Abstract
Programmed cell death protein-1 (PD-1)/programmed death protein ligand-1 (PD-L1) inhibitors for treatment of a various types of cancers have revolutionized cancer immunotherapy. However, PD-1/PD-L1 inhibitors are associated with a low response rate and are only effective on a small number of patients with cancer. Development of an anti-PD-1/PD-L1 sensitizer for improving response rate and effectiveness of immunotherapy is a challenge. The present study reviews the synergistic effects of PD-1/PD-L1 inhibitor with oncolytic virus, tumor vaccine, molecular targeted drugs, immunotherapy, chemotherapy, radiotherapy, intestinal flora and traditional Chinese medicine, to provide information for development of effective combination therapies.
Keywords: oncolytic virus, cancer vaccine, molecular targeted therapy, immunotherapy, intestinal flora, Traditional Chinese Medicine
1. Introduction
Advances in immunotherapy have revolutionized cancer therapy
Programmed death receptor-1 [programmed cell death protein 1 (PD-1)] and programmed death protein ligand-1 [programmed death-ligand 1 (PD-L1)] inhibitors, have improved tumor therapy in cancer immunotherapy (1). The combination of PD-1 and PD-L1 inhibits the activity of T cells and act as the ‘brake’ of immunity, thereby preventing effector immune cells from killing cancer cells (2). Common PD-1/PD-L1 inhibitors in clinical used include Nivolumab, Pembrolizumab, Atezolizumab, Durvalumab and Avelumab (3). PD-1/PD-LI inhibitors block PD-1/PD-L1 pathway to restore normal immune function of T cells. Effector T cells play a role in recognizing and killing tumors (4). Various PD-1/PD-L1 inhibitors have been approved by the United States Food and Drug Administration (US FDA) for the treatment of various tumors (5). PD-1/PD-L1 inhibitors are characterized by high efficacy and fewer adverse events (6). However, PD-1/PD-LI inhibitors are associated with low response rate when used as a monotherapy with few patients meeting the treatment conditions and the high cost of treatment (7). In addition, immune regulatory signaling pathways are complex, so even those patients who are initially sensitive to PD-1/PD-L1 therapy may develop resistance or relapse. Therefore, there is need to develop approaches to improve sensitivity of PD-1/PD-LI inhibitors (8).
Tumor-related gene deletions and mutations are implicated in anti-PD-1/PD-LI resistance (9). For example, Janus kinase (JAK)1, JAK2 and β2 microglobulin mutations cause antigen presentation barriers, which induce CD8-infiltrated T cells to lose major histocompatibility complex (MHC) I and reduce sensitivity to IFN-γ (10). Mutation or activation of epidermal growth factor receptor (EGFR), T cell immunoglobulin mucin 3 (Tim-3), lymphocyte activation gene-3 (LAG-3), T cell Ig and ITIM domain (TIGIT) and other T cell depletion-related protein receptors results in a gradual loss of T cell proliferative potential and effector function, thus inducing drug resistance against PD-1 inhibitors (11–13). Immune checkpoint inhibitors (ICIs) inhibit checkpoints of the immune system rather than directly enhancing immune function. A single ICI is not effective in activating immune response. Therefore, there is a requirement to explore novel alternative strategies and personalized immunotherapy strategies through a combination of PD-1/PD-L1 inhibitors with small molecular targets, chemotherapy and radiotherapy to improve sensitivity to activated anti-tumor immune response and the response rate of patients and solve the bottleneck of drug resistance (14). The present study summarizes previous studies on the anti-tumor effects of PD-1/PD-LI inhibitors combination therapy to provide information for clinical and basic research (Fig. 1).
Figure 1.
Schematic diagram of combined treatment regimen for PD-1/PD-L1 inhibitors. PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1.
2. Combination of oncolytic virus (OVs) with PD-1/PD-L1 inhibitors
Tumor virus therapy induces immunogenic death on target cells and induces immune response by releasing pathogen-associated molecular pattern and damage-associated molecular pattern (15,16). As a result, tumor virus therapy improves the sensitivity of tumor cells to immunotherapy thus improving therapeutic effect. OVs mediates clearance of cancer cells or killing of cancer cells by targeting the tumor vascular system and inducing immunity (17). Talimogene laherparepvec, a herpes simplex virus expressing granulocyte-macrophage colony stimulating factor, was the first US FDA approved oncolytic therapy (18). Local intratumoral injection of the virus into tumors improves the overall survival rate of patients (19). A previous study (7) reported on the treatment of 21 patients with advanced melanoma with Talimogene laherparepvec combined with Pembrolizumab. The study report that therapy was well tolerated, with fatigue, fevers and chills as the common adverse events. The therapy showed no dose-dependent toxic reaction and an objective response rate (ORR) of 62%. Patients who responded to the combination therapy showed an increase number of CD8+T cells (7). Vaccinia virus is a highly immunogenic oncolytic immunotherapy vector (20,21). Previous studies report that vaccinia virus attracts effector T cells in mouse model of colorectal cancer and ovarian cancer (22,23). A combination of vaccinia virus with PD-L1 inhibitor enhances the infiltration of effector CD4+ and CD8+T cells and increases granzyme B, ICOS, perforin and IFN-γ, thus improving the survival rate (23).
PD-1/PD-L1 drug resistance is a main challenge, therefore, studies are required to explore novel approaches to improve immunogenicity of tumors and overcome resistance to immunotherapy (8). Rotavirus vaccine has immunostimulatory and anti-tumor effects (24). Administration of rotavirus in tumors overcomes drug resistance against PD-L1 inhibitors and has a synergistic effect with PD-L1 inhibitors. Heat- and UV-inactivated rotaviruses have no oncolytic activity but offer a synergistic effect with immune checkpoint-targeted antibodies through upregulation of the double-stranded RNA receptor retinoic acid-induced gene 1 (25). Rotaviruses have been used clinically and can be used for clinical sensitization of anti-PD-1/PD-L1 therapy (25) (Table I).
Table I.
Combination therapy of oncolytic viruses with PD-1/PD-L1 inhibitors.
| Author(s) (year) | Interventions | Primary end point(s) | Results | (Refs.) |
|---|---|---|---|---|
| Ribas et al, 2017 | Talimogene laherparepvec + Pembrolizumab | ORR | 62% | (7) |
| CD8+ T cells | Increased | |||
| Liu et al, 2017 | Vaccinia virus + Anti-PD-L1 | Tumor burden | Reduced | (23) |
| Survival rate | Improved | |||
| Granzyme B, Perforin, IFN-γ, ICOS, Effector CD4+ and CD8+T cells | Increased | |||
| Shekarian et al, 2019 | Rotavirus vaccine + Anti-PD-L1 | Tumor size | Reduced | (25) |
| Percent survival | Improved |
PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1.
3. Combination of cancer vaccine with PD-1/PD-L1 inhibitors
Tumor vaccine enhances immunogenicity and activates the immune system of the patient, thus controlling or eliminating tumors (26). DNA vaccine, a universal and personalized cancer treatment containing multiple new antigen coding sequences, is ideal for new antigen vaccination (27). DNA vaccine induces Cytotoxicity of CD8 T cells. A single dose of DNA vaccine combined with anti-PD-1 treatment significantly delays tumor growth in tumor-bearing mice inoculated with MC38 colon cancer cell line and some of the tumors are cleared completely, with a cure rate of 25%, and this indicates that tumor vaccine works synergistically with immune checkpoint blocking therapy (28).
Lmdd-MPFG vaccine promotes expression of PD-L1 in HCC cells but re-sensitizes tumor local T cell to respond to anti-PD-1 immunotherapy (29). Lmdd-MPFG vaccine activates NF-κB pathway and autophagy pathway in tumor-associated macrophages (TAMS). In addition, it converts M2 TAMS to M1 and induces the expression of antineoplastic factors, thus restoring T cell response to PD-1 inhibitors (29). Lmdd-MPFG vaccine acts synergistically with PD-1 inhibitors in treatment of liver cancer (29).
The tumor vaccine OVA @ Mn-DAP with nano-scale coordination polymer as a carrier, prepared from Mn2+ ions, Nod1 agonist and DAP as organic ligands, promotes maturation of dendritic cells and cross-presentation of antigens. Further, it prevents occurrence of B16-OVA tumors and works synergistically with PD-1 inhibitors to inhibit tumor growth (30).
Nivolumab combined with a multi-peptide vaccine (gp100, MART-1 and NY-ESO-1 with Montanide ISA 51 VG) was investigated as adjuvant therapy in resected stage IIIC and IV melanoma patients (31). The study findings showed that the treatment strategy was well tolerated. Common adverse events observed included fatigue, rash/pruritus, nausea/diarrhea, arthralgias and endocrinopathies. Although related grade 3 events occurred in 4 out of 33 patients, they were manageable. Notably, the combination therapy significantly increases CD8+ T-cell levels and decreases PD-1 expressing T-cells. In addition, significant increases in CD25+ regulatory T cells (Tregs)/CTLA4+/CD4+ T-cell populations are observed with anti-PD-1 therapy (31). These findings imply that synergistic activity of nivolumab and anti-PD-1 therapy is mediated through CTLA-4 and/or Tregs (32).
A previous phase I study (33) evaluated a vaccine that targets ≤20 predicted personal tumor neoantigens in patients with previously untreated high-risk melanoma following surgical resection. In that study, vaccine-induced polyfunctional CD4+ and CD8+ T cells targeted 58 (60%) and 15 (16%) of the 97 unique neoantigens used across patients, respectively. These T cells discriminated mutated antigens from wild-type antigens and recognized autologous tumors. Out of the six vaccinated patients, four showed no recurrence at 25 months following vaccination, whereas two showed recurrent disease and were subsequently treated with anti-PD-1 (pembrolizumab) therapy achieving complete tumor regression, with the expansion of a repertoire of neoantigen-specific T cells (33).
A previous study (34) explored the synergistic effect of a vaccine targeting HER2Δ16 on anti-PD-1 therapy in enhancing antitumor immunity in a model of advanced HER2+ breast cancer. HER2Δ16 is a critical oncogenic pathway and spontaneous tumors driven by HER2Δ16 are reflective of clinically advanced immunosuppressive HER2+ breast cancer. Endogenous HER2Δ16+ breast cancers show no response to anti-PD-1 as a single agent. Treatment with anti-PD-1 is not effective in increasing systemic anti-HER2 T-cell responses. However, combination of anti-PD-1 with Ad-HER2Δ16-KI significantly increases survival rate, with ~30% of mice exhibiting complete tumor regression and long-term tumor-free survival. These findings show that vaccinated mice are characterized by a high IFN-γ gene signature score. In addition, the results show that HER2Δ16 vaccination induces systemic adaptive immune responses and increases HER2-specific CD8 T cells that infiltrate into tumors. Therefore, addition of anti-PD-1 effectively induces HER2-specific T cells in TME (34) (Table II).
Table II.
Combination of cancer vaccines with PD-1/PD-L1 inhibitors.
| Author(s) (year) | Interventions | Primary end point(s) | Results | (Refs.) |
|---|---|---|---|---|
| Tondini et al, 2019 | DNA vaccine + Anti-PD-1 | Tumor growth | Delayed | (28) |
| Cure rate | 25% | |||
| Xu et al, 2020 | Anti-PD-1 + Lmdd-MPFG vaccine | Percent survival | Prolonged | (29) |
| Tumor volume | Retardation | |||
| TAMS | Converted M2 TAMS to M1 | |||
| PD-L1 | Promoted | |||
| Zhao et al, 2019 | OVA@Mn-DAP vaccine + Anti-PD-1 | Tumor-infiltrating lymphocytes | Increased | (30) |
| Tumor size | Inhibited | |||
| Percent survival | Prolonged | |||
| Gibney et al, 2015 | Nivolumab + A multi-peptide vaccine | CD8+/CD25+Treg/CTLA4+/CD4+ T-cells | Increased | (31) |
| PD-1 | Decreased | |||
| Crosby et al, 2020 | Ad-HER2D16-KI + Anti-PD-1 vs. Anti-PD-1 | Survival | Prolonged | (34) |
| IFN-γ | Increased |
PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1.
4. Combination of molecular targeting drugs with PD-1/PD-L1 inhibitors
Combined application of vascular endothelial growth factor (VEGF) and PD-1/PD-L1 inhibitors
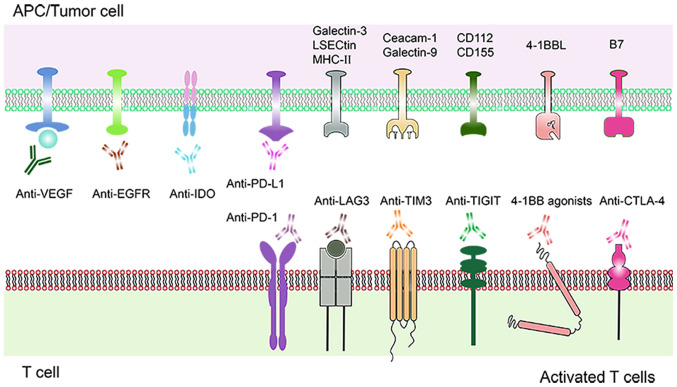
VEGF is an angiogenic factor that regulates the growth and survival of vascular endothelial cells, thereby causing immunosuppression (35) (Fig. 2). VEGF inhibitors are used to prevent angiogenesis and to promote differentiation of immune cells. Co-blocking of PD-1 and VEGF enhances efficacy of PD-1 inhibition (36). A clinical trial showed that the combination of VEGF and PD-1 inhibitors is effective in cancer treatment (NCT01472081) (37). Another study reported that PD-L1 inhibitors combined with VEGF receptor 2 (R2) small molecule inhibitors significantly downregulated the expression levels of PD-1 and PD-L1, and inhibited tumor growth by increasing tumor infiltrating lymphocytes (TILs) and decreasing Tregs and myeloid-derived suppressor cells (MDSCs) (38).
Figure 2.
Schematic diagram of a combination therapy comprising molecular targeting drugs and PD-1/PD-L1 inhibitors. Current and emerging molecular targeting drugs. Various molecular targets expressed on T cells and tumor cells are shown. Immune molecular targets such as PD-1, LAG-3, TIM-3, TIGIT, 4-1BB, CTLA-4, IDO bound to their respective specific antibodies, triggering a positive signal to T cells response. Inhibition of VEGF and EGFR mediated angiogenesis. PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1; LAG3, lymphocyte activation gene-3; TIM-3, T cell immunoglobulin mucin 3; TIGIT, T cell immunoreceptor with Ig and ITIM domains; CTLA-4, cytotoxic T-lymphocyte antigen-4; IDO, indoleamine 2,3-dioxygenase; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor.
Bevacizumab was the first antiangiogenic drug and vascular modulator used for clinical treatment of solid tumors (39). Bevacizumab binds to vascular endothelial growth factor A, blocks interaction of its receptor VEGFR-1/VEGFR-2, induces tumor vascular degradation and inhibits tumor growth. Bevacizumab confers immunomodulatory effects by inhibiting VEGF and promoting DC maturation (40). In addition, it reverses immunosuppression by increasing T cell infiltration. Furthermore, it enhances anti-tumor activity of PD-L1 antibody Atezolizumab (41). Phase III randomized controlled trials showed that Atezolizumab combined with chemotherapy and Bevacizumab improves progression-free survival (PFS) and overall survival (OS) of patients with metastatic NSCLC. Moreover, Bevacizumab monoclonal antibody increases sensitivity of Atezolizumab therapy (42) (Table III).
Table III.
Combination of molecular targeting drugs with PD-1/PD-L1 inhibitors.
| Author(s) (year) | Interventions | Primary end point(s) | Results | (Refs.) |
|---|---|---|---|---|
| Zhao et al, 2019 | PD-L1 inhibitors + VEGFR2 | TILs | Increased | (38) |
| small molecule inhibitors (apatinib) | TAMs, MDSCs, TGF-β, Tumor growth | Hindered | ||
| Decreased | ||||
| Survival | Prolonged | |||
| Reck et al, 2019 | Anti-PD-L1+ Bevacizuma + | PFS | 10.2 months vs. 6.9 months | (42) |
| Chemotherapy vs. Bevacizuma + Chemotherapy | OS | 13.3 months vs. 9.4 months | ||
| Haratani et al, 2017 | PD-1/PD-L1 inhibitors+ EGFR-TKIs | ORR | T790M-negative patients (24%) vs. T790M-positive patients (13%) | (48) |
| Yang et al, 2019 | Pembrolizumab + Erlotinib | ORR | 41.7% vs. 14.3% | (50) |
| vs. Pembrolizumab+ Gefitinib | PFS | 19.5 months vs. 1.4 months | ||
| Siu et al, 2017 | IDO1 inhibitor (BMS-986205) + Nivolumab vs. BMS-986205 | Safety | All treatment-related adverse events were grade 1/2 except three grade 3 toxicities | (52) |
| Zakharia et al, 2016 | IDO inhibitor (Indoximod) + Ipilimumab, Nivolumab or Pembrolizumab | ORR | 52% | (56) |
| Hamid et al, 2017 | IDO inhibitor (Epacadostat) + Pembrolizumab | ORR | 75% of melanoma and 4% of colorectal cancer | (57) |
| Huang et al, 2015 | Anti-LAG3 + Anti-PD-1 vs. Anti-PD-1 | Tumor clearance | 100% vs. 50% | (59) |
| Goding et al, 2013 | Anti-PD-L1 + anti-LAG-3 antibodies | Tumor area | Reduced | (60) |
| Sakuishi et al, 2010 | Co-blocking Tim-3 and PD-1 pathways | Tumor Size | Reduced | (67) |
| Friedlaender et al, 2019 | Co-blocking Tim-3 and PD-1 pathways | An ongoing phase I trials | Anti-tumor study of TIM3 and PD-L1 inhibitors is under way (NCT03099109; NCT02608268) | (71) |
| Davar et al, 2018 | Anti-Tim-3(TSR-022)+ anti-PD-1(TSR-042) | PR | 1 case of 11 evaluable patients with 100 mg dose vs. 3 cases of 20 evaluable patients with 300 mg dose | (72) |
| SD | 3 cases of 11 evaluable patients with 100 mg dose vs. 8 cases of 20 evaluable patients with 300 mg dose | |||
| Chauvin et al, 2015 | Anti-TIGIT+ anti-PD-1 vs. anti-TIGIT vs. anti-PD-1 | NY-ESO-1-specific CD8+ T cell | Anti-TIGIT+ anti-PD-1>anti- TIGIT/anti-PD-1 | (74) |
| Johnston et al, 2014 | Anti-TIGIT + anti-PD-L1 vs. anti-TIGIT vs. anti-PD-L1 | Tumor volume | Anti-TIGIT+ anti-PD-L1 <anti-TIGIT/anti-PD-L1 | (76) |
| Percent survival | Anti-TIGIT+ anti-PD-L1 >anti-TIGIT/anti-PD-L1 | |||
| Morales-Kastresana et al, 2013 | Combination of anti-4-1BB, anti-OX40 and anti-PD-L1 | Survival | Extended | (80) |
| Tumor-infiltrating lymphocytes | Increased | |||
| Tolcher et al, 2017 | 4-1BB (Utomilumab) + Pembrolizumab | Safety | Treatment-emergent adverse events were mostly grades1-2 | (83) |
| Activated memory/effector CD8+ T cells | Increased | |||
| Postow et al, 2015 | Nivolumab + Ipilimumab vs. Ipilimumab | ORR | 61% vs. 11% | (87) |
| The median reduction in tumor volume | 68.1% vs. 5.5% | |||
| Larkin et al, 2015 | Nivolumab + Ipilimumab vs. Ipilimumab vs. Nivolumab | PFS | 11.5 months vs. 2.9 months vs. 6.9 months, | (88) |
| Safety | Grade 3 or 4 adverse events: 55.0% vs. 27.3% vs. 16.3% | |||
| Omuro et al, 2018 | Nivolumab + Ipilimumab vs. Ipilimumab vs. Nivolumab | Tolerance | 80% vs. 70% vs. 90%, | (89) |
| Safety | Fatigue: 55% vs. 80% vs. 30% | |||
| Diarrhea: 30% vs. 70% vs. 10% |
PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1.
Combined application of EGFR and PD-1/PD-L1 inhibitors
EGFR is a transmembrane tyrosine kinase receptor, implicated in tumor cell proliferation, invasion and metastatic angiogenesis (43) (Fig. 2). EGFR tyrosine kinase inhibitor (EGFR-TKI) inhibits EGFR, reduces T cell apoptosis and increases production of IFN-γ (44). However, most patients develop acquired drug resistance following EGFR-TKIs treatment (45). Activation of EGFR pathway during tumorigenesis induces tumor immune escape mediated by PD-L1 (46). A previous study has explored the combination of PD-1/PD-L1 inhibitors and EGFR-TKIs for clinical use. Efficacy of PD-1/PD-L1 inhibitors combined with EGFR-TKIs in treatment of advanced EGFR mutant NSCLC has not yet been fully explored. Advanced patients with NSCLC and acquired tolerance to first or second generation of EGFR-TKIs should be treated with third generation of EGFR-TKIs before PD-1/PD-L1 inhibitors in case of a T790M mutation (47). In EGFR-TKIs-resistant EGFR mutant NSCLC, positive expression rate of PD-L1 in T790M negative patients was higher compared with that in T790M-positive patients (48). T790M negative patients were more sensitive to anti-PD-1 therapy after EGFR-TKIs treatment (48). Another clinical study reports that patients with advanced NSCLC and EGFR mutations show immune responses to PD-1/PD-L1 inhibitors following EGFR-TKIs pretreatment and chemotherapy (49).
However, a clinical study reports that EGFR inhibitors do not improve sensitivity to PD-1/PD-L1 inhibitors. Phase I/II clinical trials (NCT02039674; keynoteo-021) explored effect Erlotinib or Gefitinib combined with Pembrolizumab for treatment of advanced NSCLC patients with EGFR sensitive mutations. The results showed that combination of these drugs could not improve efficacy and showed no synergistic effect with Pembrolizumab in killing tumor cells (50) (Table III).
Combined application of indoleamine 2,3-dioxygenase (IDO) and PD-1/PD-L1 inhibitors
IDO is a rate-limiting enzyme that breaks down tryptophan, reduces the number and activity of CD8T cells and is implicated in immunosuppression (Fig. 2). Increase in IDO activity is associated with poor clinical efficacy of PD-1 inhibitors (51). A clinical trial on immunotherapy combined with IDO inhibitors showed high efficacy. A combination therapy of Bms-986205, a potent oral IDO1 inhibitor and Nivolumab resulted in grade 1–2 toxicities with the exception of 3 cases of grade 3 hepatitis, rash and hypophosphatemia (52). Phase II clinical trials of the effect of Indoximod, an IDO inhibitor on melanoma (NCT02073123) (53), pancreatic cancer (NCT02077881) (54) and castrated prostate cancer (NCT01560923) (55) are underway with promising results. The ORR of melanoma patients treated with Indoximod combined with Ipilimumab, Nivolumab or Pembrolizumab was 52% (56). Epacadostat, an oral drug targeting IDO pathway is in phase I/II clinical trials (NCT 02327078, NCT 02178722) for treatment of multiple malignant tumors. Preliminary results show that ORR for melanoma is 75 and 4% for colorectal cancer. A combination with Pembrolizumab is relatively safe, however 3% of patients stopped treatment due to adverse events (57) (Table III).
Combined application of LAG3and PD-1/PD-L1 inhibitors
LAG3 serves a protective role in autoimmunity through direct inhibition of T-helper (Th) cell response by MHCII. LAG3 has a negative regulatory effect on T cells. Continuous exposure of antigens in tumor microenvironment leads to sustained expression of LAG3 (58) (Fig. 2). LAG3 and PD-1 have synergistic effects, and a previous study has recently explored combined immunotherapy for LAG3 and PD-1 (12).
A combination of anti-LAG3 and PD-1 inhibitors yielded a 100% tumor clearance in an EG7 lymphoma model, whereas tumor clearance rate in mice treated with PD-1 inhibitors alone was 50% (59). Targeted inhibition of LAG3 and PD-1 showed significant tumor regression in B16-F10 recurrent melanoma model (60).
These findings show that LAG3 and PD-1 acts synergistically. Bispecific LAG3/PD1 antibodies are being developed to improve efficacy of PD-1 inhibitor monotherapy by inhibiting both LAG3 and PD-1 (61). BMS-986016 was the first anti-LAG3mAb to be developed. The first phase of I/IIa trial has been launched to evaluate efficacy of LAG3 inhibitors combined with Nivolumab in treatment of advanced malignant tumors (NCT01968109) (62). Merck conducted a phase I clinical trial of anti-LAG3 monoclonal antibody (MK-4280) to evaluate safety and tolerance of the drug (63). MK-4280 combined with PD-1 blocker (Pbrobrolizumab) is currently under clinical trial of 70 patients with metastatic solid tumors (NCT02720068) (58) (Table III).
Combined application of Tim-3 and PD-1/PD-L1 inhibitors
Overexpression of Tim-3 is positively associated with poor prognosis of lung, gastric, prostate and cervical cancer (64). Interaction between Tim-3 on effector T cells and Galectin-9 on tumor cells induces T cell apoptosis and suppresses immune response (Fig. 2). Blocking Tim-3 enhances T cell proliferation and immune function (65). Tim-3 is highly expressed in CD8 positive tumor infiltrating lymphocytes in solid tumor mice (66). Tim-3 (+) PD-1 (+) TILs is a severe failure phenotype, which does not proliferate to produce IL-2, TNF and IFN-γ (67). A previous study reported that blocking Tim-3 and PD-1 pathways effectively controls tumor growth through synergistic activity (67). A combination of Tim-3 inhibitor and PD-1 inhibitor in mice with lung cancer upregulates expression of TILs (68). Administration of PD-1 inhibitors only results in drug resistance promoting tumor progression. Co-administration with Tim-3 inhibitor restores anti-tumor effect and increases survival time (69,70). These findings imply that Tim-3 inhibitor may increase IFN-γ levels and increase T cell proliferation (13). Co-administration of Tim-3 and PD-1 shows synergistic effect on anti-tumor cells. An anti-tumor study on combination of Tim-3 and PD-L1 inhibitors is underway (NCT03099109 and NCT02608268) (71). Currently, only Phase I results have been reported (72) on Tim-3 antibodies (TSR-022) and PD-1 inhibitors (TSR-042) combination therapy. A total of 39 patients with NSCLC who were treated with PD-1 inhibitors were further treated with TSR-042, at a fixed dose of 500 mg combined with TSR-022 100 mg (14 cases)/3 weeks and 300 mg (25 cases)/3 weeks. Of the 11 patients who received a dose of 100 mg TSR-022, 1 case was partially responsive (PR) and 3 cases were stable disease (SD). For 20 patients who received a dose of 300 mg TSR-022, 3 cases were PR and 8 cases were SD. All reactions occurred in PD-L1 positive patients. Only 12 PD-L1 positive patients were analyzed, 4 were PR and 6 were SD (the 2 other patients were not specifically identified). The current dose was well tolerated. The disease control rate was 55% and the disease control rate was 83% in PD-L1 positive subgroups (72) (Table III).
Combined application of TIGIT and PD-1/PD-L1 inhibitors
TIGIT is a member of the immunoglobulin superfamily which is exclusively expressed in lymphocytes. When it binds to its ligand CD155, TIGIT inhibits T cell proliferation and IFN-γ production. Therefore, activation of TIGIT pathway induces tumor immune escape (73) (Fig. 2). Co-blocking of PD-1/PD-L1 and TIGIT pathways restores the function of failed CD8+T cells. In patients with melanoma, co-blocking of TIGIT and PD-1 increases the proliferation of CD8 TILs, cytogenesis and degranulation (74). In a mouse CT26 tumor model, co-inhibition of TIGIT and PD-L1 enhances CTL functions and restores CD8+T functions (75). Combination therapy induces tumor regression and tumor antigen-specific protective memory (76). TIGIT synergized with other co-suppressor molecules PD-1 and Tim-3 to inhibit effector T cell response and promote T cell dysfunction. Therefore, inhibiting TIGIT with PD-1 or Tim-3 may promote anti-tumor immunity and induce tumor regression (77). Phase I clinical trials are underway to evaluate the safety and efficacy of anti-TIGIT monoclonal antibodies (OMP-31M32; NCT 03119428) (78) (Table III).
Combined application of 4-1BB (CD137) agonists and PD-1/PD-L1 inhibitors
4-1BB (CD137) is an inducible costimulatory receptor. On binding to its ligand (4-1BBL), it triggers the proliferation and activation of immune cells (79) (Fig. 2). A combination of PD-1 inhibitors and 4-1BB agonists has a strong synergistic effect. The combination also exerts significant effects on mice cancer models with poor immunogenicity (80). Utomilumab (PF-05082566) is a human monoclonal antibody that stimulates 4-1BB (81). As an accelerator of the immune system, Utomilumab has been investigated in clinical research (82). A study has shown that the level of activated memory/effector CD8+T cells in peripheral blood increases following treatment with a combination of Utomilumab (0.45–5.0 mg/kg) and Pembrolizumab (2 mg/kg). The combination is safe and well tolerated, consistent with the expected side effects of Pembrolizumab alone (83). Urelumab (BMS-663513) was the first anti-4-1BB drug to enter clinical trials. Studies show that a combination use of Urelumab and Nivolumab is well tolerated. The overall response rate of metastatic melanoma was 47% (84) (Table III).
Combined application of cytotoxic T lymphocyte associated protein 4 (CTLA-4) and PD-1/PD-L1 inhibitors
Ipilimumab (Anti-CTLA-4) is an immunomodulatory monoclonal antibody that targets cell surface antigen CTLA-4 as an ICL (85) (Fig. 2). The use of CTLA-4 and PD-1 inhibitors, either as singly or as combinations, has been approved by US FDA for the treatment of metastatic melanoma (86).
In a phase II clinical study, the objective response rate of patients with advanced melanoma who received Nivolumab + Ipilimumab was significantly higher than that of patients who received Ipilimumab + placebo (61 vs. 11%). In the Nivolumab + Ipilimumab group, 22% of the patients showed complete response (87). A clinical study has shown that Nivolumab combined with Ipilimumab yields a PFS of 11.5 months, whereas the PFS of Ipilimumab or Nivolumab alone was 2.9 and 6.9 months, respectively. The probability of treatment-related grade 3 or 4 adverse events in Nivolumab group, Ipilimumab group and combination group was 16.3, 27.3 and 55.0%, respectively (88). Another clinical study has explored the safety and tolerance of Nivolumab with or without Ipilimumab in the treatment of recurrent glioblastoma. It has been reported that the tolerance of Nivolumab 3 mg/kg group exceeds that of Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg and Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg subgroups (90 vs. 70 vs. 80%, respectively). Fatigue and diarrhea were the most common treatment-related adverse events associated with the aforementioned drugs (30 vs. 80 vs. 55%; 10 vs. 70 vs. 30%, respectively) and no other side effects were observed. Tolerance to the combination was negatively influenced by the dose of Ipilimumab (89) (Table III).
5. Combination of chemotherapy with PD-1/PD-L1 inhibitors
Chemotherapy usually kills cancer cells by targeting their DNA synthesis and replication (90). It also promotes the presentation of tumor antigens following cancer cell death, activates tumor specific T cells, facilitates DCs maturation, stimulates type I interferon response and eliminates bone marrow-derived immunosuppressive cells (91). Appropriate combination of chemotherapeutic drugs and PD-1/PD-L1 inhibitors can enhance the efficacy of PD-1 blockers and produce a more sustained anti-tumor response, especially in tumors with poor immunogenicity and sensitivity to chemotherapy. A study has shown that Pembrolizumab combined with pemetrexed/carboplatin enhances improves symptoms of metastatic non-squamous NSCLC and has been approved by US FDA (92). Application of Pembrolizumab in combination with pemetrexed and platinum increases the PFS of metastatic NSCLC (93). For untreated patients with metastatic squamous NSCLC, the PFS and OS of Pembrolizumab combined treatment group versus the placebo group were 6.4 months vs. 4.8 months and 15.9 months vs. 11.3 months, respectively. The risk of death decreased by 36% and the risk of disease progression or death reduced by 44% in the Pembrolizumab combined treatment group (94) (Table IV).
Table IV.
Combination of chemotherapy or radiotherapy with PD-1/PD-L1 inhibitors.
| Author(s) (year) | Interventions | Primary end point(s) | Results | (Refs.) |
|---|---|---|---|---|
| Langer et al, 2016 | Pembrolizumab + Chemotherapy vs. Chemotherapy | ORR | 55% vs. 29% | (92) |
| The incidence of grade 3 or worse treatment-related adverse events | 39% vs. 26% | |||
| Gandhi et al, 2018 | Pembrolizumab + Chemotherapy vs. Placebo + Chemotherapy | Rate of Overall survival at 12 months | 69.2% vs. 49.4% | (93) |
| PFS | 8.8 months vs. 4.9 months | |||
| Paz-Ares et al, 2018 | Pembrolizumab + Chemotherapy vs. Placebo + Chemotherapy | PFS | 6.4 months vs. 4.8 months | (94) |
| OS | 15.9 months vs. 11.3 months | |||
| Deng et al, 2014 | Irradiation (IR) + Anti-PD-L1 vs. Anti-PD-L1 vs. IR | Tumor volume | 25.59±10.26 mm vs. 587.3±169.1 mm vs. 402.8±76.73 mm | (95) |
| The percentage of MDSCs in the total CD45+ cell population | 0.38±0.16% vs. 7.33±2.22% vs. 4.78±2.49% | |||
| Sharabi et al, 2015 | XRT + Anti-PD-1 | Tumor volume | Inhibited | (99) |
| T-cell infiltration | Increased | |||
| Dovedi et al, 2014 | RT + PD-1/PD-L1 blocking | Tumor volume | Inhibited | (101) |
| Percent survival | Improved | |||
| Ahmed et al, 2016 | Stereotactic radiation + Anti-PD-1 | local lesions control rates at 6 and 12 months | 91 and 85% | (102) |
| OS rates at 6 and 12 months | 78 and 55% |
PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1.
6. Combination of radiotherapy with PD-1/PD-L1 inhibitors
Radiotherapy (RT) has profound immunological effects. Basic research studies have demonstrated that RT can improve the efficacy of PD-1 inhibitors (95). Cancer cells can be killed by radiation. RT activates the immune system by triggering the release of tumor antigens (96). Basic research and clinical trials have revealed that RT synergizes with immunotherapy when applied together (97,98). A study has shown that PD-1 inhibitors combined with RT can activate CTLs and reduce immunosuppressive cells (99). A combination of RT and PD-1/PD-L1 inhibitors significantly improves the survival rate and reduces the tumor volume in mice (100). Compared with the control group, co-treatment of RT and PD-1 significantly increased the expression of PD-L1, CD8+T cells and interferon-γ in tumor cells (101). Clinical studies have provided evidence that anti-PD-1 therapy can significantly improve the control rate and OS rate of patients with melanoma brain metastasis who received stereotactic radiotherapy (102). Clinical trials of the efficacy of Nivolumab combined with RT in the treatment of NSCLC (NCT02768558) and glioblastoma (NCT02617589) are under way (103).
Immunotherapy amplifies the rare systemic effects of radiotherapy, while radiotherapy renders immune-excluded tumors quickly responsive to immunotherapy (104). MDSCs have been implicated in development of radioresistance. Accumulation of MDSC in the tumor microenvironment promotes tumor relapse by directly affecting tumor cell survival and indirectly affecting local T cell suppression (105). A combination of irradiation (IR) and anti-PD-L1 therapy enhanced the activation of CD8+ T cells and inhibition of TUBO tumor growth. CD8+ T cells induce the apoptosis of MDSCs through TNF-α following combination therapy (95). PD-L1 is upregulated in the tumor microenvironment following IR. IR-induced increases in tumor-infiltrating lymphocytes (TILs) and upregulation of PD-L1 could provide an opportunity for PD-L1 blockade (106). The combination of IR and anti-PD-L1 treatment optimizes the tumor immune microenvironment and results in tumor regression (95). Local radiotherapy significantly adds to the systemic efficacy of immunotherapy. Combining single-site stereotactic body radiotherapy (SBRT) with pembrolizumab improves response rates in metastatic NSCLC. PD-L1-negative patients benefited from SBRT (107). This study also suggested that the way to improve the effect of immunotherapy was to treat with local radiotherapy to synergize the local and systemic effects of both modalities. Immunotherapy increased the local effect of radiotherapy in all treated sites. Radiotherapy suppresses the tumor burden allowing immunotherapy better to eliminate micro-metastatic disease (108). In addition, a study demonstrated that higher single doses of RT from 12–18 Gy blunt the efficacy of anti-tumor immunity. They also reduce IFN-β production and abrogate DC-mediated CD8+T-cell priming, suggesting that RT doses below 12 Gy may be more immunogenic (109). Another study confirmed that a single dose of 15 Gy irradiation results in higher tumor immune cell infiltration than a fractionated (3 Gy × 5) schedule (110). The differences in the above results might be due to differences in the genetic backgrounds of mice, immune competence and immunogenicity of models and radiosensitivity of cell lines (111) (Table IV).
7. Combination of intestinal microflora with PD-1/PD-L1 inhibitors
Intestinal microflora influences the effects of immunotherapy in cancers. A previous study reported that oral Bifidobacterium can significantly decrease the growth rate of melanoma, promote the maturation of dendritic cells and production of IFN-γ and enhance the anti-tumor effect of PD-1 inhibitors (112). The abnormal composition of intestinal flora may affect the response of patients to cancer immunotherapy (113). Transplantation of fecal bacteria improved the anti-tumor effect of PD-1 inhibitors (114). A study has shown that the clinical response of PD-1 inhibitors is dependent on the relative abundance of Akkermansia muciniphila. Oral supplementation of Akkermansia muciniphila restores the efficacy of PD-1 inhibitors in an IL-12-dependent manner (115). In another study, intestinal microflora regulated the response of anti-PD-1 immunotherapy to melanoma patients (116).
Patients with abundant beneficial intestinal bacteria (Ruminococcaceae/Faecalibacterium) have improved antigen presentation, effector T cells function in peripheral and tumor microenvironment and strong anti-tumor immune response (117). By contrast, the intestinal harmful bacteria (Bacteroidales) weakened antigen presentation and impaired anti-tumor immune response (118,119). Response to PD-1 inhibitors is influenced by the composition of intestinal flora, but not to oral flora (120). Other studies suggest that patients with melanoma responsive to Nivolumab were rich in Fecalibacterium prausnitzii, Bacteroides thetaiotamicron, B. longum, C. aerofaciens and E. faecium. Patients who responded well to Pembrolizumab were rich in intestinal Dorea formicogenerans (121,122) (Table V).
Table V.
Combination of intestinal microflora with PD-1/PD-L1 inhibitors.
| Author(s) (year) | Interventions | Primary end point(s) | Results | (Refs.) |
|---|---|---|---|---|
| Sivan, 2015 | Bifidobacterium + Anti-PD-L1 | Tumor volume | Reduced | (112) |
| IFN-γ, DCs | Increased | |||
| Routy et al, 2018 | A. muciniphila + Anti-PD-1 vs. Anti-PD-1 | PR | 69% vs. 31% | (115) |
| SD | 58% vs. 42% | |||
| PD | 34% vs. 66% | |||
| Tumor size | A. muciniphila +Anti-PD-1< Anti-PD-1 | |||
| Frankel et al, 2017 | Ipilimumab + Nivolumab vs. Pembrolizumab | RECIST response | 67% vs. 23% | (121) |
| SD | 8% vs. 23% | |||
| Matson, 2018 | Fecal material from three responder patient donors + | IFN-γ, Tumor-infiltrating specific CD8+ T cells | R>NR | (122) |
| Anti-PD-L1(R) vs. Fecal material from three non-responder patient donors + Anti-PD-L1(NR) | Tumor volume | R<NR |
PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1.
8. Combination of Traditional Chinese Medicine with PD-1/PD-L1 inhibitors
Diosgenin is a natural steroidal saponin (123). A combination of diosgenin with PD-1 inhibitor suppresses tumor growth, increased T cell infiltration and IFN-γ expression in tumor tissues. Diosgenin stimulates the immune cells thereby improving the response rate and therapeutic effect of PD-1 inhibitors (124). Diosgenin treatment downregulates intestinal Bacteroidetes but upregulated Clostridiales, Lactobacillus and Sutterella (124).
Icariin possesses a variety of pharmacological and biological activities. Icaritin is now under clinical trial for the treatment of PD-L1 positive advanced liver cancer (NCT03236649) and advanced breast cancer (NCT01278810). Pre-clinal studies have shown that Icaritin can effectively reduce the tumor load of B16F10 melanoma and MC38 colorectal cancer in mice and its therapeutic effect is T cell-dependent. It increased CD8 T cell infiltration and the number of effector memory T cells. A combination of PD-1 inhibitor and Icaritin significantly suppressed tumor growth (125).
Rhus verniciflua Stokes (RVS) has been shown to contain a large number of bioactive phytochemicals, including alkaloids, polyphenols and flavonoids, which block the interaction between PD-1/PD-L1 and CTLA-4/CD80. Thus, RVS might be used as an immune checkpoint blocker (126).
Ganoderma lucidum reduces the proportion of PD-1 positive cells in B lymphocytes. It can, therefore, be used to develop a new type of immunomodulator for the prevention and treatment of cancer (127). The combination of Ganoderma lucidum and paclitaxel inhibits the expression of immune checkpoints (PD-1 and Tim-3) and restored TILs. The combination regulates the development of 4T1-breast cancer in mice (128) (Table VI).
Table VI.
Combination of Traditional Chinese Medicine with PD-1/PD-L1 inhibitors.
| Author(s) (year) | Interventions | Primary end point(s) | Results | (Refs.) |
|---|---|---|---|---|
| Dong et al, 2018 | Diosgenin + anti-PD-1 vs. diosgenin vs. anti-PD-1 | Mean tumor weigh | 1,980.00±861.22 mg vs. 3,203.33±641.43 mg vs. 2,530.00±584.04 mg | (124) |
| Hao et al, 2019 | Icariin + anti-PD-1 + anti- CTLA-4 vs. anti-PD-1 + anti- CTLA-4 | Average inhibition rates | 65% vs. 34.2% | (125) |
| Li et al, 2019 | Rhusverniciflua Stokes | The IC50 of blocking | 26.22 µg/ml | (126) |
| PD-1/PD-L1 interaction | ||||
| Wang et al, 2019 | Ganoderma lucidum | PD-1 | Decreased | (127) |
| Su et al, 2018 | Ganoderma lucidum + Paclitaxel | Tumor weight | Decreased | (128) |
| Tumor infiltration lymphocytes | Increased | |||
| PD-1, Tim-3 | Inhibited |
PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1.
9. Conclusion and future perspectives
The anti-tumor response rate of PD-1 inhibitors is low. Patients sensitive to PD-1/PD-L1 inhibitors develop drug resistance, tumor recurrence and disease progression and the mortality rate of patients with advanced tumor stages is high. A study has reported that patients with melanoma sensitive to anti-PD-L1 antibody treatment show increased levels of interferon-γ and related genes in blood prior to treatment (129). Anti-PD-1 therapy downregulates expression of IFN receptor-related genes and MHC I and upregulates inhibitory receptors on the surface of T cells (10). Furthermore, the inhibitory receptors inhibit the cytotoxic activity of T cells and these effects can be attributed to drug resistance against ICIs. Several basic and clinical studies are exploring effective combination and sequence of PD-1/PD-L1 inhibitors and other anti-tumor therapies to induce tumor cell immunogenicity and improve effectiveness of anti-tumor effect of PD-1/PD-L1 inhibitors. These advances will provide effective therapies for patients who are unresponsive to current treatment regimens. However, development of these combination therapies possesses several challenges.
Development of effective antineoplastic therapy should consider medical costs and adverse reactions for each treatment. Therefore, it is necessary to determine predictive biomarkers for individualized therapy, so as to predict efficacy and adverse reactions of PD-1/PD-L1 inhibitors. At present, some patients are not sensitive to PD-1/PD-L1 inhibitors. Lack of biomarkers for predicting response rate limits the effectiveness of clinical treatment strategies, thus there is need to screen novel biomarkers for predicting immunotherapy responses in patients.
In order to increase the proportion of patients benefiting from PD-1/PD-L1 inhibitors, studies should explore potential predictive biomarkers for anti-tumor treatment. PD-L1 expression is a potential biomarker for predicting effectiveness of PD-1/PD-L1 immunotherapy on patients with cancer thus identifying patients who may benefit from immunotherapy. Expression of PD-L1 is associated with several TILs and activated tumor antigen-specific T cells induces expression of PD-L1 (130,131). However, expression of PD-L1 in tumor tissues is heterogeneous and changes with tumor treatment (132,133). Several staining antibodies are used in immunohistochemical methods (IHC) to detect PD-L1 expression and the staining techniques (manual and automated) vary (134–136). Currently, effectiveness of PD-L1 detection as an anti-tumor immune response index is still controversial. The association between the expression of PD-1 or PD-L1 at the tumor site and disease outcome varies in patients with different tumors (137–139). Therefore, it is difficult to achieve consistent results with PD-L1 detection, hindering application of anti-PD-1/PD-L1 therapy as precision medicine.
TIL in tumor tissue demonstrates the presence of immune response by the body (140). TIL positive + PD-L1 positive group show improved PD-1/PD-L1 inhibitor immune response compared with TIL negative + PD-L1 positive group. This implies that the number of TIL can predict efficacy of PD-1/PD-L1 inhibitors (141). TIL mainly infiltrates into tumor nests, tumor stroma and tumor invasive margins of tumor tissues and different parts have different associations with therapeutic effects (142). Therefore, it is necessary to further determine the association between the quantity and quality of TIL and other infiltrating immune cells and tumor immune response. In addition, local radiotherapy is effective in inducing inflammation, which may benefit patients without sustained immune response (99). Radiotherapy should not be used in patients with significant tumor infiltration, as this may impair the ongoing immune response (143,144). Effects of different therapies on immune response should be considered when designing combination therapies with chemotherapy and targeted therapy.
Mismatch repair (MMR) is a set of susceptibility genes isolated from hereditary non-polyposis colorectal cancer. Mutations in these gene leads to loss of mismatch repair function, resulting in microsatellite instability (MSI) which is prone to tumors (145). Microsatellite instability high (MSI-H) attracts tumor-infiltrating lymphocytes (TILs) and upregulates PD-L1 expression in tumor epithelial cells (146). MMR deficiency (MMR-D) type solid tumors have more tumor neoantigens to enhance anti-tumor immune response and show an improved response to PD-1 monoclonal antibody, thereby improving immune suppression and restoring anti-tumor immunity (147). MMR-D is a predictor for anti-PD-D efficacy. However, MMR-D only occurs in a small number of patients. Further pre-clinical and clinical research should be performed before clinical application.
The therapeutic effect of PD-1 inhibitors is high in patients with a high mutation load of tumor mutation burden (TMB). Tumor cells with high TMB expression have higher levels of neoantigens, which stimulate a stronger anti-tumor immune response (148). TMB and PD-L1 have similar predictive function. However, TMB is not associated with PD-L1 expression. TMB is an important and independent predictive biomarker, which can predict the effectiveness of ICIs (149).
Further studies should explore ways to alleviate side effects of immunotherapy. Resistance of malignant tumors against PD-1/PD-L1 inhibitors can be overcome by use of combination therapy of PD-1/PD-L1 inhibitors (150). Notably, a combination of anti-PD-1/PD-L1 therapy is more effective compared with use of anti-PD-1/PD-L1 inhibitors alone. However, combination therapy is associated increased side effects (88). A study revealed that patients younger than 65 years old benefit more from nivolumab plus ipilimumab treatment than patients older than 65 years old. Therefore, combination therapies with ICIs should be carefully chosen for patients >65 years of age (151). Goals for treatment of patients with advanced cancer is usually palliative, prolonging survival, controlling symptoms and improving quality of life. Therefore, studies should explore combination therapies with ICIs and fully understand the toxic effects of immunotherapy, chemotherapy and radiotherapy to make sound treatment decision. Side effects such as immune disorders caused by ICIs are called immune-related adverse events. Common adverse reactions include diarrhea, fatigue, itching, rash, nausea and loss of appetite. Severe adverse reactions include severe diarrhea, colitis, myocarditis and cardiac insufficiency, liver dysfunction, pneumonia and glomerulonephritis (88,152,153). Serious side effects may require discontinuation of treatment, although patients may have an immune response thereafter. Intravenous corticosteroids or immunosuppressive drugs should be given if necessary. Some treatment-related autoimmune responses, such as rashes, are associated with improved prognosis (154). This implies that occurrence of adverse reactions is manifested by activation of immune system and represents action of PD-1/PD-L1 inhibitor, which eliminates tumors. There is an overlap between autoimmune reaction and anti-tumor immune reaction. Further studies should be performed to explore adverse drug reactions associated with immunotherapy.
Clinical application of molecular targeted drugs is associated with challenges such as acquired drug resistance and side effects which need to be minimized. Several studies are exploring the development of molecular targeted drugs with higher efficiency and fewer side effects (155,156). Studies exploring sequence, dosage and safety of PD-1 inhibitors and EGFR should be performed (157,158). Development of combination therapies will improve efficacy and reduce side effects of molecular targeted drugs. A number of clinical studies on combination therapies between chemotherapy and radiotherapy with immunotherapy are underway. An open-label, randomized phase 3 study showed that pembrolizumab+chemotherapy significantly improved OS in the total population. The data support the use of pembrolizumab+platinum +5-FU as new first-line standards of care for recurrent/metastatic head and neck squamous cell carcinoma (NCT0235803) (159). In the KEYNOTE-189 and KEYNOTE-407 studies (phase III), PFS and OS were significantly longer in patients treated with pembrolizumab and chemotherapy compared with those in patients treated with chemotherapy alone (93,94). Anti-PD-1 therapy enhances the efficacy of radiotherapy in metastatic gastric cancer treatment by increasing the CD8+ T cell/effector regulatory T cell ratio in TILs (160). Another study showed that patients with metastatic NSCLC treated with nivolumab or pembrolizumab+radiotherapy did not have increased grade 3/4 immune-related adverse events (161). The combination of chemotherapy and radiotherapy with PD-1/PD-L1 inhibitors induces lasting immune response in treatment of tumors when other treatment strategies fail.
Despite a significant number of basic and ongoing clinical trials aimed at improving effectiveness of combination therapies, intestinal flora combined with PD-1/PD-L1 inhibitors is a novel approach for cancer treatment. However, differences between basic and clinical trial results occur due to high variability of bacteria in intestinal tract and the effects produced by bacteria in the laboratory. Less diverse bacteria used in basic trials may not fully represent the complicated environment in the intestinal tract. Therefore, further studies should explore the mechanism of intestinal flora, side effects, optimal dosage and species for human use for development of effective combination therapies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- CTLs
cytotoxic T lymphocytes
- DC
dendritic cell
- IDO
indoleamine 2, 3-dioxygenase
- IFN-γ
interferon-γ
- LAG3
lymphocyte activation gene-3
- ICIs
immune checkpoint inhibitors
- IR
irradiation
- MDSCs
myeloid-derived suppressor cells
- MSI
microsatellite instability
- NSCLC
non-small cell lung cancer
- OVs
oncolytic virus
- ORR
objective response rate
- PR
partially responsive
- PD-1
programmed cell death protein-1
- PD-L1
programmed death protein ligand-1
- PFS
progression-free survival
- RT
radiotherapy
- SD
stable disease
- SBRT
stereotactic body radiotherapy
- TIL
tumor infiltrating lymphocyte
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- TMB
tumor mutation burden
- US FDA
United States Food and Drug Administration
- VEGF
vascular endothelial growth factor
Funding Statement
This study was funded by the National Natural Science Foundation of China (grant no. 81973728) and Natural Science Foundation of Tianjin (grant no. 18JCZDJC36600).
Funding
This study was funded by the National Natural Science Foundation of China (grant no. 81973728) and Natural Science Foundation of Tianjin (grant no. 18JCZDJC36600).
Availability of data and materials
Not applicable.
Authors' contributions
All authors contributed to the content development of this article. XK conceived and designed the study. PL, CL and YG reviewed literature and collated appropriate information. XK, YY, YP, FW, ZB and XD wrote the manuscript. HS generated figures and tables. JM reviewed and edited the manuscript. All authors approved the final manuscript
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing interests.
References
- 1.Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, Zhao Z, Zhao J, Chen S, Song J, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: A systematic review and meta-analysis. JAMA Oncol. 2020;6:375–384. doi: 10.1001/jamaoncol.2019.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu YM, Tsai CL, Kao JT, Hsieh CT, Shieh DC, Lee YJ, Tsay GJ, Cheng KS, Wu YY. PD-1 and PD-L1 up-regulation promotes T-cell apoptosis in gastric adenocarcinoma. Anticancer Res. 2018;38:2069–2078. doi: 10.21873/anticanres.12446. [DOI] [PubMed] [Google Scholar]
- 3.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J. 2018;24:47–53. doi: 10.1097/PPO.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrejon DY, Abril-Rodriguez G, Champhekar AS, Tsoi J, Campbell KM, Kalbasi A, Parisi G, Zaretsky JM, Garcia-Diaz A, Puig-Saus C, et al. Overcoming genetically based resistance mechanisms to PD-1 blockade. Cancer Discov. 2020;10:1140–1157. doi: 10.1158/2159-8290.CD-19-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Chen Y, Shi X, Le X, Feng F, Chen J, Zhou C, Chen Y, Wen S, Zeng H, et al. A systematic and genome-wide correlation meta-analysis of PD-L1 expression and targetable NSCLC driver genes. J Thorac Dis. 2017;9:2560–2571. doi: 10.21037/jtd.2017.07.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg AD, Agostinis P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 16.Cai J, Lin Y, Zhang H, Liang J, Tan Y, Cavenee WK, Yan G. Selective replication of oncolytic virus M1 results in a bystander killing effect that is potentiated by Smac mimetics. Proc Natl Acad Sci SA. 2017;114:6812–6817. doi: 10.1073/pnas.1701002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanSeggelen H, Tantalo DG, Afsahi A, Hammill JA, Bramson JL. Chimeric antigen receptor-engineered T cells as oncolytic virus carriers. Mol Ther Oncolytics. 2015;2:15014. doi: 10.1038/mto.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffin R. Interview with Robert Coffin, inventor of T-VEC: The first oncolytic immunotherapy approved for the treatment of cancer. Immunotherapy. 2016;8:103–106. doi: 10.2217/imt.15.116. [DOI] [PubMed] [Google Scholar]
- 19.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 20.Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, Liu W, Storkus WJ, He Y, Liu Z, Bartlett DL. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J Immunother Cancer. 2019;7:6. doi: 10.1186/s40425-018-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalsky SJ, Liu Z, Feist M, Berkey SE, Ma C, Ravindranathan R, Dai E, Roy EJ, Guo ZS, Bartlett DL. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol Ther. 2018;26:2476–2486. doi: 10.1016/j.ymthe.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Wang J, Zhang Z, Cao H, Yan W, Chu Y, Chard Dunmall LS, Wang Y. A novel vaccinia virus enhances anti-tumor efficacy and promotes a long-term anti-tumor response in a murine model of colorectal cancer. Mol Ther Oncolytics. 2020;20:71–81. doi: 10.1016/j.omto.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melero I, Gato M, Shekarian T, Aznar A, Valsesia-Wittmann S, Caux C, Etxeberrria I, Teijeira A, Marabelle A. Repurposing infectious disease vaccines for intratumoral immunotherapy. J Immunother Cancer. 2020;8:e000443. doi: 10.1136/jitc-2019-000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shekarian T, Sivado E, Jallas AC, Depil S, Kielbassa J, Janoueix-Lerosey I, Hutter G, Goutagny N, Bergeron C, Viari A, et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci Transl Med. 2019;11:eaat5025. doi: 10.1126/scitranslmed.aat5025. [DOI] [PubMed] [Google Scholar]
- 26.Shemesh CS, Hsu JC, Hosseini I, Shen BQ, Rotte A, Twomey P, Girish S, Wu B. Personalized cancer vaccines: Clinical landscape, challenges, and opportunities. Mol Ther. 2021;29:555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Zhou C, Wang D, Ma W, Lin C, Wang Y, Liang X, Li J, Guo S, Wang Y, et al. Enhancement of DNA vaccine potency by sandwiching antigen-coding gene between secondary lymphoid tissue chemokine (SLC) and IgG Fc fragment genes. Cancer Biol Ther. 2006;5:427–434. doi: 10.4161/cbt.5.4.2528. [DOI] [PubMed] [Google Scholar]
- 28.Tondini E, Arakelian T, Oosterhuis K, Camps M, van Duikeren S, Han W, Arens R, Zondag G, van Bergen J, Ossendorp F. A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control. Oncoimmunology. 2019;8:1652539. doi: 10.1080/2162402X.2019.1652539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu G, Feng D, Yao Y, Li P, Sun H, Yang H, Li C, Jiang R, Sun B, Chen Y. Listeria-based hepatocellular carcinoma vaccine facilitates anti-PD-1 therapy by regulating macrophage polarization. Oncogene. 2020;39:1429–1444. doi: 10.1038/s41388-019-1072-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, Xu J, Li Y, Guan X, Han X, Xu Y, Zhou H, Peng R, Wang J, Liu Z. Nanoscale coordination polymer based nanovaccine for tumor immunotherapy. ACS Nano. 2019;13:13127–13135. doi: 10.1021/acsnano.9b05974. [DOI] [PubMed] [Google Scholar]
- 31.Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, Eysmans C, Richards A, Schell MJ, Fisher KJ, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21:712–720. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crosby EJ, Acharya CR, Haddad AF, Rabiola CA, Lei G, Wei JP, Yang XY, Wang T, Liu CX, Wagner KU, et al. Stimulation of oncogene-specific tumor-infiltrating T cells through combined vaccine and αPD-1 enable sustained antitumor responses against established HER2 breast cancer. Clin Cancer Res. 2020;26:4670–4681. doi: 10.1158/1078-0432.CCR-20-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Tacchio M, Macas J, Weissenberger J, Sommer K, Bähr O, Steinbach JP, Senft C, Seifert V, Glas M, Herrlinger U, et al. Tumor vessel normalization, immunostimulatory reprogramming, and improved survival in glioblastoma with combined inhibition of PD-1, angiopoietin-2, and VEGF. Cancer Immunol Res. 2019;7:1910–1927. doi: 10.1158/2326-6066.CIR-18-0865. [DOI] [PubMed] [Google Scholar]
- 36.Gao F, Yang C. Anti-VEGF/VEGFR2 monoclonal antibodies and their combinations with PD-1/PD-L1 inhibitors in clinic. Curr Cancer Drug Targets. 2020;20:3–18. doi: 10.2174/1568009619666191114110359. [DOI] [PubMed] [Google Scholar]
- 37.Amin A, Plimack ER, Ernstoff MS, Lewis LD, Bauer TM, McDermott DF, Carducci M, Kollmannsberger C, Rini BI, Heng DYC, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: The CheckMate 016 study. J Immunother Cancer. 2018;6:109. doi: 10.1186/s40425-018-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, Jia Y, Shi J, Zhang L, Liu X, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 2019;7:630–643. doi: 10.1158/2326-6066.CIR-17-0640. [DOI] [PubMed] [Google Scholar]
- 39.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 40.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 43.Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20. doi: 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Han C, Dong C, Shen A, Hsu E, Ren Z, Lu C, Liu L, Zhang A, Timmerman C, et al. Hypofractionated EGFR tyrosine kinase inhibitor limits tumor relapse through triggering innate and adaptive immunity. Sci Immunol. 2019;4:eaav6473. doi: 10.1126/sciimmunol.aav6473. [DOI] [PubMed] [Google Scholar]
- 45.Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-Driven NSCLC: Implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10:910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 47.Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019;16:341–355. doi: 10.1038/s41571-019-0173-9. [DOI] [PubMed] [Google Scholar]
- 48.Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28:1532–1539. doi: 10.1093/annonc/mdx183. [DOI] [PubMed] [Google Scholar]
- 49.Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J, Chouaid C, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang JC, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, Fiore J, Saraf S, Raftopoulos H, Patnaik A. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol. 2019;14:553–559. doi: 10.1016/j.jtho.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 51.Zhai L, Bell A, Ladomersky E, Lauing KL, Bollu L, Sosman JA, Zhang B, Wu JD, Miller SD, Meeks JJ, et al. Immunosuppressive IDO in cancer: Mechanisms of action, animal models, and targeting strategies. Front Immunol. 2020;11:1185. doi: 10.3389/fimmu.2020.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siu LL, Gelmon K, Chu Q, Pachynski R, Alese O, Basciano P, Walker J, Mitra P, Zhu L, Phillips P, et al. Abstract CT116: BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Cancer Res. 2017;77(Suppl 13):CT116. [Google Scholar]
- 53.Zakharia Y, Rixe O, Ward JH, Drabick JJ, Shaheen MF, Milhem MM, Munn D, Kennedy EP, Vahanian NN, Link CJ, et al. Phase 2 trial of the IDO pathway inhibitor indoximod plus checkpoint inhibition for the treatment of patients with advanced melanoma. J Clin Oncol. 2018;36(Suppl 15):S9512. doi: 10.1200/JCO.2018.36.15_suppl.9512. [DOI] [Google Scholar]
- 54.Bahary N, Wang-Gillam A, Haraldsdottir S, Somer BG, Lee JS, O'Rourke MA, Nayak-Kapoor A, Beatty GL, Liu M, Delman D, et al. Phase 2 trial of the IDO pathway inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of patients with metastatic pancreas cancer. J Clin Oncol. 2018;36(Suppl 15):S4015. doi: 10.1200/JCO.2018.36.15_suppl.4015. [DOI] [Google Scholar]
- 55.Jha GG, Gupta S, Tagawa ST, Koopmeiners JS, Vivek S, Dudek AZ, Cooley SA, Blazar BR, Miller JS. A phase II randomized, double-blind study of sipuleucel-T followed by IDO pathway inhibitor, indoximod, or placebo in the treatment of patients with metastatic castration resistant prostate cancer (mCRPC) J Clin Oncol. 2017;35(Suppl 15):S3066. doi: 10.1200/JCO.2017.35.15_suppl.3066. [DOI] [Google Scholar]
- 56.Zakharia Y, Drabick JJ, Khleif S, McWilliams RR, Munn D, Link CJ, Vahanian NN, Kennedy E, Shaheen MF, Rixe O, Milhem MM. Updates on phase1b/2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus checkpoint inhibitors for the treatment of unresectable stage 3 or 4 melanoma. J Clin Oncol. 2016;34(Suppl 15):S3075. doi: 10.1200/JCO.2016.34.15_suppl.3075. [DOI] [Google Scholar]
- 57.Hamid O, Bauer TM, Spira AI, Smith DC, Olszanski AJ, Tarhini AA, Lara P, Gajewski T, Wasser JS, Patel SP, et al. Safety of epacadostat 100 mg bid plus pembrolizumab 200 mg Q3W in advanced solid tumors: Phase 2 data from ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35(Suppl 15):S3012. doi: 10.1200/JCO.2017.35.15_suppl.3012. [DOI] [Google Scholar]
- 58.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang RY, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6:27359–27377. doi: 10.18632/oncotarget.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, Fulton A, Tamada K, Strome SE, Antony PA. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190:4899–4909. doi: 10.4049/jimmunol.1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahlén E, Veitonmäki N, Norlén P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother. 2018;6:3–17. doi: 10.1177/2515135518763280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ascierto PA, Bhatia S, Bono P, Bono P, Sanborn RE, Lipson EJ, Callahan MK, Gajewski T, Gomez-Roca CA, Hodi FS, et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti-LAG-3; BMS-986016) in combination with nivolumab (nivo) in PTS with melanoma (MEL) previously treated with anti-PD-1/PD-L1 therapy. J Clini Oncol. 2017;35(Suppl 15):S9520. doi: 10.1200/JCO.2017.35.15_suppl.9520. [DOI] [Google Scholar]
- 63.Puhr HC, Ilhan-Mutlu A. New emerging targets in cancer immunotherapy: The role of LAG3. ESMO Open. 2019;4:e000482. doi: 10.1136/esmoopen-2018-000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yasinska IM, Sakhnevych SS, Pavlova L, Teo Hansen Selnø A, Teuscher Abeleira AM, Benlaouer O, Gonçalves Silva I, Mosimann M, Varani L, Bardelli M, et al. The Tim-3-galectin-9 pathway and its regulatory mechanisms in human breast cancer. Front Immunol. 2019;10:1594. doi: 10.3389/fimmu.2019.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 66.Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, Pawlak M, Dionne D, Xia J, Rozenblatt-Rosen O, et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1−CD8+ tumor-infiltrating T cells. Immunity. 2019;50:181–194.e6. doi: 10.1016/j.immuni.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun F, Guo ZS, Gregory AD, Shapiro SD, Xiao G, Qu Z. Dual but not single PD-1 or TIM-3 blockade enhances oncolytic virotherapy in refractory lung cancer. J Immunother Cance. 2020;8:e000294. doi: 10.1136/jitc-2019-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang TY, Huang GL, Zhang CY, Zhuang BW, Liu BX, Su LY, Ye JY, Xu M, Kuang M, Xie XY. Supramolecular photothermal nanomedicine mediated distant tumor inhibition via PD-1 and TIM-3 blockage. Front Chem. 2020;8:1. doi: 10.3389/fchem.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gestermann N, Saugy D, Martignier C, Tillé L, Fuertes Marraco SA, Zettl M, Tirapu I, Speiser DE, Verdeil G. LAG-3 and PD-1+LAG-3 inhibition promote anti-tumor immune responses in human autologous melanoma/T cell co-cultures. Oncoimmunology. 2020;9:1736792. doi: 10.1080/2162402X.2020.1736792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedlaender A, Addeo A, Banna G. New emerging targets in cancer immunotherapy: The role of TIM3. ESMO Open. 2019;4(Suppl 3):e000497. doi: 10.1136/esmoopen-2019-000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davar D, Boasberg PD, Eroglu Z, Falchook G, Gainor J, Hamilton E, Hecht R, Luke J, Pishvaian M, Ribas A, et al. Abstract O21: A phase 1 study of TSR-022, an anti-TIM-3 monoclonal antibody, in combination with TSR-042 (anti-PD-1) in patients with colorectal cancer and post-PD-1 NSCLC and melanoma. J Immuno Therapy Cancer. 2018;6(Suppl 1):S155. [Google Scholar]
- 73.Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang LL, Wu H, Bu LL, Kulkarni AB, Zhang WF, Sun ZJ. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res. 2019;7:1700–1713. doi: 10.1158/2326-6066.CIR-18-0725. [DOI] [PubMed] [Google Scholar]
- 74.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, Derangere V, Laurent PA, Thibaudin M, Fumet JD, et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: A promising new combination. J Immunother Cancer. 2019;7:160. doi: 10.1186/s40425-019-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 77.Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Finetti F, Baldari CT. The immunological synapse as a pharmacological target. Pharmacol Res. 2018;134:118–133. doi: 10.1016/j.phrs.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 80.Morales-Kastresana A, Sanmamed MF, Rodriguez I, Palazon A, Martinez-Forero I, Labiano S, Hervas-Stubbs S, Sangro B, Ochoa C, Rouzaut A, et al. Combined immunostimulatory monoclonal antibodies extend survival in an aggressive transgenic hepatocellular carcinoma mouse model. Clin Cancer Res. 2013;19:6151–6162. doi: 10.1158/1078-0432.CCR-13-1189. [DOI] [PubMed] [Google Scholar]
- 81.Fisher TS, Kamperschroer C, Oliphant T, Love VA, Lira PD, Doyonnas R, Bergqvist S, Baxi SM, Rohner A, Shen AC, et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother. 2012;61:1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Segal NH, He AR, Doi T, Levy R, Bhatia S, Pishvaian MJ, Cesari R, Chen Y, Davis CB, Huang B, et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin Cancer Res. 2018;24:1816–1823. doi: 10.1158/1078-0432.CCR-17-1922. [DOI] [PubMed] [Google Scholar]
- 83.Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, Di Gravio D, Huang B, Gambhire D, Chen Y, et al. Phase Ib study of utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res. 2017;23:5349–5357. doi: 10.1158/1078-0432.CCR-17-1243. [DOI] [PubMed] [Google Scholar]
- 84.Masserelli E, Segal NH, Ribrag V. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. J Immunother Cancer. 2016;4:O7. [Google Scholar]
- 85.Callahan MK, Postow MA, Wolchok JD. Immunomodulatory therapy for melanoma: Ipilimumab and beyond. Clin Dermatol. 2013;31:191–199. doi: 10.1016/j.clindermatol.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 87.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, Voloschin A, Ramkissoon SH, Ligon KL, Latek R, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20:674–686. doi: 10.1093/neuonc/nox208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kitao H, Iimori M, Kataoka Y, Wakasa T, Tokunaga E, Saeki H, Oki E, Maehara Y. DNA replication stress and cancer chemotherapy. Cancer Sci. 2018;109:264–271. doi: 10.1111/cas.13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 92.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 94.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 95.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lhuillier C, Rudqvist NP, Elemento O, Formenti SC, Demaria S. Radiation therapy and anti-tumor immunity: Exposing immunogenic mutations to the immune system. Genome Med. 2019;11:40. doi: 10.1186/s13073-019-0653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen D, Barsoumian HB, Yang L, Younes AI, Verma V, Hu Y, Menon H, Wasley M, Masropour F, Mosaffa S, et al. SHP-2 and PD-L1 inhibition combined with radiotherapy enhances systemic antitumor effects in an anti-PD-1-resistant model of non-small cell lung cancer. Cancer Immunol Res. 2020;8:883–894. doi: 10.1158/2326-6066.CIR-19-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 99.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dudzinski SO, Cameron BD, Wang J, Rathmell JC, Giorgio TD, Kirschner AN. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J Immunother Cancer. 2019;7:218. doi: 10.1186/s40425-019-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 102.Ahmed KA, Stallworth DG, Kim Y, Johnstone PA, Harrison LB, Caudell JJ, Yu HH, Etame AB, Weber JS, Gibney GT. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27:434–441. doi: 10.1093/annonc/mdv622. [DOI] [PubMed] [Google Scholar]
- 103.Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: A new era in cancer immunotherapy. J Intern Med. 2018;283:110–120. doi: 10.1111/joim.12708. [DOI] [PubMed] [Google Scholar]
- 104.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84:879–880. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liang H, Deng L, Hou Y, Meng X, Huang X, Rao E, Zheng W, Mauceri H, Mack M, Xu M, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun. 2017;8:1736. doi: 10.1038/s41467-017-01566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seyedin SN, Hasibuzzaman MM, Pham V, Petronek MS, Callaghan C, Kalen AL, Mapuskar KA, Mott SL, Spitz DR, Allen BG, Caster JM. Combination therapy with radiation and PARP inhibition enhances responsiveness to anti-PD-1 therapy in colorectal tumor models. Int J Radiat Oncol Biol Phys. 2020;108:81–92. doi: 10.1016/j.ijrobp.2020.07.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and immunotherapy for cancer: From ‘systemic; to ‘multisite’. Clin Cancer Res. 2020;26:2777–2782. doi: 10.1158/1078-0432.CCR-19-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 111.Kordbacheh T, Honeychurch J, Blackhall F, Faivre-Finn C, Illidge T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: Building better translational research platforms. Ann Oncol. 2018;29:301–310. doi: 10.1093/annonc/mdx790. [DOI] [PubMed] [Google Scholar]
- 112.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song P, Yang D, Wang H, Cui X, Si X, Zhang X, Zhang L. Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac Cancer. 2020;11:1621–1632. doi: 10.1111/1759-7714.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 115.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 116.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 117.Kikuchi T, Mimura K, Ashizawa M, Okayama H, Endo E, Saito K, Sakamoto W, Fujita S, Endo H, Saito M, et al. Characterization of tumor-infiltrating immune cells in relation to microbiota in colorectal cancers. Cancer Immunol Immunother. 2020;69:23–32. doi: 10.1007/s00262-019-02433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, Wang N, Zhang C, Kong L, Liu Y, et al. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front Microbiol. 2020;11:814. doi: 10.3389/fmicb.2020.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19:848–855. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sethi G, Shanmugam MK, Warrier S, Merarchi M, Arfuso F, Kumar AP, Bishayee A. Pro-apoptotic and anti-cancer properties of diosgenin: A comprehensive and critical review. Nutrients. 2018;10:645. doi: 10.3390/nu10050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dong M, Meng Z, Kuerban K, Qi F, Liu J, Wei Y, Wang Q, Jiang S, Feng M, Ye L. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018;9:1039. doi: 10.1038/s41419-018-1099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hao H, Zhang Q, Zhu H, Wen Y, Qiu D, Xiong J, Fu X, Wu Y, Meng K, Li J. Icaritin promotes tumor T-cell infiltration and induces antitumor immunity in mice. Eur J Immunol. 2019;49:2235–2244. doi: 10.1002/eji.201948225. [DOI] [PubMed] [Google Scholar]
- 126.Li W, Kim TI, Kim JH, Chung HS. Immune checkpoint PD-1/PD-L1 CTLA-4/CD80 are blocked by Rhus verniciflua stokes and its active compounds. Molecules. 2019;24:4062. doi: 10.3390/molecules24224062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang G, Wang L, Zhou J, Xu X. The possible Role of PD-1 protein in Ganoderma lucidum-mediated immunomodulation and cancer treatment. Integr Cancer Ther. 2019;18:1534735419880275. doi: 10.1177/1534735419880275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Su J, Li D, Chen Q, Li M, Su L, Luo T, Liang D, Lai G, Shuai O, Jiao C, et al. Anti-breast cancer enhancement of a polysaccharide from spore of Ganoderma lucidum with paclitaxel: Suppression on tumor metabolism with gut microbiota reshaping. Front Microbiol. 2018;9:3099. doi: 10.3389/fmicb.2018.03099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: Implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28:245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 133.Adams DL, Adams DK, He J, Kalhor N, Zhang M, Xu T, Gao H, Reuben JM, Qiao Y, Komaki R, et al. Sequential tracking of PD-L1 expression and RAD50 induction in circulating tumor and stromal cells of lung cancer patients undergoing radiotherapy. Clin Cancer Res. 2017;23:5948–5958. doi: 10.1158/1078-0432.CCR-17-0802. [DOI] [PubMed] [Google Scholar]
- 134.Hansen AR, Siu LL. PD-L1 testing in cancer: Challenges in companion diagnostic development. JAMA Oncol. 2016;2:15–16. doi: 10.1001/jamaoncol.2015.4685. [DOI] [PubMed] [Google Scholar]
- 135.Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: A review. JAMA Oncol. 2016;2:1217–1222. doi: 10.1001/jamaoncol.2016.0639. [DOI] [PubMed] [Google Scholar]
- 136.Mahoney KM, Sun H, Liao X, Hua P, Callea M, Greenfield EA, Hodi FS, Sharpe AH, Signoretti S, Rodig SJ, Freeman GJ. PD-L1 antibodies to its cytoplasmic domain most clearly delineate cell membranes in immunohistochemical staining of tumor cells. Cancer Immunol Res. 2015;3:1308–1315. doi: 10.1158/2326-6066.CIR-15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Honda Y, Otsuka A, Ono S, Yamamoto Y, Seidel JA, Morita S, Hirata M, Kataoka TR, Takenouchi T, Fujii K, et al. Infiltration of PD-1-positive cells in combination with tumor site PD-L1 expression is a positive prognostic factor in cutaneous angiosarcoma. Oncoimmunology. 2016;6:e1253657. doi: 10.1080/2162402X.2016.1253657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y, Kang S, Shen J, He J, Jiang L, Wang W, Guo Z, Peng G, Chen G, He J, Liang W. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: A meta-analysis. Medicine (Baltimore) 2015;94:e515. doi: 10.1097/MD.0000000000000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawakami F, Sircar K, Rodriguez-Canales J, Fellman BM, Urbauer DL, Tamboli P, Tannir NM, Jonasch E, Wistuba II, Wood CG, Karam JA. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer. 2017;123:4823–4831. doi: 10.1002/cncr.30937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y, Chen L. Classification of advanced human cancers based on tumor immunity in the microenvironment (TIME) for cancer immunotherapy. JAMA Oncol. 2016;2:1403–1404. doi: 10.1001/jamaoncol.2016.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Ruysscher D. Combination of radiotherapy and immune treatment: First clinical data. Cancer Radiother. 2018;22:564–566. doi: 10.1016/j.canrad.2018.07.128. [DOI] [PubMed] [Google Scholar]
- 144.Vansteenkiste J, Wauters E, Reymen B, Ackermann CJ, Peters S, De Ruysscher D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol. 2019;30:1244–1253. doi: 10.1093/annonc/mdz175. [DOI] [PubMed] [Google Scholar]
- 145.Sahin IH, Akce M, Alese O, Shaib W, Lesinski GB, El-Rayes B, Wu C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. 2019;121:809–818. doi: 10.1038/s41416-019-0599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Narayanan S, Kawaguchi T, Peng X, Qi Q, Liu S, Yan L, Takabe K. Tumor infiltrating lymphocytes and macrophages improve survival in microsatellite unstable colorectal cancer. Sci Rep. 2019;9:13455. doi: 10.1038/s41598-019-49878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Diaz LA, Jr, Le DT. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;373:1979. doi: 10.1056/NEJMc1510353. [DOI] [PubMed] [Google Scholar]
- 148.Wang X, Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. 2019;20:4. doi: 10.1186/s12865-018-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen D, Barsoumian HB, Fischer G, Yang L, Verma V, Younes AI, Hu Y, Masropour F, Klein K, Vellano C, et al. Combination treatment with radiotherapy and a novel oxidative phosphorylation inhibitor overcomes PD-1 resistance and enhances antitumor immunity. J Immunother Cancer. 2020;8:e000289. doi: 10.1136/jitc-2019-000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 153.Abdel-Rahman O, Fouad M. A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy. 2016;8:665–674. doi: 10.2217/imt-2015-0020. [DOI] [PubMed] [Google Scholar]
- 154.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun J, Lu Q, Sanmanmed MF, Wang J. Siglec-15 as an emerging target for next-generation cancer immunotherapy. Clin Cancer Res. 2021;27:680–688. doi: 10.1158/1078-0432.CCR-19-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang L, Gong X, Liao W, Lv N, Yan R. Molecular targeted treatment and drug delivery system for gastric cancer. J Cancer Res Clin Oncol. 2021 Feb 7; doi: 10.1007/s00432-021-03520-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 157.Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, Kris MG, Riely GJ, Yu HA, Hellmann MD. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30:839–844. doi: 10.1093/annonc/mdz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Uchida T, Kaira K, Yamaguchi O, Mouri A, Shiono A, Miura Y, Hashimoto K, Nishihara F, Murayama Y, Kobayashi K, Kagamu H. Different incidence of interstitial lung disease according to different kinds of EGFR-tyrosine kinase inhibitors administered immediately before and/or after anti-PD-1 antibodies in lung cancer. Thorac Cancer. 2019;10:975–979. doi: 10.1111/1759-7714.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro Junior G, Psyrri A, Rotllan NB, Neupane PC, Bratland Å, et al. LBA8_PRKEYNOTE-048: phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) Ann Oncol. 2018;29(Suppl 8):viii729. doi: 10.1093/annonc/mdy424.045. [DOI] [Google Scholar]
- 160.Sasaki A, Nakamura Y, Togashi Y, Kuno H, Hojo H, Kageyama S, Nakamura N, Takashima K, Kadota T, Yoda Y, et al. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer. 2020;23:893–903. doi: 10.1007/s10120-020-01058-4. [DOI] [PubMed] [Google Scholar]
- 161.Samuel E, Lie G, Balasubramanian A, Hiong A, So Y, Voskoboynik M, Moore M, Shackleton M, Haydon A, John T, et al. Impact of radiotherapy on the efficacy and toxicity of anti-PD-1 inhibitors in metastatic NSCLC. Clin Lung Cancer. 2020;S1525-7304:30183–30192. doi: 10.1016/j.cllc.2020.06.001. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.