Abstract
The novel human coronavirus disease (COVID-19) has been associated with vascular and thrombotic complications, some of which may result from endothelial dysfunction, including the posterior reversible encephalopathy syndrome (PRES). We report a case series of 8 patients with COVID-19 and PRES diagnosed at two academic medical centers between March and July of 2020. The clinical, laboratory and radiographic data, treatment, and short-term outcomes were retrospectively analyzed. The mean age was 57.9 ± 12 years, and 50% were women. Four patients had previous vascular comorbidities. All the patients suffered from severe pneumonia, requiring intensive care unit admission. Five patients were not hypertensive at presentation (all SBP < 127 mmHg). Neurologic symptoms included seizures in 7 patients; impaired consciousness in 5 patients; focal neurological signs in 3 patients; and visual disturbances in 1 patient. All patients underwent brain magnetic resonance imaging which indicated asymmetric T2 prolongation or diffusion changes (50%), extensive fronto-parieto-occipital involvement (25%), vascular irregularities (12.5%) and intracranial hemorrhage (25%). Four patients were treated with tocilizumab. Three patients were discharged without neurologic disability, 2 patients had persistent focal neurologic deficits and 2 expired. One patient’s prognosis remains guarded. Together, these data support the relationship between PRES and endothelial dysfunction associated with severe COVID-19. In patients with severe COVID-19, PRES can be triggered by uncontrolled hypertension, or occur independently in the setting of systemic illness and certain medications. Like other infectious processes, critically ill patients with COVID-19 may be at greater risk of PRES because of impaired vasoreactivity or the use of novel agents like Tocilizumab.
Keywords: RES, Posterior reversible encephalopathy syndrome, COVID, SARS-CoV-2, Endothelial dysfunction, Systemic inflammatory response syndrome
1. Introduction
Since Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified in Wuhan (China) at the end of 2019, over one hundred million people have been diagnosed with coronavirus disease 2019 (COVID-19), with more than two million deaths to date [1].
The majority of COVID-19 patients present with asymptomatic or mild infection, developing symptoms like fever, cough, myalgias and headache. Pneumonia is the most frequent serious manifestation of the disease, with acute respiratory distress syndrome being a major complication in severe cases [2], [3]. Neurological symptoms have been observed in over one-third of severe COVID-19 patients [4], [5], some of which have been attributed to the systemic involvement of the disease, including headache, dizziness, myalgias, or even cerebrovascular diseases that might have been triggered by a hypercoagulable state. However, other more specific manifestations have been observed, like hyposmia and hypogeusia, polyneuropathy, encephalitis, and encephalopathy that raise the possibility of direct neural invasion of SARS-CoV-2 [6].
Various reports of neurological manifestations of previous coronavirus epidemics provide a roadmap regarding potential neurological complications, but the pathophysiology of neurologic damage caused by SARS-CoV-2 is still being studied. Neurotropism of SARS-CoV-2 is not yet defined, with several theories being explored at the moment. It has been suggested that the virus might enter the brain through the transcribial route described in other CNS pathogens in the past, or by leveraging the ACE-2 receptors on endothelial and glial tissue [6], which would justify the cerebral edema detected in some COVID-19 patients [7].
In this case series we sought to review one manifestation of endothelial dysfunction associated with COVID-19, the posterior reversible encephalopathy syndrome (PRES).
2. Patients and methods
We report a case series of 8 patients diagnosed during the pandemic, from March to July 2020, with severe COVID-19 infection and PRES at Vall d'Hebron University Hospital, Barcelona, and Cooper University Hospital, Camden.
All patients had a positive SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR) test on a nasopharyngeal specimen at the moment of developing PRES, as well as COVID-19 symptomatology. Pneumonia was defined as severe if the patient had SpO2 < 94% on room air, respiratory rate > 30, PaO2/FiO2 < 300 mmHg or lung infiltrates > 50% on chest radiograph [8]. In addition to pulmonary disease, these patients all experienced septic shock and/or multiple organ dysfunction. Patients met clinical and/or radiographic criteria for PRES [9], with acute neurological symptoms in the appropriate clinical context (i.e. in the presence of pronounced hypertension, renal failure, severe infection), and brain imaging (brain CT or MRI) that revealed vasogenic edema.
The following groups of variables were collected and retrospectively analyzed: demographic data, clinical data related to PRES and COVID-19, laboratory parameters and radiological findings. Statistical analysis was performed using SPSS software ver 25.0. Categorical variables were presented as absolute values and percentages and continuous variables as means (+/− standard deviation (SD)) as indicated.
3. Results
We present 8 patients with radiographically confirmed PRES (4 at each site), who were identified out of a series of 2812 consecutively admitted patients with laboratory-confirmed COVID-19 (2.8 per 1,000 COVID-19 admissions). Four of them were women (50%), with a mean age ± SD at presentation of 57.9 ± 12 years. Four patients (50%) did not have any relevant medical history, with a mean age of 51 years. The other 4 patients (50%), with a mean age of 64.7 years, had a medical history of cardiovascular diseases and other conditions (Table 1 ).
Table 1.
Demographic and clinical data of patients with PRES and COVID-19.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | ||
|---|---|---|---|---|---|---|---|---|---|
| Age (years), sex | 49, F | 36, F | 66, M | 53, M | 55, F | 70, M | 66, F | 68, M | |
| Comorbidities | -None | -None | -None | -None | -HBP –DLP –DM –Obesity –CKD -PV | -DM -Liver transplant rejection (Tacrolimus) | -HBP –DLP –DM -DVT | -HBP –DLP –DM –OSA -Obesity | |
| Time from COVID-19 to PRES (days) | 17 | 9 | 22 | 44 | 39 | 1 | 48 | 70 | |
| COVID-19 specific treatment | 1.Lopi/Rito 2.Chloroquine 3.Corticosteroids 4.Tocilizumab 5. Not received | 1.Lopi/Rito 2.Chloroquine 3.Corticosteroids 4.Tocilizumab 5. Not received | 1.Not received 2.Chloroquine 3.Corticosteroids 4. Not received 5. Not received | 1.Lopi/Rito 2.Chloroquine 3. Not received 4.Tocilizumab 5.Convalescent plasma | 1.Lopi/Rito-Daru/Cobi 2.Chloroquine 3. Not received 4. Not received 5. Not received | 1. Not received 2. Not received 3. Corticosteroids 4. Not received 5. Not received | 1.Lopi/Rito 2.Chloroquine 3.Corticosteroids 4.Tocilizumab 5. Convalescent plasma | 1. Not received 2. Not received 3. Corticosteroids 4. Not received. 5. Not received | |
| COVID-19 complications | -Septic shock Bacterial superinfection | -Fungal superinfection | -Septic shock -Bacterial superinfection | -Septic shock-Bacterial superinfection | -Septic shock-Bacterial superinfection | -Bacterial superinfection | -Bacterial superinfection | -Septic shock-Bacterial superinfection | |
| Acute hypertension at PRES (SBP) | –No (120 mmHg) | –No (116 mmHg) | –No (127 mmHg) | –No (123 mmHg) | –No (110 mmHg) | -Yes (167 mmHg) | -Yes (210 mmHg) | -Yes (218 mmHg) | |
| Renal function at PRES (Crea mg/dL) | -Normal (0,5) | -Normal (0,56) | -AKI (4,77) | -AKI (2,64) | -CKD (2,96) | -Normal (1,06) | -AKI (1,34) | -AKI (9,38) | |
| Laboratory Findings | IL-6 (pg/mL) | 159 | 13,4 | N/A | N/A | 148 | 135 | N/A | N/A |
| Ferritin (ng/mL) | 1020 | 605 | 1470 | 1370 | 2360 | 444 | 1687 | 92 | |
| D-Dimer (ng/mL) | 4430 | 246 | 2100 | 5900 | 2044 | 660 | 1700 | 1700 | |
| Radiologic Findings | Location | Parieto-occipital | Hemispheric involvement | Parieto-occipital | Parieto-occipital | Parieto-occipital | Parieto-occipital | Parieto-occipital | Hemispheric involvement |
| Asymmetry | Asymmetric | Asymmetric | Symmetric | Symmetric | Asymmetric | Symmetric | Symmetric | Asymmetric | |
| Hemorrhage | No | Yes | No | No | Yes | No | No | No | |
| Vasoconstriction | Yes | No | No | No | No | No | No | No | |
| PRES symptoms | -Focal signs (paresis) -Visual disturbance | -Seizures-Impaired consciousness-Focal signs (Paresis) | -Seizures (status epilepticus) –Impaired consciousness | -Seizures-Impaired consciousness | -Seizures | -Seizures-Focal signs (Paresis) | -Seizures-Impaired consciousness | -Seizures (status epilepticus) -Impaired consciousness | |
| PRES treatment | Intra-arterial nimodipine | AEDs | AEDs | AEDs | AEDs | AEDs | AEDs | AEDs | |
| Clinical outcome | Focal sequel (paresis); discharged home after 38 days). | Focal sequel (paresis); discharged home after 60 days. | Remains guarded (stabilized and transferred to another acute hospital) | Neurologically asymptomatic; dischargedto a subacute nursing facility after 35 days. | Neurologically asymptomatic; discharged home asymptomatic after 87 days. | Exitus (respiratory failure) | Neurologically asymptomatic; discharged home asymptomatic after 9 days. | Exitus (status epilepticus) | |
M = Male; F = Female; HBP = High blood pressure; DLP = Dyslipidemia; DM = Diabetes mellitus; CKD = Chronic Kidney Disease; PV = Peripheral vasculopathy; DVT = Deep vein thrombosis; OSA = obstructive sleep apnea; AKI = Acute Kidney Disease.
Patients presented with the following comorbidities: high blood pressure (3 patients, 37.5%), dyslipidemia (3 patients, 37.5%), diabetes mellitus (2 patients, 25%), obesity (2 patients, 25%), obstructive sleep apnea (1 patient, 12.5%), peripheral vasculopathy (1 patient, 12.5%), deep vein thrombosis (1 patient, 12.5%). None of the patients had a history of stroke or ischemic heart disease. One patient had acute liver transplant rejection at the time of COVID-19, and was under immunosuppressive therapy with tacrolimus with a level of 8.0 ng/mL at the time of PRES.
All patients suffered from severe pneumonia, requiring intensive care unit admission. The patients transferred to the ICU required intubation, and 2 of them (25%) were proned. During their ICU stay, all patients (100%) developed superimposed nosocomial bacterial and/or fungal infections, and 6 patients (75%) developed septic shock requiring vasoactive infusions.
As a specific treatment for COVID-19, 6 patients (75%) received hydroxychloroquine, 6 patients (75%) received corticosteroids, 5 patients (62.5%) received antiretroviral agents (such as lopinavir-ritonavir and darunavir-cobicistat) and 4 patients (50%) received Tocilizumab. In 2 cases (25%) convalescent plasma was administered. All of the patients were treated with empiric antibiotic therapy for bacterial and/or fungal infections.
Serum markers of systemic inflammation were elevated in all of the patients: IL-6 mean value of 114 (SD 68) pg/mL, ferritin mean value of 1131 (SD 740) ng/mL, and D-dimer mean value of 2348 (SD 1897) ng/mL.
Acute hypertension was present in 3 patients (37.5%) with mean peak systolic blood pressure of 198 mmHg (167–218 mmHg) and mean peak diastolic blood pressure of 101 mmHg (92–111 mmHg). The 3 patients had vascular comorbidities, and 2 of them had chronic hypertension.
The mean time from COVID-19 diagnosis to PRES was 31 days (SD 23), varying from a mean of 23 days in patients without comorbidities recorded to a mean of 39.5 days for those with them. The most common clinical manifestations were seizures, which appeared in 7 patients (87.5%), with 2 patients evolving into status epilepticus. Altered mental status was present in 5 patients (62.5%), focal neurological signs in 3 patients (37.5%) and visual disturbance in 1 patient (12.5%). As per our institutional protocols, sedation is temporarily interrupted to permit frequent neurologic assessments of all patients, which permitted recognition of new neurologic symptoms and detailed examinations.
Regarding treatment of PRES, hypertensive patients were treated with afterload reduction [10] and antiepileptic drugs (AEDs) were used for seizure control. Immunosuppressive therapy was discontinued in one patient (patient 5 was receiving Tacrolimus due to liver transplant). One patient (patient 1) presented with focal signs (right hemianopsia and hemiparesis) within <4.5 h of evolution, without hypodensities or hemorrhages in the head CT (although CT angiography showed proximal stenosis in both posterior cerebral arteries). Given the initial suspicion of arterial ischemic stroke and the known relationship between COVID-19 and embolic stroke, intravenous thrombolysis was administrated. Diagnostic arteriography was performed immediately after, demonstrating the presence of luminal irregularities in the posterior cerebral arteries (Fig. 2.), and local intra-arterial nimodipine was administrated for three times, with clinical improvement in vessel caliber—suggesting vasospasm rather than atherosclerosis. Oral nimodipine therapy was administered over the next ten days.
Fig. 2.
Digital subtraction angiography demonstrates vasoconstriction towards posterior cerebral arteries in case 1.
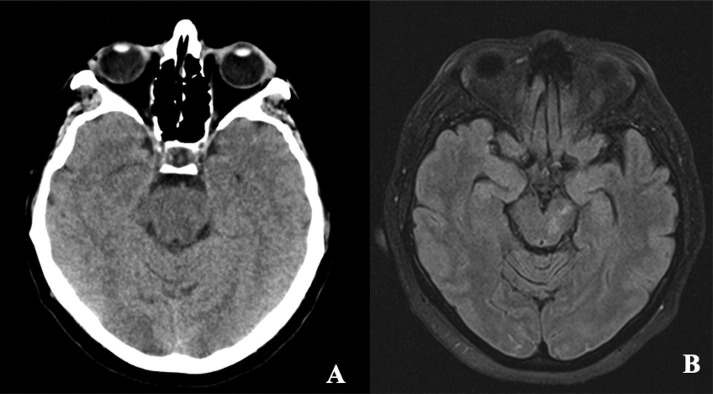
PRES was confirmed radiographically using brain CT (n = 3) or magnetic resonance imaging (n = 5) with characteristic findings in 7 patients. One patient demonstrated bilateral areas of T2/FLAIR hyperintensity with restricted diffusion in the subcortical white matter, with an atypical pattern for ischemia, but could have been consistent with hypoxic ischemic encephalopathy. However, this patient was not hypoxemic at any time during the ICU admission despite severe pneumonia. The most common location of the vasogenic edema was the parieto-occipital region (75%) with an extensive fronto-parieto-occipital involvement in 2 patients (25%) (Fig. 1 ). Lesions were asymmetric in 4 patients (50%). In one patient there was vasoconstriction associated with the vasogenic edema (Fig. 2 ), and hemorrhagic manifestations (microbleeds) were present in 2 patients (25%) (Fig. 3 ). Follow-up neuroimaging available for 4 patients based on availability and clinical stability of these patients, with significant resolution of the preexisting edema (Fig. 4 ).
Fig. 1.
Extensive asymmetric hemispheric involvement of the vasogenic edema in diffusion-weighted MRI (DWI) sequences on the left side and fluid-attenuated inversion recovery (FLAIR) sequences on the right side in cases 2 (A) and 8 (B).
Fig. 3.

Microbleeds in T2*-weighted gradient-echo MRI sequences in cases 2 (A) and 5 (B).
Fig. 4.
Case 1. Posterior hypodensities suggestive of vasogenic edema in brain CT (A) with resolution in control MRI FLAIR sequence (B).
The median length of stay at the hospital was 45.8 days (range 9–87) with 4 patients being discharged home and 1 discharged to a nursing facility. Three patients (37.5%) were neurologically asymptomatic at discharge and 2 patients (50%) had persistent focal neurologic signs, including moderate limb paresis. Two patients (25%) expired, one of whom died as a consequence of ongoing seizure activity, and another because of respiratory failure. In 1 patient, the short-term prognosis remains guarded as the patient was transferred to another hospital after stabilization.
4. Discussion
As a radiographic manifestation of many medical conditions, PRES is a heterogeneous disorder triggered by a variety of proximate causes [9], [11], [12]. The systemic illness associated with an inflammatory response to SARS-CoV-2, endothelial dysfunction, and comorbidities observed in patients with severe COVID-19 are only a few potential explanations for the development of PRES that have been reported in these patients [13], [14], [15], [16], [17], [18], [19].
PRES has been historically characterized by an impairment of brain blood vessel autoregulation after severe hypertension [9], or as a consequence of severe infections, inflammation, or vasotoxicity. A pre-existing autoimmune condition has been identified in as many as half of patients with PRES [9] suggesting a relationship with immune dysregulation. While hypertension is the most common primary cause of PRES, less than a half of our patients were severely hypertensive at the time of their neurologic manifestations (3/8 patients).
The endothelial dysfunction associated with COVID-19 [20] may also be a contributing factor for PRES and other vascular complications (e.g., myocardial infarction and ischemic stroke). Recent pre-clinical and autopsy studies support this hypothesis, as alterations of the microcirculation and vascular endothelial glycocalyx have been detected in patients with severe COVID-19 [21], [22]. The absence of severe hypertension in most patients in this case series, coupled with the significant level of systemic inflammation would support this theory.
Among patients with sepsis, the activation of the immune system can stimulate cytokine production, notably increasing levels of tumor necrosis factor α and vascular endothelial growth factor, thereby increasing the blood brain barrier permeability and promoting edema formation. This has been described particularly with sepsis [23], but also in viral infections like influenza A [24] or parainfluenza [25]. A similar complication may occur PRES associated with COVID-19. That said, most of our patients developed concomitant bacteremia or fungal infections, which may also have predisposed them to PRES independently from their SARS-CoV-2 infection.
In order to control this exaggerated immune response to COVID-19, Tocilizumab (an IL-6 antibody) has been utilized in severe cases with some improvement in severity of symptoms [26]. Cases of PRES during treatment with Tocilizumab have been reported [27], although the mechanism has not been well defined yet. Half of the patients in our case series were given Tocilizumab, 3 of whom lacked vascular comorbidities including hypertension (75%). It is possible that Tocilizumab may have contributed to this complication, and its use should be cautioned although PRES appears to be a rare complication of treatment.
Renal failure is also a significant risk factor for PRES [9], and it was a frequent comorbidity in this cohort of COVID-19 with PRES (50%). Along with the previously described risk factors for PRES, it is unlikely that renal failure alone would be responsible for this condition.
In summary, PRES remains a heterogeneous condition with many potential triggers in patients with COVID-19. The excess cytokine release and endothelial dysfunction associated with SARS-CoV-2 infection, coupled with renal insufficiency, uncontrolled hypertension and possibly treatment with immunomodulatory therapies may contribute to the development of PRES. Whether PRES is a more common complication in COVID-19 than in other critical illnesses should be confirmed in larger observational cohort studies, or by pooling of published data.
There are several limitations of our case series, most importantly the small number of cases. Additionally, not all the patients underwent MRI at the time neurologic symptoms began to manifest, which would have provided more information than CT. Also, the lack of follow-up neuroimaging in half of these patients limits our understanding as to the temporal resolution of these changes. Larger studies are required to really understand the relationship between COVID-19 and PRES.
5. Conclusions
PRES appears to be a rare but serious complication of severe COVID-19, with associated seizures and focal neurologic deficits. As SARS-CoV-2 may cause more severe disease in patients with vascular comorbidities and may lead to other complications (e.g., nosocomial infections, multi-organ dysfunction), it stands to reason that PRES may be a complication of these collateral effects of the infection rather than a consequence of direct viral invasion into the nervous system. Coupled with the impaired vasoreactivity [6] and side effects of aggressive treatments (e.g., Tocilizumab), these risk factors put patients with COVID-19 at greater risk of conditions like PRES. Despite this biologic plausibility, there remain no convincing data that patients with COVID-19 are at any greater risk of PRES than patients with other systemic illnesses.
PRES is likely to be an underappreciated complication of critically ill patients with COVID-19 given the difficulties with examining intubated/sedated patients, the prolonged hospital course of patients with severe disease, and the reduction in use of magnetic resonance imaging [27]. As many patients with PRES have clinical and/or electrographic seizures, we would encourage providers to heighten their awareness for PRES as a treatable complication in this at-risk population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
There is adherence to ethical guidelines. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr. Siegler serves as a consultant for Ceribell and has no competing financial interests.
References
- 1.Coronavirus disease (COVID-19) Pandemic. Geneva: World Health Organization. 2020. https://www.who.int/emergencies/diseases/novel- coronavirus-2019. Accessed July 31, 2020 n.d.
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus. 2020;12 doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harapan B.N., Yoo H.J. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) J Neurol. 2021;2 doi: 10.1007/s00415-021-10406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 7.van den Enden A.J.M., van Gils L., Labout J.A.M., van der Jagt M., Moudrous W. Fulminant cerebral edema as a lethal manifestation of COVID-19. Radiol Case Reports. 2020;15(9):1705–1708. doi: 10.1016/j.radcr.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Management of COVID-19. Coronavirus Disease COVID-19. NIH. Last Updated: June 11, 2020. https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19. Accessed August 26, 2020. n.d.
- 9.Fugate J.E., Rabinstein A.A. Posterior reversible encephalopathy syndrome: Clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–925. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 10.Hassinger AB, Goodman DM. Hypertensive emergencies. Pediatr Crit Care Med Vol 2 Respir Cardiovasc Cent Nerv Syst 2014;356:523–31. https://doi.org/10.1007/978-1-4471-6356-5_32.
- 11.Bartynski W.S. Posterior reversible encephalopathy syndrome, Part 2: Controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol. 2008;29(6):1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer M., Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017;264(8):1608–1616. doi: 10.1007/s00415-016-8377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschi A.M., Ahmed O., Giliberto L., Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 Infection. Am J Neuroradiol. 2020;41(7):1173–1176. doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishfy L., Casasola M., Banankhah P., Parvez A., Jan Y.J., Shenoy A.M., et al. Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe Covid-19. J Neurol Sci. 2020;414:116943. doi: 10.1016/j.jns.2020.116943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J. Rogg A. Baker G. Tung 22 2020 100808 10.1016/j.inat.2020.100808
- 16.Parauda S.C., Gao V., Gewirtz A.N., Parikh N.S., Merkler A.E., Lantos J., et al. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020;416:117019. doi: 10.1016/j.jns.2020.117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordoñez-Boschetti L., Torres-Romero C.M., Ortiz de Leo M.J. Associated posterior reversible encephalopathy syndrome (PRES) to SARS-CoV-2. Case report. Neurologia. 2020;35:696–698. doi: 10.1016/j.nrl.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Princiotta Cariddi L., Tabaee Damavandi P., Carimati F., Banfi P., Clemenzi A., Marelli M., et al. Reversible Encephalopathy Syndrome (PRES) in a COVID-19 patient. J Neurol. 2020;267(11):3157–3160. doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doo F.X., Kassim G., Lefton D.R., Patterson S., Pham H., Belani P. Rare presentations of COVID-19: PRES-like leukoencephalopathy and carotid thrombosis. Clin Imag. 2021;69:94–101. doi: 10.1016/j.clinimag.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A. Huertas D. Montani L. Savale J. Pichon L.y. Tu F. Parent et al. 56 1 2020 2001634 10.1183/13993003.01634-2020 10.1183/13993003.01634-2020.Shareable1
- 21.Yamaoka-Tojo M. Vascular endothelial glycocalyx damage in COVID-19. Int J Mol Sci. 2020;21:1–22. doi: 10.3390/ijms21249712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovas A., Osiaevi I., Buscher K., Sackarnd J., Tepasse P.-R., Fobker M., et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartynski W.S., Boardman J.F., Zeigler Z.R., Shadduck R.K., Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. Am J Neuroradiol. 2006;27:2179–2190. doi: 10.1016/s0098-1672(08)70231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartynski W.S., Upadhyaya A.R., Boardman J.F. Posterior reversible encephalopathy syndrome and cerebral vasculopathy associated with influenza a infection: Report of a case and review of the literature. J Comput Assist Tomogr. 2009;33(6):917–922. doi: 10.1097/RCT.0b013e3181993a43. [DOI] [PubMed] [Google Scholar]
- 25.Ogunneye O., Hernandez-Montfort J.A., Ogunneye Y., Ogu I., Landry D. Parainfluenza virus infection associated with posterior reversible encephalopathy syndrome: A case report. J Med Case Rep. 2012;6:1–5. doi: 10.1186/1752-1947-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegler J.E., Heslin M.E., Thau L., Smith A., Jovin T.G. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020;29(8):104953. doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed] [Google Scholar]