng et al., 2017). Human newborns display evidence of maternal recognition using olfactory and auditory cues, with early prenatal associations bolstered by olfactory, auditory, visual, and tactile experience during postnatal life (Burnham, 1993; Schaal et al., 2020). These early associations can be strengthened by shared life experiences, reciprocal social interactions, and emotional valence as the child grows, forming memories of the mother that last a lifetime (Fivush, 2011; Schaal et al., 2020). Evidence suggests that like humans, mice exhibit early maternal recognition through both prenatal and early postnatal olfactory experience (Logan et al., 2012) and that mice can discriminate between their own and an unfamiliar mother during the third postnatal week (Mogi et al., 2017). However, no studies provide evidence that mice can recognize their mother during the neonatal period. Although one study showed that dogs can recognize their mothers’ odors nearly 2 years after weaning and permanent separation (Hepper, 1994), no studies have reported whether mice maintain a memory of the mother into adulthood.
Evidence supports the role of the hippocampal CA2 region in forming, maintaining, and retrieving memories of recently encountered unrelated conspecifics (Alexander et al., 2016; Hitti and Siegelbaum, 2014; Smith et al., 2016). Additional studies have revealed that the CA2 possesses a high density of vasopressin and oxytocin receptors and that activation of these receptors facilitates social recognition in adulthood (Caldwell et al., 2008; Cilz et al., 2019; Lin et al., 2018; Raam et al., 2017). However, no studies have investigated whether the CA2 plays a role in memories of the mother during development or in adulthood. Here we show that mouse pups can discriminate between their mother and an unfamiliar dam by postnatal day (P) 3 and that memories for the mother persist into adulthood. Offspring display reversed social preference for an unfamiliar dam over the mother after weaning. We also show that despite the relative immaturity of the CA2 during the early postnatal period, this area plays a crucial role in recognizing the mother, a mechanism that persists into adulthood despite the change in social preference.
RESULTS
Mice demonstrate memory for the caregiving mother from P3 to adulthood with a switch in social preference after weaning
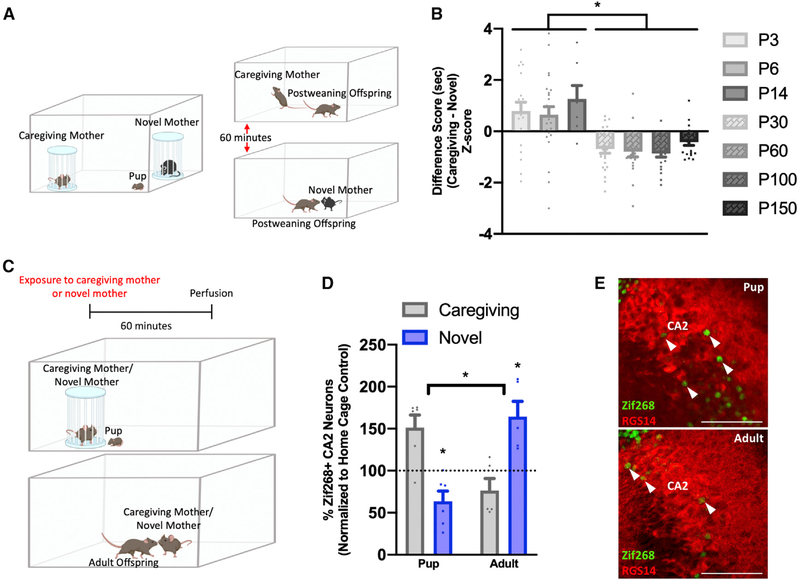
To determine whether mice develop memories of their mother within the first days of birth, we exposed P3 pups of both sexes to a modified 2-choice social test. P3 was selected because at this age, mice display olfactory-associated memories (Armstrong et al., 2006), substantially before the earliest age at which pups had been shown to discriminate between the mother and an unfamiliar dam (P17) (Mogi et al., 2017). To eliminate preference based on genetically shared odorants or uterine environment stimuli, pups were cross-fostered on the day of birth. Because interactions between neonates and mothers are often initiated by the dam, the stimulus adults were placed in wire mesh containers to study the behavior of the pup with limited interference from the dams. During testing, the caregiving foster mother (called the caregiving mother) and an unfamiliar dam (called the novel mother) were each placed in wire mesh containers in opposite corners of the testing box (Figure 1A). The novel mother was a genetically unrelated lactating female with an age-matched litter. At P3, mouse pups are not ambulatory, and their eyes and ear flaps are closed (Castelhano-Carlos et al., 2010), so investigation was scored when a pup’s nose was directed toward either mother. P3 pups demonstrated a significant preference for the caregiving mother over the novel mother (Figure 1B). Mice were also tested at P6 after the ear flaps open and at P14 after the eyes open (Castelhano-Carlos et al., 2010). Preference for the caregiving mother was observed at P6 and P14 (Figure 1B). To further investigate whether pups can distinguish the caregiving mother from the novel mother, we examined ultrasonic vocalizations (USVs), known as whistles, which pups emit when separated from the mother (Figures S1A and S1B). P5 was selected for this analysis because it is when USV separation calls are high relative to P3 and P14 (Yin et al., 2016). We found that P5 pups emit more calls when near the caregiving mother than when near a novel mother (Figure S1C), providing further evidence that pups recognize the caregiving mother.
Figure 1. Mice discriminate between the caregiving mother and a novel mother in infancy and adulthood, and exhibit reversed social preference and CA2 IEG expression.
(A) Pups placed in a neutral corner of a testing box with the caregiving mother and a novel mother in wire mesh containers for a 2-choice social test, and postweaning offspring undergoing direct social interaction testing.
(B) Offspring demonstrate a preference for the caregiving mother from P3 to P14 (P3 n = 19, P6 n = 24, P14 n = 7) and a preference for a novel mother from P30 to P150 (P30 n = 24, P60 n = 20, P100 n = 20, P150 n = 20) (F(6,127) = 10.03, p < 0.0001; Table S1).
(C) Pups were exposed to the caregiving mother or a novel mother in a wire mesh container, and postweaning offspring were exposed to the caregiving mother or a novel mother directly and perfused 1 h later.
(D) Pups exhibit more Zif268+ CA2 neurons after exposure to the caregiving mother (n = 6) than after exposure to a novel mother (n = 6), whereas adult offspring exhibit the opposite effect (caregiving n = 5, novel n = 5) (F(1,18) = 15.04, p < 0.0001; Table S1).
(E) Confocal images of Zif268+ cells (green, arrowheads) in the RGS14 labeled (red) CA2 from pup (top) and adult (bottom) exposed to a caregiving mother (pup) or novel mother, respectively. Scale bars, 200 μm. *p < 0.05 compared with groups within brackets or the same-age caregiving mother group; bars show mean + SEM; sec, seconds.
To determine whether the memory of the caregiving mother remains after the time of weaning, mice of both sexes underwent testing with the caregiving mother and a novel mother at P30, P60, P100, and P150. Because the offspring were ambulatory and engage in behaviors resembling those of the mother at these ages, we used the more sensitive direct social interaction test (Hitti and Siegelbaum, 2014). To avoid differences in investigation based on odors related to sexual receptivity, adult female mice were only used for tests when they were in metestrus or diestrus (Cora et al., 2015). Offspring were placed in a testing box with the caregiving mother or a novel mother for 5 min and then returned to the home cage. After 60 min, mice were placed in the testing box with a stimulus mouse not previously encountered for an additional 5 min (Figure 1A). Offspring demonstrated a significant preference for the novel mother over the caregiving mother at P30, P60, P100, and P150 (Figure 1B), with males and females showing no differences (Figure S2). These findings highlight the long-lasting nature of the memory of the caregiving mother and reveal a switch in social preference after weaning.
Social preference in pups is not a generalized preference for familiarity
We next set out to determine whether pups prefer the caregiving mother because of a general familiarity preference. Within hours of birth, a metallic object with a distinct nonsocial odor was placed directly in the nest so that pups had physical contact with it. This object remained in the home cage until weaning (Figure S1D). On P3, pups were given a 2-choice object test, in which they were placed in a testing box containing the object from the home cage and a novel object with a distinct nonsocial odor (Figure S1D). P3 pups displayed no overall preference for either object (Figure S1F). The objects placed in the home were counterbalanced across litters. On P60, ~40 days after the last object encounter in the home cage, mice were again tested for their ability to discriminate between the familiar object and a novel object Figure S1E). Mice showed no preference for either object (Figure S1F), despite preferring novel over familiar objects when the interval between object exposure was short (45 min) (Figure S1F). These findings suggest that although adult mice have a general novelty preference (preferring novel social stimuli and objects), pups do not exhibit a general familiarity preference but more likely prefer the caregiving mother because it is a highly salient cue.
Immediate-early gene expression in the CA2 parallels preference for the caregiving mother during development and in adulthood
Because the CA2 participates in social memory in adulthood (Hitti and Siegelbaum, 2014), we investigated whether this area responds differentially to the caregiving mother compared with a novel mother, using the immediate-early genes (IEGs) Zif268 and c-Fos as proxies for neuronal activation (Guzowski et al., 2005; Alexander et al., 2016) in developing and adult mice. We carried out the developmental experiment at P14, because by this time, neuronal activation is known to increase IEG expression in the hippocampus (Vendrell et al., 1998). P14 mice were placed in a testing box with the caregiving mother or a novel mother in a wire mesh container. After 5 min, pups were removed from the box and perfused after 60 min (Figure 1C). Pups in these groups were compared with a group that remained in the home cage. For the adult experiment, P60 offspring were placed in a testing box containing either the caregiving mother or a novel mother for 5 min (Figure 1C) and then perfused 60 min later. Adult mice in the caregiving mother and novel mother conditions were compared with an age-matched group that spent the same amount of time in an empty testing box. Pups had more Zif268+ CA2 cells after exposure to the caregiving mother, whereas adults had more Zif268+ CA2 cells after exposure to a novel mother (Figures 1D and 1E). Thus, pups and adults displayed Zif268 CA2 expression that paralleled social preference, with higher levels associated with exposure to a preferred social stimulus. The effects with c-Fos labeling were consistent in adults and pups but with smaller differences between groups (Figure S3). Other hippocampal regions that communicate directly with the CA2, including ventral CA1 (vCA1), dorsal CA1 (dCA1), and dorsal CA3 (dCA3), were examined for IEG expression to assess regional specificity. Zif268 was not assessed in the CA1, because it stains all pyramidal cells for unknown reasons (Figure S3) (Schlingensiepen et al., 1991). The number of c-Fos+ cells in the vCA1 paralleled that of the CA2 in pups and adults, with more c-Fos+ cells in pups after exposure to the caregiving mother and more c-Fos+ cells in adults after exposure to a novel mother (Figure S3). Because vCA1 receives direct connections from CA2 and participates in social memory (Okuyama et al., 2016; Pilarzyk et al., 2019; Meira et al., 2018), IEG expression in the vCA1 may arise from social-stimulus-induced CA2 neuron activation. No differences in number of IEG+ cells were observed in dCA1 or dCA3 at either age (Figure S3), consistent with findings that these areas are not required for social memory (Chiang et al., 2018).
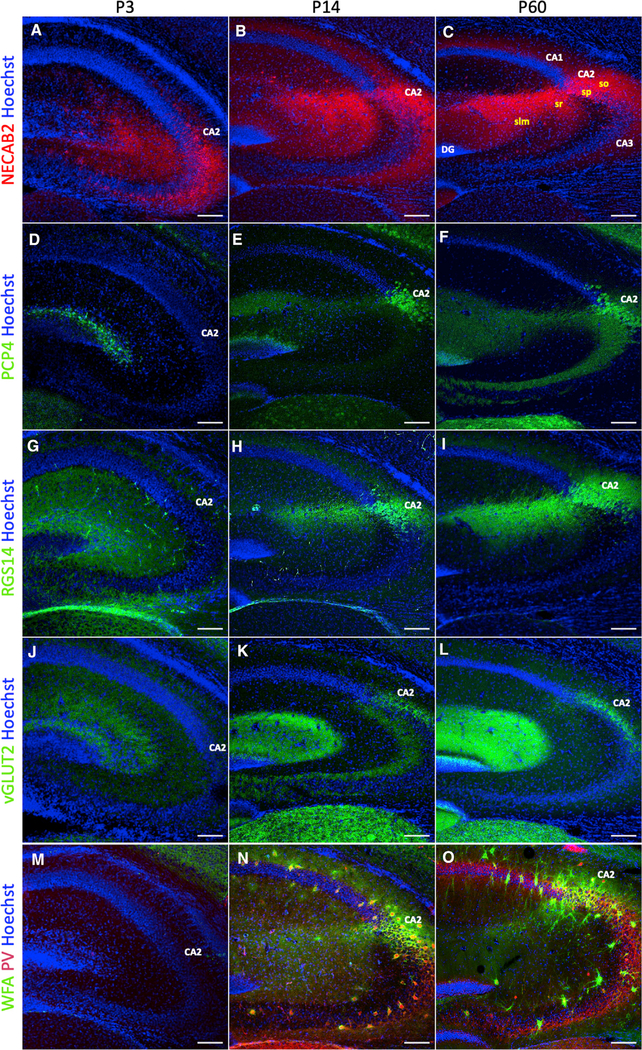
The association between Zif268 expression and preference for the caregiving mother over a novel mother in pups suggests that the CA2 may play a role in these memories during development. Neurons in the CA fields are produced during the embryonic period, but multiple developmental processes, such as dendritic growth/retraction and synapse formation/elimination, occur postnatally (Frotscher and Seress, 2007). The CA2 exhibits unique molecular characteristics, including high expression of several proteins expressed by its pyramidal neurons, such as PCP4, RGS14, NECAB2, as well as a protein originating from supramammillary afferents to the CA2, vGLUT2, and an intense concentration of perineuronal nets (PNNs), specialized extracellular matrix structures, around pyramidal neurons and some inhibitory neurons (Carstens et al., 2016; Dudek et al., 2016). To investigate the expression of these markers when we explored memories for the caregiving mother, groups of mice were perfused at P3, P14, and P60, and the CA2 was examined qualitatively. As expected, we found robust region-defining staining for all of these markers in the CA2 at P60 (Carstens et al., 2016; Dudek et al., 2016) (Figures 2C, 2F, 2I, 2L, and 2O). At P14, we found nearly adult-like staining patterns in the CA2 for all markers (Figures 2B, 2E, 2H, 2K, and 2N). At P3, no evidence of labeling for PCP4, RGS14, vGLUT2, or PNNs was observed in the CA2 (Figures 2D, 2G, 2J, and 2M), but staining for NECAB2 was present (Figure 2A), with an expression pattern not discretely localized to the CA2 (Figures 2B and 2C). These findings are consistent with previous reports about the developmental labeling of PCP4 (Carstens and Dudek, 2019), RGS14 (Evans et al., 2014), NECAB2 (McCann et al., 2019), and PNNs (Carstens and Dudek, 2019). Our results suggest that vGLUT2 shares a similar developmental pattern as PCP4, RGS14, and PNNs. Altogether, these findings suggest that although the CA2 is immature in terms of molecular markers at P3, at least one such marker (NECAB2) has begun to emerge, suggesting that even at this early age, the CA2 may have some basic function.
Figure 2. Molecular markers of the adult mouse CA2 emerge over the first 2 postnatal weeks.
(A–C) NECAB2 labeling appears in the CA2 as early as P3 but with less regional specificity than observed at P60. At P60, NECAB2 was distinctly localized to all layers of the CA2, including the stratum oriens (so), the stratum pyramidale (sp), the stratum radiatum (sr), and the stratum lacunosum moleculare (slm).
(D–F) PCP4 labeling of the CA2 is only present at P14 and P60.
(G–I) RGS14 is diffuse at P3, with specific labeling of the CA2 at P14 and P60.
(J–L) vGLUT2 immunolabeling is absent at P3 but present at P14 and P60.
(M–O) Wisteria floribunda agglutinin (WFA)+ PNNs are absent at P3, with intense staining in the CA2 around pyramidal neurons and parvalbumin (PV)+ interneurons at P14 and P60.
Scale bars, 200 μm.
Activating inhibitory DREADDs in CA2 before weaning impairs preference for the caregiving mother
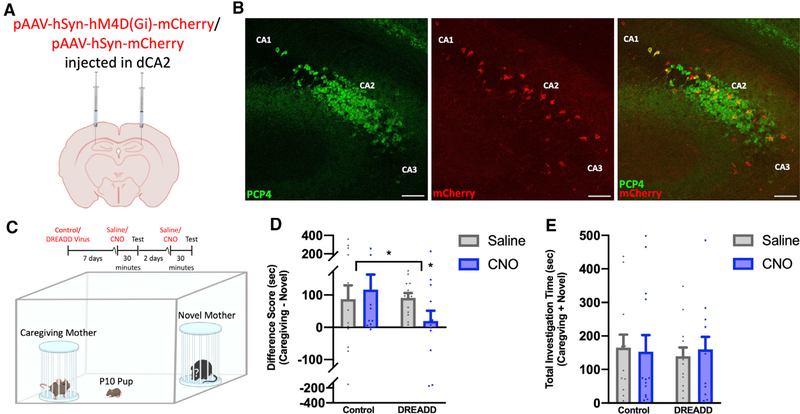
We next tested whether the CA2 is required for memories of the caregiving mother during the postnatal period. P3 male and female mice were subjected to stereotaxic bilateral injections of inhibitory DREADD virus (pAAV-hSyn-hM4D(Gi)-mCherry) or control virus (pAAV-hSyn-mCherry) in the CA2 (Figures 3A, 3B, and S4). A week after surgery, P10 mice underwent social testing in the 2-choice social test between the caregiving mother and a novel mother. Virus-injected mouse pups were administered clozapine N-oxide (CNO) or saline over a 2-day period. For each test, 30 min after CNO or saline injection, pups were placed in a neutral corner of a testing box containing the caregiving mother and a novel mother located in separate wire mesh containers (Figure 3C). Pups injected with control or DREADD virus and administered saline preferred the caregiving mother over a novel mother; pups injected with control virus also preferred the caregiving mother after CNO administration (Figure 3D). However, in DREADD-virus-injected pups, this preference disappeared with CNO administration (Figure 3D). DREADD virus pups injected with CNO did not show changes in overall investigation times (Figure 3E), suggesting that CNO did not render pups immobile or incapable of orienting toward maternal odors. Pups were perfused after the last social test, and their brains were examined for the specificity and extent of virus expression (Figures 3B and S4). Despite the relative immaturity of the CA2 during the early postnatal period, CNO treatment of mouse pups expressing inhibitory DREADDs in this region impairs social preference for the caregiving mother.
Figure 3. Activation of inhibitory DREADDs in the CA2 impairs memory of the caregiving mother in pups.
(A) P3 pups received bilateral injections of control virus (AAV5-hSyn-mCherry) (n = 13) or inhibitory DREADD virus (AAV5-hSyn-hM4D(Gi)-mCherry) (n = 14) targeting the dorsal CA2 (dCA2).
(B) PCP4 staining and endogenous mCherry expression reveal specific infection of CA2 neurons with inhibitory DREADD virus at P10 (Figure S4). Scale bars, 200 μm.
(C) Schematic demonstrating control- or DREADD-infected P10–P12 pup behavior after CNO or saline administration.
(D) Control-virus-infected pups with saline or CNO and DREADD-virus-infected pups with saline had positive difference scores (time spent investigating the caregiving mother minus time spent investigating the novel mother). DREADD-virus-infected pups with CNO did not prefer the caregiving mother over a novel mother (F(1,50) = 5.831, p = 0.0194; Table S1).
(E) No differences were observed in overall investigation times between control-virus-infected mice (n = 13) and DREADD-virus-infected mice (n = 13) with saline or CNO (F(1,48) = 0.1757, p = 0.6770; Table S1).
*p < 0.05 compared with either the groups within brackets or the DREADD-infected, saline-injected mice; bars represent mean +SEM; sec, seconds.
Silencing CA2 neurons after weaning impairs social discrimination of the caregiving mother versus a novel mother
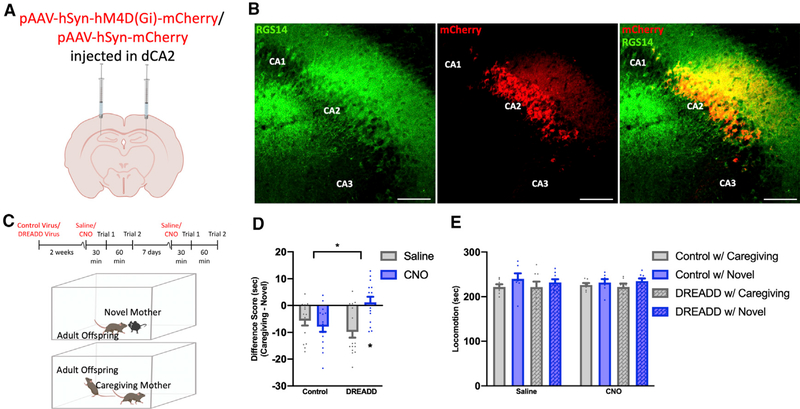
To test whether CA2 neurons are responsible for retrieving the memory of the caregiving mother in adulthood, P120 male and female mice were bilaterally injected with inhibitory DREADD virus (pAAV-hSyn-hM4D(Gi)-mCherry) or control virus (pAAV-hSyn-mCherry) in the CA2 (Figures 4A, 4B, and S4). Mice underwent direct social interaction testing 2 weeks after surgery. Virus-injected mice were administered CNO or saline, with a seven-day period between each set of tests (Figure 4C). After a 30-min delay following saline or CNO injection, mice were placed in a testing box with the caregiving mother or a novel mother for 5 min. After the 5-min social interaction, mice were returned to the home cage for 60 min and then returned to the testing box with a stimulus mouse not previously encountered and allowed to investigate for an additional 5 min (Figure 4C). Adult mice injected with control virus and administered saline or CNO, as well as those injected with DREADD virus and administered saline, preferred the novel mother over the caregiving mother, whereas adult mice injected with inhibitory DREADD virus and administered CNO had no preference for either social stimulus (Figure 4D). No differences among the groups were detected in overall locomotion times (Figure 4E). These findings suggest that activating inhibitory DREADDs in the CA2 of adult offspring impairs discrimination between the caregiving mother and a novel mother.
Figure 4. Inhibition of CA2 neurons prevents social discrimination of the caregiving mother and a novel mother in adulthood.
(A) Adult mice received bilateral injections of control virus (AAV5-hSyn-mCherry) (n = 15) or inhibitory DREADD virus (AAV5-hSyn-hM4D(Gi)-mCherry) (n = 16) targeting the dCA2.
(B) RGS14 staining and endogenous mCherry expression reveal specific infection of CA2 neurons with inhibitory DREADD virus (Figure S4). Scale bars, 250 μm.
(C) Timeline for viral infection and behavior testing and schematic demonstrating direct social interaction behavior testing in adult virus-infected mice.
(D) Adult mice injected with control virus displayed a preference for the novel mother over the caregiving mother after saline or CNO administration, as did adult mice injected with inhibitory DREADD virus and administered saline. Adult mice injected with DREADD virus and CNO did not discriminate between the caregiving mother and a novel mother (F(1,27) = 10.74, p = 0.0004; Table S1).
(E) No differences were observed in overall locomotion times between control-virus-infected mice (n = 7) and DREADD-virus-infected mice (n = 7) with saline compared with CNO (F(1,14) = 0.0003419, p = 0.9855; Table S1).
*p < 0.05 compared with either the groups within brackets or the DREADD-infected, saline-injected mice; bars represent mean + SEM; sec, seconds.
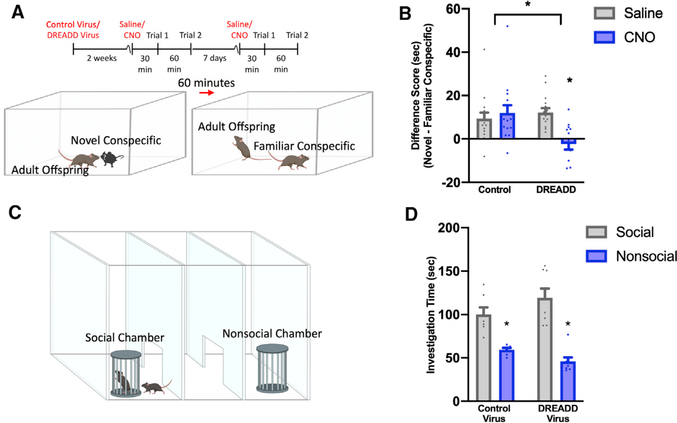
Next, we tested these same mice to determine whether activation of inhibitory DREADDs in CA2 neurons impairs memory of a recently encountered conspecific, as would be expected from previous studies (Hitti and Siegelbaum, 2014; Meira et al., 2018; Smith et al., 2016). Virus-injected mice were placed in a testing box with a novel same-age female for 5 min. After the 5-min trial, mice were returned to the home cage for 60 min and then returned to the testing box with the previous stimulus mouse, now familiar, for an additional 5 min (Figure 5A). Adult mice injected with control or DREADD virus preferred novel over familiar conspecifics after saline administration (Figure 5B); in contrast, CNO administration in mice injected with inhibitory DREADD virus in the CA2 resulted in similar investigation times for novel and familiar mice, whereas mice injected with control virus and then CNO retained a preference for the novel mouse (Figure 5B).
Figure 5. Inhibition of CA2 neurons blocks memory of familiar conspecifics in adulthood, but not sociability.
(A) Timeline for control (AAV5-hSyn-mCherry) (n = 15) or inhibitory DREADD (AAV5-hSyn-hM4D(Gi)-mCherry) (n = 14) viral infection and direct social interaction testing in adult mice and schematic demonstrating direct social interaction behavior testing.
(B) Adult mice infected with control virus and injected with saline, or CNO, preferred a novel same-age mouse, as did adult mice infected with the DREADD virus and then injected with saline. After CNO injection, mice infected with the DREADD virus showed reduced discrimination between the novel and the familiar mouse (F(1,27) = 16.41, p = 0.0004; Table S1).
(C) Schematic demonstrating the sociability 3-chamber test.
(D) Investigation times of a stimulus mouse were higher than those of an empty container in both DREADD-virus-infected mice (n = 8) and control-virus-infected mice (n = 7) injected with CNO (F(1,13) = 49.45, p < 0.0001; Table S1).
*p < 0.05 compared with either the groups within brackets in (B) or the social chamber for each virus group in (D); bars show mean + SEM; sec, seconds.
Lastly, to test whether inhibitory DREADD silencing of CA2 produced impairments specific to the memory of the caregiving mother and familiar conspecifics, not an overarching sociability deficit, virus-injected mice underwent 3-chamber sociability testing. Mice were placed in the central neutral chamber of a 3-chamber testing box (Figure 5C) and given 5 min to explore the testing box, including a neutral central chamber and 2 adjacent nonsocial chambers containing empty wire mesh containers. Mice were then reset in the neutral center chamber and a novel female was placed in the wire mesh container of an adjacent chamber. Mice were given an additional 5 min to explore the box now containing a social chamber and a nonsocial chamber. As expected, mice with control-virus-infected CA2 preferred the social chamber over the nonsocial chamber 30 min after CNO administration (Figure 5D). Mice with inhibitory DREADD-virus-infected CA2 also preferred the social chamber over the nonsocial chamber 30 min after injection of CNO (Figure 5D). Altogether, these results suggest that silencing CA2 neurons in adult offspring inhibits recognition of the caregiving mother, as well as a recently encountered conspecific, without affecting sociability. Mice were perfused after the last social test, and their brains were examined for the specificity and extent of virus expression (Figures 4B and S4).
To verify that CNO reduced neuronal activity in the DREADD-infected CA2, additional DREADD-infected mice were implanted with a four-wire electrode in the CA2 to record neuronal activity before and after CNO administration. Mice were placed in an open-field testing box while a wireless headstage recorded neuronal activity in CA2 for 2 min. The headstage was removed and mice received an injection of CNO before being returned to the home cage. After 30 min, the headstage was reattached and mice were returned to the testing box, where CA2 activity was recorded for an additional 2 min (Figure S5A). The results revealed that CNO treatment reduced local field potentials (LFPs) in the CA2 at both low (2–50 Hz) and high (50–100 Hz) frequencies, as well as the overall number of spikes (Figure S5), confirming the efficacy of DREADDs to inhibit CA2 neuronal activity when exposed to CNO. These effects are larger than those using inhibitory DREADDs to silence cells in the CA2 in previous reports (Alexander et al., 2018; Brown et al., 2020). Some reasons for these differences include that previous studies used Cre lines to express DREADDs in pyramidal neurons, whereas we used a pan-neuronal approach with a synapsin promoter, and previous electrophysiology studies used lower doses of CNO. Given that we included saline controls in our DREADD studies and evaluated overall activity levels, the impairment of memory for the caregiving mother and the age-matched conspecific likely arises from reduced CA2 neuronal activity.
DISCUSSION
Memories of the mother are evident during early postnatal life and persist into adulthood
Neonatal mice root toward lactating mice immediately after birth, a drive induced by a combination of odors from fetal life and parturition (Logan et al., 2012). This behavior suggests that maternal odor has a positive hedonic value (Perry et al., 2016; Porter and Winberg, 1999; Schaal et al., 2020; Todrank et al., 2005). However, the ability of neonatal mice to discriminate between mothers has not been established. Studies have shown that mouse pups can discriminate between their mother and a novel mother by the third postnatal week (Mogi et al., 2017), and our findings suggest this ability begins earlier, at P3. P3 pups have closed eyes and ear flaps (Castelhano-Carlos et al., 2010), which suggest that this recognition is likely mediated by olfactory cues. Because we cross-fostered pups, their ability to discriminate between mothers must be learned postnatally. Learning of novel odor associations has been demonstrated as early as P3 in mice (Armstrong et al., 2006), which likely reflects similar means by which fostered pups form a preference for their caregiving mother. Previous work has shown that mother recognition in rat pups requires a maternal diet with a distinct odor to promote discrimination between mothers (Perry et al., 2016; Sullivan et al., 1990). Our findings suggest a potential species difference, because caregiving mother and novel mother mice were maintained on the same diet but the offspring were able to discriminate between them in measures of both social investigation and USV calling. The experience-dependent aspect of this connection likely allows for malleability in the pup’s response, as has been shown through successful cross-fostering of mouse pups at later ages (Hickman and Swan, 2011; Umemura et al., 2015), as well as healthy offspring raised in communal-nest conditions (Branchi, 2009; Sayler and Salmon, 1969).
We also found evidence that memories for the caregiving mother persist into adulthood, when mice are permanently housed separately from the mother. Although studies have shown sibling recognition in adult mice (D’Amato, 1994), to our knowledge, no studies have shown that mouse offspring exhibit mother recognition after weaning. A previous study showed that dogs prefer odors of their mothers over those of novel females of the same breed up to 2 years after separation (Hepper, 1994). Our findings indicate that adult mice also recognize their mother but with a reversed preference than what is observed with dogs; adult mice have reduced preference for their caregiving mothers compared with novel mothers. The reason for this species difference remains unknown, but it may be relevant that dogs are pack animals, among whom a preference for kin, even after weaning, may improve chances of survival over the lifespan. Preference for the novel mother displayed by adult mice may reflect a generalized novelty preference, as observed for both social stimuli (Hitti and Siegelbaum, 2014) and nonsocial stimuli (Dere et al., 2007), which may enhance exploratory drive and reduce the likelihood of mating with genetically related mice.
The CA2 plays a role in developmental and adult memories of the mother
Like other subregions of the hippocampus (Frotscher and Seress, 2007), the CA2 is immature during the early postnatal period (McCann et al., 2019). Consistent with other reports (Evans et al., 2014; McCann et al., 2019; Carstens and Dudek, 2019), we found that the unique molecular signature of the CA2 is almost absent at the earliest age at which we detected evidence of caregiving mother recognition, P3. The lack of staining localized to the CA2 for most markers that robustly delineate this region in adulthood suggests that development of the unusual molecular features of the adult CA2 is not required for the earliest social memories.
At P14, we found more Zif268+ CA2 cells after exposure to the caregiving mother compared to the novel mother, a pattern that paralleled social preference. In adults, we found more Zif268+ CA2 cells after exposure to the novel mother, a pattern that also paralleled social preference. Altogether, these findings suggest that the CA2 is differentially responsive before and after weaning to the caregiving mother and raise the likelihood that this region is critically involved in memories for the caregiving mother. Our inhibitory DREADD data support this hypothesis, showing that decreasing CA2 neuronal activity prevents discrimination of the caregiving mother from the novel mother. The earliest time point we were able to test using DREADDs in our pup study was P10 (surgery at P3 with a 7-day recovery), because shorter postsurgical intervals do not allow for adequate viral expression. At this age, CNO administration in DREADD pups eliminates the ability to distinguish the caregiving mother from a novel mother. The extent to which the CA2 is required for this memory at even earlier ages remains unknown. Because DREADD pups were returned to their mother between testing with CNO or saline, it is not possible to determine whether the CA2 participates in acquisition, maintenance, and/or retrieval of the earliest social memories.
Our DREADD findings in adults also suggest that the CA2 is important for discriminating between the caregiving mother and the novel mother long after the memory has formed. Because the mice in this study encountered the caregiving mother months before surgery, and had no contact with her since weaning, our DREADD results rule out an effect on memory acquisition. In addition, counterbalancing CNO and saline showed that after discrimination between the caregiving mother and a novel mother was blocked with CNO, it returned a week later, suggesting that the CNO temporarily blocked discrimination without impairing memory maintenance. The CA2 also participates in other important social functions, including mate preference (Cymerblit-Sabba et al., 2020) and social aggression (Leroy et al., 2018). Altogether, with our findings, the CA2 may be a region that integrates social information about familiarity to determine appropriate responses (e.g., approach, avoidance, and/or aggression) given life stage and other circumstances.
Circuitry underlying memories of the mother
Evidence suggests that the ability of neonates to recognize mothers in most mammals involves olfaction (Logan et al., 2012). Previous studies have revealed that general maternal odors activate several brain regions, including the olfactory bulb, the anterior piriform cortex, and the orbitofrontal cortex (Al Aïn et al., 2017; Perry et al., 2016). Learning of odors paired with the mother requires noradrenergic afferents from the locus coeruleus to the olfactory bulb (Sullivan et al., 2000). These regions, along with the amygdala, play important roles in mediating attachment during the first postnatal weeks of life (Debiec and Sullivan, 2017; Raineki et al., 2010). Evidence suggests that other hippocampal regions, including CA1 and CA3, are activated in response to maternal odors (Perry et al., 2016). Our findings, although focused on discrimination of naturally occurring maternal odors in mice, extend these results to the CA2, which receives afferents from the CA3 and projects to the CA1 (Benoy et al., 2018; Jones and McHugh, 2011). Studies have shown that the vCA1 plays an important part in mediating social memories in mice (Okuyama et al., 2016; Pilarzyk et al., 2019; Meira et al., 2018). Our data on IEG expression in the vCA1 parallels our CA2 data, with increased number of IEG+ cells in the vCA1 after exposure to the caregiving mother compared with a novel mother during development and the reverse pattern in adulthood. These findings suggest that the CA2-vCA1 pathway may be a critical component of the circuitry supporting the earliest social memories.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Elizabeth Gould (goulde@princeton.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The datasets supporting the current study have not been deposited in a public repository but are available from the corresponding author on request. This study did not generate code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mixed groups of male and female C57BL/6J mice were used for all experiments. All animal procedures were performed in accordance with the Princeton University animal care committee’s regulations and were in accordance with the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals. Mice were housed under a 12-hr reversed (7 AM to 7 PM) light-dark cycle at 21°C and breeding occurred on site. To control for potential genetic, prenatal and parturition factors involved in the memory of the mother, pups were cross-fostered with age-matched litters on the day of birth. For each experiment, 4–5 litters of pups were used. Naive pups were tested on P3, P6, and P14. Pups were weaned at P21 and housed 2–5 in single sex cages where they received ad lib access to food and water. Postweaning mice were tested at P30, P60, P100 and P150. Stereotaxic injections were performed on P3 pups with behavioral testing on P10 and P12, or on P120 adults with behavioral testing on P134, P141 and P148. After their litters were weaned, mothers were pair-housed with unrelated primiparous females and did not breed again. All mice (mothers and offspring) were fed standard rodent chow throughout the study. All experiments had equal or near equal numbers of male and female cross-fostered offspring in each group. From P30 onward, the behavioral data were considered with sex as a variable for statistical analyses. No sex differences were observed so the data from males and females were combined for all experiments.
METHOD DETAILS
Histology and immunohistochemistry
For histology of the CA2 markers during development, P3 (n = 3), P14 (n = 5) and P60 (n = 7) mice were transcardially perfused with cold 4% paraformaldehyde. Brains were post-fixed for 48 hours in 4% paraformaldehyde followed by 48 hours in PBS with 30% sucrose for cryoprotection (or 10% sucrose for P3 and P14). Coronal sections (40 μm thick) were cut using a Leica CM3050S cryostat. Sections were washed three times in PBS, blocked in 3% normal serum with 0.3% Triton in PBS and incubated for 24 hours in combinations of the following primary solutions: rabbit anti-NECAB2 (1:500, Novus Biologicals #NBP184002), rabbit anti-PCP4 (1:500, Sigma #HPA005792), mouse anti-RGS14 (1:500, UC Davis/NIH NeuroMab #75–170), rabbit anti-VGLUT2 (1:500, Synaptic Systems #135403), mouse anti-PV (1:1000, Sigma-Aldrich #P3088) and Wisteria Floribunda Lectin (WFA) (1:500, Sigma #L1516). Following overnight incubation, sections were washed 3x in PBS and placed in PBS with 0.3% Triton where they were incubated in the secondary solutions for 1.5 hours. The following secondary solutions were used: donkey anti-rabbit (488 or 568) (1:500), donkey anti-mouse (488 or 568) (1:500), and Streptavidin 488 (1:500). All secondary antibodies were purchased from Life Technologies. After incubation in the secondary solution, sections were mounted on Superfrost Plus slides and dried overnight before being coverslipped with glycerol or Vectashield and sealed for confocal imaging. Labeled brain sections were examined on a confocal microscope (Zeiss 700 LSM) with the same settings used across ages.
Preparation of AAVs
The inhibitory DREADD virus (pAAV-hSyn-hM4D(Gi)-mCherry) was generated by the University of Pennsylvania viral core and purchased through Addgene (catalog # 50475-AAV5). This virus had a titer of ≥ 7 × 1012 genome copies per ml. The control virus (pAAV-hSyn-mCherry) was also generated by the University of Pennsylvania viral core and purchased through Addgene (catalog # 114472-AAV5). Pilot studies showed specific comparable infection of the dorsal CA2 region with undiluted DREADD virus and a 1:25 dilution of control virus, which were used for these experiments.
Stereotaxic Surgery
P3 male and female mouse pups were anesthetized by hypothermia and surrounded by ice throughout the procedure to maintain anesthesia. Pups were maintained under anesthesia with ice for no longer than 15 minutes. Pups were protected from direct contact with the ice by having their bodies inserted into the cut tip of a nitrile glove.
Anesthetized pups were placed on a Kopf stereotaxic stage, modified for use with very young pups (Chen et al., 2018; Davidson et al., 2010; Mathon et al., 2015), under a fiber optic surgical lamp to identify bregma through the scalp. Because no published studies have described stereotaxic injections into the dorsal CA2 region of the hippocampus of young mouse pups, we first carried out pilot studies to determine stereotaxic coordinates for the CA2 at P3, as well as the minimum volume of virus and duration of time to achieve adequate viral expression. Once these parameters were determined, the following study was performed. Pups received bilateral stereotaxic injections of 150 nL of inhibitory DREADD or control virus injected directly through the scalp and skull into the CA2 (coordinates −1.0 anteroposterior, ± 1.0 mediolateral, and −2.0 dorsoventral relative to bregma) with a WPI nanofil 33 gauge beveled needle. It should be noted that pups required a larger volume of virus than adults, most likely due to the fact that the promoter, synapsin, is present at low levels in the hippocampus during the first postnatal week (Chen et al., 2018; Davidson et al., 2010; Mathon et al., 2015). In addition, because our goal was to test pups during the early postnatal period, we allowed only one week after surgery for viral infection, which is one week shorter than the conventional time we used for our adult virus study. Immediately following viral injection of both hemispheres, pups were transferred to a heating pad maintained at 37°C and, after recovery from anesthesia, returned to the nest.
Adult male and female offspring mice (P120) were anesthetized using isoflurane and head-fixed in a Kopf stereotaxic apparatus. Animals were placed on an infrared heating pad (Kent Scientific) to maintain body temperature for the duration of surgery. The skull was exposed and burr holes were drilled at the appropriate coordinates. Mice received bilateral injections containing 75 nL of inhibitory DREADD or control virus in the dorsal CA2 using the following coordinates: −1.82 anteroposterior, ± 2.15 mediolateral, and −1.7 dorsoventral relative to bregma. After injections, burr holes were filled with bone wax and the scalp was sutured. Mice were placed in a clean cage on a heating pad during recovery from anesthesia.
In a subset of adult mice (P120), which were not used for behavior studies, a tetrode was inserted unilaterally at −1.82 anteroposterior, +2.15 mediolateral, and −1.7 dorsoventral relative to bregma directly after DREADD virus injection. The electrode array consisted of four platinum-iridium wires that were arranged in a square grid with a distance of 100 μm between each wire (Microprobes). Three bone screws were implanted on the contralateral anterior, contralateral posterior, and ipsilateral anterior portion of the skull. A ground screw was implanted on the ipsilateral posterior region of the skull, where it was enwrapped with a ground wire and then coated with metallic paint.
CNO or saline treatment
One week after surgery, P3 pups injected with DREADD virus or control virus received CNO (5 mg/kg) or saline (IP) and 30 min later given a two-choice social recognition test with the caregiving mother and novel mother (see below). Each pup was administered the test twice, either after CNO or saline injection with a two-day rest period between tests. Thus, one test was administered at P10 and the other at P12. The order of injections was counterbalanced.
Two weeks after surgery, adult mice injected with DREADD virus or control virus received CNO (10 mg/kg) or saline (IP) and 30 min later were exposed to a direct social investigation test (see below) with the caregiving mother and novel mother. Each mouse was administered this test twice, either after CNO or saline injection, with a 7-day rest period between tests. Previous studies have shown that activity in DREADD infected neurons returns to baseline by 10–24 hours after CNO injection in the mouse (Alexander et al., 2009, 2018; Ray et al., 2011). The order of social stimulus presentation and the order of injections were counterbalanced. CNO injection was administered to a subset of these adult mice prior to sociability testing (see below).
Behavioral testing
All tests were conducted during the dark cycle under red light. One day before behavior testing, postweaning mice were habituated to the testing room for 30 minutes before being placed in the testing apparatus for the duration of the specific test. On the day of testing, postweaning mice were habituated to the testing room for 30 minutes before the initiation of the test. All behavioral tests were videorecorded and a trained observer scored behavior testing. Testing apparatuses were cleaned with 70% ethanol after each trial.
Preweaning two-choice social recognition test
At P3, P6, P10, P12, and P14, male and female mouse pups underwent behavior testing to investigate the memory of the mother using a modified two-choice social recognition test (Moy et al., 2004). Mouse pups were placed in a 12” × 12” opaque plexiglass testing apparatus with two wire mesh containers containing the caregiving mother and a novel mother. The testing apparatus was placed on a heating pad to maintain pup body temperature comparable to that of pups in the home cage. The novel mother was a lactating mother with a litter of age-matched pups. Because at P3, P6, P10 and P12 mouse pups do not engage in substantial locomotion and have limited sensory capabilities, which are primarily olfactory, we scored investigation of one or the other social stimulus whenever the test pup’s nose was oriented toward a stimulus animal. The duration of each test was 10 minutes. At the start of each test, the pup was placed in a neutral corner equidistant from the two social stimuli with the head directed toward the center of the apparatus. The location of the mother and novel mother in different corners was counterbalanced in each test. Time spent investigating was recorded when the pup’s snout moved in the direction of one or the other social stimulus. Testing periods only occurred for 20 minutes at a time for each caregiving mother/novel mother pair, after which dams were returned to their respective home cages to allow adequate nursing of their litters. Mothers remained with their litters for 20 minutes before beginning the next round of behavior. At P14, when pups are more active and engage in walking (Castelhano-Carlos et al., 2010), investigation was characterized as sniffing within one inch of a wire mesh container holding a social stimulus, instead of just preferential nose orientation. Since the social stimulus mice were confined within wire mesh containers, no attempt was made to quantify their behaviors.
Postweaning direct social interaction test
To test the memory of the mother in postweaning offspring, a more sensitive social test, the direct social interaction test, was used (Hitti and Siegelbaum, 2014; Smith et al., 2016). To avoid the confound of altered social preference during times of sexual receptivity, the estrous cycle was tracked in the caregiving mother and novel mother, and behavioral testing only occurred when stimulus mice were in metestrus or diestrus (Cora et al., 2015). At P30, P60, P100, P135, and P150, offspring mice underwent a social interaction test in which they directly interacted with the caregiving mother, or a novel mother, for 5 minutes in a 12” by 12” opaque plexiglass testing apparatus. After a one-hour delay spent in the home cage, mice were introduced to a stimulus animal not previously encountered in the test for an additional 5 minutes. The order of stimulus exposure was counterbalanced in all tests. Bouts of investigation were characterized as direct sniffing of the stimulus mouse’s anogenital region, body, and head.
Sociability test
P140 mice underwent three-chamber sociability testing (Moy et al., 2004). The three-chamber apparatus consisted of a neutral center chamber with two adjacent chambers, each containing a single wire mesh container. All chambers were equal in size. In the first trial, mice were allowed to explore all three chambers of the apparatus for 5 minutes. Immediately following the first trial, test mice were placed in the center neutral chamber while a novel female was placed in one of the wire mesh containers in an adjacent chamber. The adjacent chamber in which the female was placed was counterbalanced between trials. In the second trial, mice were once again given 5 minutes to explore the three chambers now consisting of a neutral center chamber, a nonsocial chamber, and a social chamber containing the novel female. Sniffing within 1” of a wire mesh container was counted toward total investigation time.
Object preference test
To determine whether mouse pups have an overall preference for familiarity, a metallic object with a distinct nonsocial odor (Walmart #603937991801 or #087547110607) was placed in the home cage of newborn cross-fostered litters. The object, now referred to as the “object: odor 1” was placed directly in the nest for the first 14 days of life to make certain that mouse pups with limited motor skills received adequate exposure to the object. After P14 and until weaning (P21), the object was left in the home cage, but not directly in the nest. On P3, mice were placed in a 12” × 12” opaque plexiglass testing apparatus. The familiar object: odor 1 and a novel object “object: odor 2” were symmetrically placed near the center of the testing apparatus. Investigation was scored when the test pup’s nose was oriented toward the stimulus object. The testing apparatus was placed on a heating pad to maintain pup body temperature comparable to that of pups in the home cage. At P60, ~40 days after the last exposure to the familiar object, these mice were again tested using the same experimental design previously described, with investigation scored whenever the test mouse directly sniffed the objects. P3 and P60 object preference tests consisted of a single 10-minute trial. The objects were counterbalanced between litters to address potential differences in odor saliency. We then verified that P60 mice have the ability to distinguish between novel and familiar objects by exposing a separate group of naive P60 mice to object: odor 1 for 10 minutes, and after a 45 min intertrial interval in the home cages, testing investigation times when given a choice of object: odor 1 and object: odor 2. Data were expressed as difference scores with the amount of time spent investigating object: odor 1 subtracted from the amount of time spent investgating object: odor 2.
USV recording and analysis
USVs were recorded using an Avisoft-UltraSoundGate 116Hb kit with a single-channel condenser microphone from Avisoft Bioacoustics (item #51162). USV recordings were collected using the Avisoft RECORDER software (version 4.2.29) and were configured with the following parameters: 300kHz sampling rate, 16-bit format, 0.032 s buffer, 50% overlap and 256 FFT size. P5 mouse pups were removed from the nest and placed in the center of a 12” by 12” opaque plexiglass testing apparatus on top of a commercial heating pad maintained at 33°C to simulate nest temperature (Hofer et al., 2001). The microphone was suspended above the behavioral apparatus, approximately 6 inches away from the animal. USVs were recorded under red light for three minutes during exposure to the caregiving mother or novel mother. Following a one hour inter-trial interval during which the animals were placed back in the nest, pups were again recorded for three minutes during exposure to the novel mother or caregiving mother. The order of exposure to the caregiving mother and novel mother was counterbalanced between animals. During testing, the stimulus female was kept in a wire mesh container positioned in the top left corner of the apparatus to prevent the female from initiating interaction with the pup. Separate containers were used for the caregiving mother and novel mother.
USVs were analyzed using Avisoft SASLab Pro (version 5.2.13) from Avisoft Bioacoustics (item #10101). Sound files were put through a high-pass filter to exclude amplitude under 30kHz (Ferhat et al., 2016). Spectrograms were created with the following parameters: 512 FFT size, 100% frame rate, Hamming window, 75% temporal overlap (Vogel et al., 2019). Calls were automatically detected using a single threshold element separation of −22dB with a hold time of 14ms. All detected calls were manually assessed, and false calls caused by background noise were removed from the dataset. Calls were also excluded if they were emitted when the mouse pup fell over and had to be righted by the experimenter. The total number of calls as well as the total duration of calls in seconds were analyzed for the first 60 s of each recording.
Caregiving mother/novel mother exposure for immediate early gene analysis
To determine if exposure to the caregiving mother or a novel mother differentially altered immediate early gene expression in CA2, vCA1, proximal dCA1, and distal dCA3 regions, P14 or P60 mice were placed in a 12” × 12” opaque plexiglass testing apparatus containing the caregiving mother or the novel mother and allowed to investigate for 5 minutes. Adult animals were allowed to directly investigate the stimulus mouse whereas pups were exposed to a stimulus mouse located in a wire mesh container. At the conclusion of the five-minute trial, adult mice were transferred to the home cage, or pups to a clean cage without the caregiving mother, and perfused one hour later. The brains were processed as previously described for immunohistochemistry and stained using rabbit anti-c-Fos (1:500, Cell Signaling Technology #2250S) or rabbit anti-Zif268 (1:500, Cell Signaling Technology #4153S), combined with mouse anti-RGS14 (1:500) to identify the CA2 region. Researchers were blinded from the experimental conditions of each slide before conducting c-Fos or Zif268 cell counts. Counts were conducted using a fluorescence microscope (Olympus BX-60) with c-Fos and Zif268 cell counts taken from a one in six series of coronal sections containing CA2 or distal dCA3, and c-Fos+ cell counts taken from a one in six series of coronal sections containing vCA1 and proximal dCA1. One hemisphere was randomly selected and all counts for a given region were made from the chosen hemisphere. Only c-Fos+ and Zif268+ cells located within RGS14 labeling that passed a stringent intensity threshold using Stereo Investigator (Microbrightfield) were included in CA2 analyses. Target region volumes were determined, and the data were expressed as the density of Zif268+ cells or c-Fos+ cells per mm3. c-Fos+ and Zif268+ cell densities were normalized to respective positive cell counts from equal numbers of age-matched controls that remained in the home cage and did not undergo social interaction. c-Fos+ and Zif268+ cell densities were divided by the average densities observed in home cage controls, yielding % c-Fos+ or Zif268+ cells, relative to home cage control, after exposure to the caregiving mother or novel mother.
Electrophysiology
To verify that CNO administration reduced neuronal activity in the CA2 after infection with inhibitory DREADD virus, a subset of DREADD virus-injected mice were implanted with a four-wire electrode array in hippocampal CA2. After a two week recovery period, mice were first habituated to wearing the headstage in an open-field testing apparatus for 10 minutes, after which a 140× gain wireless headstage (Triangle Biosystems) recorded neural activity for two minutes, taken when mice were moving as well as at rest. The neural data were transmitted to a wireless receiver (Triangle Biosystems) and recorded using NeuroWare software (Triangle Biosystems). After the initial recording, the wireless headstage was removed and mice received an injection of CNO (10 mg/kg IP) before being returned to the home cage. After 30 minutes in the home cage, the wireless headstage was reattached and mice were returned to the testing apparatus where hippocampal CA2 activity was recorded for an additional two minutes. Continuous LFP data were bandpass filtered from 1–500 Hz and stored at 1,000 Hz. All recordings referenced a silver wire wrapped around a ground screw implanted in the posterior parietal bone. Neuronal data were analyzed using Neuroexplorer software (Nex Technologies).
QUANTIFICATION AND STATISTICAL ANALYSIS
All data were checked for meeting the assumptions of parametric statistics and analyzed using Graphpad Prism 8. When normally distributed, data were analyzed using unpaired t tests, paired t tests, one-way ANOVA followed by Tukey’s HSD tests or by two-way ANOVA followed by Sidak multiple comparison tests. Four datasets were determined to be unsuitable for parametric statistical analysis. Data collected from the pup DREADD experiment exhibited a non-normal distribution and underwent log transformation prior to two-way ANOVA and Sidak multiple comparisons testing. Object odor discrimination data were determined to be unsuitable for parametric statistics and a Kruskal-Wallis test with Dunn’s multiple comparisons tests were performed instead. Pup ultrasonic vocalization data were non-normally distributed and utilized a Wilcoxon matched-pairs signed rank test. Developmental social preference data were z-scored prior to one-way ANOVA, allowing for comparisons across ages. Sample sizes were chosen based on previous published data and pilot studies conducted within the laboratory. Data were expressed as mean ± SEM in all figures. Significance was determined as p < 0.05.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-NECAB2 | Novus Biologicals | Cat#NBP1-84002; RRID: AB_11028373 |
| rabbit anti-PCP4 | Sigma | Cat#HPA005792; RRID: AB_1855086 |
| mouse anti-RGS14 | UC Davis/NIH NeuroMab Facility | Cat#75-170; RRID: RRID:AB_2179931 |
| rabbit anti-VGLUT2 | Synaptic Systems | Cat#135403; RRID: AB_2864778 |
| rabbit anti-c-Fos | Cell Signaling Technology | Cat#2250S; RRID: AB_2247211 |
| rabbit anti-zif-268 | Cell Signaling Technology | Cat#4153; RRID: AB_2097038 |
| mouse anti-parvalbumin | Sigma-Aldrich | Cat# P3088, RRID:AB_477329 |
| Bacterial and virus strains | ||
| pAAV-hSyn-hM4D(Gi)-mCherry | http://www.addgene.org/50475/ | Addgene viral prep # 50475-AAV5; RRID:Addgene_50475 |
| pAAV-hSyn-mCherry | http://www.addgene.org/114472/ | Addgene viral prep # 114472-AAV5; RRID:Addgene_114472 |
| Chemicals, peptides, and recombinant proteins | ||
| CNO | NIMH Chemical Synthesis and Drug Supply Program; RTI International | Cat#C-929; batch ID # 14073-2 |
| Hoechst 33342 | Molecular Probes | Cat# H-3570 |
| Wistera Floribunda Lectin | Sigma | Cat#L1516 |
| Experimental models: organisms/strains | ||
| C57BL/6J Mus musculus | https://www.jax.org/strain/000664 | Cat# JAX:000664, RRID:IMSR_JAX:000664 |
| Software and algorithms | ||
| NeuroWare software | Triangle Biosystems | |
| NeuroExplorer software | http://www.neuroexplorer.com/ | RRID:SCR_001818 |
| Avisoft RECORDER software | Avisoft Bioacoustics | version 4.2.29 |
| Avisoft SASLab Pro | Avisoft Bioacoustics. http://www.avisoft.com/sound-analysis/ | RRID:SCR_014438 |
| GraphPad Prism | http://www.graphpad.com/ | RRID:SCR_002798 |
| ImageJ | https://imagej.nih.gov/ij/ | RRID:SCR_003070 |
Highlights.
Mice recognize their caregiving mother and distinguish her from novel mothers
Preference for the caregiving mother over a novel mother reverses after weaning
Immediate-early gene expression in the CA2 parallels social preference
The CA2 is required for this social preference across development
ACKNOWLEDGMENTS
This work was supported by NIMH grant MH118631-01 (to E.G.). Figure images were created using BioRender.com.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.108668.
REFERENCES
- Al Aïn S, Perry RE, Nuñez B, Kayser K, Hochman C, Brehman E, LaComb M, Wilson DA, and Sullivan RM (2017). Neurobehavioral assessment of maternal odor in developing rat pups: implications for social buffering. Soc. Neurosci 12, 32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Farris S, Pirone JR, Zheng C, Colgin LL, and Dudek SM (2016). Social and novel contexts modify hippocampal CA2 representations of space. Nat. Commun. 7, 10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Brown LY, Farris S, Lustberg D, Pantazis C, Gloss B, Plummer NW, Jensen P, and Dudek SM (2018). CA2 neuronal activity controls hippocampal low gamma and ripple oscillations. eLife 7, e38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, DeVito LM, and Cleland TA (2006). One-trial associative odor learning in neonatal mice. Chem. Senses 31, 343–349. [DOI] [PubMed] [Google Scholar]
- Benoy A, Dasgupta A, and Sajikumar S (2018). Hippocampal area CA2: an emerging modulatory gateway in the hippocampal circuit. Exp. Brain Res. 236, 919–931. [DOI] [PubMed] [Google Scholar]
- Blank DA, and Yang W (2017). Mother-young recognition in goitered gazelle during hiding period. Behav. Processes 142, 21–28. [DOI] [PubMed] [Google Scholar]
- Branchi I (2009). The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci. Biobehav. Rev 33, 551–559. [DOI] [PubMed] [Google Scholar]
- Brown LY, Alexander GM, Cushman J, and Dudek SM (2020). Hippocampal CA2 organizes CA1 slow and fast g oscillations during novel social and object interaction. eNeuro 7, ENEURO.0084–20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham D (1993). Visual recognition of mother by young infants: facilitation by speech. Perception 22, 1133–1153. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Wersinger SR, and Young WS 3rd. (2008). The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog. Brain Res. 170, 65–72. [DOI] [PubMed] [Google Scholar]
- Carstens KE, and Dudek SM (2019). Regulation of synaptic plasticity in hippocampal area CA2. Curr. Opin. Neurobiol 54, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, and Dudek SM (2016). Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J. Neurosci 36, 6312–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelhano-Carlos MJ, Sousa N, Ohl F, and Baumans V (2010). Identification methods in newborn C57BL/6 mice: a developmental and behavioural evaluation. Lab. Anim 44, 88–103. [DOI] [PubMed] [Google Scholar]
- Chen SY, Kuo HY, and Liu FC (2018). Stereotaxic surgery for genetic manipulation in striatal cells of neonatal mouse brains. J. Vis. Exp 137, 57270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Huang AJY, Wintzer ME, Ohshima T, and McHugh TJ (2018). A role for CA3 in social recognition memory. Behav. Brain Res. 354, 22–30. [DOI] [PubMed] [Google Scholar]
- Cilz NI, Cymerblit-Sabba A, and Young WS (2019). Oxytocin and vasopressin in the rodent hippocampus. Genes Brain Behav. 18, e12535. [DOI] [PubMed] [Google Scholar]
- Cora MC, Kooistra L, and Travlos G (2015). Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol 43, 776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cymerblit-Sabba A, Smith AS, Williams Avram SK, Stackmann M, Korgan AC, Tickerhoof MC, and Young WS (2020). Inducing partner preference in mice by chemogenetic stimulation of CA2 hippocampal subfield. Front. Mol. Neurosci 13, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR (1994). Physiological evidence for genetically mediated sibling recognition in mice. Behav. Genet 24, 493–496. [DOI] [PubMed] [Google Scholar]
- Davidson S, Truong H, Nakagawa Y, and Giesler GJ Jr. (2010). A microinjection technique for targeting regions of embryonic and neonatal mouse brain in vivo. Brain Res. 1307, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, and Sullivan RM (2017). The neurobiology of safety and threat learning in infancy. Neurobiol. Learn. Mem. 143, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, and De Souza Silva MA (2007). The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev 31, 673–704. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Alexander GM, and Farris S (2016). Rediscovering area CA2: unique properties and functions. Nat. Rev. Neurosci. 17, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Showk S, van Zweden JS, d’Ettorre P, and Sundström L (2010). Are you my mother? Kin recognition in the ant Formica fusca. J. Evol. Biol 23, 397–406. [DOI] [PubMed] [Google Scholar]
- Evans PR, Lee SE, Smith Y, and Hepler JR (2014). Postnatal developmental expression of regulator of G protein signaling 14 (RGS14) in the mouse brain. J. Comp. Neurol 522, 186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhat A-T, Torquet N, Le Sourd A-M, de Chaumont F, Olivo-Marin J-C, Faure P, Bourgeron T, and Ey E (2016). Recording mouse ultrasonic vocalizations to evaluate social communication. J. Vis. Exp 112, 53871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivush R (2011). The development of autobiographical memory. Annu. Rev. Psychol 62, 559–582. [DOI] [PubMed] [Google Scholar]
- Frotscher M, and Seress L (2007). Morphological development of the hippocampus. In The Hippocampus Book, Andersen P, Morris R, Amaral D, Bliss T, and O’Keefe J, eds. (Oxford Univ. Press; ), pp. 115–132. [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, and Barnes CA (2005). Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol 15, 599–606. [DOI] [PubMed] [Google Scholar]
- Hepper PG (1994). Long-term retention of kinship recognition established during infancy in the domestic dog. Behav. Processes 33, 3–14. [DOI] [PubMed] [Google Scholar]
- Hickman DL, and Swan MP (2011). Effects of age of pups and removal of existing litter on pup survival during cross-fostering between multiparous outbred mice. J. Am. Assoc. Lab. Anim. Sci 50, 641–646. [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, and Siegelbaum SA (2014). The hippocampal CA2 region is essential for social memory. Nature 508, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Shair HN, and Brunelli SA (2001). Ultrasonic vocalizations in rat and mouse pups. Curr. Protoc. Neurosci 17, 8.14.1–8.14.16. [DOI] [PubMed] [Google Scholar]
- Jones MW, and McHugh TJ (2011). Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci. 34, 526–535. [DOI] [PubMed] [Google Scholar]
- Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, Buss EW, Kandel ER, and Siegelbaum SA (2018). A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 564, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Hsieh TY, Tsai TC, Chen CC, Huang CC, and Hsu KS (2018). Conditional deletion of hippocampal CA2/CA3a oxytocin receptors impairs the persistence of long-term social recognition memory in mice. J. Neurosci 38, 1218–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, and Stowers L (2012). Learned recognition of maternal signature odors mediates the first suckling episode in mice. Curr. Biol 22, 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathon B, Nassar M, Simonnet J, Le Duigou C, Clemenceau S, Miles R, and Fricker D (2015). Increasing the effectiveness of intracerebral injections in adult and neonatal mice: a neurosurgical point of view. Neurosci. Bull 31, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann KE, Lustberg DJ, Shaughnessy EK, Carstens KE, Farris S, Alexander GM, Radzicki D, Zhao M, and Dudek SM (2019). Novel role for mineralocorticoid receptors in control of a neuronal phenotype. Mol. Psychiatry, Published online November 19, 2019. 10.1038/s41380-019-0598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meira T, Leroy F, Buss EW, Oliva A, Park J, and Siegelbaum SA (2018). A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun 9, 4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi K, Takakuda A, Tsukamoto C, Ooyama R, Okabe S, Koshida N, Nagasawa M, and Kikusui T (2017). Mutual mother-infant recognition in mice: The role of pup ultrasonic vocalizations. Behav. Brain Res. 325 (Pt B), 138–146. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, and Crawley JN (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, and Tonegawa S (2016). Ventral CA1 neurons store social memory. Science 353, 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Al Aïn S, Raineki C, Sullivan RM, and Wilson DA (2016). Development of odor hedonics: experience-dependent ontogeny of circuits supporting maternal and predator odor responses in rats. J. Neurosci 36, 6634–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarzyk K, Klett J, Pena EA, Porcher L, Smith AJ, and Kelly MP (2019). Loss of function of phosphodiesterase 11A4 shows that recent and remote long-term memories can be uncoupled. Curr. Biol 29, 2307–2321.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, and Winberg J (1999). Unique salience of maternal breast odors for newborn infants. Neurosci. Biobehav. Rev 23, 439–449. [DOI] [PubMed] [Google Scholar]
- Raam T, McAvoy KM, Besnard A, Veenema AH, and Sahay A (2017). Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun 8, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Pickenhagen A, Roth TL, Babstock DM, McLean JH, Harley CW, Lucion AB, and Sullivan RM (2010). The neurobiology of infant maternal odor learning. Braz. J. Med. Biol. Res 43, 914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Sarro E, Rincón-Cortés M, Perry R, Boggs J, Holman CJ, Wilson DA, and Sullivan RM (2015). Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology 40, 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, and Dymecki SM (2011). Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler A, and Salmon M (1969). Communal nursing in mice: influence of multiple mothers on the growth of the young. Science 164, 1309–1310. [DOI] [PubMed] [Google Scholar]
- Schaal B, Saxton TK, Loos H, Soussignan R, and Durand K (2020). Olfaction scaffolds the developing human from neonate to adolescent and beyond. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingensiepen KH, Lüno K, and Brysch W (1991). High basal expression of the zif/268 immediate early gene in cortical layers IV and VI, in CA1 and in the corpus striatum—an in situ hybridization study. Neurosci. Lett 122, 67–70. [DOI] [PubMed] [Google Scholar]
- Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, and Young WS (2016). Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 21, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreng L, Temime-Roussel B, Wortham H, and Mourre C (2017). Chemical identification of “maternal signature odors” in rat. Chem. Senses 42, 211–222. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, and Leon M (1990). Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res. Dev. Brain Res. 53, 243–247. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, and Wilson DA (2000). Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav. Neurosci 114, 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todrank J, Busquet N, Baudoin C, and Heth G (2005). Preferences of newborn mice for odours indicating closer genetic relatedness: is experience necessary? Proc. Biol. Sci 272, 2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura S, Imai S, Mimura A, Fujiwara M, and Ebihara S (2015). Impaired maternal behavior in usp46 mutant mice: a model for trans-generational transmission of maternal care. PLoS ONE 10, e0136016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell M, Curran T, and Morgan JI (1998). A gene expression approach to mapping the functional maturation of the hippocampus. Brain Res. Mol. Brain Res. 63, 25–34. [DOI] [PubMed] [Google Scholar]
- Vogel AP, Tsanas A, and Scattoni ML (2019). Quantifying ultrasonic mouse vocalizations using acoustic analysis in a supervised statistical machine learning framework. Sci. Rep 9, 8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Chen L, Xia Y, Cheng Q, Yuan J, Yang Y, Wang Z, Wang H, Dong J, Ding Y, and Zhao X (2016). Maternal deprivation influences pup ultrasonic vocalizations of c57bl/6j mice. PLoS ONE 11, e0160409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the current study have not been deposited in a public repository but are available from the corresponding author on request. This study did not generate code.