Abstract
Autoantibodies against granulocyte/macrophage colony-stimulating factor (GMAbs) cause autoimmune pulmonary alveolar proteinosis (PAP) and measurement of the GMAb level in serum is now commonly used to identify this disease, albeit, in a clinical research setting. The present study was undertaken to optimize and standardize serum GMAb concentration testing using a GMAb enzyme-linked immunosorbent assay (GMAb ELISA) to prepare for its introduction into routine clinical use. The GMAb ELISA was evaluated using serum specimens from autoimmune PAP patients, healthy people, and GMAb-spiked serum from healthy people. After optimizing assay components and procedures, its accuracy, precision, reliability, sensitivity, specificity, and ruggedness were evaluated. The coefficient of variation in repeated measurements was acceptable (<15%) for well-to-well, plate-to-plate, day-to-day, and inter-operator variation, and was not affected by repeated freeze-thaw cycles of serum specimens or the reference standards, or by storage of serum samples at −80°C. The lower limit of quantification (LLOQ) of the PAP patient-derived polyclonal GMAb reference standard (PCRS) was 0.78 ng/ml. Receiver operating characteristic curve analysis identified a serum GMAb level of 5 μg/ml (based on PCRS) as the optimal cut off value for distinguishing autoimmune PAP serum from normal serum. A pharmaceutical-grade, monoclonal GMAb reference standard (MCRS) was developed as the basis of a new unit of measure for GMAb concentration: one International Unit (IU) of GMAb is equivalent to 1 μg/ml of MCRS. The median [interquartile range] serum GMAb level was markedly higher in autoimmune PAP patients than in healthy people (21.54 [12.83 – 36.38] versus 0.08 [0.05 – 0.14] IU; n=56, 38; respectively; P<0.0001). Results demonstrate that serum GMAb measurement using the GMAb ELISA was accurate, precise, reliable, had an acceptable LLOQ, and could be accurately expressed in standardized units. These findings support the use of this GMAb ELISA for the routine clinical diagnosis of autoimmune PAP and introduce a new unit of measure to enable standardized reporting of serum GMAb data from different laboratories.
Keywords: Pulmonary alveolar proteinosis, Granulocyte/macrophage-colony stimulating factor, Autoimmune disease, Autoantibodies, Enzyme Linked Immunosorbent Assay, Diagnosis
1. Introduction
Autoimmune pulmonary alveolar proteinosis (autoimmune PAP)* is a rare disease characterized by alveolar surfactant accumulation, respiratory failure, and an increased risk of opportunistic infections (Trapnell et al., 2003). The disease is strongly associated with granulocyte/macrophage-colony stimulating factor autoantibodies (GMAbs) (Kitamura et al., 1999) that neutralize GM-CSF bioactivity (Kitamura et al., 1999; Uchida et al., 2004) and mediate pathogenesis by blocking signaling to alveolar macrophages (Sakagami et al., 2009; Sakagami et al., 2010) and neutrophils (Uchida et al., 2007). Alveolar macrophages require GM-CSF for terminal differentiation and constitutive regulation of functions including surfactant clearance (Shibata et al., 2001; Bonfield et al., 2003). Without pulmonary GM-CSF signaling, alveolar macrophages have impaired pulmonary surfactant clearance (Ikegami et al., 1996) resulting in the slow, progressive surfactant accumulation and the insidious onset of the clinical manifestations of PAP syndrome. Disruption of GM-CSF signaling by recessive mutations in CSF2RA or CSF2RB, which encode the GM-CSF receptor α and β chains, respectively, causes a hereditary form of PAP that is clinically, physiologically, and histologically indistinguishable from autoimmune PAP (Martinez-Moczygemba et al., 2008; Suzuki et al., 2008; Suzuki et al., 2010; Suzuki et al., 2011; Tanaka et al., 2011). PAP can also occur in a heterogeneous group of other diseases either as a consequence of an underlying clinical condition presumably affecting alveolar macrophage function (secondary PAP) or mutations in the genes involved surfactant production (e.g., SFTPB, SFTPC, ABCA3, TTF1) (congenital PAP, and PAP associated with interstitial lung disease) (Nogee, 2010).
Clinically, the diagnosis of PAP is made based on a compatible history, typical radiologic findings, and characteristic lung biopsy or bronchoalveolar lavage cytology findings. However, while this approach can determine if PAP syndrome is present, it can not identify the underlying causative disease. The strong association of a high serum GM-CSF autoantibody (GMAb) level with autoimmune PAP (Kitamura et al., 1999; Bonfield et al., 2002; Trapnell et al., 2003; Inoue et al., 2008), development of an ELISA to measure GMAbs (Schoch et al., 2002), and demonstration that GMAbs actually drive the pathogenesis of autoimmune PAP (Uchida et al., 2007; Sakagami et al., 2009; Sakagami et al., 2010), support what is now widespread use of serum GMAb measurement for the clinical research diagnosis of autoimmune PAP. The potential clinical use of the GMAb ELISA is further supported by the identification of critical threshold of serum GMAb that is associated with an increased risk of autoimmune PAP (Bendtzen et al., 2007; Uchida et al., 2009; Sakagami et al., 2010). Several GMAb ELISA-based methods have been developed for measurement of serum GMAb including a quantitative method based on use of a neutralizing, polyclonal GMAb reference standard (PCRS) (Kitamura et al., 1999; Schoch et al., 2002) and a non-neutralizing, monoclonal GMAb reference standard (MCRS) (Inoue et al., 2008) that return values in units of GMAb concentration and a serum titration method that returns values in units of GMAb titer (Kitamura et al., 1999; Bonfield et al., 2002).
The purpose of the present study was to optimize the GMAb ELISA with respect to reagents, experimental protocol, and analysis methods, and then validate it by rigorously establishing its sensitivity, accuracy, precision, and ruggedness to support its clinical use for the diagnosis of autoimmune PAP. Further, we compared the relative performance of a GMAb polyclonal reference standard (PCRS) purified from PAP patient serum against a GMAb monoclonal reference standard (MCRS) prepared under good manufacturing practices for potential use as a reference standard for calibrating the results obtained in different laboratories.
2. Materials and methods
2.1. Participants
The institutional review board of the Cincinnati Children’s Hospital Medical Center approved the study. All participants or their legal guardians gave written informed consent and minors gave assent. Participants included patients with autoimmune PAP (n = 96; 45.8 % male; 37.3 ± 15.7 years of age at evaluation) referred for evaluation or treatment. The diagnosis of autoimmune PAP was based on clinical and radiographic findings; an open lung biopsy, transbronchial lung biopsy, or cytologic analysis of bronchoalveolar lavage cells and fluid; and a positive GMAb test performed as previously described (Uchida et al., 2004). We also studied healthy people (n = 58; 22.4 % male; 30.6 ± 7.0 years of age at evaluation) who were nonsmokers with no history of major illness and symptom-free at the time of evaluation.
2.2. GMAb Polyclonal Reference Standard
A GMAb polyclonal reference standard (PCRS) was prepared as previously described (Schoch et al., 2002)from a patient with autoimmune PAP (Luisetti et al., 2009). Briefly, GMAb was isolated from plasmapheresis fluid by protein G column chromatography followed by GM-CSF affinity chromatography (Schoch et al., 2002), concentrated by ultrafiltration (Ultra-15; MWCO 30 kDa, Amicon), and re-suspended in phosphate-buffered saline (PBS). The concentration of GMAb was determined previously by comparison to a GMAb reference standard for which the IgG content had been determined by ELISA (Kitamura et al., 2000). The purity of the PCRS was assessed by polyacrylamide gel electrophoresis (ReadyGel®, 5–15% gradient, BioRad Laboratories, Hercules, CA) with 2-mercaptoethanol. A ‘PCRS Master Stock’ containing the final purified GMAb at 2 mg/ml in PBS was prepared as 10 μl aliquots in 0.5 ml polypropylene tubes (Eppendorf, Hamburg, Germany) and stored at −80°C with continuous electronic temperature monitoring as previously reported (Uchida et al., 2009). A PCRS ‘Working Standard was prepared by diluting one vial of PCRS Master Stock with ELISA dilution buffer to GMAb concentration of 100 ng/ml and stored as 250 μl frozen aliquots as above. GMAb ELISA plate concentration standards were prepared from the PCRS Working Standard by thawing one vial at room temperature and diluting a 120 μl aliquot serially (1:1) to create standard concentrations (50, 25, 12.5, 6.25, 3.125, 1.5625 ng/ml). ELISA buffer without PCRS served as a 0 ng/ml control. A serial dilution to 0.78125 ng/ml was used in some experiments to define the lower limit of quantification (LLOQ).
2.3. GMAb Monoclonal Reference Standard
A GMAb monoclonal reference standard (MCRS) was previously prepared from a PAP patient-derived, Epstein-Barr virus transformed, immortalized B lymphocyte clone. One of six initial cell clones was used to produce the MCRS by Boehringer Ingelheim pharmaceuticals (Biberach, Germany). Briefly, the B cell clone-derived cDNA was used to stably-transduce Chinese hamster ovary D644 cells (Urlaub et al., 1983). A pool of MCRS-producing cells was subjected to 10 day fed-batch cultures in a 30 and 50 liter bio-fermenter and MCRS was purified from culture supernatant using protein A chromatography (MabSelect SuRE™, GE Healthcare, Inc.). Purified MCRS was re-suspended in 25 mM sodium citrate, pH 6.0, 115 mM sodium chloride, 0.01% (w/v) polysorbate 20, pH 6. Its binding affinity for GM-CSF was determined to be 423 pM using KinExA (Sapidyne Instruments, Inc.) equilibrium experiments, which were conducted at room temperature (~ 21°C). The antibody concentration was held constant as the antigen (rGM-CSF, Biomol GmbH, Germany) was titrated in a 2-fold serial dilution. Solutions were allowed to come to equilibrium prior measurement and the free antibodies were captured by rGM-CSF-rabbit Fc coupled to beads. The final MCRS preparation (monoclonal, non-neutralizing, human anti-human GM-CSF IgG1 lambda antibody; BI 01049904, Lot 1, 05.08.2009; 500 μg/500 μl; MCRS Original Stock) was shipped on dry ice with continuous electronic temperature recording to Cincinnati, Ohio. A “MCRS Master Stock’ was prepared by thawing the Original Stock tube at room temperature, mixing thoroughly by gentle inversion, dispensing as 25 μl aliquots without dilution in 0.5 ml polypropylene tubes (Eppendorf, Hamburg, Germany) and then storing frozen as for the PCRS. A ‘MCRS Working Stock’ was prepared by diluting one vial (25 μl) of MCRS Master Stock with ELISA dilution buffer (4.975 ml; PBS, Tween-20, 1% BSA) to a final concentration of 5 μg/ml, which was dispensed as 200 μl aliquots and stored frozen as above. A ‘MCRS Working Standard was prepared by diluting one vial (200 μl) of MCRS Master Stock with ELISA dilution buffer (49.8 ml) to 20 ng/ml and stored frozen in 250 μl aliquots as above. GMAb ELISA plate concentration standards were prepared from the MCRS Working Standard as above to create standard concentrations (20, 10, 5, 2.5, 1.25, 0.63, 0.31). ELISA buffer without MCRS serves as the 0 ng/ml control.
2.4. GM-CSF capture antigen
Preparations of recombinant, human GM-CSF from several suppliers (Miltenyi Biotech, Auburn, CA, USA; Prospec-Tany Technogene Ltd, Rehovot, Israel, USA; Invitrogen Corp., Camarillo, CA, USA; Genway Biotech, Inc. San Diego, CA, USA; Genzyme, Cambridge, MA, USA) were evaluated as a capture antigen in the GMAb ELISA by measuring the baseline optical absorbance for each preparation in the absence of added serum sample. The rhGM-CSF from Miltenyi Biotech (Figure 1C, Supplier 2) was chosen for the standard GMAb ELISA and was used throughout this study except where indicated.
Fig. 1. Effects of the Capture Antigen on GMAb ELISA Performance.

A. Non-Glycosylated Versus Glycosylated GM-CSF Capture Antigen. Serum GMAb concentration measured with the GMAb ELISA using GM-CSF produced in E. Coli (non-glycosylated form) or in yeast (glycosylated form). Each symbol represents the mean of 2 determinations on serum samples from each autoimmune PAP patient or healthy person (n = 20 and 10, respectively). The GMAb concentration measured for each sample with each capture antigen was not different (P=0.796, n = 30; Mann-Whitney Rank Sum Test).
B. Bland and Altman Analysis. All data shown in panel A were evaluated by Bland and Altman analysis as described in the methods.
C. Source of E. coli-derived GM-CSF Capture Antigen. ELISA plates were coated with GM-CSF (1 μg/ml) produced in E. coli obtained from several suppliers (1–5) and then used in the GMAb ELISA with sham samples (i.e., buffer instead of serum) to determine the effect on background optical absorbance. Each bar represents the mean (±SD) of 4 separate determinations. The background absorbance was different for all comparisons except between suppliers 1, 4, and 5 (P < 0.001, n = 4 each; ANOVA with pairwise multiple comparison procedures by the Holm-Sidak method).
2.5. Human immunoglobulin G detection antibody
Two anti-human immunoglobulin G (IgG) reagents (goat anti-human IgG-specific F(ab’)2 antibody fragment, #A2290; and intact goat anti-human-IgG-Fc specific antibody, #A6029; both from Sigma-Aldrich, St. Louis, MO, USA) were evaluated as the secondary detection antibody in the GMAb ELISA. The F(ab’)2 antibody was chosen for the standard GMAb ELISA and was used throughout this study except where indicated.
2.6. GMAb ELISA
The standard GMAb ELISA used was based on an initial description (Nakata, 1999; Kitamura et al., 2000, Schoch et al., 2002) and subsequent modifications (Uchida et al., 2004) and performed as follows. Microtiter plates (96-well, Maxisorp; Nalge Nunc International, Rochester, NY) were incubated (4°C, overnight) with 50μl of capture antigen solution (1 μg/mL rhGM-CSF (Miltenyi except where noted) in phosphate buffered saline (PBS)), washed in PBS containing 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MO, USA), and incubated (room temperature, 1 hour) with blocking solution, Stabilcoat® (Surmodics, Eden Prairie, MN, USA). Serum samples (100 μl except where indicated) were mixed with sample dilution buffer (PBS, 1% [vol/vol] BSA, 0.1% [wt/vol] Tween 20) to prepare a standard dilution series (1:100, 1:3000, 1:6000 and 1:12000) for each sample. Aliquots (50 μl) of diluted serum or reference standards were pipetted into adjacent microtiter wells, incubated (room temperature, 40 minutes) to allow binding of GMAb to the capture antigen, and then washed five times with 300 μl of wash buffer (PBS, 0.1% [vol/vol] Tween 20). Horse radish peroxidase-conjugated anti–human IgG F(ab’)2 fragment (except where noted) was diluted 1/3000 with sample dilution buffer for use as the detection antibody and 50 μl was pipetted into each well. The plates were incubated (room temperature, 30 minutes) to allow binding of IgG detection antibody to captured GMAb and then rinsed 4 times with wash buffer. Chromogenic substrate solution (50 μl; 3,3’,5,5’-tetramethylbenzidine; T4444, Sigma-Aldrich) was added to each well and the plates were incubated (room temperature, 15 min) to permit color development, which was stopped by adding 50μL of 1 N H2SO4. Optical absorbance at wavelength of 450 nm was measured using a Benchmark® ELISA plate reader using Microplate Manager software, version 5.21 (Bio-Rad Laboratories, Hercules, CA, USA) as described by the manufacturer.
GMAb concentration was determined as follows. The mean optical absorbance of the PCRS in replicate wells was plotted (x-axis) against the known GMAb concentration (y-axis) and quadratic regression analysis (except where noted) was performed to determine the equation relating optical absorbance to PCRS concentration (ng/ml on plate) using Microsoft Excel (Microsoft Corp, Seattle, WA, USA). The mean optical absorbance of replicate wells for each sample was substituted for x in the regression equation to determine the concentration of GMAb in the well in ng/ml, which was multiplied by the dilution factor to determine the GMAb concentration in serum in μg/ml.
Quality control standards consisting of duplicate serum samples of known GMAb concentration were run on every plate. All results from any plate for which the quality control replicates differed by more than 15% were rejected, which occurred in one of eighty plates in this study.
2.7. Statistical Analysis
Numerical data were evaluated for a normal distribution using the Shapiro-Wilk test and for equal variance using the Levene median test. Parametric data are presented as means (±SD) and nonparametric data are presented as medians (interquartile range [IQR]). Statistical comparisons of parametric data were made with Student’s t-test for two-group comparisons and with one-way analysis of variance with post hoc analysis by the Holm-Sidak method for comparisons among three or more groups. Nonparametric data were compared with the use of the Mann-Whitney Rank-Sum test. Agreement between assay methods was made using Bland-Altman difference analysis. Receiver operating characteristic (ROC) curve analysis and other statistical analyses were done using SigmaPlot software (Version 12.3, Systat Software, San Jose, CA, USA). P values less than 0.05 were considered to indicate statistical significance. All experiments were repeated at least twice, with similar results.
3. Results
3.1. Optimization of GMAb ELISA Components and Procedure
GMAb concentration measured with the GMAb ELISA using E. coli- or yeast-derived GM-CSF as the capture antigen was similar indicating glycosylation had little effect on capture antigen function (Figure 1A, B). Because prior studies indicated some rhGM-CSF preparations had a high background absorption, GM-CSF from multiple sources was evaluated. Background absorbance in the GMAb ELISA varied significantly for rhGM-CSF from different suppliers (Figures 1C). Thus every batch of rhGM-CSF considered for use as the capture antigen must be checked for acceptability to ensure a low background absorbance since higher values will affect the cutoff between normal and disease (see below). Non-glycosylated rhGM-CSF produced in E. coli was chosen for use in the standard GMAb ELISA to minimize potential non-specific binding to the carbohydrate moiety.
Use of an F(ab’)2 fragment (#A2290) or intact IgG (#A6029) as the anti-human IgG secondary (detection) antibody in the GMAb ELISA gave similar results except at higher GMAb concentrations where the intact IgG gave slightly higher values (Figure 2A, B). Since F(ab’)2 gave good results and was used in our prior reports (Schoch et al., 2002; Trapnell et al., 2003; Uchida et al., 2004; Uchida et al., 2007; Uchida et al., 2009), it was chosen for routine use in the standard GMAb ELISA to maximize comparability to previously reported results.
Fig. 2. Effects of the Secondary Detection Antibody on GMAb ELISA Performance.
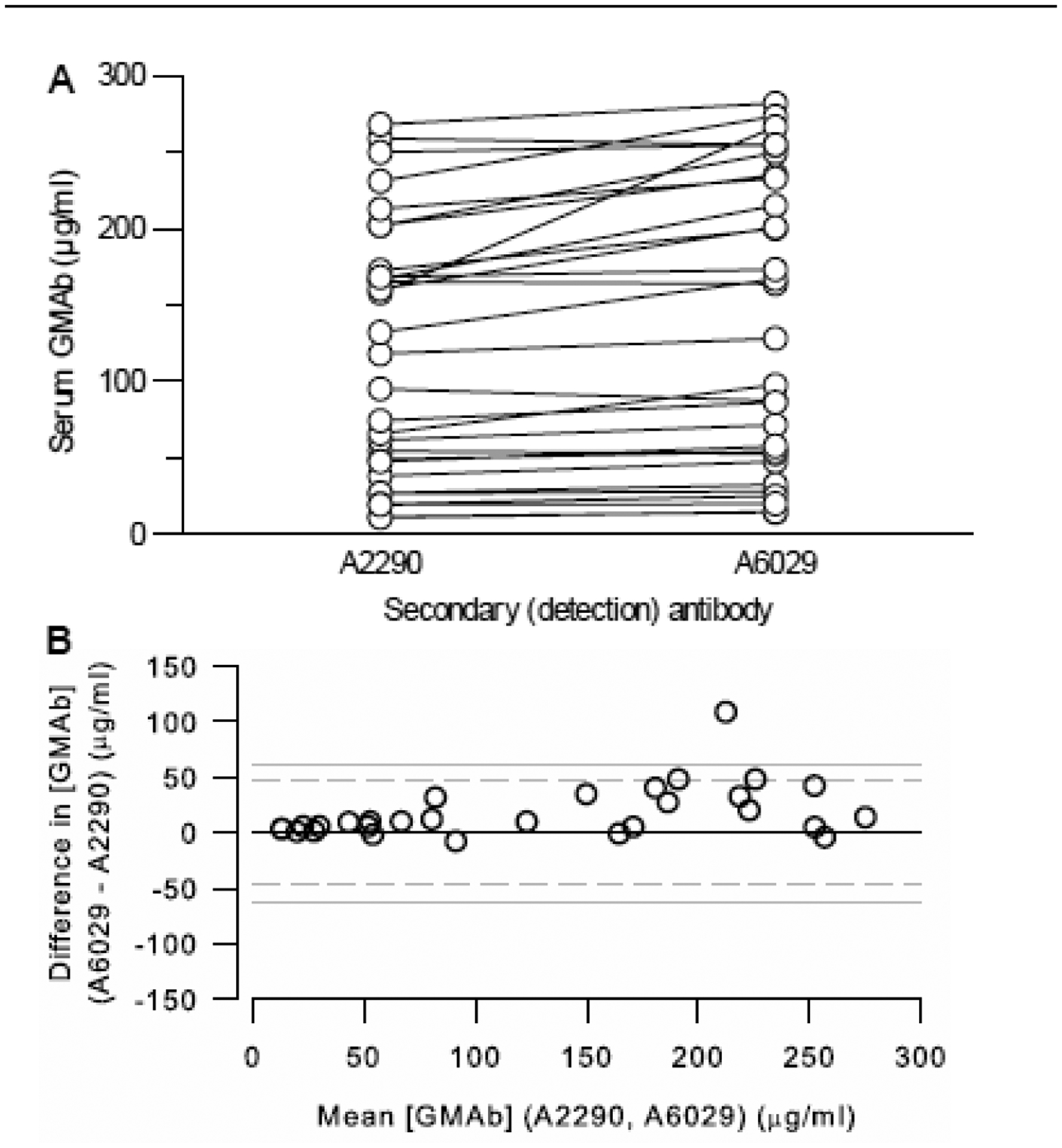
A. Use of Intact Goat-Anti-Human IgG or an F(ab’)2 Fragment as the Detection Antibody. Serum GMAb concentration measured with the GMAb ELISA in parallel using either an F(ab’)2 fragment (A2290) or intact anti-human IgG (A6029) as the detection antibody. Each symbol represents the mean of 3 determinations on the serum sample from a different autoimmune PAP patient. The median GMAb concentration (not shown) determined with the intact IgG secondary antibody was slightly higher than with the F(ab’)2 secondary antibody (128 [51 – 234] vs 118 [43 – 187] μg/mL; P< 0.001; n = 29 serum samples; Wilcoxon Signed Rank test).
B. Bland and Altman Analysis. All data shown in panel A were evaluated by Bland and Altman analysis as described in the methods.
Patient-derived GMAb PCRS contained three molecular species similar in molecular mass to IgG1, IgG2, (predominant species of roughly equal proportion) and IgG3, and IgG4 (minor species; 0.2 – 3.5%) (Figure 3A) consistent with our prior report that GMAbs from autoimmune PAP patients and healthy people are composed primarily of IgG1 and IgG2 with negligible amounts of IgG3 and IgG4 (Uchida et al., 2009). The IC50 of the PCRS was previously shown to be 10.3 molecules of GMAb per molecule of GM-CSF (Sakagami et al., 2010), similar to values reported for GMAb from a single patient (10.6), GMAb from serum pooled from 11 patients (10.3) (Uchida et al., 2007), or the mean value for GMAb from 11 patients determined individually (7.05 ± 3.81) (Uchida et al., 2004). In the GMAb ELISA, optical absorbance increased as a smooth, slightly curved function of PCRS concentration over a range from 0.781 to 50 ng/ml (Figure 3B). Quadratic regression analysis of optical absorbance versus PCRS concentration yielded an outstanding correlation coefficient and gave a lower percent deviation for repeated measurements over the entire PCRS concentration range than did either linear or log regression analysis (Figure 3C).
Fig. 3. Characterization and Performance of a PAP Patient-Derived GMAb Polyclonal Reference Standard (PCRS).
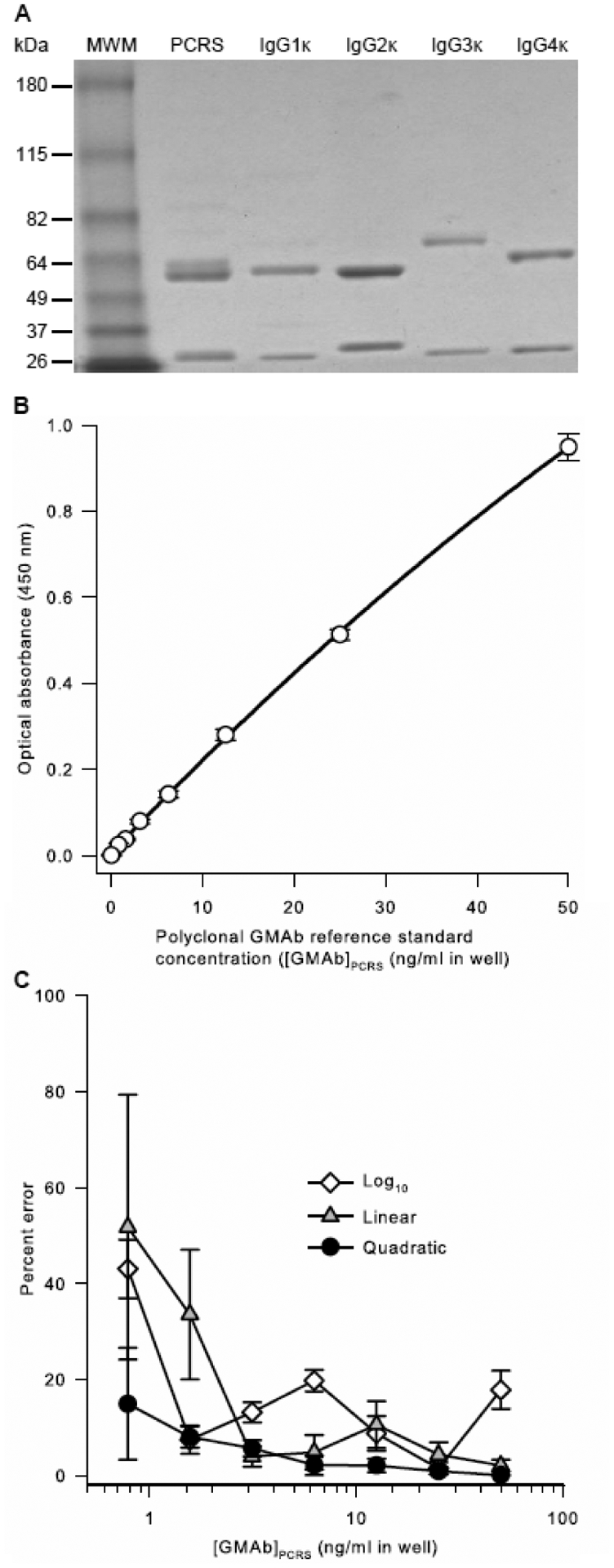
A. Purity and composition of the PCRS. PCRS, prepared as described in the methods, commercially available IgG heavy chain isotype standards (IgGκ 1, 2, 3, or 4), or molecular weight markers (MWM) were subjected to polyacrylamide gel electrophoresis under reducing conditions, Coomassie blue staining, and photography as described in the methods.
B. Optical Absorbance of the PCRS as a Function of Concentration. The PCRS was serially diluted and evaluated as the standard in the GMAb ELISA as described in the methods. Optical absorbance increased smoothly in proportion with PCRS concentration. Regression analysis using a quadratic equation yielded a correlation coefficient (R2) of 0.9998. Each point represents the mean (+SD) of 5 separate measurements.
C. Effect of Regression Method Used on Percent Error of the PCRS Curve Rit. Results from 5 independent, simultaneously conducted experiments determining the optical absorbance of serial dilutions of the PCRS were subjected individually to linear, quadratic, or logarithmic regression analysis and the mean (±SD) percent deviation at each concentration was determined. The percent error of GMAb concentration was determined as the measured value minus the expected value divided by the expected value and multiplied by 100. The mean (±SD) correlation coefficients for regression analysis of 5 separate experiments (not shown) were 0.9999 ± 0.0001 (quadratic), 0.9969 ± 0.0029 (log10), and 0.9819 ± 0.0043 (linear).
Evaluation of the initial serum aliquot volume used to prepare the standard serum dilutions for measurement in the GMAb ELISA revealed an important source of assay variability. The mean serum GMAb concentration was slightly higher with 10 μl initial serum aliquots than with 100 μl aliquots but did not reach statistical significance (Figure 4A). However, the percent deviation was significantly greater for smaller aliquots (Figure 4B). The total amount of serum transferred to the first dilution tube includes that intended for measurement (i.e., serum inside the pipet tip) plus unintentionally included serum (i.e., serum adherent to the outside and end of the pipet tip), these findings suggest that unintentionally included serum comprised a greater fraction of the total amount transferred when the initial serum aliquot was smaller. The net effect was a tendency to overestimate GMAb concentration and markedly increased variability of repeated measurements.
Fig. 4. Effect of Serum Aliquot Volume on GMAb ELISA Performance.
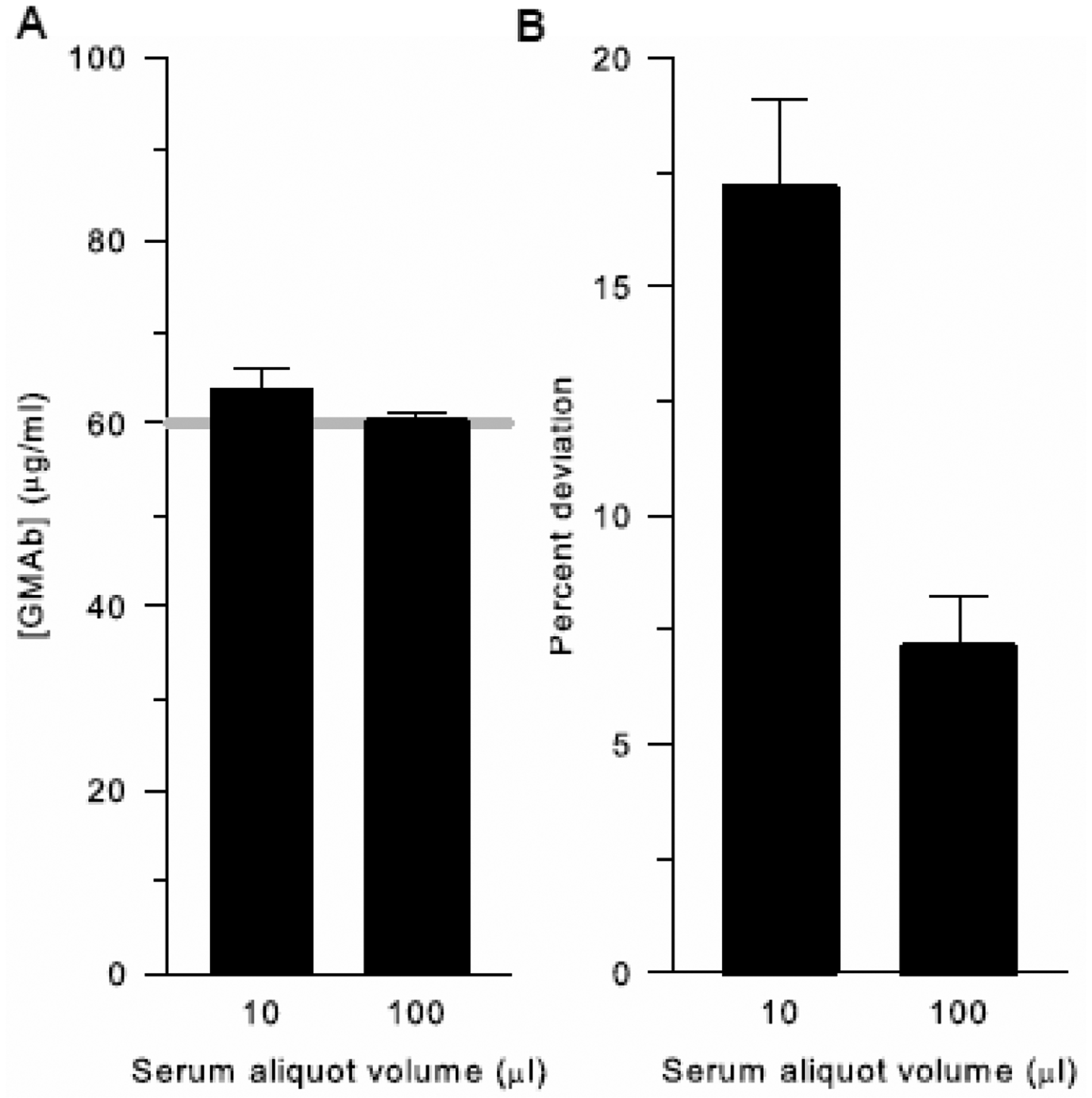
A. Effect on Serum GMAb Concentration. An initial volume of serum either 10 or 100 μl was used to measure serum GMAb concentration with the GMAb ELISA. Bars represent the mean (±SD) of 20 independent determinations using the same autoimmune patient serum. The gray line represents the mean for the data obtained using the 100 μl aliquots of serum. The difference in median (IQR) GMAb concentration for the assay performed using 10 vs 100 μl initial serum aliquots did not reach statistical significance (64.9 [52.6 – 73.4] vs 59.3 [56.0 – 64.8] μg/mL; P = 0.525, n = 20 samples; Mann-Whitney Rank Sum Test).
B. Effect on Percent Deviation of the Serum GMAb Concentration. Data from the experiment shown in panel A were used to calculate the percent deviation of the 20 determinations. The mean (±SD) percent deviation for the assay performed when using 10 μl initial serum aliquots was markedly higher than with 100 μl aliquots (17.1 ± 8.9 vs 7.1 ± 5.2; P < 0.001; n = 20 samples; Student’s t test).
Since dilution is required to reduce the optical absorbance of PAP patient serum samples on the ELISA plate into a readable range and experience has shown that the serum GMAb concentration determined can vary with the dilution used to make the measurement, we evaluated the effect of the serum dilution used for determining GMAb concentration on variability of the results for both PAP patients and healthy people. As expected, the percent deviation of GMAb concentration was higher for diluted samples with optical absorbance values falling outside the range of reference standard values (‘out-of-range’) (Figure 5A) at all dilutions (Figure 5B) in both PAP patients and healthy controls. In contrast, the percent deviation was lowest and similar at all dilutions for PAP patient serum when the absorbance was ‘in-range’ (Figure 5B). Most PAP patients had an in-range absorbance value at a dilution of 1:3000 or 1:6000, with slightly fewer at 1:12000 and none at 1:100 (Figure 5B–C). In healthy people, all dilutions greater than 1:100 had an out-of-range absorbance with the percent deviation increasing in proportion to the dilution (Figure 5B) and all had an in-range or undetectable value at a dilution of 1:100 (Figure 5C). These results indicate that any dilution can be used to determine serum GMAb concentration as long as the optical absorbance of the diluted sample is within the range of the reference standard values, or below the level of detection as in some healthy people.
Fig. 5. Effect of the Choice of Serum Dilution on Determination of GMAb concentration.
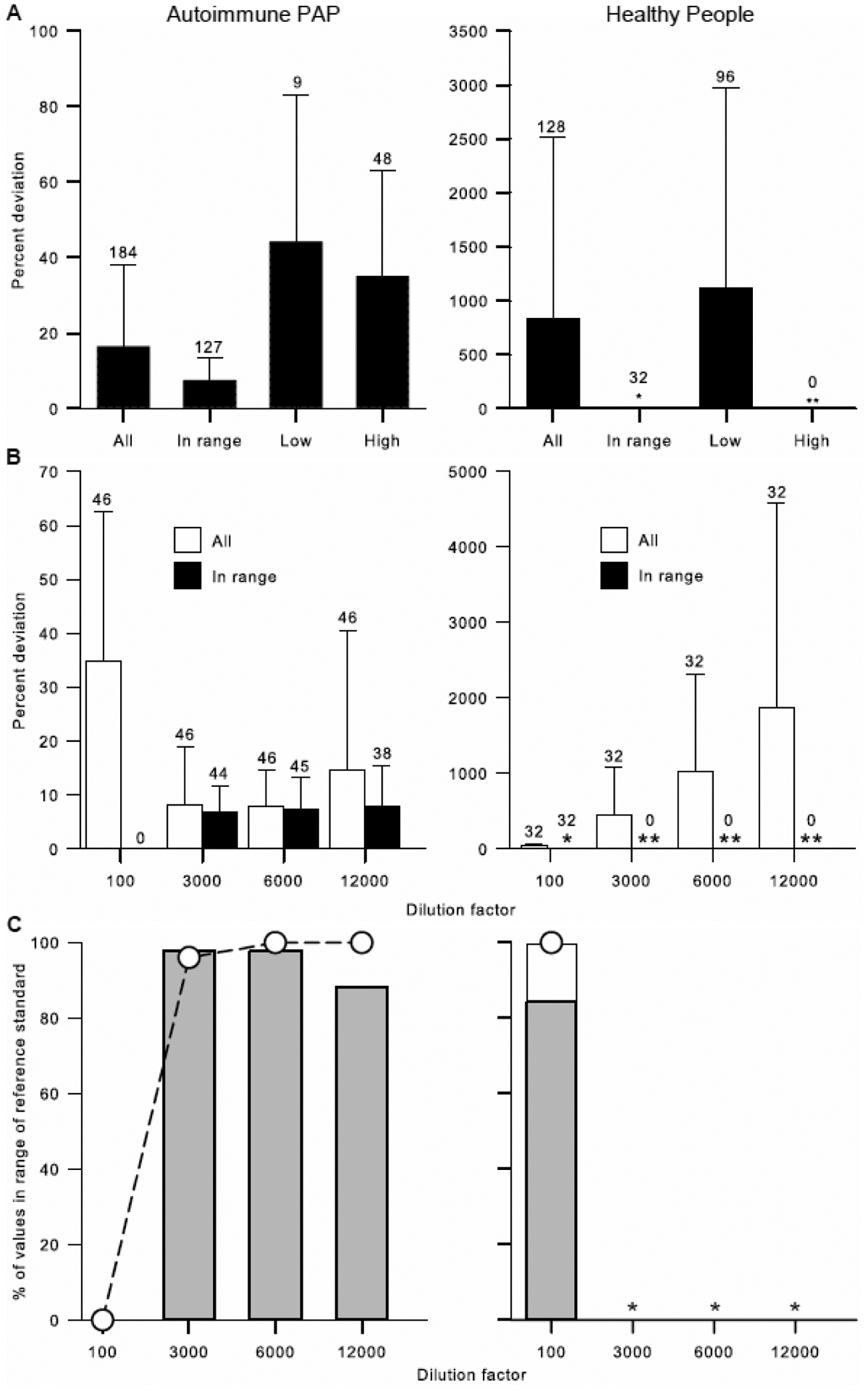
A. Effect of the Sample Absorbance to that of the Reference Standard Range. Separate series of standard dilutions (1:100, 1:3000, 1:6000, 1:12000) were prepared from serum from 46 autoimmune PAP patients and 32 healthy people and GMAb concentration was measured for each dilution (184 for autoimmune PAP and 128 for healthy controls) using the GMAb ELISA. Individual measurements were defined as ‘in-range’ if the optical absorbance for the dilution was within the range of the optical absorbance values for the reference standards, ‘low’ (and out of range) if the optical absorbance was less than that of the lowest reference standard, and ‘high’ (and out of range) if the optical absorbance was greater than that of the highest reference standard. Only ‘in-range’ values were used to calculate the mean GMAb concentration. In healthy people, the percent deviation for ‘in range’ values was small (*) and no measurements were greater than the highest reference standard (**). Bars represent the mean (±SD) and the number of determinations is shown above the error bars.
B. Effect of Serum Dilution Factor on Percent Deviation. Data are derived from the experiments shown in panel A but shown here as function of the specimen dilution. In healthy people, the percent deviation for ‘in-range’ values was small at the 1:100 dilution (*) and no determinations were in-range at higher dilutions (**). Bars represent the mean (±SD) and the number of determinations is shown above the error bars.
C. Effect of Serum Dilution on the Frequency of Obtaining ‘In range’ Measurements. Data are derived from the experiments shown in panels A and B. The percentage of determinations that are ‘in-range’ for each of the standard dilutions (1:100, 1:3000, 1:6000, and 1:12000) is shown (bars). Gray bar represent measurements that were ‘in-range’ and the clear bar represents measurements in which GMAbs were undetectable. The cumulative percent of determinations that are ‘in-range’ at each successively greater dilution is shown (open symbols). In healthy people, GMAb values below the LLOQ were accepted as normal and counted as being in-range.
The specificity of GMAb detection by the GMAb ELISA was evaluated using PAP patient serum before and after GMAb depletion by GM-CSF affinity chromatography performed as previously described (Uchida et al., 2004). GMAb was readily detected in serum before depletion, essentially undetectable in the unbound fraction (i.e., column flow-through), and approximately 60 percent of the serum value was detected in the bound fraction (i.e., bound to the column) recovered after elution with acidic buffer and neutralization (Figure 6). These results indicate that the GMAb ELISA is highly specific for detection of GMAb in serum.
Figure 6. Specificity of the GMAb ELISA.
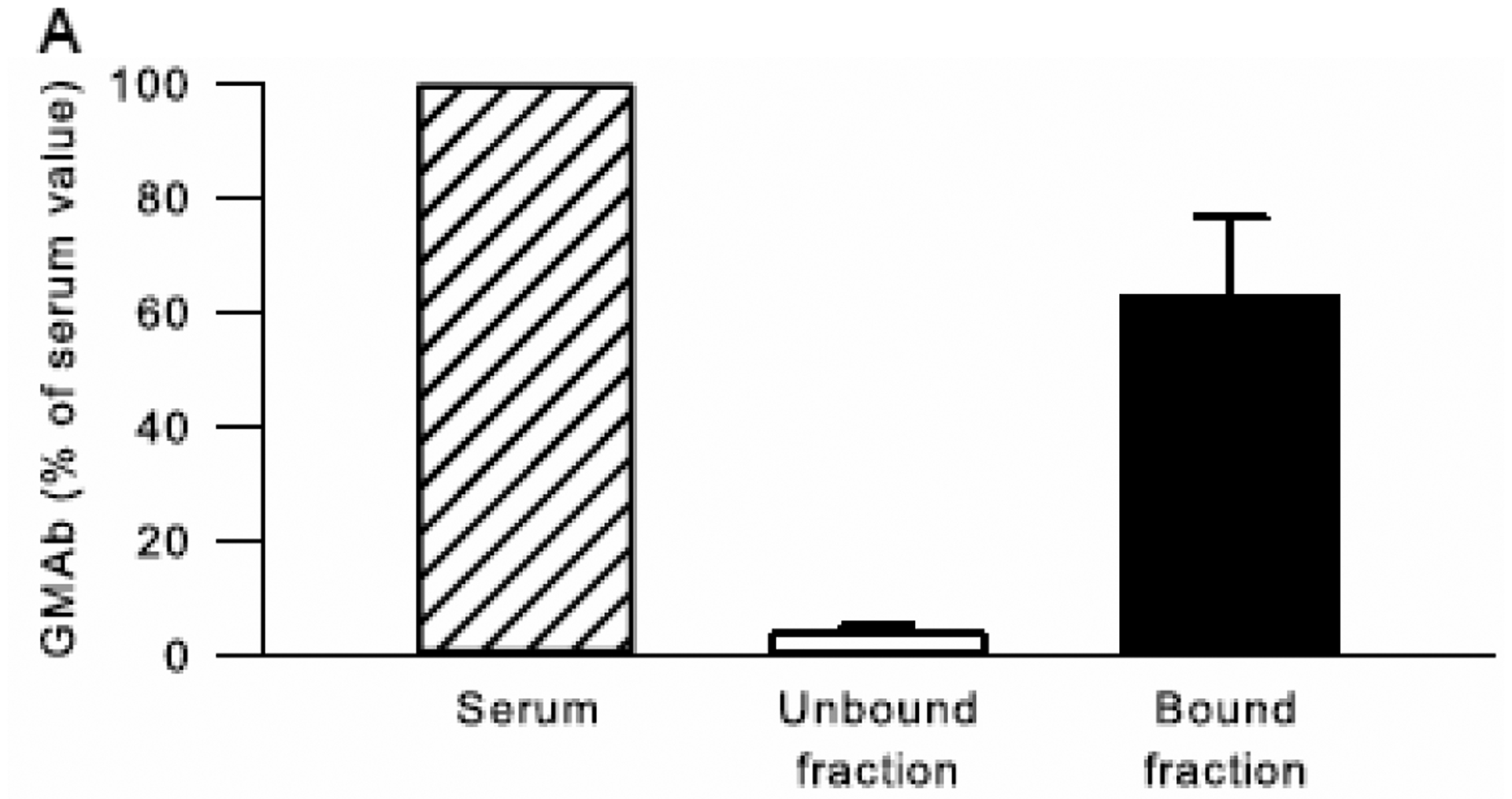
Serum from autoimmune PAP patients (n=3) was subjected to GM-CSF affinity chromatography and the unbound fraction (flow-through) and bound fraction (after elution from the column) from each was collected as previously described (Uchida et al., 2004). The GMAb concentration was measured in serum aliquots taken before chromatography and the unbound and bound fractions. All data was normalized to the serum GMAb concentration for each patient, which was set to 100%. Bars represent the mean (±SD) amount of GMAb present in serum (hatched bar), or recovered in the Unbound (open bar) and Bound (solid bar) fractions.
3.2. GMAb ELISA Performance
The accuracy of the GMAb ELISA was evaluated using the PCRS with ‘spiked’ samples containing known concentrations of exogenously added, purified GMAbs. The standard error for measurements of GMAb concentration in serial dilutions of the PCRS ranging from 50 ng/ml to 1.563 ng/ml (plate concentration) was less than 15% (Table 1) in accordance with FDA guidance regarding diagnostic assay performance (Anonymous, 2001). The standard error of the measurement at a GMAb PCRS concentration of 0.78125 ng/ml in the well was 16.7±12.1% (Table 1), which is less than the limit of 20% specified by FDA guidance to define the LLOQ for the GMAb ELISA. The standard error of measurement of GMAb concentration in ‘spiked’ human serum samples containing 10, 20, 40 ng/ml GMAb revealed a standard error of less than 7% (Table 1) in accordance with FDA guidance criteria for assay accuracy (Anonymous, 2001).
Table 1.
Concentration Response of the Calibration Curve and Accuracy of Measuring GMAb Concentration in Human Serum Using the GMAb ELISA.
| Expected concentration, ng/ml | Measured concentration, ng/ml (Mean ± SD) | Coefficient of variation (100×SD/Mean) | Standard error (%) (Mean ± SD) |
|---|---|---|---|
| Calibration curve* | |||
| 50 | 51.9 ± 2.3 | 4.39 | 4.3 ± 3.9 |
| 25 | 24.4 ± 0.7 | 2.79 | 2.5 ± 2.7 |
| 12.5 | 12.2 ± 0.4 | 5.07 | 4.0 ± 3.5 |
| 6.25 | 5.8 ± 0.4 | 6.09 | 7.7 ± 5.2 |
| 3.125 | 3.1 ± 0.2 | 6.35 | 5.3 ± 2.5 |
| 1.5625 | 1.3 ± 0.1 | 10.58 | 14.8 ± 7.0 |
| 0.78125 | 0.8 ± 0.2 | 19.41 | 16.7 ± 12.1 |
| Spiked samples† | |||
| 10 | 10.1 ± 0.6 | 6.32 | 5.4 ± 2.9 |
| 20 | 19.0 ± 1.1 | 5.89 | 6.2 ± 4.0 |
| 40 | 41.8 ± 1.7 | 4.18 | 4.5 ± 4.2 |
Prepared using the autoimmune PAP-patient derived PCRS by serial dilution in sample dilution buffer.
Spiked samples were dilution buffers spiked with known concentration of purified GMAb.
The precision of GMAb ELISA measurements with respect to well-to-well variability, plate-to-plate variability, day-to-day variability was evaluated using the three samples from PAP patients. The coefficient of variation for measurement of GMAb concentration in patient sera was less than 15% in accordance with FDA guidance criteria for assay precision (Anonymous, 2001). The coefficient of variation for measurement of GMAb concentration in spiked samples was also less than 15% (not shown). The GMAb concentrations in PAP patient serum measured repeatedly using the GMAb ELISA by two different operators were similar (Table 2).
Table 2.
Precision of Measuring GMAb Concentration in Human Serum.*
| Coefficient of variation* | Operator to operator† (Mean + SD)(mcg/ml) | |||||
|---|---|---|---|---|---|---|
| Serum samples‡ | Well-to-well | Plate-to-plate | Day-to-day | Operator 1 | Operator 2 | P value |
| PAP patient 1 | 10.3 | 11.7 | 11.6 | 100.4±4.5 | 97.8±7.0 | 0.27 |
| PAP patient 2 | 9.1 | 8.3 | 5.4 | 142.1±12.9 | 138.4±11.1 | 0.61 |
| PAP patient 3 | 4.5 | 10.0 | 7.2 | 24.0±2.5 | 25.5±2.1 | 0.47 |
Coefficient of variation was calculated as defined in the methods. Standard deviation × 100 / mean
GMAb concentration of three serum samples were evaluated to validate precision of the assay.
GMAb concentration of three serum samples were evaluated by two different operators. Data are expressed as mean.
Repeated cycles of freezing and thawing of serum had no effect on measurement of GMAb concentration at low or high GMAb concentrations (Table 3). Storage of serum samples at room temperature for six hours or at −80°C for three months had no detectable effect on measurement of GMAb concentration (Table 3). Storage of the PCRS at room temperature for six hours had no detectable effect on measurement of GMAb concentration (Table 3).
Table 3.
Stability of Samples and Reagents Used in GM-CSF Autoantibody Assay.
| Concentration (Mean ± SD) μg/ml | |||
|---|---|---|---|
| Condition / Sample* | Before | After | P Value¶ |
| Freeze-Thaw (x3) † | |||
| Low | 9.398 ± 0.9 | 10.46 ± 0.67 | 0.17 |
| High | 37.20 ± 2.44 | 37.66 ± 1.61 | 0.77 |
| Short term sample storage‡ | |||
| Low | 9.398 ± 0.9 | 9.82 ± 0.69 | 0.56 |
| High | 37.20 ± 2.44 | 38.64 ± 2.18 | 0.49 |
| Long term sample storage§ | |||
| Low | 9.398 ± 0.9 | 9.75 ± 0.05 | 0.54 |
| High | 37.20 ± 2.44 | 38.19 ± 1.10 | 0.55 |
| Short term reagent storage ║ | |||
| Diluted PCRS, low concentration | 8.45 ± 0.56 | 7.82 ± 0.75 | 0.31 |
| Diluted PCRS, high concentration | 27.85 ± 1.21 | 27.79 ± 1.09 | 0.95 |
GMAb concentration was measured in separate serum aliquots (n=3) from two patients.
P values were determined by comparison of before and after values using Student’s t-test.
GMAb concentration was measured in serum samples subjected to freezing to −80°C and thawing at room temperature three times.
GMAb concentration was measured in serum samples before and after keeping at temperature for 6 hours.
GMAb concentration was measured in serum samples before and after keeping in −80°C freezer for 3 months.
GMAb concentration was measured in aliquots of PCRS diluted to low or high concentration (indicated) before or 6 hours after being kept on the counter at room temperature for 6 hours.
The optimized GMAb ELISA was used to measure serum GMAb concentration in people previously diagnosed with autoimmune PAP and in healthy, asymptomatic people. The serum GMAb concentration in patients with autoimmune PAP (94.13 [34.64 – 158.80] μg/ml; n=44) was markedly higher than in healthy people (0.28 [0.20 – 0.51] μg/ml; n=38) (Figure 7A). Serum GMAb concentration were skewed towards higher concentrations in PAP patients but there was a clear separation between the two groups (Figure 7B). ROC curve analysis in this limited group of patients and controls showed a good sensitivity and specificity for a diagnosis of autoimmune PAP and an optimal cut off value for serum GMAb of 5.0 μg/ml (Figure 7C, D).
Figure 7. Measurement and ROC Analysis of Serum GMAb Concentration in Autoimmune PAP Patients and Healthy Controls.

A. Serum GMAb levels in Autoimmune PAP Patients and Healthy People. Serum GMAb concentration was measured using the GMAb ELISA with the PCRS in 44 autoimmune PAP patients and 38 healthy controls as described in the text.
B. Histogram of the Distribution of Serum GMAb Concentrations in Autoimmune PAP Patients and Healthy People. Data represented as a frequency distribution of serum GMAb concentrations in autoimmune PAP patients (filled bars) and healthy people (hatched bars).
C.-D. Receiver Operating Characteristic (ROC) Curve Analysis of Serum GMAb ELISA Test Results for 44 Autoimmune PAP Patients and 38 Healthy People. Standard ROC characteristic analysis was performed to determine the sensitivity and specificity for the data shown in Panel A. The area under the curve was 1.0 (C), and, at a cut off value for GMAb of 5.0 μg/ml determined by the software, the sensitivity and specificity of the GMAb ELISA were both 100% (D).
3.3. A new GMAb reference standard as the basis for a standardized unit of measure
A new immunoglobulin G1 subclass, monoclonal GMAb reference standard (MCRS) was prepared as described in the Methods and characterized as for the PCRS (Figure 3). The MCRS had a molecular mass similar to IgG1 (Figure 8A). Functional evaluation in the GMAb ELISA demonstrated a smooth, slightly curved increase in optical absorbance with increasing MCRS concentration over a range from 0.3125 to 20 ng/ml (Figure 8B). Quadratic regression analysis of the optical absorbance versus MCRS concentration yielded an outstanding correlation coefficient and gave a lower percent error over the entire MCRS concentration range than did linear or logarithmic regression analysis (Figure 8C).
Figure 8. Characterization and Performance of Pharmaceutical-Grade, GMAb Monoclonal Reference Standard (MCRS).
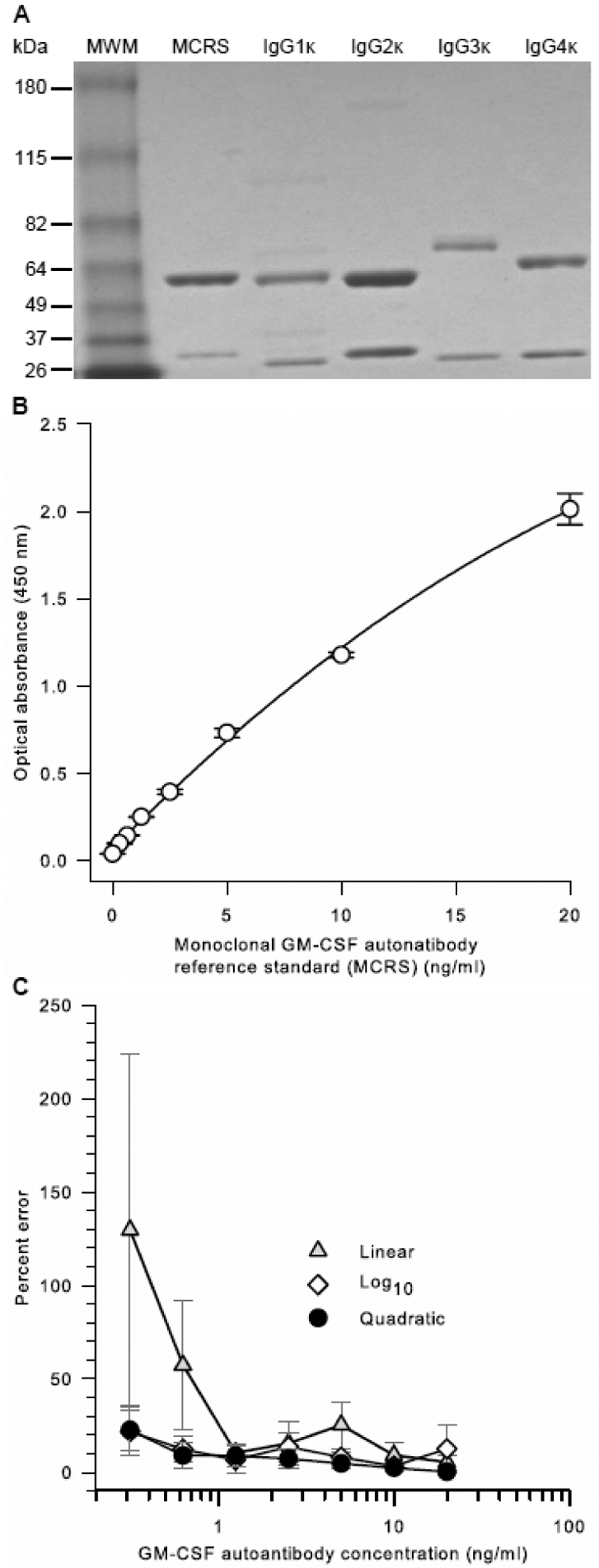
A. Purity of the MCRS. The MCRS was prepared as described in the methods. MCRS, commercially available IgG heavy chain isotype standards (IgGκ 1, 2, 3, or 4), or molecular weight markers (MWM) were subjected to polyacrylamide gel electrophoresis under reducing conditions, Coomassie blue staining, and photography as described in the methods.
B. Optical Absorbance of the MCRS as a Function of Concentration. The MCRS was serially diluted and evaluated as the standard in the GMAb ELISA as described in the methods. Optical absorbance increased smoothly in proportion with MCRS concentration. Regression analysis using a quadratic equation yielded a correlation coefficient (R2) of 0.999.
C. Effect of Regression Method used on Percent Error of the MCRS Curve Fit. Results from 6 independent, simultaneously conducted experiments determining the optical absorbance of serial dilutions of the MCRS were subjected to linear, quadratic, or logarithmic regression analysis and the percent deviation at each concentration was determined. The percent error of the [GMAb]PCRS measurement was calculated as [GMAb]PCRS minus [GMAb]MCRS divided by [GMAb]MCRS multiplied by 100; where [GMAb]PCRS are the unconverted values (None) or values after conversion using the linear, quadratic, or cubic regression equation parameters (indicated) and [GMAb]MCRS is the value actually determined using the MCRS (assumed to be the true value). The mean (±SD) correlation coefficients for regression analysis of 6 separate experiments (not shown) were 0.999 ± 0.0004 (quadratic), 0.990 ± 0.008 (log10), and 0.982 ± 0.0168 (linear).
To determine the relationship between GMAb concentration determined using the PCRS ([GMAb]PCRS) with that determined using the MCRS ([GMAb]MCRS), standard dilutions of sera from PAP patients and healthy people were measured with both reference standards included on the same plate. [GMAb]MCRS values were ~5-fold less than corresponding [GMAb]PCRS values; the ratio varied slightly with concentration resulting in a nonlinear relationship (Figure 9A). Regression analysis using linear, quadratic, and cubic fit equations was done to define the relationship between [GMAb]PCRS and [GMAb]MCRS. Cubic regression gave the best fit (Figure 9A). To confirm this, [GMAb]PCRS values were converted to values equivalent to [GMAb]MCRS using each set of regression parameters just described and the percent error was determined for each measurement. Conversion using cubic equation parameters gave the lowest for percent error, which was negligible at all GMAb concentrations >0.2 ng/ml in diluted serum samples (Figure 9B). Thus, the relationship between [GMAb]PCRS and [GMAb]MCRS was best described as cubic polynomial. To establish the parameters needed to routinely convert [GMAb]PCRS values into values equivalent of [GMAb]MCRS, six independent experiments similar to and including the one shown above (Panel A) were evaluated simultaneously, which yielded an excellent correlation coefficient (Figure 9C). Thus, the relationship between the GMAb concentration determined with these two reference standards is given by the following equation:
where x is the GMAb concentration in diluted serum in units of ng/ml determined with the PCRS (i.e., [GMAb]PCRS) and F(x) is the GMAb concentration in ng/ml determined with the MCRS (i.e., [GMAb]MCRS). To standardize reporting, one international unit (IU) of GMAb is hereby defined as a GMAb concentration equivalent to 1 μg/ml measured using the GMAb ELISA as described in the Methods with the MCRS as the reference standard.
Figure 9. Conversion of GMAb Concentration Data into International units.
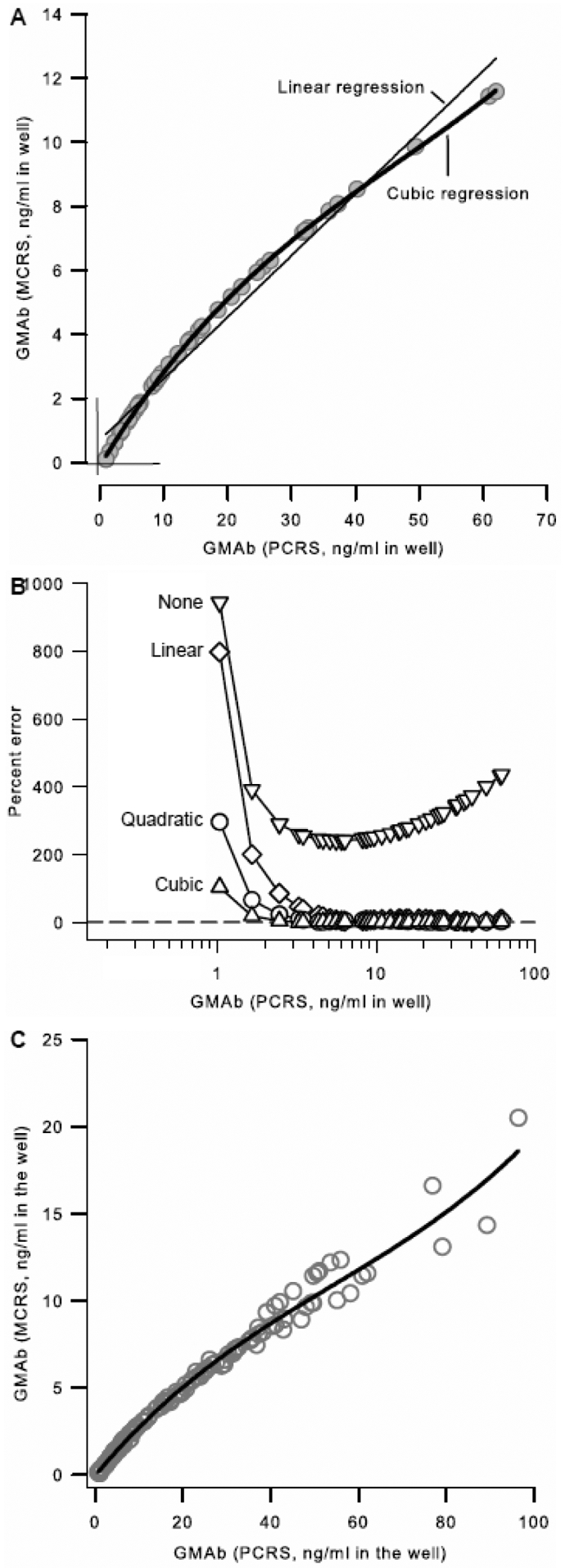
A. Comparison of Serum GMAb concentration Determined with the PCRS and MCRS. Separate sets of standard dilutions (1:100, 1:3000, 1:6000, 1:12000) were prepared from serum from 14 autoimmune PAP patients and 1 healthy person. All standard dilutions (60 total) were used to measure GMAb concentration with the PCRS ([GMAb]PCRS) and MCRS ([GMAb]MCRS) as described in the legends to Figures 3 and 7, respectively, in parallel on the same plate. All in-range values (n = 41; defined in the legend to Figure 5) are shown and were used for analysis. The relationship between GMAb concentration determined using each of the two reference standards was evaluated by linear, quadratic (not shown to improve readability), or cubic regression analysis. The correlation coefficients for regression analysis were 0.980 (linear), 0.998 (quadratic), and 1.00 (cubic).
B. Effect of Regression Method and GMAb Concentration Range on the Accuracy of Conversion of [GMAb]PCRS to International Units. Using the data and regression equation parameters determined from the linear, quadric, or cubic regression analysis described above (panel A), the values for GMAb concentration determined with the PCRS ([GMAb]PCRS) were converted mathematically to values that would have been obtained if the MCRS had been used (i.e., equivalent to [GMAb]MCRS). The percent error of the [GMAb]PCRS measurement was calculated as [GMAb]PCRS minus [GMAb]MCRS divided by [GMAb]MCRS multiplied by 100; where [GMAb]PCRS are the unconverted values (None) or values after conversion using the linear, quadratic, or cubic regression equation parameters (indicated) and [GMAb]MCRS is the value actually determined using the MCRS (assumed to be the true value). The dashed line represents a percent error of zero.
C. Determining the Equation to Convert [GMAb]PCRS Data to International units. Data from six independent experiments similar to and including the one shown in panel A were combined and cubic regression analysis was done to determine the equation needed to convert data for GMAb concentration determined using the PCRS into units equivalent to that determined using the MCRS. In total, serum samples from 55 autoimmune PAP patients and 12 healthy controls were used for determining the equation. The correlation coefficient (R2) was 0.98343.
To validate the mathematical approach for conversion of [GMAb]PCRS data to international units equivalent to values determined with the MCRS, serum GMAb was measured in 12 additional, separate autoimmune PAP patients using the PCRS (described above) and simultaneously using the MCRS. A plot of [GMAb]PCRS after conversion to IU against [GMAb]MCRS revealed a linear relationship with an outstanding correlation (Figure 10A). Bland and Altman analysis confirmed this agreement for the two values with a difference of less than 5 IU at all concentrations below 60 IU (Figure 10B).
Figure 10. Validation of the Equation to Convert [GMAb]PCRS Data to International Units.
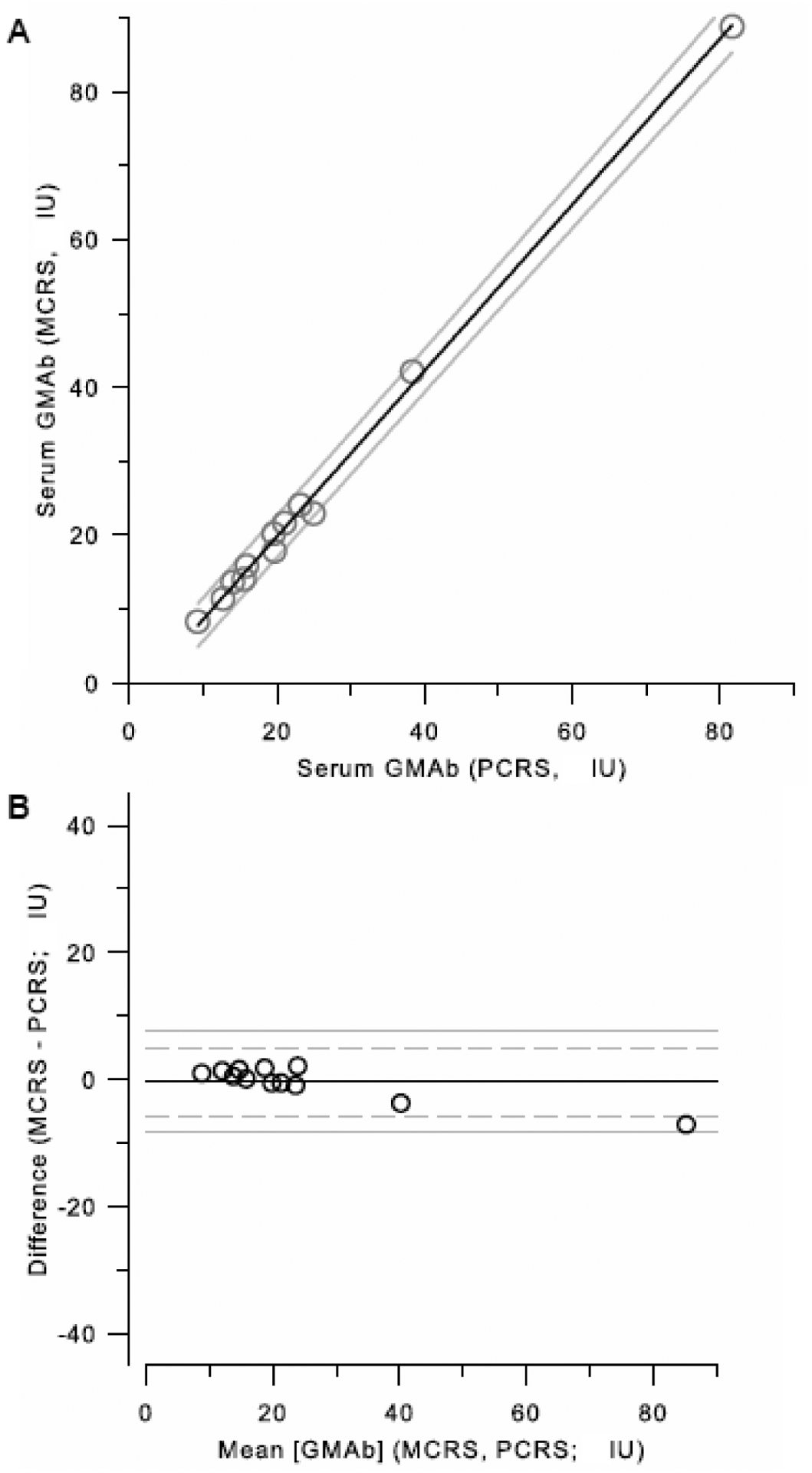
A. Comparison of [GMAb]PCRS Data Before and After Conversion to International Units with Data Determined Directly with the MCRS. The converted PCRS-based data described above was plotted against the MCRS-based data and evaluated by linear regression analysis. The correlation coefficient was 0.984. See text for further details.
B. Bland and Altman analysis. The data shown in panel A were used to evaluate the error for the comparison between [GMAb]PCRS converted to international units and [GMAb]MCRS by Bland and Altman analysis as described in the methods.
To determine the range of normal and abnormal values for GMAb concentration in international units, serum GMAb in 56 autoimmune PAP patients and 38 healthy individuals was measured using the GMAb ELISA with the PCRS and converted to international units using the equation parameters identified (Figure 11).
Figure 11.
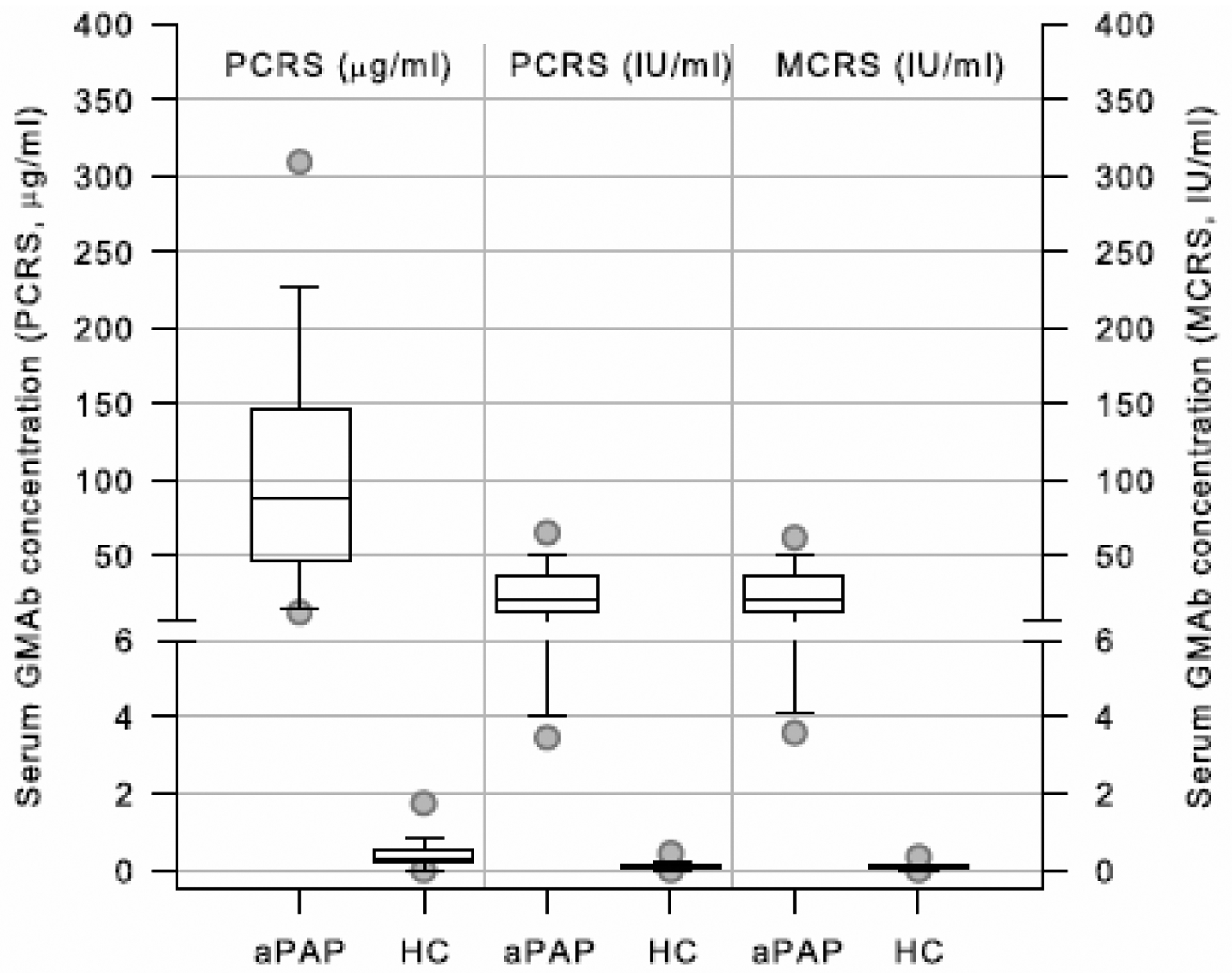
Normal and Abnormal Ranges for GMAb Concentration Expressed in Units of the PCRS, MCRS, and International Units. Serum GMAb concentration in 56 autoimmune PAP patients and 38 healthy people was measured using the GMAb ELISA with the PCRS and converted into international units using the equation parameters determined by the analysis shown in Figure 9C and was also simultaneously measured with the MCRS on the same plate. Box plots show the median (line) and IQR (box top and bottom), and 5th, 95 CI (circles) for patients with autoimmune 56 PAP patients (aPAP), and 38 healthy people (HP).
4. Discussion
In this study, we optimized a GMAb ELISA (Schoch et al., 2002) and evaluated its accuracy, precision, and reliability for measuring serum GMAb concentration. The assay performed very well in distinguishing patients with autoimmune PAP from healthy people. A monoclonal GMAb reference standard (MCRS) was developed as the basis for a new proposed international unit of measure for GMAb concentration and a method for converting GMAb data obtained with the PCRS into international units was developed. These results facilitate the comparison of serum GMAb concentration testing obtained in different laboratories thereby facilitating research on this rare disease.
Several modifications to the original GMAb ELISA were important in improving assay performance. First, identification of a capture antigen preparation with low baseline optical absorbance minimized non-specific background. Second, use of an increased initial serum aliquot volume to prepare specimen dilutions improved precision. Third, regression of reference standard data with a quadratic equation resulted in a better fit than with linear or logarithmic equations, which have been used previously. Fourth, restriction of absorbance values used for calculating serum GMAb concentration to those within range of the reference standards improved accuracy. Further, any sample dilution with an ‘in-range’ absorbance value was acceptable for determining the GMAb concentration. In contrast, some variables had little influence on GMAb ELISA performance, e.g., glycosylation of the capture antigen and use of an F(ab’)2 fragment or intact IgG as the anti-human IgG detection antibody.
These results help establish a basis for the routine clinical use of the GMAb ELISA for diagnosis of autoimmune PAP. First, the accuracy, precision, and reliability of the GMAb ELISA were within performance parameter guidelines established by the United States Food and Drug Administration (Anonymous, 2001). Second, the ranges of serum GMAb concentrations in autoimmune PAP patients and healthy people were similar to prior reports utilizing the GMAb ELISA with a PCRS (Trapnell et al., 2003; Uchida et al., 2004; Uchida et al., 2007; Uchida et al., 2009). Third, the optimal cut off value of 5 μg/ml for the upper limit of normal determined by ROC curve analysis in this study is consistent with passive immunization studies in non-human primates identifying a serum GMAb concentration of 5 μg/ml as the critical threshold above which GM-CSF bioactivity was completely neutralized and the risk of autoimmune PAP in passively immunized animals is increased (Sakagami et al., 2009; Sakagami et al., 2010). At this cut off value, the sensitivity and specificity of the assay were both 100%, which is improved compared to a prior report utilizing the assay prior to optimization (Presneill et al., 2004). This is important given the low prevalence of autoimmune PAP (Inoue et al., 2008). Notwithstanding, the present study was not designed to establish the range of normal and abnormal serum GMAb levels or the sensitivity and specificity of the GMAb ELISA for a diagnosis of autoimmune PAP. It is also necessary to establish and validate the cutoff values used to identify autoimmune PAP and to validate the use of GMAb ELISA testing and these parameters for the diagnosis of autoimmune PAP. To this end, a study designed for this purpose involving subjects from Germany, Italy, Japan, and the United States (the MICEPAP study) has been undertaken and will be subsequently reported elsewhere.
One limitation of the GMAb ELISA is that it measures both neutralizing and non-neutralizing GMAbs. This should not be a problem when the total serum GMAb levels is high as in most autoimmune PAP patients but could be for values near the cutoff as can occur in some healthy people (Uchida et al., 2009). In such cases, the measurement of serum GM-CSF neutralizing capacity using a functional assay may help determine if such increases are functionally important, i.e., disrupt GM-CSF signaling. Several cell-based assays are useful for this including inhibition of GM-CSF-dependent TF1 cell growth (Uchida et al., 2004; Uchida et al., 2009) or the GM-CSF-stimulated increase in whole blood leukocyte surface CD11b levels (Uchida et al., 2007) or GM-CSF stimulated increase in signal inducer of transcription 5 (STAT5) phosphorylation in blood leukocytes (Suzuki et al., 2008). Another limitation relates to small increases in serum GMAb observed in diseases not associated with development of PAP (Bendtzen et al., 2007; Han et al., 2009). For example, in 272 pediatric and 88 adult patients with Crohn’s disease who did not have PAP, the median serum GMAb concentrations were 2.4 and 11.7 μg/ml, respectively (Han et al., 2009). Functional testing was helpful and indicated that GM-CSF signaling was reduced but not abolished in these patients. Since the clinical symptoms of autoimmune PAP do not occur in patients without significant radiographic findings, combining GMAb testing with routine chest computed tomography will likely resolve any discrepancy potentially arising from intermediate GMAb values close to the cutoff. Further, in a typical clinical setting, GMAb testing would likely be considered after radiographic evaluation had suggested a diagnosis of PAP. Another limitation is the GMAb ELISA only detects free GMAb and not GMAb bound to GM-CSF, which could underestimate the GMAb concentration at the very low levels in individuals without PAP. This is illustrated by considering the following: 1) up to 7.8 GMAb molecules can bind to each GM-CSF molecule (Uchida et al., 2004); 2) more than 99% of serum GM-CSF is bound to GMAb in healthy people and PAP patients (Uchida et al., 2009); 3) total serum GM-CSF, i.e., unbound and GMAb-bound GM-CSF, is ~3048 pg/ml in healthy people and ~2360 in aPAP patients (Uchida et al., 2009); 4) the serum GMAb level in healthy people was 280 ng/ml (this report). Assuming 7.8 GMAb molecules bind to each GM-CSF molecule and the molecular mass for GMAb is ten times that of GM-CSF, the amount of GMAb bound to GM-CSF would be 7.8 multiplied by 3048 multiplied 10, or ~237 ng/ml. Thus, in healthy people, the percentage of GMAb detected by the ELISA would equal unbound GMAb (280 ng/ml) divided by total GMAb (unbound and GM-CSF-bound; 280 + 237 ng/ml) multiplied by 100 (conversion to percent) or approximately 54% of total GMAb. In autoimmune PAP patients, by similar calculations, ~99.8% of GMAb would be unbound and therefore detected by the GMAb ELISA. From above calculations, it is anticipated that GM-CSF bound to GMAb minimally interfere with the diagnostic threshold. Further, the lower limit of detection (0.78 ng/ml) is well below the cutoff for a positive result (5 μg/ml).
The observation that optical absorbance was greater for the MCRS than for the PCRS can be interpreted in terms of epitope differences: the PCRS has polyclonal epitopes distributed throughout the entire GM-CSF molecule while the MCRS targets a single epitope. In the GMAb ELISA, a lower optical absorbance indicates reduced secondary (detection) antibody binding, which could be caused by either 1) reduced binding of GMAb to capture antigen or 2) reduced binding of the detection antibody to captured GMAb (i.e., fewer ‘targets for detection or reduced efficiency of target detection). Assuming GMAb capture has reached equilibrium, theoretically, reduced binding of PCRS in the GMAb ELISA (compared to MCRS) could be explained by ‘epitope hiding’, perhaps of less-avid GMAb species, or by reduced access to GM-CSF adjacent to bulkier complexes of polyvalent PCRS. Neither of these mechanisms seems likely since capture antigen is in molar excess of GMAb. Alternatively, since the binding ratio can be as high as 7.8 molecules of GMAb per GM-CSF molecule for the PCRS and only one for MCRS, reduced binding of the detection antibody could be explained by increased steric hinderance for the polyvalent PCRS-antigen complexes than for monovalent MCRS-antigen complexes. While we believe the latter is most likely, these mechanisms are not mutually exclusive and additional studies would be needed to elucidate the mechanism.
The availability of a high-quality, monoclonal GMAb reference standard (e.g., MCRS) should facilitate research on autoimmune PAP, which is challenged by low prevalence like many rare diseases. Specifically, the use of a common international unit of measure for GMAb concentration based on the MCRS would improve comparability of test results from different laboratories. To this end, efforts are underway to establish the GMAb ELISA in multiple reference laboratories in Japan, Europe, Asia, and the United States. The MCRS and a common set of serum specimens will be used to calibrate serum GMAb test results between laboratories. Conceivably, these reagents could also allow GMAb data obtained by other methods, e.g., serial dilution GMAb titer data (Bonfield et al., 2002), to be expressed in a common unit of measure. Finally, the availability of a validated method for converting data obtained with other GMAb reference standards to international units may facilitate meta-analysis of published data from small cases series, which are common in rare diseases.
Acknowledgements
We thank Barbara Enenkel, Boehringher Engelheim for producing the MCRS (BI1049904), Barbara Kistler, PhD for helpful discussions during the course of this work and writing of the manuscript, and Carrie Stevens for help with collection of human blood specimens. This work was supported by grants from the National Heart Lung and Blood Institute (R01 HL085453; B.C.T.), National Center for Research Resources (U54 RR0198498, B.C.T.), the National Institute for Health and Human Development, Japan Society for the Promotion of Science (Category B international, 19390403, Y.I. and K.U.) and from the Japanese Ministry of Health, Labor, and Welfare (H24-Nanchitou-ippan-035, Y.I. and K.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: PAP, pulmonary alveolar proteinosis; PCRS, polyclonal reference standard; MCRS, monoclonal reference standard; rhGM-CSF, recombinant, human GM-CSF;
References
- Anonymous. 2001. Guidance for Industry Bioanalytical Method Validation. In: Guidence Documents, Vol. 2012. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Bethesda. [Google Scholar]
- Bendtzen K, Svenson M, Hansen MB, Busch T, Bercker S, Kaisers U, Uchida K, Beck DC and Trapnell BC, 2007, GM-CSF Autoantibodies in Pulmonary Alveolar Proteinosis. The New England journal of medicine 356, 2001–2002. [PubMed] [Google Scholar]
- Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS and Thomassen MJ, 2003, PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. American journal of physiology. Lung cellular and molecular physiology 285, L1132–6. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Russell D, Burgess S, Malur A, Kavuru MS and Thomassen MJ, 2002, Autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis. American journal of respiratory cell and molecular biology 27, 481–6. [DOI] [PubMed] [Google Scholar]
- Han X, Uchida K, Jurickova I, Koch D, Willson T, Samson C, Bonkowski E, Trauernicht A, Kim MO, Tomer G, Dubinsky M, Plevy S, Kugathsan S, Trapnell BC and Denson LA, 2009, Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology 136, 1261–71, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G and Jobe AH, 1996, Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. The American journal of physiology 270, L650–8. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Sakatani M, Krischer JP and Nakata K, 2008, Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. American journal of respiratory and critical care medicine 177, 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y and Nakata K, 1999, Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. The Journal of experimental medicine 190, 875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Uchida K, Tanaka N, Tsuchiya T, Watanabe J, Yamada Y, Hanaoka K, Seymour JF, Schoch OD, Doyle I, Inoue Y, Sakatani M, Kudoh S, Azuma A, Nukiwa T, Tomita T, Katagiri M, Fujita A, Kurashima A, Kanegasaki S and Nakata K, 2000, Serological diagnosis of idiopathic pulmonary alveolar proteinosis. American journal of respiratory and critical care medicine 162, 658–62. [DOI] [PubMed] [Google Scholar]
- Luisetti M, Rodi G, Perotti C, Campo I, Mariani F, Pozzi E and Trapnell BC, 2009, Plasmapheresis for treatment of pulmonary alveolar proteinosis. The European respiratory journal 33, 1220–2. [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, Lei JT, Moore JP, Tavana G, Lewis LR, Zhu Y, Muzny DM, Gibbs RA and Huston DP, 2008, Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. The Journal of experimental medicine 205, 2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K 1999. Anti-GM-CSF Autoantibodies and Reagents Required for Its Evaluation. In: J.P. Office (Ed.) Japan Patent Office, Number 4372904, Japan. [Google Scholar]
- Nogee LM, 2010, Genetic Basis of Children’s Interstitial Lung Disease. Pediatric allergy, immunology, and pulmonology 23, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presneill JJ, Nakata K, Inoue Y and Seymour JF, 2004, Pulmonary alveolar proteinosis. Clin Chest Med 25, 593–613, viii. [DOI] [PubMed] [Google Scholar]
- Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, Keller G, Wood RE, Wert SE, Ikegami M, Whitsett JA, Luisetti M, Davies S, Krischer JP, Brody A, Ryckman F and Trapnell BC, 2010, Patient-derived GM-CSF Autoantibodies Reproduce Pulmonary Alveolar Proteinosis in Non-human Primates. American journal of respiratory and critical care medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE, Whitsett JA, Trapnell BC and Luisetti M, 2009, Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. The New England journal of medicine 361, 2679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch OD, Schanz U, Koller M, Nakata K, Seymour JF, Russi EW and Boehler A, 2002, BAL findings in a patient with pulmonary alveolar proteinosis successfully treated with GM-CSF. Thorax 57, 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA and Trapnell BC, 2001, GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 15, 557–67. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Maranda B, Sakagami T, Catellier P, Couture C, Carey B, Chalk C and Trapnell B, 2011, Hereditary pulmonary alveolar proteinosis caused by CSF2RB mutations. The European respiratory journal 37, 201–4. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, Grabowski G, Carey BC, Stevens C, van der Loo JC and Trapnell BC, 2008, Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. The Journal of experimental medicine 205, 2703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sakagami T, Young LR, Carey BC, Wood RE, Luisetti M, Wert SE, Rubin BK, Kevill K, Chalk C, Whitsett JA, Stevens C, Nogee LM, Campo I and Trapnell BC, 2010, Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. American journal of respiratory and critical care medicine 182, 1292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Motoi N, Tsuchihashi Y, Tazawa R, Kaneko C, Nei T, Yamamoto T, Hayashi T, Tagawa T, Nagayasu T, Kuribayashi F, Ariyoshi K, Nakata K and Morimoto K, 2011, Adult-onset hereditary pulmonary alveolar proteinosis caused by a single-base deletion in CSF2RB. J Med Genet 48, 205–9. [DOI] [PubMed] [Google Scholar]
- Trapnell BC, Whitsett JA and Nakata K, 2003, Pulmonary alveolar proteinosis. The New England journal of medicine 349, 2527–39. [DOI] [PubMed] [Google Scholar]
- Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, Puchalski JT, Hauck DM and Trapnell BC, 2007, GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. The New England journal of medicine 356, 567–79. [DOI] [PubMed] [Google Scholar]
- Uchida K, Nakata K, Suzuki T, Luisetti M, Watanabe M, Koch DE, Stevens CA, Beck DC, Denson LA, Carey BC, Keicho N, Krischer JP, Yamada Y and Trapnell BC, 2009, Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood 113, 2547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, Eishi Y, Kitamura T, Yamada Y, Hanaoka K and Keicho N, 2004, High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 103, 1089–98. [DOI] [PubMed] [Google Scholar]
- Urlaub G, Kas E, Carothers AM and Chasin LA, 1983, Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell 33, 405–12. [DOI] [PubMed] [Google Scholar]


