Abstract
Background
It is currently unknown whether patients with childhood-onset Takayasu disease (c-TA) are prone to physical inactivity and poor aerobic capacity. In this study, we assessed physical activity levels and cardiorespiratory fitness along with health-related quality of life (HRQL) and various traditional and non-traditional risk factors in patients with c-TA vs. healthy controls.
Methods
c-TA patients with non-active disease (n = 17) and age- and sex-matched healthy controls (n = 17) were enrolled in the study. We assessed physical activity levels, aerobic capacity, body composition, systemic inflammation, cardiometabolic markers, disease-related parameters, and HRQL.
Results
c-TA patients showed greater time spent in sedentary behavior (P = 0.010), and lower moderate-to-vigorous physical activity (P > 0.001) and lower step counts per day (P > 0.001). VO2peak (P < 0.001) and chronotropic response (P = 0.016) were significantly lower in patients with c-TA and they had worse HRQL in physical domain (P < 0.001), lower bone mineral content and density, and higher insulin levels vs. healthy controls (all P ≤ 0.05).
Conclusions
c-TA patients exhibited reduced physical activity levels and aerobic capacity, worse cardiometabolic risk factors and HRQL parameter compared with healthy peers. Physical inactivity and aerobic deconditioning emerge as potentially novel risk factors for c-TA. The role of physical activity interventions in preventing poor outcomes and improving HRQL in c-TA remains to be explored.
Keywords: Physical activity, Pediatric rheumatology, Physical fitness, Cardiovascular risk, Cardiovascular health
Background
Childhood-onset Takayasu Arteritis (c-TA) is a very rare, granulomatous, chronic large-vessel vasculitis that involves mostly the aorta and its major branches [1]. The main pathophysiological features of this disease include increased expression of interleukin 1 (IL-1) and IL-6 in aortic tissues, and up-regulated tumor necrosis factor alpha (TNF-α) from peripheral blood mononuclear cells [2, 3]. Importantly, c-TA shows a high mortality rate (~ 35 %) [4–6] that is related to cardiovascular diseases, such as arterial occlusions and stenosis, ischemia or aneurysm formation [7].
c-TA patients show cardiometabolic abnormalities including increased cholesterol and triglycerides levels, and decreased high-density lipoprotein [HDL] cholesterol levels, impaired insulin sensitivity, and an exacerbated inflammation [4, 5]. All of these risk factors could be aggravated by an inactive lifestyle, which, in turn, could further worse clinical symptoms, traditional (e.g., diabetes, hyperlipidemia, and hypertension) and non-traditional cardiometabolic risk factors (e.g., inflammation, oxidative stress, endothelial dysfunction), physical capacity, and overall health-related quality of life (HRQL), as seen in other pediatric rheumatic diseases [8–10]. Whether due to direct limitations imposed by the disease in the cardiovascular involvement, and other indirect factors (e.g., superprotection by parents and health practitioners, low self-efficacy and, social isolation), one could surmise that patients with c-TA could also experience low physical activity levels and decreased aerobic conditioning, however, supporting evidence remains scarce.
Thus, we assessed physical activity levels and cardiorespiratory fitness along with HRQL parameters and various traditional and non-traditional cardiovascular risk factors related to physical activity and physical capacity among patients with c-TA.
Methods
Study design and patients
This was a multicenter, cross-sectional study conducted in Sao Paulo, SP, Brazil, between November 2017 and March 2019, as a part of a multicenter, randomized controlled trial aimed to test the safety and efficacy of an exercise training program in patients with c-TA (NCT03494062). The study was approved by the local ethics committees and all the procedures were in accordance with the recommendations of the Helsinki Declaration. The participants and their parents provided written informed consent before entering the study.
c-TA patients were recruited from the Division of Rheumatology of the School of Medicine and the Pediatric Rheumatology Unit of the Children and Adolescents’ Institute of the University of Sao Paulo, and from the Division of Pediatric Rheumatology of the Federal University of Sao Paulo. Inclusion criteria were: patients who fulfilled the current classification for c-TA [11] aged between 12 and 25 years old. Exclusion criteria were: pregnancy, heart failure, renal failure, cardiac, pulmonary or musculoskeletal disorders that precluded exercise training, the Pediatric Vasculitis Activity Score (PVAS) > 1 or greater [12] and acute infection in the last 30 days. Seventeen age- and sex-matched healthy controls (CTRL) were either friends referred by the patients or recruited from the Outpatient Clinics of Exercise and Sports Medicine (Clinical Hospital of the School of Medicine of the University of Sao Paulo), which follows healthy children and adolescents.
Physical activity levels
Physical activity was objectively measured using Actigraph GT3X accelerometers. All participants were instructed to wear the accelerometer during waking hours, except when bathing or swimming, for 7 consecutive days. All participants accumulated at least 10 h of valid activity recordings per day for at least 4 days. The accelerometer was worn on an elastic belt at the waistline on the right side of the hip. Participants were instructed to complete a daily time diary to record when the device was worn and removed to ensure data accuracy (e.g., to distinguish sedentary time from non-wear time). Data were exported from the device every 15-seconds for children and adolescent and, 60-seconds for adults using ActiLife 6 software. Non-wear time was defined as a minimum of 60 min of continuous zero counts and days with at least 600 min of wear time were considered valid [13]. Freedson cut points were used to define epochs for patients with c-TA aged ≥ 18 years: sedentary time (< 100 counts/minute), light-intensity physical activity (≥ 100 to < 1,952 counts/minute), and moderate-to-vigorous physical activity (MVPA) (≥ 1,952 counts/minute) [13]. Evenson cut points were used to define epochs for patients with c-TA aged < 18 years: sedentary time (< 100 counts/minute), light-intensity physical activity (≥ 100 to < 2,296 counts/minute), and MVPA (≥ 2,296 counts/minute) [14]. All participants had valid accelerometer data (a minimum of any four valid days). Data are shown as minutes/day in each domain of intensity.
Cardiorespiratory fitness test
Maximum graded exercise tests were performed on a treadmill (Centurion 200, Micromed), with increments in velocity or grade at every minute until volitional exhaustion. Heart rate (HR) was continuously recorded at rest (HRrest), during exercise and recovery using a 12-lead ECG (ErgoPC Elite, Micromed, Brazil). Resting HR (HRrest) was measured immediately before the test and HRpeak was measured at the end of the test with the subjects standing on the treadmill. Oxygen uptake (VO2) and carbon dioxide output (VCO2) were obtained through breath-by-breath sampling and expressed as a 30-second average using an indirect calorimetric system (Cortex, Metalyzer IIIB). The test was considered maximal when one of the following criteria was met: VO2plateau (i.e., < 150 ml/min increase between two consecutive stages); respiratory exchange ratio value above 1.10; heart rate no less than 10 beats below age-predicted maximal heart rate. Heart rate was continuously recorded at rest and during exercise and recovery. Peak oxygen consumption (VO2peak) and ventilatory thresholds were determined following previous description [14]. Chronotropic response was calculated through the following formula: ([HRpeak-HRrest/220-age-HRrest] x 100).
Disease and healthy‐related quality of life parameters
Age at disease onset, time since diagnosis and current medications were obtained through review of medical records and interviews. Two physicians blinded to the intervention performed the clinical assessments using the following tools: Indian Takayasu Arteritis Clinical Activity Score (ITAS2010) [15] and Pediatric Vasculitis Activity Score (PVAS) [12]. General HRQL was also assessed through SF-36, which is a multi-purpose, short-form health survey with 36 questions. It yields a profile of functional health and well-being scores as well as psychometrically-based physical and mental health summary measures, with the higher total scores indicating better health condition [16].
Bone mineral density and body composition
Participants underwent a dual-energy x-ray absorptiometry (DXA) scan (GE Healthcare®, WI, USA) to quantify bone mineral density (BMD) at lumbar spine (L1-L4), total hip and total body; bone mineral content (BMC); fat mass; percentage fat mass; lean mass Visceral adipose tissue were also measured by DXA using CoreScan™ software (enCORE version 17). All DXA measurements were carried out by the same trained technician.
Blood sampling
After 12-hour overnight fasting, 40 mL of blood was extracted into vacutainer tubes and stored for subsequent analysis of fasting glucose and insulin, blood lipids, total cholesterol, triglycerides, anti- and pro-inflammatory biomarkers, and angiogenesis markers.
IFN-γ, IL-10, IL-12p70, IL-1ra, IL-1β, IL-6, TNF-α, VEGF and PDGF were analyzed using Milliplex MAP Human Cytokine/Chemokine Magnetic Bead Panel (Millipore Corp., Billerica, MA), following the manufacturer’s instructions.
Statistical analysis
Data were presented as mean/median, standard deviation (SD) or interquartile range (IQR 25–75), between-group difference and 95 % confidence interval (95 %CI), unless otherwise indicated. Normality data was assessed by Shapiro-Wilk test. Two-tailed unpaired T-test (for normally distributed dependent variables) or Mann-Whitney U test (for non-normally distributed dependent variables) was used to compare patients with c-TA vs. controls. Data analysis were performed using Statistical Package for Social Sciences (SPSS), version 17.0 for Windows. Significance level was set at P ≤ 0.05.
Results
Seventeen patients with c-TA (64.7 % females) aged 12–25 years, with median disease duration of 9.5 years were recruited. All patients fulfilled the classification for c-TA [11] and all patients had inactive disease based on disease activity scores previously described [12, 15]. Table 1 illustrates demographic, current clinical treatment, laboratory and disease-related parameters patients’ data. Groups were comparable regarding age, gender, weight, and body mass index (BMI) (all P > 0.05).
Table 1.
Baseline, demographic and disease-related parameters in childhood-onset Takayasu arteritis (c-TA) and healthy control group (CTRL).
| c-TA (n = 17) |
CTRL (n = 17) |
|
|---|---|---|
| Demographic parameters | ||
| Age (years) | 18.4 (19) ± 3.44 (12–20) | 18.5 (20) ± 3.50 (12–21) |
| Sex, female, n (%) | 11 (64.7) | 11 (64.7) |
| Height (m) | 1.60 (1.57) ± 0.11 (1.46–1.64) | 1.65 (1.62) ± 0.12 (1.40–1.74) |
| Body weight (kg) | 56.1 (54.8) ± 10.6 (40.6–63.8) | 60.6 (62.0) ± 13.3 (31.2–71.0) |
| BMI (kg/m2) | 17.4 (17.3) ± 2.68 (13.4–18.8) | 18.2 (18.5) ± 3.39 (11.0-20.4) |
| Disease duration (years) | 9.48 (10.1) ± 4.16 (7.07–11.8) | NA |
| Age at the disease onset (years) | 9.25 (9.50) ± 4.62 (6.75-13.0) | NA |
| TA classification n, (%) | ||
| Type I | 1 (5.88) | NA |
| Type IIa | 1 (5.88) | NA |
| Type III | 2 (11.7) | NA |
| Type IV | 4 (23.5) | NA |
| Type V | 9 (52.9) | NA |
| Arterial hypertension, n (%) | 10 (58.8) | NA |
| Aortic stenosis, n (%) | 9 (52.9) | NA |
| Biological therapy, n (%) | ||
| Infliximab | 5 (29.4) | NA |
| Non-biological therapy, n (%) | ||
| Methotrexate | 8 (47.0) | NA |
| Leflunomide | 5 (29.4) | NA |
| Prednisone | 4 (23.5) | NA |
| Enalapril | 5 (29.4) | NA |
| Amlodipine | 3 (17.2) | NA |
| Disease score | ||
| ITAS score | 0.0 (0.0–0.0) | NA |
| PVAS score | 0.0 (0.0–1.0) | NA |
Data are expressed as mean (median) ± SD (IQR 25–75) or n (%). BMI (body mass index), ITAS (indian takayasu’s arteritis activity score 2010), PVAS (Pediatric Vasculitis Activity Score), NA (not applicable).
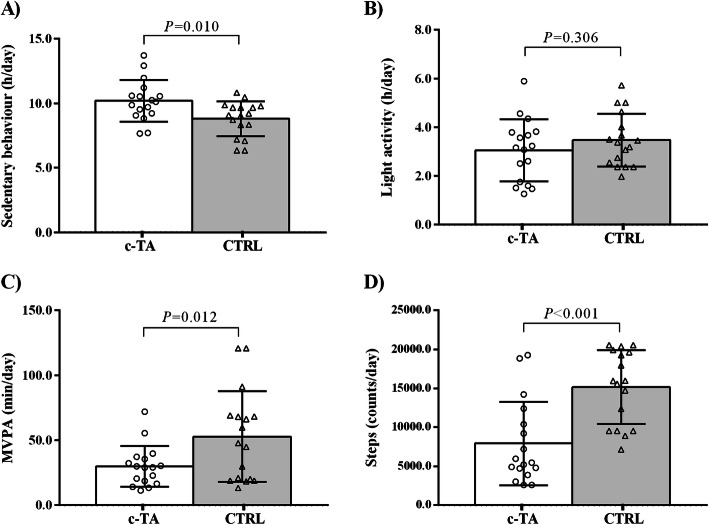
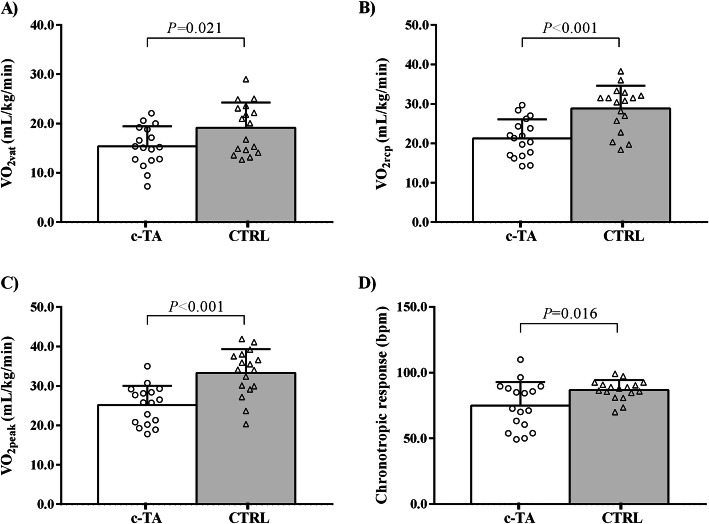
Physical activity and aerobic capacity data are shown in Figs. 1 and 2. c-TA patients exhibited greater time spent in sedentary behavior (hours per day) (P = 0.010), significantly lower moderate-to-vigorous physical activity (MVPA) (minutes per day) (P = 0.012) and step counts (P < 0.001) as compared to CTRL. c-TA patients also had lower VO2vat (P = 0.021), VO2rcp (P < 0.001), VO2peak (P < 0.001) and chronotropic response (P = 0.016) vs. CTRL.
Fig. 1.
Objectively-measured physical activity in patients with childhood-onset Takayasu Arteritis (c-TA) and healthy controls (CTRL). Panel (a) Sedentary behavior; Panel (b) Light activity; Panel (c) Moderate-to-vigorous physical activity (MVPA); Panel (d) Steps per day
Fig. 2.
Cardiorespiratory measures in patients with childhood-onset Takayasu Arteritis (c-TA) and healthy controls (CTRL). Panel (a) Ventilatory threshold (VO2vat); Panel (b) Respiratory compensation point (VO2rcp); Panel (c) Peak oxygen uptake (VO2peak); Panel (d) chronotropic response
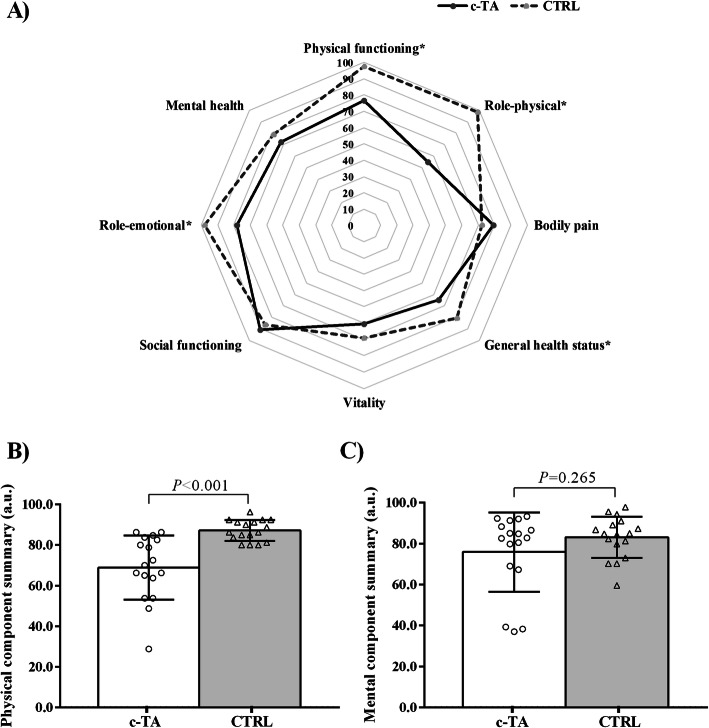
HRQL parameters are depicted in Fig. 3. c-TA patients had worse HRQL regarding the physical domain (P < 0.001) compared with CTRL. As for SF-36 subscales, functional capacity (P = 0.021), physical appearance (P < 0.001), and general health status (P < 0.001) were reduced in patients with c-TA in comparison with CTRL. Emotional aspect tended to be lower in c-TA vs. CTRL (P = 0.059).
Fig. 3.
Healthy-related quality of life in patients with childhood-onset Takayasu Arteritis (c-TA) and healthy controls (CTRL). Panel (a) Short Form-36 health survey domains between-group; b Physical health domain; Panel (c) Mental health domain
Bone mineral density and body composition measurements, systemic inflammation, and cardiometabolic risk factors are presented in Table 2. c-TA patients had significantly lower bone BMD at L1-L4 (P = 0.025), BMC total body (P = 0.023) and BMD total body (P = 0.032) than CTRL. In addition, patients exhibited significantly greater percent fat mass and lower lean mass (P = 0.024 and P = 0.029, respectively), when compared to controls. Inflammatory and angiogenesis markers did not differ between groups. However, patients with c-TA displayed worse insulin sensitivity, based on insulin fasting (P = 0.050), HOMA-IR (P = 0.033) and lower HDL cholesterol levels (P = 0.017) vs. CTRL.
Table 2.
Body composition, systemic inflammation and cardiometabolic risk factors between childhood-onset Takayasu Arteritis patients (c-TA) and healthy controls (CTRL).
| Characteristics | c-TA (n = 17) |
CRTL (n = 17) |
Between-group difference (95 % CI) | P |
|---|---|---|---|---|
| DXA measurements | ||||
| BMD (L1-L4) (g/cm2) | 0.89 ± 0.16 | 1.02 ± 0.16 | -0.13 (-0.01 to -0.24) | 0.025 |
| BMD total hip (g/cm2) | 0.89 ± 0.17 | 0.99 ± 0.15 | -0.10 (0.01 to -0.21) | 0.080 |
| BMC total body (kg) | 1.86 ± 0.48 | 2.28 ± 0.58 | -0.42 (-0.04 to -0.79) | 0.023 |
| BMD total body (kg) | 1.03 ± 0.12 | 1.13 ± 0.13 | -0.10 (-0.01 to -0.18) | 0.032 |
| Lean mass + BMC (kg) | 39.6 ± 9.03 | 49.0 ± 13.4 | -9.40 (-1.41 to -17.3) | 0.028 |
| Lean mass (kg) | 37.9 ± 8.69 | 45.3 ± 11.2 | -7.40 (-0.39 to -14.4) | 0.029 |
| Percent fat mass (kg) | 30.1 ± 8.30 | 23.2 ± 7.80 | 6.90 (1.27 to 12.5) | 0.024 |
| Visceral fat (kg) | 2.75 ± 1.30 | 2.14 ± 0.85 | 0.61 (-0.15 to 1.37) | 0.201 |
| Systemic inflammation | ||||
| C-reactive protein (mg/L) | 4.84 ± 6.63 | 2.30 ± 1.64 | 2.54 (-0.83 to 5.91) | 0.135 |
| ESR (mm/1st hour) | 9.18 ± 9.22 | 4.89 ± 2.24 | 4.29 (-0.39 to 8.97) | 0.072 |
| IFN-γ (mg/L) | 7.70 ± 13.1 | 1.70 ± 0.90 | 6.00 (-0.48 to 12.4) | 0.078 |
| IL-10 (pg/mL) | 14.8 ± 16.0 | 7.70 ± 3.77 | 7.10 (-1.02 to 15.2) | 0.081 |
| IL-12p70 (pg/mL) | 5.46 ± 3.76 | 4.74 ± 1.90 | 0.72 (2.80 to -1.36) | 0.488 |
| IL-1ra (pg/mL) | 123.6 ± 236.3 | 34.4 ± 15.6 | 89.2 (-27.7 to 206.1) | 0.126 |
| IL1-β (pg/mL) | 2.55 ± 1.25 | 2.16 ± 0.40 | 0.39 (-0.25 to 1.03) | 0.233 |
| IL-6 (pg/mL) | 21.7 ± 32.3 | 15.2 ± 28.4 | 6.50 (-14.7 to 27.7) | 0.542 |
| TNF-α (pg/mL) | 15.1 ± 7.27 | 13.2 ± 6.60 | 1.90 (-2.95 to 6.75) | 0.443 |
| VEGF (pg/mL) | 2.86 ± 4.89 | 1.67 ± 0.60 | 1.19 (-1.24 to 3.62) | 0.337 |
| PDGF (pg/mL) | 561.4 ± 1273.8 | 766.1 ± 1529.3 | -204.7 (778.2 to -1187.6) | 0.667 |
| Cardiometabolic risk factors | ||||
| SBP (mmHg) | 110.9 ± 15.3 | 104.8 ± 8.1 | 6.10 (-2.45 to 14.6) | 0.225 |
| DBP (mmHg) | 64.4 ± 9.30 | 68.3 ± 8.20 | -3.90 (2.23 to -10.0) | 0.251 |
| Glucose fasting (mg/dL) | 83.1 ± 8.70 | 82.2 ± 4.55 | 0.90 (-3.95 to 5.75) | 0.723 |
| Insulin fasting (mg/dL) | 13.9 ± 9.85 | 8.37 ± 3.80 | 5.53 (0.31 to 10.7) | 0.050 |
| HOMA-IR | 2.88 ± 2.11 | 1.67 ± 0.78 | 1.21 (0.09 to 2.32) | 0.033 |
| Total cholesterol (mg/dL) | 151.2 ± 19.5 | 156.2 ± 18.8 | -5.00 (8.38 to -18.4) | 0.479 |
| HDL cholesterol (mg/dL) | 53.4 ± 13.6 | 66.1 ± 14.1 | -12.7 (-3.02 to -22.3) | 0.017 |
| LDL cholesterol (mg/dL) | 82.0 ± 16.9 | 75.6 ± 13.4 | 6.40 (-4.25 to 17.0) | 0.271 |
| VLDL cholesterol (mg/dL) | 15.8 ± 2.98 | 14.6 ± 3.02 | 1.20 (-0.89 to 3.29) | 0.262 |
| Triglycerides (mg/dL) | 71.2 ± 25.1 | 60.5 ± 14.9 | 10.7 (-3.72 to 25.1) | 0.139 |
Values are means ± SD. DXA (dual-energy x-ray absorptiometry), BMD (bone mineral density), BMC (bone mineral content), ESR (erythrocyte sedimentation rate), IFN-γ (interferon gamma), IL-10 (interleukin 10), IL-12p70 (interleukin 12p70), IL-1ra (interleukin 1 receptor antagonist), IL-1β (interleukin 1 beta), IL-6 (interleukin 6), TNF-α (tumor necrosis factor alfa), VEGF (vascular endothelial growth factor), PDGF (platelet-derived growth factor), SBP (systolic blood pressure), DBP (diastolic blood pressure), HOMA-IR (homeostatic model assessment for insulin resistance), HDL (high-density lipoprotein), LDL (low-density lipoprotein), VLDL (very-low-density lipoprotein),
Discussion
We described, for the first time, physical activity levels and aerobic conditioning and multiple traditional and non-traditional cardiovascular risk factors associated with these variables among patients with c-TA. Confirming our hypothesis, patients with c-TA exhibited lower MVPA levels and increased sedentary behavior (step per day), decreased aerobic capacity (VO2vat, VO2rcp and VO2peak) and decreased autonomic function (chronotropic response) when compared to healthy peers. c-TA patients also showed poorer health-related quality of life and worse body composition (BMD and lean mass) and cardiometabolic parameters (insulin sensitivity surrogates and HDL cholesterol).
TA is the most common form of large-vessel vasculitis in children and adolescents that predisposes patients to a high risk of mortality and a variety of comorbidities linked to the cardiovascular system [17]. We [18, 19] and others [20–22] have shown that patients with pediatric rheumatic diseases are often hypoactive. WHO recommends that children and adolescents aged 5 to 17 should engage in at least 60 min of MVPA daily, whereas adults aged ≥ 18 years should perform at least 150 min of MVPA per week or 75 min of vigorous activity per week, or a combination of both [23]. In our sample, none of the patients met the recommendations for physical activity levels (i.e., minimum of 60 min of MVPA per day). This is in accordance with data showing a high frequency of physical inactivity among other pediatric rheumatic diseases, such as childhood-onset systemic lupus erythematosus [19], childhood-onset dermatomyositis [24] and juvenile idiopathic arthritis [25]. Of relevance, only 3 patients from CTRL achieved the guidelines; even so, CTRL group showed higher physically activity levels than c-TA, which supports the notion that hypoactivity may be a marked feature in this disease. Physical inactivity appears to be implicated in worsen clinical symptoms, increased traditional and non-traditional risks factors, and poor HRQL among pediatric rheumatic patients (for comprehensive reviews, see [8, 26, 27]). The very high proportion of physically inactive patients in the current study suggests that physical inactivity is a potentially novel risk factor for disturbances in cardiovascular, skeletal muscle and bone systems to be considered in this disease. The main factors contributing to the low physical activity levels showed by our patients could be related to the disease comorbidities and clinical features (arterial hypertension, stenosis, claudication, etc.), although other aspects could also play a role in inactivity (e.g., physical and emotional limitations imposed by the disease, parent’s superprotection, social isolation). The actual barriers for physical activity in this condition require further investigation.
Previous studies have suggested that physical inactivity can lead to decreased aerobic capacity and autonomic dysfunction, both being independent predictors of all-cause mortality and poor HRQL [9, 10, 18]. We observed that patients with c-TA displayed poor cardiorespiratory fitness and autonomic dysfunction, in consistent with data reported for other pediatric rheumatic diseases [18, 28]. To which extent these poor prognosis parameters can increase the burden of cardiovascular diseases in c-TA needs to be investigated.
c-TA patients also showed reduced total body and lumbar spine BMD, BMC and lower lean mass as compared to their heathy counterparts. Abnormalities in bone metabolism have been reported among pediatric rheumatic diseases, possibly due to the chronic inflammation and the prolonged use of glucocorticoids and/or other immunosuppressive drugs [29]. Low physical activity can also contribute to diminished bone accretion during childhood, which may result in sub-optimal bone mass in later adulthood [30]. The lack of sufficient physical activity has also a causal link with worse cardiometabolic health, including insulin resistance and dyslipidemia [31]. In the current study, we observed lower HDL cholesterol and higher insulin levels among patients with c-TA. It is also possible that hypoactivity may account for the poor quality of life (both emotional and physical aspects, see Fig. 3) reported herein. Of clinical relevance, physical activity promotion has been shown to attenuate all these poor outcomes in several pediatric rheumatic diseases [18, 32–34]. To date, however, there is no study showing the ability of physical activity interventions in yielding beneficial effects in c-TA.
This study has some limitations. First, although data were collected in three centers, the sample size was yet limited due to the very rare nature of this severe disease. Second, patients’ characteristics such as age, sex, and current treatment were relatively heterogeneous. Future studies should try to replicate these data in more homogenous samples. Third, all our patients were in disease remission and had a well-controlled disease. It is important to investigate to which extent, if any, disease activity can aggravate physical inactivity and aerobic deconditioning.
Conclusions
In conclusion, patients with c-TA in disease remission showed reduced physical activity levels and aerobic conditioning as well as poor traditional and non-traditional cardiovascular risk factors and quality of life as compared to healthy peers. These findings reveal that physical inactivity and aerobic deconditioning are potentially novel risk factors for c-TA. The role of physical activity interventions in preventing poor outcomes and improving HRQL in c-TA remains to be explored.
Acknowledgements
Not applicable.
Abbreviations
- c-TA
Childhood onset Takayasu arteritis
- IL-6
Interleukin six
- TNF-α
Tumor necrosis factor alpha
- HDL
High-density lipoprotein
- HRQL
Health-related quality of life
- PVAS
The paediatric vasculitis activity score
- CTRL
Control group
- ITAS2010
The Indian Takayasu Arteritis Score 2010
- DXA
Dual-energy x-ray absorptiometry
- BMD
Bone mineral density
- BMC
Bone mineral content
- BMI
Body mass index
- MVPA
Moderate-vigorous physical activity
Authors' contributions
CA contributed to conception, design, acquisition of data, interpretation of data, drafting original article and final approval of the version to be published. SG contributed to analysis, interpretation of data, drafting original article and final approval of the version to be published. GC contributed to conception, design, and acquisition of data. Revising original article and final approval of the version to be published. contributed to conception, design, and acquisition of data. Revising original article and final approval of the version to be published. CAS contributed to conception, design, and acquisition of data. Revising original article and final approval of the version to be published. LMAC contributed to conception, design, and acquisition of data. Revising original article and final approval of the version to be published. NEA contributed to conception, design, and acquisition of data. Revising original article and final approval of the version to be published. ALSP contributed to conception, design, and acquisition of data. Revising original article and final approval of the version to be published. RMR contributed to conception, design, and acquisition of data and analysis. Drafting original article and final approval of the version to be published. HR contributed to conception, design, and acquisition of data. Drafting original article and final approval of the version to be published. BG contributed to conception, design, acquisition of data analysis and interpretation of data. Drafting original article and final approval of the version to be published.
Funding
The authors acknowledge the support by Sao Paulo Research Foundation (FAPESP) (grant 2017/07358-9).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval
This study was approved by the local ethics committee Comissão de Ética para Análise de Projetos de Pesquisa (CAPPesq) and by the Plataforma Brasil registered in May/2017 under the number 2.070.921.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alibaz-Oner F, Koster MJ, Unal AU, et al. Assessment of the frequency of cardiovascular risk factors in patients with Takayasu’s arteritis. Rheumatol (United Kingdom) 2017;56:1939–44. doi: 10.1093/rheumatology/kex300. [DOI] [PubMed] [Google Scholar]
- 2.Alibaz-Oner F, Yurdakul S, Aytekin S, Direskeneli H. Impaired endothelial function in patients with Takayasu’s arteritis. Acta Cardiol. 2014;69:45–9. doi: 10.1080/AC.69.1.3011344. [DOI] [PubMed] [Google Scholar]
- 3.Seko Y, Minota S, Kawasaki A, et al. Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu’s arteritis. J Clin Invest. 1994;93:750–8. doi: 10.1172/JCI117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin S, Hopurcuoglu D, Bektas S, et al. Childhood - onset Takayasu arteritis: A 15 ‐ year experience from a tertiary referral center. 2018;1–8. [DOI] [PubMed]
- 5.Goel R, Kumar TS, Danda D, et al. Childhood-onset Takayasu arteritis - Experience from a tertiary care center in south India. J Rheumatol. 2014;41:1183–9. doi: 10.3899/jrheum.131117. [DOI] [PubMed] [Google Scholar]
- 6.Szugye HS, Zeft AS, Spalding SJ. Takayasu Arteritis in the pediatric population: a contemporary United States-Based Single Center Cohort. Pediatr Rheumatol [Internet] 2014;12:21. Available from: 10.1186/1546-0096-12-21. [DOI] [PMC free article] [PubMed]
- 7.Alibaz-Oner F, Yentur S, Saruhan-Direskeneli G, Direskeneli H. Serum Cytokine Profiles in Patients with Takayasu’s Arteritis. Clin Exp Rheumatol [Internet] 2014;73:1–5. Available from: 10.1136/annrheumdis-2014-eular.3382. [PubMed]
- 8.Gualano B, Bonfa E, Pereira RMR, Silva CA. Physical activity for paediatric rheumatic diseases: Standing up against old paradigms. Nat Rev Rheumatol [Internet] 2017;13:368–79. Available from: 10.1038/nrrheum.2017.75. [DOI] [PubMed]
- 9.Hardy LL, Dobbins TA, Denney-Wilson EA, Okely AD, Booth ML. Sedentariness, Small-Screen Recreation, and Fitness in Youth. Am J Prev Med [Internet] 2009 [cited 2020 Apr 8];36:120–5. Available from: . [DOI] [PubMed]
- 10.Booth R. 2014 L. Lack of exercise is a major cause of chronic diseases. 2014;2:1143–211. [DOI] [PMC free article] [PubMed]
- 11.Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis [Internet] 2010;69:798–806. Available from: 10.1136/ard.2009.116657. [DOI] [PubMed]
- 12.Dolezalova P, Price-Kuehne FE, Özen S, et al. Disease activity assessment in childhood vasculitis: Development and preliminary validation of the Paediatric Vasculitis Activity Score (PVAS) Ann Rheum Dis. 2013;72:1628–33. doi: 10.1136/annrheumdis-2012-202111. [DOI] [PubMed] [Google Scholar]
- 13.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci [Internet] 2008 [cited 2020 Mar 10];26:1557–65. Available from: . [DOI] [PubMed]
- 15.Perandini LA, Sales-de-Oliveira D, Mello SBV, et al. Exercise training can attenuate the inflammatory milieu in women with systemic lupus erythematosus. J Appl Physiol [Internet] 2014;117:639–47. Available from: 10.1152/japplphysiol.00486.2014. [DOI] [PubMed]
- 16.Misra R, Danda D, Rajappa SM, et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010) Rheumatol (United Kingdom) 2013;52:1795–801. doi: 10.1093/rheumatology/ket128. [DOI] [PubMed] [Google Scholar]
- 17.Ciconelli R, Ferraz M, Santos W, Meinão I, Quaresma M. Brazilian-Portuguese version of the SF-36: a reliable and valid quality of life outcome measure. Rev Bras Rheumatol. 1999;3:143–50. [Google Scholar]
- 18.Aeschlimann FA, Twilt M, Yeung RSM. Childhood-onset Takayasu Arteritis. Eur J Rheumatol. 2020;7:58–66. doi: 10.5152/eurjrheum.2019.19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prado DML, Benatti FB, Sá-Pinto AL de, et al. Exercise training in childhood-onset systemic lupus erythematosus: A controlled randomized trial. Arthritis Res Ther [Internet] 2013;15. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/ar4205. [DOI] [PMC free article] [PubMed]
- 20.Pinto AJ, Miyake CNH, Benatti FB, et al. Reduced Aerobic Capacity and Quality of Life in Physically Inactive Patients With Systemic Lupus Erythematosus With Mild or Inactive Disease. Arthritis Care Res. 2016;68:1780–6. doi: 10.1002/acr.22905. [DOI] [PubMed] [Google Scholar]
- 21.Takken T, Van Der Net J, Helders PJM. Anaerobic exercise capacity in patients with juvenile-onset idiopathic inflammatory myopathies. Arthritis Care Res. 2005;53:173–7. doi: 10.1002/art.21066. [DOI] [PubMed] [Google Scholar]
- 22.Brussel M, Lelieveld O, van der NETJ, Al. E. Aerobic and Anaerobic Exercise Capacity in Children With Juvenile Idiopathic Arthritis AND. 2007;57:891–7. [DOI] [PubMed]
- 23.Hulsegge G, Henschke N, McKay D, et al. Fundamental movement skills, physical fitness and physical activity among Australian children with juvenile idiopathic arthritis. J Paediatr Child Health. 2015;51:425–32. doi: 10.1111/jpc.12733. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Global recommendations on physical activity for health. 2010;. . [PubMed]
- 25.Pinto AJ, Yazigi Solis M, de Sá Pinto AL, et al. Physical (in)activity and its influence on disease-related features, physical capacity, and health-related quality of life in a cohort of chronic juvenile dermatomyositis patients. Semin Arthritis Rheum [Internet] 2016 [cited 2020 Apr 17];46:64–70. Available from: . [DOI] [PubMed]
- 26.Bohr A-H, Nielsen S, Müller K, Karup Pedersen F, Andersen LB. Reduced physical activity in children and adolescents with Juvenile Idiopathic Arthritis despite satisfactory control of inflammation. Pediatr Rheumatol [Internet] 2015;13:57. Available from: . [DOI] [PMC free article] [PubMed]
- 27.Gualano B, Sá Pinto AL, Perondi B, et al. Evidence for prescribing exercise as treatment in pediatric rheumatic diseases. Autoimmun Rev [Internet] 2010;9:569–73. Available from: 10.1016/j.autrev.2010.04.001. [DOI] [PubMed]
- 28.Klepper SE. Exercise in pediatric rheumatic diseases. Curr Opin Rheumatol. 2008;20:619–24. doi: 10.1097/BOR.0b013e32830634ee. [DOI] [PubMed] [Google Scholar]
- 29.van Brussel M, Lelieveld OTHM, van der Net J, Engelbert RHH, Helders PJM, Takken T. Aerobic and anaerobic exercise capacity in children with juvenile idiopathic arthritis. Arthritis Rheum [Internet] 2007;57:891–7. Available from: 10.1002/art.22893. [DOI] [PubMed]
- 30.Alsufyani KA, Ortiz-Alvarez O, Cabral DA, et al. Bone mineral density in children and adolescents with systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis: Relationship to disease duration, cumulative corticosteroid dose, calcium intake, and exercise. J Rheumatol. 2005;32:729–33. [PubMed] [Google Scholar]
- 31.Chilibeck PD, Sale DG, Webber CE. Exercise and Bone Mineral Density. Sport Med. 1995;19:103–22. doi: 10.2165/00007256-199519020-00003. [DOI] [PubMed] [Google Scholar]
- 32.Andersen LB, Riddoch C, Kriemler S, Hills A. Physical activity and cardiovascular risk factors in children. Br J Sports Med. 2011;45:871–6. doi: 10.1136/bjsports-2011-090333. [DOI] [PubMed] [Google Scholar]
- 33.Omori CH, Silva CAA, Sallum AME, et al. Exercise training in juvenile dermatomyositis. Arthritis Care Res (Hoboken) [Internet] 2012;. Available from: 10.1002/acr.21684. [DOI] [PubMed]
- 34.Alexanderson H, Munters LA, Dastmalchi M, et al. Resistive home exercise in patients with recent-onset polymyositis and dermatomyositis - A randomized controlled single-blinded study with a 2-year followup. J Rheumatol. 2014;41:1124–32. doi: 10.3899/jrheum.131145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.