To the editor,
Emerging evidence suggests that endothelial dysfunction plays a central role in the pathophysiology of coronavirus disease 2019 (COVID-19). Recent post-mortem studies have documented extensive endothelial damage and inflammatory infiltrates in pulmonary and extra-pulmonary capillary beds of COVID-19 patients [1, 2]. This results in loss of endothelial integrity, activation of pro-coagulant pathways, disruption of the alveolar-capillary barrier, and vascular hyperpermeability [2]. Endothelial damage is a common denominator of thrombosis (micro- and macrovascular), acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), and multiorgan failure (MOF), which are major drivers of morbidity and mortality in COVID-19 patients [3]. AKI is a common feature of COVID-19, impacting nearly half of all hospitalized patients, and is associated with high mortality, especially among those requiring renal replacement therapy [4–6]. We have recently shown that AKI may be driven in COVID-19 by a secondary thrombotic microangiopathy (TMA) phenomenon, as evidenced by low ADAMTS13 activity to von Willebrand factor (VWF:Ag) ratio [7]. However, the mechanism by which AKI occurs in COVID-19 has yet to be fully elucidated.
With the high frequency of AKI and thromboses in patients with COVID-19, biomarkers of endothelial damage/activation-related biomarkers have become of interest. Angiopoietin-1 (Ang-1) is an angiogenic growth factor that promotes vessel maturation and survival by activation of the Tie2 receptor (Tie2) on endothelial cells [8]. Ang-1 is expressed by pericytes and vascular smooth muscle cells and can stabilize endothelial functions by reducing inflammation and apoptosis of endothelial cells [9]. On the contrary, Angiopoietin-2 (Ang-2) enhances endothelial inflammation and hyperpermeability as it can act as an antagonist to Ang-1 and Tie2 signaling [9, 10]. We hypothesized that elevated Ang-2 would be associated with an increased risk for developing severe COVID-19-related AKI during the course of infection.
In this prospective observational study, adults (≥ 18 years old) presenting to the University of Cincinnati Medical Center Emergency Department (ED) with respiratory symptoms at triage suggestive of COVID-19 and with positive reverse transcription-polymerase chain reaction (RT-PCR) test for COVID-19 via nasopharyngeal swab were enrolled. This study was approved by the University of Cincinnati institutional review board (IRB) and performed under a waiver of informed consent. Blood samples were collected via routine draws for clinical indications in the ED. Circulating levels of Ang-1 and Ang-2 were determined in EDTA plasma using an enzyme-linked immunosorbent assay following the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA) using a DS2 ELISA processing system (Dynex Technologies, Inc, Chantilly, Virginia, USA). Serum creatinine was measured using a kinetic alkaline picrate (modified Jaffe) method using either a Beckman Coulter AU480 Chemistry Analyzer (Brea, California, USA) or a Beckman Coulter AU5822 Chemistry Analyzer (Brea, California, USA). Patients were monitored through hospitalization until discharge/death if admitted from the ED or for 30 days if discharged from the ED. The primary outcome of interest was the development of severe AKI, defined as Kidney Disease: Improving Global Outcomes (KDIGO) Stage 2 + 3 according to serum creatinine (SCr) criteria [11]. The secondary outcome was the need for renal replacement therapy (RRT). Ang-2 levels were correlated with white blood cell count (WBC), C-reactive protein (CRP), interleukin (IL) 6, 8, 10, tumor necrosis factor-alpha (TNF-α), plasminogen, fibrinogen, D-Dimer, ADAMTS13 activity, VWF:ag, myoglobin, plasma neutrophil gelatinase-associated lipocalin (NGAL), and serum cystatin C.
Analysis of data was carried out using R software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria). Categorical data were reported as frequencies (%), while continuous data were reported as the median and interquartile range (IQR). Comparison of baseline Ang-1 and Ang-2 levels, as well as other laboratory values between COVID-19 patients with and without severe AKI, was carried out using the Mann–Whitney U-test. Proportions were compared between groups using Fisher’s exact test. Logistic regression analysis was performed to estimate the effect of changes in Ang-1 and Ang-2 levels when adjusted for the presence of comorbidities, and variable selection was performed using the stepwise algorithm.
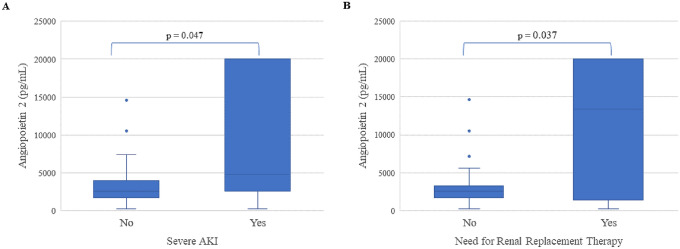
A total of 51 COVID-19 patients were included. The median age was 50.5 (IQR: 39.3–66.0) years, and 57.7% were males. Their comorbidities are shown in Table 1. A total of 12 (23.5%) COVID-19 patients developed severe AKI, 8 (66.6%) needing RRT, and 3 (25.0%) died. No significant differences were observed in Ang-1 levels (2904.1 [IQR: 737.5–5111.] vs. 2670.7 [IQR: 1321.6–4711.] pg/mL; p = 0.916) or Ang-2/Ang-1 ratio (0.45 [IQR: 0.07–1.08] vs. 1.15 [IQR: 0.53–2.47] pg/mL; p = 0.201) in those who developed severe AKI versus those who did not. Nonetheless, Ang-2 levels were found to be significantly higher in those who developed severe AKI (4715.7 [IQR: 2768.8–17,919.1] vs. 2462.4 [IQR: 1699.0–3641.8] pg/mL; p = 0.047) (Fig. 1a). Moreover, Ang-2 level was the highest in those who required RRT (13,372.7 [IQR: 3604.4–20000] vs. 2556.1 [IQR: 1699–3235] pg/mL; p = 0.037) (Fig. 1b). Ang-2 was found to be positively correlated with WBC (r = 0.596; p < 0.001), IL-6 (r = 0.280; p = 0.049), TNF-α (r = 0.316; p = 0.024), fibrinogen (r = 0.405; p = 0.009), D-dimer (r = 0.552; p = 0.008), cystatin C (r = 0.345, p = 0.019), NGAL (r = 0.431, p = 0.002), and negatively correlated with plasminogen (r = − 0.370; p = 0.007) and ADAMTS13 (r = − 0.302; p = 0.031). No correlation was observed for IL-10 (p = 0.794), CRP (p = 0.11), or VWF:ag (p = 0.427).
Table 1.
Baseline demographics of the Cincinnati emergency department COVID-19 cohort
| Variable | All patients (n = 51) | KDIGO AKI stage | p-value | |
|---|---|---|---|---|
| 0 + 1 | 2 + 3 | |||
| Age (years): median (IQR) | 50.5 (41–66) | 47 (37.5–64.0) | 66 (56.5–70.2) | 0.005 |
| Sex (male): n (%) | 30 | 23 (76.7%) | 7 (23.3%) | 1.000 |
| BMI: median (IQR) | 28.5 (24.8–33.5) | 29.5 (25.8–34.5) | 24.5 (21.6–27.5) | 0.018 |
| Race: n (%) | ||||
| Black | 21 | 12 (57.1%) | 9 (42.9%) | 0.036 |
| Hispanic | 18 | 17 (94.4%) | 1 (5.6%) | |
| White | 9 | 7 (77.8%) | 2 (22.2%) | |
| Other | 3 | 3 (100%) | 0 (0%) | |
| Comorbidities: n (%) | ||||
| Coronary artery disease | 8 | 3 (37.5%) | 5 (62.5%) | 0.012 |
| Heart failure | 9 | 3 (33.3%) | 6 (66.7%) | 0.003 |
| Hypertension | 26 | 15 (57.7%) | 11 (42.3%) | 0.002 |
| Hyperlipidemia | 15 | 11 (73.3%) | 4 (26.7%) | 0.730 |
| Diabetes | 21 | 15 (71.4%) | 6 (28.6%) | 0.738 |
| Chronic obstructive pulmonary disease | 8 | 4 (50%) | 4 (50%) | 0.076 |
| Asthma | 8 | 6 (75%) | 2 (25%) | 1.000 |
| Chronic kidney disease | 6 | 1 (16.7%) | 5 (83.3%) | 0.002 |
| Chronic liver disease | 7 | 3 (42.9%) | 4 (57.1%) | 0.044 |
| Cerebrovascular disease | 1 | 0 (0%) | 1 (100%) | 0.375 |
| Cancer | 4 | 1 (25%) | 3 (75%) | 0.036 |
| Acquired immunodeficiency (HIV, transplant) | 3 | 2 (66.7%) | 1 (33.3%) | 1.000 |
| Autoimmune disease | 2 | 2 (100%) | 0 (0%) | 1.000 |
*BMI Body Mass Index, KDIGO Kidney Disease: Improving Global Outcomes, AKI Acute Kidney Injury
p < 0.05
Fig. 1.
Angiopoietin-2 levels in patients developing severe AKI (a) and in patients requiring renal replacement therapy (RRT) (b)
In multivariate logistic regression, both pre-existing chronic kidney disease and hypertension were significantly associated with increased odds of severe AKI, with adjusted odds ratios (ORs) of 31.8 (95%CI 1.18–854.88) and 22.0 (95%CI 1.15–420.32), respectively. An increase in 1000 pg/mL of Ang-2 was associated with a 39% increase in odds of severe AKI (OR 1.39 [95%CI 1.05–1.86]). Full results are presented in Supplemental Table 1.
In this prospective study, we observed that Ang-2 levels measured at ED presentation are significantly increased in patients at risk of developing severe AKI. Moreover, we observed that elevated Ang-2 is an independent predictor of severe AKI and RRT. Our findings are in agreement with Smadja et al. [12], who reported significantly higher levels of Ang-2 in intensive care unit-admitted COVID-19 patients. They observed that patients with Ang-2 levels greater than 5000 pg/mL had ninefold higher odds of ICU admission. Our findings are also in agreement with Araujo et al. [13] who observed that elevated Ang-2 levels were significantly associated with increased odds of severe AKI and need for RRT in ICU-admitted non-COVID-19 acute respiratory distress syndrome (ARDS) patients.
Overall, our results are consistent with a picture of endothelial injury and a thrombotic microangiopathy phenomenon in COVID-19-associated AKI, further supported by the negative correlation with ADAMTS13 activity and positive correlations with fibrinogen and D-dimer. These results are consistent with elevations of Ang-2 observed in other forms of TMA [14–16]. Ang-2 was also correlated with several pro-inflammatory biomarkers, consistent with a hyperinflammatory response that can produce endothelial injury. Endothelium activation can lead to the release of Ang-2 from Weibel–Palade (WP) bodies [17]. Interestingly, however, we did not observe significant correlation between Ang-2 and VWF:ag. Philippe et al. [18] reported observing two distinct biomarker profiles, with VWF:ag increased in accordance with disease severity, while Ang-2 was elevated only in the critically ill. Taken together, this suggests that endothelial VWF secretion in COVID-19 may in part occur via pathways different than that of Ang-2. Indeed, while VWF is also secreted via WP bodies in the basal and regulated secretory pathways, the endothelium may also directly secrete VWF via a constitutive secretory pathway using small anterograde carriers [19]. Moreover, COVID-19 is associated with platelet hyperactivity, which occurs via multiple mechanisms, including spike protein binding to platelet angiotensin-converting enzyme 2 (ACE2) receptors, resulting in platelet activation and alpha granule release, which contains VWF in high molecular weight forms [20].
Ang-2 inhibits the protective anti-inflammatory Ang-1/Tie2 signaling cascade [17]. The Tie2 receptor is a central regulator in protecting the vasculature against thrombus formation in the setting of systemic inflammation, such as that seen in sepsis [21]. In a pilot study of critically ill patients with TMA and anti-glomerular basement membrane disease, plasma exchange was shown to be an effective method to remove excess circulating Ang-2, returning to almost normal values with ≤ 4 treatments [17]. As such, the investigation of the pathophysiologic role of Ang-2 in COVID-19 should be prioritized as targeting Ang-2 via plasma exchange or other inhibitory approaches are potential therapies in patients with severe COVID-19. Future longitudinal studies are needed to fully elucidate the role of Ang-2 in COVID-19 endothelial dysfunction and multiorgan injury and the specificity of Ang-2 for COVID-19 AKI.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This study was funded by the University of Cincinnati College of Medicine Special Coronavirus (COVID-19) Research Pilot Grant Program and the Lymphatic Malformation Institute.
Declarations
Conflict of interest
The authors do not have any conflicts of interest concerning this publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. The Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pons S, Fodil S, Azoulay E, et al. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Coca SG, Chan L, et al. (2020) AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. JASN [Internet]. [cited 2020 Nov 11]; Available from: https://jasn.asnjournals.org/content/early/2020/10/15/ASN.2020060897. [DOI] [PMC free article] [PubMed]
- 5.Chan L, Chaudhary K, Saha A, et al. (2020) AKI in Hospitalized Patients with COVID-19. JASN [Internet]. [cited 2020 Nov 11]; Available from: https://jasn.asnjournals.org/content/early/2020/09/02/ASN.2020050615.
- 6.Cheruiyot I, Henry B, Lippi G, et al. Acute Kidney Injury is Associated with Worse Prognosis In COVID-19 Patients: A Systematic Review and Meta-analysis. 1. 2020;91:ahead of print-ahead of print. [DOI] [PMC free article] [PubMed]
- 7.Henry BM, Benoit SW, de Oliveira MHS, et al. ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID-19: Evidence of SARS-CoV-2 induced secondary thrombotic microangiopathy. Int J Lab Hematol. 2020 doi: 10.1111/ijlh.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 9.Neuhauß A-K, Gutbier B, Friedemann T, et al. Angiopoietins: Possible biomarkers in severe pneumonia? European Respiratory Journal [Internet]. 2012 [cited 2020 Nov 5];40. Available from: https://erj.ersjournals.com/content/40/Suppl_56/P830.
- 10.Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2:a006550. doi: 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KDIGO AKI Working Group KDIGO clinical practice guideline for acute kidney injury. Kidney International Suppl. 2012;2:1. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 12.Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23(4):611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araújo CB, de Oliveira Neves FM, de Freitas DF, et al. Angiopoietin-2 as a predictor of acute kidney injury in critically ill patients and association with ARDS. Respirology. 2019;24:345–351. doi: 10.1111/resp.13464. [DOI] [PubMed] [Google Scholar]
- 14.Lukasz A, Beneke J, Thamm K, et al. Involvement of Angiopoietin-2 and Tie2 Receptor Phosphorylation in STEC-HUS Mediated by Escherichia coli O104:H4 [Internet]. Mediators of Inflammation. Hindawi; 2015 [cited 2020 Dec 29]. p. e670248. Available from: https://www.hindawi.com/journals/mi/2015/670248/. [DOI] [PMC free article] [PubMed]
- 15.Ueda N, Chihara D, Kohno A, et al. Predictive Value of Circulating Angiopoietin-2 for Endothelial Damage-Related Complications in Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2014;20:1335–1340. doi: 10.1016/j.bbmt.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Inoue N, Kuroda M, et al. Angiopoietin-1 and -2 as markers for disease severity in hemolytic uremic syndrome induced by enterohemorrhagic Escherichia coli. Clin Exp Nephrol. 2017;21:76–82. doi: 10.1007/s10157-016-1254-z. [DOI] [PubMed] [Google Scholar]
- 17.Lovric S, Lukasz A, Hafer C, et al. Removal of elevated circulating angiopoietin-2 by plasma exchange–a pilot study in critically ill patients with thrombotic microangiopathy and anti-glomerular basement membrane disease. Thromb Haemost. 2010;104:1038–1043. doi: 10.1160/TH10-02-0138. [DOI] [PubMed] [Google Scholar]
- 18.Philippe A, Chocron R, Gendron N, et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis. 2021 doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes da Silva M, Cutler DF. von Willebrand factor multimerization and the polarity of secretory pathways in endothelial cells. Blood. 2016;128:277–285. doi: 10.1182/blood-2015-10-677054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins SJ, De Ceunynck K, Kellum JA, et al. Tie2 protects the vasculature against thrombus formation in systemic inflammation. J Clin Invest. 2018;128:1471–1484. doi: 10.1172/JCI97488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.