Abstract
Background:
Information on coronavirus disease 2019 (COVID-19) infection in patients with chronic kidney disease (CKD) remains limited. To understand the influence of COVID-19 infection in patients with pre-existing CKD, we conducted a systematic review and meta-analysis to evaluate and compare the risks of all-cause mortality, hospitalization, and critical progression between patients with and without CKD.
Methods:
We selected randomized controlled trials (RCTs), prospective or retrospective observational, case-control, cross-sectional, and case-series studies analyzing outcomes of COVID-19 infection in patients with pre-existing CKD from the PubMed, Embase, and Cochrane Central Register of Controlled Trials databases published on the Internet before 16 July 2020.
Results:
A total of 27 studies comprising 77,856 patients with COVID-19 infection was identified; 3922 patients with pre-existing CKD were assigned CKD group, and 73,934 patients were assigned to the non-CKD group. The pooled analysis showed that patients with CKD had a significantly higher risk of all-cause mortality and hospitalization than those without CKD [odds ratio (OR) 2.25, 95% confidence interval (CI) 1.91–2.66, p < 0.001; OR 4.29, 95% CI 2.93–6.28, p < 0.001; respectively]. Patients with CKD had a higher risk of critically ill conditions than those without CKD in the pooled analysis of studies with multivariable adjustment (adjusted OR 2.12, 95% CI 0.95–4.77, p = 0.07) and in the analysis of all included studies (OR 1.27, 95% CI 0.71–2.26, p = 0.41), but both analyses did not attain statistical significance.
Conclusion:
COVID-19 infected patients with CKD had significantly increased risks of all-cause mortality and hospitalization compared with those without CKD.
Keywords: chronic kidney disease, COVID-19, hospitalization, meta-analysis, mortality
Introduction
A coronavirus 2019 (COVID-19) disease outbreak occurred in Wuhan, Hubei Province, China in December 2019, and has been declared a global pandemic by World Health Organization (WHO).1 COVID-19 is highly contagious by human-to-human transmission, and can cause critical condition and even death.2 There were over 20 million confirmed cases and over 800,000 deaths by the end of August 2020.3 Although underlying diseases have been considered one of the major risk factors of clinical outcomes such as acute respiratory distress syndrome (ARDS), sepsis, shock, and all-cause mortality,4,5 the relationship between the specific underlying disease, for example, kidney dysfunction, and these outcomes of patients with COVID-19 remains unclear.
The main clinical features of severe COVID-19 infection are lung destruction and respiratory failure, but some studies have shown that COVID-19 infection also involves other organs, including kidney and liver.6–8 Recent clinical evidence has revealed that COVID-19 patients with acute kidney injury (AKI) had fatal outcomes.6,9,10 This implies that patients with pre-existing chronic kidney disease (CKD), who have functional defects in their innate and adaptive immune cells, may have a higher risk of COVID-19 infection and death.11–13 Clinicians should take more notice of medical management and prevention of COVID-19 infection in this population.
Although several investigations have reported CKD in patients with COVID-19 infection, the data collected were usually restricted to a single hospital or country.14,15 Hence, we conducted a systematic review and meta-analysis to evaluate the influence of pre-existing CKD on risks of all-cause mortality, hospitalization, and critically ill condition among patients with COVID-19 infection.
Materials and methods
This systematic review and meta-analysis was established according to the PRISMA checklist (Supplemental File S1)16 and was registered with PROSPERO (registration number: CRD42020198797). Randomized controlled trials (RCTs), prospective or retrospective observational, case-control, cross-sectional, and case-series studies analyzing outcomes of COVID-19 infection in patients with pre-existing CKD were included. We excluded studies with patients younger than 18 years of age, receiving transplantation or under maintenance dialysis, animal studies, and those without abstract or full text for further data extraction. Eligible studies were identified after searching the following electronic databases: PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library for publications appearing before 16 July 2020 on the internet. We first retrieved studies by specific filters in electronic databases and then used the following keywords to identify eligible studies: “COVID-19” or “coronavirus disease 2019” combined with “Renal” or “Kidney”. We then reviewed the abstracts and obtained the full texts of studies for patients including CKD. The studies retrieved were then reviewed independently by another author. The exact search strategy of PubMed is described in Supplemental File S2. Any differences in opinion between the two assessors (Y.C.L. and T.S.L.) were then discussed with a third author in conference to achieve a consensus.
Data extraction and quality assessment
Two investigators (Y.C.L. and T.S.L.) reviewed the full texts of selected studies, integrated data for the characteristics of studies and patients, and assessed the quality of individual included studies. The outcomes extracted included all-cause mortality, hospitalization, and critical or severe condition. The critical condition consisted of respiratory failure, endotracheal tube intubation, major adverse cardiovascular events, septic shock, and any admission to the intensive care unit (ICU). All articles were reviewed to identify duplicates and determine whether subgroup analyses were used. Subgroup analysis data were included if they met the inclusion criteria and comprised our outcomes of interest. Two investigators (Y.C.L. and T.S.L.) checked the data extraction tasks and independently performed critical appraisal to confirm a random selection and accuracy of screening articles. Discrepancies in the data extraction or quality assessment of included studies between two investigators were discussed with two other senior investigators (T.S.C. and Y.K.T) to achieve a consensus if a decision could not be made. Two investigators (Y.C.L. and T.S.L.) assessed the quality of the included studies using the Newcastle-Ottawa scale (NOS).17 NOS scores of more than six suggested high-quality literature.
Study endpoints
COVID-19 infection was diagnosed as a positive result on a reverse-transcriptase-polymerase chain reaction (RT-PCR) assay.4 The primary outcome was all-cause mortality related to COVID-19 infection. Secondary outcomes were risks of any hospitalization and critically ill conditions, comprising respiratory failure, endotracheal tube intubation, major adverse cardiovascular events, sepsis, septic shock, and any admission to ICU.18,19
Statistical analysis
We used the software RevMan 5.4 (Cochrane Collaboration, 2020) to calculate a weighted estimate effect size of the individual studies. The inverse–variance weighting method was used for the fixed-effect model and the DerSimonian and Laird method for the random-effect model. The effect size measure for dichotomous outcomes, such as all-cause mortality, any hospitalization, and critical condition, is pooled odds ratio (OR) with the associated 95% confidence interval (CI). We also undertook subgroup analyses on studies with or without multivariable adjustment. The heterogeneity of effect size estimates within these studies was quantified by using the X2 (Cochran Q) statistic and the I2 test. The I2 statistic ranged from 0% to 100%, and a value of I2 > 50% was considered to indicate significant heterogeneity. We performed a pooled analysis with random effect models if heterogeneity was significant and substantial variations in baseline characteristics were found among the included studies. Sensitivity analyses by removing studies with small numbers of CKD and of lower quality (NOS score <8) were conducted. Publication bias was evaluated using a funnel plot with the Egger regression test (where at least five studies were available) and Begg’s test. Significant publication bias was determined if p < 0.05.
Results
Literature assessment and screening
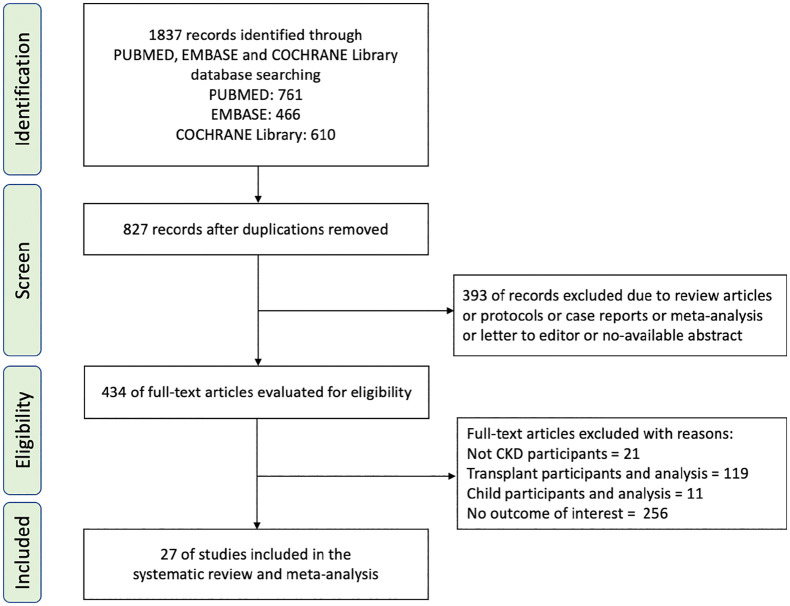
Of the 1837 screened articles, 434 relevant full-text articles were selected after the removal of duplicate articles, protocols, case reports, reviews, protocols, and unavailable abstracts. After screening, 27 articles were included for further analysis.20–46 The process of selection of the included studies is shown in Figure 1.
Figure 1.
Flow chart of the process to identify eligible studies, with reasons for inclusion or exclusion.
CKD, chronic kidney disease.
Characteristics and quality of studies
A total of 77,856 patients was identified; 3922 patients with CKD were assigned to the CKD group, and 73,934 patients were assigned to the non-CKD group. The age of patients was between 20 and 95 years and the subjects were predominantly men (42.9–85%). Among 27 included studies, there were 3 prospective cohort, 10 retrospective cohort, two cross-sectional, and 12 retrospective case series investigations. The basic characteristics of the included studies are summarized in Table 1. All included studies received NOS quality scores ranging from 6 to 9, suggesting high quality. Detailed descriptions of quality assessment of included studies are summarized in Table 2.
Table 1.
Basic characteristics of included studies in this systematic review and meta-analysis.
| Study | Publication date | Country/city | Study type | Sample size | Age (year) [median (IQR)] | Female (%) | CKD patients (%) | Baseline serum creatinine, mg/dl [median (IQR)] | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Okoh et al.20 | 2 June 2020 | US/New Jersey | Retrospective, cohort study | 251 | 62 (49–74) | 122 (49) | 46 (18.3) | NA | 1 |
| Dawei Wang et al.21 | 14 April 2020 | China/Wuhan | Retrospective, case series | 107 | 51 (36–65) | 50 (46.7) | 3 (2.8) | 0.80 (0.68–0.97) | 1 |
| Ciceri et al.22 | 10 June 2020 | Italy/Milan | Retrospective, cohort study | 410 | 65 (56–75) | 111 (27.1) | 47 (11.8) | 1.02 (0.84–1.25) | 1 |
| Grasselli et al.23 | 15 July 2020 | Italy/Milan | Retrospective, cohort study | 3988 | 63 (56–69) | 800 (20.1) | 87 (2.2) | NA | 1, 2 |
| Iaccarino et al.24 | 24 May 2020 | Italy | Cross-sectional, multicenter | 1591 | 66.5 (0.4) [mean (SD)] | 572 (36) | 87 (5.5) | NA | 1 |
| Barman et al.25 | 24 May 2020 | Turkey/Istanbul | Retrospective, cohort study | 607 | 68.5 (13.4) [mean (SD)] | 273 (44.9) | 57 (9.4) | 0.90 (0.8–2.0) | 1 |
| Nikpouraghdam et al.26 | 9 April 2020 | Iran/Tehran | Retrospective, cohort study | 2964 | NA | 1009 (34) | 18 (0.6) | NA | 1 |
| Marcello et al.27 | 23 June 2020 | US/New York | Retrospective, cohort study | 13,442 | 52.7 (39.5–64.5) | 5961 (44.0) | 809 (6.0) | NA | 1, 3 |
| Shi et al.28 | 6 May 2020 | China/Wuhan | Retrospective, cohort study | 671 | 63 (50–72) | 349 (52) | 28 (4.2) | 0.65 (0.54–0.79) | 1 |
| Gupta et al.29 | 15 July 2020 | US | Prospective, cohort study | 2215 | 60.5 (14.5) [mean (SD)] | 779 (35.2) | 280 (12.6) | 1.0 (0.8–1.5) | 1 |
| Mikami et al.30 | 11 June 2020 | US/New York | Retrospective, cohort study | 6493 | 59 (43–72) | 2955 (45.5) | 525 (8.1) | NA | 1, 3 |
| Tao Chen et al.31 | 17 March 2020 | China/Wuhan | Retrospective case series | 274 | 62 (44–70) | 103 (37) | 4 (1.4) | 0.86 (0.66–1.06) | 1 |
| TieLong Chen et al.32 | 11 April 2020 | China/Wuhan | Retrospective case series | 203 | 54 (20–91) | 95 (46.8) | 8 (3.9) | 0.98 (0.42–12.05) | 1 |
| Mani et al.33 | 21 May 2020 | US/New York | Retrospective case series | 184 | 64.7 (14.9) [mean (SD)] | 73 (39.7) | 9 (4.9) | NA | 1 |
| Yan et al.34 | 6 April 2020 | China/Wuhan | Retrospective, cohort study | 193 | 64 (49–73) | 79 (40.9) | 4 (2.1) | 0.94 (0.74–1.23) | 1 |
| Bhargava et al.35 | 30 May 2020 | US/Detroit | Retrospective, cohort study | 197 | NA | 94 (47.7) | 34 (17.3) | NA | 2 |
| Petrilli et al.36 | 14 May 2020 | US/New York | Prospective, cohort study | 5279 | 49.5 (32–69). | 2664 (50.5) | 647 (12.3) | 1.0 (0.80–1.39) | 2, 3 |
| Khamis et al.37 | 1 June 2020 | Oman | Retrospective, case series | 66 | 48 (16) [mean (SD)] | 10 (15.0) | 4 (6.4) | NA | 2 |
| Suleyman et al.38 | 16 June 2020 | US/Detroit | Retrospective, case series | 463 | 57.5 (16.8) [mean (SD)] | 259 (55.9) | 182 (39.3) | 1.1 (0.84–1.54) | 2, 3 |
| Helms et al.39 | 17 April 2020 | French | Prospective, cohort study | 222 | 63 (53–71) | 47 (21.1) | 19 (8.6) | NA | 2 |
| Luwen Wang et al.40 | 24 March 2020 | China/Wuhan | Retrospective, case series | 116 | 54 (38–69) | 49 (42.2) | 5 (4.3) | NA | 2 |
| Argenziano et al.41 | 18 May 2020 | US/New York | Retrospective, case series | 1000 | 63.0 (50.0–75.0) | 404 (40.4) | 137 (13.7) | NA | 2 |
| Salomon et al.42 | 18 May 2020 | Mexico | Retrospective, case series | 36,182 | NA | NA | 820 (2.2) | NA | 2, 3 |
| Aggarwal et al.43 | 16 April 2020 | US | Retrospective, case series | 16 | 67.0 (38–95) | 4 (25) | 6 (38) | 1.22 (0.79–7.68) | 2 |
| Zhao et al.44 | 1 April 2020 | China/Hubei | Retrospective, case series | 91 | 46.0 (NA) | 42 (46.2) | 1 (1.1) | NA | 2 |
| Killerby et al.45 | 26 June 2020 | US | Retrospective, case series | 531 | Non-hospitalized:45.0 (33–58) hospitalized:61.0 (45–70) | 303 (57.1) | 45 (8.4) | NA | 3 |
| Duanmu et al.46 | 24 April 2020 | US | Cross-sectional | 100 | 45 (32–65) | 44 (44.0) | 10 (10.0) | NA | 3 |
Outcome 1: All-cause mortality, 2: Critical or severe condition, 3: Hospitalization.
CKD, IQR, interquartile range; NA, no application; SD, standard deviation; US, United States.
Table 2.
Summary of quality assessment of included studies using NOS scores.
| Prospective and retrospective cohort studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Selection | Comparability | Outcome | Score | |||||
| Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration of interest outcome | Comparability of cohorts on the basis of the design | Assessment of outcome | follow-up duration for outcomes | Adequacy of follow up (>4 week) | ||
| Okoh et al.20 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Ciceri et al.22 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Grasselli et al.23 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Barman et al.25 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Nikpouraghdam et al.26 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | - | - | 7 |
| Marcello et al.27 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | - | 8 |
| Shi et al.28 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | - | 8 |
| Gupta et al.29 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Mikami et al.30 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | - | - | 7 |
| Yan et al.34 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Bhargava et al.35 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Petrilli et al.36 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Helms et al.39 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | ✦ | 9 |
| Retrospective case series | |||||||||
| Study | Selection | Ascertainment | Outcome | Score | |||||
| Adequate definition and number of cases (n > 30) | Population representation (multi-centers) | Adequate test | Adequate confirmation of comorbidity | Adequately reported outcome | Sufficient follow-up duration (>2 weeks) | ||||
| Dawei Wang et al.21 | ✦ | - | ✦ | ✦✦ | ✦✦ | ✦ | 7 | ||
| Tao Chen et al.31 | ✦ | - | ✦ | ✦✦ | ✦✦ | ✦ | 7 | ||
| TieLong Chen et al.32 | ✦ | - | ✦ | ✦✦ | ✦✦ | ✦ | 7 | ||
| Mani et al.33 | ✦ | - | ✦ | ✦✦ | ✦✦ | - | 6 | ||
| Khamis et al.37 | ✦ | - | ✦ | ✦✦ | ✦✦ | ✦ | 7 | ||
| Suleyman et al.38 | ✦ | ✦ | ✦ | ✦✦ | ✦✦ | ✦ | 8 | ||
| Luwen Wang et al.40 | ✦ | - | ✦ | ✦✦ | ✦✦ | ✦ | 7 | ||
| Argenziano et al.41 | ✦ | - | ✦ | ✦✦ | ✦✦ | ✦ | 7 | ||
| Salomon et al.42 | ✦ | ✦ | ✦ | ✦✦ | ✦✦ | - | 7 | ||
| Aggarwal et al.43 | - | - | ✦ | ✦✦ | ✦✦ | ✦ | 6 | ||
| Zhao et al.44 | ✦ | - | ✦ | ✦✦ | ✦✦ | ✦ | 7 | ||
| Killerby et al.45 | ✦ | ✦ | ✦ | ✦✦ | ✦✦ | ✦ | 8 | ||
| Cross-sectional studies | |||||||||
| Study | Selection | Comparability | Outcome | Scores | |||||
| Representativeness of samples | Sample size (n > 30) | Non-respondents | Ascertainment of exposure | Comparability | Assessment of outcome | Statistical test | |||
| Iaccarino et al.24 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | 8/10 | |
| Duanmu et al.46 | ✦ | ✦ | ✦ | ✦ | ✦✦ | ✦ | ✦ | 8/10 | |
NOS, Newcastle-Ottawa scale.
All-cause mortality
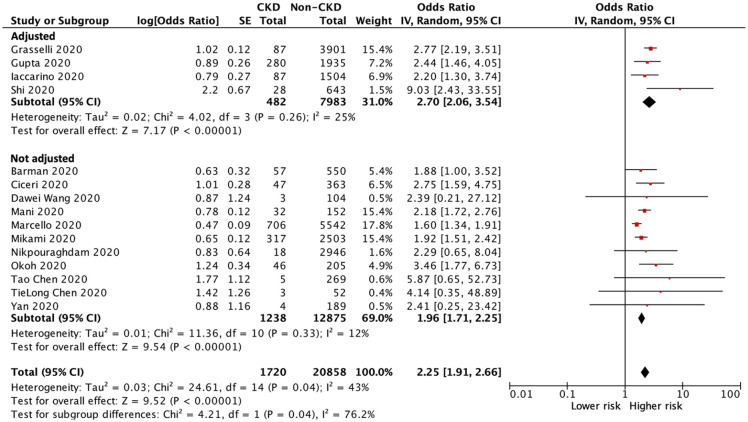
Of the 22,578 patients with COVID-19 infection in 15 of the included studies,20–34 a total of 6315 patients died during the study period: 725 patients in the CKD group and 5590 patients in the non-CKD group. The heterogeneity test showed moderate heterogeneity among these studies (I2 = 43%), and a random-effects model was used for this meta-analysis. The pooled analyses of all included studies and studies with multivariable adjustment showed a significantly higher risk of all-cause mortality in CKD patients than in those without CKD (OR 2.25, 95% CI 1.91–2.66, p < 0.001; OR 2.70, 95% CI 2.06–3.54, p < 0.001; respectively) (Figure 2).
Figure 2.
Forest plot of all-cause mortality of COVID-19 infection between CKD and non-CKD patients.
CI, confidence intervals; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; IV, inverse–variance; SE, standard error.
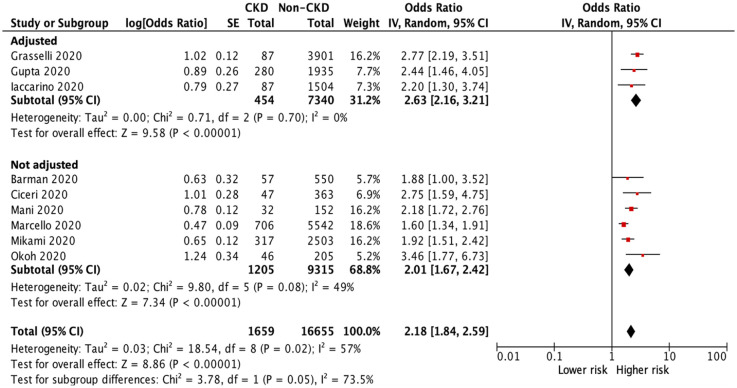
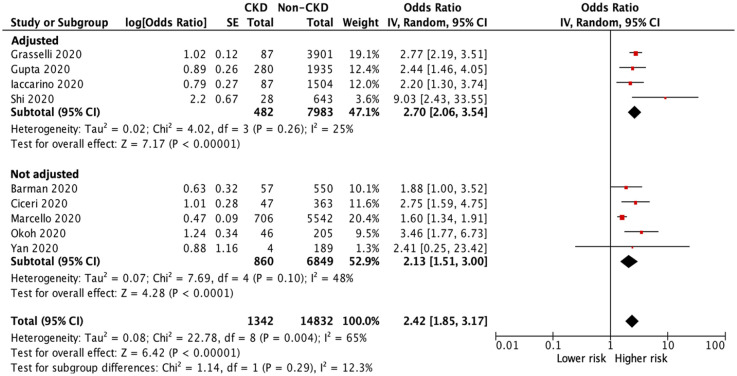
Sensitivity tests were conducted by removing small studies with fewer than 30 CKD patients each and or lower quality (NOS score <8) studies; the results of all-cause mortality remained similar (OR 2.18, 95% CI 1.84–2.59, p < 0.001, Figure 3; OR 2.42, 95% CI 1.85–3.17, p < 0.001, Figure 4).
Figure 3.
Forest plot of all-cause mortality of COVID-19 infection between CKD and non-CKD patients after a sensitivity test with removing small studies.
CI, confidence intervals; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; IV, inverse–variance; SE, standard error.
Figure 4.
Forest plot of all-cause mortality of COVID-19 infection between CKD and non-CKD patients after a sensitivity test with removing lower quality (NOS score <8) studies.
CI, confidence intervals; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; IV, inverse–variance; NOS, Newcastle-Ottawa Scale; SE, standard error.
Hospitalization
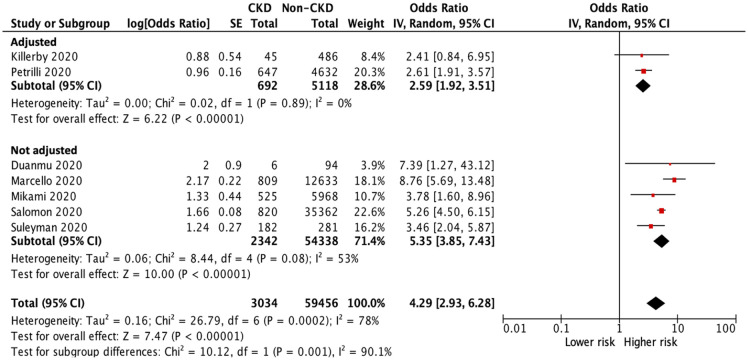
Analysis of hospitalization was performed in seven studies.27,30,36,38,42,45,46 Among 62,490 patients with COVID-19, a total of 25,906 patients were hospitalized during the study period: 2520 patients in the CKD group and 23,386 patients in the non-CKD group. The heterogeneity test showed high heterogeneity among these studies (I2 = 78%), and a random-effects model was used. The pooled analyses of all included studies and studies with multivariable adjustment showed a significantly higher risk of hospitalization in patients with CKD than in those without CKD (OR 4.29, 95% CI 2.93–6.28, p < 0.001; OR 2.59, 95% CI 1.92–3.51, p < 0.001; respectively) (Figure 5).
Figure 5.
Forest plot of hospitalization of COVID-19 infection between CKD and non-CKD groups.
CI, confidence intervals; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; IV, inverse-variance; SE, standard error.
Critically ill condition
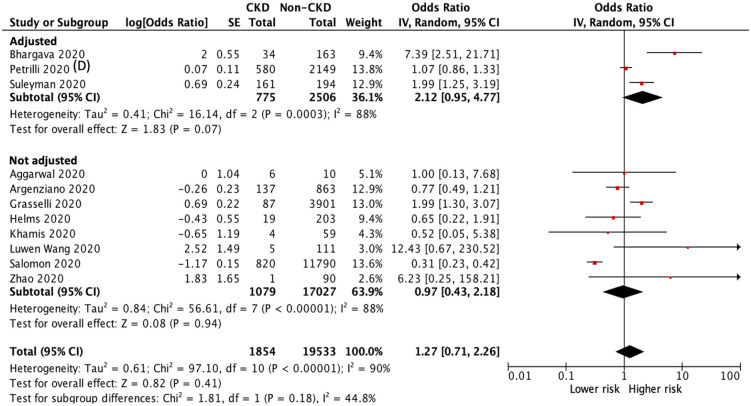
In our meta-analysis, information on the critical or severe condition was available in 11 studies.23,35–44 Of the 21,387 patients with COVID-19 infection, 5411 patients suffered from critically ill condition during the study period: 507 patients in the CKD group and 4904 patients in the non-CKD group. The heterogeneity test showed high heterogeneity among these studies (I2 = 90%), and a random-effects model was used. Patients with CKD had a higher risk of critically ill conditions than those without CKD in the pooled analysis of studies with multivariable adjustment (adjusted OR 2.12, 95% CI 0.95 to 4.77, p = 0.07) and in the analysis of all included studies (OR 1.27, 95% CI 0.71 to 2.26, p = 0.41), but both analyses did not attain statistical significance (Figure 6).
Figure 6.
Forest plot of critically ill condition (including respiratory failure, endotracheal tube intubation, major adverse cardiovascular events, sepsis, septic shock, and any admission in the ICU) of COVID-19 infection between CKD and non-CKD groups.
CI, confidence intervals; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IV, inverse-variance; SE, standard error.
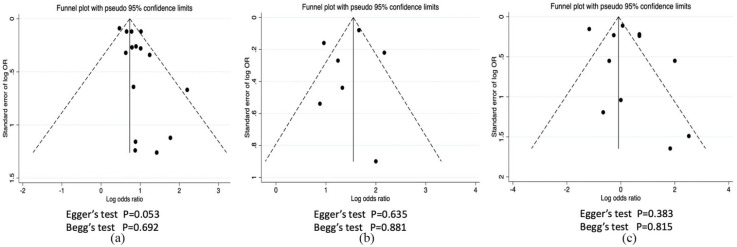
Assessment of publication bias
Publication bias was evaluated in the outcomes of all-cause mortality, hospitalization, and critically ill condition by funnel plots. Egger’s regression test and Begg’s test showed no significant publication bias (p > 0.05) (Figure 7).
Figure 7.
Funnel plot for publication bias assessment. (a) All-cause mortality, (b) hospitalization, (c) critically ill condition.
OR, odds ratio.
Discussion
As far as we know, this is the first systematic review and meta-analysis to assess outcomes of COVID-19 infection in patients with CKD in the initial pandemic period. After integration of data from the included studies, our pooled analysis of all included studies and subgroup analyses of studies with multivariable adjustment for subjects with COVID-19 infection revealed that patients with CKD had a significantly increased risk of all-cause mortality and hospitalization compared with those without CKD. Patients with CKD suffering from COVID-19 infection also had a higher risk of progressing to a critically ill condition than those without CKD in the pooled analysis of included studies and subgroup analyses of studies with multivariable adjustment, though neither result achieved statistical significance. These results indicate CKD as a strong risk factor of grave outcomes of COVID-19 infection
Patients with CKD, having a higher risk of the severe disease due to higher incidence rate of infections and cardiovascular disease than in the general population, have been regarded as having immunocompromised status due to functional defects of the innate and adaptive immune system, leading to frequent infectious complications.47,48 Changes in the immune system of patients with CKD include mainly phagocytic dysfunction of B and T cells and the increased reaction of pro-inflammatory cytokines and inflammatory monocytes; these may gradually progress as renal function declines.49,50 Some investigations have shown that this chronic systemic inflammation contributes to higher morbidity and mortality in CKD patients.12,51,52 In our study, patients with CKD also had a higher risk of mortality, hospitalization, and critical condition. Hence, clinicians should pay more medical attention to this population in COVID-19 infection and may consider earlier hospitalization to help prevent disease deterioration and spread.
Renal involvement has been reported in patients with COVID-19 infection, supported by the findings of PCR products of coronavirus in both blood and urine samples.6–8 The etiology of kidney involvement in patients with COVID-19 may be associated with direct cytopathic effects through the angiotensin-converting enzyme 2 (ACE2)-dependent pathway, with increased expression of ACE2 in patients with CKD, as a cell entry receptor of coronavirus, and indirect effect of viral infection related cytokines or mediators on renal tissues.4,53,54 Of note, predisposed CKD is recognized as a risk factor for the development of AKI, which is associated with an increased risk of progressive CKD, end-stage renal disease (ESRD), and mortality.55 Thus, early detection of kidney injury in COVID-19 infection should be made in patients with CKD, thereby allowing timely adequate medical strategies to prevent progression to poor outcomes.
Due to the lack of approved vaccine or specific therapies against COVID-19 in the initial pandemic period of COVID-19 infection, the principle of management for COVID-19 infection in patients with CKD was supportive care, similar to the general population.56,57 Besides being quarantined at the initial COVID-19 infection, maintenance of stable hemodynamic status, adequate oxygenation supply, closely following up changes in renal function, and prevention using renal toxicity medicine should be performed in COVID-19 infected patients with CKD.58 More large clinical trials are needed to clarify the effect and safety of medicine against COVID-19, such as a vaccines, and antiviral or anti-inflammatory agents, particularly in patients with CKD due to their insufficient ability to excrete drugs.
Although our study analyzed clinical outcomes with a large sample size of patients, some limitations still exist. First, the total sample size of patients with CKD was relatively small in some included studies, and this may reduce the significance of the results. Second, the causes of pre-existing CKD and serum creatinine level were not clearly stated in the included studies. Therefore, our study did not explore the potential difference in outcomes among various etiologies or stages of CKD. Third, the exact information of other coexisting illnesses beyond CKD, such as diabetes mellitus, hypertension, cardiovascular disease, and detailed characteristics, such as age or sex, in included studies was insufficient. To examine the impact of potential confounding, we conducted subgroup analyses of studies with or without multivariable adjustment, which comprises age, sex, diabetes mellitus, hypertension, cardiovascular disease, etc. We observed small differences in the pooled estimates between adjusted and non-adjusted studies. More large-scale and higher-quality studies are needed to further confirm the influence of COVID-19 on outcomes in patients with CKD in the future.
In summary, patients with CKD had a significantly increased risk of all-cause mortality and hospitalization compared with those without CKD. Clinicians should pay more medical attention to this population in COVID-19 infection and apply timely adequate medical strategies to prevent progression to poor outcomes.
Supplemental Material
Supplemental material, sj-doc-1-taj-10.1177_2040622321998860 for Outcomes of coronavirus 2019 infection in patients with chronic kidney disease: a systematic review and meta-analysis by Yi-Chih Lin, Tai-Shuan Lai, Shuei-Liong Lin, Yung-Ming Chen, Tzong-Shinn Chu and Yu-Kang Tu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pdf-2-taj-10.1177_2040622321998860 for Outcomes of coronavirus 2019 infection in patients with chronic kidney disease: a systematic review and meta-analysis by Yi-Chih Lin, Tai-Shuan Lai, Shuei-Liong Lin, Yung-Ming Chen, Tzong-Shinn Chu and Yu-Kang Tu in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors thank the Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan and Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan, for their analytical support in this study.
Footnotes
Authors’ contributions: Research idea and study design: Y.-C.L., T.-S.L., Y.-K.T.; data acquisition: Y.-C.L., T.-S.L. S.-L.L., Y.-M.C., T.-S.C.; data analysis and interpretation: Y.-C.L., T.-S.L., Y.-K.T.; statistical analysis: Y.-C.L., Y.-K.T.; supervision or mentorship: T.-S.L., Y.-K.T.. Y.-C.L., T.-S.L., Y.-K.T. provided critical contributions to the data analysis and were the major contributors in writing the manuscript. All authors read and approved the final manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was partially funded by a grant from the Ministry of Science & Technology in Taiwan (grant number: MOST 109-2314-B-002-150-MY3).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Yi-Chih Lin  https://orcid.org/0000-0002-7813-3423
https://orcid.org/0000-0002-7813-3423
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yi-Chih Lin, Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei; Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei; Department of Medicine, National Taiwan University Hospital Jinshan Branch, New Taipei City.
Tai-Shuan Lai, Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital, No. 7, Chung-Shan South Road, Taipei 100.
Shuei-Liong Lin, Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei; Graduate Institute of Physiology, National Taiwan University College of Medicine; Department of Integrated Diagnostics and Therapeutics, National Taiwan University Hospital; Research Center for Developmental Biology and Regenerative Medicine, National Taiwan University.
Yung-Ming Chen, Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei.
Tzong-Shinn Chu, Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei.
Yu-Kang Tu, Institute of Epidemiology and Preventive Medicine, National Taiwan University, Room 501, No. 17, Xu-Zhou Road, Taipei 100; Department of Dentistry, National Taiwan University Hospital, Taipei; Research Center of Big Data and Meta-Analysis, Wan Fang Hospital, Taipei Medical University, Taipei.
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382: 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200824-weekly-epi-update.pdf?sfvrsn=806986d1_2.
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim JH, Park SH, Jeon Y, et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu KH, Tsang WK, Tang CS, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 2005; 67: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol 2013; 9: 255–265. [DOI] [PubMed] [Google Scholar]
- 12. Sibbel S, Sato R, Hunt A, et al. The clinical and economic burden of pneumonia in patients enrolled in Medicare receiving dialysis: a retrospective, observational cohort study. BMC Nephrol 2016; 17: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit Health 2020; 2: e201–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kang SH, Kim SW, Kim AY, et al. Association between chronic kidney disease or acute kidney injury and clinical outcomes in COVID-19 patients. J Korean Med Sci 2020; 35: e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. Epub ahead of print 19 September 2020. DOI: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 350: g7647. [DOI] [PubMed] [Google Scholar]
- 17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 18. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 20. Okoh AK, Sossou C, Dangayach NS, et al. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health 2020; 19: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care 2020; 24: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol 2020; 217: 108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iaccarino G, Grassi G, Borghi C, et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian society of hypertension. Hypertension 2020; 76: 366–372. [DOI] [PubMed] [Google Scholar]
- 25. Barman HA, Atici A, Sahin I, et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. Epub ahead of print 19 June 2020. DOI: 10.1097/MCA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nikpouraghdam M, Jalali Farahani A, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: a single center study. J Clin Virol 2020; 127:104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalyanaraman Marcello R, Dolle J, Grami S, et al. Characteristics and Outcomes of COVID-19 Patients in New York City’s Public Hospital System. medRxiv. Epub ahead of print 2 June 2020. DOI: 10.1101/2020.05.29.20086645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi S, Qin M, Cai Y, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020; 41: 2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mikami T, Miyashita H, Yamada T, et al. Risk factors for mortality in patients with COVID-19 in New York city. J Gen Intern Med. Epub ahead of print 30 June 2020. DOI: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci 2020; 75: 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mani VR, Kalabin A, Valdivieso SC, et al. New York inner city hospital COVID-19 experience and current data: retrospective analysis at the epicenter of the American Coronavirus outbreak. J Med Internet Res 2020; 22: e20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care 2020; 8:e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhargava A, Fukushima EA, Levine M, et al. Predictors for severe COVID-19 infection. Clin Infect Dis 2020; 71: 1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369: m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khamis F, Al-Zakwani I, Al Naamani H, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health 2020; 13: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open 2020; 3: e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 2020; 51: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020; 369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wollenstein-Betech S, Cassandras CG, Paschalidis IC. Personalized predictive models for symptomatic COVID-19 patients using basic preconditions: hospitalizations, mortality, and the need for an ICU or ventilator. medRxiv.Epub ahead of print 8 May 2020. DOI: 10.1101/2020.05.03.20089813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aggarwal S, Garcia-Telles N, Aggarwal G, et al. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis. 2020; 7: 91–96. [DOI] [PubMed] [Google Scholar]
- 44. Zhao XY, Xu XX, Yin HS, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis 2020; 20: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Killerby ME, Link-Gelles R, Haight SC, et al. Characteristics associated with hospitalization among patients with COVID-19 - metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duanmu Y, Brown IP, Gibb WR, et al. Characteristics of emergency department patients with COVID-19 at a single site in Northern California: clinical observations and public health implications. Acad Emerg Med 2020; 27: 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens 2012; 21: 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernández-Fresnedo G, Ramos MA, González-Pardo MC, et al. B lymphopenia in uremia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2. Nephrol Dial Transplant 2000; 15: 502–510. [DOI] [PubMed] [Google Scholar]
- 49. Heine GH, Ortiz A, Massy ZA, et al. Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol 2012; 8: 362–369. [DOI] [PubMed] [Google Scholar]
- 50. Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health 2020; 25: 278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. D’Marco L, Puchades MJ, Romero-Parra M, et al. Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J 2020; 13: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen-Hagai K, Rozenberg I, Korzets Z, et al. Upper respiratory tract infection among dialysis patients. Isr Med Assoc J 2016; 18: 557–560. [PubMed] [Google Scholar]
- 53. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shi C, Lu K, Xia H, et al. Alteration and association between serum ACE2/ angiotensin(1-7)/Mas axis and oxidative stress in chronic kidney disease: a pilot study. Medicine 2020; 99: e21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vanmassenhove J, Kielstein J, Jörres A, et al. Management of patients at risk of acute kidney injury. Lancet 2017; 389: 2139–2151. [DOI] [PubMed] [Google Scholar]
- 56. Li J, Xu G. Lessons from the experience in Wuhan to reduce risk of COVID-19 infection in patients undergoing long-term hemodialysis. Clin J Am Soc Nephrol 2020; 15: 717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Watnick S, McNamara E. On the frontline of the COVID-19 outbreak: keeping patients on long-term dialysis safe. Clin J Am Soc Nephrol 2020; 15: 710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Naicker S, Yang CW, Hwang SJ, et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020; 97: 824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-taj-10.1177_2040622321998860 for Outcomes of coronavirus 2019 infection in patients with chronic kidney disease: a systematic review and meta-analysis by Yi-Chih Lin, Tai-Shuan Lai, Shuei-Liong Lin, Yung-Ming Chen, Tzong-Shinn Chu and Yu-Kang Tu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pdf-2-taj-10.1177_2040622321998860 for Outcomes of coronavirus 2019 infection in patients with chronic kidney disease: a systematic review and meta-analysis by Yi-Chih Lin, Tai-Shuan Lai, Shuei-Liong Lin, Yung-Ming Chen, Tzong-Shinn Chu and Yu-Kang Tu in Therapeutic Advances in Chronic Disease