Abstract
Zinc ions play an important role in numerous crucial biological processes in the human body. The ability to image the function of Zn2+ would be a significant asset to biomedical research for monitoring various physiopathologies dependent on its fate. To this end, we developed a novel Gd3+ chelate that can selectively recognize Zn2+ over other abundant endogenous metal ions and alter its paramagnetic properties. More specifically, this lanthanide chelate displayed an extraordinary increase in longitudinal relaxivity (r 1) of over 400 % upon interaction with Zn2+ at 7 T and 25 °C, which is the greatest r 1 enhancement observed for any of the metal ion‐responsive Gd‐based complexes at high magnetic field. A “turn‐on” mechanism responsible for these massive changes was confirmed through NMR and luminescence lifetime studies on a 13C‐labeled Eu3+ analogue. This molecular platform represents a new momentum in developing highly suitable magnetic resonance imaging contrast agents for functional molecular imaging studies of Zn2+.
Keywords: gadolinium, magnetic resonance imaging, responsive contrast agents, zinc
A highly potent MRI contrast agent based on paramagnetic Gd3+ was developed to selectively recognize Zn2+ over other abundant endogenous metal ions. The interaction with Zn2+ modulates the hydration of the complex, leading to a significant increase in its longitudinal relaxivity. The new responsive platform is highly suitable for studying the Zn‐related physiological processes at high magnetic fields.
Zinc ions are found in all cells in the human body, either in free or protein‐bounded forms. [1] As the second most abundant transition metal ion, Zn2+ plays an important role in many essential biological processes. [2] For example, it is involved in numerous aspects of cellular metabolism such as the mediation of enzymes activity, the conveyance of neural signals and the transcription of genes. Both an excess and deficiency of Zn2+ causes different symptoms and pathologies, such as hair loss, brain or prostate cancer. [3] Therefore, it is essential for healthy organs that the concentration of Zn2+ is perfectly balanced by transporters and metallochaperones. Magnetic resonance imaging (MRI), a non‐invasive technique with high spatial resolution, is one of the highly suitable methods for investigating the biological role of Zn2+ and providing early‐stage disease detection, particularly in combination with the use of contrast agents (CA). [4]
Application of CAs in MRI guarantees higher contrast images through the shortening of the T 1 (spin‐lattice) and T 2 (spin‐spin) relaxation times of the water molecule that interact with the CA. [4a] Complexes of gadolinium with different polydentate chelating ligands are most frequently chosen for such purposes,[ 4a , 5 ] as they can shorten the T 1 and T 2 relaxation times of water protons by rapid exchange of inner‐sphere water molecules with the bulk solvent and thus enhance the MR image contrast. [6] A variant of these complexes, so‐called bioresponsive or “smart” contrast agents (SCAs), are well suitable for the visualization of numerous biological processes through functional MRI (fMRI) studies, as they can alter their signal upon interaction with the desired target (e.g. metal ion of interest).[ 4c , 7 ] To this end, development of SCAs to specifically distinguish Zn2+ over other metal ions was initiated in the pioneering studies from Nagano and co‐workers in 2001. [8] Meanwhile, many approaches and a large number of SCAs sensitive to Zn2+ have been reported, based mainly on Gd3+ complexes,[ 4c , 9 ] while the Zn‐responsive probes for other imaging modalities have also been developed. [10] Recently, significant progress in performing MRI in vivo to study the function of Zn2+ has been made by Sherry and co‐workers. In these studies, the SCAs can only be trigged by Zn2+ and human serum albumin (HSA) together, resulting in a longitudinal r 1 relaxivity enhancement of ≈60 % at high magnetic field (9.4 T). A greater change in r 1 (≈270 %) can only be measured at low magnetic field (0.5 T). [9a] Nevertheless, the former level of changes in r 1 was sufficient to perform a set of important in vivo experiments to study the role of Zn2+ in β‐cell function and insulin release at high field.[ 9a , 11 ]
To push the limits of Zn2+ detection at high magnetic fields with SCAs, we approached the problem by using a structural motif we recently discovered, which shows an excellent “turn‐on” luminescence emission response to Zn2+. [12] The SCA molecule we designed was based on the di‐(2‐picolyl)amine (DPA) as the Zn2+ recognition moiety, 1,4,7,10‐tetraazacyclododecane‐1,4,7‐tricarboxylic acid (DO3A) as chelator for Gd3+ and a tyrosine (Tyr) unit as a spacer. Herein, we protected the carboxylate of Tyr as the methyl ester, and deliberately appended an acetate moiety on the phenolic oxygen, which can act as a bridge between the DPA moiety and the DO3A chelator. Positioned in such way, the phenoxyacetic acid can interact and coordinate with either Zn2+ or Gd3+, thus playing an important role in potential alterations of relaxivity.
The preparation of the desired complex GdL (Figure 1) was done in analogy to the recently reported luminescence chemosensor. [12] Additionally, phenoxyacetate was installed by the alkylation of the phenolic ‐OH of 1 at room temperature to smoothly provide 2 within 12 h (Scheme S1 in SI). The chelator H4L was obtained by treating 2 with TFA, followed by purification with HPLC (synthetic procedure in SI). In parallel, we prepared the chelator H4L*, labeled with 13C isotopes on the phenoxyacetate group. Here, we used 1,2‐13C‐tert‐butyl bromoacetate in the alkylation step, instead of the molecule with normal isotope abundance. The final complexes GdL, TbL or EuL* were prepared by treating H4L/H4L* with the respective metal ion salt.
Figure 1.
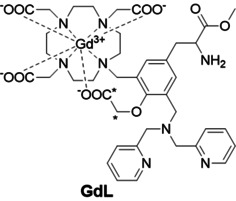
Chemical structure of GdL. Asterisks show the positions labeled with 13C isotopes in the respective EuL* complex.
To evaluate the response of GdL toward Zn2+ and its potential as a MRI contrast agent, a series of 1H NMR relaxometric titrations were performed at 7 T and 25 or 37 °C. Both r 1 and r 2 were measured after every addition of Zn2+, resulting in an unprecedented change in r 1 relaxivity from 2.05 to 10.30 mM−1 s−1 (≈400 %) at 25 °C and 1.51 to 6.04 mM−1 s−1 (≈300 %) at 37 °C upon saturation with Zn2+ (Figure 2 a and S1a in SI). Such a significant increase in r 1 upon the addition of 1 equiv of Zn2+ is, to the best of our knowledge, the highest change reported thus far for a metal cation‐sensitive Gd‐based CA operating at the high magnetic fields. These observations seemingly indicate substantial changes in the coordination geometry around the Gd3+ center that lead to changes in the inner‐sphere hydration (vide infra). Indeed, when the HEPES buffer was exchanged for PBS, the overall r 1 increased from 1.82 to 7.37 mM−1 s−1 (≈300 %) and 1.47 to 5.58 mM−1 s−1 (≈280 %) for 25 and 37 °C, respectively. These indicated still an outstanding r 1 response, however slightly affected by the formation of ternary complexes between the phosphates and Gd3+. [13] The changes in r 2 values in both buffered media followed similar trends (Figure S1 in SI).
Figure 2.
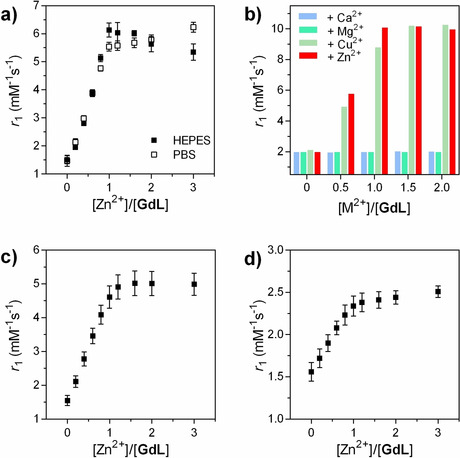
Longitudinal relaxivity of GdL at 7 T. a) r 1 in the presence of various concentrations of ZnCl2 in HEPES (50 mM) or PBS (50 mM) ([GdL]=3 mM, pH 7.4 and 37 °C); b) r 1 in the presence of different quantities of Ca2+, Mg2+, Cu2+ or Zn2+ ([GdL]=3 mM, 50 mM HEPES, pH 7.4 and 25 °C). c,d) r 1 in the presence of various concentrations of ZnCl2 in c) HSA (0.6 mM) or d) human serum (both [GdL]=1 mM, pH 7.4 and 37 °C). Values in (a), (c) and (d) represent the mean and standard deviation from 3 independent measurements.
The selectivity of GdL towards Zn2+ was tested in separate experiments with metal ions commonly present in biological systems, such as Ca2+, Mg2+ and Cu2+ (Figure 2 b). No obvious response of GdL toward any other cation was observed, with the exception of Cu2+. However, such potential competition can be omitted because the concentration of free Cu2+ in living cells is very low. [14]
Finally, the relaxometric behavior of GdL was probed in more complex environments at physiological pH and 37 °C. The r 1 enhancement in the presence of HSA (0.6 mM in PBS) exceeded 200 % upon saturation with ZnCl2 (Figure 2 c). The r 1 and r 2 titrations of HSA with GdL or GdLZn complex showed that interaction of GdL/GdLZn with the protein is not pronounced and is slightly notable only in the sub‐equimolar amounts; however, the bicarbonates (25 mM) have remarkable influence on both r 1 and r 2 values, as expected (Figure S1b,c in SI). Nevertheless, the titration of GdL with ZnCl2 in human serum resulted in the increase in r 1 of around 60 % (Figure 2 d), which is the highest change observed so far for any of the Zn‐sensitive SCA. [9a]
The specificity toward Zn2+ and its strong relaxivity enhancement, suggest that GdL could be a highly promising molecular probe for the imaging of this biomarker. We therefore performed additional characterization of the complex to assess its coordination properties and estimate its potential for MRI applications. The binding affinity of GdL towards Zn2+ was determined by means of isothermal titration calorimetry in both HEPES and PBS (Figure S2 in SI). The obtained dissociation constant resulted in the values K d(GdZnL)=543±39 and 552±76 nM in HEPES and PBS, respectively. This is in line with the values we obtained for the luminescence sensor with a similar structure; [12] also, the results indicate that the binding affinity is not affected by the ternary complex formation between the phosphates and Gd3+ (vide supra).
The stability of GdL was investigated in a transmetallation experiment against Zn2+, a major potential competitor for the displacement of Gd3+. [15] For this, GdL was exposed to 2 equiv of Zn2+ in a phosphate buffer (50 mM, pH 7.0) at 25 and 37 °C. The replacement of Gd3+ for Zn2+ was monitored by measuring the relaxation rate of the solution over a period of 120 h (Figure S3 in SI). Subsequently, a “thermodynamic index” was calculated as the ratio of the paramagnetic relaxation rate after a given period, compared with the starting value. For GdL, this index resulted in values 90, 81 and 75 % after 24, 72 and 120 h for the temperature 37 °C, respectively, indicating high stability of the investigated SCA.
To confirm the binding pattern of Zn2+ with GdL, an analogous Eu3+ complex EuL* was prepared with the 13C‐labeled phenoxyacetate group (vide supra). Thereafter, a series of 13C NMR spectra of 15 mM EuL* were recorded with increasing concentrations of ZnCl2 (Figure 3 a and S4 in SI), in analogy to the experiments previously conducted by Meade and co‐workers. [16] In the absence of Zn2+, the spectrum showed two broad signals at 93.5 ppm and 211.7 ppm. Once Zn2+ was gradually added to the sample, these signals slowly disappeared, while two sharp doublets at 71.7 ppm and 175.6 ppm appeared, owing to the coupling interactions with the neighboring isotopic carbon atom. After the addition of one equiv of Zn2+, the broader signals at 93.5 ppm and 211.7 ppm disappeared completely. Furthermore, the doublets at 71.7 ppm and 175.6 ppm experienced a small shift with further additions of Zn2+, indicating the formation of the second type of species in the excess of Zn2+; no further changes in 13C NMR spectra were observed beyond the second equiv of Zn2+.
Figure 3.
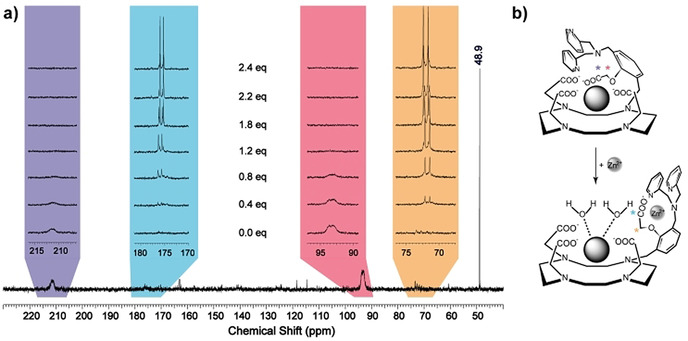
a) 13C NMR spectra of EuL* (15 mM) in the presence of 0–2.4 equiv of Zn2+ at 25 °C and 75 MHz (note: 48.9 ppm is the referent signal of 13CH3OH). b) Proposed interaction of the phenoxyacetate group with the paramagnetic metal center (top) and Zn2+ (bottom), which leads to an increase in hydration number and the “turn‐on” response.
This specific experiment provided the strongest ground for the mechanism responsible for changes in the coordination around the paramagnetic metal ion that are caused by Zn2+. Namely, broad and shifted signals in the Zn‐free state indicated coordination of phenoxyacetate group to the paramagnetic Eu3+. Moreover, the amplitude of the shifts (≈36 and ≈22 ppm for the carboxylate and methylene group, respectively), suggests the carboxylate being much closer to Eu3+, hence the larger shift. Upon addition of Zn2+, the phenoxyacetate group flips from Eu3+ to Zn2+, forming a Zn2+ complex with the DPA moiety (Figure 3 b). Consequently, the 13C signals of the carbons in the phenylacetate recover in intensity, multiplicity and the usual frequency shift in 13C NMR for the respective functional groups. Moreover, the minor change in the shift of doublets between 1–2 equiv of Zn2+ indicates that EuL*:Zn2+ stoichiometry likely moves from 1:1 to 1:2 complex formation, which was also observed in the case of the luminescent phenoxy analogue. [12] However, it is obvious that, irrespective of the type of formed species (1:1 or 1:2), already the first equiv of Zn2+ causes the decoordination of the phenoxyacetate from the paramagnetic metal center. We note that 1H NMR of EuL* at 25 °C displays sharp resonances, suggesting the existence of only one of the isomers, namely the twisted antisquare prismatic species (TSAP, Figure S5 in SI).
The coordination features of this system were also studied by means of the luminescence lifetime experiments. For this purpose, the luminescence lifetimes of EuL* and TbL in D2O or H2O with and without Zn2+ were measured at pH 7.4 and 25 °C. Subsequently, the number of the water molecules coordinated to the Eu3+/Tb3+ center (q) was estimated (Table 1).
Table 1.
Luminescence lifetimes of the EuL* (5 mM) and TbL (1 mM) in H2O and D2O with and without Zn2+, and the calculated q values.
|
|
LnL only |
LnL + Zn2+ (2 equiv) |
|
||||
|---|---|---|---|---|---|---|---|
|
|
τ [ms] |
τ [ms] |
q |
τ [ms] |
τ [ms] |
q |
Δq |
|
EuL* |
0.89 |
1.25 |
0.1 |
0.42 |
1.02 |
1.4 |
1.3 |
|
TbL |
1.91 |
1.96 |
0.0 |
1.30 |
2.37 |
1.5 |
1.5 |
In the absence of Zn2+, both EuL* or TbL are non‐hydrated, explaining the very low initial r 1 value of GdL (vide supra). Upon Zn2+ addition, the estimated q values are 1.4 and 1.5 for EuL* and TbL, respectively. In parallel, the r 1 value of GdL upon Zn2+ binding dramatically increases, which matches this observation. These results confirm the mechanism that assumes complete coordination of the Gd3+ with DO3A and DPA units, leading to q=0 in the absence of Zn2+. Once Zn2+ is added, the whole DPA unit including the phenoxyacetate is involved in coordination with Zn2+, giving rise to higher hydration of the paramagnetic chelate and therefore the boost in r 1 (Figure 3 b). Additionally, this coordination probably causes a concurrent decrease in τ R with an increase in the outer‐sphere hydration, which contribute to the overall r 1, as previously observed on structurally similar systems that are Ca2+ sensitive. These also exhibited high r 1 values, while being monohydrated complexes. [17]
The potential of this complex to serve as a T 1‐weighted SCA was demonstrated in vitro in an MRI experiment on tube phantoms. Six tubes containing GdL alone or with added Zn2+, Mg2+ or Ca2+ were imaged in a 7 T MRI scanner. The results indicated a great enhancement in the MR signal intensity for tubes with GdL and 0.5 or 1.0 equiv of Zn2+, whereas no obvious difference was observed in tubes where Ca2+ or Mg2+ were added (Figure 4 and Table S1 in SI). The collected MR data also confirmed that a selective “turn‐on” response of GdL can be visualized in a Zn‐rich environment.
Figure 4.
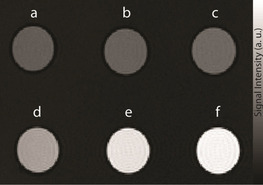
T 1‐weighted MR images of tube phantoms at 7 T of a 1 mM solution of GdL in 50 mM HEPES buffer (pH 7.4 and ≈22 °C). The tubes were positioned in the following order: a) GdL only, b)+1.0 equiv Mg2+, c)+1.0 equiv Ca2+, d)+0.5 equiv Zn2+, e)+1.0 equiv Zn2+, f)+2.0 equiv Zn2+.
In summary, we report a novel paramagnetic complex appended with DPA as a Zn2+ recognition moiety and phenoxyacetate as a trigger for the “turn‐on” relaxometric response. The overall r 1 relaxivity enhancement reached 400 % upon Zn2+ addition, which is, to the best of our knowledge, the highest r 1 change observed to date for this type of ion‐sensitive SCA at high magnetic fields. The additional experiments demonstrated high binding affinity and specificity of the complex toward Zn2+ and confirmed the existence of the “turn‐on” mechanism. Indeed, this system displays the most desirable properties for a SCA, which encompass high q modulation, followed by a massive increase in relaxivity. These features are highly preferred for the development of potent probes for the molecular imaging of biomarkers. With the new paramagnetic system presented in this work, the field of functional imaging of Zn2+ is receiving an important tool to enable substantial and faster progress.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank Drs. Tanja and Giuseppe Gambino for MRI tube phantom measurements. The financial support of China Scholarship Council (PhD fellowship to Gaoji Wang), the German Federal Ministry of Education and Research (BMBF, e:Med program: FKZ: 01ZX1503) and the Shanghai Municipal Science and Technology Major Project (Grant No. 2019SHZDZX02) is gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
G. Wang, G. Angelovski, Angew. Chem. Int. Ed. 2021, 60, 5734.
References
- 1. Krężel A., Maret W., Arch. Biochem. Biophys. 2016, 611, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.
- 2a. Vallee B. L., Auld D. S., Biochemistry 1990, 29, 5647–5659; [DOI] [PubMed] [Google Scholar]
- 2b. Costello L. C., Fenselau C. C., Franklin R. B., J. Inorg. Biochem. 2011, 105, 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills C. F., Zinc in human biology, Springer, London, 1988. [Google Scholar]
- 4.
- 4a. Wahsner J., Gale E. M., Rodríguez-Rodríguez A., Caravan P., Chem. Rev. 2019, 119, 957–1057; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Jordan M. V. C., Lo S. T., Chen S. W., Preihs C., Chirayil S., Zhang S. R., Kapur P., Li W. H., De Leon-Rodriguez L. M., Lubag A. J. M., Rofsky N. M., Sherry A. D., Proc. Natl. Acad. Sci. USA 2016, 113, E5464–E5471; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Bonnet C. S., Coord. Chem. Rev. 2018, 369, 91–104. [Google Scholar]
- 5. Clough T. J., Jiang L., Wong K.-L., Long N. J., Nat. Commun. 2019, 10, 1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heffern M. C., Matosziuk L. M., Meade T. J., Chem. Rev. 2014, 114, 4496–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H., Meade T. J., J. Am. Chem. Soc. 2019, 141, 17025–17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanaoka K., Kikuchi K., Urano Y., Nagano T., J. Chem. Soc. Perkin Trans. 2 2001, 1840–1843. [Google Scholar]
- 9.
- 9a. Yu J., Martins A. F., Preihs C., Jordan V. C., Chirayil S., Zhao P. Y., Wu Y. K., Nasr K., Kiefer G. E., Sherry A. D., J. Am. Chem. Soc. 2015, 137, 14173–14179; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b. Stasiuk G. J., Minuzzi F., Sae-Heng M., Rivas C., Juretschke H. P., Piemonti L., Allegrini P. R., Laurent D., Duckworth A. R., Beeby A., Rutter G. A., Long N. J., Chem. Eur. J. 2015, 21, 5023–5033; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Major J. L., Parigi G., Luchinat C., Meade T. J., Proc. Natl. Acad. Sci. USA 2007, 104, 13881–13886; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9d. Zhang X. A., Lovejoy K. S., Jasanoff A., Lippard S. J., Proc. Natl. Acad. Sci. USA 2007, 104, 10780–10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Haas K. L., Franz K. J., Chem. Rev. 2009, 109, 4921–4960; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. McRae R., Bagchi P., Sumalekshmy S., Fahrni C. J., Chem. Rev. 2009, 109, 4780–4827; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10c. Price T. W., Firth G., Eling C. J., Kinnon M., Long N. J., Sturge J., Stasiuk G. J., Chem. Commun. 2018, 54, 3227–3230. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Lubag A. J. M., De Leon-Rodriguez L. M., Burgess S. C., Sherry A. D., Proc. Natl. Acad. Sci. USA 2011, 108, 18400–18405; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11b. Martins A. F., Jordan V. C., Bochner F., Chirayil S., Paranawithana N., Zhang S. R., Lo S. T., Wen X. D., Zhao P. Y., Neeman M., Sherry A. D., J. Am. Chem. Soc. 2018, 140, 17456–17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang G., Platas-Iglesias C., Angelovski G., ChemPlusChem 2020, 85, 806–814. [DOI] [PubMed] [Google Scholar]
- 13. Dickins R. S., Aime S., Batsanov A. S., Beeby A., Botta M., Bruce J., Howard J. A. K., Love C. S., Parker D., Peacock R. D., Puschmann H., J. Am. Chem. Soc. 2002, 124, 12697–12705. [DOI] [PubMed] [Google Scholar]
- 14. Zhang S. W., Adhikari R., Fang M. X., Dorh N., Li C., Jaishi M., Zhang J. T., Tiwari A., Pati R., Luo F. T., Liu H. Y., ACS Sens. 2016, 1, 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laurent S., Vander Elst L., Henoumont C., Muller R. N., Contrast Media Mol. Imaging 2010, 5, 305–308. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Major J. L., Boiteau R. M., Meade T. J., Inorg. Chem. 2008, 47, 10788–10795; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Matosziuk L. M., Leibowitz J. H., Heffern M. C., MacRenaris K. W., Ratner M. A., Meade T. J., Inorg. Chem. 2013, 52, 12250–12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verma K. D., Forgács A., Uh H., Beyerlein M., Maier M. E., Petoud S., Botta M., Logothetis N. K., Chem. Eur. J. 2013, 19, 18011–18026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


