Abstract
Objectives
The study aimed to estimate the incidence and prevalence of feline lymphoma in cats attending primary‐care practices across the UK and to identify patient‐based and environmental (radon and pesticide exposure) risk factors.
Materials and Methods
Case records from the VetCompass programme from primary‐care veterinary practices in the UK were searched for a diagnosis of lymphoma in cats in 2016. Cases were required to have had an external laboratory confirmed diagnosis based on cytology and/or histopathology. A nested case–control study design was used to identify risk factors for lymphoma using multivariable logistic regression.
Results
From a cohort of 562,446 cats under veterinary care at VetCompass participating practices in 2016, a total of 271 lymphoma cases were identified (prevalence: 48/100,000, 95% confidence interval (CI) 44 to 56/100,000; incidence 32/100,000, 95% CI 26 to 35/100,000). There were 180 incident lymphoma cases and 803 controls, all aged 2 years and older. Male (odds ratio (OR) 1.7, 95% CI 1.2 to 2.4), insured (OR 3.6, 95% CI 2.3 to 5.6) and older cats (compared to cats 2 to <5 years, OR 5.0, 95% CI 2.8 to 8.8) were associated with increased odds of lymphoma diagnosis. Vaccinated cats were associated with decreased odds (OR 0.7, 95% CI 0.5 to 1.0) compared to unvaccinated cats, although the type of vaccination received was not statistically significant. Breed and environmental factors studied were not associated with a diagnosis of lymphoma.
Clinical Significance
This is the first study to estimate the frequency and report risk factors for lymphoma in cats attending UK primary‐care practice.
INTRODUCTION
Feline lymphoma is a neoplastic disorder of the haemopoietic system that has been reported as the most commonly diagnosed neoplasia in domestic cats (Dorn 1967, Dorn et al. 1968). Haematopoietic tumours have been estimated to account for one‐third of all feline tumours with lymphoma comprising 50 to 90% of these (Hardy 1981, Haga et al. 1988). However, there is as yet no information regarding the incidence and prevalence of feline lymphoma in the UK.
Lymphoma in cats typically presents in older domestic crossbreed cats with a median age at diagnosis of 10 years (Gabor et al. 1998, Sato et al. 2014). Alimentary lymphoma was described as the most common anatomical location, representing 40 to 50% of feline lymphomas in previous studies, with the majority of cats testing negative on blood tests for feline leukaemia virus (FeLV) (Gabor et al. 1998, Sato et al. 2014). Previously reported risk factors for feline lymphoma include breed (in particular Siamese and Oriental breeds) (Gabor et al. 1998, Louwerens et al. 2005, Fabrizio et al. 2014), FeLV and feline immunodeficiency virus (FIV) positive status (Shelton et al. 1990, Louwerens et al. 2005). A male predisposition has been suggested in a few reports (Gabor et al. 1998, Vail et al. 1998, Meichner et al. 2012) although other studies have failed to demonstrate an association between lymphoma, sex and neutering status (Haga et al. 1988, Louwerens et al. 2005, Sato et al. 2014).
Domestic environmental influences as risk factors for feline lymphoma have become of interest since the publication of a USA study (Bertone et al. 2002), showing that cats exposed to tobacco smoke had increased risk of developing lymphoma compared to cats from non‐smoking households. Other environmental risk factors for lymphoma in both human and domestic species include exposure to radon and agricultural chemicals (Zahm et al. 1990, Hayes et al. 1991, Zahm & Blair 1992, Baris et al. 1998, Gavazza et al. 2008, Ha et al. 2017). However, conflicting evidence in both the human and canine literature, in addition to the limited studies involving cats, warrants further investigation of the role of radon and pesticide exposure and their association with lymphoma (Hayes et al. 1991, Zahm & Blair 1992, Baris et al. 1998, Forastiere et al. 1998, Gavazza et al. 2008, Ha et al. 2017).
To date, there have been no studies exploring radon and exposure to agricultural chemicals as risk factors for feline lymphoma. Additionally, no study has evaluated incidence, prevalence or risk factors for feline lymphoma using nationwide primary‐care practice data in the UK. Previous veterinary research has so far been restricted to studying cats from referral practices, which may have limited relevance to the wider UK cat population (Bartlett et al. 2010). The current study aimed to evaluate the frequency of feline lymphoma and its associated risk factors, including signalment, vaccination status, FeLV status, FIV status and environmental risk factors, in cats registered to UK primary‐care veterinary practices during 2016. The hypotheses for this study were based on current literature that older cats and Siamese/Oriental breeds would have higher odds of lymphoma and cats with FeLV or FIV would be at increased odds. Environmental factors and vaccination status were explored as additional potential risk factors.
MATERIALS AND METHODS
Data were collected from the VetCompass programme, which collates electronic patient records (EPRs) from primary‐care veterinary practices in the UK (VetCompass 2020). The denominator population included cats in the VetCompass cohort that were under veterinary care in 2016. Ethical approval was provided by the Royal Veterinary College Ethics and Welfare Committee (SR2018‐1652).
Candidate cases of lymphoma from the VetCompass database were identified by searching within the clinical notes using the search terms: lymphoma~1, lymphosarc*, COP, LGAL, HGAL, cyclop*, vincr*, lomust*, chlorambucil~1, doxorubicin~1. Confirmed cases of lymphoma were defined as veterinary diagnosed cases within 1 January 2016 and 31 December 2016; either prevalent or incident. “Prevalent” cases were those first diagnosed before the study period and “incident” cases were those first diagnosed during the study period. Cases were required to have had an external laboratory confirmed diagnosis based on cytology and/or histopathology. In some cases, PCR for Antigen Receptor Rearrangements (PARR) or immunohistochemistry/immunocytochemistry was used as an additional confirmatory test. Cases with a diagnosis of leukaemia where lymphoid neoplasia appeared to primarily affect the bone marrow or blood, without obvious lymph node involvement/lymphoid masses, were excluded.
A case–control study design nested within the cohort of cats under veterinary care at VetCompass particpating practices in 2016 was used to identify risk factor association with lymphoma diagnosis. Only cats aged 2 years and older in 2016 were evaluated in the risk factor analysis for both cases and controls. Cases without a diagnosis date and those that were “prevalent” were excluded from the risk factor analysis (incident cases only were used). Control cats were selected from the “non‐cases” within the denominator population using the random generator (RAND) in Excel (Microsoft 2019). All cats that did not fit the case definition were identified as a “non‐case” and were eligible for selection as controls.
Data that were routinely available included demographic (sex, date of birth, clinic ID veterinary group, owner partial postcode) and clinical data (free‐text clinical notes, VeNom diagnostic terms). The EPRs of cats identified as lymphoma cases were then further examined to extract information on histological/cytological grade, cell size, anatomical location, FeLV status, FIV status and vaccination status. Grade and cell size were extracted from the pathology report in the clinical notes when provided. Grade classifiation represented the grade given by pathologist based either on cytology or histology results and was categorised as either “low” or “intermediate/high” and cell size was categorised as “small” or “intermediate/large.” Information in the clinical notes regarding physical examination, ultrasound scan results, radiographs and exploratory surgical evaluation was used to classify the anatomical location of lymphoma cases and categorise them according to a modified version of a published anatomical definition (Gabor et al. 1998) (Table 1). FeLV status and FIV status were categorised separately as “Positive,” “Negative” or “Not Tested.” “Positive” diagnosis of FeLV or FIV was determined by an antigen‐ or antibody‐based screening test, respectively, which could be performed “in‐house” or at an external laboratory. FeLV and FIV status of the selected controls were manually assessed using the same method and definition criteria described for lymphoma cases.
Table 1.
Classification of feline lymphoma based on anatomical tissue involvement as described by Gabor et al. (1998)
| Anatomical Location | Subcategory | Organ/tissue involvement |
|---|---|---|
| Mediastinal | Any structure within the mediastinal space | |
| Abdominal | Alimentary | Gastrointestinal tract and associated lymph nodes excluding liver and pancreas |
| Renal | Involving one or both kidneys | |
| Hepatic | Sole involvement of liver | |
| Splenic | Sole involvement of spleen | |
| Combination | Combination of two or more of the abdominal subcategories | |
| Nodal | Solitary | One peripheral lymph node only |
| Regional | A chain of adjacent lymph nodes involving lymph nodes on one side of the diaphragm | |
| Multinodal | Many or all peripheral lymph nodes involving lymph nodes on both sides of the diaphragm | |
| Atypical | Respiratory | A structure involving any of the respiratory passages (e.g. nasal cavity, pharynx, larynx, trachea) |
| Other | Other non‐lymphoid tissues such as the CNS and skin | |
| Mixed | Combination of two or more of the above categories |
Vaccination status of cases and controls was analysed statistically in two ways. The first method categorised cats as “vaccinated” or “no evidence of vaccination.” Classification as “vaccinated” required evidence of vaccination in their EPR before confirmatory diagnosis of lymphoma for the cases or 31 December 2016 for the controls. The second method used to assess vaccination status grouped cats depending on the type of vaccinations received based on available treatment data for each cat. Cases and controls were categorised into four groups depending on the type of vaccinations present in their clinical records before the confirmatory diagnosis of cases or December 31, 2016 for controls: (1) “Not vaccinated,” (2) “Vaccinated Unknown,” (3) “Vaccinated: no FeLV,” (4) “Vaccinated: FeLV.” “Vaccinated Unknown” referred to cats who had evidence of vaccination in their clinical notes but no information on the type of vaccinations given. “Vaccinated: no FeLV” were cats with known vaccinations which did not include FeLV vaccination. “Vaccinated: FeLV” were cats vaccinated against FeLV with or without other vaccinations. It was not recorded in this study whether vaccinations were “up to date.”
The age (years) of cases was calculated using their date of birth and date at which a first laboratory‐confirmed diagnosis of lymphoma was obtained. When an exact date for laboratory diagnosis was not available, date at which the pathology sample was sent plus 3 days was used to estimate age. Age of controls was calculated using their date of birth and the date midway through the study period, June 30, 2016. Age was restricted to include only those aged 2 years and above to make the age‐distribution of the case and control populations more similar in order to help control for time‐related confounding variables such as exposure to environmental factors. Age (years) was categorised into four groups for analysis: 2 to <5, 5 to <8, 8 to <11 and ≥11. Cats aged 2 to <5 years were used as the baseline in the analysis. Cats were categorised into a “Breed” variable using VeNom standardised breed terms (VeNom coding group, 2017). Breeds with three or more cases were included individually as a breed category while all remaining purebreds were grouped as “Other Purebreds.” All non‐purebred cats were classified as crossbred. A binary purebred variable was also evaluated in the analysis and categorised cats as “purebred” or “crossbred.” Sex and insurance status were examined as categorical variables. Neutering status was deemed poorly reliable after data checking and was therefore not included in the study.
Partial postcodes (postcode sector) of owner home location from cases and controls were used to gather data on potential environmental risk factors. Pesticide (fungicide and herbicide) levels equated to kilogrammes of pesticide applied per census ward on agricultural land as reported from a national survey of a sample of farms and were supplied by the UK Small Area Health Statistics Unit for England in 2000 at 1998 census ward level. Pesticide potential exposure levels were categorised into three groups: A group with no known exposure and two groups split into roughly equal sizes to form “low” and “high” exposure groups. Potential fungicide exposure levels were (usage per census ward, in kg): Zero (“no exposure”), 1 to <150 (“low exposure”) and ≥150 (“high exposure”). Herbicide levels were (usage per census ward, kg): Zero (“no exposure”), 1 to <230 (“low exposure”) and ≥230 (“high exposure”)(Schofield et al. 2019). A combined pesticide variable was created with four groups describing the level of potential exposure to both fungicide and herbicide: Low‐low (fungicide <150 kg and herbicide <230), low‐high (fungicide <150 kg and herbicide >230), high‐ low (fungicide >150 kg and pesticide <230) and high‐high (fungicide >150 kg and herbicide >230) (Schofield et al. 2019). Data for radon were provided by Public Health England for England and Wales for the year 2007 at a resolution of 1 km2 (UK radon 2018). Potential radon exposure was classified based on the percentage of households estimated to exceed the recommended radon action level of 200 Bq/m3 (2018, Schofield et al. 2019). Radon exposure for cases and controls was categorised into three groups: ≤1%, >1 to <2% and ≥3.0%.(Zahm et al. 1990).
Statistical analysis
Annual incidence risk and prevalence risk with 95% confidence intervals were calculated using the proportion of incident cases, and incident and prevalent cases respectively within the study cohort population during 2016. Cats of all ages were included in both the incidence and prevalence risk estimation with 95% confidence intervals for these estimates calculated using standard methods (Kirkwood et al. 2003).
For the evaluation of risk factors, data checking and cleaning were performed in Microsoft Excel (2019) producing one record per cat which were then imported into IBM SPSS version 25 statistical software for analysis. Categorical data were summarised with count and percentage. Medians and interquartile range (IQRs) were calculated for continuous variables. Univariable analysis tested associations between risk factors and a diagnosis of lymphoma using univariable logistic regression. Variables which were broadly significant at the univariable analysis (P ≤ 0.2) were carried forward for multivariable evaluation. Collinearity was assessed between all variables taken forward for multivariable consideration using either chi‐square or Fishers exact tests. A manual backwards stepwise elimination method was used for development of the logistic regression model. Final variables were evaluated for pairwise interaction. Receiver operating characteristic (ROC) curve analysis was carried out to evaluate the area under the curve (AUC) for the final model using predicted probability. Mixed effect logistic regression with clinic ID as a random effect was assessed for in both the univariable and multivariable analysis.
RESULTS
Prevalence and incidence estimate
The study included 562,446 cats registered to 626 clinics across the UK between January 1, 2016 and December 31, 2016. The intial search revealed 10,260 possible candidate lymphoma cases. After manual assessment, 271(2.6%) cats met the case definitions and inclusion criteria. Of these lymphoma cases, 86 (32%) were prevalent and 185 (68%) were incident cases. Therefore, the annual prevalence risk of feline lymphoma was estimated as 48/100,000 cats per year (95% CI 44.2 to 55.8/100,000) and the annual incidence risk as 32/100,000 cats per year (95% CI 25.5 to 34.5/100,000).
Descriptive statistics
Histological/cytological grade was reported in 88 (48%) incident cases with 73% reported as intermediate/high grade (64/88) and 27% reported as low grade (24/88). Cell size was reported in 92 (51%) incident cases with 71% reported as intermediate/large (65/92), 24% small (22/92) and 5% large granular (5/92) (Fig. 1). Abdominal lymphoma was the most common anatomical type (118 cases, 64%), followed by nodal (15, 8%) and respiratory (13, 7%) (Table 2 and Fig. 2). Ten cats had no information regarding their anatomical locations and were grouped as a separate category.
FIG 1.
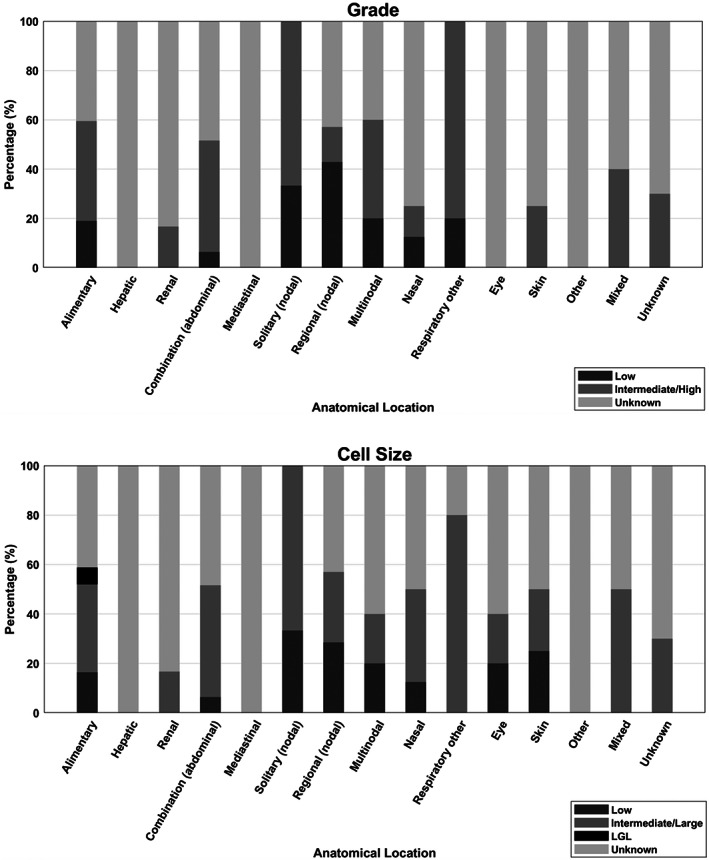
Anatomical locations of feline lymphoma cases according to grade (histological/cytological) and cell size in cats attending primary‐care practices in the UK 2016. Percentages correspond to the percent of total number of cats with each grade/cell size within each anatomical location category. *“LGL” refers to large granular lymphoma
Table 2.
Descriptive statistics for incident cases of feline lymphoma (n = 185) showing their anatomical locations/subcategories and patient characteristics from cats attending primary‐care practices in the UK in 2016
| Anatomical location | Case no. (%) | Age at diagnosis in years, median (range) | Breed distribution, n (%) | Subcategory | Case, n (%) | Age at diagnosis in years, median (range) |
|---|---|---|---|---|---|---|
| Abdominal | 118 (63.8) | 10.9 (0.7 to 18.2) |
102 (86.4) Domestic 3 (2.5) Persian 3 (2.5) British Short Hair 2 (1.7) British Blue 2 (1.7) Crossbred 1 (0.8) Siamese 1 (0.8) Oriental 1 (0.8) Bengal 1 (0.8) Ragdoll 1 (0.8) Russian blue 1 (0.8) Birman |
Alimentary | 73 (39.5) | 10.7 (2.6 to 18.2) |
| Hepatic | 2 (1.1) | 12.7 (10.1 to 15.3) | ||||
| Renal | 12 (6.5) | 8.7 (0.7 to 15.4) | ||||
| Combination | 31 (16.8) | 12.0 (0.94 to 17.4) | ||||
| Mediastinal | 7 (3.8) | 5.6 (0.7 to 12.1) |
6 (85.7) Domestic 1 (14.3) Crossbred |
7 (3.8) | 5.6 (0.7 to 12.1) | |
| Nodal | 15 (8.1) | 11.3 (2.0 to 18.7) |
14 (93.3) Domestic 1 (6.7) Siamese |
Solitary | 3 (1.6) | 7.9 (5.1 to 17.2) |
| Regional | 7 (3.8) | 13.0 (10.0 to 18.7) | ||||
| Multinodal | 5 (2.7) | 7.1 (2.0 to 11.7) | ||||
| Respiratory | 13 (7.0) | 9.4 (5.7 to 16.8) |
11 (84.6) Domestic 1 (7.7) Maine Coon 1 (7.7) Burmilla |
Nasal | 8 (4.3) | 10.7 (6.3 to 16.8) |
| Other | 5 (2.7) | 9.0 (5.7 to 14.2) | ||||
| Atypical/Other | 12 (6.5) | 12.0 (5.5 to 15.9) |
9 (75.0) Domestic 1 (8.3) Siamese 1 (8.3) British Short Hair 1 (8.3) Norwegian Forest |
Eye | 5 (2.7) | 12.5 (8.1 to 14.6) |
| Skin | 4 (2.2) | 12.7 (5.5 to 15.9) | ||||
| Other | 3 (1.6) | 11.6 (7.5 to 13.0) | ||||
| Mixed | 10 (5.4) | 10.7 (2.0 to 15.1) |
7 (70.0) Domestic 1 (10.0) Exotic 1 (10.0) Russian Blue 1 (10.0) Siamese |
10 (5.4) | 10.7 (2.0 to 15.1) | |
| Unknown | 10 (5.4) | 13.1 (5.0 to 16.1) |
8 (80.0) Domestic 1 (10.0) Birman 1 (10.0) British Short Hair |
10 (5.4) | 13.1 (5.0 to 16.1) |
FIG 2.
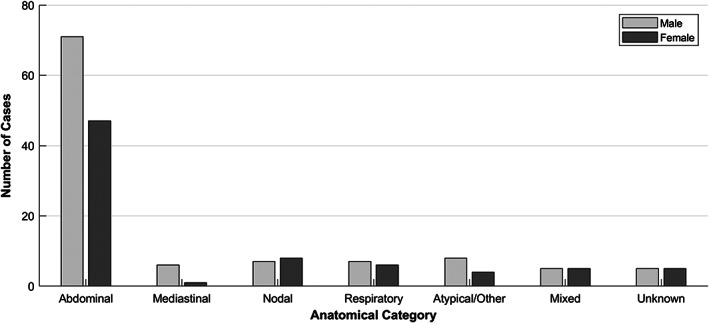
Anatomical locations of feline lymphoma cases according to sex in cats attending primary‐care practices in the UK 2016
Thirty‐two percent of the incident cases were tested for FeLV (59 cases), of which only three tested postive (5%) (two mediastinal, one not mentioned anatomical site). Thirty‐one percent were tested for FIV (58), of which, seven tested positive (12%) (three abdominal, two respiratory, one mixed, one atypical). No incident cases tested positive for both FeLV and FIV. Two percent of controls were tested for FeLV and FIV (n = 17) with only one control testing positive for FIV (6%, 1/17) and the remaining testing negative for both FIV and FeLV (94%, 16/17).
Case–control study
Out of the 185 incident cases, five were removed from the risk factor analysis to include only cats aged 2 years and above resulting in 180 lymphoma cases taken forward to the case–control study. These were compared to 803 controls in the risk factor analysis, all aged 2 years and above. Descriptive statistics for cases included in the risk factor analysis were similar to those described for incident cases above and are summarised into Table 3. The distribution of incident cases and controls were very similar for crossbred and purebred cats with 86% (153) of incident cases being crossbred compared to controls where 87% (686) were crossbred cats. Of the incident cases, the most common breed types were Domestic (83.8%, 151/180), Siamese (2.2%, 4/180) and British Blue (1.6%, 3/180). There was a greater distribution of males in the incident cases (59%, 109) compared to controls (46%, 366) whereas a slightly smaller proportion of cats were vaccinated in incident cases (59%, 106) compared to controls (66%, 527). Of the incident cases, 42% (75) were insured compared to only 13% (108) of cats in controls. The median age at diagnosis for incident cases was 11.0 years (IQR 8.1 to 14.0). Date of birth was not provided for one incident case. The median age reported for controls was 7.3 years (IQR 4.3 to 11.5).
Table 3.
Descriptive and univariable logistic regression results for risk factors for diagnosis of lymphoma in cats attending UK primary‐care practices in 2016. Only incident cases aged 2 years and older were included here
| Variable | Category | Case, n (%) | Control, n (%) | Odds ratio | 95% CI | Variable P‐value |
|---|---|---|---|---|---|---|
| Purebred Status | Crossbred | 153 (85.5) | 686 (85.4) | Base | 0.539 | |
| Purebred | 26 (14.4) | 100 (12.5) | 1.2 | 0.7 to 1.9 | ||
| Not recorded | 1 (0.1) | 17 (2.1) | ||||
| Breed | Domestic | 151 (83.8) | 665 (82.8) | Base | 0.116 | |
| British Blue | 3 (1.6) | 2 (0.2) | 6.6 | 1.1 to 39.9 | ||
| Persian | 3 (1.6) | 9 (1.1) | 1.5 | 0.4 to 5.9 | ||
| Siamese | 4 (2.2) | 9 (1.1) | 2.0 | 0.6 to 6.4 | ||
| Cross breed | 2 (1.1) | 21 (2.6) | 0.4 | 0.1 to 1.8 | ||
| Other purebreds | 16 (8.8) | 80 (10.0) | 0.9 | 0.5 to 1.5 | ||
| Not recorded | 1 (0.5) | 17 (2.1) | ||||
| Age (years) | 2.0 to 4.9 | 14 (7.8) | 254 (31.6) | Base | < 0.001 | |
| 5.0 to 7.9 | 26 (14.5) | 186 (23.2) | 2.5 | 1.3 to 5.0 | ||
| 8.0 to 10.9 | 47 (26.3) | 146 (18.2) | 5.8 | 3.1 to 11.0 | ||
| ≥11.0 | 92 (51.4) | 217 (27.0) | 7.7 | 4.3 to 13.9 | ||
| Sex | Female | 74 (41.1) | 435 (54.3) | Base | 0.001 | |
| Male | 106 (58.9) | 366 (45.7) | 1.7 | 1.2 to 2.4 | ||
| Insurance status | Insured | 75 (41.7) | 108 (13.4) | 4.5 | 3.2 to 6.6 | < 0.001 |
| No evidence/Non‐insured | 105 (58.3) | 695 (86.6) | Base | |||
| Vaccination status 1 | Not vaccinated | 74 (41.1) | 276 (34.4) | Base | 0.088 | |
| Vaccinated | 106 (58.9) | 527 (65.6) | 0.8 | 0.5 to 1.0 | ||
| Vaccination status 2 | Not vaccinated | 74 (41.1) | 276 (34.4) | Base | 0.258 | |
| Vaccinated Unknown | 26 (14.4) | 111 (13.8) | 1.4 | 0.9 to 2.0 | ||
| Vaccinated: no FeLV | 3 (1.7) | 11 (1.4) | 1.2 | 0.8 to 2.0 | ||
| Vaccinated: FeLV | 77 (42.8) | 405 (50.4) | 1.4 | 0.4 to 5.3 | ||
| Fungicide (kg usage per census ward) | “No exposure” (0) | 99 (55.0) | 437 (54.4) | Base | 0.512 | |
| 1 to 150 | 16 (8.9) | 97 (12.1) | 0.7 | 0.4 to 1.3 | ||
| ≥150 | 27 (15.0) | 127 (15.8) | 0.9 | 0.6 to 1.5 | ||
| Not available | 38 (21.1) | 142 (17.7) | 1.1 | 0.8 to 1.8 | ||
| Herbicide (kg usage per census ward) | “No exposure” (0) | 96 (53.3) | 418 (52.1) | Base | 0.548 | |
| 1 to 230 | 21 (11.7) | 108 (13.4) | 0.8 | 0.5 to 1.4 | ||
| ≥230 | 25 (13.9) | 135 (16.8) | 0.8 | 0.5 to 1.3 | ||
| Not available | 38 (21.1) | 142 (17.7) | 1.2 | 0.8 to 1.8 | ||
| Pesticide | Low‐low | 112 (62.2) | 514 (64.0) | Base | 0.502 | |
| High‐high | 22 (12.2) | 115 (14.3) | 0.9 | 0.5 to 1.4 | ||
| High‐ low | 5 (2.8) | 12 (1.5) | 1.9 | 0.6 to 5.5 | ||
| Low‐ high | 3 (1.7) | 20 (2.5) | 0.7 | 0.2 to 2.4 | ||
| Not available | 38 (21.1) | 142 (17.7) | 1.2 | 0.8 to 1.9 | ||
| Vet Groups | Vet Group 1 | 82 (45.6) | 206 (25.7) | Base | < 0.001 | |
| Vet Group 2 | 6 (3.3) | 5 (0.6) | 3.0 | 0.9 to 10.2 | ||
| Vet Group 3 | 65 (36.1) | 485 (60.4) | 0.3 | 0.2 to 0.5 | ||
| Vet Group 4 | 27 (15.0) | 107 (13.3) | 0.6 | 0.4 to 1.0 |
Data on potential exposure to radon and pesticides for the risk factor analysis were available for 142 cases (78%) and 661 controls (82%). Exposure to radon and pesticide for cases and controls are summarised within Table 3.
Univariable analysis identified age (P = <0.001), sex (P = <0.001), insurance status (P = <0.001) and veterinary group (P = <0.001) as being associated with feline lymphoma (Table 3). Purebred status, breed, vaccination status, pesticide, fungicide, herbicide and radon were not significantly associated with presence of feline lymphoma. FeLV and FIV status were not included in the univariable and multivariable analysis as the numbers where this information was recorded were considered to be insufficient for robust statistical analysis.
Final multivariable logistic regression model without clinic ID as a random effect (fixed effect) and with clinic ID as a random effect (mixed effect) was performed. Veterinary group was included in both final multivariable models as a fixed effect to account for clustering at a practice level as it was highly significant as a predictor variable in the univariable analysis. The final multivariable models identified age, sex, insurance status and vaccination status as significantly associated with a diagnosis of feline lymphoma (Table 4 and Fig. 4). In the mixed effect final model, cats over 11 years had 5.0 times the odds of being diagnosed compared to cats aged 2 to 5 years (95% CI 2.8 to 8.8). Insured cats were at 3.6 times the odds (95% CI 2.3 to 5.6) and male cats were at 1.7 times the odds (95% CI 1.2 to 2.4) of lymphoma compared to uninsured and female cats respectively. Vaccinated cats had decreased odds (OR 0.7, 95% CI 0.5 to 1.0) of having lymphoma compared to unvaccinated cats, however this was significant in the fixed effect model (P = 0.043) and only weakly significant in the mixed effect model (P = 0.054), once clustering at a clinic level was adjusted for (Table 4). No major difference was found between the two final models and no significant interaction between final model variables was found. The AUC for the fixed effect multivariable model was 0.776 (95% CI 0.739 to 0.813) and 0.781 (95% CI 0.744 to 0.818) for the mixed effect multivariable model.
Table 4.
Final multivariable logistic regression model without clinic ID as a random effect (fixed effect) and with clinic ID as a random effect (mixed effect) for risk factors for diagnosis of feline lymphoma in cats aged over 2 years attending UK primary‐care practices in 2016
| Variable | Category | Fixed effect | Mixed effect | ||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐value | Odds ratio | 95% CI | P‐value | ||
| Age | 2.0 to 4.9 | Base | <0.001 | Base | <0.001 | ||
| 5.0 to 7.9 | 2.3 | 1.1 to 4.5 | 1.8 | 1.0 to 3.5 | |||
| 8.0 to 10.9 | 5.1 | 2.7 to 9.8 | 4.0 | 2.2 to 7.5 | |||
| ≥11.0 | 6.3 | 3.4 to 11.6 | 5.0 | 2.8 to 8.8 | |||
| Sex | Female | Base | 0.003 | Base | 0.004 | ||
| Male | 1.7 | 1.2 to 2.5 | 1.7 | 1.2 to 2.4 | |||
| Insurance status | Non‐insured | Base | <0.001 | Base | <0.001 | ||
| Insured | 3.7 | 2.4 to 5.8 | 3.6 | 2.3 to 5.6 | |||
| Evidence of vaccination | Not vaccinated | Base | 0.043 | Base | 0.054 | ||
| Vaccinated | 0.7 | 0.5 to 1.0 | 0.7 | 0.5 to 1.0 | |||
| Veterinary group | Vet Group 1 | Base | 0.129 | Base | 0.223 | ||
| Vet Group 2 | 0.3 | 0.8 to 1.1 | 0.3 | 0.1 to 1.1 | |||
| Vet Group 3 | 0.3 | 0.1 to 1.4 | 0.3 | 0.1 to 1.3 | |||
| Vet Group 4 | 0.5 | 0.1 to 1.7 | 0.4 | 0.1 to 1.6 | |||
| Random effect | Interclass correlation | Variance = 4.05 × 10−10 | |||||
FIG 4.
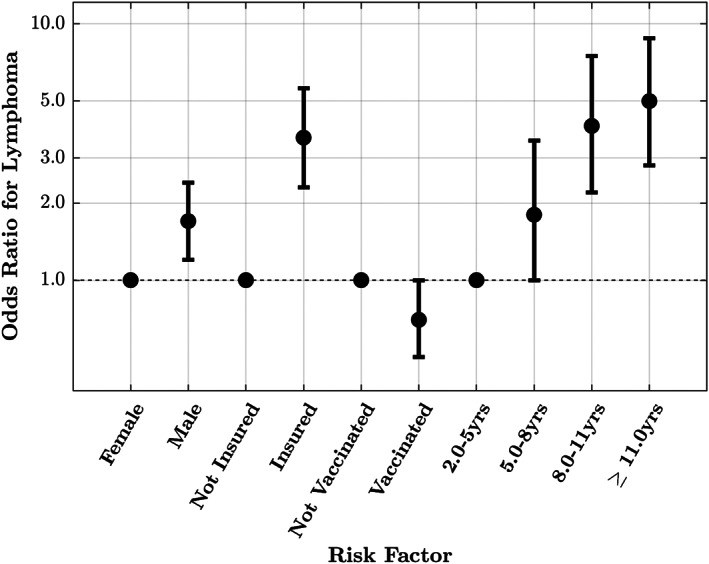
Risk factors for feline lymphoma from final multivariable binary logistic regression model with clinic ID included as a random effect (mixed effect). Veterinary group was included as a fixed effect. Odds ratio for lymphoma with corresponding 95% confidence intervals are reported. Baseline category reported first
DISCUSSION
The current study is the first to report incidence and prevalence values and risk factors associated with lymphoma in cats registered to primary‐care practices in the UK. Previous veterinary research is dominated by small populations of cats from referral practice with a focus on specific anatomical forms of lymphoma and their response to chemotherapy (Moore et al. 1996, Henderson et al. 2004, Sfiligoi et al. 2007). These studies may have limited relevance to the wider UK cat population(Bartlett et al. 2010). Instead, the current study explored cases diagnosed in primary‐practice by evaluating the EPR of 562,446 cats and identified 185 lymphoma cases, of which 180, all aged 2 years and over, were taken forward to the risk factor analysis. The current study identified a greater risk of feline lymphoma with older aged, insured, unvaccinated and male cats in the multivariable analysis.
Alimentary lymphoma was the most common anatomical location followed by the abdominal combination classification and renal lymphoma. This is consistent with other studies where alimentary lymphoma was reported in 40 to 50% of lymphoma cases (Gabor et al. 1998, Sato et al. 2014). The mediastinal anatomical subgroup had the youngest reported median age of 5.6 years compared to other anatomical subgroups. This is comparable with a relatively recent UK referral study where the median age of mediastinal lymphoma in cats was reported as 3 years (Fabrizio et al. 2014). This observation has also been frequently reported in older studies (Mooney et al. 1989, Gabor et al. 1998). In the current study, the “grade” classification represented the grade given by the pathologist based either on cytology or histology results, whereas most previous studies report histological grade exclusively. Nevertheless, 73% of lymphoma cases were classified as intermediate/high grade and 27% were low grade, which is comparable with other studies reporting 65 to 90% of feline lymphomas being intermediate/high grade (Gabor et al. 1998, Sato et al. 2014). Proportions described for lymphoma grade were similar to those reported for cell size in this study with 71% of lymphoma cases reported as intermediate/large and 24% small.
The annual prevalence risk of feline lymphoma in primary‐care UK veterinary practices was 48/100,000 cats per year and the annual incidence risk was 32/100,000 cats per year in the current study. This incidence rate was slightly lower than that reported by a USA study (Dorn et al. 1968) over 50 years ago where 44/100,000 cats at risk per year was reported. Differences described between the two studies may be due to variations between the cat populations (e.g. geographical or genetic factors), study period and other methods of data collection (e.g. inclusion criteria).
Age was identified as a risk factor for feline lymphoma in the current study, with increased odds associated with increasing age. Previous studies have also found increased age associated with increased risk of feline lymphoma, with a reported median age at diagnosis of 10 years (Gabor et al. 1998, Sato et al. 2014). A bimodal age distribution for feline lymphoma has been described, with peaks seen at less than 2 years and greater than 8 years, with FeLV‐associated lymphoma reported to be responsible for the peak seen at <2 years (Rezanka et al. 1992, Gabor et al. 1998, Fabrizio et al. 2014). This was not observed in in the current study (Fig. 3), likely due to the overall low prevalence of FeLV‐positive cats in the current study.
FIG 3.
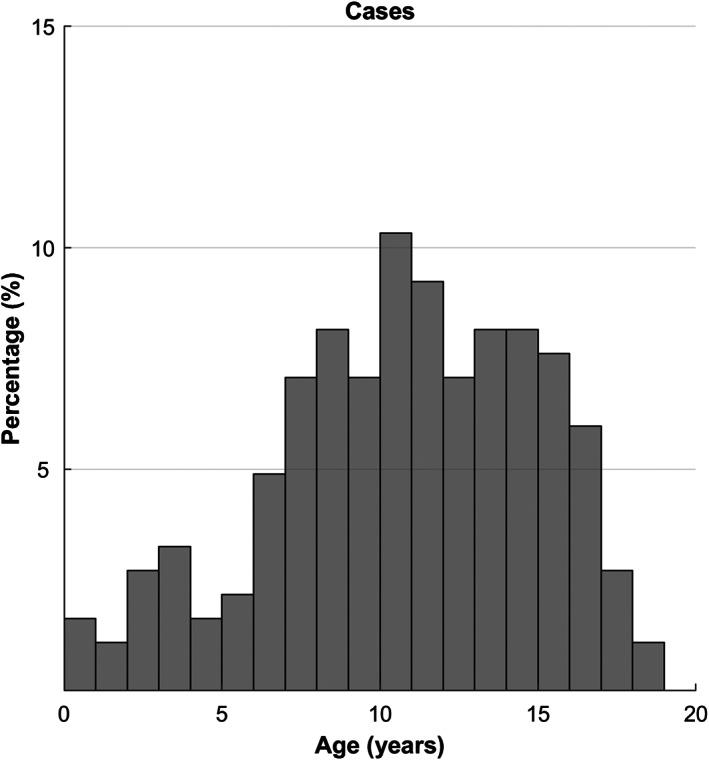
Ages of feline lymphoma cases (n = 185) in cats attending primary‐care veterinary practices in England in 2016
Overall, only 5% of lymphoma cases were reported as retrovirus‐positive, similar to a multi‐institutional referral UK study which reported 7% (10/149) of lymphoma cases being FeLV or FIV positive (Taylor et al. 2009). Interestingly, in the current study, only a minority of cases were tested for FeLV and FIV (32%) suggesting the prevalence of FeLV or FIV in cases could be higher than actually recorded. The low number of lymphoma cases tested for FeLV or FIV may reflect cost implications for owners but may also indicate a need for further awareness of the value of FeLV status for prognosis of lymphoma patients. Current literature highlights the importance of FeLV status on prognosis of lymphoma patients with FeLV positive patients being reported to have a poorer prognosis (Mooney et al. 1989, Vail et al. 1998). FeLV and FIV status were not included in statistical analysis due to the small numbers of cats tested for cases and controls, therefore no conclusions can be drawn in the current study on its role as a risk factor for feline lymphoma.
Vaccinated cats had decreased odds of lymphoma diagnosis compared to unvaccinated cats, once other variables were adjusted for in the final model. This is the first time this has been described in the literature and might suggest vaccination has a protective effect. The decreased risk of lymphoma in vaccinated cats in the current study is not easily explained. Several studies indicate that the introduction of routine FeLV vaccination programmes has decreased the frequency of lymphoma cases that test serum antigen positive suggesting FeLV vaccination has a protective effect against FeLV associated lymphoma (Louwerens et al. 2005, Graf et al. 2015). Recent studies suggest cats can be FeLV‐antigenemia negative but still test positive for the FeLV provirus DNA thereby increasing likelihood of lymphoma through insertional mutagenesis (Hofmann‐Lehmann et al. 2006, Cattori & Hofmann‐Lehmann 2008, Fujino et al. 2008). Interestingly, studies to date have reported that vaccination against FeLV does not provide a protective role against the presence of FeLV proviral DNA in feline tissues following FeLV exposure (Hofmann‐Lehmann et al. 2006, Hofmann‐Lehmann et al. 2007) and therefore does not reduce the risk of insertional mutagenesis and malignant transformation. This could explain why lymphoma cases are rising despite FeLV antigenemia‐associated lymphoma cases decreasing (Louwerens et al. 2005). The type of vaccinations which cats received was not found to be statistically significant in the current study due to incomplete recording of the type of vaccination administered, limiting the interpretation of these findings. Further, it was not recorded whether vaccinations were up to date. Evidence of vaccination could additionally reflect other factors such as owners’ dedication to the overall care of their pets which may influence the diet, environment and access to veterinary care which these cats receive. Future studies further exploring the role of FeLV vaccination and presence of FeLV provirus DNA in feline lymphoma are required.
Sex was significantly associated with a diagnosis of feline lymphoma in the current study with male cats at increased odds compared to females. Similar findings have been reported in a few studies where male cats have been reported with an increased risk of 2.3 in one study (Dorn et al. 1968) and a 1.5:1 male to female ratio was reported in other studies (Gabor et al. 1998, Vail et al. 1998, Meichner et al. 2012). Other studies have failed to report an assocation between lymphoma and sex (Meincke et al. 1972, Haga et al. 1988, Louwerens et al. 2005). The male predisposition in the current study appeared highest in the alimentary and atypical anatomical subgroups though anatomical subtypes were not evaluated in the risk factor analysis. The cause for a sex predisposition has not been determined; however, similar findings are reported in human lymphoma (Ghazawi et al. 2019) which might suggest a role for hormonal influence.
Insured cats were at increased odds of a lymphoma diagnosis compared to non‐insured cats in this study. Insurance may be an indication of the “human‐animal bond” where owners are more likely to undergo further diagnostic testing to confirm lymphoma and meet the inclusion criteria for the study (Egenvall et al. 2009).
Limitations in the present study are worth noting. Underestimation of feline lymphoma prevalence and incidence in this study is probable, due to the stated case definition as only some owners are able or willing to afford the diagnostic tests needed to reach a lymphoma diagnosis. In addition, it is difficult to apply the present study's prevalence and incidence estimates to the wider cat population that do not attend VetCompass practices. No association between breed and lymphoma was observed in the current study despite several studies which report Siamese/Oriental breeds being over‐represented amongst cats with lymphoma (Gabor et al. 1998, Louwerens et al. 2005, Fabrizio et al. 2014). It is possible that the small sample sizes of cases within any specific breed group limited the ability to detect an association. Similarly, no association between feline lymphoma and radon or pesticide exposure was found. The relatively small number of cases may have prevented a subtle association between lymphoma and the environmental factors from being detected, in addition, it was not recorded whether cats were mainly indoor or outdoor which would affect their exposure to environmental factors. Furthermore, bias due to missing data for the environmental variables is highly probable due to the absence of sufficient public records for some districts. Although FeLV and FIV status were rarely recorded, it was considered less likely that this was subject to bias due to missing not at random as these tests are generally considered chargeable items and hence should have been reliably recorded in the EPR. Nonetheless, due to the sparcity of recording of these variables they were not retained in the models as discussed above. Finally, over interpretation of age and its association with feline lymphoma should be avoided due to the use of age categories in the current study. Age categories were selected in the present study according to biological categories and therefore the reader should appreciate the increase in risk of feline lymphoma as the cat ages from young, middle‐aged and older‐aged rather than based on the exact category boundaries.
This study reported the prevalence of UK feline lymphoma cases as 48/100,000 cats per year and the incidence as 32/100,000 cats per year. This investigation supports previously reported risk factors for feline lymphoma where age and sex were associated with an increased risk. However, this study also idenitfied an increased risk of feline lymphoma diagnosis in insured cats and a decreased risk of feline lymphoma in vaccinated cats. No association with environmental factors were found. This study suggests older aged, males, insured and unvaccinated cats are at increased risk of being diagnosed with lymphoma in primary practice. Future studies are needed to further explore the role of vaccination and sex in the development of lymphoma in cats in order to further improve our understanding of this disease.
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
Thanks to Noel Kennedy (RVC) for VetCompass software and programming development. We acknowledge the Medivet Veterinary Partnership, Vets4Pets/Companion Care, Goddard Veterinary Group, Independent Vet Care, CVS Group, Beaumont Sainsbury Animal Hospital, Vets Now and the other UK practices who collaborate in VetCompass. We are grateful to The Kennel Club, The Kennel Club Charitable Trust and Agria Pet Insurance for supporting VetCompass. We thank Dr Daniela Fecht and colleagues at Imperial College for access to the environmental data.
References
- Baris, D. , Zahm, S. H. , Cantor, K. P. , et al. (1998) Agricultural use of DDT and risk of non‐Hodgkin's lymphoma: pooled analysis of three case‐control studies in the United States. Occupational and Environmental Medicine 55, 522‐527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, P. C. , Van Buren, J. W. , Neterer, M. , et al. (2010) Disease surveillance and referral bias in the veterinary medical database. Preventive Veterinary Medicine 94, 264‐271 [DOI] [PubMed] [Google Scholar]
- Bertone, E. R. , Snyder, L. A. & Moore, A. S. (2002) Environmental tobacco smoke and risk of malignant lymphoma in pet cats. American Journal of Epidemiology 156, 268‐273 [DOI] [PubMed] [Google Scholar]
- Cattori, V. & Hofmann‐Lehmann, R. (2008) Absolute quantitation of feline leukemia virus proviral DNA and viral RNA loads by TaqMan real‐time PCR and RT‐PCR. Methods in Molecular Biology 429, 73‐87 [DOI] [PubMed] [Google Scholar]
- Dorn, C. R. (1967) The epidemiology of cancer in animals. California Medicine 107, 481‐489 [PMC free article] [PubMed] [Google Scholar]
- Dorn, C. R. , Taylor, D. O. , Schneider, R. , et al. (1968) Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. Journal of the National Cancer Institute 40, 307‐318 [PubMed] [Google Scholar]
- Egenvall, A. , Nodtvedt, A. , Penell, J. , et al. (2009) Insurance data for research in companion animals: benefits and limitations. Acta Veterinaria Scandinavica 51, 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio, F. , Calam, A. E. , Dobson, J. M. , et al. (2014) Feline mediastinal lymphoma: a retrospective study of signalment, retroviral status, response to chemotherapy and prognostic indicators. Journal of Feline Medicine and Surgery 16, 637‐644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forastiere, F. , Sperati, A. , Cherubini, G. , et al. (1998) Adult myeloid leukaemia, geology, and domestic exposure to radon and gamma radiation: a case control study in central Italy. Occupational and Environmental Medicine 55, 106‐110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino, Y. , Ohno, K. & Tsujimoto, H. (2008) Molecular pathogenesis of feline leukemia virus‐induced malignancies: insertional mutagenesis. Veterinary Immunology and Immunopathology 123, 138‐143 [DOI] [PubMed] [Google Scholar]
- Gabor, L. J. , Malik, R. & Canfield, P. J. (1998) Clinical and anatomical features of lymphosarcoma in 118 cats. Australian Veterinary Journal 76, 725‐732 [DOI] [PubMed] [Google Scholar]
- Gavazza, A. , Lubas, G. , Valori, E. , et al. (2008) Retrospective survey of malignant lymphoma cases in the dog: clinical, therapeutical and prognostic features. Veterinary Research Communications 32(Suppl 1), S291‐S293 [DOI] [PubMed] [Google Scholar]
- Ghazawi, F. M. , Alghazawi, N. , Le, M. , et al. (2019) Environmental and other extrinsic risk factors contributing to the pathogenesis of cutaneous T cell lymphoma (CTCL). Frontiers in Oncology 9, 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf, R. , Gruntzig, K. , Hassig, M. , et al. (2015) Swiss feline cancer registry: a retrospective study of the occurrence of tumours in cats in Switzerland from 1965 to 2008. Journal of Comparative Pathology 153, 266‐277 [DOI] [PubMed] [Google Scholar]
- Ha, M. , Hwang, S. S. , Kang, S. , et al. (2017) Geographical correlations between indoor radon concentration and risks of lung cancer, non‐Hodgkin's lymphoma, and leukemia during 1999‐2008 in Korea. International Journal of Environmental Research and Public Health 14, 3‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga, T. , Yokomori, K. , Nakayama, H. , et al. (1988) Canine and feline lymphoid and myeloid tumors encountered in Tokyo. Japanese Journal of Veterinary Science 50, 809‐813 [DOI] [PubMed] [Google Scholar]
- Hardy, W. D. (1981) Hematopoietic tumors of cats. Journal of the American Animal Hospital Association 17, 921‐940 [Google Scholar]
- Hayes, H. M. , Tarone, R. E. , Cantor, K. P. , et al. (1991) Case‐control study of canine malignant lymphoma: positive association with dog owner's use of 2,4‐dichlorophenoxyacetic acid herbicides. Journal of the National Cancer Institute 83, 1226‐1231 [DOI] [PubMed] [Google Scholar]
- Henderson, S. M. , Bradley, K. , Day, M. J. , et al. (2004) Investigation of nasal disease in the cat – a retrospective study of 77 cases. Journal of Feline Medicine and Surgery 6, 245‐257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann‐Lehmann, R. , Tandon, R. , Boretti, F. S. , et al. (2006) Reassessment of feline leukaemia virus (FeLV) vaccines with novel sensitive molecular assays. Vaccine 24, 1087‐1094 [DOI] [PubMed] [Google Scholar]
- Hofmann‐Lehmann, R. , Cattori, V. , Tandon, R. , et al. (2007) Vaccination against the feline leukaemia virus: outcome and response categories and long‐term follow‐up. Vaccine 25, 5531‐5539 [DOI] [PubMed] [Google Scholar]
- Kirkwood, B. R. , Sterne, J. A. C. & Kirkwood, B. R. (2003) Essential Medical Statistics. Blackwell Science, Malden, MA, USA: [Google Scholar]
- Louwerens, M. , London, C. A. , Pedersen, N. C. , et al. (2005) Feline lymphoma in the post‐feline leukemia virus era. Journal of Veterinary Internal Medicine 19, 329‐335 [DOI] [PubMed] [Google Scholar]
- Meichner, K. , Kruse, D. B. , Hirschberger, J. , et al. (2012) Changes in prevalence of progressive feline leukaemia virus infection in cats with lymphoma in Germany. The Veterinary Record 171, 348 [DOI] [PubMed] [Google Scholar]
- Meincke, J. E. , Hobbie, W. V. Jr. & Hardy, W. D. Jr. (1972) Lymphoreticular malignancies in the cat: clinical findings. Journal of the American Veterinary Medical Association 160, 1093‐1098 [PubMed] [Google Scholar]
- Mooney, S. C. , Hayes, A. A. , Macewen, E. G. , et al. (1989) Treatment and prognostic factors in lymphoma in cats: 103 cases (1977‐1981). Journal of the American Veterinary Medical Association 194, 696‐702 [PubMed] [Google Scholar]
- Moore, A. S. , Cotter, S. M. , Frimberger, A. E. , et al. (1996) A comparison of doxorubicin and COP for maintenance of remission in cats with lymphoma. Journal of Veterinary Internal Medicine 10, 372‐375 [DOI] [PubMed] [Google Scholar]
- Rezanka, L. J. , Rojko, J. L. & Neil, J. C. (1992) Feline leukemia virus: pathogenesis of neoplastic disease. Cancer Investigation 10, 371‐389 [DOI] [PubMed] [Google Scholar]
- Sato, H. , Fujino, Y. , Chino, J. , et al. (2014) Prognostic analyses on anatomical and morphological classification of feline lymphoma. Journal of Veterinary Medical Science 76, 807‐811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield, I. , Stevens, K. B. , Pittaway, C. , et al. (2019) Geographic distribution and environmental risk factors of lymphoma in dogs under primary‐care in the UK. The Journal of Small Animal Practice 60, 746‐754 [DOI] [PubMed] [Google Scholar]
- Sfiligoi, G. , Theon, A. P. & Kent, M. S. (2007) Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Veterinary Radiology & Ultrasound 48, 388‐393 [DOI] [PubMed] [Google Scholar]
- Shelton, G. H. , Grant, C. K. , Cotter, S. M. , et al. (1990) Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968‐1988). Journal of Acquired Immune Deficiency Syndromes 3, 623‐630 [PubMed] [Google Scholar]
- Taylor, S. S. , Goodfellow, M. R. , Browne, W. J. , et al. (2009) Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. The Journal of Small Animal Practice 50, 584‐592 [DOI] [PubMed] [Google Scholar]
- UKradon . (2018). www.ukradon.org. [accessed in March 2018]
- Vail, D. M. , Moore, A. S. , Ogilvie, G. K. , et al. (1998) Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. Journal of Veterinary Internal Medicine 12, 349‐354 [DOI] [PubMed] [Google Scholar]
- VETCOMPASS . (2020). Veterinary companion animal surveillance system. https://www.rvc.ac.uk/vetcompass. Accessed June 1, 2018
- Zahm, S. H. & Blair, A. (1992) Pesticides and non‐Hodgkin's lymphoma. Cancer Research 52, 5485s‐5488s [PubMed] [Google Scholar]
- Zahm, S. H. , Weisenburger, D. D. , Babbitt, P. A. , et al. (1990) A case‐control study of non‐Hodgkin's lymphoma and the herbicide 2,4‐dichlorophenoxyacetic acid (2,4‐D) in eastern Nebraska. Epidemiology 1, 349‐356 [DOI] [PubMed] [Google Scholar]


