Abstract
Background
The feasibility and outcomes of concomitant atrioventricular node ablation (AVNA) and leadless pacemaker implant are not well studied. We report outcomes in patients undergoing Micra implant with concomitant AVNA.
Methods
Patients undergoing AVNA at the time of Micra implant from the Micra Transcatheter Pacing (IDE) Study, Continued Access (CA) study, and Post‐Approval Registry (PAR) were included in the analysis and compared to Micra patients without AVNA. Baseline characteristics, acute and follow‐up outcomes, and electrical performance were compared between patients with and without AVNA during the follow‐up period.
Results
A total of 192 patients (mean age 77.4 ± 8.9 years, 72% female) underwent AVNA at the time of Micra implant and were followed for 20.4 ± 15.6 months. AVNA patients were older, more frequently female, and tended to have more co‐morbid conditions compared with non‐AVNA patients (N = 2616). Implant was successful in 191 of 192 patients (99.5%). The mean pacing threshold at implant was 0.58 ± 0.35 V and remained stable during follow‐up. Major complications within 30 days occurred more frequently in AVNA patients than non‐AVNA patients (7.3% vs. 2.0%, p < .001). The risk of major complications through 36‐months was higher in AVNA patients (hazard ratio: 3.81, 95% confidence interval: 2.33–6.23, p < .001). Intermittent loss of capture occurred in three AVNA patients (1.6%), all were within 30 days of implant and required system revision. There were no device macrodislodgements or unexpected device malfunctions.
Conclusion
Concomitant AVN ablation and leadless pacemaker implant is feasible. Pacing thresholds are stable over time. However, patient comorbidities and the risk of major complications are higher in patients undergoing AVNA.
Keywords: AV node ablation, leadless pacemaker, Micra
1. INTRODUCTION
Atrioventricular nodal ablation (AVNA) and pacing (“ablate and pace”) is sometimes required for the treatment of atrial fibrillation (AF) complicated by medically refractory tachycardia. 1 , 2 This approach is largely reserved for patients who fail catheter ablation or who are not considered candidates for AF ablation, 3 which is not uncommon in octogenarians and in patients with advanced structural heart disease. 3 , 4 While an ablate and pace approach can be very effective, lead dislodgment and device infection in patients who were rendered pacemaker dependent remain important concerns. 5
Leadless pacemakers (LP) reduce lead and pocket related complications encountered with transvenous pacemakers (TV‐PPM). 6 , 7 , 8 This potential benefit over TV‐PPM in addition to the advantage of using a single femoral venous puncture to implant a LP and concomitantly perform an AVNA makes the use of leadless pacing as part of the “ablate and pace” approach an appealing strategy. Small studies have reported on the safety and efficacy of a simultaneous LP and AVNA. 9 , 10 Accordingly, we sought to assess the safety and efficacy of concomitant AVNA and Micra pacemaker implantation in a large cohort of patients enrolled in clinical trials.
2. METHODS
2.1. Patient cohort and setting
Patients enrolled in the Micra Transcatheter Pacing (IDE) Study, Continued Access (CA) study, and Post‐Approval Registry (PAR) were included in this analysis and stratified by the presence or absence of AVNA at the time of Micra implantation. The methodologies and results of these studies have been previously published. 6 , 7
These studies were sponsored by Medtronic and enrolled patients with Class I or II pacing indications 11 with no co‐morbidity restrictions. The Micra IDE study was designed to obtain regulatory FDA approval for the Micra Transcatheter Pacing System (TPS) 7 and excluded patients with an existing cardiac implantable electronic devices (CIEDs). The long‐term results showed excellent safety and efficacy of Micra. 12 The Micra CA study allowed continued access to Micra while under review by FDA. The Micra PAR studied the performance of Micra in a real‐world setting, in clinical practice outside of clinical trials. Both the Micra CA and PAR studies allowed participation of patients with pre‐existing CIEDs. Initial results of the PAR were published confirming the safety and efficacy of Micra in a large patient population. 6 , 13 The 9‐year follow‐up period in the Micra PAR is ongoing. The study protocols were approved by the ethics committee at each center, and all patients provided written informed consent. An independent committee of physicians adjudicated all adverse events.
Baseline demographics, medical comorbidities, procedural characteristics, and study outcomes were collected on study case report forms. Device diagnostic data contained in interrogation files were obtained from study centers as they were collected.
2.2. Objective
The objective of this analysis was to assess the safety and performance of Micra when AVNA was performed at the time of device implant. The safety outcomes included major complications and system revisions through 36 months postimplant. In brief, major complications were defined as any events related to Micra device or procedure that resulted in death, permanent loss of device function, hospitalization, prolonged hospitalization, or system revision. 7 Device electrical performance was also assessed through 24‐months following implant.
2.3. Statistical analysis
Patients enrolled in the Micra IDE, CA, or PAR studies with AVNA were identified. The Micra IDE and CA databases were locked before reporting their results. The Micra PAR database was frozen for this analysis on March 2, 2020. Baseline characteristics and co‐morbidities were compared between those with and without AVNA using t‐tests or the Wilcoxon Rank sum test (continuous variables) or Fisher's exact test (categorical variables). A logistic regression model was used to compare the rate of acute (within 30 days of implant) major complications between groups. Cox proportional hazards models were used to compare the risk for major complication and system revisions through the 36‐months postimplant follow‐up period. Logistic regression models and Cox models using propensity score overlap weights were used to adjust for differences in co‐morbid conditions between groups. Specifically, these weights estimate the probability of treatment with the opposing therapy based on observed characteristics. To calculate the propensity score, the probability for each patient to receive AVNA was estimated using a logistic‐regression model that included baseline characteristics found in Table S1. The resulting propensity scores were used to derive a weight for each patient to adjust for patient differences between AVNA status. Analyses using propensity score overlap weights place the most emphasis on patients who are considered the most “exchangeable” (i.e., patients with similar or equal probability of receiving [or not receiving] AVNA based on observed characteristics) and the least emphasis on patients who are least likely to be treated with the opposing therapy. 14 Standardized mean differences were used to assess balance among covariates included in the propensity model following weighting.
Device electrical parameters including pacing impedance and pacing capture thresholds (PCTs) were obtained from follow‐up visits and device interrogation uploads. The Micra device stores the minimum and maximum weekly pacing impedance and pacing capture threshold in device memory. For electrical parameters obtained from interrogation uploads, the mean weekly pacing impedance was used for analysis, while the maximum weekly PCT was used for analysis. All PCTs were standardized to a pulse duration of 0.24 ms (nominal setting) using the Micra strength duration curve. Device electrical parameters were compared at implant and follow‐up visits by AVNA status using a mixed effects linear regression model. Additionally, the mean, 10th and 90th percentile for maximum PCT was computed weekly during the first 12‐weeks following implant for the subset of patients with AVNA.
p‐values less than .05 were considered statistically significant. All analyses were performed using SAS v9.4 (SAS Institute) or R v4.0.2 (www.r-project.org).
3. RESULTS
3.1. Patients
The cohort included a total of 2817 patients who underwent a Micra implant attempt across the three studies of which 192 had AVNA, 2616 did not have AVNA. In nine patients the AVNA status was unknown and these subjects were excluded from further analysis. Of the 192 patients with AVNA, 40, 42, and 110 came from the Micra IDE, CA, and PAR studies, respectively, representing 5.5%, 15.2%, and 6.1% of each study's population. Patients with AVNA had an average follow‐up of 20.4 ± 15.6 months with 34 (17.7%) patients having more than or equal to 36 months of follow‐up compared with an average of 25.2 ± 15.3 for patients without AVNA. Patients with AVNA were more likely female, and had more co‐morbidities including heart failure, chronic obstructive pulmonary disease, and hypertension compared with patients without AVNA (Table 1). One (0.5%) of the 192 patients with AVNA had a prior cardiac implantable electronic device compared with 11.8% of patients without AVNA (p < .001) and 14.1% and 19.8% of patients, respectively had a condition precluding transvenous pacemaker implant (p = .06).
Table 1.
Baseline medical history and implant characteristics
| Patient characteristic | AVNA (N = 192) | No AVNA (N = 2616) | p‐valuea |
|---|---|---|---|
| Age | |||
| Mean ± standard deviation | 77.4 ± 8.9 | 75.5 ± 13.0 | .53 |
| Median | 78.0 | 78.0 | |
| 25th Percentile ‐ 75th Percentile | 73.0–84.0 | 71.0–84.0 | |
| Female | 71.9% (138/192) | 37.5% (980/2615) | <.001 |
| Co‐morbidities | |||
| Atrial tachyarrhythmias | 98.4% (189/192) | 75.1% (1963/2615) | <.001 |
| CHF | 29.7% (57/192) | 14.6% (383/2615) | <.001 |
| COPD | 27.6% (53/192) | 10.2% (268/2615) | <.001 |
| CAD | 27.1% (52/192) | 24.9% (651/2615) | .49 |
| Hypertension | 79.7% (153/192) | 68.9% (1803/2615) | .001 |
| Diabetes | 27.1% (52/192) | 27.5% (719/2615) | .93 |
| Renal dysfunction | 26.6% (51/192) | 21.1% (551/2615) | .08 |
| Dialysis | 7.8% (15/192) | 7.0% (184/2615) | .66 |
| Prior CIED | 0.5% (1/192) | 11.8% (308/2615) | <.001 |
| Condition that precludes the use of TV‐PPM | 14.1% (27/192) | 19.8% (519/2616) | .06 |
| Pacing indication (%) | |||
| Bradyarrhythmia with AF | 94.3% (181/192) | 60.9% (1591/2611) | < 0.001 |
| Sinus node dysfunction | 3.1% (6/192) | 12.5% (327/2611) | |
| AV Block | 1.6% (3/192) | 12.9% (338/2611) | |
| Syncope | 0.0% (0/192) | 11.3% (294/2611) | |
| Other | 1.0% (2/192) | 2.3% (61/2611) | |
| Not reported | 0.0% (0/192) | 0.0% (0/2611) | |
| LVEF b | |||
| Mean ± standard deviation | 56.7 ± 8.0 | 57.1 ± 9.3 | .20 |
| Median | 55.0 | 59.0 | |
| 25th–75th percentile | 50.0–60.0 | 53.0–62.0 | |
| Implant success | 99.5% (191/192) | 99.2% (2596/2616) | 1.00 |
| Follow‐up (months) | |||
| Mean ± standard deviation | 20.4 ± 15.6 | 25.2 ± 15.3 | |
| Median | 19.0 | 24.5 | |
| 25th–75th Percentile | 5.8–30.0 | 14.3–35.5 | |
| Procedure duration (min) | |||
| Mean ± standard deviation | 47.6 ± 30.9 | 32.8 ± 24.2 | <.001 |
| Median | 41.0 | 27.0 | |
| 25th–75th percentile | 27.0–62.0 | 20.0–39.0 | |
| Fluoroscopy duration (min) | |||
| Mean ± standard deviation | 11.7 ± 28.7 | 9.2 ± 15.2 | .001 |
| Median | 8.0 | 6.4 | |
| 25th–75th percentile | 5.0–12.1 | 4.1–10.5 | |
| Anticoagulation strategy (%) | |||
| Not on OAC | 22.6% (43/190) | 34.9% (910/2604) | <.001 |
| Interrupted OAC | 50.0% (95/190) | 38.6% (1005/2604) | |
| Continued OAC | 27.4% (52/190) | 26.5% (689/2604) |
Abbreviations: AF, atrial fibrillation; AVNA, atrioventricular nodal ablation; CAD, coronary artery disease; CHF, congestive heart failure; CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; OAC, oral anticoagulation; TV‐PPM, transvenous pacemaker.
p‐value from Wilcoxon Rank sum test (age, LVEF, procedure duration, fluoroscopy duration), or Fisher's exact test (categorical variables).
LVEF was available for 129 patients with AVNA and 1805 patients without AVNA.
Among the 2808 patients where AVNA status was known, 99.5% of the 192 patients with AVNA were successfully implanted compared with 99.2% of patients without AVNA (p = 1.00). Median procedure time was significantly greater in patients with AVNA compared with those without (41 min, interquartile range [IQR]: 27.0–62 min vs. 27 min, IQR: 20–39 min, p < .001). Similar results were observed for fluoroscopy duration (8.0 min, IQR: 5.0–12.1 min vs. 6.4 min, IQR 4.1–10.5 min, p = .001). The median percentage of RV pacing was 99.7% (IQR 98.6%–99.9%) in the AVNA group as compared with 59.1% (IQR: 10.0%–97.2%) in the non‐AVNA group (p < .001).
3.2. Electrical performance
Pacing capture threshold (PCT) at implant was 0.58 ± 0.35 V at a pulse duration of 0.24 ms among patients with AVNA compared to 0.65 ± 0.49 in patients without AVNA (p = .12). The maximum weekly PCT among AVNA patients increased to an average of 0.89 V with a lower 10th and upper 90th percentile of 0.38 V and 1.60 V, respectively, at 3 weeks postimplant and declined to an average of 0.64 V (10th–90th percentile: 0.38 V–1.12 V) by Week 12 among those patients with electrical data available for analysis (Figure 1). Pacing capture thresholds and impedance were not different between patients with and without AVNA through 24‐months postimplant (Figure 2A,B) where electrical data were available for analysis.
Figure 1.
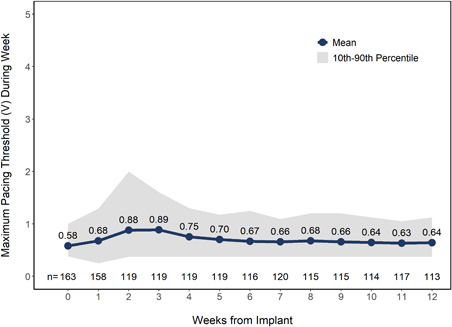
Maximum weekly pacing capture thresholds in patients with concomitant atrioventricular nodal ablation following implant procedure. Mean pacing capture thresholds standardized to a pulse duration of 0.24 ms. Gray shaded area represents 10th–90th percentile intervals. N values represent the number of patients with data available at each timepoint
Figure 2.
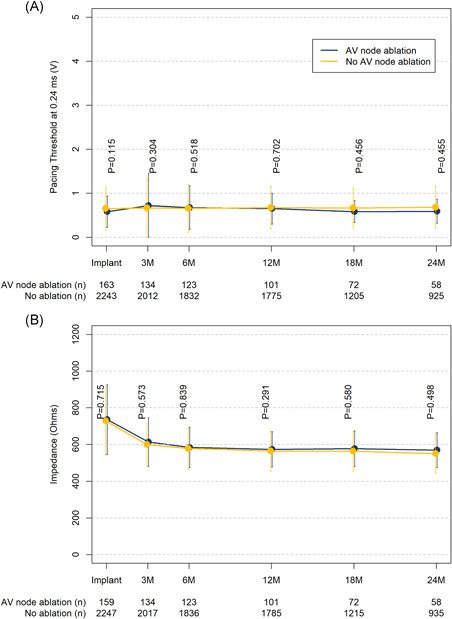
Electrical performance over time by concomitant atrioventricular nodal ablation status. (A) Pacing capture thresholds standardized to a pulse duration of 0.24 ms. (B) Impedance. Error bars represent the standard deviation. n values represent the number of patients with data available at each timepoint
3.3. Safety
Table 2 displays major complications by AVNA status. The majority of major complications (72%) in both groups occurred within 30‐days of implant. The rate of acute major complications in the 192 patients with AVNA was 7.3% compared with 2.0% in the 2616 patients without AVNA (p < .001). The difference in major acute complications was driven by a higher rate of cardiac effusions, thrombosis events, pacing issues and other major complications (which included heart failure, acute myocardial infarction, pacemaker syndrome, etc.) in patients with AVNA compare with those without AVNA (Table 2). The weighted analysis balanced the baseline characteristics used in the propensity model in the subset of 2764 patients with all baseline measurements available for constructing the overlap weights (Table S1). Similar results were observed after adjusting for differences in baseline patient condition (Table S2, 6.8% vs. 1.9%, p = .046).
Table 2.
Major complications by concomitant AVNA status
| No. events (no. patients, %) | Concomitant AVNA (n = 192) | No AVNA (n = 2616) | ||||
|---|---|---|---|---|---|---|
| Adverse event | Acute | Totala | Acute | Totala | p‐value (acute)b | p‐value (total)c |
| Total major complications | 15 (14, 7.3%) | 21 (20, 11.8%) | 61 (53, 2.0%) | 85 (77, 3.3%) | <.001 | <.001 |
| Cardiac effusion/perforation | 3 (3, 1.6%) | 3 (3, 1.6%) | 15 (15, 0.6%) | 16 (16, 0.6%) | .112 | .132 |
| Events at groin puncture site | 1 (1, 0.5%) | 2 (2, 1.1%) | 15 (15, 0.6%) | 16 (16, 0.6%) | .926 | .460 |
| Thrombosis | 2 (2, 1.0%) | 2 (2, 1.1%) | 3 (3, 0.1%) | 3 (3, 0.1%) | .016 | .014 |
| Pacing issuesd | 5 (5, 2.6%) | 5 (5, 3.1%) | 15 (13, 0.5%) | 21 (19, 0.8%) | .002 | .008 |
| Cardiac rhythm disorder | 0 (0, 0.0%) | 0 (0, 0.0%) | 1 (1, < 0.1%) | 3 (3, 0.1%) | .356 | .996 |
| Infection | 1 (1, 0.5%) | 1 (1, 0.6%) | 2 (2, 0.1%) | 4 (4, 0.2%) | .117 | .240 |
| Othere | 3 (3, 1.6%) | 8 (8, 5.3%) | 10 (10, 0.4%) | 22 (22, 1.0%) | .032 | <.001 |
Abbreviations: AVNA, atrioventricular nodal ablation; MI, mycocardial infarction.
Total percentages are estimated from the Cox regression model.
p‐value comparing acute major complications from the logistic regression model.
p‐value for comparing total major complications from Cox regression model at 36‐months postimplant.
Includes device loss of capture, undersensing, device dislodgement, and device embolization.
Others include congestive heart failure, acute MI, pacemaker syndrome, syncope, pulmonary edema, etc.
The risk for major complication through 36‐months was significantly higher in the AVNA group compared with patients without AVNA (Figure 3A, hazard ratio: 3.81, 95% confidence interval [CI]: 2.33–6.23, p < .001) and this difference persisted after adjustment for differences in baseline characteristics (Figure 3B). There were three cardiac effusions in the AVNA group, two cases required pericardiocentesis, and one case required no intervention. Among patients without ANVA, there were 16 cardiac effusions, four required surgical repair of which two resulted in death, nine required pericardiocentesis, and in the remaining three cases, no interventions were taken. Details of the deaths have previously been reported. 6 There were no cases of device dislodgement or embolization in the AVNA group during the follow‐up period (Table S3). However, there were three instances of device micro‐dislodgment and one device embolization in patients without AVNA, of which all four events occurred either during the implant procedure (device embolization) or within the acute period. There were no macrodislodgments.
Figure 3.
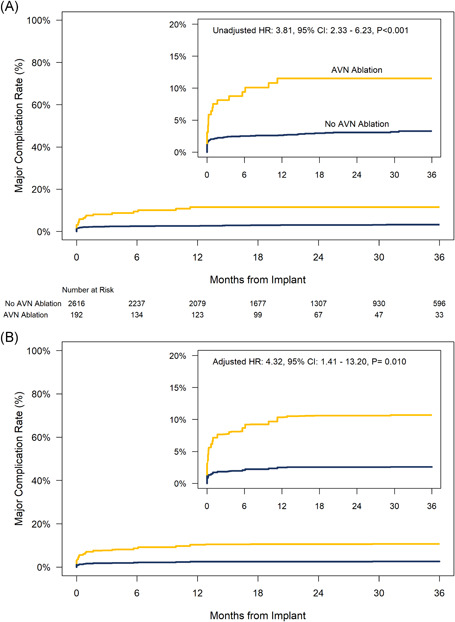
Major complication rates through 36‐months postimplant for patients with and without concomitant atrioventricular nodal ablation. (A) Unadjusted (univariate) comparison. (B) Comparison adjusted for differences in baseline characteristics using overlap weights. HR = hazard ratio from Cox model. CI = confidence interval
There were three intermittent loss of capture events that all occurred within 30 days of implant in patients with AVNA (Table S3). All three resulted in a revision (percutaneous retrieval) of the Micra device, of which two patients received a new Micra device and one patient received a transvenous system. Loss of capture was reported in eight cases in seven patients without AVNA of which one occurred before tether release, five occurred within 30 days of implant and two occurred after 30 days. Six of the eight events required revision of the Micra device.
There were six procedure‐related deaths, of which two occurred in patients with AVNA. One death was caused by metabolic acidosis in a 77‐year‐old female patient with end stage renal disease. The second death was caused by retroperitoneal bleeding in a 92‐year‐old female with low body mass index (19.2 kg/m2), hypertension, and COPD. The remaining four deaths occurred in patients without AVNA. Two resulted from cardiac tamponade, one from RV failure (possible from acute infarct), and one from pulmonary edema in a patient with severe aortic valve disease. All six procedure‐related deaths have been described previously in detail. 6 , 7
3.4. System revisions
There were 10 system revisions for any reason in 9 (4.7%) of the patients with AVNA and 64 system revisions in 62 (2.4%) of the patients without AVNA (Table 3). The risk for system revision for any reason through 36‐months was 2.72 times higher on a univariate basis (95% CI: 1.34–5.51, p = .005) in patients with AVNA compared with those without AVNA and trended higher after adjustment for differences in baseline characteristics (Figure 4A,B). The most common reasons for system revision in both groups were device upgrade or high pacing thresholds (including loss of capture). The most common action taken with the Micra device was to leave the device in place and program it to OOO mode. There were three system revisions for infection in patients without AVNA. In two patients the Micra was prophylactically removed following prosthetic valve endocarditis. The third patient had their Micra implant following removal of an infected cardiac resynchronization therapy (CRT)‐pacemaker. After the infection cleared, their Micra was programmed to OOO and following implant of a new CRT‐pacemaker system. However, their new transvenous system became infected and was removed necessitating resumption of pacing therapy from the Micra device.
Table 3.
System revision by concomitant AVNA status
| System revisions | AVNA (n = 192) | No AVNA (n = 2616) |
|---|---|---|
| Total system revisions | 10 (9, 4.7%) | 64 (62, 2.4%) |
| Reason for system revision | ||
| Device upgrade | 4 (4, 2.1%) | 25 (25, 1.0%) |
| Transvenous single chamber IPG | 0 (0, 0.0%) | 1 (1, < 0.1%) |
| Transvenous dual chamber IPG | 0 (0, 0.0%) | 4 (4, 0.2%) |
| ICD | 0 (0, 0.0%) | 1 (1, < 0.1%) |
| CRT‐pacemaker | 4 (4, 2.1%) | 13 (13, 0.5%) |
| CRT‐ICD | 0 (0, 0.0%) | 6 (6, 0.2%) |
| High threshold | 5 (4, 2.1%) | 19 (18, 0.7%) |
| Pacemaker syndrome | 0 (0, 0.0%) | 5 (5, 0.2%) |
| Battery ERIa | 0 (0, 0.0%) | 3 (3, 0.1%) |
| Cardiac failure | 1 (1, 0.5%) | 2 (2, 0.1%) |
| Infection | 0 (0, 0.0%) | 3 (3, 0.1%) |
| Dislodgement | 0 (0, 0.0%) | 2 (2, 0.1%) |
| Heart transplant | 0 (0, 0.0%) | 1 (1, < 0.1%) |
| Other | 0 (0, 0.0%) | 4 (4, 0.2%) |
| Type of system revision | ||
| Programmed to OOO | 5 (5, 2.6%) | 44 (44, 1.7%) |
| Explanted | 3 (3, 1.6%) | 11 (11, 0.4%) |
| Programmed to backup | 2 (2, 1.0%) | 4 (4, 0.2%) |
| Heart transplant | 0 (0, 0.0%) | 1 (1, < 0.1%) |
| Repositioned | 0 (0, 0.0%) | 1 (1, < 0.1%) |
| Therapy resumed | 0 (0, 0.0%) | 1 (1, < 0.1%) |
| Not reported | 0 (0, 0.0%) | 2 (2, 0.1%) |
Note: Numbers reflect the number of events (number of unique patients, percentage of unique patients).
Abbreviations: AVNA, atrioventricular nodal ablation; CRT, cardiac resynchronization therapy; ERI, elective replacement indicator.
ERI due to elevated pacing thresholds.
Figure 4.
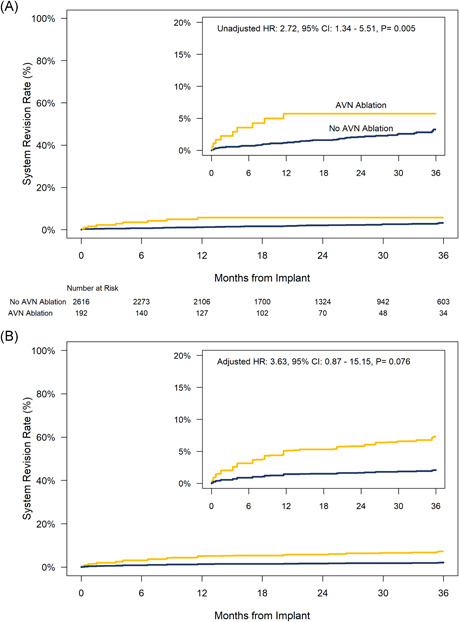
System revision rates for any reason through 36‐months postimplant for patients with and without concomitant atrioventricular nodal ablation. (A) Unadjusted (univariate) comparison. (B) Comparison adjusted for differences in baseline characteristics using overlap weights. HR = hazard ratio from Cox model. CI = confidence interval
4. DISCUSSION
This is the largest study to date examining simultaneous leadless pacemaker implant and AVNA. The results show that simultaneous LP/AVNA appears to be safe and feasible, despite the higher rate of observed complications and device revision as compared with patients undergoing LP implant alone.
The complications observed in the LP/AVNA group appear to be mostly related to the LP implantation and do not involve the simultaneous performance of the AVNA. For example, the rate of pericardial effusion in the simultaneous LP/AVNA group was 1.6% versus 0.6% in the LP group. Similarly, it is unlikely that the higher rate of microdislodgment or changes in pacing capture thresholds over time are due to the AVNA itself, particularly since the mean capture threshold was numerically lower in the LP/AVNA patients.
The increased rate of complications in the AVNA cohort is not entirely unexpected, as those undergoing LP/AVNA were older and had more risk factors known to be associated with complications including female sex, concomitant AF (requiring attendant anticoagulation), heart failure, end stage renal disease, and COPD. 15 Despite adjusting for baseline comorbidities, the overall rate of complications remained higher in the LP/AVNA group. This could be due to either the added complexity in a LP/AVNA procedure or the difference could be due to residual confounding that is not entirely corrected by the propensity weighting techniques.
Simultaneous LP implantation and AVNA via a single femoral venous puncture is a strategy with potential advantages over TV‐PPM and AVNA. LPs are associated with lower dislodgment rates and infection rate as compared to TV‐PPMs. These two complications have devastating consequences in pacemaker dependent patients. It is likely that performing two simultaneous procedures will always carry higher risk than performing one procedure, but one must weigh this against the risk of performing two procedures that requires similar access entry point separately. Patients referred for an ablate and pace approach are typically an elderly population with multiple comorbidities as seen in this study. Despite adjusting for baseline comorbidities, the total complication rates remained greater in the AVNA group. As mentioned previously, it is likely that there are some confounding factors that we did not account for that could explain the higher rate of complications in this group. It is interesting to note that the individual adjusted major complication rates (rates of pericardial effusion, groin complications, and pacing issues) were not different between the two groups (Table S3), though propensity weighting resulted in a lower effective sample size.
There are a few previously published reports regarding the safety and feasibility of this approach. 9 , 10 , 16 Similar to our international cohort, these small reports also suggest that this approach is safe and feasible. For instance, Martinez‐Sande et. al 16 reported on 27 patients who received simultaneous LP and AVNA. Three patients experienced complications (11%) as compared with 5.5% complication rate in their cohort (127 patients) of patients that received LP without a concomitant AVNA. None of these complications were related to the AVNA procedure.
It is also worth noting that the higher rate of system revisions in the LP/AVNA group is not only related to changes in thresholds but also due to heart failure and requirement for upgrade to a CRT system. Patients with AVNA typically have higher rate of RV pacing as compared with patients receiving LP who are not pacemaker dependent. High burden of RV pacing predisposes to heart failure and left ventricular dysfunction. 17 In this study, the median percentage of RV pacing was 99.7% (IQR 98.7%–99.9%) in the AVNA group as compared with 59.1% (IQR: 10.0%–97.2%) in the non‐AVNA group (p < .001). In patients with an expected high percentage of RV pacing, an approach of utilizing physiologic pacing or CRT as a first‐line strategy for pacing may be beneficial, especially in patients with an LVEF ≤ 50%. 18 It is worth noting that the mean LVEF in the AVNA group was 56.7 ± 8.0%, which may explain physicians’ choice of a leadless system rather than CRT or a pacing system that provides physiologic pacing. Although three of the 129 patients in the AVNA group had an LVEF ≤ 40%, none required CRT upgrade or underwent system revision. The higher rate of system revision due to rise in thresholds in the LP/AVNA group may also be partly explained by the lower threshold for revising a pacing system in pacemaker dependent patients.
In this study, no patients in either group died due to loss of capture. However, 2.1% of patients had a system revision for elevated thresholds in the LP/AVNA group during follow‐up. Therefore, it is an imperative recommendation for new implanters or in the case of a challenging implant with suboptimal electrical characteristics 19 to delay the AVNA until the LP is mature and thresholds are stable.
4.1. Limitations
Despite adjusting for baseline measured differences with propensity‐based methods; residual confounding due to unmeasured factors cannot be ruled out in an observational study. Also, the effective sample size of our adjusted analysis was small due to the large differences in patients’ profiles between the two groups. This led to wider confidence intervals for our adjusted comparison. It is also important to note that this study does not have a comparison group of patients undergoing AVNA weeks to months post LP implant. Therefore, we cannot compare a strategy of simultaneous versus staggered LP/AVNA. Similarly, there was not a comparator group of patients undergoing concomitant AVNA with TV‐PPM implant; therefore, it is unknown whether such patients also experience a higher rate of complications. Nevertheless, this is the largest multicenter study evaluation the safety of concomitant LP and AVNA.
5. CONCLUSION
Simultaneous LP and AVNA appears to be a safe approach for treating a subgroup of patients with AF. Thresholds remain stable over time with a low risk of needing a system revision. Patients referred for an ablate and pace have a higher comorbidity burden and performing LP with AVNA concomitantly is associated with a higher complication rate than solely implanting a LP without AVNA.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The Micra Post‐Approval Registry (ClinicalTrials.gov identifier: NCT02536118), Micra Continued Access Study (ClinicalTrials.gov identifier: NCT02488681), and Micra Transcatheter Pacing Study (ClinicalTrials.gov identifier: NCT02004873) are funded by Medtronic, Inc.
El‐Chami MF, Shinn T, Bansal S, et al. Leadless pacemaker implant with concomitant atrioventricular node ablation: Experience with the Micra transcatheter pacemaker. J Cardiovasc Electrophysiol. 2021;32:832–841. 10.1111/jce.14881
Disclosures: Mikhael F. El‐Chami is a Consultant: Medtronic, Boston Scientific, and Biotronik; Timothy Shinn is a Consultant for Biotronik; Sundeep Bansal compensation for services: Abbott, Merit Medical; Jose L. Martinez‐Sande consulting fees: Medtronic; Nicolas Clementy compensation for services: Medtronic; Ralph Augostini research funding and speaking fees: Medtronic; Bipin Ravindran, Venkata Sagi, and Hemanth Ramanna nothing to disclose; Christophe Garweg research funding and speaker/consultancy fees from Medtronic; Paul R. Roberts honoraria from Medtronic and Boston Scientific; Kyoko Soejima speaking fees: Medtronic, Abbott Japan; Kurt Stromberg employee/shareholder: Medtronic; Dedra H. Fagan employee/shareholder: Medtronic; Nicky Zuniga employee/shareholder: Medtronic; Jonathan P. Piccini is supported by R01HL128595 from the National Heart, Lung and Blood Institute. He receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Phillips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Myokardia, Sanofi, and Phillips.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Lee SH, Chen SA, Tai CT, et al. Comparisons of quality of life and cardiac performance after complete atrioventricular junction ablation and atrioventricular junction modification in patients with medically refractory atrial fibrillation. J Am Coll Cardiol. 1998;31(3):637‐644. [DOI] [PubMed] [Google Scholar]
- 2. Kay GN, Ellenbogen KA, Giudici M, et al. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT Investigators. J Interv Card Electrophysiol. 1998;2(2):121‐135. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmayer KS, Scheinman M. Current role of atrioventricular junction (AVJ) ablation. Pacing Clin Electrophysiol. 2013;36(2):257‐265. [DOI] [PubMed] [Google Scholar]
- 4. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235‐2245. [DOI] [PubMed] [Google Scholar]
- 5. Cantillon DJ, Exner DV, Badie N, et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC Clin Electrophysiol. 2017;3(11):1296‐1305. [DOI] [PubMed] [Google Scholar]
- 6. El‐Chami MF, Al‐Samadi F, Clementy N, et al. Updated performance of the micra transcatheter pacemaker in the real‐world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15:1800‐1807. [DOI] [PubMed] [Google Scholar]
- 7. Reynolds DW, Ritter P. A Leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374(26):2604‐2605. [DOI] [PubMed] [Google Scholar]
- 8. Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373(12):1125‐1135. [DOI] [PubMed] [Google Scholar]
- 9. Okabe T, El‐Chami MF, Lloyd MS, et al. Leadless pacemaker implantation and concurrent atrioventricular junction ablation in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2018;41(5):504‐510. [DOI] [PubMed] [Google Scholar]
- 10. Yarlagadda B, Turagam MK, Dar T, et al. Safety and feasibility of leadless pacemaker in patients undergoing atrioventricular node ablation for atrial fibrillation. Heart Rhythm. 2018;15(7):994‐1000. [DOI] [PubMed] [Google Scholar]
- 11. Kusumoto FM, Schoenfeld MH, Barrett C, et al. ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol. 2018:2018. [DOI] [PubMed] [Google Scholar]
- 12. Duray GZ, Ritter P, El‐Chami M, et al. Long‐term performance of a transcatheter pacing system: 12 month results from the Micra Transcatheter Pacing Study. Heart Rhythm. 2017;14:702‐709. [DOI] [PubMed] [Google Scholar]
- 13. Roberts PR, Clementy N, Al Samadi F, et al. A leadless pacemaker in the real‐world setting: the Micra Transcatheter Pacing System Post‐Approval Registry. Heart Rhythm. 2017;14:1375‐1379. [DOI] [PubMed] [Google Scholar]
- 14. Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390‐400. [Google Scholar]
- 15. Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374(6):533‐541. [DOI] [PubMed] [Google Scholar]
- 16. Martínez‐Sande JL, Rodríguez‐Mañero M, García‐Seara J, et al. Acute and long‐term outcomes of simultaneous atrioventricular node ablation and leadless pacemaker implantation. Pacing Clin Electrophysiol. 2018;41(11):1484‐1490. [DOI] [PubMed] [Google Scholar]
- 17. Merchant FM, Mittal S. Pacing induced cardiomyopathy. J Cardiovasc Electrophysiol. 2020;31(1):286‐292. [DOI] [PubMed] [Google Scholar]
- 18. Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368(17):1585‐1593. [DOI] [PubMed] [Google Scholar]
- 19. Tolosana JM, Guasch E, San Antonio R, et al. Very high pacing thresholds during long‐term follow‐up predicted by a combination of implant pacing threshold and impedance in leadless transcatheter pacemakers. J Cardiovasc Electrophysiol. 2020;31(4):868‐874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


