Abstract
Recurrent pregnancy loss (RPL) is usually characterized as ≥3 miscarriages before 20 weeks of gestation. Patients with RPL may have autoimmune abnormalities or alloimmune problems. Vitamin D has a major function on the mechanism of immunomodulation at the maternal-fetal interface. However, whether vitamin D can be used as an effective method to treat patients with RPL requires investigation. It has been reported that vitamin D could prevent the occurrence of antiphospholipid syndrome (APS) by reducing the expression levels of anti-β2 glycoprotein and tissue factor in RPL cases with APS. In addition, there is an opposite relationship between vitamin D and thyroid peroxidase antibody levels in autoimmune thyroid disease cases with RPL. Vitamin D changes the ratio of T helper (Th) 1/Th2 and regulatory T cell/Th17 to a certain extent, as well as affects the activity of natural killer cells and the production of cytokines to reduce the incidence of RPL. The objective of the current review was to address the research progress of vitamin D in RPL in recent years, which could facilitate the use of vitamin D treatment to enhance the pregnancy outcome of RPL. Collectively, it was suggested that vitamin D may be used as an important and effective immunotherapeutic agent for patients with RPL.
Keywords: vitamin D, RPL, autoimmunity, cellular immunity, NK cells
1. Introduction
Recurrent pregnancy loss (RPL) is usually characterized as ≥3 miscarriages before 20 weeks of gestation, but an increasing number of researchers describe it as ≥2 spontaneous abortions (1). The etiology of RPL is multifactorial (2), including chromosomal abnormalities, genetic issues (3), congenital uterine defects, acquired or hereditary thrombotic diseases, endocrine issues, infections, autoimmune diseases and male factors (4). However, ~50% of patients with RPL do not have a definite etiology (5). Patients with RPL may also have autoimmune abnormalities or alloimmune problems (2). The former mainly includes antiphospholipid antibodies (APAs) (6), antithyroid antibodies (7) and antinuclear antibodies (ANAs) (8,9), while the latter mainly refers to cellular immune problems, such as increased natural killer (NK) cells (10–12) or decreased inhibitory T cells (13). Therefore, the success of pregnancy depends to a large degree on the development of an appropriate immune response (14).
It has been suggested that in patients, RPL, is largely associated with immune abnormalities (14), thereby contributing to the development and use of different forms of immunomodulatory therapies (1). Prednisone has been reported to help women who have multiple consecutive abortions by reducing inflammation and inhibiting the function of multiples types of immune cells, including T cells (15,16). Moreover, plaquenil can be useful in the treatment of RPL as a drug with comprehensive protection identified during pregnancy (17). The therapeutic role of plaquenil is associated with pharmacological properties, such as antithrombotic activity, vascular defense, immunomodulation, improved glucose resistance, hypolipidemic activity and anti-infection function (17). Several studies have revealed that intravenous immunoglobulin modulated immune dysfunction and contributed to positive pregnancy outcomes in women with RPL, although controversial results have been reported (18–23).
In humans, vitamin D is involved in the metabolism of numerous elements, such as calcium and phosphorus (24). Vitamin D is a crucial modulator of essential biological effects, such as immune function and hormone secretion via the vitamin D receptor (VDR) (25). Vitamin D affects the innate and acquired immune response (26), as well as exerts an inhibitory function on the adaptive immune system (27). In comparison, it inhibits T helper (Th)1 cytokines (such as IFN-γ), and promotes the response of Th2 by both downregulating IFN-γ and upregulating IL-4 (26). As VDR is expressed in the placenta, and it has been suggested that vitamin D has a major function on the mechanism of immunomodulation at the maternal-fetal interface (28). In the clinical setting, multiple patients with RPL have vitamin D deficiency (VDD) (29). As a result, these women have impaired cellular immune systems, including elevated peripheral NK levels, NK cytotoxicity and higher Th1/Th2 ratios (30–32). Moreover, women with low vitamin D (VDL) levels are more susceptible to autoimmune defects (29). For example, the decreased expression of vitamin D is associated with a higher occurrence of APAs, ANAs, anti-single stranded DNA and thyroid peroxidase antibodies (anti-TPO) in patients with RPL (27).
Few foods contain vitamin D (33), and vitamin D supplementation could be applied as a natural therapy to minimize the risk of spontaneous early abortion (4). However, excessive vitamin D intake can have adverse effects (34). For example, excessive vitamin D molecules can affect the production of oocytes and the quality of embryos due to their anti-estrogen effect (34). It is also important to periodically measure vitamin D levels and change the therapy during pregnancy (4). In clinical trials, doctors may neglect vitamin D intake partly due to the lack of knowledge of the mechanism of vitamin D in RPL (4). The objective of the present review was to address the research progress of vitamin D in RPL in recent years, which could facilitate the use of vitamin D treatment to enhance the pregnancy outcome of RPL. In total, two independent researchers searched for articles in PUBMED with the following medical subject heading: ‘Vitamin D’, ‘RPL’, ‘recurrent miscarriages’, ‘autoimmunity’, ‘cellular immunity’ or ‘1,25-dihydroxy vitamin D’. All articles were published in English between January 1995 and August 2020. Review manuscripts and letters were excluded.
2. Vitamin D and antiphospholipid syndrome (APS) in RPL
As an autoimmune disease, APS is closely associated with adverse obstetrical outcomes (32,35). APS is a systemic autoimmune disease characterized by thrombosis (36). It has been well documented that the increased susceptibility to the thrombosis of blood vessels in APS may lead to microvascular thrombus in the placenta, as well as the reduction of blood flow at the maternal-fetal interface (6,37). A significant crosstalk between inflammation and coagulation involves the complement system and tissue factor (TF), and both mice and humans experience APS-related pregnancy complications (38–40).
As aforementioned, complement activation serves a critical function in adverse outcomes of pregnancy, including RPL with APS in both mice and humans (41–46). Vitamin D can enhance the level of complement inhibitor CD55 in human monocytes (47). Moreover, the related inhibitory effect of complement activation can prevent preterm birth observed in APS (47). In patients with APS, vitamin D exerts a suppressive effect on anti-β2 glycoprotein expression, thus decreasing the risk of thrombosis (48,49). Furthermore, as shown in in vitro studies, APA-induced TF expression was suppressed by vitamin D (36,48). Another study reported that vitamin D regulated TF in vascular smooth muscle cells (50). In addition, abnormal TF/protease activated receptor 2 signaling was considered to be involved in the pathogenesis of pregnancy-associated complications, which included abortions in an APS murine model (38). The frequency of APS antibodies in women with RPL is 15–20% (51). Researchers have confirmed that VDD in APS women with RPL was more common relative to normal controls (49.5 vs. 30%; P<0.05) (48). Additionally, women with VDD were found to have enhanced levels of several autoantibodies, including APAs (29). The prevalence of total APA in women with RPL was substantially increased in VDL relative to a normal vitamin D (VDN) group (39.7 vs. 22.9%; P<0.05; odds ratio = 2.22; 95% CI, 1.0–4.7) (29). In brief, VDD is more common in RPL cases with APS. Moreover, an increased percentage of patients with RPL and VDD are at risk of autoimmune abnormalities, including APS (29).
3. Vitamin D and autoimmune thyroid disease (AITD) in RPL
Accumulating evidence has suggested that thyroid autoimmunity is the cause of miscarriage during pregnancy (52,53). An increased morbidity of VDD was observed among patients with AITDs, in particular, Hashimoto's thyroiditis (54). Although the mechanisms underlying the relation between vitamin D and AITDs are not fully understood, the causes may be associated with anti-inflammatory and immunomodulatory functions (55). An opposite relationship between vitamin D and anti-TPO levels has been identified in women with AITD (56). Decreased levels of anti-TPO result in lower incidence of preterm birth and reduced rates of pregnancy loss (57). Antithyroid antibodies are more prevalent in patients with VDD compared with those with high levels of vitamin D (43 vs. 17%, respectively; P<0.001) (54). Women with abnormal thyroid autoantibodies also had lower levels of vitamin D compared with healthy controls (54). Lower thyroid stimulating hormone levels are directly associated with a higher vitamin D level (54). Ozkan et al (58) revealed that 25(OH)-D in follicular fluid could independently predict successful pregnancy during the in vitro fertilization cycle. For women whose vitamin D levels in follicular fluid were <10 ng/ml, the presence of clinical pregnancy reduced significantly, while for patients whose vitamin D levels in follicular fluid increased by 1 ng/ml, the rate of clinical pregnancy was enhanced by 6% (58). Moreover, vitamin D can activate HOX genes, including HOXA10, which is essential for the process of implantation (59).
4. Vitamin D and Th1/Th2 ratio in RPL
Vitamin D suppresses the proliferation of Th1 cells and restricts the secretion of cytokines, including IFN-γ, IL-2 and TNF-α (29). Moreover, vitamin D postpones subsequent antigen presentation and the accumulation of T lymphocytes by suppressing the transcription of IFN-γ, which is the main positive response symbol for antigen-presenting cells (60). Vitamin D also prevents the activation and spread of IL-2, which is the autocrine growth factor for T lymphocytes (61). By decreasing the synthesis of IL-2 and IFN-γ and inducing the polarization of CD4+ T lymphocytes to a Th2 reaction, as well as finally reducing the autoimmune response, vitamin D increases the secretion of Th2 cytokines (62), including IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13 (63,64). In a trial with mice, there was an increased accumulation of Th2 cells and higher counts of cytokines (IL-4, IL-5 and IL-10) when 1,25(OH)2D3 was administered (65). The 1,25(OH)2D3-related functions on the augment of Th2 cells were mainly regulated via IL-4 (62). The phenomenon could also be confirmed in the mouse model (66).
Excessive inflammatory response and lower levels of vitamin D are found in women with RPL (67). As observed in a previous study, patients with VDD showed an notable higher percentage of TNF-α-secreting Th cells relative to individuals with normal vitamin D levels (35.1±10.2 vs. 28.3±4.8%; P<0.05), whereas the difference between vitamin D insufficient group (VDI) and VDN was negligible (2). In women with elevated vitamin D concentrations, the mean serum TNF-α was substantially lower compared with those with low vitamin D expression (0.79±0.11 vs. 1.22±0.11 pg/ml; P=0.02) (68).
In a retrospective cross-sectional analysis conducted by Ota et al (29), it was observed that external vitamin D promoted Th2 polarization in a dose-dependent manner. In total, 70 women with VDN and 63 women with VDL were analyzed in this previous study (29). T cell cultures were supplied with 100 nM vitamin D for 16 h, and it was found that the TNF-α/IL-10 expressed CD3+/CD4+ cell ratio was substantially decreased (31.3±9.4) relative to the controls (40.4±11.3; P<0.05) (29). However, when 10 nM vitamin D was applied to T cells, no statistically significant difference was observed relative to the controls (29). After treatment with 100 nM vitamin D, the IFN-γ/IL-10 expressed CD3+/CD4+ cell ratio was also notably decreased compared with the controls (12.1±4.0 vs. 14.8±4.6; P<0.05) (29), whereas there was no significant difference after adding 10 nM vitamin D compared with controls (29). During this study (29), no significant difference was found in the Th1/Th2 cytokine-expressed CD3+/CD4+ Th cell ratio among VDL cases with RPL compared with VDN cases with RPL. Since it has been reported that only the treatment of 100 nM vitamin D3 can affect the Th1/Th2 shift in vitro, the difference in average vitamin D levels between VDL and VDN for the study may not be sufficient to change the ratio of Th1/Th2 in vivo (29). There is a relatively small difference in immunological biomarkers of normal vitamin D cases compared with low vitamin D cases in this study, and they may not have a strong clinical correlation with vitamin D levels.
Other studies have suggested that vitamin D could exert a vital influence on preventing excessive adaptive immune response among patients with RPL (2,29,69). In one study, women with RPL were classified into three groups based on vitamin D level in serum: VDN, VDI and VDD groups (2). The definition of VDD was a level of 25(OH)D at <20 ng/ml, and VDI was defined as levels between 20–30 ng/ml, according to previously published literature (70). In 35 VDI cases, 0.5 µg/day 1,25(OH)2D was applied for 2 months, and two patients with VDD were treated with the same amount of 1,25(OH)2D. There was a substantial decrease in the proportion of TNF-α-producing Th cells following treatment compared with that observed before treatment, whereas there was no obvious difference in the proportion of IFN-γ-producing Th cells between pre- and post-treatment (2). However, other research regarding CD4+ T cells in human has indicated that 1,25(OH)2D could decrease both the production of IFN-γ and the numbers of IFN-γ+ CD4+ T cells (71,72).
5. Vitamin D and regulatory T cell (Treg)/Th17 ratio in RPL
Decreased Treg cells and increased Th17 cells serve a vital role in the pathogenesis of RPL (73). Due to VDD in pregnancy, the activity of Treg cells is disrupted (74). It has been reported that population of Treg cells was lower (maternal, 0.2±0.01; cord, 0.63±0.03) in 25(OH)D3 deficient pregnant women (≤19 ng/ml; n=80) compared with insufficient (20–29 ng/ml; n=55; maternal, 0.34±0.01; cord, 1.05±0.04) and sufficient (≥30 ng/ml; n=18; maternal, 0.45±0.02; cord, 1.75±0.02) pregnant women (P<0.05) (74). When compared with insufficient and sufficient vitamin D levels, TGF-β and IL-10 levels were decreased in the 25(OH)D3 deficient pregnancy group (74). Other studies have revealed that 1,25 vitamin D treatment significantly augmented the percentage of Tregs from baseline in the patients with RPL compared with the same percentage in the control group (69,75,76).
A double-blind placebo-controlled analysis identified that vitamin D3 reduced the frequency of Th17, as well as decreased the Th17/Treg ratio in peripheral blood of women with RPL (77). In total, 44 women with RPL were involved in this research and were randomly assigned to the treatment [n=22; one 300,000 IU dose of intramuscular vitamin D3 and Lymphocyte Immune Therapy (LIT)] and control (n=22; one dose of packaged inert placebo and LIT) groups (77). The findings revealed that the average proportion of Th17 cells and Th17/Treg ratio was significantly decrease in the treatment group (average proportion of Th17; 0.93 vs. 0.43%; P=0.001; ratio of Th17/Treg, 0.48 vs. 0.12; P=0.001) and the control group (mean percentage of Th17, 0.92 vs. 0.65%; P=0.001; ratio of Th17/Treg, 0.32 vs. 0.17; P=0.001) when compared with those before therapy (77). The decline in the number of Th17 cell displayed a substantial increase in the treatment group compared with the control group relative to the baseline values, and the decline in the ratio of Th17/Treg showed a significant enhancement in the treatment group when compared with the controls (77).
Further research revealed an association between the ratio of Treg/Th17 and vitamin D, as well as the impact on the balance between Treg and Th17, among patients with RPL who were given vitamin D (76). This study found that the expression of vitamin D had a strong association with the proportion of Treg cells, a negative association with the proportion of Th17 cells and a positive association with the ratio of Treg to Th17 in the RPL group (76). Moreover, the results were reversed in the control group (76). It has also been revealed that, relative to the control decidual tissues, 25(OH) D was significantly decreased among patients with RPL (78). Furthermore, correlation analysis indicated that there was a significantly negative correlation between 25(OH) D and IL-23. On the contrary, IL-23 increased the expression of IL-17 (78). Vitamin D can also be a beneficial medication by decreasing the amount of IL-23 (78). In addition, a study analyzing the impact of women with RPL given vitamin D found that vitamin D could decrease not only serum IL-23 but also the frequency of miscarriage (79).
6. Vitamin D and peripheral NK cells in RPL
Increased NK cells in the peripheral blood can lead to RPL (80). A previous retrospective cross-sectional study has reported that VDD may regulate cell immunity (29). Moreover, VDD may serve regulatory roles in peripheral NK cells (29). Significant differences have been identified in CD56+ NK cell levels from the peripheral blood between VDL (n=63) and VDN (n=70) cases with RPL (P<0.05) (29). In VDD (n=22), a significant increase of peripheral NK cell cytotoxicity has been indicated compared with that in the VDN group (n=70) (29). After the vitamin D supplement, the cytotoxicity of NK cells from peripheral blood was significantly inhibited in a dose-dependent manner, when compared with the controls (29). Another study suggested that 1,25(OH)2D3 exerted an immunomodulatory impact on NK cell cytotoxicity, the secretion of cytokines, including Toll-like receptor 4 expression, and the degranulation process in RPL peripheral blood samples (27). In women with RPL, 1,25(OH)2D3 supplementation substantially decreased peripheral NK cell cytotoxicity compared with that of the vehicle group which was given reconstitution agent ethanol (P<0.01) (27). Previous research has also observed a substantial rise in peripheral NK cytotoxicity between VDI (n=51) and VDN (n=35) cases (2). Moreover, peripheral NK cell cytotoxicity was significantly decreased after 1,25(OH)2D3 treatment compared with the levels prior to treatment (2). However, no obvious distinction was found in the percentage of CD3−CD56+ NK cells among three groups, VDN, VDI and VDD, before and after treatment (2). Therefore, it can be concluded that the effect of vitamin D on the count or cytotoxicity of NK cells tends to be independent (2). Taken together, a sufficient amount of vitamin D was found to be critical in regulating NK cell cytotoxicity in peripheral blood from patients with RPL.
7. Vitamin D and cytokines in RPL
It has been reported that the levels of VEGF and granulocyte colony-stimulating factor (G-CSF) can be induced by vitamin D in NK cells (29). These two factors may induce weak angiogenesis and complications of pregnancy; for instance, in RPL, NK cells decrease the production of VEGF at the maternal fetal interface (29). The receptor of G-CSF exists in the trophoblast cells. It has been identified that G-CSF exerts significant impacts on autocrine and paracrine activities in both the decidua and placenta, and increased G-CSF markedly decreased the risk of pregnancy loss in a human trial (81). Vitamin D could also be a possible therapeutic alternative to avoid RPL by increasing the levels of VEGF and G-CSF.
8. Conclusions
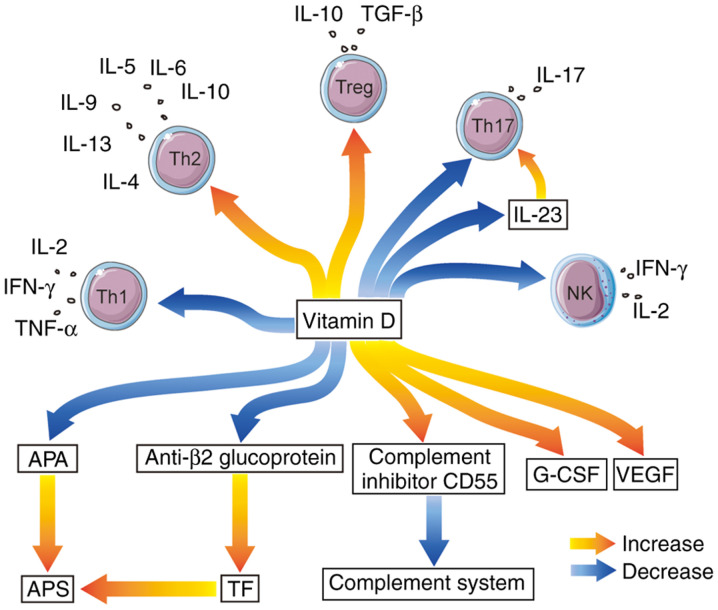
In conclusion, vitamin D can significantly affect both autoimmunity and cellular immunity in RPL based on previously published studies (Table I; Fig. 1). Vitamin D is proposed to be available as a potential therapy for RPL. For patients with RPL or high risk factors, appropriate vitamin D supplement could be given, and the serological level of vitamin D should be detected regularly to obtain favorable maternal and fetal outcomes. In the future, a larger multicenter, prospective controlled study with a larger sample size is required.
Table I.
Studies investigating vitamin D and RPL.
| Authors (year) (Ref.) | Research objective | Research type | Number of experimental group vs. control group | Type of samples | Vitamin D supplementation | Conclusions |
|---|---|---|---|---|---|---|
| Tavakoli et al (2011) (33) | To assess the influence of 1,25 (OH)2D3 on cytokines. | Case control study (in vitro) | n=8 women with RPL vs. n=8 healthy women | Endometrium | 10−7 M 1,25(OH)2D3 for 6 h | Vitamin D3 lowered IFN-γ/IL-10 ratio and reduced IL-6, TGF-β and IL-10 production. |
| Ibrahim et al (2013) (72) | To evaluate the role of vitamin D3 in prevention of RPL. | Randomized controlled trial (in vitro) | 40 pregnant women with RPL, n=20 (study group) vs. n=20 (control group) | Peripheral blood | 0.25 mcg vitamin D3 given twice daily after pregnancy was documented till delivery. | Vitamin D3 supplementation resulted in a lower risk of pregnancy loss among women with RPL. |
| Ota et al (2014) (29) | To investigate the relationship between autoimmune or cellular immunity and vitamin D. | Retrospective cross sectional study (in vitro) | 133 females with RPL: VDN (n=70) vs. VDL (n=63) | Peripheral blood | 10 and 100 nM vitamin D3 | VDD was associated with autoimmune or cellular immune abnormalities in RPL. |
| Ota et al (2015) (27) | To study the influence of vitamin D upon NK cells. | Case-control study (in vitro) | 18 women with RPL vs. 16 healthy women | Peripheral blood | 10 and 100 nM 1,25(OH)2D3 | 1,25 (OH)2D3 has immunomodulatory influence upon NK cell cytotoxicity, cytokine production, the process of degranulation and TLR4 expression. |
| Rafiee et al (2015) (77) | To research the impact of vitamin D3 upon Th17 and Treg cells. | A double-blind placebo-controlled study (in vivo) | 44 women with RPL: n=22 (experimental group) vs. n=22 (control group) | Peripheral blood | 300,000 IU vitamin D3 | Vitamin D3 decreased the number of Th17 cells and the ratio of Th17/Treg in RPL. |
| Chen et al (2016) (2) | To study the function of vitamin D on cellular immunity in RPL. | Prospective study (in vivo) | 99 women with RPL: VDN (n=35) vs. VDI (n=51) vs. VDD (n=13). | Peripheral blood | 1,25(OH)2D 0.5 µg/day for 2 months | Abnormal cellular immune reactions were shown in RPL cases with low vitamin D levels. |
| Samimi et al (2017) (79) | To examine the influence of vitamin D supplementation on RPL. | A double-blind randomized and controlled clinical trial (in vivo) | 77 pregnant women with RPL: n=39 (experimental group) vs. n=38 (control group) | Peripheral blood | 400 IU/day | Vitamin D3 leads to decreased IL-23 and lower morbidity of abortion among patients with RPL. |
| Ji et al (2019) (76) | To identify the relationship between vitamin D and Treg/Th17. | Clinical trial (in vivo) and a case control study (in vitro) | Patients with RPL (n=107) vs. healthy pregnant women (n=48) | Peripheral blood | In vivo: 2,000 IU/day for 2 months; In vitro: 1, 10 and 100 nmol/l for 4.5 days | The Treg/Th17 imbalance observed in patients with RPL can be restored by vitamin D supplementation. |
| Abdollahi et al (2020) (75) | To study the function of 1,25(OH)2D3 on Tregs and Th17. | Case control study | Non-pregnant women with RPL (n=20) vs. healthy non-pregnant women (n=20) | Peripheral blood | 1,25(OH)2D3 50 nM for 16 h | 1,25 (OH)2D3 supplementation substantially enhanced the proportion of Treg cells in patients with RPL. |
VDN, vitamin D normal; VDI, vitamin D insufficient; VDD, vitamin D deficiency; VDL, vitamin D low; TLR4, Toll like receptor 4.; Treg, regulatory T; Th, T helper; RPL, recurrent pregnant loss; NK, natural killer.
Figure 1.
Immune impact of vitamin D on RPL. Vitamin D suppresses cytokines produced by Th1 cells and promotes cytokines secreted by Th2 cells. Moreover, vitamin D increases the function of Treg cells, whereas it reduces the number of Th17 cells, which secrete IL-17. Vitamin D is negatively correlated with IL-23, while IL-23 is positively associated with IL-17. Peripheral NK cell activation and its cytotoxic actions are inhibited by vitamin D. Vitamin D inhibits peripheral NK cytotoxicity by suppressing IFN-γ and IL-2. VEGF and G-CSF production are stimulated by vitamin D. Vitamin D promotes the proliferation of the complement inhibitor CD55 and inhibits anti-β2 glycoprotein. By inhibiting TF and APAs, vitamin D can prevent the occurrence of APS in RPL. RPL, recurrent pregnant loss; G-CSF, granulocyte colony-stimulating factor; TF, tissue factor; APS, antiphospholipid syndrome; APAs, antiphospholipid antibodies; Treg, regulatory T; Th, T helper; NK, natural killer.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- RPL
recurrent pregnancy loss
- APAs
antiphospholipid antibodies
- ANAs
antinuclear antibodies
- NK cells
natural killer cells
- VDR
vitamin D receptor
- VDD
vitamin D deficiency
- VDL
low vitamin D
- APS
antiphospholipid syndrome
- TF
tissue factor
- VDN
normal vitamin D
- AITD
autoimmune thyroid disease
- anti-TPO
thyroid peroxidase antibodies
- VDI
vitamin D insufficient
- Treg
regulatory T cell
- G-CSF
granulocyte colony-stimulating factor
Funding Statement
This manuscript was funded by Natural Science Foundation of Liaoning Province (grant no. 2020-MS-167).
Funding
This manuscript was funded by Natural Science Foundation of Liaoning Province (grant no. 2020-MS-167).
Availability of data and materials
Not applicable.
Authors' contributions
HZ and XW wrote the manuscript, and XY revised this manuscript. All authors read and approved the final version.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Muyayalo KP, Li ZH, Mor G, Liao AH. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2018;80:e13018. doi: 10.1111/aji.13018. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Yin B, Lian RC, Zhang T, Zhang HZ, Diao LH, Li YY, Huang CY, Liang DS, Zeng Y. Modulatory effects of vitamin D on peripheral cellular immunity in patients with recurrent miscarriage. Am J Reprod Immunol. 2016;76:432–438. doi: 10.1111/aji.12585. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Yang E, Wang WJ, He Q, Jubiz G, Katukurundage D, Dambaeva S, Beaman K, Kwak-Kim J. Decreased HLA-C1 alleles in couples of KIR2DL2 positive women with recurrent pregnancy loss. J Reprod Immunol. 2020;142:103186. doi: 10.1016/j.jri.2020.103186. [DOI] [PubMed] [Google Scholar]
- 4.Yan X, Wang L, Yan C, Zhang X, Hui L, Sheng Q, Xue M, Yu X. Decreased expression of the vitamin D receptor in women with recurrent pregnancy loss. Arch Biochem Biophys. 2016;606:128–133. doi: 10.1016/j.abb.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Jeve YB, Davies W. Evidence-based management of recurrent miscarriages. J Hum Reprod Sci. 2014;7:159–169. doi: 10.4103/0974-1208.142475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rand JH, Wu XX, Andree HA, Lockwood CJ, Guller S, Scher J, Harpel PC. Pregnancy loss in the antiphospholipid-antibody syndrome-a possible thrombogenic mechanism. N Engl J Med. 1997;337:154–160. doi: 10.1056/NEJM199707173370303. [DOI] [PubMed] [Google Scholar]
- 7.Mecacci F, Parretti E, Cioni R, Lucchetti R, Magrini A, La Torre P, Mignosa M, Acanfora L, Mello G. Thyroid autoimmunity and its association with non-organ-specific antibodies and subclinical alterations of thyroid function in women with a history of pregnancy loss or preeclampsia. J Reprod Immunol. 2000;46:39–50. doi: 10.1016/S0165-0378(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 8.Cavalcante MB, Cavalcante C, Sarno M, da Silva ACB, Barini R. Antinuclear antibodies and recurrent miscarriage: Systematic review and meta-analysis. Am J Reprod Immunol. 2020;83:e13215. doi: 10.1111/aji.13215. [DOI] [PubMed] [Google Scholar]
- 9.Sakthiswary R, Rajalingam S, Norazman MR, Hussein H. Antinuclear antibodies predict a higher number of pregnancy loss in unexplained recurrent pregnancy loss. Clin Ter. 2015;166:e98–e101. doi: 10.7417/CT.2015.1827. [DOI] [PubMed] [Google Scholar]
- 10.Kwak JY, Beaman KD, Gilman-Sachs A, Ruiz JE, Schewitz D, Beer AE. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol. 1995;34:93–99. doi: 10.1111/j.1600-0897.1995.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 11.Aoki K, Kajiura S, Matsumoto Y, Ogasawara M, Okada S, Yagami Y, Gleicher N. Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet. 1995;345:1340–1342. doi: 10.1016/S0140-6736(95)92539-2. [DOI] [PubMed] [Google Scholar]
- 12.Yougbaré I, Tai WS, Zdravic D, Oswald BE, Lang S, Zhu G, Leong-Poi H, Qu D, Yu L, Dunk C, et al. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat Commun. 2017;8:224. doi: 10.1038/s41467-017-00269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 14.Stricker RB, Steinleitner A, Winger EE. Intravenous immunoglobulin (IVIG) therapy for immunologic abortion. Clin Appl Immunol Rev. 2002;2:187–199. doi: 10.1016/S1529-1049(02)00046-6. [DOI] [Google Scholar]
- 15.Bansal AS, Bajardeen B, Thum MY. The basis and value of currently used immunomodulatory therapies in recurrent miscarriage. J Reprod Immunol. 2012;93:41–51. doi: 10.1016/j.jri.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Novac N, Baus D, Dostert A, Heinzel T. Competition between glucocorticoid receptor and NFkappaB for control of the human FasL promoter. FASEB J. 2006;20:1074–1081. doi: 10.1096/fj.05-5457com. [DOI] [PubMed] [Google Scholar]
- 17.Pasquier E, de Saint-Martin L, Marhic G, Chauleur C, Bohec C, Bretelle F, Lejeune-Saada V, Hannigsberg J, Plu-Bureau G, Cogulet V, et al. Hydroxychloroquine for prevention of recurrent miscarriage: Study protocol for a multicentre randomised placebo-controlled trial BBQ study. BMJ Open. 2019;9:e025649. doi: 10.1136/bmjopen-2018-025649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SK, Kim JY, Han AR, Hur SE, Kim CJ, Kim TH, Cho BR, Han JW, Han SG, Na BJ, Kwak-Kim J. Intravenous immunoglobulin G improves pregnancy outcome in women with recurrent pregnancy losses with cellular immune abnormalities. Am J Reprod Immunol. 2016;75:59–68. doi: 10.1111/aji.12442. [DOI] [PubMed] [Google Scholar]
- 19.Kim DJ, Lee SK, Kim JY, Na BJ, Hur SE, Lee M, Kwak-Kim J. Intravenous immunoglobulin G modulates peripheral blood Th17 and Foxp3(+) regulatory T cells in pregnant women with recurrent pregnancy loss. Am J Reprod Immunol. 2014;71:441–450. doi: 10.1111/aji.12208. [DOI] [PubMed] [Google Scholar]
- 20.Kwak JY, Quilty EA, Gilman-Sachs A, Beaman KD, Beer AE. Intravenous immunoglobulin infusion therapy in women with recurrent spontaneous abortions of immune etiologies. J Reprod Immunol. 1995;28:175–188. doi: 10.1016/0165-0378(94)00918-W. [DOI] [PubMed] [Google Scholar]
- 21.Han AR, Ahn H, Vu P, Park JC, Gilman-Sachs A, Beaman K, Kwak-Kim J. Obstetrical outcome of anti-inflammatory and anticoagulation therapy in women with recurrent pregnancy loss or unexplained infertility. Am J Reprod Immunol. 2012;68:418–427. doi: 10.1111/j.1600-0897.2012.01178.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 23.Hutton B, Sharma R, Fergusson D, Tinmouth A, Hebert P, Jamieson J, Walker M. Use of intravenous immunoglobulin for treatment of recurrent miscarriage: A systematic review. BJOG. 2007;114:134–142. doi: 10.1111/j.1471-0528.2006.01201.x. [DOI] [PubMed] [Google Scholar]
- 24.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brannon PM. Vitamin D and adverse pregnancy outcomes: Beyond bone health and growth. Proc Nutr Soc. 2012;71:205–212. doi: 10.1017/S0029665111003399. [DOI] [PubMed] [Google Scholar]
- 26.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota K, Dambaeva S, Kim MW, Han AR, Fukui A, Gilman-Sachs A, Beaman K, Kwak-Kim J. 1,25-dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur J Immunol. 2015;45:3188–3199. doi: 10.1002/eji.201545541. [DOI] [PubMed] [Google Scholar]
- 28.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202:429.e1–e9. doi: 10.1016/j.ajog.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ota K, Dambaeva S, Han AR, Beaman K, Gilman-Sachs A, Kwak-Kim J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum Reprod. 2014;29:208–219. doi: 10.1093/humrep/det424. [DOI] [PubMed] [Google Scholar]
- 30.Lee SK, Na BJ, Kim JY, Hur SE, Lee M, Gilman-Sachs A, Kwak-Kim J. Determination of clinical cellular immune markers in women with recurrent pregnancy loss. Am J Reprod Immunol. 2013;70:398–411. doi: 10.1111/aji.12137. [DOI] [PubMed] [Google Scholar]
- 31.Fukui A, Kwak-Kim J, Ntrivalas E, Gilman-Sachs A, Lee SK, Beaman K. Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures. Fertil Steril. 2008;89:157–165. doi: 10.1016/j.fertnstert.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Ford HB, Schust DJ. Recurrent pregnancy loss: Etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2:76–83. [PMC free article] [PubMed] [Google Scholar]
- 33.Tavakoli M, Jeddi-Tehrani M, Salek-Moghaddam A, Rajaei S, Mohammadzadeh A, Sheikhhasani S, Kazemi-Sefat GE, Zarnani AH. Effects of 1,25(OH)2 vitamin D3 on cytokine production by endometrial cells of women with recurrent spontaneous abortion. Fertil Steril. 2011;96:751–757. doi: 10.1016/j.fertnstert.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 34.Lagana AS, Vitale SG, Ban Frangez H, Vrtacnik-Bokal E, D'Anna R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur Rev Med Pharmacol Sci. 2017;21:4243–4251. [PubMed] [Google Scholar]
- 35.Chighizola CB, de Jesus GR, Branch DW. The hidden world of anti-phospholipid antibodies and female infertility: A literature appraisal. Autoimmun Rev. 2016;15:493–500. doi: 10.1016/j.autrev.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Cyprian F, Lefkou E, Varoudi K, Girardi G. Immunomodulatory effects of Vitamin D in pregnancy and beyond. Front Immunol. 2019;10:2739. doi: 10.3389/fimmu.2019.02739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carp HJ, Shoenfeld Y. Anti-phospholipid antibodies and infertility. Clin Rev Allergy Immunol. 2007;32:159–161. doi: 10.1007/s12016-007-0010-2. [DOI] [PubMed] [Google Scholar]
- 38.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118:3453–3461. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobaldini LQ, Arantes FT, Saraiva SDS, Mazetto BM, Colella MP, de Paula EV, Annichino-Bizzachi J, Orsi FA. Circulating levels of tissue factor and the risk of thrombosis associated with antiphospholipid syndrome. Thromb Res. 2018;171:114–120. doi: 10.1016/j.thromres.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 40.Tedesco F, Borghi MO, Gerosa M, Chighizola CB, Macor P, Lonati PA, Gulino A, Belmonte B, Meroni PL. Pathogenic role of complement in antiphospholipid syndrome and therapeutic implications. Front Immunol. 2018;9:1388. doi: 10.3389/fimmu.2018.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oku K, Atsumi T, Bohgaki M, Amengual O, Kataoka H, Horita T, Yasuda S, Koike T. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis. 2009;68:1030–1035. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- 42.Breen KA, Seed P, Parmar K, Moore GW, Stuart-Smith SE, Hunt BJ. Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thromb Haemost. 2012;107:423–429. doi: 10.1160/TH11-08-0554. [DOI] [PubMed] [Google Scholar]
- 43.De Carolis S, Botta A, Santucci S, Salvi S, Moresi S, Di Pasquo E, Del Sordo G, Martino C. Complementemia and obstetric outcome in pregnancy with antiphospholipid syndrome. Lupus. 2012;21:776–778. doi: 10.1177/0961203312447866. [DOI] [PubMed] [Google Scholar]
- 44.Reggia R, Ziglioli T, Andreoli L, Bellisai F, Iuliano A, Gerosa M, Ramoni V, Tani C, Brucato A, Galeazzi M, et al. Primary anti-phospholipid syndrome: Any role for serum complement levels in predicting pregnancy complications? Rheumatology (Oxford) 2012;51:2186–2190. doi: 10.1093/rheumatology/kes225. [DOI] [PubMed] [Google Scholar]
- 45.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–1654. doi: 10.1172/JCI200318817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G. Tissue factor: A link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izban MG, Nowicki BJ, Nowicki S. 1,25-Dihydroxyvitamin D3 promotes a sustained LPS-induced NF-κB-dependent expression of CD55 in human monocytic THP-1 cells. PLoS One. 2012;7:e49318. doi: 10.1371/journal.pone.0049318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agmon-Levin N, Blank M, Zandman-Goddard G, Orbach H, Meroni PL, Tincani A, Doria A, Cervera R, Miesbach W, Stojanovich L, et al. Vitamin D: An instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann Rheum Dis. 2011;70:145–150. doi: 10.1136/ard.2010.134817. [DOI] [PubMed] [Google Scholar]
- 49.van den Hoogen LL, van Roon JA, Radstake TR, Fritsch-Stork RD, Derksen RH. Delineating the deranged immune system in the antiphospholipid syndrome. Autoimmun Rev. 2016;15:50–60. doi: 10.1016/j.autrev.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Moreno JM, Herencia C, Montes de Oca A, Muñoz-Castañeda JR, Rodríguez-Ortiz ME, Díaz-Tocados JM, Peralbo-Santaella E, Camargo A, Canalejo A, Rodriguez M, et al. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J. 2016;30:1367–1376. doi: 10.1096/fj.15-272872. [DOI] [PubMed] [Google Scholar]
- 51.Santos TDS, Ieque AL, de Carvalho HC, Sell AM, Lonardoni MVC, Demarchi IG, de Lima Neto QA, Teixeira JJV. Antiphospholipid syndrome and recurrent miscarriage: A systematic review and meta-analysis. J Reprod Immunol. 2017;123:78–87. doi: 10.1016/j.jri.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Matalon ST, Blank M, Ornoy A, Shoenfeld Y. The association between anti-thyroid antibodies and pregnancy loss. Am J Reprod Immunol. 2001;45:72–77. doi: 10.1111/j.8755-8920.2001.450202.x. [DOI] [PubMed] [Google Scholar]
- 53.Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol. 2007;66:309–321. doi: 10.1111/j.1365-2265.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 54.Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dankó K, Szekanecz Z, Langevitz P, Shoenfeld Y. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol. 2011;8:243–247. doi: 10.1038/cmi.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D'Aurizio F, Villalta D, Metus P, Doretto P, Tozzoli R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun Rev. 2015;14:363–369. doi: 10.1016/j.autrev.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Shin DY, Kim KJ, Kim D, Hwang S, Lee EJ. Low serum vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med J. 2014;55:476–481. doi: 10.3349/ymj.2014.55.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharyya R, Mukherjee K, Das A, Biswas MR, Basunia SR, Mukherjee A. Anti-thyroid peroxidase antibody positivity during early pregnancy is associated with pregnancy complications and maternal morbidity in later life. J Nat Sci Biol Med. 2015;6:402–405. doi: 10.4103/0976-9668.160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94:1314–1319. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocrine Rev. 2006;27:331–355. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- 60.Cippitelli M, Santoni A. Vitamin D3: A transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 61.Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J Immunol. 1998;160:209–218. [PubMed] [Google Scholar]
- 62.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol. 2000;109:30–33. doi: 10.1016/S0165-5728(00)00299-X. [DOI] [PubMed] [Google Scholar]
- 64.Adams JS, Hewison M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 66.Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, Laureys J, Bouillon R, Mathieu C. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543) Diabetes. 2000;49:1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 67.Gonçalves DR, Braga A, Braga J, Marinho A. Recurrent pregnancy loss and vitamin D: A review of the literature. Am J Reprod Immunol. 2018;80:e13022. doi: 10.1111/aji.13022. [DOI] [PubMed] [Google Scholar]
- 68.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdollahi E, Saghafi N, Rezaee SA, Rastin M, Jarahi L, Clifton V, Rafatpanah H. Evaluation of 1,25(OH)2D3 Effects on FOXP3, ROR-γt, GITR, and CTLA-4 Gene expression in the PBMCs of Vitamin D-Deficient Women with unexplained recurrent pregnancy loss (URPL) Iran Biomed J. 2020;24:295–305. doi: 10.29252/ibj.24.5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 71.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibrahim ZM, madany EH, Abdel Aal RM, El Biely MM. Role of 1,25-dihydroxyvitamin D (vitamin D3) as immunomodulator in recurrent missed miscarriage. Middle East Fertility Soc J. 2013;18:171–176. doi: 10.1016/j.mefs.2013.04.002. [DOI] [Google Scholar]
- 73.Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148:13–21. doi: 10.1111/imm.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vijayendra Chary A, Hemalatha R, Seshacharyulu M, Vasudeva Murali M, Jayaprakash D, Dinesh Kumar B. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J Steroid Biochem Mol Biol. 2015;147:48–55. doi: 10.1016/j.jsbmb.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Abdollahi E, Rezaee R, Saghafi N, Rastin M, Clifton V, Sahebkar A, Rafatpanah H. Evaluation of the effects of 1,25 vitamin D3 on regulatory T cells and T helper 17 cells in Vitamin D-deficient women with unexplained recurrent pregnancy loss. Curr Mol Pharmacol. 2020;13:306–317. doi: 10.2174/1874467213666200303130153. [DOI] [PubMed] [Google Scholar]
- 76.Ji J, Zhai H, Zhou H, Song S, Mor G, Liao A. The role and mechanism of vitamin D-mediated regulation of Treg/Th17 balance in recurrent pregnancy loss. Am J Reprod Immunol. 2019;81:e13112. doi: 10.1111/aji.13112. [DOI] [PubMed] [Google Scholar]
- 77.Rafiee M, Gharagozloo M, Ghahiri A, Mehrabian F, Maracy MR, Kouhpayeh S, Pieper IL, Rezaei A. Altered Th17/Treg ratio in recurrent miscarriage after treatment with paternal lymphocytes and Vitamin D3: A Double-blind placebo-controlled study. Iran J Immunol. 2015;12:252–262. [PubMed] [Google Scholar]
- 78.Li N, Wu HM, Hang F, Zhang YS, Li MJ. Women with recurrent spontaneous abortion have decreased 25(OH) vitamin D and VDR at the fetal-maternal interface. Braz J Med Biol Res. 2017;50:e6527. doi: 10.1590/1414-431x20176527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samimi M, Foroozanfard F, Amini F, Sehat M. Effect of Vitamin D supplementation on unexplained recurrent spontaneous abortion: A double-blind randomized controlled trial. Global J Health Sci. 2017;9:95–102. doi: 10.5539/gjhs.v9n3p95. [DOI] [Google Scholar]
- 80.Yang X, Gilman-Sachs A, Kwak-Kim J. Ovarian and endometrial immunity during the ovarian cycle. J Reprod Immunol. 2019;133:7–14. doi: 10.1016/j.jri.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Scarpellini F, Sbracia M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: A randomised controlled trial. Hum Reprod. 2009;24:2703–2708. doi: 10.1093/humrep/dep240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.