Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
A triplet regimen associating plasma exchange, immunosuppression, and caplacizumab reduces unfavorable outcomes in immune-mediated TTP.
The triplet regimen alleviates the burden of care.
Abstract
The anti–von Willebrand factor nanobody caplacizumab was licensed for adults with immune-mediated thrombotic thrombocytopenic purpura (iTTP) based on prospective controlled trials. However, few data are available on postmarketing surveillance. We treated 90 iTTP patients with a compassionate frontline triplet regimen associating therapeutic plasma exchange (TPE), immunosuppression with corticosteroids and rituximab, and caplacizumab. Outcomes were compared with 180 historical patients treated with the standard frontline treatment (TPE and corticosteroids, with rituximab as salvage therapy). The primary outcome was a composite of refractoriness and death within 30 days since diagnosis. Key secondary outcomes were exacerbations, time to platelet count recovery, the number of TPE, and the volume of plasma required to achieve durable remission. The percentage of patients in the triplet regimen with the composite primary outcome was 2.2% vs 12.2% in historical patients (P = .01). One elderly patient in the triplet regimen died of pulmonary embolism. Patients from this cohort experienced less exacerbations (3.4% vs 44%, P < .01); they recovered durable platelet count 1.8 times faster than historical patients (95% confidence interval, 1.41-2.36; P < .01), with fewer TPE sessions and lower plasma volumes (P < .01 both). The number of days in hospital was 41% lower in the triplet regimen than in the historical cohort (13 vs 22 days; P < .01). Caplacizumab-related adverse events occurred in 46 patients (51%), including 13 major or clinically relevant nonmajor hemorrhagic events. Associating caplacizumab to TPE and immunosuppression, by addressing the 3 processes of iTTP pathophysiology, prevents unfavorable outcomes and alleviates the burden of care.
Visual Abstract
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a devastating disease characterized by the association of a microangiopathic hemolytic anemia, a profound thrombocytopenia, organ involvement of variable severity, and a severe (<10% of normal activity) deficiency in the von-Willebrand factor (vWF) cleaving protease ADAMTS13 (A disintegrin and metalloproteinase with thrombospondin-1 motifs, 13th member). Left untreated, iTTP is almost always fatal. However, prompt diagnosis and treatment allow survival rates of up to 85%.1,2 Historically, the standard iTTP treatment consisted of the association of daily therapeutic plasma exchange (TPE) and steroids.3,4 Rituximab, a B-cell–depleting therapy, progressively evolved from a second-line agent in patients with unfavorable outcomes under standard care to become a frontline strategy.5-8 Rituximab was shown to shorten the duration of daily TPE, especially in long-term responders, and led to durable platelet responses within 30 days as a result of a faster and more durable improvement in ADAMTS13 activity.5,6,9,10 However, rituximab efficacy is only observed after a mean time of 2 weeks after its first infusion; during this period, patients are consequently exposed to unfavorable outcomes, especially death, typically heralded by refractoriness and exacerbations.5,6,11,12 Recently, caplacizumab (Cablivi, Ablynx, a Sanofi Company), a nanobody directed against the A1 domain of ultra-large vWF multimers, has been evaluated in iTTP through 2 pivotal trials with the aim to prevent the formation of microthrombi and subsequent microcirculation occlusion and ischemic organ damage. In both trials, iTTP patients were treated with caplacizumab in association with the standard treatment (TPE/corticosteroids and rituximab as per clinician’s practice). Patients who received caplacizumab recovered platelet counts more rapidly and more durably compared with patients in the placebo group, despite the early use of rituximab.13-15 Neither death nor refractoriness was observed with caplacizumab, although the difference with the placebo arm could not reach the statistical significance level.13,14 Caplacizumab treatment appeared to be safe, with side effects mostly consisting in minor mucocutaneous bleeding.13,14 Although caplacizumab was approved for the treatment of iTTP in conjunction with TPE and immunosuppression by the European Medicines Agency in Europe (September 2018) and the US Food and Drug Administration (February 2019), uncertainties remain regarding the role of caplacizumab in the therapeutic arsenal of iTTP and postmarketing studies are urgently needed. Recently the very first report of real world experience, although uncontrolled and unplanned, confirmed that caplacizumab allows a rapid recovery of the disease, whether used frontline or as a salvage therapy in patients experiencing a refractory disease or an exacerbation.16
France has been one of the first countries to allow a caplacizumab compassionate use program for iTTP patients, starting in September 2018. We hereby report findings from the follow-up of patients treated by caplacizumab and reported to the French Reference Center for thrombotic microangiopathies (CNR-MAT) since the onset of this program.
Methods
Study design
The use of caplacizumab for iTTP patients was allowed in France in a compassionate use program in September 2018; from February 2019 onward, caplacizumab is authorized in France for initial treatment of iTTP. To rapidly improve our experience in its use, the CNR-MAT (www.cnr-mat.fr) recommended, based on a consensus of all participating centers, a triplet regimen comprised of (1) daily TPE, (2) immunosuppression with corticosteroids and rituximab, and (3) caplacizumab.8 In 2011, in France, rituximab obtained a transient recommendation of use as a salvage therapy in iTTP patients with a suboptimal response to TPE/corticosteroids; in 2019, this recommendation was obtained for iTTP patients as a frontline therapy to prevent long-term responses to TPE/corticosteroids treatment and 1- to 2-year relapses5,6,9,10 (https://ansm.sante.fr/var/ansm_site/storage/original/application/d793fd3d291b2f91af34e27cfea51786.pdf; page 11; document in French; translated in supplemental Table 1 available on the Blood Website). Therefore, the use of rituximab as frontline therapy in iTTP has become part of the standard of care in our country.
The primary outcome was the prevalence of a composite of 2 crucial outcomes within 30 days since diagnosis: death and refractoriness, usually heralding death11,12 (see definition in supplemental Table 2). Key secondary outcomes were refractoriness, death, exacerbations, the time to durable platelet count recovery, the number of TPE, the volume of plasma required to achieve durable platelet count recovery, the length of hospitalization, and caplacizumab-related adverse events. We considered that the triplet regimen would represent an improvement in iTTP management if the primary outcome was at least 3 times lower than historically observed (10%).5,6,9,10 To achieve this goal, we estimated that at least 65 patients were needed for the present study. The study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology methodology.
Patients and data collection
All data from French patients with a clinical diagnosis of iTTP and treated according to our triplet regimen were analyzed beginning in September 2018. The clinical diagnosis of iTTP was considered in patients with features of thrombotic microangiopathy and a French score of 1 or 2.8,17 The French score was calculated in patients with features of thrombotic microangiopathy and no associated condition (cancer, chemotherapy, pregnancy, transplantation, severe disseminated intravascular coagulopathy). Patients with a French score of 0 (platelet count ≥ 30 × 103/mm3 and serum creatinine ≥ 200 µmol/L [2.27 mg/dL]) were considered having an alternative diagnosis, mostly hemolytic and uremic syndrome, and were not considered here. A French score of 2 (platelet count < 30 × 103/mm3 and serum creatinine < 200 µmol/L [2.27 mg/dL]) was highly suggestive of iTTP. Patients with only 1 of these 2 measures were considered having probable iTTP, and daily TPE with corticosteroids and caplacizumab was immediately started; in this scenario, however, rituximab was started only after iTTP diagnosis was confirmed (ie, if ADAMTS13 activity was <10%). The final diagnosis of iTTP was confirmed in patients with a severe acquired ADAMTS-13 deficiency (<10% of activity with anti-ADAMTS13 antibodies ≥ 15 U/mL).18 After TPE cessation, ADAMTS13 activity was assessed weekly until normalization (activity ≥ 50%).
Severity of iTTP at baseline was assessed using cerebral involvement (including confusion, stupor, coma, or focal deficiency), age, and lactate dehydrogenase (LDH) level (reflecting mostly organ injury). Patients were classified into 2 groups: low-intermediate and high risk of early death, according to age, cerebral involvement, and very high LDH levels.12 Cardiac involvement was defined as an increase of troponin and/or electrocardiographic abnormalities. However, troponin assessment was not performed homogeneously through all centers; consequently, troponin was used for initial prognostic evaluation, but detailed prognostic analyses were not performed.
To more accurately address improvements in the disease burden caused by caplacizumab, the outcome of patients treated with the triplet regimen (triplet regimen cohort) was compared with a historical cohort of iTTP patients (historical cohort) managed with the standard regimen (ie, daily TPE and steroids in association with salvage rituximab in patients experiencing refractoriness or an exacerbation of the disease). Patients from the triplet regimen were compared on a 1-to-2 ratio with the more recent patients of the historical cohort. The choice of a 1-to-2 ratio with the historical cohort was driven by the rarity of iTTP and the desire to include patients with standardized management.
Treatment
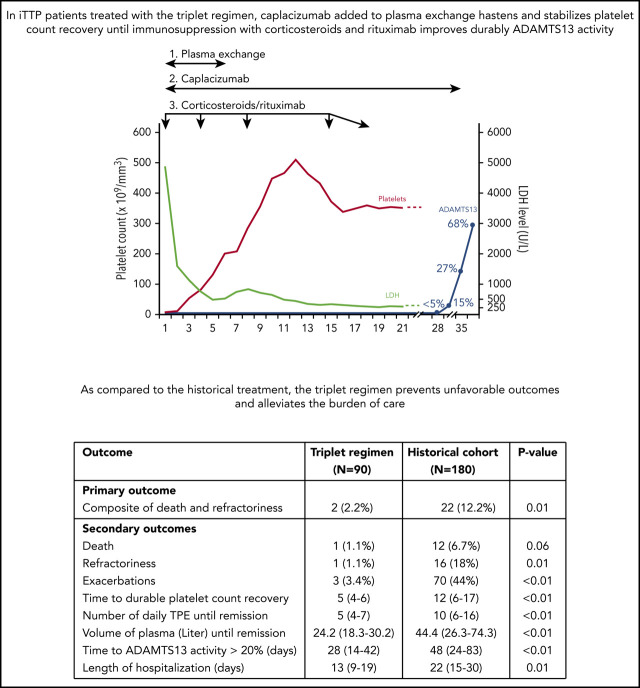
In all centers, daily TPE, corticosteroids (prednisone 1.0 mg/kg per day [maximal dose, 100 mg/day]), and caplacizumab (10 mg intravenous loading dose followed by daily 10 mg subcutaneous doses) were started as soon as the clinical diagnosis of iTTP was suspected based on the French score. Rituximab (375 mg/m2) was administered intravenously on a day1-4-8-15 schedule.5 It could be started from day 1 if the French score was highly suggestive for the diagnosis of iTTP (French score = 2)8,14,17 or alternatively by day 4 of the management once severe ADAMTS13 deficiency was ascertained (French score = 1; Figure 1). Caplacizumab was continued for 30 days after TPE cessation and could be extended until ADAMTS13 improvement (ie, activity ≥ 20%).19,20 TPE were performed daily until 2 days of a normal platelet count (≥150 × 103/mm3) and interrupted with no maintenance. Corticosteroids were pursued for 3 weeks, as previously described.5,6,9,10
Figure 1.
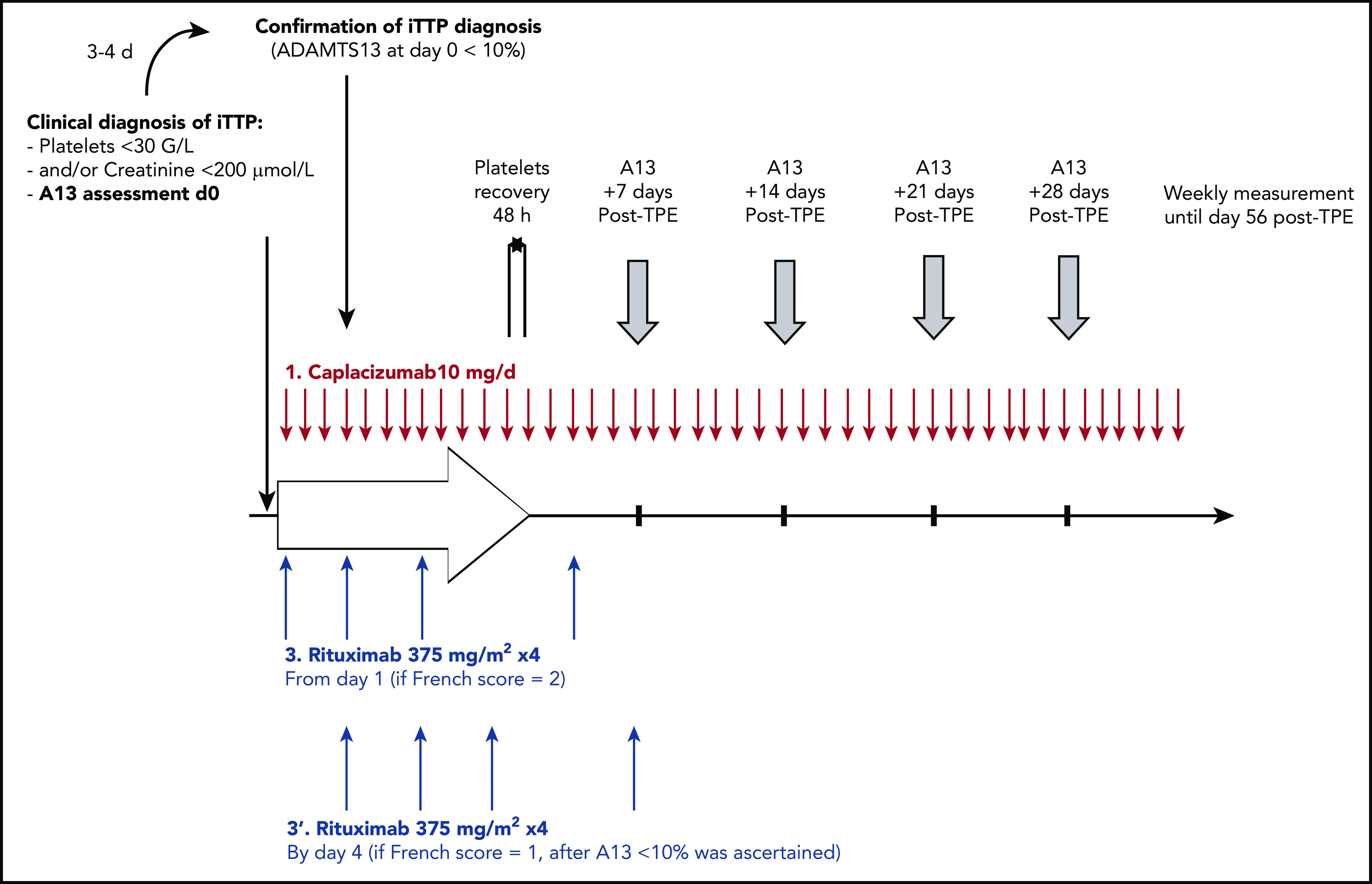
The CAPLAVIE regimen. ADAMTS13 activity was assessed weekly until normalization or day 56.
Outcomes
Assessment of response to treatment was performed as previously described1 (supplemental Table 2). Adverse events related to caplacizumab were reported. The definition of major bleeding and clinically relevant nonmajor bleeding events is adapted from the International Society on Thrombosis and Hemostasis.21
Ethics
This study was part of the Thrombotic Microangiopathy program study approved by our institutional review board (CPP04807) in accordance with the Declaration of Helsinki and the French Data Protection Authority.
Statistics
Cohorts are described by absolute numbers, with percentages for nominal variables and otherwise by the median with first and third quartiles (Q1-Q3). To assess whether cohorts differed at treatment start, the Mann-Whitney-Wilcoxon test was used for ordinal variables, and Freeman-Halton’s test was used otherwise. Because follow-up time varied between patients, Poisson regression was used to reach the end points. Patient time under risk was estimated using the actuarial method. P < .05 was considered statistically significant. Analyses were performed using SAS 9.4 statistical software (SAS institute Inc, Cary, NC).
Results
Population characteristics and iTTP presentation
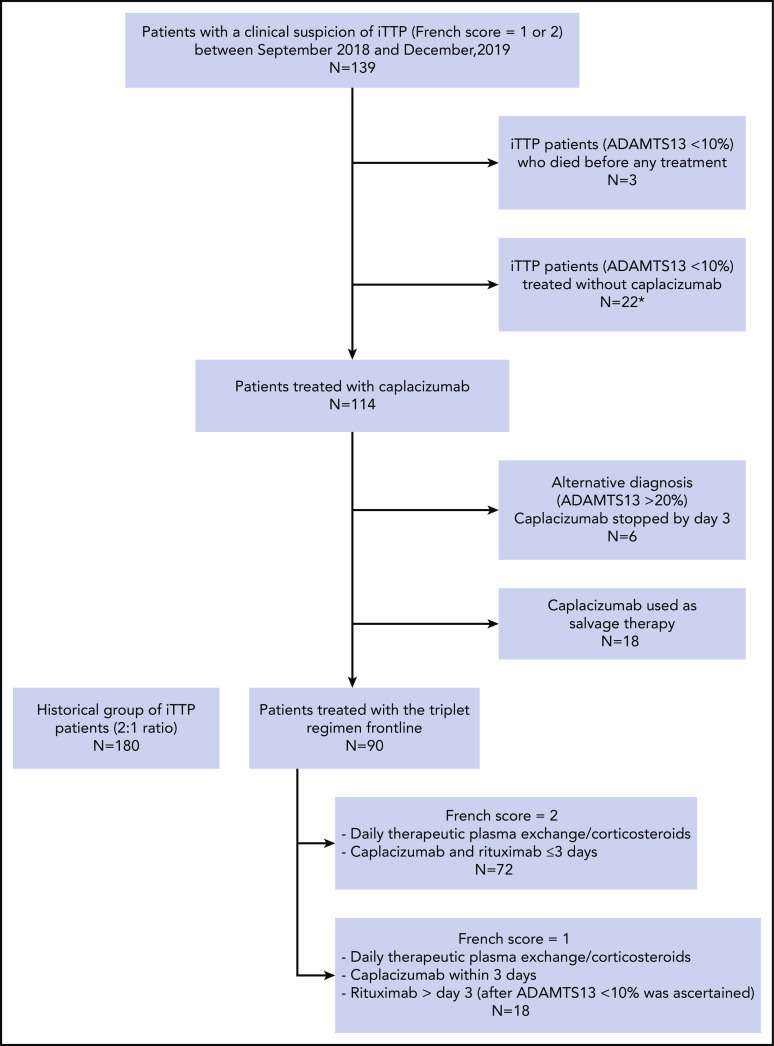
Between September 2018 (date of caplacizumab availability for iTTP in France) and December 2019, 139 patients were diagnosed with iTTP. Twenty-two patients who had a confirmed diagnosis of iTTP were managed without caplacizumab, mostly because of unawareness of practitioners about the availability of the compound (18 cases) or because patients were considered at risk of bleeding (1 case of recent surgery and 3 cases of active bleeding). Three other patients with retrospectively confirmed iTTP died at the time of diagnosis before any therapeutic measure could be enacted. Caplacizumab was therefore administered in a total of 114 patients with a clinical diagnosis of iTTP. In 6 patients who had a French score of 1, caplacizumab was stopped by day 3 because ADAMTS13 activity was suggestive of an alternative diagnosis (ie, activity ≥ 20%). Eighteen additional patients with confirmed iTTP (ADAMTS13 activity < 10%) received caplacizumab on a different schedule (mainly as salvage therapy, ie, not frontline) and were not further considered here. Finally, 90 patients with confirmed iTTP were treated according to the triplet regimen; they were compared with a historical cohort of 180 patients, registered in the French cohort between September 2018 and June 2015 (flowchart in Figure 2). During this period, patients of the historical cohort were managed consensually and homogeneously; particularly, rituximab was systematically performed in patients experiencing refractoriness or exacerbation based on national recommendations.22,23 Patients treated according to the triplet regimen were consecutively recruited from 32 centers, including 8 centers in Ile-de-France (Paris and suburbs). Centers included a median of 2 patients (interquartile range, 1-3; extremes: 1-12).
Figure 2.
Flowchart of the study. *Mostly because of unawareness of practitioners about the availability of the compound (18 cases) or because patients were considered at risk of bleeding (1 case of recent surgery and 3 cases of active bleeding).
Clinical presentation of patients of the triplet regimen cohort was comparable to this of patients in the historical cohort, except for LDH level (P = .01), providing evidence that our cohort of studied patients is fully representative of the whole iTTP population. Especially, 19% of patients from the triplet regimen and 14% from the historical cohort (P = .37) were considered to be at higher risk of death (Table 1). Moreover, the clinical presentation of patients who were not treated with caplacizumab was comparable to patients of the triplet regimen cohort, except for a higher percentage of previous iTTP episodes, suggesting that these patients did not have a more severe disease (supplemental Table 3).
Table 1.
Clinical features and concomitant treatment of patients on diagnosis according to the treatment regimen
| Characteristic | Triplet regimen (N = 90) | Historical cohort (N = 180) | P |
|---|---|---|---|
| Age (y) | 45 (34-57) | 43 (30-57) | 1.00 |
| Female sex | 63 (70%) | 127 (70%) | .30 |
| Weight (kg) | 71 (60-91) | 71 (60-86) | .83 |
| Body mass index | 27.2 (22.7-32.2) | 26.6 (23-31.7) | .68 |
| Ethnicity | .39 | ||
| White | 74 | 149 | |
| African-West Indies | 10 | 25 | |
| Asian | 6 | 6 | |
| Ongoing antiplatelet agent/anticoagulation | 9 (10%) | 16 (8.9%) | .77 |
| Antiplatelet agent | 7 | 11 | |
| Anticoagulant | 2 | 5 | |
| Relapse | 12 (13.3%) | 21 (11.7%) | .70 |
| Cerebral involvement | 55 (61%) | 111 (62%) | .91 |
| Headache | 19 | 58 | |
| Confusion | 22 | 36 | |
| Seizure | 10 | 15 | |
| Coma | 2 | 5 | |
| Focal deficiency | 20 | 26 | |
| Cardiac involvement | 51 (56%) | 86 (47%) | .15 |
| Hemoglobin (g/dL) | 8.9 (7.5-10.2) | 8.6 (7.3-10.1) | .54 |
| Platelet count (×103/mm3) | 12 (10-20) | 12 (8-23) | .88 |
| LDH level xN (U/L) | 5.1 (4.0-6.5) | 3.7 (2.4-5.6) | .01 |
| Serum creatinine level (µmol/L) | 92 (71-120) | 86 (68-133) | .17 |
| GFR (mL/min per 1.73 m2) (MDRD) | 74 (51-108) | 80 (46-120) | .85 |
| ADAMTS13 activity (%) | <10% | <10% | — |
| Anti-ADAMTS13 antibodies (U/mL) | 78 (39-91) | 80 (36-100) | .44 |
| French Severity score | .37 | ||
| 0-2 | 72 (81%)* | 145 (87%)† | |
| 3-4 | 17 (19%) | 21 (13%) | |
| Immunosuppressive therapy | |||
| Corticosteroids | 88 (98%) | 166 (92%) | .10 |
| Rituximab | 90 (100%) | 123 (68%) | <.01 |
| Time between first infusion and first TPE | 2 (1-3) | 7 (4-10) | <.01 |
| Other therapies | 0 | 25 (13.9%) | <.01 |
| Twice-daily TPE | 20 | ||
| Cyclophosphamide | 4 | ||
| Splenectomy | 2 | ||
| Vincristine | 3 | ||
| Bortezomib | 1 | ||
| >1 salvage therapy | 4 |
Data are given as median (25th-75th percentile) for quantitative variables and as n (%) for qualitative variables. Severe ADAMTS13 activity was defined as an activity <10% (normal range for ADAMTS13 activity: 50%-100%). The positivity threshold for anti-ADAMTS13 immunoglobulin G (IgG) was 15 U/mL, according to the manufacturer’s instructions (Technoclone). Cardiac involvement was defined as an increase of troponin and/or electrocardiographic abnormalities. Patients at high risk of early death of iTTP were defined by a French severity score ≥ 3 (cerebral involvement: yes = 1/no = 0, LDH: >10×ULN = 1/≤10×ULN = 0, age: >60 y = 2/>40 and ≤60 y = 1/≤40 y = 0).12
Data from 89 patients.
Data from 166 patients.
iTTP treatment
Daily TPE and corticosteroids were started immediately after the clinical diagnosis of iTTP. Most patients received caplacizumab within 3 days after TPE/corticosteroids initiation (median time, 0 days [range, 0-1; extremes, 0-4]). In 47 patients, caplacizumab was started on the same day as TPE (day +0); in the others, it was started at day +1 (24 patients), day +2 (8 patients), day +3 (6 patients), or day +4 (5 patients). The total duration of caplacizumab treatment was 33 days (range, 29-38 days); caplacizumab was continued for 32 days (range, 28-37 days) after TPE. In 12 patients, caplacizumab was interrupted empirically when ADAMTS13 activity reached ≥20%, without further event. Most patients (80%) had a French score of 2 and started rituximab within 3 days (before ADAMTS13 activity was available), whereas the 18 others had a French score of 1 and started rituximab by day 4, after severe ADAMTS13 deficiency was ascertained. In the historical cohort, 68% of patients were treated with rituximab (Table 1). Prophylactic anticoagulation and/or prophylactic antiplatelet aggregation was performed in 33 patients (37%) when platelet count increased above 50 × 103/mm3.
Primary outcome
The percentage of patients in the triplet regimen with the composite primary outcome was 2.2% vs 12.2% in patients with the historical treatment (P = .01). Therefore, patients treated with the triplet regimen were 6.2 times less likely to have refractory iTTP or iTTP-related death than patients from the historical cohort (95% confidence interval [CI], 1.4-26.3; P = .013). As expected, the death rate was 6.7% (n = 12) in the historical cohort, whereas in the triplet regimen cohort, only 1 patient died (1.1%, P = .06). This 83 year old died at day 9; the cause of death reported by the practitioner was a massive pulmonary embolism. She had cardiac involvement (cardiac troponin, 0.51 µg/L) without cerebral involvement at the time of diagnosis, and her treatment consisted in the frontline triplet regimen from day 1. After an initial improvement, she subsequently presented an exacerbation of her disease at day 5 and died of cardiogenic shock despite salvage thrombolysis. She did not receive thromboprophylaxis when platelet count improved. Whether this complication resulted from a complication of the central venous catheter insertion could not be ascertained. Only 1 patient was classified as having a refractory disease because platelet count did not double after 4 days of treatment; however, response to treatment occurred slowly thereafter, whereas no intensification was needed. In contrast, 16 patients experienced this outcome (18%, P = .01) in the historical treatment. Of note, death was preceded by refractoriness in 6 patients (50% of cases).
Key secondary outcomes
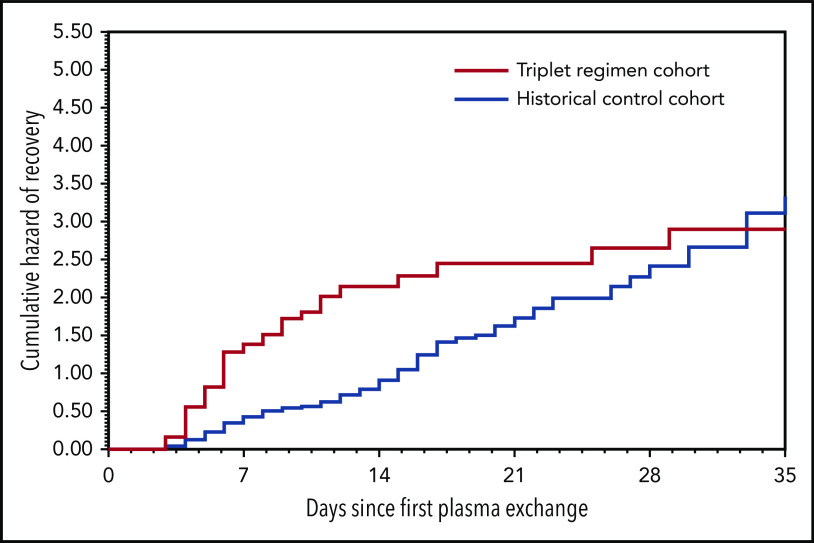
A significant decrease in exacerbations was noted in the triplet regimen cohort compared with the historical cohort (3.4% vs 44%, P < .01). Therefore, exacerbations were 16.4 times more frequent in the historical cohort than in the triplet regimen cohort (95% CI, 5.2-52.1; P < .01). The cause of the 3 exacerbations of the triplet regimen cohort remains unclear; no triggering factor (ie, infectious event) could be identified. None of the 3 patients had their caplacizumab therapy interrupted; all had a favorable outcome in the following days with no organ involvement, and no treatment intensification was required.24 As a result, patients in this cohort recovered durable platelet count 1.8 times more rapidly than those of the historical cohort (5 [4-6] vs 12 [6-17] days; 95% CI, 1.41-2.36; P < .01; Figure 3). Patients in the triplet regimen cohort needed a median of 5 days (range, 4-7 days) of TPE compared with 10 days (range, 6-16 days) of TPE in the historical cohort (P < .01). Consequently, the corresponding median volume of plasma was 24.2 L (range, 18.3-30.2 L) for patients in the triplet regimen cohort compared with 44.4 L (range, 26.3-74.3 L) for patients in the historical cohort (P < .01), corresponding to a 45% reduction. In addition, the length of hospitalization was reduced by 41% in the triplet regimen cohort (Table 2). After a median follow-up of 127 days (range, 47-200 days), only 1 clinical relapse occurred in the triplet regimen cohort. In this patient, caplacizumab was interrupted 33 days after TPE while ADAMTS13 activity was still <10%. Relapse occurred at day 6 after caplacizumab interruption. The outcome was rapidly favorable after TPE and caplacizumab were resumed, and ADAMTS13 activity improved at day 49 after TPE of the first episode. One additional patient experienced severe ADAMTS13 deficiency at 14 months of follow-up that resolved after a single infusion of rituximab.
Figure 3.
Cumulative daily rate of platelet count recovery after first therapeutic plasma exchange within 35 days by cohort.
Table 2.
Primary and secondary outcomes according to the treatment regimen
| Outcome | Triplet regimen (N = 90) | Historical cohort (N = 180) | P |
|---|---|---|---|
| Primary outcome | |||
| Composite of death and refractoriness | |||
| All patients | 2 (2.2%) | 22 (12.2%)* | .01 |
| According to French Severity score | |||
| 0-2 | 2 (2.8%) | 15 (8.3%) | <.01 |
| 3-4 | 0 | 7 (33%) | |
| Secondary outcomes | |||
| Death | 1 (1.1%) | 12 (6.7%) | .06 |
| Refractoriness | 1 (1.1%) | 16 (18%)† | .01 |
| Exacerbations | 3 (3.4%) | 70 (44%) | <.01 |
| Time to durable platelet count recovery | 5 (4-6) | 12 (6-17) | <.01 |
| Number of daily TPE until remission | 5 (4-7) | 10 (6-16) | <.01 |
| Volume of plasma (L) until remission | 24.2 (18.3-30.2) | 44.4 (26.3-74.3) | <.01 |
| Time to ADAMTS13 activity > 20% (days) | 28 (14-42) | 48 (24-83) | <.01 |
| Length of hospitalization (days) | 13 (9-19) | 22 (15-30) | .01 |
| Thromboembolic events | 11 (12%) | 20 (11.1%) | .79 |
Data are given as median (25th-75th percentile) for quantitative variables and as n (%) for qualitative variables. Patients at high risk of early death of iTTP were defined by a French severity score ≥ 3 (cerebral involvement: yes = 1/no = 0, LDH: >10×ULN = 1/≤10×ULN = 0, age: >60 y = 2/>40 and ≤60 y = 1/≤40 y = 0).12
Includes 10 refractory patients who survived (only 1 event per patient was considered).
Includes 6 deaths.
Other outcomes
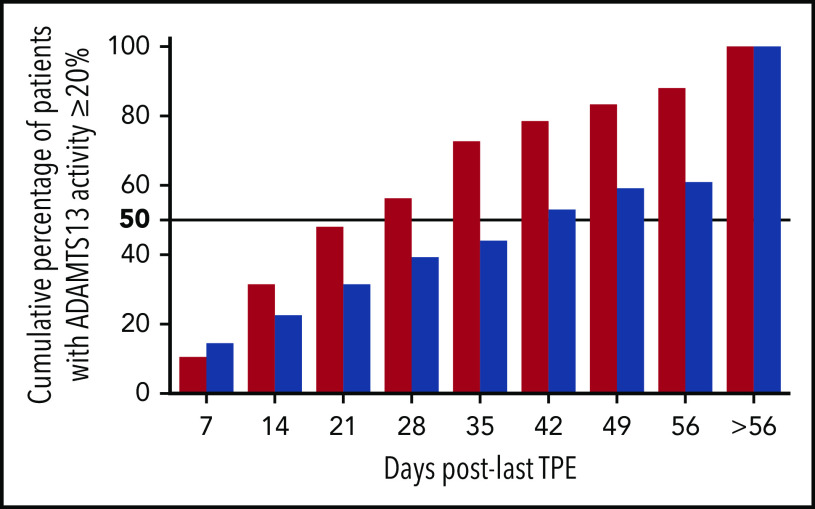
ADAMTS13 activity was assessed weekly after TPE. In the triplet regimen, there was a great disparity in the time to ADAMTS13 activity improvement (activity ≥ 20%; median, 28 days; interquartile range, 14-42; extremes, 7-164), but >50% patients recovered ADAMTS13 activity at day 28, although in 10 cases, this threshold was reached after day 56. Those patients with a slow ADAMTS13 response improved all their activity ≥20% within days 64 and 164 after TPE. In 2 of them, an additional course of 4-infusion rituximab was performed during this period, whereas the others improved with no further action.
One patient relapsed clinically after caplacizumab was stopped, whereas the 9 others continued caplacizumab until ADAMTS13 improvement without relapse. In the historical cohort, where rituximab was not systematically performed, >50% of patients improved ADAMTS13 activity at day 42 (Figure 4); moreover, up to 40% improved ADAMTS13 activity after day 56. As a result, patients in the triplet regimen recovered ADAMTS13 activity of >20% 4 times faster than those in the historical cohort (95% CI, 3.03-5.26; P < .01).
Figure 4.
Cumulative percentage of patients who achieved ADAMTS13 activity ≥ 20% after the last therapeutic plasma exchange in the triplet regimen (red columns) and the historical cohort (blue columns). ADAMTS13 activity was assessed weekly until normalization or day 56.
Outcome according to severity cohorts
Consistent with our previous findings, the percentage of patients with the composite primary outcome in the historical cohort was 8.3% for iTTP patients with a severity score of 0 to 2, whereas in patients with more severe disease (severity score ≥ 3), the composite primary outcome was significantly higher (33%, P < .01). However, only 2 patients treated with the triplet regimen (both with a severity score of 0-2) reached the composite primary outcome, whereas all 17 patients with a severity score ≥ 3 recovered from their disease (Table 2).
Safety
Ultimately, 46 (51%) patients experienced at least 1 drug-related adverse event in the triplet regimen cohort (Table 3). Hemorrhagic events were mostly drug related (30 patients, 33%), with epistaxis and gingival bleeding being the most prevalent. All these events resolved without specific intervention. Major bleeding events and clinically relevant nonmajor bleeding events were observed in 2 and 11 patients, respectively. One major bleeding event from the digestive tract was complicated by hemorrhagic shock of favorable outcome with symptomatic measures and the interruption of caplacizumab. No patient required infusions of vWF concentrates or any coagulation concentrate. No temporal relationship between the occurrence of bleeding and the duration of exposure to caplacizumab was observed. Six patients (6.7%) developed an inflammatory reaction consisting of swelling at the caplacizumab injection sites, which typically occurred by the end of the treatment course; however, no premature interruption of the treatment was needed. Nineteen patients (21%) developed mild to severe transient thrombocytosis (platelet count > 450 × 103/mm3) along with platelet count recovery (Table 3; supplemental Table 4), although the association with the use of caplacizumab remains questionable.
Table 3.
Caplacizumab-related adverse events
| Adverse event | Number of adverse events | Description |
|---|---|---|
| Major bleeding | 2 | One with hemorrhagic shock with lower digestive bleeding |
| One with abundant menorrhagia with a decrease in hemoglobin level of 2.5 g/dL | ||
| Clinically relevant nonmajor bleeding | 11 | Three with macroscopic gastrointestinal hemorrhage |
| Seven with epistaxis | ||
| One with subcutaneous hematoma larger than 25 cm2 | ||
| Non–clinically relevant nonmajor bleeding | 17 | Nine with ecchymosis or small hematoma |
| Six with gingival bleedings | ||
| Two with catheter site hemorrhage | ||
| Inflammatory reaction | 6 | Inflammatory swelling at the injection site, especially at the end of the treatment course |
| Thrombocytosis | 19 | Platelet count (×103/mm3) |
| >450-600: 11 cases | ||
| >600-900: 7 cases | ||
| >900: 1 case |
Thromboembolic events
Eleven patients (12%) of the triplet regimen cohort experienced a thromboembolic event (TEE), consisting in pulmonary embolism without apparent deep venous thrombosis or central venous catheter thrombosis (5 cases), lower limb deep venous thrombosis (3 cases), and central venous catheter-related thrombosis (4 cases). One patient had both a central venous catheter-related thrombosis associated with a more distal deep venous thrombosis. None of them had received thromboprophylaxis when platelet count increased above 50 × 103/mm3. On the other hand, we found no clear association between the occurrence of TEE and thrombocytosis (thrombocytosis was present in 3 [27%] patients with TEE vs 16 [21%] patients without TEE). Moreover, the prevalence of TEE was similar in both cohorts (P = .79).
Discussion
We report the outcome of iTTP patients managed with a triplet strategy associating caplacizumab with frontline rituximab and the historical TPE/corticosteroids treatment. This regimen, established in the HERCULES trial and submitted to patients with a clinical diagnosis of iTTP,14 is aimed at addressing all 3 aspects of iTTP pathophysiology: to replenish the missing ADAMTS13 enzyme, to suppress the production of anti-ADAMTS13 antibodies, and to inhibit the excessive vWF-platelet interaction leading to microthrombi formation, organ failure, and death.8 In this prospective study performed in real-life conditions, we confirm previous findings from both pivotal trials and uncontrolled observational reports in unselected patients homogeneously treated with a standardized approach: the triplet regimen.13,14,16 We showed that early treatment with caplacizumab allows significantly preventing unfavorable outcomes during the very acute phase of iTTP as only 1 case of death and 1 slow response were identified, whereas very few cases of exacerbations were observed. Although the rarity of events for death led to a trend to less death in the triplet regimen, a difference may have emerged with a larger cohort of patients. By contrast, in our historical cohort of patients, these events were more prevalent and consistent with previous reports.5,17 Importantly, the prevention of such events also alleviated the burden of the disease that translates into a dramatic reduction of plasma volumes and TPE sessions and a shortened hospital stay. From these results, the triplet regiment is now considered the best standard of care in France.
One limitation of our study lies in the different modalities of use of rituximab, which was used frontline in the triplet regimen but as a salvage therapy in the historical cohort. However, our work was not only aimed at demonstrating the superiority of the addition of caplacizumab to the standard treatment; instead, we wished to show here that a strategy addressing the 3 aspects of iTTP pathophysiology simultaneously from diagnosis is superior to more sequential/escalating strategies5,6,9,10 while the risk/benefit effect remains acceptable.
Although frontline rituximab was reported to prevent slow responders to TPE and prevent 1- to 2-year relapses, it scarcely improved survival and other early unfavorable outcomes in acute iTTP.6,22 Therefore, the substantial improvement of iTTP prognosis we observed here, reflected by a rapid and durable platelet count recovery, is likely because of the early administration of caplacizumab. Consequently, caplacizumab and rituximab should be considered complementary, nonredundant agents in the management of iTTP. By preventing the neoformation of microthrombi, caplacizumab allows a rapid and stable platelet count recovery with organ protection, especially during very early management of iTTP during which most deaths occur and during which rituximab is not efficient yet.6,12 On the other hand, by suppressing the autoimmune response against ADAMTS13, rituximab improves and stabilizes durable ADAMTS13 activity usually after a 2- to 5-week period.14 The combination of both agents (ie, a standard course of 4 infusions of rituximab to improve ADAMTS13 activity and daily caplacizumab to prevent additional microthrombi formation until ADAMTS13 improvement) should therefore prevent clinical relapses when caplacizumab is interrupted while ADAMTS13 activity is still undetectable.13,14 Here, the systematic use of rituximab frontline in the triplet cohort accounts for the faster improvement of ADAMTS13 activity than with the historical regimen where rituximab was only introduced later as a salvage therapy.
We provide evidence that patients treated with the triplet regimen have a favorable outcome irrespective of disease severity on diagnosis,12 suggesting that the negative impact of cerebral involvement and very high LDH level is suppressed by caplacizumab. However, the benefit of caplacizumab on prognosis needs further evaluation in the elderly, because the single case of fatal outcome and the most serious adverse events were observed in this population. Moreover, the usual prevalence of TEE was not apparently reduced with the use of caplacizumab.25 One explanation would be a more prevalent omission of thromboprophylaxis (in 63% of patients) with the use of caplacizumab, as clinicians considered here that the association of caplacizumab and anticoagulation could have exposed patients to an increased risk of bleeding. Consequently, iTTP patients should be considered at risk of TEE despite the use of caplacizumab, and thromboprophylaxis should be more systematically offered, especially when platelet count increases above 50 × 103/mm3.
A main obstacle to the use of caplacizumab may be its high cost. In this way, medico-economic studies are still pending to address whether the outcomes realized with caplacizumab-containing regimens, including the dramatic decrease in the burden of care, are sufficiently superior to be cost effective. On the other hand, the price of caplacizumab, recognizing its innovative nature, will have to be balanced with the improvement of survival with usually no sequelae in a disease involving young patients.
This postmarketing surveillance study confirms the results of caplacizumab trials in unselected iTTP patients. A triplet strategy systematically associating TPE, immunosuppression with corticosteroids and rituximab, and caplacizumab prevents unfavorable outcomes of the disease and substantially alleviates the burden of care in these patients.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
Patients were recruited with the help of the members of the Reference Center for Thrombotic Microangiopathies. We thank S. Thouzeau, S. Capdenat, S. Savigny (Laboratoire d'Hématologie, Hôpital Lariboisière, AP-HP, Paris), and Raïda Bouzid (Centre de Référence des Microangiopathies Thrombotiques, Hôpital Saint-Antoine, Assistance Publique–Hôpitaux de Paris, Paris) for technical assistance.
This work was partly funded by a grant from the French Ministry of Health (Projet Hospitalier de Recherche Clinique, P120118, AOM12259). This work was also supported by the National Plan for Rare Diseases of the French Ministry of Health (Direction Générale de l’Offre de Soin).
Footnotes
For original data, please e-mail the corresponding author at paul.coppo@aphp.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.C. and Y.B. designed the study, interpreted the results, and wrote the manuscript; M.B. performed the statistical analysis of the French Registry for Thrombotic Microangiopathies; P.C., E.A., N.B., L.G., P.P., F.P., M.N., C.P., O.M., R.B., A.D., A.S., M.H., A.W., Y.D., J.-F.A., P.P., V.R., C.B., F.L., M.U., A.C.R., S.d.W., T.K., A.V., and Y.B. enrolled patients and collected clinical and laboratory information; S.M. collected the data from all patients; and all authors critically reviewed and substantially improved the manuscript.
Conflict-of-interest disclosure: P.C. is member of the clinical advisory board for Alexion, Sanofi, Shire, and Octapharma. Y.B., P.P., A.W., Y.D., C.P., and A.V. participated in advisory boards for Sanofi. The remaining authors declare no competing financial interests.
A complete list of members of the Reference Center for Thrombotic Microangiopathies appears in the supplemental Appendix.
Correspondence: Paul Coppo, Service d’Hématologie, Centre de Référence des Microangiopathies Thrombotiques (CNR-MAT), Hôpital Saint-Antoine, AP-HP, 75571 Paris, France; e-mail: paul.coppo@aphp.fr.
REFERENCES
- 1.Scully M, Cataland S, Coppo P, et al. ; International Working Group for Thrombotic Thrombocytopenic Purpura . Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312-322. [DOI] [PubMed] [Google Scholar]
- 2.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. [DOI] [PubMed] [Google Scholar]
- 3.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325(6):398-403. [DOI] [PubMed] [Google Scholar]
- 4.Rock GA, Shumak KH, Buskard NA, et al. ; Canadian Apheresis Study Group . Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325(6):393-397. [DOI] [PubMed] [Google Scholar]
- 5.Froissart A, Buffet M, Veyradier A, et al. ; Experience of the French Thrombotic Microangiopathies Reference Center . Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Crit Care Med. 2012;40(1):104-111. [DOI] [PubMed] [Google Scholar]
- 6.Scully M, McDonald V, Cavenagh J, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118(7):1746-1753. [DOI] [PubMed] [Google Scholar]
- 7.Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol. 2007;136(3):451-461. [DOI] [PubMed] [Google Scholar]
- 8.Coppo P, Cuker A, George JN. Thrombotic thrombocytopenic purpura: toward targeted therapy and precision medicine. Res Pract Thromb Haemost. 2018;3(1):26-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2016;127(24):3092-3094. [DOI] [PubMed] [Google Scholar]
- 10.Mazepa AM, Evans M, Davis E, et al. Differential effect of rituximab on relapse-free survival in de novo and relapse immune thrombotic thrombocytopenic purpura in African-American and Caucasian populations. Blood. 2019;134(suppl 1):190. [Google Scholar]
- 11.Patton JF, Manning KR, Case D, Owen J. Serum lactate dehydrogenase and platelet count predict survival in thrombotic thrombocytopenic purpura. Am J Hematol. 1994;47(2):94-99. [DOI] [PubMed] [Google Scholar]
- 12.Benhamou Y, Assié C, Boelle PY, et al. ; Thrombotic Microangiopathies Reference Center . Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Haematologica. 2012;97(8):1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyvandi F, Scully M, Kremer Hovinga JA, et al. ; TITAN Investigators . Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. [DOI] [PubMed] [Google Scholar]
- 14.Scully M, Cataland SR, Peyvandi F, et al. ; HERCULES Investigators . Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346. [DOI] [PubMed] [Google Scholar]
- 15.Knoebl P, Cataland S, Peyvandi F, et al. Efficacy and safety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study. J Thromb Haemost. 2020;18(2):479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Völker LA, Kaufeld J, Miesbach W, et al. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020;4(13):3085-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppo P, Schwarzinger M, Buffet M, et al. ; French Reference Center for Thrombotic Microangiopathies . Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One. 2010;5(4):e10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prevel R, Roubaud-Baudron C, Gourlain S, et al. Immune thrombotic thrombocytopenic purpura in older patients: prognosis and long-term survival. Blood. 2019;134(24):2209-2217. [DOI] [PubMed] [Google Scholar]
- 19.Hie M, Gay J, Galicier L, et al. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura: experience of the French Thrombotic Microangiopathies Reference Center. Blood. 2014;124(2):204-210. [DOI] [PubMed] [Google Scholar]
- 20.Jestin M, Benhamou Y, Schelpe AS, et al. ; French Thrombotic Microangiopathies Reference Center . Preemptive rituximab prevents long-term relapses in immune-mediated thrombotic thrombocytopenic purpura. Blood. 2018;132(20):2143-2153. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. [DOI] [PubMed] [Google Scholar]
- 22.Froissart A, Veyradier A, Hié M, Benhamou Y, Coppo P; French Reference Center for Thrombotic Microangiopathies . Rituximab in autoimmune thrombotic thrombocytopenic purpura: A success story. Eur J Intern Med. 2015;26(9):659-665. [DOI] [PubMed] [Google Scholar]
- 23.Benhamou Y, Paintaud G, Azoulay E, et al. ; French Reference Center for Thrombotic Microangiopathies . Efficacy of a rituximab regimen based on B cell depletion in thrombotic thrombocytopenic purpura with suboptimal response to standard treatment: Results of a phase II, multicenter noncomparative study. Am J Hematol. 2016;91(12):1246-1251. [DOI] [PubMed] [Google Scholar]
- 24.Soucemarianadin M, Benhamou Y, Delmas Y, et al. Twice-daily therapeutical plasma exchange-based salvage therapy in severe autoimmune thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Eur J Haematol. 2016;97(2):183-191. [DOI] [PubMed] [Google Scholar]
- 25.Vendramin C, McGuckin S, Alwan F, Westwood JP, Thomas M, Scully M. A single-center prospective study on the safety of plasma exchange procedures using a double-viral-inactivated and prion-reduced solvent/detergent fresh-frozen plasma as the replacement fluid in the treatment of thrombotic microangiopathy. Transfusion. 2017;57(1):131-136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.