Abstract
Aim
Although eye‐tracking technology expands beyond capturing eye data just for the sole purpose of ensuring participants maintain their gaze at the presented fixation cross, gaze technology remains of less importance in clinical research. Recently, impairments in visual information encoding processes indexed by novel gaze metrics have been frequently reported in patients with schizophrenia. This work undertakes a scoping review of research on saccadic dysfunctions and exploratory eye movement deficits among patients with schizophrenia. It gathers promising pieces of evidence of eye movement abnormalities in attention‐demanding tasks on the schizophrenia spectrum that have mounted in recent years and their outcomes as potential biological markers.
Methods
The protocol was drafted based on PRISMA for scoping review guidelines. Electronic databases were systematically searched to identify articles published between 2010 and 2020 that examined visual processing in patients with schizophrenia and reported eye movement characteristics as potential biomarkers for this mental illness.
Results
The use of modern eye‐tracking instrumentation has been reported by numerous neuroscientific studies to successfully and non‐invasively improve the detection of visual information processing impairments among the screened population at risk of and identified with schizophrenia.
Conclusions
Eye‐tracking technology has the potential to contribute to the process of early intervention and more apparent separation of the diagnostic entities, being put together by the syndrome‐based approach to the diagnosis of schizophrenia. However, context‐processing paradigms should be conducted and reported in equally accessible publications to build comprehensive models.
Keywords: biomarker, eye‐tracking dysfunction, gaze metrics parameters, schizophrenia, visual information processing
‘Nam ut imago est animi voltus sic indices oculi (…)’
Cicero, Orator
Uncovering the evidence: The eye and the brain
Given that the retina develops from the same tissue as the brain, it is the only part of the central nervous system that can be seen with the naked eye in its natural state in living organisms. Therefore, it should not be a surprise that the ability to mirror the brain's integrity of sensory function and its structure has crowned the eyes with the sobriquet of windows to the mind. Although philosophers defined the eyes as the interpreters of one's mind long before neuroscientists, more recently, it has been scientifically confirmed (through vastly improving retinal imagining techniques) that several well‐defined neurodegenerative conditions, as well as psychiatric disorders, find their manifestations in the detailed structures of the human eye. 1 , 2 Patients with schizophrenia (SZ) possess an anomalous eye structure; thus, specific retinal findings, such as thickness mapping of retinal layers, 3 reduced retinal axon layer, macula thickness and volume measurements, in patients with schizophrenia 4 can serve as structural biomarkers of neural pathology and disease progression. 5 , 6 , 7 , 8
Undoubtedly, the eye's microarchitectural information can be used as a valuable structural model to study psychiatric disorders to improve the non‐invasive identification of high‐risk individuals. 9 , 10 , 11 Therefore, structural biomarkers remain an increasingly popular topic in clinical applications. However, severe limitations hamper the everyday use of structural targets as far‐reaching and cost‐effective markers for SZ. Meanwhile, a misperception that SZ research findings related to vision are considered minor casts a shadow over gaze metrics as potential neurophysiological biomarkers interpreting the highly heterogeneous brain in patients with SZ. 12 , 13 , 14 , 15 , 16 , 17
Eye‐tracking devices are characterized by relatively cost‐effective assessments and extraordinary flexible data collection locations that can occur in any comfortable environment, and are not restricted to the surrounding of a hospital or psychiatry clinic. Therefore, an eye‐tracking tool introduces a significant promise of accessibility. Also, in the light of a noticeable shift in focus of research in the SZ spectrum from classical symptomatology to context‐processing impairments 2 , 18 , 19 , 20 , 21 , 22 , 23 and cognitive remediation for treating cognitive dysfunction, 24 , 25 , 26 , 27 , 28 the study of cognitive impairments has become a high‐priority area of interest. Finally, following the line of evidence that vision in SZ does matter, 29 an intriguing trend to study the visual processing structure by re‐examining paradigms with the inclusion of modern eye‐tracking devices started to emerge. 30
Eye‐tracking datasets obtained from the unconscious movements of their beholders give powerful insights regarding the information processing patterns, and provide accurate instrumentation to measure cognition (and its deficits) at the same time indicating and predicting disease processes. 1 Although it is possible to attend covertly to a spatial location, 31 , 32 it is more effective to fixate the eyes on what one chooses to attend to. Therefore, the fundamental point of eye behavior reflecting one's attention (and consequently thoughts) 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 is attractive as a measurable indicator (biomarker) of SZ, detected through an analysis of gaze metrics. 42 , 43 , 44
To systematically present recent contributions of eye‐tracking‐based methodology in research on SZ, a scoping review has been conducted. A research question, which aimed to draw both clinical and psychological scientific communities’ attention to investigate information processing patterns, was formulated. What is known about eye movement impairments in patients with SZ, and what experimental paradigms disclose their presence? Furthermore, saccadic dysfunctions and exploratory eye movements were highlighted as potential biomarkers for SZ. Finally, we identified an inefficient translation of dynamic experimental paradigms expansion (in vision science research) into clinical research, creating a widening gap between scientific evidence and potential clinical application.
Vision science as a new practice
Eye movements reflect the human thought processes; so the observer's thought may be followed to some extent from records of eye movements. It is easy to determine from these records which elements attract the observer's eye (and, consequently, his thought), in what order, and how often. 45 (p. 190)
In 1879, by merely observing the eyes through a system of mirrors, Louis Javal noted that while reading, the eyes do not sweep smoothly across the page, but rather, make a series of brief stops on individual words. These brief stops became known as fixations, while the rapid eye movements between fixations became known as saccades. Later on, Guy T. Buswell (How people look at pictures) 46 and Alfred Lukyanovich Yarbus (Eye Movements and Vision) 45 pioneered the study of cognitive influences acting on visual exploration, researching the interplay between fixation and interest. Deliberately, we mention these two now‐classic, however, surprisingly excluded from clinical research monographs, to inspire scientists to investigate the influence of task' instruction on visual exploration patterns among clinical populations, a topic yet uncovered by neurocognitive research.
Modern eye‐tracking technology is recognized for its sophisticated mathematical image analysis, with its ability to detect viewers’ pupils and calculate the corneal reflection patterns. By probing one's information processing, which requires a synchronized activity of lower and higher‐order brain structures, gaze can mirror the brain's integrity. It has been reported that patients with psychiatric disorders show significant attention deficits in comparison with healthy controls (HC). 2 , 47 , 48 , 49 , 50 , 51 Eye‐tracking studies may facilitate understanding of the neurobiology of populations with mental disorders, and evaluate mechanisms involved in attention processes. 52 , 53 , 54 , 55 , 56 For example, reflexive saccades are considered to be type of cognitive parameter that evaluates attention. Furthermore, to unveil insights regarding the complexity of one's eye movement behavior (to process given information), researchers start to record exploratory scan paths during free‐viewing exploration tasks. 53 , 57 , 58 , 59 , 60 , 61 Clarifying aspects of brain circuitry by illustrating the complex interplay between vision and cognition, 62 eye movement measurements are actively performed in interdisciplinary laboratories that integrate contributions from psychology, neuroscience, economy, philosophy and computer science. A vast number of efficient and well‐grounded behavioral experimental paradigms have been generated regarding sincerity, 63 magic‐tricks, 64 , 65 judgmental tasks, 66 , 67 , 68 , 69 , 70 , 71 moral dilemma, 72 reading, 73 , 74 , 75 , 76 visual search, 77 , 78 , 79 gambling, 80 daily‐life activities, 81 , 82 , 83 , 84 , 85 , 86 problem‐solving, 39 , 87 , 88 , 89 learning 90 and discrete choice in clinical decision‐making. 91 Vision science becomes actively adopted in neuromarketing, aiming to reveal valuable information about consumers’ decisions, as well as answer the question of how to hack the consumer's internal ‘purchase button’. 92 , 93 , 94 , 95 , 96
Despite such immense focus on the gaze within cognitive neuroscience and an active import of eye‐tracking paradigms to other fields, vision science has not resulted in a similar focus in mental illness research. 97 , 98 Findings of significant importance show that gaze metrics parameters are abnormal in psychiatric diseases, such as depression, bipolar disorder and SZ. Consequently, we state that cognitively informative behavior paradigms might have useful implications for clinical researchers. Namely, to clarify aspects of disturbed brain circuity and visual impairments that manifest among patients with SZ. Of great importance is the thoroughly reported fact that schizophrenia/schizoaffective disorder (SZ/SA) patients are usually accompanied by a broad spectrum of oculomotor alterations. 99 Previously, Chapman et al. investigated the subjective reports of visual perception in early‐stage SZ individuals, where he reported that patients showed perceptual disturbances, which included impaired visual experiences while showing no symptoms of depressive mood. 100 This has been the most frequent initial behavioral symptom of SZ appearing over a period of 4 years before the first admission, being later followed by negative symptoms and functional impairment. 101 Another retrospective survey revealed that most participants experienced distortions (in contrast, shape, color and motion) before being officially diagnosed. 102 In modern research, several independent research groups unanimously reported that patients with SZ have poor ability to maintain their attention, which in turn led to their overall poor perception. 48 , 103 , 104
Gaze metrics are successfully implemented into paradigms that exercise patients’ abnormalities in motion processing, 105 contrast sensitivity, 106 , 107 surround suppression, 108 , 109 perceptual organization, 98 facial emotion recognition 110 and color processing. 111 , 112 Deeper knowledge regarding visual information processing impairments among patients starts to unveil potential neurophysiological markers and facilitate early diagnosis of mental disorders, such as SZ, characterized by misinterpretation in thinking processes, especially delusions (beliefs not based on reality) and hallucinations (inability to differentiate between what is real and what is not). 42 , 53 , 57 , 113 , 114 Recent pieces of evidence highlight patients’ exploratory eye movement measurements to be potential trait‐linked markers of vulnerability to the SZ (single eye‐movement test, 115 , 116 multiple eye‐movement tests 42 , 57 , 117 , 118 ).
Objectives
An overall misperception that findings related to gaze metrics are considered minor in SZ research has been previously emphasized. 119 Consequently, to systematically map the findings, a scoping review was conducted that referred to studies showing the potential value of eye‐tracking technology as a way of analyzing cognitive function – following the underlying hypothesis that fixation tends to follow attention. This review is a preliminary attempt to draw readers’ attention to eye‐tracking technology advantages in clinical studies (e.g. experimental paradigms regarding SZ patients). Furthermore, state‐of‐the‐art articles related to visual processing in patients with SZ, reporting abnormal gaze metrics, were identified, and clinical concepts that implement eye‐tracking technology in visual exploration experimental paradigms were discussed. The next decade of SZ research is likely to witness eye movement‐related measurements (gaze metrics) being actively used as potential biological markers. The necessity to include tasks that incorporate cognitive features to currently exercised experiments has also been highlighted. Such interdisciplinary combinations could be beneficial to patients by finding novel ways for early diagnosis of SZ. Finally, the authors undertook the present study to emphasize the importance of a multifaceted medical research team that includes cognitive and computational neuroscience specialists.
Methods
Our protocol was drafted using PRISMA guidelines, revised by the authors and laboratory members to solicit additional feedback. 120 Articles published between 2010 and 2020, reporting attention deficits or abnormal exploratory patterns among patients with SZ, were identified by Mendeley and PubMed searches. To narrow the result of over 1100 journal articles, search terms ‘eye‐tracking patterns’, ‘gaze metrics’ and ‘information processing’ were added (in total 75). Journal articles that reported eye movement measurement tests as potential biomarkers for psychotic disorders were localized and added to the scoping review. 42 , 57 , 60 , 116 , 117 , 118 , 121 , 122 Reference lists of all in‐scope‐articles have been additionally screened for relevant publications. To interlock the current trend of eye tracking with SZ research findings, non‐clinical open access articles were included in this scoping review. The research synthesis starts with an introduction of gaze metrics used to study cognition while disclosing cognitive disturbances in psychotic populations. Then, saccadic and scan path dysfunctions are highlighted as potential biological markers of symptoms of SZ. Finally, we call attention to eye movement tests having considerable power to discriminate SZ cases from HC.
(Eye‐)tracking the cognition
Gaze metrics derived from the eye position data are commonly used to study cognition. For example, fixation duration gives insights regarding the looking time at an object, representing the relative engagement and the time dedicated to processing binding features that belong to an object (information). Fixation duration has been used to gain insights into cognitive control capacity; for example, what we remember, 123 , 124 how we perform mental computations, 125 read, 126 solve problems, 89 , 127 , 128 , 129 , 130 , 131 , 132 , 133 compare specific stimuli and make decisions based on integrated pieces of information. 134 Eye‐tracking technology also provides knowledge about regions of interest, and therefore answers essential questions of when and how information is being captured and processed during vision‐oriented tests. 92 , 135 , 136 , 137 , 138 , 139 , 140 Of great interest is the time spent on an image; this gaze metric has been widely covered in the scientific literature and is associated with the visual expression of preference. 141 , 142 , 143 , 144
In the context of SZ, cognitive factors (increased attention, reflexive saccades suppression, intrusive saccades presence) are studied in experimental tasks; that is, gaze tracking of a moving target (horizontal pursuit paradigm) and gaze maintenance on a fixation target presented in the center of the monitor (fixation stability test). In contrast, some research questions require analysis of scan paths, rather than quantification of fixation and saccade measures. 60 , 145 , 146 , 147 Thus, the interest in exercising patients’ visual engagement driven by an individual's preferences, beliefs and intuition started to emerge. 148 , 149 , 150 Although free‐viewing tests are currently applied to objectively illustrate patient's visual exploration paths, a minor amount of clinical paradigms implement higher‐order cognitive components that relate to abstract thinking, perspective‐taking (more commonly referred to as the theory of mind) and decision‐making. To investigate the interplay between cognition and vision, more complex information processing paradigms that reveal spontaneous, task‐oriented gaze patterns are required. 33 , 112 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158
Furthermore, throughout the years, human eye behavior has been exercised with a vast amount of different types of stimuli (i.e. geometrical figures, paintings, moral picture database, computerized faces, photographs of human real faces, naturalistic food images) applied in various context‐processing paradigms. 32 , 35 , 41 In the context of SZ, patients have been reported to avoid looking at salient regions of the face. 159 Such a restricted visual scan path is associated with lower emotion recognition accuracy. 159 However, these findings relate to ‘bottom‐up’ sensory processing, where ‘bottom‐up’ atypicalities may result from atypical eye‐gaze patterns or deficits of attentional modulation of neural activity. Further studies are required to investigate sensory processing, in the context of visual scan paths to elucidate whether an atypical function of sensory regions can genuinely be considered a ‘bottom‐up’ deficit in SZ. In particular, the use of visual illusions stimuli may demonstrate how the brain uses highly adaptive mechanisms that allow top‐down perceptual mechanisms. In SZ, this top‐down modulation of social perception has been reported as abnormal (patients demonstrate abnormally strong influences of prior expectations). 159 The opposite appeared to be true among autism patients, characterized by abnormally weak top‐down modulation. 159 , 160 The addition of high‐ and low‐level illusion stimuli to future cross‐diagnostic paradigms may contribute to the current knowledge of what symptomologies in SZ have in common, and clarify the understanding of visual distortion symptoms rather than hallucinations.
Cognitive disturbances as crucial features of schizophrenia
For the reason that almost every area of the brain plays a role related to the ocular motor control system, gaze metrics have been applied to fields of exploration, such as social behavior, design, marketing and consumer sciences, becoming essential for neuroscientists who look at decision‐making through the lens of information processing. 143 , 161 , 162 Although suited to today's challenges, eye‐tracking technology was cut out from clinical studies, mostly by event‐related potential and oscillatory measures, as a way to discover the mechanisms that underlie patients’ behavior, as well as a tool supporting mental illnesses diagnosis. Furthermore, the dynamic expansion of cognitively informative paradigms in vision science is inefficiently translated into psychiatry, widening the gap between scientific evidence and potential application. At the same time, cognitive deficits are repeatedly named to be key features of SZ. It has been scientifically reported that a large proportion of individuals at clinical high risk has poor ability to maintain their attention. 163 , 164 , 165 In recent studies, valuable shreds of evidence regarding cognitive deficits have been confirmed to be highly prevalent in SZ, 166 , 167 , 168 and present even before the onset of the disease. 169 It has been reported that by the time of the onset of psychosis, where patients experience their first episode of psychosis, attentional impairments are typically present and of moderate severity. 170 Furthermore, of high importance are studies that report longitudinal persistence of cognitive deficits, thus, suggesting that information processing abnormalities are probably related to the core of the SZ. 171 , 172 , 173 , 174 In comparison with bipolar disorder or those with drug‐induced psychosis, it has been reported that after the acute psychosis abates, information‐processing deficits persist selectively in SZ patients, 175 dramatically impoverishing the patient's real‐world functioning.
Notably, most SZ patients and high‐risk states for psychosis individuals demonstrate at least some cognitive impairments, which remain relatively stable within the same patient over time. Therefore, cognitive deficits seem to be unique due to their general consistency in severity and topography across the individual's fluctuating clinical status. 12 , 24 , 116 , 176
The relatively inexpensive, safe and far‐reaching eye movement technology can mirror cognitive deficits in psychotic patients. Therefore, it is necessary to undertake detailed investigations of visual information processing patterns among psychiatric disorder patients, especially those with SZ spectrum disorder. 29 , 177 , 178 Recent essential signs of progress, such as the use of integrated eye movement measurements to distinguish patients with SZ from HC, support the statement that the interpretations of one's attention in the form of eye movement measurements should be actively studied in SZ research. 1 , 42 , 47 , 54 , 62 , 98 , 179 , 180 , 181 , 182 Finally, studies of vision in SZ have an enormous potential to identify sensitive probes of neural functioning that can be used as neurophysiological biomarkers.
The next part of our scoping review attempts to describe abnormalities in eye movements and visual information processing among patients with SZ, providing a disorder‐specific marker value. Furthermore, currently implemented clinical viewing tests (i.e. free viewing, horizontal pursuit, fixation stability) distinguishing patients with SZ from the HC have been outlined. Finally, we underlined the necessity to incorporate cognitive features to generally exercised experimental paradigms, as extracting task‐relevant information from an external input and acting accordingly to internal preferences and/or given experimental tasks requires a balance between receptor structures (in order to, e.g., visually scan the environment) and the central circuit (to process and interpret the incoming information). 183 Thus, to understand the real‐life interplay between cognition and eye behavior, clinical researchers may consider introducing ecologically valid situations into experimental paradigms, monitoring the gaze during monitor display and social communication mode. Gained knowledge of how information is being extracted and further manipulated may serve as a background for future comparisons across various psychiatric disorders.
Clinical concepts that implement eye‐tracking technology
In 1903, the first study of eye movements in SZ (dementia praecox patients at that time) had been published, in which Kraepelin reported an incomplete perception of very briefly exposed stimuli. In 1908, psychiatrist Allen Diefendorf and experimental psychologist Raymond Dodge collaborated to study ocular motor function in psychiatric patients. 184 To capitalize on over‐learned visual behavior, they chose to study smooth pursuit and reflexive saccades that avoid any confounding effects of tasks that could be ‘too complicated’ or have ‘unusual demands’ for patients (hence, any found deficits would suggest disease‐related dysfunction in a potentially informative neural system). Diefendorf and Dodge's landmark study of eye movements in psychiatric patients laid the foundation for further investigations. However, with Bleuler's report of being unable to replicate Kraepelin's findings (in Dementia Praecox or the Group of Schizophrenias 1911), research on visual processing in SZ diminished. It peaked during the ‘cognitive revolution’ (in the 1950s and 1960s), when the number of cognition‐based experiments increased and the interest in oculomotor disturbances associated with psychoses re‐emerged. 47 , 180 , 181 , 185 , 186
The independent rediscovery by Holzman et al. revealed an eye‐tracking dysfunction (or smooth pursuit eye movement impairment) not only in patients with SZ, but also in almost half of their clinically unaffected first‐degree biological relatives. 180 This research team made a great contribution to the field by: (i) replicating and extending previous findings of Diefendorf and Dodge; and (ii) directing the focus of SZ research primarily on motion perception tasks. Afterwards, a vast number of studies reported biological motion sensitivity delineating patients with SZ from those with bipolar disorder (for a comprehensive review, refer to Okruszek et al. 187 ). Until now, albeit other factors that have been scientifically reported to be abnormal in patients with SZ, eye‐tracking dysfunction remains above all as a trait abnormality limited to the risk for SZ. 15 , 105 , 116 , 188 , 189 , 190 , 191 , 192
The smooth pursuit eye‐movement serves to keep swinging in a harmonic motion object foveated in the field of one's vision. 193 Although the smooth pursuit task provides information about the patient's voluntary behavior, in the sense that the observer can decide whether or not to track a moving stimulus, it basically relies on visual cues indicating motion of the visual field. Hence, aiming to investigate the sensory system coordinating movement (prioritized information search) with balance (stabilizing the eyes on a stationary figure reflecting cognitive processes), researchers start to record eye movements during free‐viewing visual exploration tasks and unfold key findings (i.e. abnormal gaze behavior) in SZ patients. Finally, more than 100 years after Kraepelin's work, evidence of saccadic and scanning patterns dysfunctions among SZ patients start to be highlighted in scientific publications. Here, we aim to outline these abnormalities as potential biological markers of symptoms of SZ, as well as highlight eye movement tests discriminating SZ cases from HC.
Saccadic dysfunctions among patients with schizophrenia
Over the past three decades, the majority of studies among psychiatric patient groups focused on saccadic performance. Much of the impetus for such focus came from the fact that saccades provide accessible means of investigating psychomotor functioning, higher‐order cognitive processes and their underlying neural mechanisms, remaining non‐invasive at the same time. Furthermore, saccadic eye movements can be measured reliably and precisely. As a result, a considerable body of knowledge regarding the neurophysiology of the saccadic eye movement subsystem has been shaped, suggesting that saccadic eye movements in psychiatric groups provide a ‘window into the brain’ of affected individuals.
Within 30 years after Holzman's studies, the crucial finding of eye‐tracking dysfunction in SZ has been consistently reported in an extensive number of replications, 180 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 where frequent small saccades were pointed out to be the distractors of eye behavior. Oculomotor control in psychiatric populations has been studied with a range of saccade paradigms, such as prosaccades (saccades to target sequences), antisaccades (saccades away from targets) and saccades to remembered targets, demanding an increase of attention. 48 , 55 , 202 , 203 , 204 , 205 Orienting toward a stimulus is reflexive, and moving one's eyes to the opposite location requires inhibitory control and maintenance of the task's instruction. Poor inhibition has been linked with low impulse control, agitation, excitement and hostility, as well as impairment in the dorsolateral prefrontal cortex. 206 , 207 Thus, the antisaccade task (used to explore one's inhibitory control) generates the most frequently observed volitional saccade abnormality among SZ patients. 208 Combined with an increased rate of errors reflecting impairments in the suppression of reflexive saccades, antisaccades represent one of the most replicable findings in SZ research, 209 drawing a line between HC and SZ.
Of course, regarding the literature on gaze metrics in psychiatric research, it has been strongly underlined that a single task may not serve as a reliable diagnostic tool. A combination of gaze metrics obtained by multiple tasks, however, may increase the classification accuracy to distinguish patients from HC, as well as characterize particular clinical dimensions of SZ. 42 , 117
A gripping saccade‐oriented study, recently reported by Obyedkov et al., included patients divided into three subgroups. 54 First with predominantly negative symptoms, second with predominantly positive symptoms and third with predominantly disorganization symptoms. Horizontal eye movements, peak velocity, latency and accuracy were recorded in all saccade‐oriented tasks (i.e. prosaccade, antisaccade and predictive saccade tasks). Additionally, in the antisaccade task, the error rates were measured. In agreement with previous reports, all SZ patients performed worse than HC across all tasks. 210 , 211 The results of Obyedkov et al. revealed that all three groups of SZ patients made a higher rate of errors (in the antisaccade task) than HC; which reflects a failure of response suppression. Notably, patients with disorganization symptoms made more errors in the antisaccade task than the two other subgroups, which in turn confirmed that disorganization symptoms are associated with (i) attentional dysfunction and (ii) failure to suppress inappropriate responses. 209 Furthermore, patients with negative symptoms were characterized with different oculomotor parameters than in other subgroups. This result has found consistency with the available literature regarding motor, cognitive and neuropathological differences in patients with prominent negative symptoms when compared with patients without negative symptoms (for involvement of the prefrontal cortex in pathological processes in patients with predominantly negative symptoms that may result in the abnormalities of saccadic eye movements see Obyedkov et al. 54 ). Furthermore, latencies compared with accuracies were reported to be more closely associated with negative symptoms of SZ. Notably, prolonged latencies among patients with positive symptoms appeared in both predictive and reflexive tasks, which in turn provided valuable feedback that latencies might serve as markers of negative symptoms of SZ.
The provided reports have raised the possibility that abnormal saccadic eye movements might be an alternative manifestation of SZ. 54 , 197 , 212 Furthermore, the necessity to study clinically unaffected relatives of SZ patients has been emphasized, as an elevated rate of eye‐tracking dysfunctions in clinically unaffected relatives and clinically discordant co‐twins has been frequently reported. 48 , 54 To date, only a small number of studies have investigated performance on saccadic tasks in high‐risk clinical populations. It is essential to mention that the group of clinically unaffected relatives has been reported to differ significantly from HC. In particular, the performance on the antisaccade task among high‐risk clinical participants has been reported to be close to that of SZ patients. Therefore, saccadic measures may have the potential to become biomarkers in translational medicine of psychosis. 54
Abnormal exploratory eye movements among patients with SZ
By recording gaze parameters, such as eye fixations (their number and sequence), scanning path (total and mean length) and responsive search score (RSS), eye movement patterns in patients with SZ are reported to be different from those observed among mood disorder patients and HC ( 115 , 116 reported by Morita et al. 42 ). Previously, pioneering exploratory eye movement experiments, conducted with HC, have shown that observers’ fixations reflect their cognitive processes. 45 , 145 , 213 Following the knowledge that the choice of one's visual fixation is influenced by the aspects of the target, as well as peripheral characteristics of the stimulus, a significant number of studies inspected SZ patients while freely viewing stationery ‘S’ shaped figures (happening to reflect visuocognitive dysfunction). 113 , 121 , 193 , 214 , 215 , 216 , 217
By showing a horizontal ‘S’ shaped figure to chronic SZ patients and their parents, and recording their exploratory eye‐movements, Moriya et al. found that SZ patients made fewer movements in comparison with HC. 218 Also, the range of eye movements among SZ patients was narrower. Interestingly, their parents’ results were reported to lie mid‐way between SZ patients and HC. Moriya et al. concluded that exploratory eye movement dysfunction reflects the trait of Sz. 216 In 1990, Kojima et al. introduced participants to a figure, which was slightly different from the initially shown ‘S’ shaped target. 214 The research team recorded exploratory eye movements (EEMs) of the original and slightly changed targets, and found that SZ patients (chronic, acute and those in remission) made fewer eye movements in response to the verbal stimuli in the comparison test (‘Are there any other differences?’). Researchers stated that the RSS represents the visual behavior that anticipates and confirms the difference in both ‘S’ shaped figures. Furthermore, it has been reported that RSS correlates to symptoms associated with SZ (blunted affect, emotional withdrawal and avolition/apathy). 115 , 214 In 1998, Matsushima et al. reported RSS and the number of eye fixations (NEFs) to differentiate between patients with and without SZ, with a sensitivity of 76.7% and specificity of 81.4%. 193 The following formula was used: D = 10.265 – (0.065 × NEF + 0.871 × RSS), whereby substituting RSS and NEF values for each participant, a positive result for D indicated SZ. The validity of the mentioned discriminant function was confirmed in 2001, when exploratory eye movements made on horizontal ‘S’ shaped figures, and a slightly different ‘S’ shaped figure displayed alternately, were recorded in seven World Health Organization collaborative centers. 121 The RSS was confirmed by Kojima et al. in 2001 as an exploratory eye movement parameter, which detects SZ irrespective of culture and race (i.e. patients with SZ were discriminated from depressed patients and HC with a sensitivity of 89.0% and specificity of 86.7%). 121
In 2009, Suzuki et al. performed a discriminant analysis between SZ and non‐SZ participants, using only EEM data. Although all parameters (i.e. NEFs, total eye scanning length [TESL], mean eye scanning length and RSS) were reported to differ between SZ and non‐SZ participants significantly, the authors performed a discriminant analysis using two parameters, namely: RSS and TESL. 217 Utilizing the following discriminant formula: D = 4.100–(0.001 × TESL + 0.332 × RSS), 184 of the 251 clinically diagnosed SZ patients were identified as true positives (with a sensitivity of 73.3%). Whereas 308 of the 389 clinically diagnosed non‐SZ participants (250 HC, 111 mood disorder patients and 28 patients with neurotic disorder) were identified as true negatives (with a specificity of 79.2%). 217 Hence, Suzuki et al. considered EEM parameters as being useful for clinical diagnosis of SZ. 217 However, as 184 of the 251 clinically diagnosed SZ patients were identified as having SZ, the remaining 67 SZ patients were wrongly discriminated as being non‐SZ. Therefore, in a follow‐up study in 2012, Suzuki et al. compared demographic and symptomatic characteristics of identified SZ patients with SZ patients identified as not having SZ. 113 Suzuki et al. concluded that EEM parameters might potentially detect SZ patients with severe symptoms related to excitement/hostility, negative symptoms and disorganization. However, to provide stronger evidence for an exploratory eye movement test simplifying the heterogeneity of SZ patients, further experiments (involving more participants) need to be performed. 113
The listed investigations showed that exploratory eye movement dysfunction appears to be specific to SZ. 115 , 121 , 193 , 217 , 219 , 220 , 221 Of course, as SZ is considered to be a cluster of several biologically different conditions, the clinical community cannot expect to cover all aspects by a single marker, 193 following an important note made by Matsushima et al., ‘(…) exploratory eye movements alone cannot be used to pick up all schizophrenics’. (p. 294). 193 However, investigating the correlation between exploratory eye movements (especially RSS) and structural characteristics of the brain (e.g. gray matter cytoarchitectural characteristics) may contribute to the classification accuracy in distinguishing SZ patients from HC. Also, such an interdisciplinary approach may help to characterize particular clinical dimensions of SZ. 222
As RSS is an integrated measure of the ability for (i) discrimination, (ii) sustained and selective attention, (iii) perception, and (iv) working memory, any impairment related to SZ would reflect by significantly reducing this particular score. At the same time, the available literature suggests that abnormality in brain structure is one of the most critical pathological substrates that underlie SZ. In 2005, Tsunoda et al., combined these two facts and conducted a pioneering study, exploring the relationship between EEM and the brain's morphology. 223 The findings concluded that among schizophrenia spectrum patients, RSS is significantly correlated with gray matter density in the right frontal eye field and right inferior frontal region. 223
Following the study of Tsunoda et al., Qiu et al. reported that patients with SZ showed lower RSS (in comparison with HC). 221 Furthermore, their crucial finding showed that SZ patients’ decreased RSS is significantly associated with gray matter density reduction (in the occipito‐temporal‐frontal circuitry, involved in processing visual information and the control of eye movements). These findings underline that lower gray matter density is related to the EEM disturbances among SZ patients. Therefore, the impaired RSS can be used as a biomarker (phenotype and vulnerability) for SZ. 219 , 221 In 2018, Qiu et al. reported that hallucination severity among SZ patients was significantly negatively correlated with both RSS and gray matter volume. 224 Importantly, in HC, no significant association between RSS and the gray matter volume of any brain regions could be shown. 224 Investigating the correlations between RSS of EEM, hallucination severity and gray matter volume supported the hypothesis of Qiu et al. regarding the RSS being not only a potential biomarker of SZ, but also predicting hallucination severity. 221 , 224
Overall, brain volume alterations, such as gray and white matter volume loss, enlarged ventricles, and change in the volume of the anterior cingulate cortex, insula and thalamus, have been identified in SZ patients. 221 , 224 , 225 , 226 Notably, cognitive impairments that determine the quality of life and social functioning among patients with SZ have been reported to be related to brain structures. 222 , 226 , 227 , 228 , 229 , 230 However, of great importance is the fact that brain structure shows maturational changes; 231 for example, gray matter cortical thickness decreases as a function of age. Age‐related changes are observed for white matter, with an age‐related increase in fractional anisotropy due to the myelination of white matter. In conclusion, brain imaging findings report an effect of brain maturation on diagnostic markers. 231 The strong correlation between age and brain measures may limit the ability to detect distinct structure–function associations. In future studies, large samples with a small age range should be of interest (i.e. reducing the influence of maturation by restricting age), providing better structure–function estimates. 231
Based on these findings, several independent research groups underlined the necessity to incorporate vision research into brain imaging studies; to investigate the relationship between eye movements, and structural and functional brain abnormalities in cognitive subgroups of SZ. 225 , 226 , 227 , 232 , 233 Furthermore, to show eye movement abnormalities among SZ patients and their relationship with patients’ social/intellectual functioning, 182 , 234 researchers actively studied the association between eye movement abnormalities and (i) work hours (obtained through the Social Activity Assessment), 235 and (ii) the Wechsler Adult Intelligence Scale. 44 , 182 , 230 However, further research questions regarding the relevance between exploratory eye movements and everyday life functioning (e.g. Utena's Brief Objective Measures, developed to assess psychophysiological functions proximal to real‐world functioning among individuals with psychiatric disorders, including SZ 236 ) need to be studied in longitudinal investigations with large samples of a small age range (reducing the influence of maturational changes). 44 , 231
Scan paths can be a valuable addition to eye movement trait markers, drawing a line between SZ patients and HC. Gazing at only one section of presented stimulus, patients with SZ were reported to tend to shift their fixation less frequently than HC, who view pictures widely. 112 , 237 Multiple research teams, who used free‐viewing tasks and re‐examined eye movement patterns, confirmed SZ patients’ scanning patterns to be abnormal, and their regions of interest to be atypical. 57 , 113 , 117 , 121 , 217 , 238 The free‐viewing experimental construct is considered beneficial, as it provides information about the participant's visual scanning behavior, in a sense that the observer may freely redirect the point of fixation according to subjective preferences, goals and beliefs (facts, opinions, uncertainties). Hence, performing free‐viewing paradigms may be useful to investigate visual scanning patterns and understand the patient's actions in a real‐life functioning context. 53 , 112 , 237
Following well‐established reports of face‐scanning patterns among HC (for a description of cyclic fixation behavior while viewing pictures of faces refer to Yarbus 45 ), some clinical studies have shown that SZ patients do not pay attention to remarkable facial characteristics while viewing pictures of recognizable and not recognizable faces. 239 , 240 , 241 , 242 , 243 , 244 , 245 To summarize, patients with SZ tend to have significantly fewer fixation points in comparison with HC. In addition, unlike HC, they show inattention of salient facial features (i.e. eyes, nose and mouth). 159 These results suggest that atypical visual scanning might impair complex object cognition, such as face perception.
Therefore, another example are studies that focus on the SZ patients’ scanning behavior during facial recognition and facial affect recognition tasks. In 1997, Streit et al. reported that patients with SZ show atypical scan paths during the facial affect recognition test. 239 In two more recent studies, where the relationship between facial affect recognition and scan path features in patients with SZ has been examined, Loughland et al. observed patients with SZ showing restricted (characterized with fewer fixations) and abnormal (inattention to significant silent features) scanning styles. 240 , 241 Furthermore, both studies provided another valuable insight; namely, that first‐degree relatives of patients with SZ show restricted exploratory patterns, which again pointed out an earlier conviction that examining not only patients with SZ, but also their relatives, is of great necessity.
To break the pattern of face recognition investigation, 242 , 243 , 244 , 245 Sprenger et al. 60 conducted a multisite study to investigate visual exploration patterns in patients with SZ. The research group used six situational pictures, containing cognitive and emotional elements. The authors compared changes in exploration behavior, cluster analyses, attentional landscapes and analyses of scan path similarities between two groups (HC and SZ). Patients with SZ showed longer fixation times and fewer fixation shifts than HC. They also seemed to generally use a more focal processing mode with longer fixations on distinct features in the center of a scene, in contrast to a more ambient processing of context information (seen in HC). Furthermore, despite visual alterations, patients with SZ appeared to be able to adapt their visual exploration strategies to changes in cognitive complexity, physical properties and emotional strain (as HC did). Interestingly, the study showed that cognitive and emotional elements of the presented stimulus significantly affected the visual exploration of both groups.
Another study stressed the possible existence of a relationship between exploratory scanning patterns and the symptomatic dimensions (positive/negative) of SZ. In a free‐viewing task, Gaebel et al. used pictures showing an interpersonal communication situation within the context of a proverb. 147 In this particular study, patients who manifested more negative symptoms were reported to use a staring‐scanning method, whereas patients with more positive symptoms disclosed an extensive‐scanning method.
Visual information processing abnormalities in schizophrenia may have a disorder‐specific‐marker value
Indeed, one may say that atypical visual scanning patterns have been shown not only in SZ patients, but in individuals with other psychiatric disorders, including social phobia, 246 , 247 bipolar affective disorder, 248 attention‐deficit/hyperactivity disorder, 131 , 249 , 250 generalized anxiety disorder, and with persecutory delusions, 251 Asperger syndrome 252 and autism (e.g. superficially normal attentional preference for social information in adults with autism spectrum disorders [ASD] 253 , 254 , 255 ). However, the abnormalities found in the aforementioned psychiatric disorders differ from those found in individuals with SZ. Brakemeier et al. reported that sensorimotor and cognitive processing impairments are relatively specific to those with a history of psychosis. 122
With an advantage, studies of eye movements in SZ have investigated visual scanning pattern paradigms that instructed the participants to identify the displayed emotional expression. The study of Bestelmayer et al., where the participants were asked to view presented images freely, replicated the well‐established finding of restricted visual scanning of faces in SZ. 248 The most remarkable result to emerge from the data was that a restricted visual scanning effect was present in all types of used stimuli (landscape, fractal and noise stimulus). Previous findings by Loughland et al., who suggested individuals with SZ were more impaired on facial stimuli compared with images without social content, were not supported in the study. 240 , 241 Instead, the visual scanning abnormalities in patients with SZ are suggested to reflect a global scanning impairment (Fig. 1).
Fig. 1.
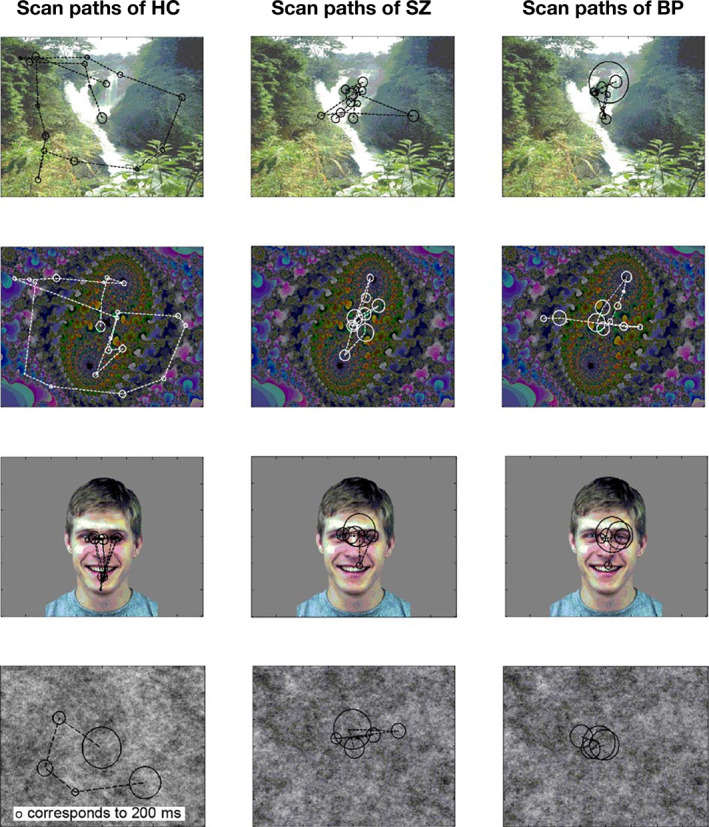
Representative visual scan paths to each stimulus type for one participant from each of the three groups (healthy control, schizophrenia and bipolar disorder). Reproduced from Bestelmayer et al., 248 with permission.
Furthermore, the sample of patients with bipolar disorder showed a more homogeneous spatial distribution of scanning of social stimuli compared with the sample of patients with SZ (the SZ within‐group variation was reported to be significantly different from the bipolar disorder within‐group variation on all but face images). As previously highlighted, the investigation of scanning patterns may expose differences within the psychoses not necessarily detectable by temporal measures alone. 248 Therefore, further investigations are required to determine the value of spatial measures as biological markers for stratifying the functional psychoses.
Notably, several imaging studies have reported an overlap of the impaired brain circuit among SZ patients with that of individuals with autism. 256 , 257 , 258 , 259 , 260 As autism spectrum disorder and SZ patients (without positive symptoms) share common cognitive symptoms, to differentiate them and state a precise diagnosis has been reported to be arduous. This has led Fukushima to examine voluntary control of saccadic and smooth pursuit eye movements among adults with SZ and autism spectrum disorder, and compare the results with the performance of a typically developed (healthy) group. 261 Although 38% of the autism spectrum disorder participants were reported to have higher error rates in the antisaccade task and expected gains in horizontal sinusoidal smooth pursuit, approximately 70% of patients with SZ showed abnormalities in the antisaccade task. Furthermore, in the smooth pursuit task, 70% of adults with SZ showed a lower gain than HC. Abnormalities in eye movement tasks have been reported to be stronger among patients with SZ rather than ASD. 261 In conclusion, eye‐tracking evidence was reported to offer insight into circuit‐level alterations, which have become promising biomarkers of autism and SZ disorder.
Following Fukushima's conclusions that eye‐tracking deficits have been localized in individuals with SZ rather than ASD, 261 a more recent study was conducted by Shiino et al. that aimed to understand the similarities and differences in eye movement abnormalities across patients diagnosed with SZ and those with ASD. 262 Out of 75 collected eye movement characteristics, the research group successfully pointed out five characteristics where individuals with SZ showed significant differences from the individuals with ASD (4 during the free viewing task: number of fixations; number of saccades; scan‐path length; number of blinks; and 1 during the smooth pursuit eye movements task: horizontal position gain; Fig. 2). Visual information processing abnormalities have been confirmed to have a disorder‐specific marker value in SZ patients. Further gaze measuring paradigms may uncover global similarities in eye behavior abnormality patterns among various groups of patients, as well as state differences between them. However, to establish such comparison across various psychiatric disorders, intensive investigations are required.
Fig. 2.
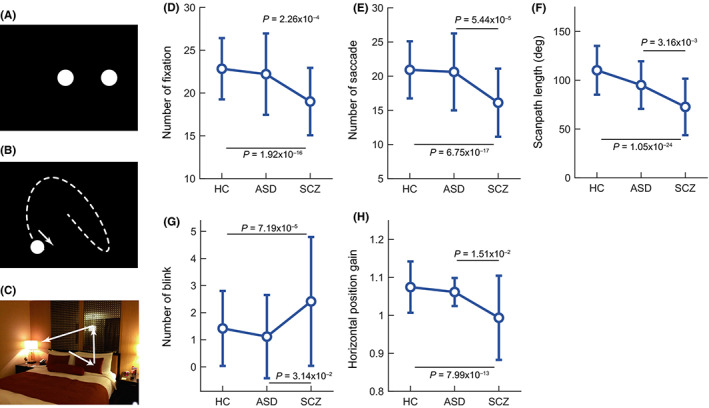
Schematic diagrams of stimulus paradigms: (a) fixation task, (b) smooth pursuit task, (c) free viewing task and comparisons among groups in five characteristics – (d) number of fixations, (e) number of saccades, (f) scan path length and (g) number of blinks – in the free viewing eye movements and the (h) horizontal position gain in the smooth pursuit eye movements. ASD, autism spectrum disorder; HC, healthy control; SCZ, schizophrenia. From Shiino et al., 262 with permission.
Modern clinical concepts that implement viewing tests
More and more modern studies, with the use of state‐of‐the‐art eye trackers, corroborate the fact that a reduced integration of visual information contributes to altered visual experiences in patients with SZ. 1 , 48 , 60 , 104 Importantly, perception deficits seem to persist exclusively (selectively) in SZ. Therefore, in recent years, some independent research groups have started to actively record eye movements to differentiate patients with SZ from HC. 23 , 42 , 54 , 117 , 219 , 238 , 263 , 264 , 265 Such an approach marks a new beginning of promising continuous years of research on visual disturbances in SZ patients, with eye‐tracking technology revolutionizing mental health screening, even with the use of one's smartphone. 266 , 267 , 268 Pioneering studies, which significantly contributed to the development of discriminant analysis that incorporates eye movement parameters to distinguish SZ cases from HC, are presented in Table 1. Whereas, modern clinical concepts, published between 2010 and 2020, which implement viewing tests that significantly differ between participants with and without SZ, are chronologically described below.
Table 1.
List of pioneering and modern studies distinguishing schizophrenia patients from healthy controls by eye‐movement parameters measured across (i) tests with oscillating targets, (ii) tests with table targets and (iii) a combination of eye‐movement tests
| Author(s) | No. participants | Suggested eye‐movement discriminator(s) | Stimuli | Test | Summary of key findings |
|---|---|---|---|---|---|
| (i) Oscillating stimulus | |||||
| Holzman et al., 1973 179 |
25 psychotic patients (18 SZ) 8 psychiatric controls 33 HC |
SPEM | Oscillating pendulum | Smooth pursuit | SZ patients show smooth pursuit eye‐tracking patterns that differ strikingly from the generally smooth eye‐tracking observed in HC and in non‐SZ probands. These deviations refer to oculomotor involvement, having a critical relevance for perceptual dysfunction in SZ. |
|
Holzman et al., 1974 180 |
69 SZ 6 schizoaffective disorders 9 manic‐depressives 19 non‐psychotic patients 34 relatives of SZ 19 relatives of non‐SZ 72 HC |
SPEM |
Oscillating pendulum | Smooth pursuit |
Smooth‐pursuit eye movement test disclosed a striking association between deviant eye‐tracking and clinically diagnosed SZ. Disruptions of smooth pursuit eye movements (SPEM abnormality) were found in 52% of recent and 86% of chronic SZ patients as well as 44% of their first‐degree relatives. SZ probands and their biological family members have a deficit in an accurate smooth pursuit. The eye‐tracking dysfunction may represent a genetic marker, highly useful for studying the transmission of a vulnerability to SZ. |
| Holzman et al., 1984 195 |
80 SZ 46 manic‐depressives 59 parents of SZ patients 48 HC |
SPEM |
Oscillating pendulum (yellow cross) | Smooth pursuit |
Eye‐tracking dysfunctions are reliably found in 50–85% of SZ patients, about 40% of manic‐depressive patients, and about 8% of the HC. Deviant eye‐tracking in pursuit of eye‐movements reported among family members of SZ. Parental eye‐movement dysfunctions are significantly related to the patient's diagnosis. In the absence of other CNS diseases, ETD represents a familial marker of vulnerability to SZ. |
| (ii) Stable stimulus | |||||
|
Moriya et al., 1972 218 |
24 chronic SZ 20 HC |
‐NR‐ † | Human figures and ‘S’ shaped figures | Free viewing | Chronic SZ patients show atypical scanning patterns (fewer fixations and a narrower range of eye‐movements in chronic SZ in comparison to HC). |
| Moriya 1979 216 |
80 chronic SZ 80 parents of SZ (relatives) 80 HC |
‐NR‐ † | ‘S’ shaped figure | Free viewing |
In most chronic SZ cases, the eye movements were inactive (the SZ patients tend to stick to one location). The range of movement within points of fixation for chronic SZ and their relatives was limited (for chronic SZ, more limited than that of their relatives). Fixation times of SZ patients and their parents were longer, compared to HC (fixation times of SZ patients longer than of their parents). A correlation between low eye movement and a high rating of the overall severity of symptoms was observed. |
| Kojima et al., 1990 214 |
10 acute SZ 50 chronic SZ 20 remitted SZ 25 methamphetamine psychosis 21 temporal lobe epileptics with left‐sided spike focus 12 temporal lobe epileptics with right‐sided spike focus 50 HC |
EEM (especially MESL and RSS) |
‘S’ shaped figures | ‘S’‐shaped figure procedure (retention and comparison tasks) |
Short MESL can be an indicator of a chronic process of SZ. The chronic SZ patients have a significantly shorter MESL than acute SZ, remitted SZ, methamphetamine psychotics, temporal lobe epileptics with left‐sided spike focus, temporal lobe epileptics with right‐sided spike focus and HC. SZ groups have significantly lower RSS than the non‐SZ patient groups and the HC. Therefore, lowering of the RSS may be a nosologically specific indicator for SZ. A significant negative correlation between the RSS and negative symptoms (in the chronic SZ group). |
|
Matsushima et al., 1992 283 |
20 SZ 18 patients with frontal lobe lesions 20 HC |
EEM (especially RSS) | ‘S’ shaped figure | ‘S’‐shaped figure procedure |
Patients with right frontal lobe lesions and SZ patients have lower scores than HC for the NEFs, total TESL and MESL. The RSS is low only among patients with SZ. |
|
Matsushima et al., 1998 193 |
Group A and B, each comprising: 30 SZ 70 non‐SZ subjects (i.e. 10 each of patients with depression, methamphetamine psychosis, alcohol psychosis, anxiety disorder, temporal lobe epilepsy, frontal lobe lesions, and HC) |
NEFs and RSS | ‘S’ shaped figures | ‘S’‐shaped figure procedure (retention and comparison tasks) |
Obtained discriminant function: D = 10.265 − (0.065 × NEF + 0.871 × RSS) used to separate outpatients with SZ from individuals with depression, methamphetamine psychosis, anxiety disorders, temporal lobe epilepsy or frontal lobe lesions and HC. Sensitivity and specificity (in group A): 76.7% and 81.4% respectively; Sensitivity and specificity (in group B): 73.3% and 84.3% respectively. |
| Kojima et al., 2001 121 |
145 SZ 116 patients with depression 124 HC |
NEFs and RSS |
‘S’ shaped figures |
‘S’‐shaped figure procedure (retention and comparison tasks) |
Using the discriminant formula from Matsushima et al., 1998 194 : D = 10.265 − (0.065 × NEF + 0.871 × RSS), patients with SZ could be discriminated from depressed patients and HC with a sensitivity of 89.0% and a specificity of 86.7%. The RSSs of patients with SZ are significantly lower than those of depressed patients or HC (with no significant difference existing between the RSSs for depressed patients and those for HC). |
| Suzuki et al., 2009 217 |
251 SZ 389 non‐SZ (i.e. 111 patients with mood disorders 28 patients with neurotic disorders 250 HC) |
TESL and RSS | ‘S’ shaped figures | ‘S’‐shaped figure procedure (retention and comparison tasks) |
The stepwise regression analysis selected the TESL and RSS as valid parameters for discriminating between SZ and non‐SZ. Using the RSS and TESL as prediction parameters, D = 4.100 − (0.001 × TESL + 0.332 × RSS), patients with SZ could be discriminated from non‐SZ with a 73.3% sensitivity and a specificity of 79.2%. |
| Sprenger et al., 2013 60 |
32 SZ 33 HC |
Number, location and duration of fixations; number and amplitude of saccades; scan path length (6 in total) |
Pictures regarding daily situations | Free viewing |
Longer fixation times and fewer fixations independent of cognitive or emotional content |
| (iii) Combination of eye‐movement tests | |||||
| Arolt et al., 1998 117 |
14 SZ (residual subtype) 17 HC |
Mean gain; number of saccades; number of errors (3 in total) |
(Exp.1.) Moving target in a triangular trajectory over a white background; (Exp.2.) Moving target in a triangular trajectory with a visual distractor (picture of the New York skyline i.e. numerous white spots on a black background); no target for voluntary saccadic task |
Smooth pursuit task without (Exp.1.) and with a visual distraction (Exp.2.); Voluntary saccadic eye‐movement task |
A stepwise procedure selected the three most essential parameters concerning the discrimination result, namely mean gain (%) and the number of saccades (both parameters obtained from smooth pursuit tasks), and the number of errors (obtained from the voluntary saccadic experiment). The correct classification rates were 90.3% (re‐substitution method) and 83.9% (jackknifed classification). A straightforward suggestion that not by one single task, but possibly by their combination, eye movements might serve as a diagnostic tool. Moreover, paradigms that require more substantial involvement of cognitive factors may have a more excellent heuristic value. |
| Benson et al., 2012 57 |
Training set: 88 SZ 88 HC Retest group: 26 SZ 8 HC Novel group: 36 SZ 52 HC |
Combination of machine learning models with gaze parameters (obtained from eye movement tasks) |
Faces, landscapes, unfamiliar computer‐generated images (fractals) |
Free viewing; smooth pursuit task; fixation stability test (with flanking distractor) |
Authors used algorithms to model the eye‐movement data, including two forms of supervised machine learning, i.e. GBDTs and PNNs. The GBDT model achieved separation of the 88 SZ cases from 88 HC; its predictive validity on retest and novel groups was 87.8%. With a trained PNN model (that used data from all subjects), it was possible to discriminate SZ cases from healthy controls with 98.3%. |
| Miura et al., 2014 118 |
40 SZ 69 HC |
Scan path length; V‐position gain; fixation number; fixation duration; S/N ratio (5 in total) |
Images of natural environments, buildings, everyday items, foods, faces, animals, fractal patterns; In the smooth pursuit eye movement test: target moving horizontally and on Lissajous trajectories |
Free viewing test; Smooth pursuit test (fast Lissajous and horizontal pursuit paradigms); Fixation stability test (far distracter paradigm) |
Canonical discriminant analysis with a stepwise procedure was conducted. 5 of the 65 eye movement variables were selected to create an integrated eye movement score (Y), which effectively represents eye‐movement abnormality. Y = 0.03 × {scan path length (free viewing)} + 2.01 × {V‐Position gain (fast Lissajous)} + 0.03 × {Fixation number (fast Lissajous)} + 0.37 × {Fixation duration (far distracter)} − 1.53 × {S/N ratio (horizontal pursuit)} − 4.92 The discriminant analysis rate was 89.9% (re‐substitution method) and 88.1% (leave‐one‐out cross‐validation method), with a sensitivity and specificity of 0.78 and 0.94, respectively. |
| Morita et al., 2017 42 |
85 SZ 252 HC |
Scan‐path length Horizontal position gain Fixation duration (3 in total) |
Images of fractal patterns, foods, natural environments and animals (selected from the International Affective Pictures System), faces |
Free viewing; Smooth pursuit test (fast Lissajous paradigm within); Fixation stability test (far distractor paradigm) |
Eye movement measures, with the largest group differences specified by Bonferroni corrected P‐values, were chosen as candidates for the integrated eye movement score. The stepwise canonical discriminant analysis reduced seven measures to 3. Used discriminant function (y): y = 0.0312 × {scan path length} + 5.614 × {horizontal position gain} + 0.0000203 × {duration of fixations} − 9.637 The eye movement score correctly classified 82.5% of the participants (in the re‐substitution method) and 81.9% (in the leave‐one‐out cross‐validation method). The sensitivity and specificity of the score were reported to be 0.79 and 0.84, respectively. |
‐NR‐ Although Moriya, 216 , 218 did not suggest a specific eye movement discriminator, his studies pointed out atypical scanning patterns, present among chronic schizophrenia (SZ) patients.
This significantly contributed to the SZ research domain. Therefore, the Authors have included these studies on the list.
CNS, central nervous system; EEM, exploratory eye movement; ETD, eye‐tracking dysfunction; GBDT, gradient boosted decision trees; HC, health controls; MESL, mean eye scanning length; NEFs, number of eye fixations; PNN, probabilistic neural networks; RSS, responsive search score; S/N ratio, signal‐to‐noise ratio; SPEM, smooth pursuit eye movements; TESL, total eye scanning length.
In 2012, Benson et al. examined a large group of SZ patients and HC using a free‐viewing task (combination of pictures included e.g. luminance‐balanced natural and manmade environments showing information at different spatial scales; expressive, neutral and occluded faces; animals; and unfamiliar computer‐generated images); smooth pursuit tracking, which involved tracking a 0.5° circular target for 20 s as it moved sinusoidally on the horizontal meridian or in Lissajous patterns (in horizontal and vertical space). In addition, as a proxy for saccadic inhibition, the authors conducted steady fixation tasks, where individuals were required to maintain their gaze only on a central circular target for 5 s and to ignore a flanking distracter appearing either 1.43° or 2.86° to the left or right of the central fixation. Notably, the authors reported being able to obtain data from both groups, as all participants could complete the mentioned tasks. Extracted eye movement performance measures supported the concept of highly accurate classifier models. Data from 298 assessments were used to train a network model, which resulted in predicted classification accuracy of 98.3%. 57 The same group highlighted that eye movement abnormalities are stable traits, independent of the neuroleptic medication and mental state at the time of testing. Furthermore, eye movement abnormalities show no correlation with the presence or absence of cigarette smoking. 57
Scan path patterns provides valuable information when and where a subject shifts attention during visual exploration, by recording and analyzing fixation sequences. 53 Sprenger et al. reported that scan path patterns differ significantly between HC and patients with SZ. 60 In their study, seven gaze metrics parameters were determined: (i) fixations (their location, number and duration); (ii) saccades (their number and amplitudes); and (iii) the scan path length. Additionally, to test for changes over the exploration time, the exploration time of 20 s was divided into four intervals (5 s each). In line with earlier conclusions, fewer but longer fixations, as well as smaller saccades (resulting in shorter scan path length), were reported in SZ patients. 53 , 240 , 248 , 269 , 270 , 271 , 272 , 273 , 274 Cluster analyses showed that patients fixated on fewer areas of interest (making fewer fixations per cluster) and had a longer total fixation time within each cluster. Nearly all patients reported to have had enough time to grasp the content of the presented stimuli, nevertheless, they gathered less visual information than HC. Therefore, these longer fixations may reflect a more general problem of disengaging attention. Together, the findings underline the hypothesis of a specific exploration behavior in patients that differs considerably from that in HC independently of the cognitive or emotional content of a presented image. The use of scan path similarities in discriminant analysis resulted in 85% of participants being classified correctly to either the patient or control group.
In 2014, another independent research group under Miura's lead conducted a discrimination analysis of SZ patients with a linear classifier. 118 An integrated eye movement score, significantly different between patients with SZ and HC, was introduced that might be useful in the early diagnosis of SZ or its prodrome phase, where subjective symptoms are relatively obscure. Therefore, it might effectively assist physicians’ diagnosis. Five of the 65 eye movement variables were selected: (i) scan path length (free viewing test); (ii) position gain; (iii) the number of fixations (fast Lissajous paradigm within smooth pursuit test); (iv) S/N ratio (horizontal pursuit paradigm within smooth pursuit test); and (iv) fixation duration (far distracter paradigm within fixation stability test). The correct rate of the discriminant analysis of the selected five variables was 89.9% (re‐substitution method) and 88.1% (leave‐one‐out cross‐validation method) with a sensitivity and specificity of 0.78 and 0.94, respectively. Such accuracy was comparable or even better than the previously reported classifications ( 275 , 276 , 277 , 278 cited by Miura et al. 118 ).
In 2017, Morita et al. reported an integrated eye score that could distinguish patients with SZ from HC with 82% accuracy. 42 Three of 75 eye movement variables have been selected: (i) scan path length (obtained through free viewing test); (ii) horizontal position gain (obtained through fast Lissajous paradigm within smooth pursuit test); and (iii) fixation duration (obtained through far distracter paradigm within fixation stability test). The aforementioned study again confirms the utility of gaze metrics in the identification process of SZ. The authors suggest that the use of multiple eye movement measures, to classify patients with SZ, is highly efficient and results in a discriminative accuracy of 80–90%.
Furthermore, to show eye movement abnormalities and their relationship with a patient's social/intellectual functioning, 182 , 234 Morita et al. started to actively study the association between eye movement abnormalities among patients with SZ and working hours (obtained through the Social Activity Assessment), and factors related to intelligence and education (years of education, estimated premorbid IQ and Wechsler Adult Intelligence Scale full‐scale IQ). 44 , 182 , 235 Furthermore, following the findings of a present correlation between exploratory eye movements and brain structure, 221 , 224 Morita et al. extended their research. In 2019, Morita et al. showed that eye movement characteristics were positively correlated with cortical thickness (of the left pars opercularis) among patients with SZ. 226 To gain more insights into social/intellectual deficits and visual abnormalities among patients with SZ and individuals in the high‐risk state for psychosis, further investigations of the relevance between exploratory eye movements, individuals’ brain structures and everyday life functioning need to be undertaken in future studies. 44 , 231
The aforementioned modern studies provide strong evidence that a multivariate eye movement phenotype (such as the integrated eye movement score, for example) may accurately distinguish SZ cases from HC at a rate far beyond any single gaze parameter measure. In conclusion, eye movement abnormalities appear to be stable traits of SZ. Tests that implement eye movement variables may supplement current symptom‐based diagnoses; hence, they have considerable power to discriminate schizophrenia patients from HC individuals. Analysis of scan paths might reveal differences within the psychoses not necessarily detectable by temporal measures alone. Furthermore, eye‐tracking tools are cost‐effective and can be a relief to the medical staff, as, compared with time‐consuming neuropsychological assessments carried out by highly‐qualified individuals, eye movement recordings can be performed by trained assistants. Although the currently available eye movement scores are not meant to replace criteria that rely on clinical observations and self‐reports, future studies should test the possibility of gaze metrics distinguishing SZ from other psychiatric disorders until a set of biomarkers of SZ, increasing the chance of early intervention, will raise.
Real‐life versus laboratory experiments: Gazing toward the future
It is essential to mention that experimental paradigms influence what the scientific community accepts as truth. Dowiasch et al. reported that some of the results obtained from a real‐life study were contradictory to previous findings from laboratory experimental settings. 86 More experiments comparing the gaze metrics under ecologically valid conditions, supported with wearable eye trackers, need to be undertaken. Until now, eye measurements are collected indoors through visual tests, such as free viewing, smooth pursuit and fixation stability. To better understand the interplay between cognition and vision in psychiatric disorder, introducing real‐life situations into clinical experimental paradigms that use cognitively informative paradigms, grouped under the umbrella terms of learning and decision‐making, needs to be addressed. Capturing ecologically relevant features of decision‐making, for example, may reveal cognitive deficits not apparent in ‘simpler tasks’. 151 Following the thought that extraction task‐relevant information and choice selection (according to internal preferences and/or given experimental task) require a balance between receptor structures and the central circuit, information processing experimental paradigms may allow the clinical community to gain insight into the decision‐constructing process (through gaze metrics) and establish a comparison across various psychiatric disorders. 279 , 280 , 281 Furthermore, studying decisional processes should be of great importance for SZ research, as patients commonly have motivational problems. 236
Seemingly subtle visual impairments happen to influence real‐world functioning and impoverish an individual's life quality drastically. It is crucial to intensively replicate now‐classic paradigms, as well as design novel real‐life inspired experimental paradigms to successfully transfer gained knowledge into diagnosis protocols, characterized with high sensitivity and specificity. Therefore, combining eye movement measurements (serving as an indirect output of cognitive processes) with behavior paradigms may help the scientific community to isolate key processes that underlie psychotic disorders. 2 As the argument over generalized impairments is clouded by the fact that until now there is no clear neuropsychological signature of SZ, information‐processing‐based investigations, supported with modern eye‐tracking devices, have the potential to bring the medical community closer to the core of SZ. 24 , 62
Discussion and Summary of Evidence
Vision science is the most coherent, integrated and prosperous branch of cognitive science. 29 , 30 , 98 Eye gaze metrics, probing human underlying believes, intentions, behavior and choices, spawn the field of neurocognition, where the merging of vision science and cognitively informative paradigms produces an array of scientifically recognized paradigms to study cognition. Eye movements promote understanding of how patterns of retinal activation are transformed into meaningful visual experiences, which in turn may be labeled with a specific reaction (behavior). However, while capturing mental processes of interest in cognitive psychology progresses at a relatively fast pace, the progress made in understanding the underlying mechanisms of psychiatric disorders has been surprisingly slow. In this scoping review, the authors identified journal articles published between 2010 and 2020 that addressed eye movement measurement methodology in SZ research and diagnostic field.
Smooth pursuit, free viewing and fixation stability tests offer an individual contribution to future studies of the neurobiological disturbances not only in SZ, but in other disorders. 282 Data from multiple gaze metrics have confirmed the existence of significant differences between patients with SZ and HC. 42 , 57 , 112 , 115 , 116 , 117 , 118 , 237 Furthermore, measurements of individuals’ exploratory eye‐movements (while comparing stationary displayed ‘S’ shaped figures) provide important results. For example, in comparison with HC, patients with SZ show fewer eye fixations, longer mean duration of fixation and shorter mean scanning length (narrower range of eye movements). 121 , 221 , 283 It has also been reported that the results of their parents were mid‐way between those of HC and SZ patients. 193 , 216 Several studies concluded that EEMs are a potential biomarker of SZ. 113 , 115 , 121 , 193 , 214 , 284 Importantly, among the five commonly used parameters obtained from exploratory eye movement tests, including the NEFs, mean eye scanning length, TESL, cognitive search score and RSS; only the RSS, which measures the pattern of eye fixations after the question, (‘Are there any other differences?’, has been pointed out to be vulnerable to SZ. 115 , 121 , 219 , 224
Furthermore, free viewing tests that involved various types of stimuli (i.e. landscapes, situations of interpersonal communication, normal and degraded faces) have shown that patients with SZ are characterized with abnormal scan path length and restricted scanning style. Thus, atypical scan path deficits and saccadic impairments are considered a trait marker of SZ. 53 , 285
Given that eye movement assessments are non‐invasive for patients, a promising future clinical research area is developing to evaluate potential relationships between disease characteristics and social functioning in patients with SZ. 1 , 2 , 20 , 61 The growing number of reports aiming to understand the relationship between eye movement characteristics, intellectual functioning and differences in brain structures across patients with SZ provides valuable conclusions regarding cognitive impairments and social skills deficits, 221 , 222 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 286 which in turn may be useful for therapeutics. 17 , 20 , 182 , 225 , 228 , 287 , 288
Consequently, the next decade of SZ research is likely to witness eye movement measurements being actively used as potential biomarkers and applied to cognitively informative experimental paradigms. Future research questions will require a more profound analysis of gaze metrics during viewing tasks rather than quantification of stabilized fixations. For this reason, more complex experimental paradigms (competitive cognitive tasks embracing attention‐demanding components) should be translated from the well‐grounded neuroscientific methods and carried out to study visual exploration among individuals with SZ. Cognitively informative paradigms, which (i) demand to act on and manipulate a given problem/task/information; (ii) provide information about patients’ behavior (in laboratory and real‐life environment); and (iii) disclose patients’ visual scanning patterns, are likely to offer an individual contribution to future studies of the neurobiological disturbances involved in SZ.
Until now, clinicians cannot draw a solid line between patients with different underlying pathologies, experiencing differing types and degrees of impairment depending on the nature of given task. With this in mind, multidisciplinary contributions of cutting‐edge experimental paradigms that use eye‐tracking methodologies are required. This will require a coordinated effort of multiple scientific disciplines, including psychiatry, psychology, neuroscience and cognitive science. Such joined effort will enable us to illustrate the characteristics of one's information processes and gaze metrics patterns under various environmental conditions. Such combination will be particularly useful in gaining insights into cognitive deficits and visual abnormalities among patients with different dimensions of SZ and individuals in the high‐risk state for psychosis.
Limitations
Our scoping review is likely generalizable to journal articles and systematic reviews acquired through the Kyushu University Open Access Policy.
Disclosure statement
The authors have no conflicts of interest to declare.
Author contributions
Y.H. encouraged A.W. to investigate eye movement abnormalities in patients with schizophrenia and supervised this scoping review's findings. A.W. wrote the manuscript. K.U. and Y.H. revised the manuscript. All authors contributed to and approved the final manuscript.
Acknowledgments
This pioneering work was funded, in part, by KAKENHI Grant Number JP19F19307 under the Postdoctoral Fellowship scholarship (A.W.) from the Japan Society for the Promotion of Science (JSPS); a Grant‐in‐Aid for Scientific Research C: JP15K09836 (Y.H.), JP18K07604 (Y.H.), JP19H03579 (Y.H.) and Fund for the Promotion of Joint International Research (Fostering Joint International Research B): JP 20KK0193 (Y.H.) from the JSPS; and SIRS Research Fund Award (Y. H.) from the Schizophrenia International Research Society.
Alexandra Wolf and Yoji Hirano contributed equally.
Contributor Information
Alexandra Wolf, Email: olaokami@gmail.com.
Yoji Hirano, Email: yhouji@mac.com.
References
- 1. Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophr. Res. Cogn. 2015; 2: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eckstein MK, Guerra‐Carrillo B, Miller Singley AT, Bunge SA. Beyond eye gaze: What else can eyetracking reveal about cognition and cognitive development? Dev. Cogn. Neurosci. 2017; 25: 69–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loduca AL, Zhang C, Zelkha R, Shahidi M. Thickness mapping of retinal layers by spectral‐domain optical coherence tomography. Am. J. Ophthalmol. 2010; 150: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee WW, Tajunisah I, Sharmilla K, Peyman M, Subrayan V. Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: Evidence from optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013; 54: 7785–7792. [DOI] [PubMed] [Google Scholar]
- 5. Jerotić S, Marić N. Structural retinal abnormalities as potential markers for psychosis spectrum disorders. Med. Podml. 2018; 69: 41–47. [Google Scholar]
- 6. Asanad S, Ross‐Cisneros FN, Nassisi M, Barron E, Karanjia R, Sadun AA. The retina in alzheimer's disease: Histomorphometric analysis of an ophthalmologic biomarker. Investig. Ophthalmol. Vis. Sci. 2019; 60: 1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asanad S, Ross‐Cisneros FN, Barron E et al. The retinal choroid as an oculovascular biomarker for Alzheimer's dementia: A histopathological study in severe disease. Alzheimers Dement. (Amst) 2019; 11: 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marziani E, Pomati S, Ramolfo P et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral‐domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013; 54: 5953–5958. [DOI] [PubMed] [Google Scholar]
- 9. Adams SA, Nasrallah HA. Multiple retinal anomalies in schizophrenia. Schizophr. Res. 2018; 195: 3–12. [DOI] [PubMed] [Google Scholar]
- 10. Almonte MT, Capellàn P, Yap TE, Cordeiro MF. Retinal correlates of psychiatric disorders. Ther. Adv. Chronic Dis. 2020; 11: 2040622320905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kazakos CT, Karageorgiou V. Retinal changes in schizophrenia: A systematic review and meta‐analysis based on individual participant data. Schizophr. Bull. 2020; 46: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoon JH, Sheremata SL, Rokem A, Silver MA. Windows to the soul: Vision science as a tool for studying biological mechanisms of information processing deficits in schizophrenia. Front. Psychol. 2013; 4: 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Darke H, Peterman JS, Park S, Sundram S, Carter O. Are patients with schizophrenia impaired in processing non‐emotional features of human faces? Front. Psychol. 2013; 4: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giersch A, Wilquin H, Capa RL, Delevoye‐Turrell YN. Combined visual and motor disorganization in patients with schizophrenia. Front. Psychol. 2013; 4: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim J, Park S, Blake R. Perception of biological motion in schizophrenia and healthy individuals: A behavioral and Fmri study. PLoS One 2011; 6: e19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sergi MJ, Rassovsky Y, Widmark C et al. Social cognition in schizophrenia: Relationships with neurocognition and negative symptoms. Schizophr. Res. 2007; 90: 316–324. [DOI] [PubMed] [Google Scholar]
- 17. Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am. J. Psychiatry 2006; 163: 448–454. [DOI] [PubMed] [Google Scholar]
- 18. Uhlhaas PJ, Phillips WA, Silverstein SM. The course and clinical correlates of dysfunctions in visual perceptual organization in schizophrenia during the remission of psychotic symptoms. Schizophr. Res. 2005; 75: 183–192. [DOI] [PubMed] [Google Scholar]
- 19. Green MF, Horan WP. Social cognition in schizophrenia. Curr. Dir. Psychol. Sci. 2010; 19: 243–248. [Google Scholar]
- 20. Billeke P, Aboitiz F. Social cognition in schizophrenia: From social stimuli processing to social engagement. Front. Psych. 2013; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frith CD, Singer T. The role of social cognition in decision making. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008; 363: 3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao H‐I, Yeh S‐L, Shimojo S. Novelty vs. familiarity principles in preference decisions: Task‐context of past experience matters. Front. Psychol. 2011; 2: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shishido E, Ogawa S, Miyata S, Yamamoto M, Inada T, Ozaki N. Application of eye trackers for understanding mental disorders: Cases for schizophrenia and autism spectrum disorder. Neuropsychopharmacol. Rep. 2019; 39: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006; 2: 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: Effects on cognition, functional competence, and real‐world behavior. Am. J. Psychiatry 2012; 169: 710–718. [DOI] [PubMed] [Google Scholar]
- 26. Holshausen K, Bowie CR, Mausbach BT, Patterson TL, Harvey PD. Neurocognition, functional capacity, and functional outcomes: The cost of inexperience. Schizophr. Res. 2014; 152: 430–434. [DOI] [PubMed] [Google Scholar]
- 27. Bowie CR, Grossman M, Gupta M, Holshausen K, Best MW. Action‐based cognitive remediation for individuals with serious mental illnesses: Effects of real‐world simulations and goal setting on functional and vocational outcomes. Psychiatr. Rehabil. J. 2017; 40: 53–60. [DOI] [PubMed] [Google Scholar]
- 28. Bowie CR, Bell MD, Fiszdon JM et al. Cognitive remediation for schizophrenia: An expert working group white paper on core techniques. Schizophr. Res. 2020; 215: 49–53. [DOI] [PubMed] [Google Scholar]
- 29. Silverstein S, Keane BP, Blake R, Giersch A, Green M, Kéri S. Vision in schizophrenia: Why it matters. Front. Psychol. 2015; 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Császár N, Kapócs G, Bókkon I. A possible key role of vision in the development of schizophrenia. Rev. Neurosci. 2019; 30: 359–379. [DOI] [PubMed] [Google Scholar]
- 31. Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Res. 1996; 36: 1827–1837. [DOI] [PubMed] [Google Scholar]
- 32. Shepherd M, Findlay JM, Hockey RJ. The relationship between eye movements and spatial attention. Q. J. Exp. Psychol. A. 1986; 38: 475–491. [DOI] [PubMed] [Google Scholar]
- 33. Tatler BW, Wade NJ, Kwan H, Findlay JM, Velichkovsky BM. Yarbus, eye movements, and vision. Iperception. 2010; 1: 7–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferreira F, Apel J, Henderson JM. Taking a new look at looking at nothing. Trends Cogn. Sci. 2008; 12: 405–410. [DOI] [PubMed] [Google Scholar]
- 35. Theeuwes J, Belopolsky A, Olivers CNL. Interactions between working memory, attention and eye movements. Acta Psychol. (Amst) 2009; 132: 106–114. [DOI] [PubMed] [Google Scholar]
- 36. Just MA, Carpenter PA, Miyake A. Neuroindices of cognitive workload: Neuroimaging, pupillometric and event‐related potential studies of brain work. Theor. Issues Ergon. Sci. 2003; 4: 59–88. [Google Scholar]
- 37. Carpenter P, Just M. Eye fixations and cognitive processes. Cogn. Psychol. 1976; 8: 441–480. [Google Scholar]
- 38. Just MA, Carpenter PA. A theory of reading: From eye fixations to comprehension. Psychol. Rev. 1980; 87: 329–354. [PubMed] [Google Scholar]
- 39. Thomas LE, Lleras A. Moving eyes and moving thought: On the spatial compatibility between eye movements and cognition. Psychon. Bull. Rev. 2007; 14: 663–668. [DOI] [PubMed] [Google Scholar]
- 40. Van Der Stigchel S, Theeuwes J. The influence of attending to multiple locations on eye movements. Vision Res. 2005; 45: 1921–1927. [DOI] [PubMed] [Google Scholar]
- 41. Van der Stigchel S, Meeter M, Theeuwes J. Eye movement trajectories and what they tell us. Neurosci. Biobehav. Rev. 2006; 30: 666–679. [DOI] [PubMed] [Google Scholar]
- 42. Morita K, Miura K, Fujimoto M et al. Eye movement as a biomarker of schizophrenia: Using an integrated eye movement score. Psychiatry Clin. Neurosci. 2017; 71: 104–114. [DOI] [PubMed] [Google Scholar]
- 43. Morita K, Kawakami S, Morita S et al. Eye movement abnormalities and their role in neurocognition and clinical recovery. Early Interv. Psychiatry 2018; 12: 104–232. [Google Scholar]
- 44. Morita K, Miura K, Kasai K, Hashimoto R. Eye movement characteristics in schizophrenia: A recent update with clinical implications. Neuropsychopharmacol. Rep. 2020; 40: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yarbus AL. Eye Movements and Vision. Springer US, Boston, MA, 1967. [Google Scholar]
- 46. Buswell GT. How People Look at Pictures. Chicago: University of Chicago Press, 1935. [Google Scholar]
- 47. Lipton RB, Levy DL, Holzman PS, Levin S. Eye movement dysfunctions in psychiatric patients: A review. Schizophr. Bull. 1983; 9: 13–32. [DOI] [PubMed] [Google Scholar]
- 48. Levy DL, Sereno AB, Gooding DC, O'Driscoll GA. Eye tracking dysfunction in schizophrenia: Characterization and pathophysiology. Curr. Top. Behav. Neurosci. 2010; 4: 311–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luna B, Velanova K, Geier CF. Development of eye‐movement control. Brain Cogn. 2008; 68: 293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bittencourt J, Velasques B, Teixeira S et al. Saccadic eye movement applications for psychiatric disorders. Neuropsychiatr. Dis. Treat. 2013; 9: 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luna B, Marek S, Larsen B, Tervo‐Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015; 38: 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pietrzak R, Mollica C, Maruff P, Reviews PS‐&B. Cognitive effects of immediate‐release methylphenidate in children with attention‐deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 2006; 30: 1225–1245. [DOI] [PubMed] [Google Scholar]
- 53. Beedie SA, St Clair DM, Benson PJ. Atypical scanpaths in schizophrenia: Evidence of a trait‐ or state‐dependent phenomenon? J. Psychiatry Neurosci. 2011; 36: 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Obyedkov I, Skuhareuskaya M, Skugarevsky O et al. Saccadic eye movements in different dimensions of schizophrenia and in clinical high‐risk state for psychosis. BMC Psychiatry 2019; 19: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kleineidam L, Frommann I, Ruhrmann S et al. Antisaccade and prosaccade eye movements in individuals clinically at risk for psychosis: Comparison with first‐episode schizophrenia and prediction of conversion. Eur. Arch. Psychiatry Clin. Neurosci. 2019; 269: 921–930. [DOI] [PubMed] [Google Scholar]
- 56. Harris MSH, Reilly JL, Thase ME, Keshavan MS, Sweeney JA. Response suppression deficits in treatment‐naïve first‐episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res. 2009; 170: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Benson PJ, Beedie SA, Shephard E, Giegling I, Rujescu D, Clair DS. Simple viewing tests can detect eye movement abnormalities that distinguish schizophrenia cases from controls with exceptional accuracy. Biol. Psychiatry 2012; 72: 716–724. [DOI] [PubMed] [Google Scholar]
- 58. Silberg JE, Agtzidis I, Startsev M et al. Free visual exploration of natural movies in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2019; 269: 407–418. [DOI] [PubMed] [Google Scholar]
- 59. Fasshauer T, Sprenger A, Silling K et al. Visual exploration of emotional faces in schizophrenia using masks from the Japanese Noh theatre. Neuropsychologia 2019; 133: 107193. [DOI] [PubMed] [Google Scholar]
- 60. Sprenger A, Friedrich M, Nagel M, Schmidt CS, Moritz S, Lencer R. Advanced analysis of free visual exploration patterns in schizophrenia. Front. Psychol. 2013; 4: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bae SM, Lee SH, Park YM, Hyun MH, Yoon H. Predictive factors of social functioning in patients with schizophrenia: Exploration for the best combination of variables using data mining. Psychiatry Investig. 2010; 7: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koychev I, El‐Deredy W, William Deakin JF. New visual information processing abnormality biomarkers for the diagnosis of schizophrenia. Expert Opin. Med. Diagn. 2011; 5: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bonensteffen F, Zebel S, Giebels E. Sincerity is in the eye of the beholder: Using eye tracking to understand how victims interpret an offender's apology in a simulation of victim‐offender mediation. Front. Psychol. 2020; 11: 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kuhn G, Tatler BW. Magic and fixation: Now you don't see it, now you do. Perception 2005; 34: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 65. Macknik SL, King M, Randi J et al. Attention and awareness in stage magic: Turning tricks into research. Nat. Rev. Neurosci. 2008; 9: 871–879. [DOI] [PubMed] [Google Scholar]
- 66. Mitsuda T, Glaholt MG. Gaze bias during visual preference judgements: Effects of stimulus category and decision instructions. Vis. Cogn. 2014; 22: 11–29. [Google Scholar]
- 67. Pärnamets P, Johansson R, Gidlöf K, Wallin A. How information availability interacts with visual attention during judgment and decision tasks. J. Behav. Decis. Mak. 2016; 29: 218–231. [Google Scholar]
- 68. Kühn S, Strelow E, Gallinat J. Multiple “buy buttons” in the brain: Forecasting chocolate sales at point‐of‐sale based on functional brain activation using fMRI. Neuroimage 2016; 136: 122–128. [DOI] [PubMed] [Google Scholar]
- 69. Wolf A, Ounjai K, Takahashi M, Kobayashi S, Matsuda T, Lauwereyns J. Evaluative processing of food images: A conditional role for viewing in preference formation. Front. Psychol. 2018; 9: 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wolf A, Ounjai K, Takahashi M, Kobayashi S, Matsuda T, Lauwereyns J. Evaluative processing of food images: Longer viewing for indecisive preference formation. Front. Psychol. 2019; 10: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leon FAD, Spers EE, de Lima LM. Self‐esteem and visual attention in relation to congruent and non‐congruent images: A study of the choice of organic and transgenic products using eye tracking. Food Qual. Prefer. 2020; 84: 103938. [Google Scholar]
- 72. Pärnamets P, Johansson P, Hall L, Balkenius C, Spivey MJ, Richardson DC. Biasing moral decisions by exploiting the dynamics of eye gaze. Proc. Natl. Acad. Sci. U.S.A 2015; 112: 4170–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Clark JJ, O'Regan JK. Word ambiguity and the optimal viewing position in reading. Vision Res. 1999; 39: 843–857. [DOI] [PubMed] [Google Scholar]
- 74. Kaakinen JK, Hyönä J. Task effects on eye movements during reading. J. Exp. Psychol. Learn. Mem. Cogn. 2010; 36: 1561–1566. [DOI] [PubMed] [Google Scholar]
- 75. Reichle ED, Rayner K, Pollatsek A. The E‐Z reader model of eye‐movement control in reading: Comparisons to other models. Behav. Brain Sci. 2003; 26: 445–476. [DOI] [PubMed] [Google Scholar]
- 76. Borji A, Itti L. Defending yarbus: Eye movements reveal observers' task. J. Vis. 2014; 14: 29. [DOI] [PubMed] [Google Scholar]
- 77. Torralba A, Oliva A, Castelhano MS, Henderson JM. Contextual guidance of eye movements and attention in real‐world scenes: The role of global features in object search. Psychol. Rev. 2006; 113: 766–786. [DOI] [PubMed] [Google Scholar]
- 78. Henderson JM. Human gaze control during real‐world scene perception. Trends Cogn. Sci. 2003; 7: 498–504. [DOI] [PubMed] [Google Scholar]
- 79. Castelhano MS, Mack ML, Henderson JM. Viewing task influences eye movement control during active scene perception. J. Vis. 2009; 9: 6.1–6.15. [DOI] [PubMed] [Google Scholar]
- 80. Zommara NM, Takahashi M, Ounjai K, Lauwereyns J. A gaze bias with coarse spatial indexing during a gambling task. Cogn. Neurodyn. 2018; 12: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Land MF, Tatler BW. Steering with the head: The visual strategy of a racing driver. Curr. Biol. 2001; 11: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 82. Victor TW, Harbluk JL, Engström JA. Sensitivity of eye‐movement measures to in‐vehicle task difficulty. Transp. Res. Part F Traffic Psychol. Behav. 2005; 8: 167–190. [Google Scholar]
- 83. Coen‐Cagli R, Coraggio P, Napoletano P, Schwartz O, Ferraro M, Boccignone G. Visuomotor characterization of eye movements in a drawing task. Vision Res. 2009; 49: 810–818. [DOI] [PubMed] [Google Scholar]
- 84. Doshi A, Trivedi MM. Head and eye gaze dynamics during visual attention shifts in complex environments. J. Vis. 2012; 12: 1–16. [DOI] [PubMed] [Google Scholar]
- 85. Land MF. Eye movements and the control of actions in everyday life. Prog. Retin. Eye Res. 2006; 25: 296–324. [DOI] [PubMed] [Google Scholar]
- 86. Dowiasch S, Backasch B, Einhäuser W, Leube D, Kircher T, Bremmer F. Eye movements of patients with schizophrenia in a natural environment. Eur. Arch. Psychiatry Clin. Neurosci. 2016; 266: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Crespi S, Robino C, Silva O, De'Sperati C. Spotting expertise in the eyes: Billiards knowledge as revealed by gaze shifts in a dynamic visual prediction task. J. Vis. 2012; 12: 30. [DOI] [PubMed] [Google Scholar]
- 88. Epelboim J, Suppes P. A model of eye movements and visual working memory during problem solving in geometry. Vision Res. 2001; 41: 1561–1574. [DOI] [PubMed] [Google Scholar]
- 89. Chen Z, Honomichl R, Kennedy D, Tan E. Aiming to complete the matrix: Eye‐movement analysis of processing strategies in children's relational thinking. Dev. Psychol. 2016; 52: 867–878. [DOI] [PubMed] [Google Scholar]
- 90. Heisz JJ, Shore DI. More efficient scanning for familiar faces. J. Vis. 2008; 8: 9.1–9.10. [DOI] [PubMed] [Google Scholar]
- 91. Spinks J, Mortimer D. Lost in the crowd? Using eye‐tracking to investigate the effect of complexity on attribute non‐attendance in discrete choice experiments clinical decision‐making, knowledge support systems, and theory. BMC Med. Inform. Decis. Mak. 2016; 16: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gidlöf K, Wallin A, Dewhurst R, Holmqvist K. Using eye tracking to trace a cognitive process: Gaze behaviour during decision making in a natural environment. J. Eye Mov. Res. 2013; 6: 1–14. [Google Scholar]
- 93. van der Laan LN, Hooge ITC, De Ridder DTD, Viergever MA, Smeets PAM. Do you like what you see? The role of first fixation and total fixation duration in consumer choice. Food Qual. Prefer. 2015; 39: 46–55. [Google Scholar]
- 94. Giebelhausen MD, Robinson SG, Cronin JJ. Worth waiting for: Increasing satisfaction by making consumers wait. J. Acad. Mark. Sci. 2011; 39: 889–905. [Google Scholar]
- 95. Constantinescu M, Orindaru A, Pachitanu A, Rosca L, Caescu SC, Orzan MC. Attitude evaluation on using the neuromarketing approach in social media: Matching company's purposes and consumer's benefits for sustainable business growth. Sustainability 2019; 11: 7094. [Google Scholar]
- 96. Peng‐Li D, Byrne DV, Chan RCK, Wang QJ. The influence of taste‐congruent soundtracks on visual attention and food choice: A cross‐cultural eye‐tracking study in Chinese and Danish consumers. Food Qual. Prefer. 2020; 85: 103962. [Google Scholar]
- 97. Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol. Psychiatry 2008; 64: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: Review of research from 2005 to 2010. Schizophr. Bull. 2011; 37: 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Keane BP, Cruz LN, Paterno D, Silverstein SM. Self‐reported visual perceptual abnormalities are strongly associated with core clinical features in psychotic disorders. Front. Psych. 2018; 9: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chapman J. The early symptoms of schizophrenia. Br. J. Psychiatry 1966; 112: 225–251. [DOI] [PubMed] [Google Scholar]
- 101. Häfner H, Maurer K, Trendler G, An Der Heiden W, Schmidt M, Könnecke R. Schizophrenia and depression: Challenging the paradigm of two separate diseases—a controlled study of schizophrenia, depression and healthy controls. Schizophr. Res. 2005; 77: 11–24. [DOI] [PubMed] [Google Scholar]
- 102. Phillipson OT, Harris JP. Perceptual changes in schizophrenia: A questionnaire survey. Psychol. Med. 1985; 15: 859–866. [DOI] [PubMed] [Google Scholar]
- 103. Matsumoto Y, Takahashi H, Murai T, Takahashi H. Visual processing and social cognition in schizophrenia: Relationships among eye movements, biological motion perception, and empathy. Neurosci. Res. 2015; 90: 95–100. [DOI] [PubMed] [Google Scholar]
- 104. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998; 12: 426–445. [DOI] [PubMed] [Google Scholar]
- 105. Chen Y. Abnormal visual motion processing in schizophrenia: A review of research progress. Schizophr. Bull. 2011; 37: 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kiss I, Fábián Á, Benedek G, Kéri S. When doors of perception open: Visual contrast sensitivity in never‐medicated, first‐episode schizophrenia. J. Abnorm. Psychol. 2010; 119: 586–593. [DOI] [PubMed] [Google Scholar]
- 107. Kelemen O, Kiss I, Benedek G, Kéri S. Perceptual and cognitive effects of antipsychotics in first‐episode schizophrenia: The potential impact of GABA concentration in the visual cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013; 47: 13–19. [DOI] [PubMed] [Google Scholar]
- 108. Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr. Biol. 2005; 15: R822–R824. [DOI] [PubMed] [Google Scholar]
- 109. Tibber MS, Anderson EJ, Bobin T et al. Visual surround suppression in schizophrenia. Front. Psychol. 2013; 4: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia:When and why does it go awry? Schizophr. Res. 2007; 94: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vollmer‐Larsen A, Handest P, Parnas J. Reliability of measuring anomalous experience: The Bonn scale for the assessment of basic symptoms. Psychopathology 2007; 40: 345–348. [DOI] [PubMed] [Google Scholar]
- 112. Kogata T, Iidaka T. A review of impaired visual processing and the daily visual world in patients with schizophrenia. Nagoya J. Med. Sci. 2018; 80: 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Suzuki M, Takahashi S, Matsushima E et al. Relationships between exploratory eye movement dysfunction and clinical symptoms in schizophrenia. Psychiatry Clin. Neurosci. 2012; 66: 187–194. [DOI] [PubMed] [Google Scholar]
- 114. Bolding MS, Lahti AC, White D et al. Vergence eye movements in patients with schizophrenia. Vision Res. 2014; 102: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kojima T, Matsushima E, Ando K et al. Exploratory eye movements and neuropsychological tests in schizophrenic patients. Schizophr. Bull. 1992; 18: 85–94. [DOI] [PubMed] [Google Scholar]
- 116. Lencer R, Sprenger A, Reilly JL et al. Pursuit eye movements as an intermediate phenotype across psychotic disorders: Evidence from the B‐SNIP study. Schizophr. Res. 2015; 169: 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Arolt V, Teichert HM, Steege D, Lencer R, Heide W. Distinguishing schizophrenic patients from healthy controls by quantitative measurement of eye movement parameters. Biol. Psychiatry 1998; 44: 448–458. [DOI] [PubMed] [Google Scholar]
- 118. Miura K, Hashimoto R, Fujimoto M et al. An integrated eye movement score as a neurophysiological marker of schizophrenia. Schizophr. Res. 2014; 160: 228–229. [DOI] [PubMed] [Google Scholar]
- 119. Shic F. Eye tracking as a behavioral biomarker for psychiatric conditions: The road ahead. J. Am. Acad. Child Adolesc. Psychiatry 2016; 55: 267–268. [DOI] [PubMed] [Google Scholar]
- 120. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 121. Kojima T, Matsushima E, Ohta K et al. Stability of exploratory eye movements as a marker of schizophrenia ‐ A WHO multi‐center study. Schizophr. Res. 2001; 52: 203–213. [DOI] [PubMed] [Google Scholar]
- 122. Brakemeier S, Sprenger A, Meyhöfer I et al. Smooth pursuit eye movement deficits as a biomarker for psychotic features in bipolar disorder—Findings from the PARDIP study. Bipolar Disord. 2019; 22: 602–611. [DOI] [PubMed] [Google Scholar]
- 123. Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron 2009; 63: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hannula DE, Althoff RR, Warren DE, Riggs L, Cohen NJ, Ryan JD. Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Front. Hum. Neurosci. 2010; 4: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Green HJ, Lemaire P, Dufau S. Eye movement correlates of younger and older adults' strategies for complex addition. Acta Psychol. (Amst) 2007; 125: 257–278. [DOI] [PubMed] [Google Scholar]
- 126. Rayner K. Eye guidance in reading: Fixation locations within words. Perception 1979; 8: 21–30. [DOI] [PubMed] [Google Scholar]
- 127. Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia 2006; 44: 2259–2269. [DOI] [PubMed] [Google Scholar]
- 128. Funahashi S, Bruce CJ, Goldman‐Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 1989; 61: 331–349. [DOI] [PubMed] [Google Scholar]
- 129. Munoz DP. Commentary: Saccadic eye movements: Overview of neural circuitry. Prog. Brain Res. 2002; 140: 89–96. [DOI] [PubMed] [Google Scholar]
- 130. Luna B, Velanova K. Development from reflexive to controlled eye movements. In: The Oxford Handbook of Eye Movements. Oxford, United Kingdom: Oxford University Press; 2012. [Google Scholar]
- 131. Fried M, Tsitsiashvili E, Bonneh YS et al. ADHD subjects fail to suppress eye blinks and microsaccades while anticipating visual stimuli but recover with medication. Vision Res. 2014; 101: 62–72. [DOI] [PubMed] [Google Scholar]
- 132. Schwab S, Jost M, Altorfer A. Impaired top‐down modulation of saccadic latencies in patients with schizophrenia but not in first‐degree relatives. Front Behav. Neurosci. 2015; 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Munoz DP, Everling S. Look away: The anti‐saccade task and the voluntary control of eye movement. Nat. Rev. Neurosci. 2004; 5: 218–228. [DOI] [PubMed] [Google Scholar]
- 134. Thibaut JP, French RM. Analogical reasoning, control and executive functions: A developmental investigation with eye‐tracking. Cogn. Dev. 2016; 38: 10–26. [Google Scholar]
- 135. Oh J, Chun J‐W, Lee JS, Kim J‐J. Relationship between abstract thinking and eye gaze pattern in patients with schizophrenia. Behav. Brain Funct. 2014; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bekele E, Bian D, Peterman J, Park S, Sarkar N. Design of a virtual reality system for affect analysis in facial expressions (VR‐SAAFE); application to schizophrenia. IEEE Trans. Neural Syst. Rehabil. Eng. 2017; 25: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bradley ER, Seitz A, Niles AN et al. Oxytocin increases eye gaze in schizophrenia. Schizophr. Res. 2019; 212: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Reisinger DL, Shaffer RC, Horn PS et al. Atypical social attention and emotional face processing in autism spectrum disorder: Insights from face scanning and pupillometry. Front. Integr. Neurosci. 2019; 13: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cercenelli L, Tiberi G, Bortolani B et al. Gaze Trajectory Index (GTI): A novel metric to quantify saccade trajectory deviation using eye tracking. Comput. Biol. Med. 2019; 107: 86–96. [DOI] [PubMed] [Google Scholar]
- 140. Kacur J, Polec J, Smolejova E, Heretik A. An analysis of eye‐tracking features and modelling methods for free‐viewed standard stimulus: Application for schizophrenia detection. IEEE J. Biomed. Heal. Informatics 2020; 24: 3055–3065. [DOI] [PubMed] [Google Scholar]
- 141. Shimojo S, Simion C, Shimojo E, Scheier C. Gaze bias both reflects and influences preference. Nat. Neurosci. 2003; 6: 1317–1322. [DOI] [PubMed] [Google Scholar]
- 142. Glaholt MG, Reingold EM. Stimulus exposure and gaze bias: A further test of the gaze cascade model. Atten. Percept. Psychophys. 2009; 71: 445–450. [DOI] [PubMed] [Google Scholar]
- 143. Glaholt MG, Reingold EM. Eye movement monitoring as a process tracing methodology in decision making research. J. Neurosci. Psychol. Econ. 2011; 4: 125–146. [Google Scholar]
- 144. Bird GD, Lauwereyns J, Crawford MT. The role of eye movements in decision making and the prospect of exposure effects. Vision Res. 2012; 60: 16–21. [DOI] [PubMed] [Google Scholar]
- 145. Noton D, Stark L. Scanpaths in eye movements during pattern perception. Science 1971; 171: 308–311. [DOI] [PubMed] [Google Scholar]
- 146. Noton D, Stark L. Scanpaths in saccadic eye movements while viewing and recognizing patterns. Vision Res. 1971; 11: 929–IN8. [DOI] [PubMed] [Google Scholar]
- 147. Gaebel W, Ulrich G, Frick K. Visuomotor performance of schizophrenic patients and normal controls in a picture viewing task. Biol. Psychiatry 1987; 22: 1227–1237. [DOI] [PubMed] [Google Scholar]
- 148. Dijkstra KA, van der Pligt J, van Kleef GA, Kerstholt JH. Deliberation versus intuition: Global versus local processing in judgment and choice. J. Exp. Soc. Psychol. 2012; 48: 1156–1161. [Google Scholar]
- 149. Mega LF, Volz KG. Intuitive face judgments rely on holistic eye movement pattern. Front. Psychol. 2017; 8: 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sabater‐Grande G, Haro G, García‐Gallego A, Georgantzís N, Herranz‐Zarzoso N, Baquero A. Risk‐taking and fairness among cocaine‐dependent patients in dual diagnoses: Schizophrenia and anti‐social personality disorder. Sci. Rep. 2020; 10: 10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Scholl J, Klein‐Flügge M. Understanding psychiatric disorder by capturing ecologically relevant features of learning and decision‐making. Behav. Brain Res. 2018; 355: 56–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Dang J, Xiao S, Liu Y, Jiang Y, Mao L. Individual differences in dopamine level modulate the ego depletion effect. Int. J. Psychophysiol. 2016; 99: 121–124. [DOI] [PubMed] [Google Scholar]
- 153. Królak A, Strumiłło P. Eye‐blink detection system for human‐computer interaction. Univers. Access Inf. Soc. 2012; 11: 409–419. [Google Scholar]
- 154. Bacher LF, Smotherman WP. Spontaneous eye blinking in human infants: A Review. Dev. Psychobiol. 2004; 44: 95–102. [DOI] [PubMed] [Google Scholar]
- 155. DeAngelus M, Pelz JB. Top‐down control of eye movements: Yarbus revisited. Vis. Cogn. 2009; 17: 790–811. [Google Scholar]
- 156. Cerf M, Frady EP, Koch C. Faces and text attract gaze independent of the task: Experimental data and computer model. J. Vis. 2009; 9: 10.1–10.15. [DOI] [PubMed] [Google Scholar]
- 157. Martinez‐Conde S, Macknik SL. From exploration to fixation: An integrative view of Yarbus's vision. Perception 2015; 44: 884–899. [DOI] [PubMed] [Google Scholar]
- 158. Barkley‐Levenson E, Galván A. Eye blink rate predicts reward decisions in adolescents. Dev. Sci. 2017; 20: e12412. [DOI] [PubMed] [Google Scholar]
- 159. King DJ, Hodgekins J, Chouinard PA, Chouinard VA, Sperandio I. A review of abnormalities in the perception of visual illusions in schizophrenia. Psychon. Bull. Rev. 2017; 24: 734–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Uhlhaas PJ, Phillips WA, Schenkel LS, Silverstein SM. Theory of mind and perceptual context‐processing in schizophrenia. Cogn. Neuropsychiatry 2006; 11: 416–436. [DOI] [PubMed] [Google Scholar]
- 161. Krajbich I, Armel C, Rangel A. Visual fixations and the computation and comparison of value in simple choice. Nat. Neurosci. 2010; 13: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 162. Lai ML, Tsai MJ, Yang FY et al. A review of using eye‐tracking technology in exploring learning from 2000 to 2012. Educ. Res. Rev. 2013; 10: 90–115. [Google Scholar]
- 163. Cornblatt BA, Lenzenweger M, Dworkin RE‐KL. Positive and negative schizophrenic symptoms, attention, and information processing. Schizophr. Bull. 1985; 11: 397–408. [DOI] [PubMed] [Google Scholar]
- 164. Erlenmeyer‐Kimling L, Rock D, Roberts SA et al. Attention, memory, and motor skills as childhood predictors of schizophrenia‐related psychoses: The New York high‐risk project. Am. J. Psychiatry 2000; 157: 1416–1422. [DOI] [PubMed] [Google Scholar]
- 165. Carrión RE, McLaughlin D, Goldberg TE et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiat. 2013; 70: 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Saykin AJ, Gur RC, Gur RE et al. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Arch. Gen. Psychiatry 1991; 48: 618–624. [DOI] [PubMed] [Google Scholar]
- 167. Keefe RSE, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol. Psychiatry 2005; 57: 688–691. [DOI] [PubMed] [Google Scholar]
- 168. Kahn RS. On the specificity of continuous cognitive decline in schizophrenia. Am. J. Psychiatry 2019; 176: 774–776. [DOI] [PubMed] [Google Scholar]
- 169. Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: A meta‐analytic review. Am. J. Psychiatry 2008; 165: 579–587. [DOI] [PubMed] [Google Scholar]
- 170. Caspi A, Reichenberg A, Weiser M et al. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr. Res. 2003; 65: 87–94. [DOI] [PubMed] [Google Scholar]
- 171. Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr. Res. 2005; 78: 27–34. [DOI] [PubMed] [Google Scholar]
- 172. Walter Heinrichs R. The primacy of cognition in schizophrenia. Am. Psychol. 2005; 60: 229–242. [DOI] [PubMed] [Google Scholar]
- 173. Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: Evidence from a prospective birth cohort 28 year follow‐up study. J. Clin. Exp. Neuropsychol. 2006; 28: 225–242. [DOI] [PubMed] [Google Scholar]
- 174. Trotta A, Murray RM, Maccabe JH. Do premorbid and post‐onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta‐analysis. Psychol. Med. 2015; 45: 381–394. [DOI] [PubMed] [Google Scholar]
- 175. Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr. Bull. 1993; 19: 233–259. [DOI] [PubMed] [Google Scholar]
- 176. Bachman P, Reichenberg A, Rice P et al. Deconstructing processing speed deficits in schizophrenia: Application of a parametric digit symbol coding test. Schizophr. Res. 2010; 118: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze‐Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry 2001; 58: 158–164. [DOI] [PubMed] [Google Scholar]
- 178. Silverstein SM, Fradkin SI, Demmin DL. Schizophrenia and the retina: Towards a 2020 perspective. Schizophr. Res. 2020; 219: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Holzman PS, Proctor LR, Hughes DW. Eye‐tracking patterns in schizophrenia. Science 1973; 181: 179–181. [DOI] [PubMed] [Google Scholar]
- 180. Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye‐tracking dysfunctions in schizophrenic patients and their relatives. Arch. Gen. Psychiatry 1974; 31: 143–151. [DOI] [PubMed] [Google Scholar]
- 181. Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking and schizophrenia: A selective review. Schizophr. Bull. 1994; 20: 47–62. [DOI] [PubMed] [Google Scholar]
- 182. Morita K, Miura K, Fujimoto M et al. Eye movement abnormalities and their association with cognitive impairments in schizophrenia. Schizophr. Res. 2019; 209: 255–262. [DOI] [PubMed] [Google Scholar]
- 183. Roux P, Brunet‐Gouet E, Passerieux C, Ramus F. Eye‐tracking reveals a slowdown of social context processing during intention attribution in patients with schizophrenia. J. Psychiatry Neurosci. 2016; 41: E13–E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Diefendorf AR, Dodge R. An experimental study of the ocular reactions of the insane from photographic records. Brain 1908; 31: 451–489. [Google Scholar]
- 185. Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking dysfunction and schizophrenia: A critical perspective. Schizophr. Bull. 1993; 19: 461–536. [DOI] [PubMed] [Google Scholar]
- 186. Levy DL, Yasillo NJ, Dorus E et al. Relatives of unipolar and bipolar patients have normal pursuit. Psychiatry Res. 1983; 10: 285–293. [DOI] [PubMed] [Google Scholar]
- 187. Okruszek Ł, Pilecka I. Biological motion processing in schizophrenia – Systematic review and meta‐analysis. Schizophr. Res. 2017; 190: 3–10. [DOI] [PubMed] [Google Scholar]
- 188. Kim J, Doop ML, Blake R, Park S. Impaired visual recognition of biological motion in schizophrenia. Schizophr. Res. 2005; 77: 299–307. [DOI] [PubMed] [Google Scholar]
- 189. Elahipanah A, Christensen BK, Reingold EM. Attentional guidance during visual search among patients with schizophrenia. Schizophr. Res. 2011; 131: 224–230. [DOI] [PubMed] [Google Scholar]
- 190. Chen Y, Levy DL, Sheremata S, Holzman PS. Bipolar and schizophrenic patients differ in patterns of visual motion discrimination. Schizophr. Res. 2006; 88: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kim J, Norton D, McBain R, Ongur D, Chen Y. Deficient biological motion perception in schizophrenia: Results from a motion noise paradigm. Front. Psychol. 2013; 4: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kaliuzhna M, Stein T, Sterzer P, Seymour KJ. Examining motion speed processing in schizophrenia using the flash lag illusion. Schizophr. Res. Cogn. 2020; 19: 100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Matsushima E, Kojima T, Ohta K et al. Exploratory eye movement dysfunction in patients with schizophrenia: Possibility as a discriminator for schizophrenia. J. Psychiatr. Res. 1998; 32: 289–295. [DOI] [PubMed] [Google Scholar]
- 194. Holzman PS. Eye movement dysfunctions and psychosis. Int. Rev. Neurobiol. 1985; 27: 179–205. [DOI] [PubMed] [Google Scholar]
- 195. Holzman PS, Solomon CM, Levin S, Waternaux CS. Pursuit eye movement dysfunctions in schizophrenia: Family evidence for specificity. Arch. Gen. Psychiatry 1984; 41: 136–139. [DOI] [PubMed] [Google Scholar]
- 196. Levin S, Luebke A, Zee DS, Hain TC, Robinson DA, Holzman PS. Smooth pursuit eye movements in schizophrenics: Quantitative measurements with the search‐coil technique. J. Psychiatr. Res. 1988; 22: 195–206. [DOI] [PubMed] [Google Scholar]
- 197. Holzman PS, Kringlen E, Levy DL, Proctor LR, Haberman SJ, Yasillo NJ. Abnormal‐pursuit eye movements in schizophrenia: Evidence for a genetic indicator. Arch. Gen. Psychiatry 1977; 34: 802–805. [DOI] [PubMed] [Google Scholar]
- 198. Holzman PS, Levy DL, Proctor LR. Smooth pursuit eye movements, attention, and schizophrenia. Arch. Gen. Psychiatry 1976; 33: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 199. Holzman PS, Kringlen E, Levy DL, Haberman SJ. Deviant eye tracking in twins discordant for psychosis: A replication. Arch. Gen. Psychiatry 1980; 37: 627–631. [DOI] [PubMed] [Google Scholar]
- 200. Abel LA, Friedman L, Jesberger J, Malki A, Meltzer HY. Quantitative assessment of smooth pursuit gain and catch‐up saccades in schizophrenia and affective disorders. Biol. Psychiatry 1991; 29: 1063–1072. [DOI] [PubMed] [Google Scholar]
- 201. Abel LA, Levin S, Holzman PS. Abnormalities of smooth pursuit and saccadic control in schizophrenia and affective disorders. Vision Res. 1992; 32: 1009–1014. [DOI] [PubMed] [Google Scholar]
- 202. Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology 2006; 43: 302–313. [DOI] [PubMed] [Google Scholar]
- 203. Bey K, Meyhöfer I, Lennertz L et al. Schizotypy and smooth pursuit eye movements as potential endophenotypes of obsessive‐compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2019; 269: 235–243. [DOI] [PubMed] [Google Scholar]
- 204. Fukumoto‐Motoshita M, Matsuura M, Ohkubo T et al. Hyperfrontality in patients with schizophrenia during saccade and antisaccade tasks: A study with fMRI. Psychiatry Clin. Neurosci. 2009; 63: 209–217. [DOI] [PubMed] [Google Scholar]
- 205. Combs DR, Tosheva A, Penn DL, Basso MR, Wanner JL, Laib K. Attentional‐shaping as a means to improve emotion perception deficits in schizophrenia. Schizophr. Res. 2008; 105: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Winograd‐Gurvich C, Fitzgerald PB, Georgiou‐Karistianis N, Millist L, White O. Inhibitory control and spatial working memory: A saccadic eye movement study of negative symptoms in schizophrenia. Psychiatry Res. 2008; 157: 9–19. [DOI] [PubMed] [Google Scholar]
- 207. Nishimura Y, Takizawa R, Muroi M, Marumo K, Kinou M, Kasai K. Prefrontal cortex activity during response inhibition associated with excitement symptoms in schizophrenia. Brain Res. 2011; 1370: 194–203. [DOI] [PubMed] [Google Scholar]
- 208. Nieman DH, Bour LJ, Linszen DH et al. Neuropsychological and clinical correlates of antisaccade task performance in schizophrenia. Neurology 2000; 54: 866–871. [DOI] [PubMed] [Google Scholar]
- 209. Gooding DC, Basso MA. The tell‐tale tasks: A review of saccadic research in psychiatric patient populations. Brain Cogn. 2008; 68: 371–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Calkins ME, Iacono WG, Ones DS. Eye movement dysfunction in first‐degree relatives of patients with schizophrenia: A meta‐analytic evaluation of candidate endophenotypes. Brain Cogn. 2008; 68: 436–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Radant AD, Dobie DJ, Calkins ME et al. Successful multi‐site measurement of antisaccade performance deficits in schizophrenia. Schizophr. Res. 2007; 89: 320–329. [DOI] [PubMed] [Google Scholar]
- 212. Iacono WG, Lykken DT. Eye tracking and psychopathology: New procedures applied to a sample of normal monozygotic twins. Arch. Gen. Psychiatry 1979; 36: 1361–1369. [DOI] [PubMed] [Google Scholar]
- 213. Mackworth NH, Bruner JS. How adults and children search and recognize pictures. Hum. Dev. 1970; 13: 149–177. [DOI] [PubMed] [Google Scholar]
- 214. Kojima T, Matsushima E, Nakajima K et al. Eye movements in acute, chronic, and remitted schizophrenics. Biol. Psychiatry 1990; 27: 975–989. [DOI] [PubMed] [Google Scholar]
- 215. Kojima T, Potkin SG, Kharazmi M, Matsushima E, Herrera J, Shimazono Y. Limited eye movement patterns in chronic schizophrenic patients. Psychiatry Res. 1989; 28: 307–314. [DOI] [PubMed] [Google Scholar]
- 216. Moriya H. A study of eye movements in patients with chronic schizophrenia and in their relatives, using an eye‐mark recorder. Psychiatr. Neurol. Jpn. 1979; 81: 523–558. [PubMed] [Google Scholar]
- 217. Suzuki M, Takahashi S, Matsushima E et al. Exploratory eye movement dysfunction as a discriminator for schizophrenia: A large sample study using a newly developed digital computerized system. Eur. Arch. Psychiatry Clin. Neurosci. 2009; 259: 186–194. [DOI] [PubMed] [Google Scholar]
- 218. Moriya H, Ando K, Kojima T et al. Eye movements during perception of pictures in chronic schizophrenia. Psychiatry Clin. Neurosci. 1972; 26: 189–199. [DOI] [PubMed] [Google Scholar]
- 219. Takahashi S, Tanabe E, Yara K, Matsuura M, Matsushima E, Kojima T. Impairment of exploratory eye movement in schizophrenia patients and their siblings. Psychiatry Clin. Neurosci. 2008; 62: 487–493. [DOI] [PubMed] [Google Scholar]
- 220. Kojima T, Matsushima E. Cognitive dysfunction in schizophrenia—analysis using exploratory eye movements. Seishin Shinkeigaku Zasshi 2000; 102: 445–458. [PubMed] [Google Scholar]
- 221. Qiu L, Tian L, Pan C et al. Neuroanatomical circuitry associated with exploratory eye movement in schizophrenia: A voxel‐based morphometric study. PLoS One 2011; 6: e25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Padmanabhan JL, Tandon N, Haller CS et al. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar i disorders. Schizophr. Bull. 2015; 41: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Tsunoda M, Kawasaki Y, Matsui M et al. Relationship between exploratory eye movements and brain morphology in schizophrenia spectrum patients: Voxel‐based morphometry of three‐dimensional magnetic resonance imaging. Eur. Arch. Psychiatry Clin. Neurosci. 2005; 255: 104–110. [DOI] [PubMed] [Google Scholar]
- 224. Qiu L, Yan H, Zhu R et al. Correlations between exploratory eye movement, hallucination, and cortical gray matter volume in people with schizophrenia. BMC Psychiatry 2018; 18: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Yasuda Y, Okada N, Nemoto K et al. Brain morphological and functional features in cognitive subgroups of schizophrenia. Psychiatry Clin. Neurosci. 2020; 74: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Morita K, Miura K, Fujimoto M et al. Eye‐movement characteristics of schizophrenia and their association with cortical thickness. Psychiatry Clin. Neurosci. 2019; 73: 508–509. [DOI] [PubMed] [Google Scholar]
- 227. Koshiyama D, Fukunaga M, Okada N et al. Role of subcortical structures on cognitive and social function in schizophrenia. Sci. Rep. 2018; 8: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Wolf RC, Höse A, Frasch K, Walter H, Vasic N. Volumetric abnormalities associated with cognitive deficits in patients with schizophrenia. Eur. Psychiatry 2008; 23: 541–548. [DOI] [PubMed] [Google Scholar]
- 229. Cannon TD. The inheritance of intermediate phenotypes for schizophrenia. Curr. Opin. Psychiatry 2005; 18: 135–140. [DOI] [PubMed] [Google Scholar]
- 230. Fujino H, Sumiyoshi C, Yasuda Y et al. Estimated cognitive decline in patients with schizophrenia: A multicenter study. Psychiatry Clin. Neurosci. 2017; 71: 294–300. [DOI] [PubMed] [Google Scholar]
- 231. Edgar JC. Identifying electrophysiological markers of autism spectrum disorder and schizophrenia against a backdrop of normal brain development. Psychiatry Clin. Neurosci. 2020; 74: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. van Erp TGM, Hibar DP, Rasmussen JM et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 2016; 21: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Okada N, Fukunaga M, Yamashita F et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 2016; 21: 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: Modeling the role of ability and motivation. Arch. Gen. Psychiatry 2012; 69: 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Morita K, Miura K, Fujimoto M et al. Abnormalities of eye movement are associated with work hours in schizophrenia. Schizophr. Res. 2018; 202: 420–422. [DOI] [PubMed] [Google Scholar]
- 236. Sawada K, Sakakibara E, Kanehara A et al. Is Utena's Brief Objective Measures (UBOM) useful in real‐world behavioral assessment of functioning? Validity and utility testing in patients with schizophrenia. Psychiatry Clin. Neurosci. 2020; 74: 40–48. [DOI] [PubMed] [Google Scholar]
- 237. Lakhlifi M, Laprevote V, Schwan R, Schwitzer T. Free viewing exploration in schizophrenia: Review of evidence from laboratory settings to natural environment. Encephale. 2020; 46: 115–122. [DOI] [PubMed] [Google Scholar]
- 238. Campana A, Duci A, Gambini O, Scarone S. An artificial neural network that uses eye‐tracking performance to identify patients with schizophrenia. Schizophr. Bull. 1999; 25: 789–799. [DOI] [PubMed] [Google Scholar]
- 239. Streit M, Wölwer W, Gaebel W. Facial‐affect recognitionand visual scanning behaviour in the course of schizophrenia. Schizophr. Res. 1997; 24: 311–317. [DOI] [PubMed] [Google Scholar]
- 240. Loughland CM, Williams LM, Gordon E. Visual scanpaths to positive and negative facial emotions in an outpatient schizophrenia sample. Schizophr. Res. 2002; 55: 159–170. [DOI] [PubMed] [Google Scholar]
- 241. Loughland CM, Williams LM, Harris AW. Visual scanpath dysfunction in first‐degree relatives of schizophrenia probands: Evidence for a vulnerability marker? Schizophr. Res. 2004; 67: 11–21. [DOI] [PubMed] [Google Scholar]
- 242. Frith CD, Stevens M, Johnstone EC, Owens DG, Crow TJ. Integration of schematic faces and other complex objects in schizophrenia. J. Nerv. Ment. Dis. 1983; 171: 34–39. [DOI] [PubMed] [Google Scholar]
- 243. Gordon E, Coyle S, Anderson J et al. Eye movement response to a facial stimulus in schizophrenia. Biol. Psychiatry 1992; 31: 626–629. [DOI] [PubMed] [Google Scholar]
- 244. Phillips ML, David AS. Visual scan paths are abnormal in deluded schizophrenics. Neuropsychologia 1997; 35: 99–105. [DOI] [PubMed] [Google Scholar]
- 245. Williams LM, Loughland CM, Gordon E, Davidson D. Visual scanpaths in schizophrenia: Is there a deficit in face recognition? Schizophr. Res. 1999; 40: 189–199. [DOI] [PubMed] [Google Scholar]
- 246. Horley K, Williams L, Gonsalvez C, Gordon E. Face to Face: Visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Res. 2004; 127: 43–53. [DOI] [PubMed] [Google Scholar]
- 247. Horley K, Williams LM, Gonsalvez C, Gordon E. Social phobics do not see eye to eye: A visual scanpath study of emotional expression processing. J. Anxiety Disord. 2003; 17: 33–44. [DOI] [PubMed] [Google Scholar]
- 248. Bestelmeyer PEG, Tatler BW, Phillips LH, Fraser G, Benson PJ, Clair DS. Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophr. Res. 2006; 87: 212–222. [DOI] [PubMed] [Google Scholar]
- 249. Karatekin C, Asarnow RF. Exploratory eye movements to pictures in childhood‐onset schizophrenia and attention‐deficit/hyperactivity disorder (ADHD). J. Abnorm. Child Psychol. 1999; 27: 35–49. [DOI] [PubMed] [Google Scholar]
- 250. Türkan BN, Amado S, Ercan ES, Perçinel I. Comparison of change detection performance and visual search patterns among children with/without ADHD: Evidence from eye movements. Res. Dev. Disabil. 2016; 49‐50: 205–215. [DOI] [PubMed] [Google Scholar]
- 251. Freeman D, Garety PA, Phillips ML. An examination of hypervigilance for external threat in individuals with generalized anxiety disorder and individuals with persecutory delusions using visual scan. Q. J. Exp. Psychol. A. 2000; 53: 549–567. [DOI] [PubMed] [Google Scholar]
- 252. Baron‐Cohen S, Wheelwright S, Jolliffe T. Is there a “language of the eyes”? Evidence from normal adults, and adults with autism or Asperger syndrome. Vis. Cogn. 1997; 4: 311–331. [Google Scholar]
- 253. Fletcher‐Watson S, Leekam SR, Benson V, Frank MC, Findlay JM. Eye‐movements reveal attention to social information in autism spectrum disorder. Neuropsychologia 2009; 47: 248–257. [DOI] [PubMed] [Google Scholar]
- 254. Robertson CE, Baron‐Cohen S. Sensory perception in autism. Nat. Rev. Neurosci. 2017; 18: 671–684. [DOI] [PubMed] [Google Scholar]
- 255. Startsev M, Dorr M. Classifying autism spectrum disorder based on scanpaths and saliency. In: Proceedings ‐ 2019 IEEE International Conference on Multimedia and Expo Workshops, ICMEW 2019. Institute of Electrical and Electronics Engineers Inc.; 2019; 633–636.
- 256. Dalton KM, Nacewicz BM, Johnstone T et al. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005; 8: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. Am. J. Psychiatry 2002; 159: 895–908. [DOI] [PubMed] [Google Scholar]
- 258. Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry 2002; 59: 809–816. [DOI] [PubMed] [Google Scholar]
- 259. Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J. Autism Dev. Disord. 2002; 32: 249–261. [DOI] [PubMed] [Google Scholar]
- 260. Rutherford MD, Towns AM. Scan path differences and similarities during emotion perception in those with and without autism spectrum disorders. J. Autism Dev. Disord. 2008; 38: 1371–1381. [DOI] [PubMed] [Google Scholar]
- 261. Fukushima J. Neurophysiological studies in autism spectrum disorders—comparison with those in schizophrenia. Seishin Shinkeigaku Zasshi 2012; 114: 335–348 (in Japanese). [PubMed] [Google Scholar]
- 262. Shiino T, Miura K, Fujimoto M et al. Comparison of eye movements in schizophrenia and autism spectrum disorder. Neuropsychopharmacol. Rep. 2020; 40: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Holzman PS. Eye movements and the search for the essence of schizophrenia. Brain Res. Rev. 2000; 31: 350–356. [DOI] [PubMed] [Google Scholar]
- 264. Bansal S, Bray LCJ, Schwartz BL, Joiner WM. Transsaccadic perception deficits in schizophrenia reflect the improper internal monitoring of eye movement rather than abnormal sensory processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018; 3: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Jurišić D, Ćavar I, Sesar A, Sesar I, Vukojević J, Ćurković M. New insights into schizophrenia: A look at the eye and related structures. Psychiatr. Danub. 2020; 32: 60–69. [DOI] [PubMed] [Google Scholar]
- 266. Itti L. New eye‐tracking techniques may revolutionize mental health screening. Neuron 2015; 88: 442–444. [DOI] [PubMed] [Google Scholar]
- 267. Wang S, Jiang M, Duchesne XM et al. Atypical visual saliency in autism spectrum disorder quantified through model‐based eye tracking. Neuron 2015; 88: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Brousseau B, Rose J, Eizenman M. Hybrid eye‐tracking on a smartphone with CNN feature extraction and an infrared 3D model. Sensors (Basel). 2020; 20: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Phillips ML, Young AW, Senior C et al. A specific neural substrate for perceiving facial expressions of disgust. Nature 1997; 389: 495–498. [DOI] [PubMed] [Google Scholar]
- 270. Phillips ML, David AS. Abnormal visual scan paths: A psychophysiological marker of delusions in schizophrenia. Schizophr. Res. 1998; 29: 235–245. [DOI] [PubMed] [Google Scholar]
- 271. Phillips ML, Williams L, Senior C et al. A differential neural response to threatening and non‐threatening negative facial expressions in paranoid and non‐paranoid schizophrenics. Psychiatry Res. 1999; 92: 11–31. [DOI] [PubMed] [Google Scholar]
- 272. Williams LM, Loughland CM, Green MJ, Harris AWF, Gordon E. Emotion perception in schizophrenia: An eye movement study comparing the effectiveness of risperidone vs. haloperidol. Psychiatry Res. 2003; 120: 13–27. [DOI] [PubMed] [Google Scholar]
- 273. Unema PJA, Pannasch S, Joos M, Velichkovsky BM. Time course of information processing during scene perception: The relationship between saccade amplitude and fixation duration. Vis. Cogn. 2005; 12: 473–494. [Google Scholar]
- 274. Benson PJ, Leonards U, Lothian RM, Clair DM, Merlo MCG. Visual scan paths in first‐episode schizophrenia and cannabis‐induced psychosis. J. Psychiatry Neurosci. 2007; 32: 267–274. [PMC free article] [PubMed] [Google Scholar]
- 275. Perry W, Braff DL. A multimethod approach to assessing perseverations in schizophrenia patients. Schizophr. Res. 1998; 33: 69–77. [DOI] [PubMed] [Google Scholar]
- 276. Arango C, Bartko JJ, Gold JM, Buchanan RW. Prediction of neuropsychological performance by neurological signs in schizophrenia. Am. J. Psychiatry 1999; 156: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 277. Price GW, Michie PT, Johnston J et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biol. Psychiatry 2006; 60: 1–10. [DOI] [PubMed] [Google Scholar]
- 278. John JP, Arunachalam V, Ratnam B, Isaac MK. Expanding the schizophrenia phenotype: A composite evaluation of neurodevelopmental markers. Compr. Psychiatry 2008; 49: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Leahy RL. Decision making and personality disorders. J. Cogn. Psychother. 2002; 16: 209–225. [Google Scholar]
- 280. Kishida KT, King‐Casas B, Montague PR. Neuroeconomic approaches to mental disorders. Neuron 2010; 67: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Wischniewski J, Brune M. Moral reasoning in schizophrenia: An explorative study into economic decision making. Cogn. Neuropsychiatry 2011; 16: 348–363. [DOI] [PubMed] [Google Scholar]
- 282. Nikitova N, Keane BP, Demmin D, Silverstein SM, Uhlhaas PJ. The Audio‐Visual Abnormalities Questionnaire (AVAQ): Development and validation of a new instrument for assessing anomalies in sensory perception in schizophrenia spectrum disorders. Schizophr. Res. 2019; 209: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Matsushima E, Kojima T, Ohbayashi S, Ando H, Ando K, Shimazono Y. Exploratory eye movements in schizophrenic patients and patients with frontal lobe lesions. Eur. Arch. Psychiatry Clin. Neurosci. 1992; 241: 210–214. [DOI] [PubMed] [Google Scholar]
- 284. Nakajima K, Kojima T, Matsushima E et al. Eye movements in patients with temporal lobe epilepsy—Comparison with schizophrenics and normal controls. Psychiatry Clin. Neurosci. 1988; 42: 632–634. [DOI] [PubMed] [Google Scholar]
- 285. Beedie SA, Clair DM, Rujescu DP, Benson PJ. Smooth pursuit and visual scanpaths: related or independent deficits in schizophrenia? Schizophr. Res. 2010; 117: 247–248. [Google Scholar]
- 286. Czepielewski LS, Wang L, Gama CS, Barch DM. The relationship of intellectual functioning and cognitive performance to brain structure in schizophrenia. Schizophr. Bull. 2017; 43: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Daban C, Martinez‐Aran A, Torrent C et al. Specificity of cognitive deficits in bipolar disorder versus schizophrenia: A systematic review. Psychother. Psychosom. 2006; 75: 72–84. [DOI] [PubMed] [Google Scholar]
- 288. van't Wout M, Sanfey AG. Interactive decision‐making in people with schizotypal traits: A game theory approach. Psychiatry Res. 2011; 185: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


