ABSTRACT
Apathy, the loss of motivation, is a common problem in Parkinson's disease (PD) and often observed following deep brain stimulation (DBS) of the subthalamic nucleus (STN). The aim of this meta‐analysis was to determine the occurrence of apathy following STN DBS in literature. Relevant articles were searched in PubMed/Medline, SCOPUS, EMBASE, and Web of Sciences electronic databases. Studies were included if they reported apathy scores pre‐ and post‐DBS or the cross‐sectional difference between PD patients receiving STN DBS and patients receiving medication only. Thirty‐three articles were included in the meta‐analyses from 6,658 screened articles by two authors independently. A total of 1,286 patients were included with a mean age (±standard deviation [SD]) of 58.4 ± 8.5 years and a disease duration of 11.0 ± 5.8 years. The apathy score measured by means of the Apathy Evaluation Scale (AES), Starkstein Apathy Scale (SAS), and the Lille Apathy Rating Scale (LARS) was significantly higher after DBS than pre‐operatively (g = 0.34, 95% confidence interval [CI] = 0.19–0.48, P < 0.001). An equal, significant difference in severity of apathy was found between STN DBS and medication only (g = 0.36, 95% CI = 0.03–0.65; P = 0.004). Statistical heterogeneity was moderately high, but the effects stood strong after multiple analyses and were independent of tapering off dopaminergic medication. The findings of this meta‐analysis indicate that apathy is increased after STN DBS compared to the pre‐operative state and to medication only (systematic review registration number: PROSPERO CRD42019133932). © 2020 Universiteit van Amsterdam. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: apathy, Parkinson, deep brain stimulation, subthalamic nucleus
Parkinson's disease (PD) is a neurodegenerative disorder characterized by bradykinesia, rigidity, and rest tremor. 1 Of PD patients, 60%–90% will develop non‐motor symptoms such as cognitive decline, anxiety, and depression. 2 Although dopaminergic drugs treat the motor manifestations effectively, they may be accompanied by side‐effects such as response fluctuations, dyskinesias, and impulse control disorders. 3 Deep brain stimulation (DBS) of the subthalamic nucleus (STN) and the globus pallidus internus (GPi) are effective treatments for PD. 4 , 5 , 6 As a result of motor improvement after STN DBS, dopaminergic medication can usually be reduced. 7
Apathy is an increasingly recognized non‐motor manifestation of PD, commonly described as loss of motivation, decreased initiative, interest, and energy, and an emotional indifference with flattened affect. 2 , 8 Apathy has received more interest in recent years and validated clinical diagnostic criteria have been published. 9 Furthermore, apathy is frequently measured in studies in PD and has a high impact on quality of life (QoL). 10 , 11 Contrary to most non‐motor symptoms, apathy may worsen after STN DBS in up to 71% of cases. 10 , 12 , 13 , 14 , 15 The results of the meta‐analysis by Wang et al 16 were among the same lines. However, this meta‐analysis had methodological limitations, including the narrow search strategy, the use of the fixed effects model, and the inclusion of studies with overlapping samples. 17 , 18
Possible causes for increased apathy are reduced dopaminergic stimulation after medication reduction following STN DBS or collateral stimulation of adjacent regions to the motor territory of the STN. 19 , 20 , 21
We performed a systematic review and meta‐analysis to test the hypothesis that apathy increases in PD patients treated with STN DBS compared to either a pre‐operative state or to a control group by including newer and larger trials.
Methods
The systematic review and meta‐analysis were designed according to the PRISMA Guidelines. 22 A clinical librarian (J.D.) developed the search strategy for the meta‐analysis (Supplementary Appendix S1).
Search
The search included studies that published apathy scores in PD patients with STN DBS in a longitudinal or cross‐sectional design, were written in English, reported apathy scores in original data or this information could be reconstructed, and used one of the apathy scales that were recommended by the International Parkinson and Movement Disorders Society (MDS) — ie, Apathy Evaluation Scale (AES), Starkstein Apathy Scale (SAS), Lille Apathy Rating Scale (LARS), and the Apathy Inventory (AI). 23 , 24 , 25 , 26 , 27
Studies were excluded if the results consisted of non‐original research, less than six patients were reported, the study was part of an intervention trial for apathy and the last assessment of apathy took place earlier than 2 weeks post‐operative. Additionally, studies with a cross‐sectional design were excluded if the study had no control group consisting of PD patients treated with medication alone. We chose 2 weeks post‐operatively as the lower threshold for assessing apathy. Hereby, we were able to analyze whether STN‐DBS has an effect on the apathy scores over time.
Relevant published articles were searched in PubMed/Medline, SCOPUS, EMBASE, and Web of Sciences electronic databases. The electronic databases were searched up to September 4th, 2020 in three separate subsets, one on PD and STN DBS, one on PD and apathy, and the third on PD, STN DBS, and apathy. The titles and abstracts were independently screened by two authors (T.Z. and G.B.) for inclusion in full‐text appraisal. Similarly, these two authors independently appraised the full texts of these studies after excluding duplicate articles. Discrepancies were resolved through discussion and when consensus could not be achieved, a third author (GvR) would have the final decision on the inclusion in the meta‐analyses and systematic review.
Data Collection Process
The screening authors extracted the data and discussed accuracy routinely throughout the extraction phase. Authors were contacted when studies lacked sufficient methodological information or to provide additional data. When the screening process revealed multiple publications on the same data set, the study with the largest number of participants was used.
The following variables were collected from the included studies: authors, publication date, study design, total number of participants, population characteristics (ie, age, sex, disease duration), months of follow‐up, whether apathy was the primary outcome, apathy scale, apathy scores, depression scores, anxiety scores, QoL scores, levodopa (l‐dopa) equivalent daily dosage (LEDD), cognitive tests, unilateral or bilateral stimulation, Unified Parkinson Disease Rating Scale (UPDRS), and the MDS‐UPDRS. 28 , 29 The quality of articles was assessed using the adapted Newcastle Ottawa Scale (NOS) for observational studies (range 0–8). 30 A NOS score of five or less is indicative for a high risk of bias.
Meta‐Analysis
We performed three separate meta‐analyses using a DerSimonian and Laird random‐effects model: one pooling longitudinal data (change in apathy score from before to after STN DBS), one pooling cross‐sectional data (difference between a post‐operative STN DBS group with a control group), and one pooling cross‐sectional longitudinal data (pre‐post change scores of a STN DBS group compared with pre‐post change scores of a control group). 29 , 31 , 32 , 33 , 34 , 35 , 36 Studies with longitudinal and cross‐sectional apathy scores were included in all meta‐analyses. Case control studies with only longitudinal data were categorized as longitudinal studies. In longitudinal studies with multiple recordings of apathy, the closest measurement to 6 months post‐operative was used because the incidence of DBS‐related apathy is thought to be highest in the early months after STN DBS. 15 The principal summary measure for each meta‐analysis was an effect size expressed as Hedges g with a statistical significance level derived from the mean and standard deviation (SD) or F scores. If the mean, SDs, and F scores were unavailable, the mean and SDs were reconstructed by simple statistics in the case of normally distributed data. 37 , 38 , 39 , 40 All statistical analyses were performed using R with packages “meta” and “metafor.” 41
Small study effects or publication bias were assessed using the funnel plot test and Egger's statistics and a trim‐and‐fill analysis was performed when the Egger's test was positive.
The heterogeneity between studies was quantified by the index of heterogeneity (I2). A P value of <0.05 was considered as evidence of heterogeneity. Meta‐regressions were carried out on common variables such as the exclusion of patients suffering from apathy, depression, and/or other neuropsychiatric illnesses apathy based on clinical evaluation or the cut‐off of the appropriate scale at baseline. Subgroup analyses were performed on the study design, different scales, UPDRS, LEDD, disease duration, and age as grouping variables for their relation to apathy.
Results
Study Selection
The flow chart of the study selection process is presented in Figure 1. The search yielded a total of 6,658 articles and 1,319 of these were considered eligible. Subsequently, 1,263 studies were excluded because of lack of a validated apathy scale or inappropriate interventions and control groups. Authors were contacted with a high response rate of 82.4% to identify studies with overlapping data sets or to provide additional information, after which 23 additional studies were excluded and 33 remained, 23 with a longitudinal design and 13 with a cross‐sectional design. Three studies had both a longitudinal and cross‐sectional design, and these studies were also combined in a separate meta‐analysis. 42 , 43 , 44
FIG. 1.
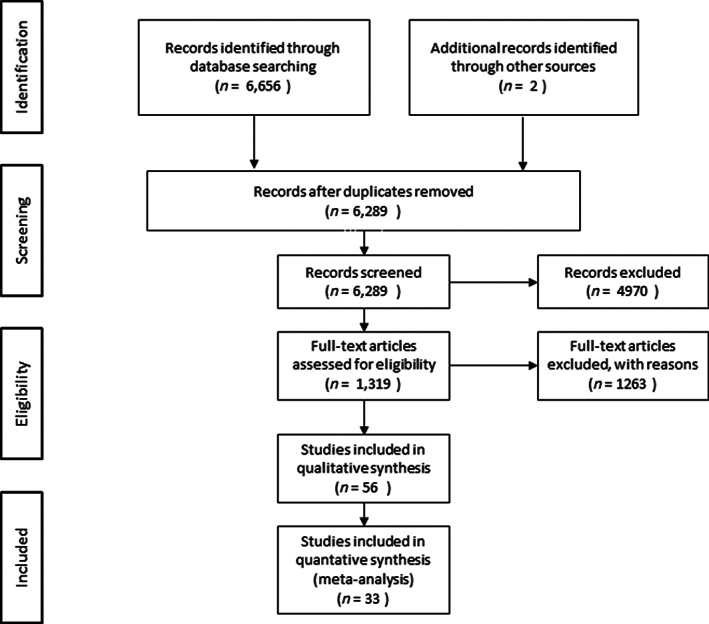
Flow diagram of study selection.
Study Characteristics
A total of 1,286 PD patients were included with a mean age (±SD) of 58.4 ± 8.5 years and a mean disease duration of 11.0 ± 5.8 years. Study characteristics of the longitudinal and cross‐sectional studies are presented in Tables 1 and 2, respectively. The AES was used in 10 studies, the SAS in 22 studies, and the LARS in three studies. 23 For uniformity, the SAS was prioritized for analyses if studies reported two scales. 46 , 47 The mean apathy scores at baseline were: SAS 5.4 to 18.8, AES 27.5 to 39.1, and LARS −32.6 to −24.0. 13 , 56 For uniformity, the SAS was prioritized for analyses in studies that reported two scales. 44 , 67 The risk of bias was high (NOS score ≤5) in 8 studies (24.4%) and low (NOS score >5) in 25 studies (75.8%). Two studies had a unique design; one with a l‐dopa/carbidopa intestinal gel control group and one investigated effects of unilateral STN‐DBS. 43 , 72
TABLE 1.
Longitudinal studies characteristics
| Study | Total sample | Age (y) | Disease duration (y) | Follow‐up (mo) | Newcastle‐Ottawa score | Apathy scale | Pre‐operative score | Post‐operative score | Mean change in LEDD (%) |
| Ardouin et al 44 | 7 | 54.0 ± 9.0 | NR | 3 | 7 | SAS | 9.5 ± 3.0 | 9.8 ± 6.3 | −73.6 |
| Castelli et al 45 | 19 | 62.1 ± 4.2 | 14.7 ± 5 | 17 | 7 | SAS | 11.6 ± 4.1 | 12.6 ± 5.3 | −52.1 |
| Castrioto et al 46 | 36 | 56.8 ± 8.3 | 9.3 ± 4.9 | 12 | 5 | SAS | 11.1 ± 4.8 | 10.4 ± 5.3 | −60.3 |
| Chou et al 47 | 10 | 62.1 ± 6.5 | 9.1 ± 5.8 | 6 | 7 | SAS | 13.2 ± 8.6 | 13.6 ± 7.4 | −51.2 |
| Dafsari et al 48 | 36 | 62.8 ± 9.1 | 9.6 ± 5.3 | 5 | 4 | AES | 28.9 ± 7.1 | 29.6 ± 6.7 | −53.3 |
| Dos Santos et al 49 | 19 | 60(6.5) | 93(3.5) | 12 | 7 | SAS | 6.9 ± 2.7 | 9.5 ± 7.7 | −39.6 |
| Drapier et al 43 | 30 | 59.7 ± 7.6 | 12.2 ± 2.8 | 6 | 7 | SAS & AES | 13.0 ± 6.5 | 18.8 ± 9.7 | −22.2 |
| Foley et al 50 | 28 | 57.5 ± 7.3 | 18.8 ± 6.1 | 19.5 | 6 | SAS | 10.8 ± 6.0 | 14.0 ± 11.2 | NR |
| Gesquiere‐Dando et al 51 | 34 | 62.7 ± 8.1 | 9.9 ± 4.3 | 12 | 6 | LARS | −32.6 ± 3.6 | −24.4 ± 12.0 | −39.4 |
| Higuchi et al 13 | 25 | 50.4 ± 9.8 | 12.5 ± 7 | 1 | 7 | SAS | 5.4 ± 3.1 | 9.6 ± 9.9 | −61.1 |
| Langner‐Lemercier et al 52 | 40 | 56.5 ± 7.8 | 12.0 ± 4.6 | 12 | 5 | AES | 30.9 ± 6.3 | 33.0 ± 8.9 | −38.9 |
| Le Jeune et al 53 | 12 | 57.4 ± 8 | 11.2 ± 2.4 | 3 | 6 | AES | 30.9 ± 4.1 | 39.1 ± 6.1 | −33.6 |
| Lhommee et al 54 | 73 | 57.3 ± 7 | 10.8 ± 2.9 | 12 | 7 | SAS | 6.4 ± 3.3 | 9.7 ± 4.6 | −69.7 |
| Lhommee et al 41 | 251 | 52.5 ± 6.3 | 7.5 ± 2.9 | 24 | 8 | SAS | 9.9 (0.7) | 12.7 (0.5) | −37.6 |
| Lilleeng et al 55 | 16 | 60.0 ± 8.1 | 12.9 ± 5.7 | 4.5 | 8 | SAS | 14.7 ± 4.1 | 16.9 ± 5.2 | −22.9 |
| Maier et al 56 | 30 | 61.2 ± 8.7 | 12.0 ± 6.79 | 3 | 7 | AES | 34.8 ± 10.9 | 34.6 ± 9.4 | −55.9 |
| Mosley et al 57 a | 64 | 62.2 ± 9.5 | 9.0 ± 5.2 | 1.5 | 7 | SAS | F‐score: 0.838 | NR | |
| Nimura et al 58 | 39 | 62.6 ± 6.7 | 13.3 ± 9.4 | 6 | 5 | SAS | 12.2 ± 7.7 | 12.0 ± 7.2 | NR |
| Pham et al 59 | 40 | 63.4 ± 6.4 | 12.1 ± 3.8 | 3 | 6 | AES | 30.6 ± 5.9 | 32.2 ± 6.6 | −47.7 |
| Robert et al 17 | 44 | 56.3 ± 7.5 | 11.4 ± 4.1 | 3 | 6 | AES | 31.4 ± 6.4 | 31.6 ± 7.1 | −30.5 |
| Seifried et al 60 | 11 | 63.0 ± 7 | 14.0 ± 4 | 6 | 4 | SAS | 10.8 ± 7.1 | 12.5 ± 8.6 | −51.5 |
| Valldeoriola et al 42 | 23 | 57.9 ± 4.8 | 13.7 | 6 | 5 | LARS | −24 ± 19.9 | −27 ± 21.6 | −21.4 |
| Voruz et al 61 | 29 | 56.5 ± 8.0 | 11.2 ± 4.2 | 3 | 6 | AES | 31.4 ± 6.5 | 32.9 ± 8.7 | −44.0 |
Follow‐up, apathy assessment follow‐up in months after the STN DBS operation. All studies used bilateral stimulation. Studies with the variance marked as ± reported standard devations, studies with parentheses reported the standard error. Abbreviations: LEDD, levodopa equivalent daily dosage; SAS, Starkstein Apathy Scale; AES, Apathy Evaluation Scale; LARS, Lille Apathy Rating Scale; NR, not reported.
F statistic was provided only.
TABLE 2.
Cross‐sectional studies characteristics
| Study | Total sample | Age | Disease duration | Months post‐operative | Newcastle‐Ottawa score | Apathy scale | Score STN DBS group | Score control group | LEDD difference (%) |
| Crespo‐Burillo et al 62 | 22 | 65.4 ± 7.7 | 21.2 ± 13.1 | 3 | 6 | SAS | 11.6 ± 7.1 | 11.4 ± 5.5 | NR |
| Czernecki et al 63 | 41 | 57.8 ± 1.8 | 13.9 ± 1.6 | 10 | 8 | SAS | 11.2(0.9) | 11.0(1.5) | −86.4 |
| Drapier et al 43 | 30 | 59.7 ± 7.6 | 12.2 ± 2.8 | 6 | 7 | SAS & AES | 18.8 ± 9.7 | 13.0 ± 6.5 | −22.2 |
| Enrici et al 64 | 38 | 60.3 ± 7.6 | 12.0 ± 6.8 | NR | 6 | SAS | 11.9 ± 3.6 | 12.8 ± 5.6 | −29.2 |
| Evens et al 65 | 66 | 65.5 ± 7.3 | 11.3 ± 6.2 | 3 | 6 | SAS | 15.5 ± 6.4 | 8.9 ± 4.7 | +6.3 |
| Hindle Fisher et al 66 | 60 | 66.3 ± 3.1 | 10.3 | 6 | 8 | SAS & LARS | 13.8 ± 4.7 | 12.1 ± 6.3 | −15.1 |
| Houvenaghel et al 67 | 50 | 57.8 ± 7.7 | 12.2 ± 3.3 | 30 | 5 | AES | 30.3 ± 8.8 | 27.5 ± 6.7 | −30.2 |
| Kojovic et al 68 | 20 | 59.3 | 9.4 ± 5 | NR | 5 | AES | 38.5 ± 2.2 | 32.2 ± 2.8 | −18.6 |
| Leimbach et al 69 | 24 | 63.6 ± 11.3 | NR | 3 | 8 | SAS | 15.0 ± 5.5 | 10.5 ± 5.3 | NR |
| Lhommee et al 41 | 251 | 52.5 ± 6.3 | 7.5 ± 2.9 | 24 | 8 | SAS | 12.7(0.5) | 11.4(0.5) | −48.2 |
| Mcdonald et al 70 | 34 | 57.4 ± 6.5 | 13.5 ± 5.2 | 14.5 | 8 | SAS | 13.0 ± 11.6 | 10.3 ± 6.4 | −12.6 |
| Okun et al 71 a | 30 | 59 ± 8.6 | 11.8 ± 3.9 | 6 | 8 | SAS | 16.4 ± 9.3 | 13.1 ± 6.0 | −17.2 |
| Valldeoriola et al 42 | 23 | 57.9 ± 4.8 | 13.7 | 6 | 5 | LARS | −27 ± 21.6 | −9 ± 15.8 | −21.4 |
Follow‐up, apathy assessment follow‐up in months after the STN DBS operation. Studies with the variance marked as ± reported standard deviations, studies with brackets () reported the standard error. Abbreviations: LEDD, levodopa equivalent daily dosage; SAS, Starkstein Apathy Scale; AES, Apathy Evaluation Scale; LARS, Lille Apathy Rating Scale; NR, not reported.
One study used unilateral stimulation, all other studies used bilateral stimulation.
Synthesis of Results
The forest plots of the meta‐analyses of the longitudinal studies are shown in Figure 2. A significant higher apathy score is found post‐operatively than before DBS treatment (g = 0.34, 95% confidence interval [CI] = 0.19–0.48, P < 0.001, Î2 = 34%). Studies that excluded patients with apathy at baseline found greater values of apathy after STN DBS (g = 0.79, P < 0.001). Studies that reported apathy as a main outcome also reported a higher mean apathy score following STN DBS (g = 0.46, P < 0.001). A higher pre‐operative UPDRS III on‐medication score (F = 6.32, P = 0.03) and a higher pre‐operative Beck depression inventory (BDI) score (F = 7.29, P = 0.04) are associated with higher apathy scores after STN DBS. The follow‐up UPDRS III on‐medication score and BDI score were not associated with apathy outcomes. The meta‐analysis for cross‐sectional studies showed a similar difference in apathy (g = 0.36, 95% CI = 0.03–0.65; P = 0.004, Î2 = 58%). Please see Figure 3 for the respective forest plot. The heterogeneity could be improved by excluding the two studies with the unique designs (Î2 = 42.8%). 43 , 72 If the studies that did not report apathy as the main outcome were analyzed separately, there was no statistically significant effect (g = 0.31, P = 0.25). The forest plots of the three studies that had pre‐ and post‐operative apathy assessments in both an STN DBS and a control group are shown in Figure 4. The combined studies did not demonstrate a statistically significant difference in apathy between the two treatment arms (g = 0.20, 95% CI = −0.27–0.67, P = 0.40).
FIG. 2.
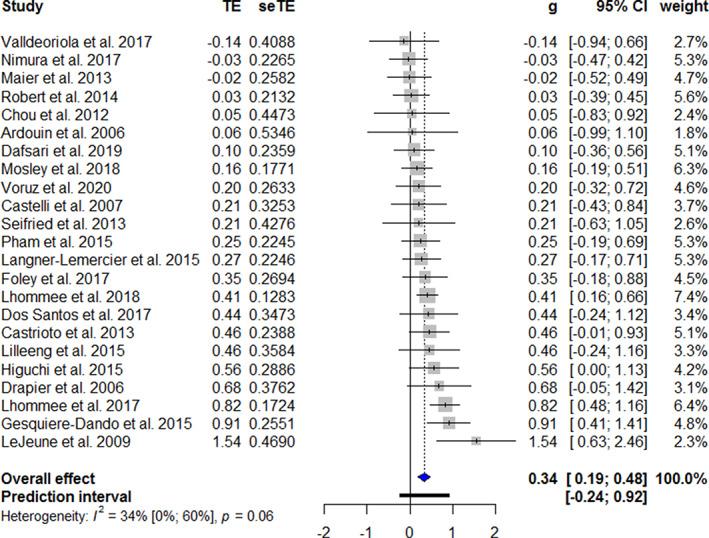
Forest plot of apathy after STN DBS in longitudinal studies. Treatment effects to the right favors more apathy. TE, treatment effect; seTE, standard error of the treatment effect; g, Hedges’ g; CI, confidence interval. [Color figure can be viewed at wileyonlinelibrary.com]
FIG. 3.
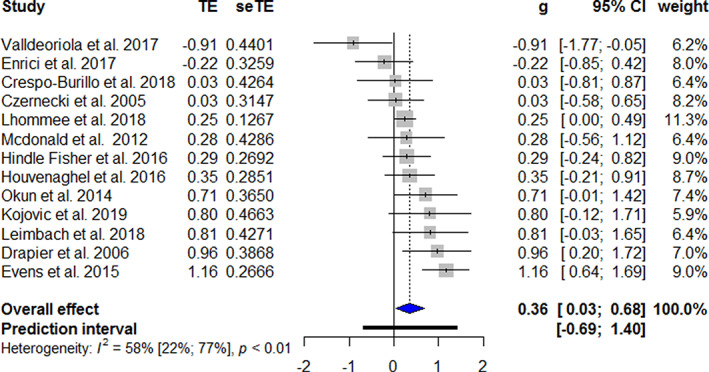
Forest plot of apathy after STN DBS in cross‐sectional studies. Treatment effects to the right favors more apathy. TE, treatment effect; seTE, standard error of the treatment effect; g, Hedges’ g; CI, confidence interval. [Color figure can be viewed at wileyonlinelibrary.com]
FIG. 4.
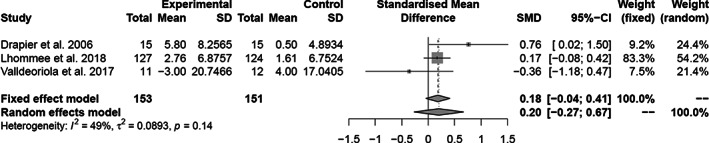
Forest plot longitudinal and cross‐sectional studies. Treatment effects to the right favors more apathy. SMD, standardized mean difference; CI, confidence interval.
Additional Analyses
The Egger's tests provided no evidence for publication bias and there was no small effects bias (Fig. 5A,B). Using meta‐regression, there were no relations between effect size and LEDD reduction (P = 0.96 for longitudinal; P = 0.23 for the cross‐sectional studies), disease duration, and age on the overall effect in all meta‐analyses. The mean increase of apathy score after STN‐DBS on the SAS and AES were +2.03 for the longitudinal studies and +2.96 for the cross‐sectional studies.
FIG. 5.
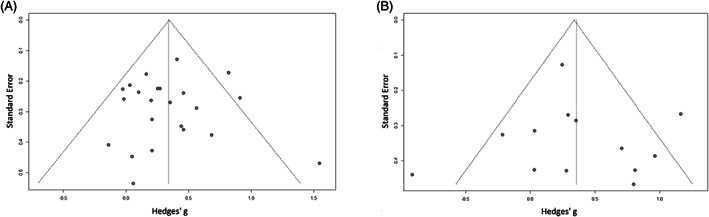
(A) Funnel plot of the longitudinal studies. (B) Funnel plot of the cross‐sectional studies.
Discussion
The main purpose of this systematic review and meta‐analysis was to determine whether STN DBS resulted in higher apathy scores in PD patients. The main result of our meta‐analysis is that apathy scores are higher after STN DBS for PD compared with the pre‐operative state and compared with PD patients on medication alone. This result is relevant for clinical care to allow for careful consideration of the benefits and drawbacks of STN DBS for PD patients. Interestingly, increase in apathy appeared to be present regardless of reduction of dopaminergic medication, disease progression, and other neuropsychiatric symptoms.
The overall effect was roughly the same for longitudinal studies and cross‐sectional studies. The smaller sample size in the cross‐sectional meta‐analysis led to more confounding variables than the longitudinal meta‐analysis. We found no evidence that coincidence findings would be more often reported because of the absence of small‐study effects. Studies that listed apathy as a main outcome had higher apathy scores than studies that did not primarily focus on non‐motor symptoms, a possible explanation could be a more thorough examination of apathy symptoms. Furthermore, studies that excluded apathetic patients at baseline reported a higher difference in apathy scores between the pre‐ and post‐operative assessment. This finding suggests that there may be a ceiling effect where already apathetic patients do not experience the same increase in symptom severity. A possible explanation is that apathy is related to PD severity and the decrease of dopaminergic medication, allowed by the effect of DBS, returns the apathy severity towards an untreated state. This is supported by the association between apathy and the pre‐operative UPDRS on‐medication score, a marker for dopaminergic unresponsive symptoms.
The difference in the severity of apathy between the STN DBS and best medical treatment groups was highest in studies with a follow‐up shorter than 2 years. With longer follow‐up, apathy increased also in the best medical treatment group and the difference in apathy between the groups decreased. 19 The SAS and AES showed some divergence in scores although scales have mostly overlapping questions and are possibly interchangeable in clinical use. Numerous studies also reported the Non‐Motor Symptoms Scale and the Non‐Motor Symptoms Questionnaire or UPDRS item 4. 73 , 74 , 75 Although these scales showed a correlation with apathy and have sub‐scores related to apathy, they lack the specificity of the scales listed by the MDS for the assessment of apathy. 23
The pathophysiology of apathy occurring in patients that are treated with STN‐DBS is still under debate. The most notable hypotheses are that apathy increases with longer disease duration, reduction of dopaminergic medication, and DBS of areas adjacent to the motor subregions of STN or spillover of current into these areas. 10 , 14 , 19 The literature regarding the direct effects of DBS‐current on apathy is inconsistent; some studies found an increase of apathy, 14 , 15 , 54 whereas others found that euphoria increases and apathy is reduced. 64 , 76 Interestingly, the only randomized controlled trial directly assessing apathy—the EARLYSTIM‐trial—did not detect a difference in apathy scores between the STN DBS and best medical treatment group. In the EARLYSTIM‐trial, both the STN DBS and best medical treatment group had an increase of apathy scores during follow‐up. 42 The dopaminergic medication is generally reduced in the weeks following STN DBS surgery. The reduced availability of mesolimbic and mesocortical dopamine accompanying the postoperative reduction in the use of dopaminergic medication is commonly theorized as the main contributing factor for apathy. 15 , 77 , 78 However, our meta‐analysis revealed no effect from dopaminergic medication reduction on apathy scores on a group level. Three articles separated dopamine agonists use from other medications and these studies suggest that higher daily doses of dopamine agonists, which have a higher affinity to the limbic D3 dopamine receptor, are accompanied by lower apathy scores. 53 , 55 , 66 , 78
Another factor for the development of non‐motor features and apathy could be the severity of PD. Although the UPDRS on‐medication score at baseline was associated with the occurrence of apathy, neither disease duration nor UPDRS III off‐medication score were associated with the increase in apathy. The effect of DBS on apathy scores and level of statistical significance does not change when correcting for UPDRS on‐medication score.
Furthermore, cognitive decline is also prominent in advanced PD because of age related illnesses (eg, Alzheimer's disease and cerebrovascular disease) and PD dementia. 79 , 80 However, literature is biased as surgical candidates are selected for absence of dementia. Moreover, most studies only used basic cognitive testing with screening instruments at a single point during the trials. As such, no relationship was found between apathy and neurocognitive functioning in our meta‐analysis. This meta‐analysis was also unable to establish a relationship between apathy and depression, anxiety, quality of life, social support, and other variables as the data on these factors was scarce and most studies did not report subscales.
This meta‐analysis succeeded the meta‐analysis of Wang et al 16 that concluded that apathy was more prevalent after STN DBS. Our meta‐analysis was able to address some of the limitations of the earlier meta‐analysis, added extra articles and the random effects demonstrates with a higher degree of confidence that our findings are relevant for the general PD population. Nevertheless, Wang et al 16 found an effect size of the same order as the effect sizes in our meta‐analyses.
Our study had some limitations that need to be acknowledged. First, the heterogeneity of the studies was high in the longitudinal and the cross‐sectional meta‐analysis and the combined meta‐analysis showed a divergence of the results. This reflects the different methodological procedures that were followed in the included studies and limits the reliability of our results. Subgroup analysis were performed and studies with a high impact on the heterogeneity were excluded, resulting in a higher overall effect remaining statistically significant. Second, we calculated the apathy scores in some studies by the estimation of a weighted mean and SD, without having access to the original data. Although these variables are less precise, we argue that the inclusion of these studies strengthened our meta‐analysis. Third, several studies were at risk of bias based on the NOS. Sensitivity of these studies did not detect any outliers and the influence on the overall effect was not distinct from other studies. Fourth, we included the closest apathy measurement point to 6 months post‐operatively in both meta‐analyses. The STN‐DBS treatment may not be optimal at that time because of suboptimal electrode settings and medication adjustments. Fifth, an important finding is the lack of relation between reduction of dopaminergic treatment and apathy at group level. It would have been informative to relate LEDD‐reduction with patients scoring above the cut‐off of the scales, but this information was not available. Finally, we could not distinguish apathy from PD progression or other neuropsychiatric symptoms. Meta‐regression found several impacting variables but there was little consistency. For example, only one specific UPDRS score was related to an increase in apathy, but the other UPDRS scores in on‐ and off‐medication, pre‐ and post‐operatively, showed no relationship with apathy scores.
Conclusion
The main result of this meta‐analysis is that apathy increases after STN DBS, compared to the pre‐operative state or to control groups managed only with medication. This effect was independent of confounding variables, including the reduction of dopaminergic medication. These findings are of clinical relevance to the increasing population of PD patients that will become reliant on STN DBS in the future, and demand further research on the subject.
Author’ Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2). Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
T.J.C.Z.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
G.vR.: 1A, 1B, 1C, 2C, 3A, 3B
G.M.F.C.B.: 1B, 1C
I.O.B.: 2A, 2B, 2C, 3A
J.G.D.: 1A, 1C
P.K.: 3A, 3B
D.A.J.P.D.: 3A, 3B
R.M.A.dB.: 1A, 1B, 2A, 2C, 3A, 3B
Funding
There was no funding for this project and the primary support was received from the corresponding authors on a collaborative basis.
Financial Disclosures of All Authors (for the Preceding 12 Months)
D.A.J.P.D. and I.O.B. received grants from ZonMw and Boston Scientific (in kind) for a trial on deep brain stimulation for depression. P.K. reports grants from Swiss National Science Foundation, Roger De Spoelberch Foundation, Bertarelli Foundation, Michael J. Fox Foundation, Annemarie Opprecht Foundation, Parkinson Schweiz, research grants from Boston Scientific, and Aleva, lecturing fees paid to employing institution from Boston Scientific, as well as reimbursement of travelling expenses to scientific meeting by Zambon, all support outside the submitted work. R.M.A.dB. received research grants from ZonMw, Parkinson Vereniging, GE Health, Medtronic, Lysosomal therapeutics, all outside the submitted work and paid to the institution. All other authors report no conflicting interests for this systematic review and meta‐analysis.
Supporting information
Appendix S1: Supporting information
Acknowledgments
We would like to express our gratitude for the rapid and thorough response of many of the authors that were contacted, including but not limited to: Marcelo Merello, Dawn Bowers, Michael Okun, Kelvin Chou, Francesc Valldeoriola, Marjan Jahanshahi, Panagiotis Bargiotas and Franziska Maier.
References
- 1. Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386(9996):896–912. [DOI] [PubMed] [Google Scholar]
- 2. Schapira AHV, Chaudhuri KR, Jenner P. Non‐motor features of Parkinson disease. Nat Rev Neurosci 2017;18(8):509. [DOI] [PubMed] [Google Scholar]
- 3. Muller T. Drug therapy in patients with Parkinson's disease. Transl Neurodegener 2012;1(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hitti FL, Ramayya AG, McShane BJ, Yang AI, Vaughan KA, Baltuch GH. Long‐term outcomes following deep brain stimulation for Parkinson's disease. J Neurosurg 2019;132 205–210. [DOI] [PubMed] [Google Scholar]
- 5. Wong JK, Cauraugh JH, Ho KWD, et al. STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: a systematic review and meta‐analysis. Parkinsonism Relat Disord 2019;58:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 2013;12(1):37–44. [DOI] [PubMed] [Google Scholar]
- 7. Alexoudi A, Shalash A, Knudsen K, et al. The medical treatment of patients with Parkinson's disease receiving subthalamic neurostimulation. Parkinsonism Relat Disord 2015;21(6):555–560; discussion. [DOI] [PubMed] [Google Scholar]
- 8. Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991;3(3):243–254. [DOI] [PubMed] [Google Scholar]
- 9. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry 2009;24(2):98–104. [DOI] [PubMed] [Google Scholar]
- 10. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24(11):1641–1649. [DOI] [PubMed] [Google Scholar]
- 11. Martinez‐Fernandez R, Pelissier P, Quesada JL, et al. Postoperative apathy can neutralise benefits in quality of life after subthalamic stimulation for Parkinson's disease. J Neurol Neurosurg Psychiatry 2016;87(3):311–318. [DOI] [PubMed] [Google Scholar]
- 12. Starkstein SE. Apathy in Parkinson's disease: diagnostic and etiological dilemmas. Mov Disord 2012;27(2):174–178. [DOI] [PubMed] [Google Scholar]
- 13. Higuchi MA, Tsuboi Y, Inoue T, et al. Predictors of the emergence of apathy after bilateral stimulation of the subthalamic nucleus in patients with Parkinson's disease. Neuromodulation 2015;18(2):113–117. [DOI] [PubMed] [Google Scholar]
- 14. Ricciardi L, Morgante L, Epifanio A, et al. Stimulation of the subthalamic area modulating movement and behavior. Parkinsonism Relat Disord 2014;20(11):1298–1300. [DOI] [PubMed] [Google Scholar]
- 15. Thobois S, Ardouin C, Lhommee E, et al. Non‐motor dopamine withdrawal syndrome after surgery for Parkinson's disease: predictors and underlying mesolimbic denervation. Brain 2010;133(Pt 4):1111–1127. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Li Y, Zhang X, Xie A. Apathy following bilateral deep brain stimulation of subthalamic nucleus in Parkinson's disease: a meta‐analysis. Parkinsons Dis 2018;2018:9756468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Houvenaghel JF, Le Jeune F, Dondaine T, et al. Reduced verbal fluency following subthalamic deep brain stimulation: a frontal‐related cognitive deficit? PLoS One 2015;10(10):e0140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robert GH, Le Jeune F, Lozachmeur C, et al. Preoperative factors of apathy in subthalamic stimulated Parkinson disease: a PET study. Neurology 2014;83(18):1620–1626. [DOI] [PubMed] [Google Scholar]
- 19. Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol 2015;14(5):518–531. [DOI] [PubMed] [Google Scholar]
- 20. Zoon TJ, de Bie RM, Schuurman PR, van den Munckhof P, Denys D, Figee M. Resolution of apathy after dorsal instead of ventral subthalamic deep brain stimulation for Parkinson's disease. J Neurol 2019;266(5):1267–1269. [DOI] [PubMed] [Google Scholar]
- 21. Castrioto A, Lhommee E, Moro E, Krack P. Mood and behavioural effects of subthalamic stimulation in Parkinson's disease. Lancet Neurol 2014;13(3):287–305. [DOI] [PubMed] [Google Scholar]
- 22. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 23. Leentjens AF, Dujardin K, Marsh L, et al. Apathy and anhedonia rating scales in Parkinson's disease: critique and recommendations. Mov Disord 2008;23(2014):2004–2014. [DOI] [PubMed] [Google Scholar]
- 24. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res 1991;38(2):143–162. [DOI] [PubMed] [Google Scholar]
- 25. Zahodne LB, Young S, Kirsch‐Darrow L, et al. Examination of the Lille apathy rating scale in Parkinson disease. Mov Disord 2009;24(5):677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1992;4(2):134–139. [DOI] [PubMed] [Google Scholar]
- 27. Robert PH, Clairet S, Benoit M, et al. The apathy inventory: assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. Int J Geriatr Psychiatry 2002;17(12):1099–1105. [DOI] [PubMed] [Google Scholar]
- 28. FS. MUDC. The unified Parkinson's disease rating scale. In: Fahn SMC, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Vol. 153–163. Florham Park, NJ: Macmillan Healthcare Information; 1987:293–304. [Google Scholar]
- 29. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 30. Luchini C. Assessing the quality of studies in meta‐analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta‐Anal 2017;5(4):80–84. [Google Scholar]
- 31. Hamilton M. Rating depressive patients. J Clin Psychiatry 1980;41(12 Pt 2):21–24. [PubMed] [Google Scholar]
- 32. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck depression inventories ‐IA and ‐II in psychiatric outpatients. J Pers Assess 1996;67(3):588–597. [DOI] [PubMed] [Google Scholar]
- 33. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 34. Jenkinson C, Dummett S, Kelly L, et al. The development and validation of a quality of life measure for the carers of people with Parkinson's disease (the PDQ‐Carer). Parkinsonism Relat Disord 2012;18(5):483–487. [DOI] [PubMed] [Google Scholar]
- 35. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 36. Borenstein MHL, Hedges LV, Higgins JPT, Rothstein HR, et al. Chapter 13: Fixed‐Effect versus Random‐Effects Models. Introduction to meta‐analysis. Hoboken, New Jersey: John Wiley & Sons Inc; 2009:77–86. [Google Scholar]
- 37. DG A . Statistics with confidence second edition. In: DM, editor. 2000. p. 28–31.
- 38. SP H . Estimating the mean and variance from the median, range, and the size of a sample. In: BB, editor. BMC Med Res Methodol 2005. p. 13. [DOI] [PMC free article] [PubMed]
- 39. Wan X, Wang WQ, Liu JM, Tong TJ. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14(135):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morris SB, DeShon RP. Combining effect size estimates in meta‐analysis with repeated measures and independent‐groups designs. Psychological Methods 2002;7(1):105–125. [DOI] [PubMed] [Google Scholar]
- 41. Rstudio Team RStudio: Integrated Development for R. 250 Northern Ave, Boston, MA: Rstudio; 2013. [Google Scholar]
- 42. Lhommee E, Wojtecki L, Czernecki V, Witt K, Maier F, Tonder L, et al. Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson's disease with early motor complications (EARLYSTIM trial): secondary analysis of an open‐label randomised trial. Lancet Neurol 2018;17(3):223–131. [DOI] [PubMed] [Google Scholar]
- 43. Valldeoriola F, Santacruz P, Rios J, et al. L‐Dopa/carbidopa intestinal gel and subthalamic nucleus stimulation: effects on cognition and behavior. Brain Behav 2017;7(11):e00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drapier D, Drapier S, Sauleau P, et al. Does subthalamic nucleus stimulation induce apathy in Parkinson's disease? J Neurol 2006;253(8):1083–1091. [DOI] [PubMed] [Google Scholar]
- 45. Ardouin C, Voon V, Worbe Y, et al. Pathological gambling in Parkinson's disease improves on chronic subthalamic nucleus stimulation. Mov Disord 2006;21(11):1941–1946. [DOI] [PubMed] [Google Scholar]
- 46. Castelli L, Lanotte M, Zibetti M, et al. Apathy and verbal fluency in STN‐stimulated PD patients. An observational follow‐up study. J Neurol 2007;254(9):1238–1243. [DOI] [PubMed] [Google Scholar]
- 47. Castrioto A, Volkmann J, Krack P. Postoperative management of deep brain stimulation in Parkinson's disease. Handbook of Clinical Neurology. Vol. 116. Amsterdam, Netherlands: Elsevier; 2013:129–146. [DOI] [PubMed] [Google Scholar]
- 48. Chou KL, Persad CC, Patil PG. Change in fatigue after bilateral subthalamic nucleus deep brain stimulation for Parkinson's disease. Parkinsonism Relat Disord 2012;18(5):510–513. [DOI] [PubMed] [Google Scholar]
- 49. Dafsari HS, Ray‐Chaudhuri K, Mahlstedt P, et al. Beneficial effects of bilateral subthalamic stimulation on alexithymia in Parkinson's disease. Eur J Neurol 2019;26(2):222–e17. [DOI] [PubMed] [Google Scholar]
- 50. Flores Alves Dos Santos J, Tezenas du Montcel S, Gargiulo M, et al. Tackling psychosocial maladjustment in Parkinson's disease patients following subthalamic deep‐brain stimulation: a randomised clinical trial. PLoS One 2017;12(4):e0174512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Foley JA, Foltynie T, Zrinzo L, Hyam JA, Limousin P, Cipolotti L. Apathy and reduced speed of processing underlie decline in verbal fluency following DBS. Behav Neurol 2017;2017:7348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gesquiere‐Dando A, Guedj E, Loundou A, et al. A preoperative metabolic marker of parkinsonian apathy following subthalamic nucleus stimulation. Mov Disord 2015;30(13):1767–1776. [DOI] [PubMed] [Google Scholar]
- 53. Langner‐Lemercier S, Drapier S, Naudet F, et al. Preoperative brain metabolism and quality of life after subthalamic nucleus stimulation in Parkinson's disease. J Neurol 2015;262(4):881–889. [DOI] [PubMed] [Google Scholar]
- 54. Le Jeune F, Drapier D, Bourguignon A, et al. Subthalamic nucleus stimulation in Parkinson disease induces apathy: a PET study. Neurology 2009;73(21):1746–1751. [DOI] [PubMed] [Google Scholar]
- 55. Lhommee E, Boyer F, Wack M, et al. Personality, dopamine, and Parkinson's disease: insights from subthalamic stimulation. Mov Disord 2017;32(8):1191–1200. [DOI] [PubMed] [Google Scholar]
- 56. Lilleeng B, Gjerstad M, Baardsen R, Dalen I, Larsen JP. The long‐term development of non‐motor problems after STN‐DBS. Acta Neurol Scand 2015;132(4):251–258. [DOI] [PubMed] [Google Scholar]
- 57. Maier F, Lewis CJ, Horstkoetter N, et al. Patients' expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson's disease: a mixed‐method approach. J Neurol Neurosurg Psychiatry 2013;84(11):1273–1281. [DOI] [PubMed] [Google Scholar]
- 58. Mosley PE, Breakspear M, Coyne T, Silburn P, Smith D. Caregiver burden and caregiver appraisal of psychiatric symptoms are not modulated by subthalamic deep brain stimulation for Parkinson's disease. NPJ Parkinsons Dis 2018;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nimura T, Nagamatsu KI, Ando T, Matsumoto A, Hisanaga K, Tominaga T. An investigation into the effects and prognostic factors of cognitive decline following subthalamic nucleus stimulation in patients with Parkinson's disease. J Clin Neurosci 2017;44:164–168. [DOI] [PubMed] [Google Scholar]
- 60. Pham UH, Andersson S, Toft M, et al. Self‐reported executive functioning in everyday life in Parkinson's disease after three months of subthalamic deep brain stimulation. Parkinsons Dis 2015;2015:461453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seifried C, Boehncke S, Heinzmann J, Baudrexel S, Weise L, Gasser T, et al. Diurnal variation of hypothalamic function and chronic subthalamic nucleus stimulation in Parkinson's disease. Neuroendocrinology 2013;97(3):283–290. [DOI] [PubMed] [Google Scholar]
- 62. Voruz P, Le Jeune F, Haegelen C, et al. Motor symptom asymmetry in Parkinson's disease predicts emotional outcome following subthalamic nucleus deep brain stimulation. Neuropsychologia 2020;144:1–14. [DOI] [PubMed] [Google Scholar]
- 63. Crespo‐Burillo JA, Rivero‐Celada D, Saenz‐de Cabezon A, Casado‐Pellejero J, Alberdi‐Vinas J, Alarcia‐Alejos R. Deep brain stimulation for patients with Parkinson's disease: effect on caregiver burden. Neurologia 2018;33(3):154–159. [DOI] [PubMed] [Google Scholar]
- 64. Czernecki V, Pillon B, Houeto JL, et al. Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson's disease? J Neurol Neurosurg Psychiatry 2005;76(6):775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Enrici I, Mitkova A, Castelli L, Lanotte M, Lopiano L, Adenzato M. Deep brain stimulation of the subthalamic nucleus does not negatively affect social cognitive abilities of patients with Parkinson's disease. Sci Rep 2017;7(1):9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Evens R, Stankevich Y, Dshemuchadse M, et al. The impact of Parkinson's disease and subthalamic deep brain stimulation on reward processing. Neuropsychologia 2015;75:11–19. [DOI] [PubMed] [Google Scholar]
- 67. Hindle Fisher I, Pall HS, Mitchell RD, Kausar J, Cavanna AE. Apathy in patients with Parkinson's disease following deep brain stimulation of the subthalamic nucleus. CNS Spectr 2016;21(3):258–264. [DOI] [PubMed] [Google Scholar]
- 68. Houvenaghel JF, Duprez J, Argaud S, Naudet F, Dondaine T, Robert GH, et al. Influence of subthalamic deep‐brain stimulation on cognitive action control in incentive context. Neuropsychologia 2016;91:519–530. [DOI] [PubMed] [Google Scholar]
- 69. Kojovic M, Higgins A, Mir P, Jahanshahi M. Enhanced motivational modulation of motor behaviour with subthalamic nucleus deep brain stimulation in Parkinson's disease. Parkinsons Dis 2019;2019:3604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leimbach F, Georgiev D, Litvak V, et al. Deep brain stimulation of the subthalamic nucleus does not affect the decrease of decision threshold during the choice process when there is no conflict, time pressure, or reward. J Cogn Neurosci 2018;30(6):876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McDonald LM, Page D, Wilkinson L, Jahanshahi M. Deep brain stimulation of the subthalamic nucleus improves sense of well‐being in Parkinson's disease. Mov Disord 2012;27(3):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Okun MS, Wu SS, Fayad S, et al. Acute and chronic mood and apathy outcomes from a randomized study of unilateral STN and GPi DBS. PLoS One 2014;9(12):e114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chaudhuri KR, Martinez‐Martin P, Brown RG, et al. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22(13):1901–1911. [DOI] [PubMed] [Google Scholar]
- 74. Romenets SR, Wolfson C, Galatas C, et al. Validation of the non‐motor symptoms questionnaire (NMS‐quest). Parkinsonism Relat Disord 2012;18(1):54–58. [DOI] [PubMed] [Google Scholar]
- 75. Kirsch‐Darrow L, Zahodne LB, Hass C, et al. How cautious should we be when assessing apathy with the unified Parkinson's disease rating scale? Mov Disord 2009;24(5):684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Funkiewiez A, Ardouin C, Krack P, et al. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson's disease. Mov Disord 2003;18(5):524–530. [DOI] [PubMed] [Google Scholar]
- 77. Czernecki V, Schupbach M, Yaici S, Levy R, et al. Apathy following subthalamic stimulation in Parkinson disease: a dopamine responsive symptom. Mov Disord 2008;23(7):964–969. [DOI] [PubMed] [Google Scholar]
- 78. Thobois S, Lhommee E, Klinger H, et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain 2013;136(Pt 5):1568–1577. [DOI] [PubMed] [Google Scholar]
- 79. Ulla M, Thobois S, Lemaire JJ. Manic behaviour induced by deep‐brain stimulation in Parkinson's disease: evidence of substantia nigra implication? J Neurol Neurosurg Psychiatry 2006;77(12):1363–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Poewe W, Gauthier S, Aarsland D, et al. Diagnosis and management of Parkinson's disease dementia. Int J Clin Pract 2008;62(10):1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


