Abstract
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma originating from the nasopharyngeal mucosal tissue and is highly prevalent in southeast Asia. Galectin-3 (gal-3) serves crucial roles in many cancers but its role in NPC remains to be elucidated. The aim of the present study was to investigate the role of gal-3 in NPC. Immunohistochemistry and ELISA were used to determine the expression level of gal-3 in patients with NPC or chronic rhinitis (CR). Gal-3 short hairpin (sh)RNA was established to knockdown gal-3 in 5–8F and 6–10B cells, allowing for the evaluation of the roles of gal-3 in proliferation, migration and apoptosis in NPC cell lines. Immunohistochemistry staining of IL-6 and IL-8 was applied to access the inflammatory state of tumor tissues, and the correlation between the inflammatory state and gal-3 was analyzed. The results demonstrated that gal-3 was upregulated in patients with NPC compared with patients with CR. Knockdown of gal-3 inhibited proliferation and migration in 5-8F and 6-10B cells, as well as promoted apoptosis in these cells. The expression levels of MMP-9 and IL-8 were also decreased in 5-8F and 6-10B cells after transfection with gal-3 shRNA. A positive correlation was identified between the expression level of gal-3 and the inflammatory state of NPC. The phosphorylation levels of ERK1/2 and Akt were downregulated after knockdown of gal-3 in 5-8F and 6-10B cells. In conclusion, the expression level of gal-3 was upregulated in patients with NPC and was positively correlated with the inflammatory state of NPC. The results suggested that gal-3 promoted the proliferation and migration of 5-8F and 6-10B cells, while inhibiting the apoptosis of these cells. Moreover, activation of ERK1/2 and Akt may be the underlying mechanism of the effects of gal-3 on NPC.
Keywords: galectin-3, nasopharyngeal carcinoma, ERK1/2, Akt, inflammation
Introduction
Nasopharyngeal carcinoma (NPC), an epithelial carcinoma originating from the nasopharyngeal mucosal tissue, is widely distributed in Southeast Asia, and especially in southern China (1–3). The incidence of NPC in southern China is 60 per 100,000 individuals, and the corresponding mortality in 2015 was 34 per 100,000 individuals (4,5). Metastasis usually occurs in the early stage of NPC and is the main cause of patient mortality (6). Therefore, it is urgent for researchers to examine the underlying mechanism of the development and metastasis of NPC and to identify potential therapeutic targets for NPC.
Galectin-3 (gal-3), a member of the β-galactoside-binding protein family, is characterized by a specific chimeric structure containing a unique carbohydrate recognition domain and multifunctional N-terminal domain (7). Gal-3 is involved in cellular homeostasis, organogenesis, angiogenesis, tumor invasion and metastasis (8). Research has indicated that the upregulation of gal-3 promotes neoplastic transformation and contributes to the phenotype maintenance of malignant breast cells (9,10). In addition, patients with tumor metastasis exhibited significantly higher concentrations of circulating gal-3 compared with healthy individuals (11). Gal-3 was also found to induce the formation of new capillaries in vivo and sustain the angiogenic capability of tumor-associated macrophages (12,13). Thus, it was hypothesized that gal-3 may be associated in the development and metastasis of NPC. ERK1/2 and Akt signaling pathways are crucial regulators in the development and metastasis of NPC (14,15). However, whether gal-3 is involved in the activation of ERK1/2 and Akt signaling pathways remains unknown and requires further investigation.
In the present study, tumor tissue samples from patients with NPC and nasopharyngeal tissues from patients with chronic rhinitis (CR) were collected, and the difference in the expression levels of gal-3 was investigated. Subsequently, the NPC cell lines, 5-8F and 6-10B, and the nasopharyngeal epithelium cell line, NP69, were used to examine the function and potential mechanism of gal-3 in NPC.
Materials and methods
Collection of human samples
The study was approved by the Ethics Committee of Xiangya Hospital Central South University. In total, 40 tumor specimens and 37 serum samples (5 ml) were obtained from 40 patients with NPC, and 15 nasopharyngeal tissues and 26 serum samples were collected from 26 patients with CR in Xiangya Hospital Central South University between January 2015 and January 2018. Written informed consent was obtained from all patients prior to inclusion in the study. Characteristics of the patients, including sex, age and TNM stage, were recorded. The tissues were fixed in 4% formalin at room temperature for 24 h and embedded in paraffin for subsequent histological experiments, and the serum was stored at −80°C.
Cell culture
The NPC cell line, 5-8F, was kindly provided by the Cancer Research Institute of Central South University. The NPC cell line, 6-10B, and the immortalized nasopharyngeal epithelium cell line, NP69, were purchased from Hunan Fenghui Biotechnology Co., Ltd. 5-8F and 6-10B cells were cultured in high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin. NP69 cells were cultured in keratinocyte serum-free medium (Invitrogen; Thermo Fisher Scientific, Inc.). All cells were cultured in a 37°C incubator with 5% CO2.
Gal-3 short hairpin (sh)RNA (1,000 ng/µl) and control shRNA (1,000 ng/µl) (Shanghai Genechem Co., Ltd.) were transfected into cells at room temperature for 15 min using the FuGENE HD transfection reagent (Promega Corporation), according to the manufacturer's recommendation. After a month and a half of screening, subsequent experiments were performed. The sequences of the gal-3 shRNA and control shRNA are presented in Tables SI and SII.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using RNAiso Plus reagent (Takara Bio, Inc.) and reverse transcribed using the PrimeScript RT Reagent kit with gDNA Eraser (Takara Bio, Inc.). The temperature and duration of reverse transcription was: 37°C for 15 min, 85°C for 5 sec. Relative gene expression levels were measured using SYBR Premix Ex Taq II (Takara Bio, Inc.). The thermocycling conditions were: Holding stage, 95°C for 30 sec; PCR stage, 95°C for 5 sec, 58°C for 30 sec, 40 cycles; melt curve stage, 95°C for 15 sec, 60°C for 60 sec and 95°C for 15 sec. The results were quantitatively analyzed according to the 2−ΔΔCq method (16). The sequences of the primers used for RT-qPCR analysis are presented in Table SIII.
Immunoblotting
For immunoblotting, whole cell lysates were prepared in RIPA buffer (Sigma-Aldrich; Merck KGaA) containing protease inhibitor and phosphatase inhibitor (Roche Diagnostics). Protein concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Then, protein samples (20 µg) were separated by SDS-PAGE and subsequently transferred to PVDF membranes (EMD Millipore). The membranes were blocked in 5% milk for 1 h at 37°C before incubation with primary mouse monoclonal anti-gal 3 antibody (1:1,000; cat. no. 60207-1-lg; ProteinTech Group, Inc.), rabbit monoclonal anti-phosphorylated (p)-Akt (S473) antibody (1:1,000; cat. no. BS9913M; Bioworld Technology, Inc.), rabbit polyclonal anti-Akt antibody (1:1,000; cat. no. BS1810; Bioworld Technology, Inc.), rabbit monoclonal anti-p-ERK1/2 antibody (1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.), rabbit monoclonal anti-ERK1/2 antibody (1:1,000; cat. no. 4695; Cell Signaling Technology, Inc.) and rabbit polyclonal anti-GAPDH antibody (1:5,000; cat. no. 10494-1-AP; ProteinTech Group, Inc.) overnight at 4°C. Then, the membranes were incubated with HRP-coupled secondary antibodies (Goat Anti-Rabbit IgG(H+L), HRP conjugate, 1:10,000; cat. no. SA00001-2 and Goat Anti-mouse IgG(H+L), HRP conjugate, 1:10,000; cat. no. SA00001-2; ProteinTech Group, Inc.) for 1 h at 37°C. After washing with PBS, the membranes were visualized using ECL reagent (cat. no. p10100; NCM Biotech) and imaged using X-ray film. ImageJ software was used for densitometry (version number: 1.51v, http://imagej.net/downloads).
ELISA
The concentration of gal-3 in serum was determined using the Human Galectin-3 ELISA kit (cat. no. E-EL-H1470c; Elabscience, Inc.) according to the manufacturer's recommendation.
Immunohistochemical assay
Paraffin-embedded sections (tissues fixed in 4% formalin at room temperature for 24 h; section thickness, 4 µm) were deparaffinized and hydrated (100, 95, 80 and 70%). After antigen retrieval, sections were blocked using normal goat serum (cat. no. ZLI-9022; OriGene Technologies, Inc.) at room temperature for 30 min and then were incubated with primary antibodies against gal-3 (1:500; cat. no. 60207-1-lg; ProteinTech Group, Inc.), IL-6 (1:200, cat. no. ab6672; Abcam) and IL-8 (1:400; cat. no. 27095-1-AP; ProteinTech Group, Inc.). Then, sections were stained using a detection kit (cat. no. PV9000; OriGene Technologies, Inc.) at room temperature for 60 sec and hematoxylin at room temperature for 30 sec (both from OriGene Technologies, Inc.). Images (10 fields for each section) were obtained using light microscope with a digital camera (Olympus Corporation) at ×400 magnification. According to the method described by Hara and Okayasu (17), the present study blindly analyzed the expression levels of gal-3, IL-6 and IL-8 in tissues. The immunohistochemistry score was determined as follows: Staining percentage: No positive cells, 0; ≤25% positive cells, 1; 26–50% positive cells, 2; 51–75% positive cells, 3; and >75% positive cells, 4; and staining intensity: No staining, 0; weak staining, 1; moderate staining, 2; and dark staining, 3. Comprehensive score=staining percentage + staining intensity. Comprehensive scores of gal-3, IL-6 and IL-8 ≥4 were classified as high expression; scores of <4 were classified as low expression.
Cell proliferation
5-8F and 6-10B cells were seeded in 96-well plates at a density of 4,000 cells per well. After 24 h, 20 µl MTT solution was added. Then, 4 h later, MTT was removed, 100 µl DMSO was added and the absorbance at 490 nm was measured (Varioskan Flash; Thermo Scientific, Inc.).
Hoechst staining
5-8F and 6-10B cells (1×103) were cultured in 96-well plates and incubated at 37°C with 5% CO2 for 24 h. The cells were washed twice with PBS and incubated at room temperature with glacial acetic acid/methanol mixture (glacial acetic acid: Methanol=1:3) for 30 min. After washing with PBS, the cells were incubated with 1 µg/ml Hoechst 33258 solution for 10 min in the dark at 37°C. Images were then acquired using a fluorescent PerkinElmer Operetta CLS system (PerkinElmer, Inc.) at ×400 magnification; six fields were randomly chosen.
5-Ethynyl-2′-deoxyuridine (Edu) labeling Assay
5-8F and 6-10B cells (1×103) were cultured in 96-well plates and incubated at 37°C with 5% CO2 for 24 h. Cells were incubated with EdU solution (50 µM; B8010; Beijing Solarbio Science & Technology Co., Ltd.) for 2 h at room temperature and then was fixed using 4% paraformaldehyde at room temperature for 30 min. Cells were incubated with glycine (50 µl; 2 mg/ml; cat. no. ST085; Beyotime Institute of Biotechnology) for 5 min at room temperature and washed with PBS thrice. After that, cells were permeated with 0.5% Triton X-100 (cat. no. ST797; Beyotime Institute of Biotechnology) for 10 min at room temperature and stained with Apollo staining solution (cat. no. CA1170; Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at room temperature. Subsequently, cells were washed with PBS thrice and stained with Hoechst 33342 solution (cat. no. B8040; Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at room temperature. Images were then acquired using a fluorescent PerkinElmer Operetta CLS system (PerkinElmer, Inc.) at ×400 magnification; six fields were randomly chosen.
Scratch assay
5-8F and 6-10B cells were seeded in 6-well plates at a density of 2×105/ml and incubated at 37°C with 5% CO2. For the scratch assay, cells were scratched using 10-µl pipette tips. During cultivation, the medium was replaced with high-glucose DMEM containing 1% FBS. Images of cells were taken at ×100 magnification after 0, 24 and 48 h (Olympus Corporation). The wound closure rate [(initial wound area-wound area at 24 h)/initial wound area] was analyzed using Image Pro Plus 6.0 software (Media Cybernetics, Inc.) (18).
Migration assay
Cells were collected and resuspended in serum-free DMEM. Then, ~5×104 cells in 200 µl serum-free DMEM were seeded in the upper chamber, while the lower chamber was filled with 600 µl DMEM containing 15% FBS. After 24 h incubation at 37°C with 5% CO2, the cells on the upper face were wiped off, while those that migrated to the lower face were fixed using 4% paraformaldehyde at room temperature for 20 min and stained with crystal violet solution (cat. no. C0121; Beyotime Institute of Biotechnology) for 5 min. Images were obtained using a microscope (Olympus Corporation). The number of migrated cells was counted in three random fields (×200 magnification).
Statistical analysis
All experiments were repeated three times. All analyses were performed using SPSS 18.0 (SPSS, Inc.) and GraphPad Prism 8 (GraphPad Software, Inc.). The numerical data are presented as the mean ± SEM, and were analyzed using an unpaired Student's t-test or one-way ANOVA followed by Tukey's multiple comparisons test post hoc test. The Fisher's exact test and Pearson's correlation were used to analyze the difference in the expression levels of gal-3 and the relationship between gal-3 and inflammation cytokines. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of gal-3 are upregulated in patients with NPC and in NPC cell lines
The expression levels of gal-3 in tumor tissues and nasopharyngeal tissues were determined via immunochemistry. The results demonstrated that gal-3 was mainly expressed in the cytoplasm and was significantly upregulated in patients with NPC compared with patients with CR (Fig. 1A; Table I). Furthermore, the results of the ELISA showed that the concentration of circulating gal-3 in patients with NPC was significantly higher compared with that of patients with CR (Fig. 1B). The mRNA expression levels of gal-3 in the NPC cell lines, 5-8F and 6-10B, were significantly higher compared with those of NP69, a nasopharyngeal epithelium cell line (Fig. 1C). In addition, immunoblotting demonstrated that the expression levels of gal-3 protein in 5-8F and 6-10B cells were significantly upregulated compared with those of NP69 cells (Fig. 1D). However, there was no correlation between the expression levels of gal-3 in tumor tissues and the TNM stage of patients with NPC (Table SIV).
Figure 1.
Expression levels of gal-3 are upregulated in patients with NPC and in NPC cell lines. (A) Representative images of immunochemistry staining of gal-3 in nasopharyngeal tissues and tumor tissues at ×400 magnification. (B) Concentrations of serum gal-3 in patients with NPC and patients with CR were determined via ELISA. #P<0.05. (C) mRNA expression levels of gal-3 in NP69, 5-8F and 6-10B cells were detected using reverse transcription-quantitative PCR and normalized to GAPDH. (D) Protein expression levels of gal-3 in NP69, 5-8F and 6-10B cells were detected via western blotting, using GAPDH as the loading control. **P<0.01, ***P<0.001 vs. NP69 cells. Gal-3, galectin-3; NPC, nasopharyngeal carcinoma; CR, chronic rhinitis.
Table I.
Expression levels of gal-3 in patients with NPC compared with patients with CR.
| Expression levels of gal-3 | ||||
|---|---|---|---|---|
| Group | Cases (n) | High expression | Low expression | P-value |
| CR | 15 | 7 | 8 | 0.0015 |
| NPC | 40 | 36 | 4 | |
The Fisher's exact test was used to analyze the expression levels of gal-3 in patients with NPC and patients with CR. CR, chronic rhinitis; NPC, nasopharyngeal carcinoma; Gal-3, galectin-3.
Knockdown of gal-3 inhibits the proliferation and migration of NPC cell cells and induces their apoptosis
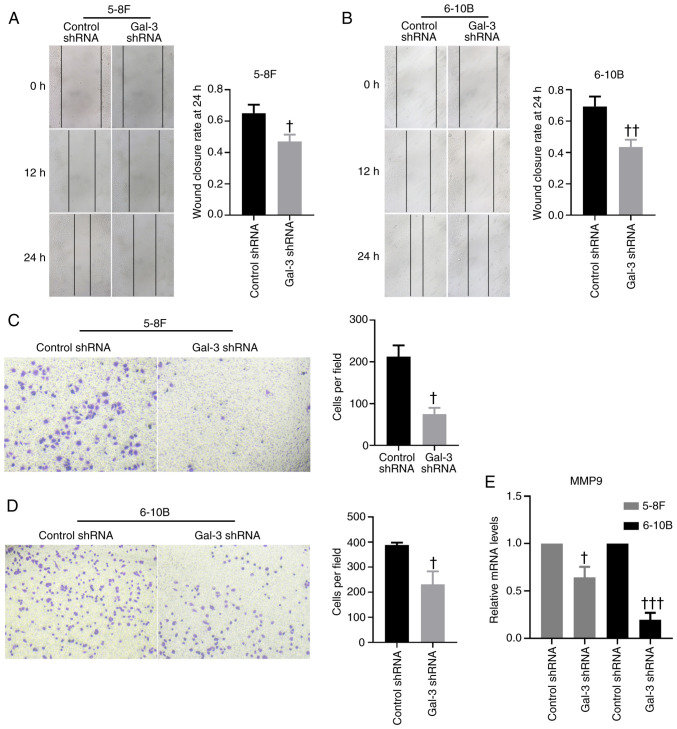
Transfection of gal-3 shRNA was capable of downregulating the protein and mRNA expression levels of gal-3 in 5-8F and 6-10B cells (Fig. 2A and B). The MTT proliferation assay demonstrated that, after transfection with gal-3 shRNA, 5-8F and 6-10B cells exhibited significantly impaired proliferative capabilities compared with cells transfected with control shRNA (Fig. 2C). The results of EdU staining indicated that knockdown of gal-3 significantly inhibited the proliferation of 5-8F and 6-10B cells, compared with cells transfected with control shRNA, while the results of Hoechst 33258 staining suggested that knockdown of gal-3 promoted apoptosis in 5-8F and 6-10B cells (Fig. 2D and E). On the other hand, based on the scratch assay used to determine the mobility of 5-8F and 6-10B cells, knockdown of gal-3 in NPC cells significantly decreased their migratory capability compared with the results of cells transfected with control shRNA (Fig. 3A and B). A Transwell migration assay further validated these findings (Fig. 3C and D). In addition, the mRNA expression levels of MMP-9 in 5-8F and 6-10B cells were significantly downregulated after transfection with gal-3 shRNA (Fig. 3E).
Figure 2.
Knockdown of gal-3 inhibits the proliferation of 5-8F and 6-10B cells, while inducing their apoptosis. (A) Protein expression levels of gal-3 in 5-8F and 6-10B cells transfected with control shRNA or gal-3 shRNA were determined via western blotting, using GAPDH as the loading control. (B) mRNA expression levels of gal-3 in 5-8F and 6-10B cells transfected with control shRNA or gal-3 shRNA were determined via reverse transcription-quantitative PCR. (C) A MTT proliferation assay using was used to investigate the proliferation of 5-8F and 6-10B cells transfected with control shRNA or gal-3 shRNA. Representative images of Hoechst 33258 and EdU staining of (D) 5-8F and (E) 6-10B cells transfected with control shRNA or gal-3 shRNA to show their apoptosis and proliferation (magnification, ×400). †P<0.05, ††P<0.01, †††P<0.001 vs. control shRNA. shRNA, short hairpin RNA; Gal-3, galectin-3; EdU, 5-Ethynyl-2′-deoxyuridine; O.D, optical density.
Figure 3.
Knockdown of gal-3 inhibits the migration of 5-8F and 6-10B cells. Representative images of the scratch assay demonstrating the mobility of (A) 5-8F and (B) 6-10B transfected with control shRNA or gal-3 shRNA (magnification, ×100). Representative images of a Transwell assay showing the capability of migration of (C) 5-8F and (D) 6-10B transfected with control shRNA or gal-3 shRNA (magnification, ×200). (E) mRNA expression levels of MMP-9 in 5-8F and 6-10B cells transfected with control shRNA or gal-3 shRNA were investigated via reverse transcription-quantitative PCR. †P<0.05, ††P<0.01, †††P<0.001 vs. control shRNA. shRNA, short hairpin RNA; Gal-3, galectin-3.
Gal-3 is associated with the inflammatory state of NPC
The expression levels of the inflammatory cytokines, IL-6 and IL-8, were measured using a immunohistochemical assay to assess the inflammatory state of the tumor tissue. The results suggested that IL-6 and IL-8 were mainly expressed in the cytoplasm and extracellular matrix. The relationship between gal-3 and the inflammatory cytokines was analyzed, and the results demonstrated that the expression level of gal-3 was positively correlated with the expression levels of IL-6 and IL-8, which suggests that gal-3 was associated with the inflammatory state of NPC (Fig. 4A). Furthermore, 5-8F and 6-10B cells transfected with gal-3 shRNA exhibited significantly lower expression levels of IL-8 compared with cells transfected with control shRNA (Fig. 4B).
Figure 4.
Gal-3 is associated with the inflammatory state of NPC. (A) Representative images of IHC staining gal-3, IL-6 and IL-8 in tumor tissues (magnification, ×400). The Spearman's correlation analyzation indicated that the expression level of gal-3 was positively correlated with the expression levels of IL-6 and IL-8 in tumor tissues. (B) mRNA expression levels of IL-8 in 5-8F and 6-10B cells transfected with control shRNA or gal-3 shRNA were determined using reverse transcription-quantitative PCR. ††P<0.01, †††P<0.001 vs. control shRNA. shRNA, short hairpin RNA; Gal-3, galectin-3; IHC, immunohistochemistry.
Knockdown of gal-3 suppresses the ERK1/2 and Akt signaling pathways
The ERK1/2 and Akt signaling pathways are involved in the processes of tumor survival and migration (19). After transfection with gal-3 shRNA, the phosphorylation levels of ERK1/2 and Akt were significantly decreased in 5-8F and 6-10B cells, compared with cells transfected with control shRNA (Fig. 5A and B).
Figure 5.
Knockdown of gal-3 suppresses the ERK1/2 and Akt signaling pathways. Phosphorylation levels of ERK1/2 and Akt in (A) 5-8F and (B) 6-10B cells transfected with control shRNA or gal-3 shRNA were detected via western blotting, using GAPDH as the loading control. †P<0.05, ††P<0.01, †††P<0.001 vs. control shRNA. shRNA, short hairpin RNA; Gal-3, galectin-3; p-, phosphorylated.
Discussion
Compared with other types of cancer, NPC is relatively rare, accounting for only 0.7% of all cancer types in 2018 (20). However, >70% of new NPC cases are diagnosed in Southeast Asia, indicating an extremely unbalanced global distribution (20). In addition, NPC is one of the most aggressive cancer types and is characterized by frequent lymph node metastasis (21). Therefore, most patients with NPC are diagnosed at the advanced stage (22). Radiotherapy and chemotherapy are the conventional treatments for NPC, but the prognosis for patients at the advanced stage remains poor (23). Under such circumstances, it is important to investigate the underlying mechanism of occurrence and development of NPC.
Gals are carbohydrate-binding proteins, and members of the galectin family serve important roles in different types of cancer (24). Using proteomic analysis, our previous study reported that gas-1 acted as a biomarker for NPC (25). Gal-3 exhibits a specific chimeric structure, and is associated with the development of tumors and metastasis (8). Gal-3 is also significantly upregulated in Caco2 and DLD-1 cells, which are both human colon cancer cell lines, and the inhibition of gal-3 can suppress the mobility of these cells (26). Under hypoxic conditions, the secretion of gal-3 is significantly upregulated in tumor-associated macrophages, and the upregulation of gal-3 can result in the progression of breast cancer (27). Moreover, forkhead box D1 and gal-3 form a positive regulatory loop to modulate the aggressiveness of lung cancer (28). In head and neck adenoid cystic carcinoma, gal-3 was found to have a close relationship with distant metastasis (29). Thus, it was suggested that gal-3 may be associated with the development and metastasis of NPC.
In the present study, tumor tissues and nasopharyngeal tissues were obtained from patients with NPC and CR, respectively, and the immunochemistry results indicated that gal-3 was significantly upregulated in the tumor tissues from patients with NPC compared with the nasopharyngeal tissues from patients with CR. Furthermore, the concentration of circulating gal-3 in patients with NPC was significantly higher compared with that of patients with CR. These results implied that gal-3 may be involved in the development and metastasis of NPC. The NPC cell lines, 5-8F and 6-10B, and immortalized nasopharyngeal epithelium cell line NP69 were also used to examine the role of gal-3 in NPC. Consistent with the expression level of gal-3 in vivo, 5-8F and 6-10B cells exhibited significantly greater expression levels of gal-3 compared with NP69.
In the subsequent experiment, gal-3 shRNA was established to investigate the roles of gal-3 in NPC cells. After transfection with gal-3 shRNA, the proliferation and migration of 5-8F and 6-10B cells were significantly inhibited, while apoptosis in 5-8F and 6-10B cells was significantly activated. In addition, the expression level of MMP-9 was upregulated in cells transfected with gal-3 shRNA. MMPs are involved in the modulation of cell-cell interaction, cell-matrix interaction and extracellular matrix remodeling, and are associated with tumor metastasis (30). MMP-9 acts as a crucial regulator of metastasis of NPC and may be a potential therapeutic target for patients with NPC (31,32). The findings of the present study suggested that gal-3 served important roles in the proliferation, migration and apoptosis of NPC cells, and a regulate the development and metastasis of NPC in vivo. However, analysis of the correlation between the expression level of gal-3 and the TNM stage of patients with NPC suggested that there was no significant correlation between the two (Table SIV). This may be due to the relatively small number of cases in the present study (40 cases). Another explanation may be that the majority of the patients were at TNM stage III–IV (Table SIV).
The NF-κB signaling pathway regulates the development of NPC, and the dysregulation of NF-κB is regarded as a vital component of NPC tumorigenesis (33). NF-κB mediates the inflammatory response inside tumors and leads to the accumulation of proinflammatory cytokines in tumor tissues, which contributes to the tumor microenvironment, and eventually, tumorigenesis (34). ILs are multifunctional inflammatory cytokines that can regulate inflammatory responses and are considered important oncogenic mediators (35). Gal-3 is known to provoke an inflammatory reaction in acute inflammatory diseases, such as pneumonia, while inducing wound healing and fibrosis in chronic inflammatory diseases (36,37). The role of gal-3 in the inflammation state of NPC, however, requires further investigation. In the present study, the expression levels of IL-6 and IL-8 in tumor tissue samples from patients with NPC were determined using immunohistochemistry to assess the inflammatory state of these tissues. It was found that the expression level of gal-3 in tumor tissues was positively correlated with the expression levels of IL-6 and IL-8 in tumor tissues. Furthermore, after transfection with gal-3 shRNA, the expression level of IL-8 in 5-8F and 6-10B cells was significantly downregulated compared with that of cells transfected with control shRNA. These results suggested that gal-3 may be involved in the inflammatory response of NPC.
Finally, the current study sought to define the underlying mechanism of the effect of gal-3 on NPC cells. The activation of the ERK1/2 and Akt signaling pathways is involved in the proliferation, migration, invasion, metastasis and radiosensitivity of NPC (38–40). However, whether gal-3 affects the ERK1/2 and Akt signaling pathways of NPC cell lines remains unknown. Thus, in the present study, the phosphorylation levels of ERK1/2 and Akt were investigated in 5-8F and 6-10B cells transfected with gal-3 shRNA or control shRNA, and the results of immunoblotting indicated that gal-3 knockdown leads to the suppression of the ERK1/2 and Akt signaling pathways in 5-8F and 6-10B cells. These results suggest that activation of the ERK1/2 and Akt signaling pathways may be the potential mechanism of the effect of gal-3 on NPC cells.
In conclusion, the present study demonstrated that the expression level of gal-3 was significantly upregulated in patients with NPC compared with patients with CR, and that the expression level of gal-3 was associated with the inflammatory state of tumor tissues from patients with NPC. Knockdown of gal-3 in 5-8F and 6-10B cells using gal-3 shRNA led to the inhibition of cell proliferation and migration, promotion of cell apoptosis and downregulation of MMP-9 and IL-8 expression levels. Moreover, knockdown of gal-3 suppressed the phosphorylation of ERK1/2 and Akt, which may highlight the underlying mechanism of gal-3 in NPC. Therefore, gal-3 may be a potential therapeutic target for NPC.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 81974112), the National Natural Science Foundation of China (grant no. 81873494), the National Natural Science Foundation of China (grant no. 81922012), the Hunan Natural Science Foundation (grant no. 2018JJ2665) and the Hunan Natural Science Foundation (grant no. 2017JJ2396).
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81974112), the National Natural Science Foundation of China (grant no. 81873494), the National Natural Science Foundation of China (grant no. 81922012), the Hunan Natural Science Foundation (grant no. 2018JJ2665) and the Hunan Natural Science Foundation (grant no. 2017JJ2396).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ML and YBC completed the experiments and wrote the manuscript. FL, JQQ and LCR collected the tissues, conducted the immunohistochemical assay, evaluated the expression levels of gal-3, IL-6, and IL-8 in tissues, and recorded the patients' data. JC and CET designed the study, confirmed the authenticity of all the raw data and helped finalize the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethic Committee of Xiangya Hospital Central South University. Written informed consent was obtained from all patients prior to inclusion into the study.
Patient consent for publication
Patients agreed with the publication of this study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y. EBV based cancer prevention and therapy in nasopharyngeal carcinoma. NPJ Precis Oncol. 2017;1:10. doi: 10.1038/s41698-017-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu M, Li X, Li X, Li G. Signaling transduction network mediated by tumor suppressor/susceptibility genes in NPC. Curr Genomics. 2009;10:216–222. doi: 10.2174/138920209788488481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 6.Kontny U, Franzen S, Behrends U, Bührlen M, Christiansen H, Delecluse H, Eble M, Feuchtinger T, Gademann G, Granzen B, et al. Diagnosis and treatment of nasopharyngeal carcinoma in children and adolescents - recommendations of the GPOH-NPC study group. Klin Padiatr. 2016;228:105–112. doi: 10.1055/s-0041-111180. [DOI] [PubMed] [Google Scholar]
- 7.Newlaczyl AU, Yu LG. Galectin-3-a jack-of-all-trades in cancer. Cancer Lett. 2011;313:123–128. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Sciacchitano S, Lavra L, Morgante A, Ulivieri A, Magi F, De Francesco GP, Bellotti C, Salehi LB, Ricci A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int J Mol Sci. 2018;19:379. doi: 10.3390/ijms19020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922–34930. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 10.Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down- regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–668. [PubMed] [Google Scholar]
- 11.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–1393. [PubMed] [Google Scholar]
- 12.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Méndez-Huergo SP, Blidner AG, Rabinovich GA. Galectins: Emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr Opin Immunol. 2017;45:8–15. doi: 10.1016/j.coi.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Han YY, Liu K, Xie J, Li F, Wang Y, Yan B. LINC00114 promoted nasopharyngeal carcinoma progression and radioresistance in vitro and in vivo through regulating ERK/JNK signaling pathway via targeting miR-203. Eur Rev Med Pharmacol Sci. 2020;24:2491–2504. doi: 10.26355/eurrev_202003_20517. [DOI] [PubMed] [Google Scholar]
- 15.Lv B, Li F, Liu X, Lin L. The tumor-suppressive role of microRNA-873 in nasopharyngeal carcinoma correlates with downregulation of ZIC2 and inhibition of AKT signaling pathway. Cancer Gene Ther. 2020;28:74–88. doi: 10.1038/s41417-020-0185-8. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Hara A, Okayasu I. Cyclooxygenase-2 and inducible nitric oxide synthase expression in human astrocytic gliomas: Correlation with angiogenesis and prognostic significance. Acta Neuropathol. 2004;108:43–48. doi: 10.1007/s00401-004-0860-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Liu F, Han F, Lv L, Tang CE, Xie Z, Luo F. Omentin-1 is associated with atrial fibrillation in patients with cardiac valve disease. BMC Cardiovasc Disord. 2020;20:214. doi: 10.1186/s12872-020-01478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moloudizargari M, Moradkhani F, Hekmatirad S, Fallah M, Asghari MH, Reiter RJ. Therapeutic targets of cancer drugs: Modulation by melatonin. Life Sci. 2021;267:118934. doi: 10.1016/j.lfs.2020.118934. [DOI] [PubMed] [Google Scholar]
- 20.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 21.Bruce JP, Yip K, Bratman SV, Ito E, Liu FF. Nasopharyngeal cancer: Molecular landscape. J Clin Oncol. 2015;33:3346–3355. doi: 10.1200/JCO.2015.60.7846. [DOI] [PubMed] [Google Scholar]
- 22.Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS, Zeng YX, Jia WH. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374:22–30. doi: 10.1016/j.canlet.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104:272–278. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim Biophys Acta. 2015;1855:235–247. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Tang CE, Tan T, Li C, Chen ZC, Ruan L, Wang HH, Su T, Zhang PF, Xiao ZQ. Identification of Galectin-1 as a novel biomarker in nasopharyngeal carcinoma by proteomic analysis. Oncol Rep. 2010;24:495–500. [PubMed] [Google Scholar]
- 26.Wu KL, Kuo CM, Huang EY, Pan HM, Huang CC, Chen YF, Hsiao CC, Yang KD. Extracellular galectin-3 facilitates colon cancer cell migration and is related to the epidermal growth factor receptor. Am J Transl Res. 2018;10:2402–2412. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Li YS, Yu LG, Zhang XK, Zhao L, Gong FL, Yang XX, Guo XL. Galectin-3 expression and secretion by tumor-associated macrophages in hypoxia promotes breast cancer progression. Biochem Pharmacol. 2020;178:114113. doi: 10.1016/j.bcp.2020.114113. [DOI] [PubMed] [Google Scholar]
- 28.Li CH, Chang YC, Hsiao M, Liang SM. FOXD1 and Gal-3 form a positive regulatory loop to regulate lung cancer aggressiveness. Cancers (Basel) 2019;11:1897. doi: 10.3390/cancers11121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teymoortash A, Pientka A, Schrader C, Tiemann M, Werner JA. Expression of galectin-3 in adenoid cystic carcinoma of the head and neck and its relationship with distant metastasis. J Cancer Res Clin Oncol. 2006;132:51–56. doi: 10.1007/s00432-005-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chien MH, Lin CW, Cheng CW, Wen YC, Yang SF. Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin Ther Targets. 2013;17:203–216. doi: 10.1517/14728222.2013.740012. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Zhuang R. Dione-thiophene conjugate inhibits proliferation and metastasis of nasopharyngeal carcinoma cells through calcium binding protein-P down-regulation. Eur J Med Chem. 2019;168:199–206. doi: 10.1016/j.ejmech.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 32.Lan YY, Chang FH, Tsai JH, Chang Y. Epstein-Barr virus Rta promotes invasion of bystander tumor cells through paracrine of matrix metalloproteinase 9. Biochem Biophys Res Commun. 2018;503:2160–2166. doi: 10.1016/j.bbrc.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Li YY, Chung GT, Lui VW, To KF, Ma BB, Chow C, Woo JK, Yip KY, Seo J, Hui EP, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-kappaB pathway activating mutations. Nat Commun. 2017;8:14121. doi: 10.1038/ncomms14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia Y, Shen S, Verma IM. NF-kappaB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Yang J, Bian Z, Shi D, Cao Z. Long noncoding RNA DANCR promotes nasopharyngeal carcinoma progression by interacting with STAT3, enhancing IL-6/JAK1/STAT3 signaling. Biomed Pharmacother. 2019;113:108713. doi: 10.1016/j.biopha.2019.108713. [DOI] [PubMed] [Google Scholar]
- 36.Farnworth SL, Henderson NC, Mackinnon AC, Atkinson KM, Wilkinson T, Dhaliwal K, Hayashi K, Simpson AJ, Rossi AG, Haslett C, Sethi T. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Friess H, Zhu Z, Frigeri L, Zimmermann A, Korc M, Berberat PO, Büchler MW. Galectin-1 and galectin-3 in chronic pancreatitis. Lab. Invest. 2000;80:1233–1241. doi: 10.1038/labinvest.3780131. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Lv Y, Cheng C, Huang Y, Yang L, He J, Tao X, Hu Y, Ma Y, Su Y, et al. SPEN induces miR-4652-3p to target HIPK2 in nasopharyngeal carcinoma. Cell Death Dis. 2020;11:509. doi: 10.1038/s41419-020-2699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng P, Chen X, Xie J, Chen X, Lin S, Ye L, Chen L, Lin J, Yu X, Zheng M. Capn4 is induced by and required for Epstein-Barr virus latent membrane protein 1 promotion of nasopharyngeal carcinoma metastasis through ERK/AP-1 signaling. Cancer Sci. 2020;111:72–83. doi: 10.1111/cas.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Liu G, Mao J, Xie M, Zhao M, Guo X, Liang S, Li H, Li X, Wang R. IGF-1R inhibition suppresses cell proliferation and increases radiosensitivity in nasopharyngeal carcinoma cells. Mediators Inflamm. 2019;2019:5497467. doi: 10.1155/2019/5497467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.