Abstract
Objective
Sleep complaints are frequent after acute COVID-19. Aim of this study was to videopolysomnographically evaluate sleep and sleep disorders after SARS-Cov2 infection.
Methods
Patients with suspected sleep disorders after acute COVID-19 underwent video-polysomnography (v-PSG) at the Sleep Disorders Clinic, Department of Neurology, Medical University Innsbruck. V-PSG was conducted 4.2 (SD = 1.3) months after diagnosis of SARS-CoV-2 infection.
Results
Eleven patients [nine men, age 52.5 (SD = 11.7) years; BMI 29 (SD = 5.2) kg/m2] were included. At 60 days follow-up after diagnosis, persisting breathing complaints were present in 7/11 (64%) patients. After v-PSG four patients (36%) were diagnosed with obstructive sleep apnea (OSA). Respiratory frequency during sleep was normal and no tachypnea, thoracoabdominal asynchrony, or periodic deep sighing were detected. Four patients (36%) showed REM sleep without atonia (RWA), and two additional patients showed an RWA index within the highest range of normality.
Conclusion
We report videopolysomnographic findings in a series of eleven patients after acute COVID-19. A major finding of this study was the presence of isolated RWA, a recognized prodromal stage of RBD, in more than one third of the patients. Future videopolysomnographic investigations including quantification of RWA in patients after COVID-19 will give more insights into a possible acute or post-infectious CNS pathology related to the SARS-CoV-2 infection.
Keywords: Polysomnography, SARS-Cov2, Sleep apnea, REM sleep without atonia, Neurodegeneration
1. Introduction
Sleep disturbances are frequent after acute COVID-19 [1]. To date, evaluation of sleep in these patients has been performed using questionnaires or by clinical history. We aimed to polysomnographically assess sleep disorders in post-acute COVID-19 patients. In particular, we focused I) on sleep related breathing disorders, as the main target of SARS-CoV-2 is the respiratory tract; and II) on REM sleep without atonia (RWA), as possible sign of involvement of the CNS.
2. Patients/methods
Patients with suspected sleep disorders were recruited from the prospective multicenter observational CovILD [Development of interstitial lung disease (ILD) in patients with SARS-CoV-2 infection] cohort (NCT04416100) [2]. Diagnosis of COVID-19 was based on positive SARS-CoV-2-PCR from a nasopharyngeal swab and typical clinical presentation (as defined by the WHO) [3]. Patients’ symptoms, including dyspnea assessed with the modified British Medical Research Council (mMRC) questionnaire [4], during acute COVID-19 and at 60 (63, SD = 23) days after the diagnosis of COVID-19 were evaluated. Additionally, at follow-up pulmonary function tests were carried out, to assess the presence of persistent breathing complaints.
V-PSG was conducted and scored according to the American Academy of Sleep Medicine (AASM) current recommendations [5]. Nasal pressure was recorded with cannula and oronasal airflow with thermistor. Respiratory movements were registered with thoracic and abdominal piezo strain gauge belts. Respiratory frequency and pattern were analyzed during NREM and REM sleep. EMG of the upper extremities on the flexor digitorum superficialis (FDS) muscles bilaterally was performed in all participants. REM sleep without atonia (RWA) was quantified for 3-s mini-epochs using a validated algorithm [4] with manual artifact correction. Phasic, tonic and “any” EMG activity indices in the chin were calculated, as well as phasic EMG activity index in the FDS bilaterally and the SINBAR index (“any” chin and/or phasic FDS) [6].
3. Results
Eleven patients were included and underwent video-polysomnography (v-PSG) (nine men, age 52.5, SD = 11.7 years, BMI 29, SD = 5.2 kg/m2). V-PSG was conducted 4.2 (SD = 1.3) months after diagnosis of SARS-CoV-2 infection. At 60 days follow-up visit after diagnosis, persisting breathing complaints were present in 7/11 (64%) patients.
Sleep variables are reported in Table 1 . Apnea-hypopnea index (AHI) was >5/h in five patients (45%) and above 15/h in three (27%). Four patients (36%) were diagnosed with obstructive sleep apnea (OSA). One patient with OSA and obstructive snoring (AHI 19.5/h) showed decreased mean oxygen saturation (88.9%) and minimal saturation of 80%. Another patient with OSA (AHI = 34,9/h) had a central apneas index of 6.7/h. Respiratory frequency during sleep was normal without tachypnea and thoracoabdominal asynchrony, and periodic deep sighing was not detected (Table 1).
Table 1.
Sleep variables.
| Sleep architecture | |
| Time in bed (min) | 475 (440–537) |
| Total sleep time (min.) | 375 (290–462) |
| Wake after sleep onset (min.) | 70 (26.5–172) |
| Sleep efficiency (%) | 82.2 (60.2–94.4) |
| Sleep onset latency (min.) | 90.5 (43.5–316.5) |
| N1%/SPT | 13.5 (7.1–18.4) |
| N2%/SPT | 45.9 (34.9–60.8) |
| N3%/SPT | 7.8 (0–19.6) |
| REM %/SPT | 13.4 (7.2–22.8) |
| Respiratory Variables | |
| Apnea-hypopnea index (events/h) | 3.8 (0–34.9) |
| Central apnea index (event/h) | 0.3 (0–6.7) |
| Obstructive apnea index (event/h) | 0 (0–7.3) |
| Hypopnea index (event/h) | 2.3 (0–28.2) |
| Oxygen desaturation index (events/h) | 4.2 (0–27.4) |
| Mean oxygen saturation (%) | 94.8 (88.9–96.6) |
| Respiratory rate REM (/min) | 16.25 (10–19) |
| Respiratory rate NREM (/min) | 16 (10–19.5) |
| REM sleep without atonia (RWA)ab | |
| SINBAR (any mentalis and/or phasic FDS) (%) | 25.3 (1.7–41.0) |
| Above published cut off, n (%) | 1/11 (9%) |
| Any mentalis only (%) | 12.9 (1.4–24.1) |
| Above cut off, n (%) | 2/11 patients (18%) |
| Phasic mentalis only (%) | 8.8 (1–20.2) |
| Above cut off, n (%) | 2/11 patients (18%) |
| Tonic mentalis (%) | 0 (0–4.0) |
| Above cut off, n (%) | 0/11 patients (0%) |
| Phasic FDS right and left (%) | 11.6 (0.7–26.5) |
| Above cut off, n (%) | 3/11 patients (27%) |
| PLM | |
| PLMS index, (events/h) | 20.6 (0–72.7) |
| Above >15/h | 7/11 patients (63%) |
Data are provided as median (range). Abbreviations: BMI: body mass index, REM: rapid-eye-movement, RWA: REM sleep without atonia, FDS: flexor digitorum superficialis muscle, PLMS: periodic limb movements during sleep, SPT: sleep period time, SINBAR: Sleep Innsbruck Barcelona.
One subject can exceed multiple cut off values.
Cut off values: SINBAR (any mentalis and/or phasic FDS): 31.9%; any mentalis only: 18.2%, phasic mentalis: 16.3%; tonic mentalis: 9.6%; phasic FDS right and left: 16.8% [6].
Four patients (36%) showed RWA (Fig. 1 ), with abnormal EMG activity exceeding at least one cut-off for RWA [6] (Table 1). None of them met diagnostic criteria for REM sleep behavior disorder (RBD). Two additional patients showed an RWA index within the highest range of normality (one for phasic muscle activity in the FDS and one for phasic activity in the chin). There was no correlation between AHI and any of the RWA indices. RWA indices were consistently lower in those patients who underwent v-PSG after a longer time from COVID-19 diagnosis, with negative correlation between RWA index and time from COVID-19 diagnosis (p = 0.021, rs = −0,7 for SINBAR RWA index). There was no correlation between SINBAR RWA index and age. None of these patients had a premorbid neurological or psychiatric disorder and none of these patients was treated with antidepressants or any other psychoactive substances at the time of PSG. Three of these patients complained of insomnia during or after COVID-19. Sleep parameters (sleep stages, respiratory variables, PLMS index) did not show any significant difference between patients with and without RWA.
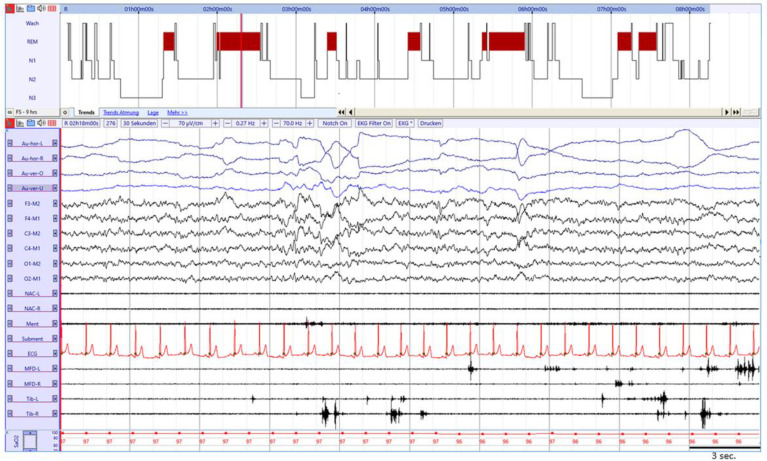
Fig. 1.
Example of 30 s. REM sleep epoch with RWA in a 53 year old male after SARS-Cov2 infection with phasic muscle activity in the mentalis and both FDS muscles. Legend: Au-hor-L: left horizontal electrooculogram (EOG); Au-hor-R: right horizontal EOG; Au-ver-O: vertical upper EOG; Au-ver-U: vertical lower EOG; NAC-L: splenius capitis muscle left; NAC-R: splenius capitis muscle right; Ment: mentalis muscle; Subment: submentalis muscle; ECG: electrocardiogram; MDF-l: left flexor digitorum superficialis (FDS) muscle; MDF-r: right flexor digitorum superficialis muscle; Tib-L: left anterior tibialis muscle; Tib-R: right anterior tibialis muscle; SaO2: oxygen saturation.
4. Discussion/conclusion
We report videopolysomnographic findings in eleven patients with suspected sleep disorder after acute COVID-19. A major finding of this study was isolated RWA, a recognized prodromal stage of RBD [7,8], in 4/11 (36%) of the patients, and high values just below the cutoff in two additional subjects. Physiological REM sleep atonia is maintained through neuronal circuits localized in the brainstem, including the subcoeruleus nucleus [9]. The described abnormal muscle activity during REM sleep therefore likely reflects the involvement of the central nervous system (CNS) in COVID-19. In fact, anosmia is a common symptom of SARS-CoV-2 infection and sometimes persists after COVID-19 [10], in line with translational models suggesting that coronaviruses can be neuro-invasive with an olfactory route [11]. Even though the main target of SARS-CoV-2 is the respiratory tract, COVID-19 is a systemic disease affecting multiple organs including the CNS. Neuropathological studies showed that neuroinflammatory changes were most prominent in the brainstem, providing a possible explanation for our findings of RWA [11]. If RWA in these patients is secondary to COVID-19 or if this was a premorbid state cannot be answered with this study. As isolated RWA (ie, prodromal RBD) is an early marker of neurodegenerative disease [6,7], follow-up investigations are needed to elucidate I) if RWA persists, increases, decreases (or may even re-increase after an initial decrease) over time, and II) if patients with RWA post COVID-19 will develop a neurodegenerative disease (such as Parkinson's disease, dementia with Lewy bodies or multiple system atrophy), as case reports (eg Cohen et al., Méndez-Guerrero et al.) of probable PD after COVID-19 seemingly increase [12,13].
V-PSG showed OSA in 4/11 (36%) patients but otherwise breathing pattern were normal. These findings suggest that pulmonary limitation post COVID-19 might only be relevant during wakefulness and physical exercise, but not during sleep. Elevated residual volumes and hypocapnia during wakefulness present in 82% and 18% of the patients respectively, suggest that dysfunctional breathing might contribute to respiratory daytime symptoms. However, typical patterns for dysfunctional breathing, such as paradoxical breathing, tachypnea, or periodic sighing [14] were not present during v-PSG. A potential limitation is the use of thoracic and abdominal strain gauge belts with piezo technology (as opposed to induction plethysmography), which may underestimate periods of altered breathing pattern; additionally, transcutaneous pCO2 measurements were not performed.
PLMS index >15/h was present in 63% of patients. Of them, 36% had restless legs syndrome.
Moreover, these reported findings might have been pre-existent, as no v-PSG previous to COVID-19 was performed in these subjects.
Future videopolysomnographic investigations in larger cohorts including quantification of RWA in patients after COVID-19 will provide more insights into a possible acute or post-infectious CNS pathology related to the SARS-CoV-2 infection.
Author’s contribution
AH, TS, BH, JL-R, and AS were responsible for conception of the work, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be submitted; AI, MC and IT contributed to data collection, data analysis and interpretation and critical revision of the article and final approval of the version to be submitted; EB, MB added input to data analysis and interpretation and commited critical revision of the article and final approval of the version to be submitted.
Acknowledgment
We acknowledge the dedication, commitment, and sacrifice of the staff, providers, and personnel in our institutions through the COVID-19 crisis.
Footnotes
The authors declared no conflict of interest related to this study.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.01.051.
Conflict of interest
The following is the supplementary data related to this article:
References
- 1.Huang C., Huang L., Wang Y., et al. Lancet; London, England: 2021. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonnweber TS S., Pizzini A., Luger A., et al. Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J. 2020 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. 2020. https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2 Available from:
- 4.Bestall J.C., Paul E.A., Garrod R., et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry R.B., Quan S.F., Abreu A.R., et al. American Academy of Sleep Medicine; 2020. The AASM manual for the scoring of sleep and asscociated events: rules, terminology and technical specifications. For the American Academy of sleep medicine. Version 2.6 Darien. [Google Scholar]
- 6.Frauscher B., Iranzo A., Gaig C., et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35(6):835–847. doi: 10.5665/sleep.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefani A., Gabelia D., Högl B., et al. Long-term follow-up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: a pilot study. J Clin Sleep Med. 2015;11(11):1273–1279. doi: 10.5664/jcsm.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Högl B., Stefani A., Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat Rev Neurol. 2018;14(1):40–55. doi: 10.1038/nrneurol.2017.157. [DOI] [PubMed] [Google Scholar]
- 9.Luppi P.H., Clément O., Sapin E., et al. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011;15(3):153–163. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Morbini P., Benazzo M., Verga L., et al. JAMA Otolaryngol Head Neck Surg; 2020. Ultrastructural evidence of direct viral damage to the olfactory complex in patients testing positive for SARS-CoV-2. [DOI] [PubMed] [Google Scholar]
- 11.Matschke J., Lütgehetmann M., Hagel C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020 Nov;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen M.E., Eichel R., Steiner-Birmanns B., et al. A case of probable Parkinson's disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19(10):804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Méndez-Guerrero A., Laespada-García M.I., Gómez-Grande A., et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95(15):e2109–e2118. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- 14.Boulding R., Stacey R., Niven R., et al. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev. 2016;25(141):287–294. doi: 10.1183/16000617.0088-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.