Abstract
Various advances have been made in the treatment of retinal diseases, including new treatment strategies and innovations in surgical devices. However, the treatment of degenerative retinal diseases, such as retinitis pigmentosa (RP) and age‐related macular degeneration (AMD), continues to pose a significant challenge. In this review, we focus on the use of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) to treat retinal diseases by harnessing the ability of stem cells to differentiate into different body tissues. The retina is a tissue specialized for light sensing, and its degradation leads to vision loss. As part of the central nervous system, the retina has very low regenerative capability, and therefore, treatment options are limited once it degenerates. Nevertheless, innovations in methods to induce the generation of retinal cells and tissues from ESCs/iPSCs enable the development of novel approaches for these irreversible diseases. Here we review some historical background and current clinical trials involving the use of stem‐cell‐derived retinal pigment epithelial cells for AMD treatment and stem cell‐derived retinal cells/tissues for RP therapy. Finally, we discuss our future vision of regenerative treatment for retinal diseases with a partial focus on our studies and introduce other interesting approaches for restoring vision.
Keywords: regenerative medicine, retinal organoid, retinal pigment epithelium, stem cell, transplantation
Current perspectives and future direction of stem‐cell‐based therapy for retinal degenerative diseases.
1. INTRODUCTION
Regenerative medicine in the field of ophthalmology began with corneal epithelial transplants in the 1990s, focusing on corneal epithelial diseases such as Stevens–Johnson syndrome and corneal epithelial stem cell exhaustion. The treatment utilized corneal epithelial stem cells or cultured epithelial sheets derived from the oral mucosa. Patients with corneal diseases were the first to benefit from regenerative therapies because of their easy accessibility to the ocular surface and the relatively small number of cells required for treatment. As a result, this treatment approach has been successful for years in modern clinical practice. The retina and retinal pigment epithelium (RPE), on the other hand, are located at the back of the eye, and as part of the central nervous system, are considered to have very low regenerative potential. Indeed, similar to other parts of the brain, it is generally considered impossible to reconstruct the function of the retina once it degenerates. Some publications indicated early on the potential benefit of transplanting fetal retinal cells or tissue in patients with retinal degeneration (Aramant & Seiler, 2002; Radtke et al., 2008); however, these grafts were limited due to ethical concerns regarding the use of fetal tissue, and it was only after the establishment of protocols to differentiate substantial quantities of retinal cells and organoids from embryonic stem cells (ESCs) that the application of regenerative medicine to the posterior eye became a practical goal in clinical medicine. The advent of induced pluripotent stem cell (iPSC) technology at the beginning of the 21st century (Takahashi & Yamanaka, 2006) further accelerated this trend, and researchers in the field have since contributed to building a foundation for stem‐cell‐based therapies for retinal degenerative diseases. One of the major focuses of the use of these technologies is a kind of cell‐based therapy that involves RPE, which is composed of nonneural monolayered cells that support the function of the retina. After the success of a clinical study using human ESC‐derived RPE (hESC‐RPE), in another clinical study conducted in Japan in 2014 by us, the patient's own iPSC‐derived RPE sheet was used for transplantation; subsequently, several clinical studies tested the use of human ESC/iPSC‐derived RPE cells and sheets (Table 1). These studies collectively verified the practicality and safety of these stem‐cell‐based therapies. The next global goal is the development of regenerative therapy for neural retinal diseases, which is more difficult because it requires the functional integration of transplanted tissue/cells into the host neural network.
TABLE 1.
Summary of ongoing and concluded clinical trials of stem‐cell‐derived RPE transplantation
| References | Source cells | HLA matching | Immunosuppression | Form | Dose/size | Scaffold | Disease | Participants | NCT or UMIN number | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| Schwartz et al. (2012), Schwartz et al. (2015) | hESC | No | Oral immunosuppression | Suspension |
50,000 100,000 150,000 cells |
No |
Dry AMD Stargardt's |
9 9 |
Completed | |
| Song et al. (2015) | hESC | No | Oral immunosuppression | Suspension |
50,000 100,000 150,000 200,000 cells |
No | Dry AMD | 12 | NCT01674829 | Active, not recruiting |
| Mehat et al. (2018) | hESC | No | Oral immunosuppression | Suspension |
50,000 100,000 150,000 200,000 cells |
No | Stargardt's | 12 | NCT01469832 | Completed |
| Liu et al. (2018) | hESC | No | Oral immunosuppression | Suspension | 100,000 cells | No | Dry AMD | 15 | NCT02749734 | Unknown |
| Sung et al. (2020) | hESC | No | Oral immunosuppression | Suspension | 50,000 cells | No | Stargardt's | 3 | NCT01625559 | Unknown |
| Sugita et al. (2020) | hiPSC | Yes (HLA homozygote donor) | Local prednisolone (Intravitreous, sub‐tenon) | Suspension | 250,000 cells | No | Wet AMD | 5 | UMIN 000026003 | Completed |
| Mandai et al. (2017) | hiPSC | Yes (autologous) | Local prednisolone (topical) | Sheet |
1.3 mm × 3 mm 17,550 ~ 113,110 cells |
No | Wet AMD | 1 |
UMIN 000011929 |
Completed |
| Da Cruz et al. (2018) | hESC | No |
Local immunosuppression Oral prednisolone Intraocular steroid implants |
Sheet |
6 mm × 3 mm ~100,000 cells |
10‐μm‐thick PET | Dry AMD | 2 | NCT01691261 | Completed |
| Kashani et al. (2018) | hESC | No | Oral immunosuppression | Sheet |
3.5 mm × 6.25 mm ~100,000 cells |
6‐μm‐thick parylene (CPCB‐RPE) |
Dry AMD | 16 | NCT02590692 | Active, not recruiting |
| Ben M'Barek et al. (2017, 2020) | hESC | No | Oral immunosuppression | Sheet |
3 mm × 5 mm 14.5 mm2 |
Amitotic membrane | RP | 12 | NCT03963154 | Recruiting |
| Sharma et al. (2019) | hiPSC | Yes (Autologous) | Unknown | Sheet | 4 mm × 2 mm | PLGA | Dry AMD | 20 | NCT04339764 | Recruiting |
Studies identified based on a search in PubMed NIH at the end of October 2020.
Abbreviations: AMD, age‐related macular degeneration; CPCB‐RPE, California Project to Cure Blindness–Retinal Pigment Epithelium; PET, polyethylene terephthalate; PLGA, poly lactic‐co‐glycolic acid; RP, retinitis pigmentosa.
2. BASIC STRUCTURE AND FUNCTION OF THE EYE IN THE PERSPECTIVE OF REGENERATIVE THERAPIES
2.1. Basic structure of the eye
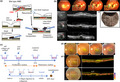
Among the five senses, vision is especially important for primates. The basic structure of the human eye is often compared to that of a camera. Indeed, the way the lens focuses on a target object is analogous to how images are projected onto a film in a camera (Figure 1a). Next, the retina converts light signals to electronic signals for further processing via transmission over a neural network. The neurons composing the retina can be broadly classified into three layers: photoreceptor cells; inner layer cells, including bipolar cells, as secondary neurons; and ganglion cells, which send the encoded visual information to the brain through long‐reaching axons that form a bundle of nerve fibers called the optic nerve (Figure 1b). Facing photoreceptor cells, a monolayer sheet of RPE is essential for the function of photoreceptors and for the protection of the ocular environment, as described below.
FIGURE 1.
Schematic images of the basic structure of the healthy human eye, retina, and retinal pigment epithelium (RPE). (a) Basic structure of the human eye. Eyeballs have a structure similar to that of a camera consisting of a lens and film parts. The cornea and lens are clear tissues that can be crossed by light. The iris adjusts light intensity. The retina is the light sensory part of the eye, supported by RPE, choroidal capillaries, and sclera. Retinal ganglion cells (RGC), located at the innermost part of the retina, extend and bundle their axons to form the optic nerve, which reaches the visual cortex. (b) Hematoxylin and eosin staining of the primate retina. The neural retina is divided into three layers: the photoreceptor cell layer, the inner cell layer, and the RGC layer. (c) RPE faces the outer part of the neural retina and plays a variety of roles in photoreceptor metabolism, phagocytosis of photoreceptor extracellular nodes, supply of oxygen and nutrients from the choroidal side, vascular maintenance, and secretion of protective factors such as pigment epithelium‐derived factor (PEDF) and vascular endothelial growth factor (VEGF). (d) Imaging of the primate macula through a color fundus photo and a sectional view obtained by optical coherent tomography (OCT). The foveal zone is the densest area of cone photoreceptors in the retina and contributes to fine spatial resolution in photopic conditions. GCL, ganglion cell layer; INL, inner nuclear cell layer; ONL, outer nuclear layer; PEDF, pigment epithelium‐derived factor; RPE, retinal pigment epithelium; VEGF, vascular endothelial growth factor
2.2. RPE is important to maintain the function of the sensory retina
The roles of RPE include: (a) the constituents of the outer blood–retinal barrier (BRB); (b) recovery of the visual pigment rhodopsin, (c) phagocytosis of photoreceptor outer segments, and (d) secretion of neurotrophic and growth factors for the retina and choroid capillaries (Figure 1c).
RPE is a neuroectodermal derivative located between the retina and choroid capillaries. These cells contribute to reducing the oxidative stress caused by strong light energy that leads to the generation of free radicals (Beatty et al., 2000). RPE cells have a characteristic hexagonal morphology that enables the formation of tight junctions between cells to prevent the inflow of extraretinal substances from the choroidal tissues. This process contributes to the separation of the inner retinal environment and to the constitution of an outer BRB. This barrier consists of inner and outer parts: the inner BRB is formed by the endothelial cells of retinal vessels, while the outer BRB is formed by RPE (Campochiaro, 2015). RPE also controls the unique immunological environment of the eye, known as immune privilege, by suppressing the infiltration of immune cells and by secreting immunomodulating factors, such as transforming growth factor beta, to suppress destructive activities by immune cells (Streilein, 2003; Sugita et al., 2006).
The photoreceptor outer segments consist of piles of disc‐like structures that carry visual pigments on the disc membrane. Visual pigments consist of opsins and 11‐cis‐retinal, with the latter changing its conformation into all‐trans‐retinal when hit by light. RPE contains the enzymes retinal pigment epithelium‐specific 65‐kDa protein (RPE65) and lecithin retinol acyltransferase (LRAT) that restore visual pigments through trans‐to‐cis isomerization (Kiser et al., 2012). Damaged discs of outer segments continually turn over by shedding and are removed by phagocytosis. RPE secretes pigment epithelium‐derived factor (PEDF) apically to protect photoreceptor cells and vascular endothelial growth factor (VEGF) basally to maintain the choroidal vascular network.
Given all these important roles, RPE dysfunction leads to impaired photoreceptor function and often results in the degeneration of photoreceptor cells, such as that observed in retinitis pigmentosa (RP) with mutations in the coding genes MER proto‐oncogene, tyrosine kinase (MERTK), RPE65, LRAT, and bestrophin 1 (BEST1).
2.3. Photoreceptor cells as a sensory device
Photoreceptor cells are specialized in converting light stimuli to signals for synaptic transmission in the retina and to the brain. There are two types of photoreceptor cells: rods and cones. Moreover, various subtypes of cones respond to different ranges of wavelengths to enable color vision. In primates, cones are densely packed in the central fovea, surrounded by the macular zone of the retina, and contribute to visual resolution in daylight (Figure 1d). Conversely, rods are more densely distributed outside the macular area, and because they are extremely sensitive to light, they contribute to dim‐light or night vision.
3. ESC/IPSC‐RPE THERAPY FOR AGE‐RELATED MACULAR DEGENERATION (AMD)
3.1. Previous therapeutic approaches for AMD
The first targets of stem‐cell‐based therapy using hESC/iPSC‐derived RPE were AMD and Stargardt disease. AMD is a major cause of blindness among the elderly population in developed countries, and currently affects almost 200 million people; moreover, the number of AMD patients is predicted to reach nearly 300 million by 2040 (Wong et al., 2014). Several etiologic factors, such as oxidative stress, chronic immune activation, and lipid dysregulation, have been suggested to trigger the development of AMD (Zarbin & Rosenfeld, 2010). Overall, AMD is a multifactorial progressive disease under the influence of the environment, genetic background, and most importantly, aging (Lambert et al., 2016). These factors can trigger RPE degeneration, leading to secondary degeneration of photoreceptors and vision loss. AMD is categorized into two types: wet AMD, which accompanies ectopic choroidal neovascularization (CNV) sub‐RPE and/or in the subretinal space; and dry AMD, which is considered to be a kind of RPE degeneration without neovascularization. In the treatment of wet AMD, surgical removal or laser photocoagulation of CNV has been conducted to terminate the exudative changes, but these therapies often lead to RPE cell loss, which results in unsatisfactory visual outcomes. More recently, intraocular injections of anti‐VEGF drugs have become the first‐line treatment for suppressing disease progression, but many patients must continue intensive treatment for life to reduce the risk of persistent CNV recurrence.
Given these factors, one ideal therapeutic approach would involve the removal of CNV and the replacement of damaged cells with healthy RPE cells.
3.2. Induction of RPE cells from mammalian ESCs/IPSCs
Kawasaki et al. elaborated a protocol for RPE cell differentiation based on the stromal cell‐derived inducing activity (SDIA) method, using which these authors differentiated primate ESCs into midbrain dopaminergic neurons upon culturing with PA6 mouse stromal cells (Kawasaki et al., 2002). Around the same time, Klimanskaya et al. (2004) obtained differentiated RPE cells through a system that did not require coculture with animal cells or factors, which they described as “zoonoses‐free RPE cells.” However, the induction rate of RPE cells was initially low (Kawasaki et al., 2002: 8 ± 4% after 3 weeks of differentiation; Klimanskaya et al., 2004: <1%). Furthermore, Ikeda et al. (2005) adapted another neural differentiation protocol, based on serum‐free floating culture of embryoid body‐like aggregates (SFEB) and exploiting the WNT antagonist DKK1 and the nodal antagonist Lefty A to induce a rostral brain field. Next, these authors revised this protocol by combining the use of fetal calf serum and activin‐A (SFEB and DKK1/Lefty A/FCS/activin method) to enhance the generation of RX+/PAX6+ retinal progenitor cells from mouse ESCs. Afterward, differentiated RPE cells were successfully obtained from monkey and human ESCs through the SFEB and DKK1/Lefty A protocol, which was subsequently applied to human iPSC‐derived RPE (hiPSC‐RPE) (Osakada et al., 2008; Osakada, Ikeda, et al., 2009). The use of small molecules, such as the ALK4,5,7 inhibitor SB‐431542 and the casein kinase inhibitor CKI‐7, was also found to be effective for RPE differentiation (Osakada, Jin, et al., 2009).
For clinical application, Kamao et al. (2014) further developed a protocol for RPE monolayer sheet formation without using any synthetic scaffold. In this protocol, the cells, seeded on collagen I on Transwell inserts, self‐produced their basement membrane consisting of collagen IV and laminin, and the self‐formed sheet was released upon collagenase treatment. The RPE cells in the sheet displayed structural characteristics similar to those of microvilli and tight junctions, expressed RPE markers, and showed phagocytic activity, suggesting their maturation and function. These hiPSC‐RPE cells also expressed RPE65, which was shown to be functional both in vitro and in vivo (Maeda et al., 2013).
Other promotive methods for RPE generation include the addition of nicotinamide and activin A (Idelson et al., 2009) or the activation of the canonical/β‐catenin WNT pathway through the glycogen synthase kinase 3 beta inhibitor CHIR99021 (Leach et al., 2015). A number of research groups have also reported induction protocols for clinical‐grade RPEs (Ben M'Barek et al., 2017, 2020; Schwartz et al., 2012; Sharma et al., 2019; Song et al., 2015). Based on these RPE derivatives, several clinical trials have been conducted in the United States, France, Korea, and Japan (Table 1).
3.3. History of transplantation approaches using allogenic or autologous RPE cells
The idea of treating AMD by replacing damaged RPE cells with healthy ones can be traced back to the 1990s. However, allogenic RPE sheets were rapidly rejected by wet AMD patients in absence of immune suppression (Algvere et al., 1999; Peyman et al., 1991). To avoid the risk of rejection, a number of research groups conducted autologous translocation of RPE with or without choroid (Binder et al., 2004; MacLaren et al., 2005); For example, Van Zeeburg et al. (2012) reported the long‐term outcome of autologous RPE‐choroid translocation in 133 eyes affected by wet AMD, some of which retained good visual acuity for as long as 7 years. However, the major disadvantage of autologous translocation is the invasiveness of the surgical procedure. Conversely, the preparation of hESC/iPSC‐RPE cells yielded a stable and safe supply of grafts by in vitro procedures, to be used even for autologous transplants.
3.4. Clinical trials using hESC‐RPE cells
The clinical trials that have been conducted with hESC/iPSC‐RPE cells are summarized in Table 1. These clinical trials were carried out in patients with dry and wet AMD or Stargardt disease. In addition, last year a clinical trial (NCT03963154) was launched for RP patients with monogenetic mutations affecting RPE65, LRAT, and MERTK, genes involved in the visual signaling process specifically at the RPE level.
Interestingly, in hESC‐RPE suspension transplantation, subretinal pigmentation or an RPE layer‐like reflex was observed by fundus photography and optical coherence tomography, indicating the integration of transplanted RPE cells (Liu et al., 2018; Mehat et al., 2018; Schwartz et al., 2015; Song et al., 2015; Sung et al., 2020). Surgical techniques may be further developed to prevent postoperative formation of epiretinal membrane by graft cells that may leak from the subretinal space, and to control the localization of transplanted RPE cells.
Furthermore, the delivery of a large RPE sheet using different types of biomaterial scaffolds (described in Table 1) in order to cover the entire macular area is a strategy that has been pursued by several groups; For instance, Da Cruz et al. (2018) reported successful transplantation of a large RPE sheet in two wet AMD patients with massive subretinal hemorrhage. The RPE sheet was cultured on plasma‐derived human vitronectin coated with a 10‐μm‐thick polyethylene terephthalate membrane. In both patients, the remaining photoreceptors were secured, and visual acuity and reading speed improved. Moreover, in vivo examination showed that the transplanted RPE sheets covered the full area characterized by dark pigment and showed uneven autofluorescence, which suggested functional RPE phagocytosis. Another group transplanted RPE sheets on parylene (CPCB‐RPE) into dry AMD patients, covering the atrophic area of the transplanted eye. One eye improved 17 letters, and two eyes demonstrated improvement in the vision fixation test (Kashani et al., 2018).
All these studies verified the overall safety and practicality of hESC‐RPE cell‐based therapies, in which any major complications could be primarily attributed to the use of immunosuppressants during allogenic transplantation.
3.5. Clinical trial using autologous hiPSC‐RPE sheet transplantation for a wet AMD patient
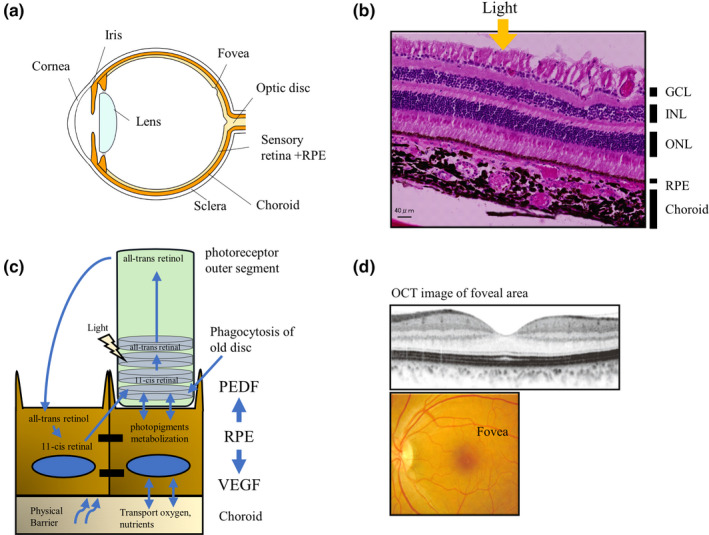
The transplantation of autologous iPSC‐RPE sheets represents an alternative option to avoid the risk of rejection and the use of immunosuppressants; For instance, Mandai et al. (2017) conducted a clinical trial in which an autologous iPSC‐derived RPE sheet was transplanted into a wet AMD patient without systemic immunosuppression. The patient's own iPSC clones were established from skin fibroblast cells, and then differentiated into RPE cells to create a 1.3 × 3 mm2 self‐formed sheet by laser dissection as illustrated in Figure 2a–c (Kamao et al., 2014). The sheet was gently inserted subfoveally after the removal of CNV. The grafted sheet was crumpled immediately after transplantation but expanded and flattened after 2 months to subsequently cover approximately 1.5 times the area of the original sheet size, possibly due to migration and proliferation of graft cells. There was no sign of rejection or unexpected growth of the graft, and the sheet remained stable over 4 years (Figure 2c). After 4 years, visual acuity remained stable with no additional anti‐VEGF injections. The presence of hiPSC‐RPE physiological function in the graft was suggested by the preservation of choroidal thickness at the graft site compared with that in the surrounding bare RPE area after CNV removal, and the characteristic RPE morphologies were also identified at the graft marginal area using an adaptive optical camera (Takagi et al., 2019). The major problem with autologous iPSC‐RPE cell therapy was the high expense and extensive preparation time needed for each individual patient (which was 10 months in this clinical case). Additionally, although the removal of CNV with RPE sheet transplantation is a straightforward therapeutic approach, repeated anti‐VEGF injections often result in minimal formation of fibrotic CNV, which is not adequate for surgical removal. In these cases, a less invasive transplantation approach using cell suspension would be more appropriate (Figure 2a). Based on these results, we used stocked iPSC‐RPE cells in suspension and expanded previous indications in our recent clinical study, which we describe in the next section.
FIGURE 2.
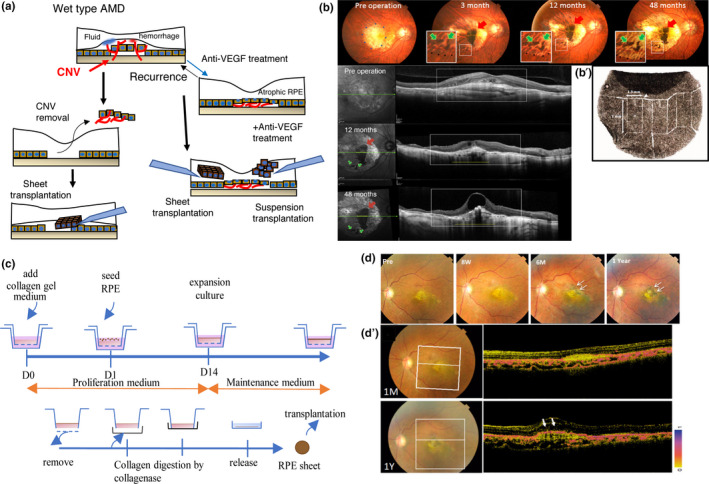
Summary of the process of induced pluripotent stem cell retinal pigment epithelium (iPSC‐RPE) transplantation to a wet‐type age‐related macular degeneration (AMD) patient. (a) Stem‐cell‐derived RPE transplantation strategies for wet‐type AMD patients in which the choroidal neovascular (CNV) membrane grows into the subretinal space in the macular area. Cell‐based RPE replacement of damaged RPE with healthy cells, either after CNV removal, or along with the administration of anti‐VEGF drugs in the presence of non‐fibrotic CNV, may represent a new treatment strategy. (b–b′) Color fundus photos (upper panel) and sectional views by optical coherent tomography (OCT) imaging (lower panels) before, and 12 and 48 months after transplantation of an autologous iPSC‐RPE sheet. CNV was removed before iPSC‐RPE sheet transplantation. Red arrows indicate the main body of the sheet, and green arrows indicate some fragmented parts of the pigmented graft (b). A 1.3 × 3 mm iPSC‐RPE sheet was prepared by laser dissection for grafting (b′) (adapted from Mandai et al., 2017; Takagi et al., 2019). (c) Preparation of an RPE sheet without a synthetic scaffold. RPE cells were seeded on collagen gels on a Transwell membrane. The RPE cell sheet growing in the Transwell culture dish was then released from the membrane after collagen digestion by collagenase (adapted from Kamao et al., 2014). (d–d′) Fundus images before and after transplantation of a human leukocyte antigen (HLA)‐matched iPSC‐RPE suspension into a wet‐type AMD patient combined with anti‐VEGF injection. The transplanted graft survived and expanded 1 year post transplantation (white arrows) (d). Representative images by polarization‐sensitive OCT, showing a layer of melanin‐containing RPE cells covering the surface 1 year after transplantation (white arrows) (d′) (adapted from Sugita et al., 2020). CNV, choroidal neovascular membrane; RPE, retinal pigment epithelium
3.6. Clinical trial using human leukocyte antigen (HLA)‐matched homologous hiPSC‐RPE
To reduce the time and cost of graft preparation and yet avoid the risk of graft rejection, we planned to use RPE cell stocks differentiated from HLA‐matched homologous iPSC lines that were prepared at the Center for iPS Cell Research Application (CiRA). CiRA started offering iPSC stocks for regenerative medicine in 2015, based on the idea that the ten cell lines that carry the most frequent three HLA homologous loci (HLA‐A, ‐B, and ‐DR) can cover 50% of the Japanese population in terms of avoiding rejection risk in cell‐based therapies (Umekage et al., 2019). The iPSCs with the six most frequent HLA homology loci in Japan, estimated to cover 17% of the Japanese population, were the first to be available, and the frequency of matches to this HLA combination was similar (18.8%) among AMD patients (Takagi et al., 2018). Therefore, in our second clinical study, we recruited five patients with the most frequent HLA type at these six loci, who were not eligible for CNV removal but suffered from RPE atrophy, and injected homologous hiPSC‐RPE cells subretinally together with anti‐VEGF. Additional anti‐VEGF injections were administered as needed for the background disease during the follow‐up period. Transplanted RPE cells survived for over 1 year in all cases, without the need for systemic immune suppression. Moreover, although one patient showed mild signs of rejection during in vivo and in vitro assays using peripheral blood mononuclear cells, these clinical signs were well controlled with the local administration of a steroid (Figure 2d; Sugita et al., 2020). In this clinical trial, however, the use of cell suspensions resulted in an insufficient number of cells delivered to the targeted area, another problem that should be solved.
3.7. Future directions in hESC/iPSC‐RPE cell transplantation
Thus far, clinical studies using hESC/iPSC‐derived RPE cells have consistently demonstrated the safety of this therapeutic approach, and that HLA matching is one strategy to reduce the risk of rejection even without the application of immune suppression. Recently, the CRISPR/Cas9 system has been applied for the production of iPSCs with selective HLA gene disruption (Xu et al., 2019), which will be very useful when considering large‐scale RPE transplantation. Currently, we are at the stage of exploring and evaluating which diseases or pathologies are best suited for the use of hESC/iPSC‐derived RPE cell‐based therapies. One example is a recent clinical trial for a specific type of RP with mutations in disease‐causing genes that affect RPE function (NCT0963154 in Table 1). In particular, this clinical trial aimed to restore RPE function and protect photoreceptors from degeneration by targeting diseased tissue at a relatively early stage.
4. REGENERATIVE THERAPY USING PHOTORECEPTOR CELL TRANSPLANTATION FOR RETINAL DEGENERATION
4.1. Photoreceptor cells primarily degenerate in RP patients
RP is a group of diseases characterized by mutations in different genes that lead to the primary degeneration of rod photoreceptor cells. It is currently the second leading cause of blindness in Japan, with a prevalence rate of approximately 1 in 3,000 people (Morizane et al., 2019; Wako et al., 2014).
More than 100 genes were reported to be responsible for RP. Rod photoreceptor degeneration affects night vision and leads to a progressive loss of peripheral vision. The speed of disease progression differs according to the causal genes, but in advanced cases, severe loss of visual acuity can result from the secondary degeneration of cone photoreceptors (Hartong et al., 2006). Reduced secretion of rod‐derived cone viability factor (RDCVF) by rod photoreceptors is considered to be a candidate source of secondary cone degeneration (Aït‐Ali et al., 2015). Therefore, it would be ideal to supply viable photoreceptors while the host photoreceptor cells are gradually lost but other retinal cells remain functional. Interestingly, the innovation of retinal organoid technology enabled this cell transplantation approach, and it is possible to choose to transplant only purified photoreceptors or parts of retinal sheet dissected from the organoid.
4.2. Retinal cells/organoid induction from ESCs/IPSCs
RX+/PAX6+ mouse retinal progenitor cells were first generated using SFEB and the DKK1/Lefty A‐based method (Ikeda et al., 2005); coculture with embryonic retinal cells was required for low‐efficiency differentiation of photoreceptors. On the other hand, Lamba et al. (2006) successfully induced a CRX+ photoreceptor precursor through the combination of SFEB and adhesive culture. Specifically, cells were cultured for 3 days together with three factors: noggin, DKK1, and insulin‐like growth factor (IGF)‐1. About 80% of cells expressed eye field transcription factors, and 20% of cells expressed CRX markers after three weeks of culture in six‐well plates coated with Matrigel. Moreover, in 2011, Eiraku et al. (2011) reported a breakthrough strategy to produce three‐dimensional structural retinal organoids (3D retina) from mouse ESCs using the SFEB method with quick aggregation (SFEBq).This protocol was adapted for hESCs (Nakano et al., 2012), and efficient methods such as the timed treatment of bone morphogenic protein 4 (BMP4) have been published (Kuwahara et al., 2015, 2019; Atsus). Other methods for 3D organoid formation have also been reported, including a protocol in which 3D organoids were mechanically picked up from two‐dimensional (2D) cultures during differentiation (Meyer et al., 2011; Reichman et al., 2014; Zhong et al., 2014), which was further optimized using a xeno/feeder‐free two‐step culture system for hiPSCs (Reichman et al., 2017). Organoid technologies can contribute to disease modeling or preclinical studies through the use of gene editing (Deng et al., 2018; Parfitt et al., 2016; Shimada et al., 2017) as well as for the preparation of substantial amounts of high‐quality retinal organoids for clinical application.
4.3. Retinal cell transplantation
In 2006, MacLaren et al. reported that suspended photoreceptor precursors transplanted from NRL‐GFP‐labeled developing mice at postnatal day 4–8 into the wild‐type retina could robustly integrate into the host photoreceptor layer with a complete mature morphology. Furthermore, these transplanted integrated cells could restore visual function by improving optokinetic head tracking and the results of water maze tests in Gnat1 −/− mice (characterized by the present but dysfunctional rod photoreceptors), suggesting their potential to contribute to functional recovery in blind mice (Pearson et al., 2012). Subsequently, it was reported that transplantation therapy using mouse and human ESC/iPSC‐derived purified photoreceptor precursors as an alternative for fetal mice was also effective for restoring vision in retinal degeneration models (Gonzalez‐Cordero et al., 2013; Lamba et al., 2009; Tucker et al., 2011). In recent years, however, these observations were revealed to originate mostly from a phenomenon known as “material transfer,” whereby biomaterial such as proteins and/or mRNA is transferred from donor to host photoreceptors, thereby restoring some visual function by rescuing remaining photoreceptor cells (Decembrini et al., 2017; Ortin‐Martinez et al., 2017; Pearson et al., 2016; Santos‐Ferreira et al., 2016; Singh et al., 2016). The problem with this phenomenon is that the transfer of labeled fluorescent proteins, such as GFP, to host photoreceptors was interpreted as a sign of integration of donor cells to host neural networks, and thus donor cells have been falsely thought to contribute to the host functional recovery. This phenomenon calls into question the interpretation of previous studies, but also suggests the potential of a new therapeutic approach in which a controlled material exchange mechanism exists between donor and host cells (Ortin‐Martinez et al., 2017).
4.4. Retinal sheet transplantation
Transplantation of retinal cells and pluripotent stem‐cell‐derived retinal cells showed potential for restoring vision in mice with end‐stage retinal degeneration with no outer nuclear layer (ONL) (Barnea‐Cramer et al., 2016; Singh et al., 2013); however, it would be difficult for retinal cells to survive for a long time or form robust synapse connectivity when transplanted into a severe retinal degeneration model with depleted ONL. Conversely, the advantages of retinal sheets are their long survival time and the ability to supply mature photoreceptor cells in a structured manner, even in end‐stage models. Clinical trials using human fetal retinal tissue with/without RPE or suspended fetal retinal cells for RP patients with severe degeneration have been conducted in India and the United States (Das et al., 1999; Radtke, 2004; Radtke et al., 1999). However, since the preparation of embryonic retinas was ethically difficult, it was not realistic for this approach to become a standard treatment; nevertheless, the overall results showed the safety of transplantation therapy as well as some promising effects, including a possible trophic effect. Fortunately, the notable progress in technology for in vitro differentiation of 3D retina from pluripotent stem cells has offered the opportunity to refocus this therapeutic approach. As a proof of principle, we prepared a retinal sheet as a graft from a mouse ESC (mESC)/iPSC‐derived 3D retina and transplanted it into the subretinal space of an end‐stage retinal degeneration mouse model (rd1), which is used as a model of rapid photoreceptor degeneration. The transplanted graft, often in the form of a rosette, enabled the observation of photoreceptor maturation with formation of outer segments (OS). Some transplant grafts showed close contact between the OS of grafted photoreceptors and the host RPE. This study provided a proof of concept (POC) for the retinal sheet transplantation approach (Assawachananont et al., 2014). For a more detailed analysis of host–graft synaptic connectivity, we transplanted a gene‐labeled graft expressing Ctbp2‐tdTomato at the photoreceptor synaptic terminal as a presynaptic marker into a rd1 mouse lineage that carried GFP‐labeled rod bipolar cells and observed direct contact between donor and host cell terminals. The restoration of visual function after transplantation of retinal sheets in rd1 mice was also suggested by the examination of shock avoidance behavior and ex vivo multiple electrode array (MEA) recordings (Figure 3a–c) (Mandai et al., 2017). In addition, transplantation of retinal sheets derived from hESCs/iPSCs into an immunodeficient mouse, nude rat, and RP‐induced monkey model showed robust survival of graft photoreceptors, sometimes in a well‐orientated manner, in the absence of tumorigenesis and severe immune rejection; moreover, the potential of this approach for functional recovery was demonstrated by multielectrode array (MEA) recordings and a visual field test based on visual‐guided saccade (Figure 3d) (Iraha et al., 2018; Shirai et al., 2016; Tu et al., 2019). These data support the usefulness of hESC/iPSC‐derived retinal sheet transplantation for clinical applications.
FIGURE 3.
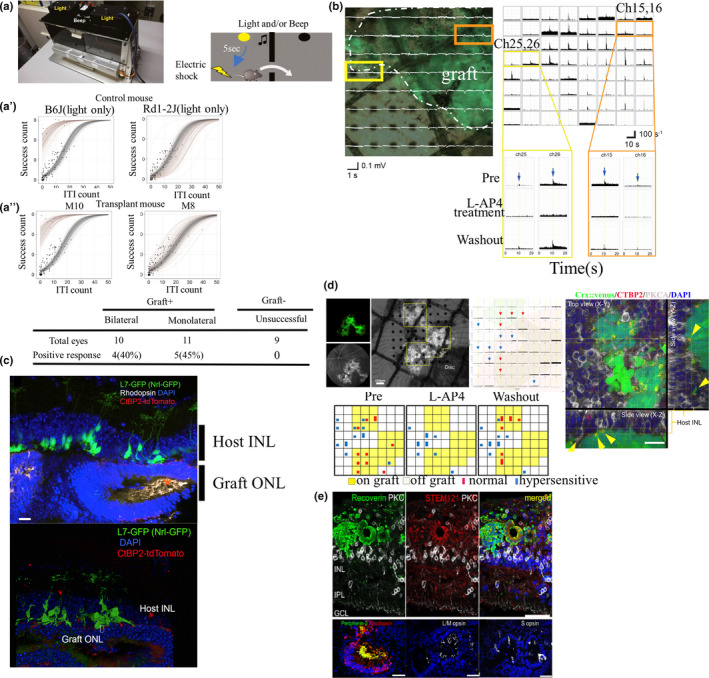
Proof‐of‐concept studies of stem‐cell‐derived retinal sheet transplantation in animal models of retinal degeneration. (a–a′′) Shuttle avoidance system (SAS) behavioral test after transplantation. Electric shock transmitted after 5 s of continuous light with or without beeping (a). Representative results of SAS tests of a wild‐type mouse (C57BL/6J) and blind mouse (Rd1‐2J), with the former showing a deviation of the behavioral pattern as success count > intertrial interval (ITI) count (i.e., signal‐associated escape > random escape) (a′ top). Representative positive (M10) and negative (M8) results after transplantation (a′ below). The light‐associated behavioral pattern (positive response) was observed in some transplanted mice (a′′). (b) Ex vivo electrophysiology experiments using a multi‐electrode array (MEA) recording system. The left panel shows a retina with an NRL‐GFP graft with overlayed electroretinogram (ERG) responses. The right panels show the light responses from a host retinal ganglion cell (RGC). Representative channels (yellow and orange squares) show spikes that were abolished by L‐AP4, specific agonists of mGluR6 but recovered after washout, indicating that the responses originated from photoreceptors. (c) Graft miPSC‐retina expressing CTBP2‐tdTomato matured subretinally with rhodopsin‐positive outer segment‐like structures in L7‐green fluorescent protein (GFP)/rd1 mice. tdTomato‐labeled graft presynaptic markers contacted the tips of the dendrites of host bipolar cells labeled with GFP, suggesting host–graft synapse formation (adapted from Mandai et al., 2017). (d) In vivo imaging of a transplanted eye at postoperative day 112 (differentiation day: DD176) with fluorescent angiography, showing Crx::Venus‐positive grafted photoreceptors under the retinal vessels. The same retina (graft DD204) was placed on the MEA electrodes with RGCs on the electrode side. Peri‐stimulus histograms from each channel, with red arrows indicating normal light‐responses and with blue arrows with hypersensitive responses. The bottom panel shows the number of RGCs recorded by each electrode, showing normal (red bars) or hypersensitive (blue bars) light responses (one cell: square, two cells: rectangle) before, during, and after removal of the ONL‐blocker LAP4. Yellow boxes represent graft area. In the right panel, yellow arrowheads mark the potential synaptic connections between PKCα‐positive host rod bipolar cells and the presynaptic marker CTBP2 on Crx::Venus‐positive grafted photoreceptor cells (adapted from Iraha et al., 2018). (e) Human iPSC‐derived retinal sheets, implanted in a local photoreceptor degeneration model by laser coagulation, matured and expressed the photoreceptor maturation markers peripherin 2 and S and L/M opsin (adapted from Tu et al., 2019). INL, internuclear layer; ITI, intertrial interval; ONL, outer nuclear layer. Scale bars: c, 20 μm; d, 20 μm; d′, 50 μm
5. FUTURE PROSPECTS FOR REGENERATION THERAPY USING ESC/IPSC‐DERIVED RETINAL CELLS
Recently, the first clinical study using hiPSC‐derived retinal sheets for the treatment of RP patients started in Japan as a safety study (jRCTa050200027). However, the optimization of regenerative cell therapy for retinal degeneration is still in progress; For example, in our retinal sheet transplantation, grafts also contain inner retinal cells connected to graft photoreceptor cells, sometimes appearing to prevent direct host–graft synaptic contact. To solve this problem, genetically engineered ESC/iPSC‐derived retinas were produced, in which rod bipolar cells degenerate as the graft retina matures after transplantation. The rationale behind this novel tool is to eliminate the photoreceptor partners in the graft at the time of synapse formation between graft photoreceptor cells and host bipolar cells, thereby enhancing this process (Tu et al., 2018). In addition, contact with the RPE is essential for photoreceptor cells to function properly; therefore, new strategies should be found to prevent rosette formation and to transplant novel RPE at the same time when the original one degenerates. Other attempts have been made to facilitate efficient network formation with host retinal cells by seeding purified photoreceptor cells onto biomaterial sheets and transplanting them (Jung et al., 2018). Another approach to cell‐based therapy is to introduce optical sensors into grafted photoreceptor cells to make them function stably and independently of the RPE (Garita‐Hernandez et al., 2019).
The collaboration of various experts in engineering, cell biology, genetics, and clinical medicine is essential for the development of these types of cellular therapies. Counting on appropriate collaborations, we expect further progress in the development of treatment approaches for retinal degenerative diseases.
CONFLICT OF INTEREST
The authors declare that this review was written in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
ACKNOWLEDGEMENTS
We thank Take Matsuyama, Suguru Yamasaki, and Hung‐Ya Tu for their valuable comments on the review.
Uyama H, Mandai M, Takahashi M. Stem‐cell‐based therapies for retinal degenerative diseases: Current challenges in the establishment of new treatment strategies. Develop Growth Differ. 2021;63:59–71. 10.1111/dgd.12704
Communicating Editor: Makoto Ikeya
REFERENCES
- Aït‐Ali, N. , Fridlich, R. , Millet‐Puel, G. , Clérin, E. , Delalande, F. , Jaillard, C. , Blond, F. , Perrocheau, L. , Reichman, S. , Byrne, L. C. , Olivier‐Bandini, A. , Bellalou, J. , Moyse, E. , Bouillaud, F. , Nicol, X. , Dalkara, D. , van Dorsselaer, A. , Sahel, J.‐A. , & Léveillard, T. (2015). Rod‐derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell, 161(4), 817–832. 10.1016/j.cell.2015.03.023 [DOI] [PubMed] [Google Scholar]
- Algvere, P. V. , Gouras, P. , & Dafgård Kopp, E. (1999). Long‐term outcome of RPE allografts in non‐immunosuppressed patients with AMD. European Journal of Ophthalmology, 9(3), 217–230. 10.1177/112067219900900310 [DOI] [PubMed] [Google Scholar]
- Assawachananont, J. , Mandai, M. , Okamoto, S. , Yamada, C. , Eiraku, M. , Yonemura, S. , Sasai, Y. , & Takahashi, M. (2014). Transplantation of embryonic and induced pluripotent stem cell‐derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports, 2(5), 662–674. https://www.ncbi.nlm.nih.gov/pubmed/24936453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramant, R. B. , & Seiler, M. J. (2002). Retinal transplantation—advantages of intact fetal sheets. Progress in Retinal and Eye Research, 21(1), 57–73. 10.1016/s1350-9462(01)00020-9 [DOI] [PubMed] [Google Scholar]
- Barnea‐Cramer, A. O. , Wang, W. , Lu, S.‐J. , Singh, M. S. , Luo, C. , Huo, H. , McClements, M. E. , Barnard, A. R. , MacLaren, R. E. , & Lanza, R. (2016). Function of human pluripotent stem cell‐derived photoreceptor progenitors in blind mice. Scientific Reports, 6(1), 29784. 10.1038/srep29784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty, S. , Koh, H.‐H. , Phil, M. , Henson, D. , & Boulton, M. (2000). The role of oxidative stress in the pathogenesis of age‐related macular degeneration. Survey of Ophthalmology, 45(2), 115–134. 10.1016/s0039-6257(00)00140-5 [DOI] [PubMed] [Google Scholar]
- Ben M’Barek, K. , Habeler, W. , Plancheron, A. , Jarraya, M. , Regent, F. , Terray, A. , Yang, Y. , Chatrousse, L. , Domingues, S. , Masson, Y. , Sahel, J.‐A. , Peschanski, M. , Goureau, O. , & Monville, C. (2017). Human ESC–derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Science Translational Medicine, 9(421), eaai7471. 10.1126/scitranslmed.aai7471 [DOI] [PubMed] [Google Scholar]
- Ben M'Barek, K. , Bertin, S. , Brazhnikova, E. , Jaillard, C. , Habeler, W. , Plancheron, A. , Fovet, C.‐M. , Demilly, J. , Jarraya, M. , Bejanariu, A. , Sahel, J.‐A. , Peschanski, M. , Goureau, O. , & Monville, C. (2020). Clinical‐grade production and safe delivery of human ESC derived RPE sheets in primates and rodents. Biomaterials, 230, 119603. 10.1016/j.biomaterials.2019.119603 [DOI] [PubMed] [Google Scholar]
- Binder, S. , Krebs, I. , Hilgers, R.‐D. , Abri, A. , Stolba, U. , Assadoulina, A. , Kellner, L. , Stanzel, B. V. , Jahn, C. , & Feichtinger, H. (2004). Outcome of transplantation of autologous retinal pigment epithelium in age‐related macular degeneration: a prospective trial. Investigative Opthalmology & Visual Science, 45(11), 4151. 10.1167/iovs.04-0118 [DOI] [PubMed] [Google Scholar]
- Campochiaro, P. A. (2015). Molecular pathogenesis of retinal and choroidal vascular diseases. Progress in Retinal and Eye Research, 49, 67–81. 10.1016/j.preteyeres.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cruz, L. , Fynes, K. , Georgiadis, O. , Kerby, J. , Luo, Y.H. , Ahmado, A. , Vernon, A. , Daniels, J.T. , Nommiste, B. , Hasan, S.M. & Gooljar, S.B. (2018). Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age‐related macular degeneration. Nature Biotechnology, 36(4), 328–337. 10.1038/nbt.4114 [DOI] [PubMed] [Google Scholar]
- Das, T. , del Cerro, M. , Jalali, S. , Rao, V. S. , Gullapalli, V. K. , Little, C. , Loreto, D. A. D. , Sharma, S. , Sreedharan, A. , del Cerro, C. , & Rao, G. N. (1999). The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: Results of a long‐term safety study. Experimental Neurology, 157(1), 58–68. 10.1006/exnr.1998.6992 [DOI] [PubMed] [Google Scholar]
- Decembrini, S. , Martin, C. , Sennlaub, F. , Chemtob, S. , Biel, M. , Samardzija, M. , Moulin, A. , Behar‐Cohen, F. , & Arsenijevic, Y. (2017). Cone Genesis Tracing by the Chrnb4 ‐EGFP mouse line: evidences of cellular material fusion after cone precursor. Transplantation, 25(3), 634–653. 10.1016/j.ymthe.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W.‐L. , Gao, M.‐L. , Lei, X.‐L. , Lv, J.‐N. , Zhao, H. , He, K.‐W. , Xia, X.‐X. , Li, L.‐Y. , Chen, Y.‐C. , Li, Y.‐P. , Pan, D. , Xue, T. , & Jin, Z.‐B. (2018). Gene correction reverses ciliopathy and photoreceptor loss in iPSC‐derived retinal organoids from retinitis pigmentosa patients. Stem Cell Reports, 10(4), 1267–1281. 10.1016/j.stemcr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku, M. , Takata, N. , Ishibashi, H. , Kawada, M. , Sakakura, E. , Okuda, S. , Sekiguchi, K. , Adachi, T. & Sasai, Y. (2011). Self‐organizing optic‐cup morphogenesis in three‐dimensional culture. Nature, 472(7341), 51–56. https://www.ncbi.nlm.nih.gov/pubmed/21475194 [DOI] [PubMed] [Google Scholar]
- Garita‐Hernandez, M. , Lampič, M. , Chaffiol, A. , Guibbal, L. , Routet, F. , Santos‐Ferreira, T. , Gasparini, S. , Borsch, O. , Gagliardi, G. , Reichman, S. , Picaud, S. , Sahel, J.‐A. , Goureau, O. , Ader, M. , Dalkara, D. , & Duebel, J. (2019). Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nature Communications, 10(1), 4524. 10.1038/s41467-019-12330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Cordero, A. , West, E. L. , Pearson, R. A. , Duran, Y. , Carvalho, L. S. , Chu, C. J. , Naeem, A. , Blackford, S. J. I. , Georgiadis, A. , Lakowski, J. , Hubank, M. , Smith, A. J. , Bainbridge, J. W. B. , Sowden, J. C. , & Ali, R. R. (2013). Photoreceptor precursors derived from three‐dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nature Biotechnology, 31(8), 741–747. 10.1038/nbt.2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong, D. T. , Berson, E. L. , & Dryja, T. P. (2006). Retinitis pigmentosa. The Lancet, 368(9549), 1795–1809. 10.1016/s0140-6736(06)69740-7 [DOI] [PubMed] [Google Scholar]
- Idelson, M. , Alper, R. , Obolensky, A. , Ben‐Shushan, E. , Hemo, I. , Yachimovich‐Cohen, N. , Khaner, H. , Smith, Y. , Wiser, O. , Gropp, M. , Cohen, M. A. , Even‐Ram, S. , Berman‐Zaken, Y. , Matzrafi, L. , Rechavi, G. , Banin, E. , & Reubinoff, B. (2009). Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell, 5(4), 396–408. 10.1016/j.stem.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Ikeda, H. , Osakada, F. , Watanabe, K. , Mizuseki, K. , Haraguchi, T. , Miyoshi, H. , Kamiya, D. , Honda, Y. , Sasai, N. , Yoshimura, N. , Takahashi, M. , & Sasai, Y. (2005). Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 102(32), 11331–11336. 10.1073/pnas.0500010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraha, S. , Tu, H.‐Y. , Yamasaki, S. , Kagawa, T. , Goto, M. , Takahashi, R. , Watanabe, T. , Sugita, S. , Yonemura, S. , Sunagawa, G. A. , Matsuyama, T. , Fujii, M. , Kuwahara, A. , Kishino, A. , Koide, N. , Eiraku, M. , Tanihara, H. , Takahashi, M. , & Mandai, M. (2018). Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC‐derived retinal sheets after transplantation. Stem Cell Reports, 10(3), 1059–1074. 10.1016/j.stemcr.2018.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, Y. H. , Phillips, M. J. , Lee, J. , Xie, R. , Ludwig, A. L. , Chen, G. , Zheng, Q. , Kim, T. J. , Zhang, H. , Barney, P. , Min, J. , Barlow, K. , Gong, S. , Gamm, D. M. , & Ma, Z. (2018). 3D microstructured scaffolds to support photoreceptor polarization and maturation. Advanced Materials, 30(39), 1803550. 10.1002/adma.201803550 [DOI] [PubMed] [Google Scholar]
- Kamao, H. , Mandai, M. , Okamoto, S. , Sakai, N. , Suga, A. , Sugita, S. , Kiryu, J. , & Takahashi, M. (2014). Characterization of human induced pluripotent stem cell‐derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports, 2(2), 205–218. 10.1016/j.stemcr.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani, A. H. , Lebkowski, J. S. , Rahhal, F. M. , Avery, R. L. , Salehi‐Had, H. , Dang, W. , Lin, C.‐M. , Mitra, D. , Zhu, D. , Thomas, B. B. , Hikita, S. T. , Pennington, B. O. , Johnson, L. V. , Clegg, D. O. , Hinton, D. R. , & Humayun, M. S. (2018). A bioengineered retinal pigment epithelial monolayer for advanced, dry age‐related macular degeneration. Science Translational Medicine, 10(435), eaao4097. 10.1126/scitranslmed.aao4097 [DOI] [PubMed] [Google Scholar]
- Kawasaki, H. , Suemori, H. , Mizuseki, K. , Watanabe, K. , Urano, F. , Ichinose, H. , Haruta, M. , Takahashi, M. , Yoshikawa, K. , Nishikawa, S.I. & Nakatsuji, N. (2002). Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell‐derived inducing activity. Proceedings of the National Academy of Sciences, 99(3), 1580–1585. 10.1073/pnas.032662199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser, P. D. , Golczak, M. , Maeda, A. , & Palczewski, K. (2012). Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochimica Et Biophysica Acta (BBA) ‐ Molecular and Cell Biology of Lipids, 1821(1), 137–151. 10.1016/j.bbalip.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimanskaya, I. , Hipp, J. , Rezai, K. A. , West, M. , Atala, A. , & Lanza, R. (2004). Derivation and Comparative Assessment of Retinal Pigment Epithelium from Human Embryonic Stem Cells Using Transcriptomics. Cloning and Stem Cells, 6(3), 217–245. 10.1089/clo.2004.6.217 [DOI] [PubMed] [Google Scholar]
- Kuwahara, A. , Ozone, C. , Nakano, T. , Saito, K. , Eiraku, M. , & Sasai, Y. (2015). Generation of a ciliary margin‐like stem cell niche from self‐organizing human retinal tissue. Nature Communications, 6, 6286. 10.1038/ncomms7286 [DOI] [PubMed] [Google Scholar]
- Kuwahara, A. , Yamasaki, S. , Mandai, M. , Watari, K. , Matsushita, K. , Fujiwara, M. , Hori, Y. , Hiramine, Y. , Nukaya, D. , Iwata, M. , Kishino, A. , Takahashi, M. , Sasai, Y. , & Kimura, T. (2019). Preconditioning the initial state of feeder‐free human pluripotent stem cells promotes self‐formation of three‐dimensional retinal tissue. Scientific Reports, 9(1), 18936. 10.1038/s41598-019-55130-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba, D. A. , Gust, J. , & Reh, T. A. (2009). Transplantation of human embryonic stem cell‐derived photoreceptors restores some visual function in Crx‐deficient mice. Cell Stem Cell, 4(1), 73–79. 10.1016/j.stem.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba, D. A. , Karl, M. O. , Ware, C. B. & Reh, T. A. (2006). Efficient generation of retinal progenitor cells from human embryonic stem cells. Proceedings of the National Academy of Sciences, 103(34), 12769–12774. 10.1073/pnas.0601990103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, N. G. , ElShelmani, H. , Singh, M. K. , Mansergh, F. C. , Wride, M. A. , Padilla, M. , Keegan, D. , Hogg, R. E. , & Ambati, B. K. (2016). Risk factors and biomarkers of age‐related macular degeneration. Progress in Retinal and Eye Research, 54, 64–102. 10.1016/j.preteyeres.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach, L. L. , Buchholz, D. E. , Nadar, V. P. , Lowenstein, S. E. , & Clegg, D. O. (2015). Canonical/ ‐catenin Wnt pathway activation improves retinal pigmented epithelium derivation from human embryonic stem cells. Investigative Ophthalmology & Visual Science, 56(2), 1002–1013. 10.1167/iovs.14-15835 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Xu, H. W. , Wang, L. , Li, S. Y. , Zhao, C. J. , Hao, J. , Li, Q. Y. , Zhao, T. T. , Wu, W. , Wang, Y. I. , Zhou, Q. I. , Qian, C. , Wang, L. , & Yin, Z. Q. (2018). Human embryonic stem cell‐derived retinal pigment epithelium transplants as a potential treatment for wet age‐related macular degeneration. Cell Discovery, 4(1), 50. 10.1038/s41421-018-0053-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren, R. E. , Bird, A. C. , Sathia, P. J. , & Aylward, G. W. (2005). Long‐term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age‐related macular degeneration. Ophthalmology, 112(12), 2081–2087. 10.1016/j.ophtha.2005.06.029 [DOI] [PubMed] [Google Scholar]
- Maeda, T. , Lee, M. J. , Palczewska, G. , Marsili, S. , Tesar, P. J. , Palczewski, K. , & Maeda, A. (2013). Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymesin vitroandin vivo. Journal of Biological Chemistry, 288(48), 34484–34493. 10.1074/jbc.m113.518571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai, M. , Fujii, M. , Hashiguchi, T. , Sunagawa, G. A. , Ito, S.‐I. , Sun, J. , Kaneko, J. , Sho, J. , Yamada, C. , & Takahashi, M. (2017). iPSC‐derived retina transplants improve vision in rd1 end‐stage retinal‐degeneration mice. Stem Cell Reports, 8(1), 69–83. 10.1016/j.stemcr.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai, M. , Watanabe, A. , Kurimoto, Y. , Hirami, Y. , Morinaga, C. , Daimon, T. , Fujihara, M. , Akimaru, H. , Sakai, N. , Shibata, Y. , & Terada, M. (2017). Autologous Induced Stem‐Cell–Derived Retinal Cells for Macular Degeneration. New England Journal of Medicine, 376(11), 1038–1046. https://www.nejm.org/doi/full/10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- Mehat, M. S. , Sundaram, V. , Ripamonti, C. , Robson, A. G. , Smith, A. J. , Borooah, S. , Robinson, M. , Rosenthal, A. N. , Innes, W. , Weleber, R. G. , Lee, R. W. J. , Crossland, M. , Rubin, G. S. , Dhillon, B. , Steel, D. H. W. , Anglade, E. , Lanza, R. P. , Ali, R. R. , Michaelides, M. , & Bainbridge, J. W. B. (2018). Transplantation of human embryonic stem cell‐derived retinal pigment epithelial cells in macular degeneration. Ophthalmology, 125(11), 1765–1775. 10.1016/j.ophtha.2018.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, J. S. , Howden, S. E. , Wallace, K. A. , Verhoeven, A. D. , Wright, L. S. , Capowski, E. E. , Pinilla, I. , Martin, J. M. , Tian, S. , Stewart, R. , Pattnaik, B. , Thomson, J. A. , & Gamm, D. M. (2011). Optic vesicle‐like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells, 29(8), 1206–1218. 10.1002/stem.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane, Y. , Morimoto, N. , Fujiwara, A. , Kawasaki, R. , Yamashita, H. , Ogura, Y. , & Shiraga, F. (2019). Incidence and causes of visual impairment in Japan: The first nation‐wide complete enumeration survey of newly certified visually impaired individuals. Japanese Journal of Ophthalmology, 63(1), 26–33. 10.1007/s10384-018-0623-4 [DOI] [PubMed] [Google Scholar]
- Nakano, T. , Ando, S. , Takata, N. , Kawada, M. , Muguruma, K. , Sekiguchi, K. , Saito, K. , Yonemura, S. , Eiraku, M. , & Sasai, Y. (2012). Self‐formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell, 10(6), 771–785. 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Ortin‐Martinez, A. , Tsai, E. L. S. , Nickerson, P. E. , Bergeret, M. , Lu, Y. , Smiley, S. , Comanita, L. , & Wallace, V. A. (2017). A reinterpretation of cell transplantation: GFP transfer from donor to host photoreceptors. Stem Cells, 35(4), 932–939. 10.1002/stem.2552 [DOI] [PubMed] [Google Scholar]
- Osakada, F. , Ikeda, H. , Mandai, M. , Wataya, T. , Watanabe, K. , Yoshimura, N. , Akaike, A. , Sasai, Y. , & Takahashi, M. (2008). Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nature Biotechnology, 26(2), 215–224. 10.1038/nbt1384 [DOI] [PubMed] [Google Scholar]
- Osakada, F. , Ikeda, H. , Sasai, Y. , & Takahashi, M. (2009). Stepwise differentiation of pluripotent stem cells into retinal cells. Nature Protocols, 4(6), 811–824. 10.1038/nprot.2009.51 [DOI] [PubMed] [Google Scholar]
- Osakada, F. , Jin, Z.‐B. , Hirami, Y. , Ikeda, H. , Danjyo, T. , Watanabe, K. , Sasai, Y. , & Takahashi, M. (2009). In vitro differentiation of retinal cells from human pluripotent stem cells by small‐molecule induction. Journal of Cell Science, 122(17), 3169–3179. 10.1242/jcs.050393 [DOI] [PubMed] [Google Scholar]
- Parfitt, D. A. , Lane, A. , Ramsden, C. M. , Carr, A.‐J. , Munro, P. M. , Jovanovic, K. , Schwarz, N. , Kanuga, N. , Muthiah, M. N. , Hull, S. , Gallo, J.‐M. , da Cruz, L. , Moore, A. T. , Hardcastle, A. J. , Coffey, P. J. , & Cheetham, M. E. (2016). Identification and correction of mechanisms underlying inherited blindness in human iPSC‐derived optic cups. Cell Stem Cell, 18(6), 769–781. 10.1016/j.stem.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, R. A. , Barber, A. C. , Rizzi, M. , Hippert, C. , Xue, T. , West, E. L. , Duran, Y. , Smith, A. J. , Chuang, J. Z. , Azam, S. A. , Luhmann, U. F. O. , Benucci, A. , Sung, C. H. , Bainbridge, J. W. , Carandini, M. , Yau, K.‐W. , Sowden, J. C. , & Ali, R. R. (2012). Restoration of vision after transplantation of photoreceptors. Nature, 485(7396), 99–103. 10.1038/nature10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, R. A. , Gonzalez‐Cordero, A. , West, E. L. , Ribeiro, J. R. , Aghaizu, N. , Goh, D. , Sampson, R. D. , Georgiadis, A. , Waldron, P. V. , Duran, Y. , Naeem, A. , Kloc, M. , Cristante, E. , Kruczek, K. , Warre‐Cornish, K. , Sowden, J. C. , Smith, A. J. , & Ali, R. R. (2016). Donor and host photoreceptors engage in material transfer following transplantation of post‐mitotic photoreceptor precursors. Nature Communications, 7, 13029. 10.1038/ncomms13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyman, G. A. , Blinder, K. J. , Paris, C. L. , Alturki, W. , Nelson, N. C. Jr , & Desai, U. (1991). A technique for retinal pigment epithelium transplantation for age‐related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surgery, 22(2), 102–108. [PubMed] [Google Scholar]
- Radtke, N. D. (2004). Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa. Archives of Ophthalmology, 122(8), 1159. 10.1001/archopht.122.8.1159 [DOI] [PubMed] [Google Scholar]
- Radtke, N. D. , Aramant, R. B. , Petry, H. M. , Green, P. T. , Pidwell, D. J. , & Seiler, M. J. (2008). Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. American Journal of Ophthalmology, 146(2), 172–182.e171. 10.1016/j.ajo.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Radtke, N. D. , Aramant, R. B. , Seiler, M. , & Petry, H. M. (1999). Preliminary report: indications of improved visual function after retinal sheet transplantation in retinitis pigmentosa patients. American Journal of Ophthalmology, 128(3), 384–387. 10.1016/S0002-9394(99)00250-0 [DOI] [PubMed] [Google Scholar]
- Reichman, S. , Slembrouck, A. , Gagliardi, G. , Chaffiol, A. , Terray, A. , Nanteau, C. , Potey, A. , Belle, M. , Rabesandratana, O. , Duebel, J. , Orieux, G. , Nandrot, E. F. , Sahel, J.‐A. , & Goureau, O. (2017). Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno‐free and feeder‐free conditions. Stem Cells, 35(5), 1176–1188. 10.1002/stem.2586 [DOI] [PubMed] [Google Scholar]
- Reichman, S. , Terray, A. , Slembrouck, A. , Nanteau, C. , Orieux, G. , Habeler, W. , Nandrot, E. F. , Sahel, J.‐A. , Monville, C. , & Goureau, O. (2014). From confluent human iPS cells to self‐forming neural retina and retinal pigmented epithelium. Proceedings of the National Academy of Sciences of the United States of America, 111(23), 8518–8523. 10.1073/pnas.1324212111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐Ferreira, T. , Llonch, S. , Borsch, O. , Postel, K. , Haas, J. , & Ader, M. (2016). Retinal transplantation of photoreceptors results in donor–host cytoplasmic exchange. Nature Communications, 7(1), 13028. 10.1038/ncomms13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, S. D. , Hubschman, J.‐P. , Heilwell, G. , Franco‐Cardenas, V. , Pan, C. K. , Ostrick, R. M. , Mickunas, E. , Gay, R. , Klimanskaya, I. , & Lanza, R. (2012). Embryonic stem cell trials for macular degeneration: A preliminary report. The Lancet, 379(9817), 713–720. 10.1016/s0140-6736(12)60028-2 [DOI] [PubMed] [Google Scholar]
- Schwartz, S. D. , Regillo, C. D. , Lam, B. L. , Eliott, D. , Rosenfeld, P. J. , Gregori, N. Z. , Hubschman, J.‐P. , Davis, J. L. , Heilwell, G. , Spirn, M. , Maguire, J. , Gay, R. , Bateman, J. , Ostrick, R. M. , Morris, D. , Vincent, M. , Anglade, E. , Del Priore, L. V. , & Lanza, R. (2015). Human embryonic stem cell‐derived retinal pigment epithelium in patients with age‐related macular degeneration and Stargardt's macular dystrophy: Follow‐up of two open‐label phase 1/2 studies. The Lancet, 385(9967), 509–516. 10.1016/s0140-6736(14)61376-3 [DOI] [PubMed] [Google Scholar]
- Sharma, R. , Khristov, V. , Rising, A. , Jha, B. S. , Dejene, R. , Hotaling, N. , Li, Y. , Stoddard, J. , Stankewicz, C. , Wan, Q. , Zhang, C. , Campos, M. M. , Miyagishima, K. J. , McGaughey, D. , Villasmil, R. , Mattapallil, M. , Stanzel, B. , Qian, H. , Wong, W. , … Bharti, K. (2019). Clinical‐grade stem cell–derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Science Translational Medicine, 11(475), eaat5580. 10.1126/scitranslmed.aat5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, H. , Lu, Q. , Insinna‐Kettenhofen, C. , Nagashima, K. , English, M. A. , Semler, E. M. , Mahgerefteh, J. , Cideciyan, A. V. , Li, T. , Brooks, B. P. , Gunay‐Aygun, M. , Jacobson, S. G. , Cogliati, T. , Westlake, C. J. , & Swaroop, A. (2017). In vitro modeling using ciliopathy‐patient‐derived cells reveals distinct cilia dysfunctions caused by CEP290 mutations. Cell Reports, 20(2), 384–396. 10.1016/j.celrep.2017.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai, H. , Mandai, M. , Matsushita, K. , Kuwahara, A. , Yonemura, S. , Nakano, T. , Assawachananont, J. , Kimura, T. , Saito, K. , Terasaki, H. , Eiraku, M. , Sasai, Y. , & Takahashi, M. (2016). Transplantation of human embryonic stem cell‐derived retinal tissue in two primate models of retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America, 113(1), E81–E90. 10.1073/pnas.1512590113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, M. S. , Balmer, J. , Barnard, A. R. , Aslam, S. A. , Moralli, D. , Green, C. M. , Barnea‐Cramer, A. , Duncan, I. , & MacLaren, R. E. (2016). Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nature Communications, 7, 13537. 10.1038/ncomms13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, M. S. , Charbel Issa, P. , Butler, R. , Martin, C. , Lipinski, D. M. , Sekaran, S. , Barnard, A. R. , & MacLaren, R. E. (2013). Reversal of end‐stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proceedings of the National Academy of Sciences of the United States of America, 110(3), 1101–1106. 10.1073/pnas.1119416110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W. K. , Park, K.‐M. , Kim, H.‐J. , Lee, J. H. , Choi, J. , Chong, S. Y. , Shim, S. H. , Del Priore, L. V. , & Lanza, R. (2015). Treatment of macular degeneration using embryonic stem cell‐derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Reports, 4(5), 860–872. 10.1016/j.stemcr.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein, J. W. (2003). Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nature Reviews Immunology, 3(11), 879–889. 10.1038/nri1224 [DOI] [PubMed] [Google Scholar]
- Sugita, S. , Futagami, Y. , Smith, S. B. , Naggar, H. , & Mochizuki, M. (2006). Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Experimental Eye Research, 83(6), 1459–1471. 10.1016/j.exer.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Sugita, S. , Mandai, M. , Hirami, Y. , Takagi, S. , Maeda, T. , Fujihara, M. , Matsuzaki, M. , Yamamoto, M. , Iseki, K. , Hayashi, N. , Hono, A. , Fujino, S. , Koide, N. , Sakai, N. , Shibata, Y. , Terada, M. , Nishida, M. , Dohi, H. , Nomura, M. , … Takahashi, M. (2020). HLA‐matched allogeneic iPS cells‐derived RPE transplantation for macular degeneration. Journal of Clinical Medicine, 9(7), 2217. 10.3390/jcm9072217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, Y. , Lee, M. J. , Choi, J. , Jung, S. Y. , Chong, S. Y. , Sung, J. H. , Shim, S. H. , & Song, W. K. (2020). Long‐term safety and tolerability of subretinal transplantation of embryonic stem cell‐derived retinal pigment epithelium in Asian Stargardt disease patients. British Journal of Ophthalmology. 10.1136/bjophthalmol-2020-316225 [DOI] [PubMed] [Google Scholar]
- Takagi, S. , Mandai, M. , Gocho, K. , Hirami, Y. , Yamamoto, M. , Fujihara, M. , Sugita, S. , Kurimoto, Y. , & Takahashi, M. (2019). Evaluation of transplanted autologous induced pluripotent stem cell‐derived retinal pigment epithelium in exudative age‐related macular degeneration. Ophthalmology Retina, 3(10), 850–859. 10.1016/j.oret.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Takagi, S. , Mandai, M. , Hirami, Y. , Sugita, S. , Takahashi, M. , & Kurimoto, Y. (2018). Frequencies of human leukocyte antigen alleles and haplotypes among Japanese patients with age‐related macular degeneration. Japanese Journal of Ophthalmology, 62(5), 568–575. 10.1007/s10384-018-0611-8 [DOI] [PubMed] [Google Scholar]
- Takahashi, K. , & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Tu, H.‐Y. , Matsuyama, T. , Sun, J. , Hashiguchi, T. , Sho, J. , Sunagawa, G. A. , & Mandai, M. (2018). Genetically engineered iPSC‐retina for improved retinal reconstruction after transplantation. Investigative Ophthalmology & Visual Science, 59(9), 1987. [Google Scholar]
- Tu, H.‐Y. , Watanabe, T. , Shirai, H. , Yamasaki, S. , Kinoshita, M. , Matsushita, K. , Hashiguchi, T. , Onoe, H. , Matsuyama, T. , Kuwahara, A. , Kishino, A. , Kimura, T. , Eiraku, M. , Suzuma, K. , Kitaoka, T. , Takahashi, M. , & Mandai, M. (2019). Medium‐ to long‐term survival and functional examination of human iPSC‐derived retinas in rat and primate models of retinal degeneration. EBioMedicine, 39, 562–574. 10.1016/j.ebiom.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, B. A. , Park, I.‐H. , Qi, S. D. , Klassen, H. J. , Jiang, C. , Yao, J. , Redenti, S. , Daley, G. Q. , & Young, M. J. (2011). Transplantation of adult mouse iPS cell‐derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One, 6(4), e18992. 10.1371/journal.pone.0018992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekage, M. , Sato, Y. , & Takasu, N. (2019). Overview: An iPS cell stock at CiRA. Inflammation and Regeneration, 39(1), 17. 10.1186/s41232-019-0106-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zeeburg, E. J. , Maaijwee, K. J. , Missotten, T. O. , Heimann, H. & van Meurs, J.C. (2012). A Free Retinal Pigment Epithelium–Choroid Graft in Patients With Exudative Age‐Related Macular Degeneration: Results up to 7 Years. American Journal of Ophthalmology, 153(1), 120–127. https://dx.doi.org/10.1016/j.ajo.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Wako, R. , Yasukawa, T. , Kato, A. , Omori, T. , Ishida, S. , Ishibashi, T. , & Ogura, Y. (2014). Causes and prevalence of visual impairment in Japan. Nippon Ganka Gakkai Zasshi, 118(6), 495–501. [PubMed] [Google Scholar]
- Wong, W. L. , Su, X. , Li, X. , Cheung, C. M. G. , Klein, R. , Cheng, C.‐Y. , & Wong, T. Y. (2014). Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta‐analysis. The Lancet Global Health, 2(2), e106–e116. 10.1016/s2214-109x(13)70145-1 [DOI] [PubMed] [Google Scholar]
- Xu, H. , Wang, B. O. , Ono, M. , Kagita, A. , Fujii, K. , Sasakawa, N. , Ueda, T. , Gee, P. , Nishikawa, M. , Nomura, M. , Kitaoka, F. , Takahashi, T. , Okita, K. , Yoshida, Y. , Kaneko, S. , & Hotta, A. (2019). Targeted disruption of HLA genes via CRISPR‐Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell, 24(4), 566–578.e567. 10.1016/j.stem.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Zarbin, M. A. , & Rosenfeld, P. J. (2010). Pathway‐based therapies for age‐related macular degeneration. Retina, 30(9), 1350–1367. 10.1097/iae.0b013e3181f57e30 [DOI] [PubMed] [Google Scholar]
- Zhong, X. , Gutierrez, C. , Xue, T. , Hampton, C. , Vergara, M. N. , Cao, L.‐H. , Peters, A. , Park, T. S. , Zambidis, E. T. , Meyer, J. S. , Gamm, D. M. , Yau, K.‐W. , & Canto‐Soler, M. V. (2014). Generation of three‐dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications, 5(1), 4047. 10.1038/ncomms5047 [DOI] [PMC free article] [PubMed] [Google Scholar]


