Abstract
SARS-CoV-2 has a high risk of outbreak in long-term skilled nursing facilities (SNF). Coronavirus disease (COVID-19) has high mortality rates among the elderly with chronic health conditions. Following identification of COVID-19 index case in a SNF, serial point-prevalence was implemented with reverse transcription–polymerase chain reaction (RT-PCR) and immunochromatographic assays. Active surveillance and early isolation of infected patients were implemented. Out of 23 SNF residents and 26 healthcare workers (HCW), 18 (78%) and 12 (46%) tested positive for SARS-CoV-2, respectively. High proportion (38%) of positive patients were asymptomatic and RT-PCR was positive up to six days before symptoms. Five (21.74%) residents were hospitalized with COVID-19, and 2 (9%) died; only 1 (4%) HCW needed to be hospitalized and no staff members died. Active surveillance helped COVID-19 control and management in a SNF. Testing symptomatic individuals only may fail to identify and isolate all persons contributing to transmission. In high-risk elderly, only symptoms screening may not be enough for outbreak control.
Keywords: Outbreak, 2019 novel coronavirus disease, COVID-19, SARS-CoV-2, Long-term care facilities, Skilled nursing facility
Introduction
On December 2019, in Wuhan, China, a group of patients with cough, fever, and dyspnea was diagnosed with pneumonia from an unknown cause that later was identified as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection.1, 2, 3
This new infection, called Coronavirus Disease 2019 (COVID-19), encompasses a broad spectrum of clinical manifestations ranging from asymptomatic to severe respiratory disease with multi-organ failure and death.2, 4, 5 Due to widespread global transmission of COVID-19, the World Health Organization declared it to be a pandemic on March 11, 2020.5 The first case in Brazil was identified on February 26 in a resident of São Paulo city who had traveled to Italy (Lombardy region) in early February 2020.6
SARS-CoV-2 infection has caused a high rate of morbidity and mortality among older adults. Therefore, long-term care facilities, such as skilled nursing facilities (SNF), are vulnerable to SARS-CoV-2 transmission and COVID-19 outbreaks. Additionally, advanced age and multiple comorbidities of their residents can be associated with poor outcomes.7, 8, 9
On April, a staff member of a SNF in middle-sized city in Sao Paulo State, Brazil, tested positive for COVID-19 and was hospitalized. One week later, three residents of the facility had respiratory symptoms and an outbreak of COVID-19 was identified. Herein we report a successful outbreak investigation and management, based on serial point-prevalence for early detection and control.
Material and methods
Study design
This was an observational, cross-sectional study conducted at the city of Serrana, SP, from April 4th to May 14th, 2020.
Study population
The SNF has four bedrooms with five beds and one bedroom with three beds (total, 23 beds) and an area for collective meals and activities. During the outbreak, 23 long-term residents were living at the SNF, and 26 healthcare workers (HCW) were working there in different shifts.
Description of the index case and outbreak
On April 13, 2020, a 39-year-old female nurse working at long-term SNF was admitted to the Serrana State Hospital, SP, Brazil, with fever, cough, malaise, headache, and anosmia. She tested positive for SARS-CoV-2 on April 14 and was defined as the index case. Symptom onset was on April 9 and the last day she worked in the long-term SNF was April 8. She received support therapy with no need for oxygen supplementation and was discharged five days after admission.
Surveillance and control measures against SARS-CoV-2 infection were triggered, which were focused on early identification of symptomatic and asymptomatic cases and contact tracing. Staff were trained on the correct use of personal protective equipment (PPE) during the care of the residents (masks, goggles, gowns, and gloves). Masks were recommended during the entire work shift, and visits and group activities were canceled.
Residents and HCW completed a questionnaire about COVID-19 symptoms in the preceding 14 days. Every six hours, HCW evaluated the residents for symptoms and fever. HCW also were screened daily at the beginning of each shift, and workers with symptoms were referred to seek medical attention. Professionals who worked in another health institution in contact with COVID-19-suspected patients were refrained from their activities at the SNF.
Between April 18 and 20, three residents presented signs and symptoms of COVID-19 and were admitted to the Serrana State Hospital for evaluation. They tested positive for SARS-CoV-2 on April 20.
Prevalence surveys
After COVID-19 confirmation in the three additional residents, a more active intervention was performed with testing of all employees and residents. The first survey included a symptom questionnaire, comorbidities assessment and SARS-COV-2 testing using reverse transcription polymerase chain reaction (RT-PCR) and immunochromatographic assay methods.
All individuals testing positive for COVID-19 were isolated for 14 days and inquired daily for COVID-19-related symptoms (cough, shortness of breath, sore throat, and other respiratory and systemic symptoms), according to local and Federal protocols. Positive residents were isolated in separate rooms in a local hospital, and positive HCW were isolated in their house. Three weeks later, residents and HCW were reassessed with RT-PCR and serological tests.
Residents were classified as symptomatic if they had symptoms at the date of testing. Those who developed symptoms during the next seven days were classified as presymptomatic. Asymptomatic was defined as residents who did not develop symptoms in the following seven days.
Laboratory testing
Residents and HCW were submitted to RT-PCR and serological testing twice, three weeks apart, on April 21–22 and May 14. Nasopharyngeal and oropharyngeal swabs were collected, stored in a sterile tube with viral transport media between 2 °C and 8 °C, and processed following the CDC guideline for detection of SARS-CoV-2.10
Immunochromatographic assays identified specific IgM and IgG antibodies against SARS-CoV-2 and were performed with a commercial kit, following manufacturer’s instruction (Asan Easy Test® COVID-19 IgM/IgG, Asan Pharmaceutical Co.).
Statistical analysis and ethical aspects
Descriptive analysis of the variables was expressed as absolute values, percentage or median (range). The local research ethics committee approved this analysis as a public health investigation and surveillance and waived the requirement for informed consent (CAAE: 38868820.3.0000.5440).
Results
Molecular and serological evaluation for SARS-CoV-2 as well as symptom questionnaires were completed for all residents and staff (49 individuals). The median age of residents was 75.3 years (range: 62–98) and 44.3 years (range: 26–74) of HCW; 57% residents and 77% HCW were women. Most (96%) residents had chronic underlying health conditions, with hypertension (48%), cognitive impairment (39%), diabetes mellitus (22%), and cerebrovascular disease (22%) being most common. Among HCW, hypertension (31%), obesity (19%) and asthma (11%) were the most common underlying diseases (Table 1).
Table 1.
Demographic, clinical characteristics and reported symptoms of residents and healthcare workers in a long-term skilled nursing facility, Serrana, SP, Brazil, April 2020.
| Characteristic | Residents (N = 23) | Health care personnel (N = 26) |
|---|---|---|
| Median age (range) — yr | 75.3 (62–98) | 44.3 (26–74) |
| Sex — no. (%) | ||
| Male | 10 (43.48%) | 6 (23.08%) |
| Female | 13 (56.52%) | 20 (76.92%) |
| Hospitalized — no. (%) | ||
| Yes | 5 (21.74%) | 1 (3.85%) |
| No | 20 (86.96%) | 25 |
| Died — no. (%) | ||
| Yes | 2 (8.70%) | 0 |
| No | 21 (91.30%) | 26 (100%) |
| Chronic underlying conditions — no. (%) | ||
| Hypertension | 11 (47.83%) | 8 (30,77%) |
| Cognitive impairment | 9 (39.13%) | 0 |
| Diabetes mellitus | 5 (21.74%) | 2 (7,69%) |
| Cerebrovascular disease | 5 (21.74%) | 0 |
| Specific symptoms | ||
| Fever | 2 (8.70%) | 2 (7.70%) |
| Sore throat | 0 | 3 (11.54%) |
| Cough | 8 (34.78%) | 2 (7.70%) |
| Shortness of breath | 3 (13.04%) | 2 (7.70%) |
On the first evaluation, RT-PCR was positive in 12 (52%) residents and in 8 (31%) HCW; IgM and IgG antibodies were positive only in 6 (26%) residents, being 3 (13.04%) residents positive for serology and RT-PCR (Table 2).
Table 2.
Reverse transcription–polymerase chain reaction (RT-PCR) and immunochromatographic assays results of residents and health care workers in a long-term skilled nursing facility, Serrana, SP, Brazil, April and May 2020.
| Residents (N = 23) |
Health care personnel (N = 26) |
|||
|---|---|---|---|---|
| First evaluation | Three weeks later | First evaluation | Three weeks later | |
| RT-PCR | ||||
| Positive | 12 (52.17%) | 9 (39.13%) | 8 (30.77%) | |
| Serological | ||||
| IgM positive | 0 | 1 (4.35%) | 0 | 2 (7.69%) |
| IgG positive | 0 | 1 (4.35%) | 0 | 0 |
| IgM and IgG positive | 6 (26.09%) | 11 (47.83%) | 0 | 9 (34.62%) |
| IgM or IgG positive | 6 (26.09%) | 13 (56.52%) | 0 | 11 (42.30%) |
| Positive RT-PCR or serological | 15 (65.22%) | 18 (78.26%) | 8 (30.77%) | 12 (46.15%) |
Among residents with positive RT-PCR, 3 (25%) were symptomatic at the date of testing and 8 (67%) were presymptomatic. Only one resident with positive RT-PCR remained asymptomatic. One (13%) out of 8 HCW with a positive RT-PCR was symptomatic and the other 7 (87%) were presymptomatic.
After the first testing, state and local health departments provided training focused on the correct use of PPE, suspended visits, and reviewed environmental cleaning and disinfection practices. Persons positive for SARS-CoV-2 were isolated from other residents.
Three weeks later, 9 (39%) residents persisted with a positive RT-PCR for SARS-CoV-2, and IgM or IgG antibodies were detected in 13 (56%) residents and 11 (42%) HCW (Table 2). All residents and HCW diagnosed with COVID-19 only by a positive serological test were asymptomatic.
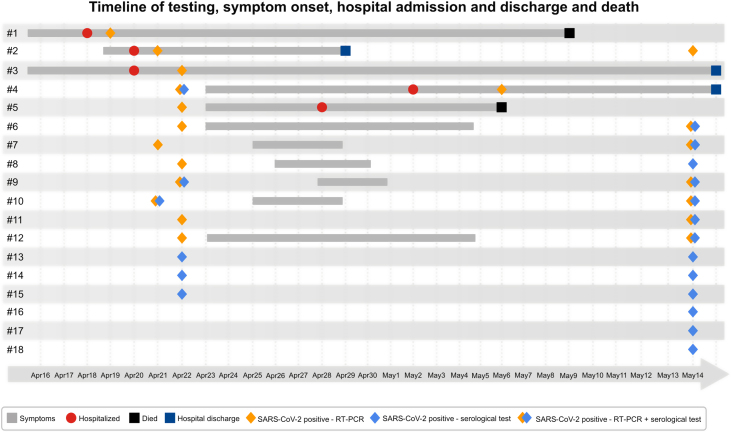
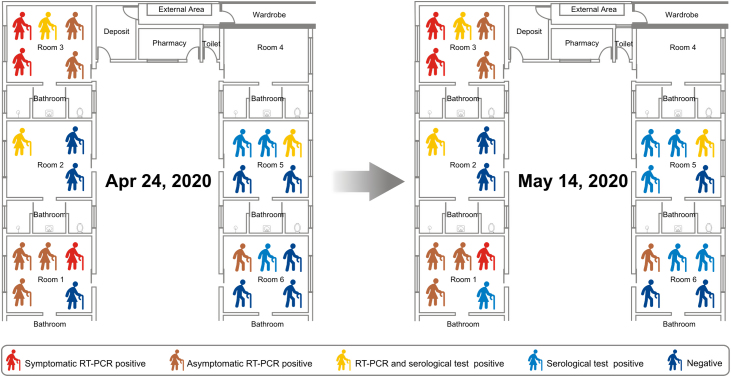
Daily symptoms onset was plotted on a timeline (Fig. 1) and positive laboratory results for each resident were entered according to their room (Fig. 2).
Fig. 1.
Timeline of testing, symptom onset, hospital admission and discharge and death among confirmed RT-PCR and/or serological test for SARS-CoV-2-infected residents.
Fig. 2.
Sketch of the skilled nursing facility (site of COVID-19 outbreak) with the distribution of residents in the rooms and test results.
Thus, out of 23 SNF residents and 26 HCW, 18 (78%) and 12 (46%) tested positive for SARS-CoV-2, respectively. Moreover, out of residents and HCW positive for SARS-CoV-2, 83% and 91% did not have any symptom on day of testing, respectively. Except for the room with accommodations for three residents, the majority of residents living in accommodations for five residents was affected by the COVID-19 (Fig. 2) showing the importance of crowding for SARS-CoV-2 transmission.
Clinical presentations ranged from mild to severe illness, mainly respiratory symptoms. The main symptoms were cough (63%) and diarrhea (37%), and the median time to symptom onset was three days (range: 1–6) (Fig. 1). Five (21.74%) residents were hospitalized with COVID-19, and 2 (9%) died; only 1 (4%) HCW needed to be hospitalized and no staff members died.
Median Ct values for the 12 RT-PCR positive residents was 22.46 (range: 15.51–36.52). Symptomatic, presymptomatic and asymptomatic residents had similar median CT values (17.84; 23.66; 19.41; respectively) at the time of diagnosis and during the course of the infection.
Discussion
SARS-CoV-2 has a significant risk of outbreak in SNF. In the present study we showed that early diagnosis and surveillance after exposure to infected persons with SARS-CoV-2 may help COVID-19 control and management. The majority of residents and HCW were asymptomatic at the time of testing, confirming that only symptoms screening may not be enough for outbreak control.11, 12, 13
Asymptomatic transmission of SARS-CoV-2 is a significant obstacle to prevent an outbreak. Unlike SARS-CoV-1, which replicates mainly in the lower respiratory tract,14, 15 SARS-CoV-2 replicates in upper respiratory tract, even in presymptomatic patients.2, 16 Additional strategies to diagnose asymptomatic people, especially high-risk older adults, might be necessary. Hellewell et al., using a mathematical model, showed that effective contact tracing and case isolation are important measures to control COVID-19 outbreaks.17 The combined diagnostic approach (serological test plus RT-PCR) increased sensitivity and helped identify the index case retrospectively and initiate appropriate preventive measures.
Similarly, previous studies have shown that SARS-CoV-2 in the elderly is often asymptomatic, delaying diagnosis and contributing to transmission.8, 9, 11, 18 In our study, Ct values were similar between asymptomatic and symptomatic residents, suggesting their similar potential for transmission.18 As suggested by Gandhi et al., mass testing should be implemented for residents and HCW, as they may behave as vectors to transmit respiratory viruses,19 and asymptomatic transmission might be responsible for significant number of positive cases.
In this study, at the first time-point of the investigation, 65% of residents and 30% of HCW were already positive for SARS-CoV-2, reinforcing the importance of asymptomatic transmission. Three weeks later, the second evaluation revealed a similar spread of infection in residents and HCW (increase of 12% in residents and 16% in HCW). There are several possible explanations for this. First, this survey occurred rapidly after the first HCW was hospitalized, enabling early identification and isolation of cases. Therefore, we reduced the time interval that asymptomatic residents were in contact with others. Second, positive residents were isolated in a separate room, thus reducing transmission. Finally, several measures, such as training of PPE usage, staff screening, and visits reduction were implemented. Interestingly, infection spread was lower in the room with less beds, reinforcing again that close contact is paramount for efficient SARS-CoV-2 transmission.
Although most residents had chronic and severe underlying conditions, only 22% needed to be hospitalized, and 9% died. In a similar study, Patel et al. evaluated 126 residents with RT-PCR and symptoms assessments, and demonstrated that 27.8% confirmed SARS-CoV-2, 10.3% were hospitalized, and 7.9% died.20 These results contrast with other studies. In Washington, Arons et al. reported that 64% of residents tested positive for SARS-CoV-2, with hospitalization and mortality rates of 45.5% and 33.7%, respectively.11 The differences observed in the disease transmission, severity, and death could be explained by residents' comorbidities, room occupancy in the facilities, the quality and timely fashion of contingency plans implementation.
This study highlights several important points in the management of COVID-19 outbreak. The need for adequate testing in asymptomatic persons, residents, and staff in long-term care and assisted living facilities, and the active surveillance for new cases. Testing based only on symptom screening might fail to identify and isolate all persons contributing to transmission.20 Another challenge to outbreak control is the lack of staff training and appropriate utilization of PPE. Adequate HCW training may help infection control.21 Hence, this study shows the importance of active surveillance and early isolation of residents positive for SARS-CoV-2 infections. It is our impression that if this active surveillance was not in place, the mortality rate among the residents of this SNF would have been much higher.
Our study has at least four limitations. First, although preventive measures were implemented rapidly, we evaluated residents and staff 13 days after the index case developed symptoms. Some asymptomatic persons might have had mild symptoms before testing. Second, 39% of residents had cognitive impairment, which may have interfered with symptom interpretation. Third, we were not able to ascertain all risk factors for COVID-19, this is particularly true for obesity as we did not investigate it objectively. Finally, although several measures, such as active surveillance, serial point-prevalence, and early isolation of infected patients, were implemented rapidly, it was an observational study.
The finding that SARS-CoV-2 has the potential to spread rapidly and widely into SNF underscores the importance of early identification and adequate outbreak management with prevention and control measures.
Funding
#2020/05367-3, São Paulo Research Foundation (FAPESP).
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 5.WHO . World Health Organization; 2020. Coronavirus disease (COVID-2019) situation reports. [Google Scholar]
- 6.Brazil . Ministério da Saúde; 2020. Painel coronavírus. [Google Scholar]
- 7.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roxby A.C., Greninger A.L., Hatfield K.M., Lynch J.B., Dellit T.H., James A., et al. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevention CfDCa . 2020. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) [Google Scholar]
- 11.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dora A.V., Winnett A., Jatt L.P., Davar K., Watanabe M., Sohn L., et al. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans — Los Angeles, California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:651–655. doi: 10.15585/mmwr.mm6921e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez G.V., Biedron C., Fink L.R., Hatfield K.M., Polistico J.M.F., Meyer M.P., et al. Initial and repeated point prevalence surveys to inform SARS-CoV-2 infection prevention in 26 skilled nursing facilities — Detroit, Michigan, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:882–886. doi: 10.15585/mmwr.mm6927e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S., et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan K.H., Poon L.L., Cheng V.C., Guan Y., Hung I.F., Kong J., et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 17.Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W., et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K., et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility — King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel M.C., Chaisson L.H., Borgetti S., Burdsall D., Chugh R.K., Hoff C.R., et al. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020;71:2920–2926. doi: 10.1093/cid/ciaa763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faíco-Filho K.S., Carvalho J.M.A., Conte D.D., de Souza Luna L.K., Bellei N. COVID-19 in health care workers in a university hospital during the quarantine in São Paulo city. Braz J Infect Dis. 2020;24:462–465. doi: 10.1016/j.bjid.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]