Abstract
Objective
The objective was to summarize pregnancy and fetal/postnatal outcomes following maternal perampanel exposure using preclinical and clinical data, and to use physiologically based pharmacokinetic (PBPK) modeling to improve understanding of perampanel pharmacokinetics (PK) during pregnancy.
Methods
Preclinical developmental studies with perampanel were conducted in pregnant rats and rabbits. Clinical data were collated from the Eisai global perampanel safety database, comprising reports of perampanel exposure during pregnancy from routine clinical settings, interventional studies, and non‐interventional post‐marketing studies, searched for events coded to Medical Dictionary for Regulatory Activities (MedDRA) high‐level group terms of Pregnancy, Labor, Delivery, and Postpartum Conditions and/or the Standardized MedDRA Query terms of Congenital, Familiar, and Genetic Disorders. A PBPK model was used to predict clinical perampanel PK throughout pregnancy.
Results
Preclinical studies indicated that perampanel may be linked with post‐implantation loss and/or some specific physical development delays but not fertility and early embryonic development. As of August 31, 2018, 96 pregnancies in 90 women receiving perampanel had been reported. No concomitant medications were reported in 26 (28.9%) women taking perampanel. Overall, 43 pregnancies reached full term (all normal live births), 28 did not reach term (induced abortion, n = 18; spontaneous miscarriage, n = 6; incomplete spontaneous miscarriage, n = 2; premature delivery, n = 1; stillbirth [Fallot’s tetralogy], n = 1), 18 were lost to follow‐up, and seven were ongoing at data cut‐off. Adverse events were reported in five full‐term neonates (low Apgar score, n = 2; fatal neonatal aspiration, n = 1; cystic fibrosis and congenital deafness, n = 1; poor sucking reflex and shallow breathing, n = 1). PK simulations predicted perampanel exposure decreases throughout pregnancy and is up to four‐ and three‐fold lower towards the end of pregnancy compared with non‐pregnant women for total and unbound perampanel, respectively.
Significance
These data provide preliminary information on perampanel use during pregnancy and should be interpreted with caution. Further outcome data are required to estimate the prevalence of adverse pregnancy outcomes with perampanel exposure.
Keywords: adverse events, epilepsy, perampanel, physiologically based pharmacokinetics, pregnancy
Key Points.
Preclinical data indicated that perampanel may be associated with post‐implantation loss and/or some specific physical development delays
Of 96 pregnancies evaluated in 90 women exposed to perampanel, 43 reached full term; 26 (28.9%) women reported no concomitant ASM use
Of 71 pregnancies with a known outcome, there was one report of stillbirth with Fallot’s tetralogy (concomitant levetiracetam reported)
Pharmacokinetic simulations suggested a possible three‐ to four‐fold decrease in perampanel exposure over the course of a pregnancy
The small size and 19% lost to follow‐up in this preliminary sample require more data to estimate prevalence of adverse pregnancy outcomes
1. INTRODUCTION
Women with epilepsy have been estimated to account for up to 0.7% of pregnant women. 1 Due to the likelihood of seizures and associated risks therewith, and an increased risk of pregnancy‐related complications compared with women without epilepsy, 2 , 3 women with epilepsy frequently take anti‐seizure medication (ASM) during pregnancy. 4 , 5 , 6 Therefore, it is important to assess the safety of exposure to ASMs in utero, so that treatment with ASMs during pregnancy can balance adequate seizure control with the potential risk of adverse effects on the exposed fetus. 2 Pharmacokinetic (PK) changes of ASMs during pregnancy, such as enhanced drug elimination and decreased drug exposure compared with non‐pregnant women, are important to consider, as they may impact both fetal exposure and maternal seizure control. 5 , 7 However, there is limited information about PK changes of many of the newer ASMs during pregnancy. 5 , 7 , 8
Moreover, use of ASMs in pregnant women is increasing, with a five‐fold increase in the use of newer ASMs (i.e., those approved in the United States since 1993) by pregnant women during the early‐to‐mid 2000s, whereas use of older ASMs has remained generally stable. 6 Additionally, some ASMs may also be used to treat non‐epilepsy indications, including bipolar disorder, neuropathic pain, and fibromyalgia, 9 and a substantial increase in the frequency of ASM use in pregnant women for non‐epilepsy indications, including psychiatric diagnoses and pain disorders, has been observed during the past 2 decades. 6 , 10 The prospective European Registry of Antiepileptic Drugs and Pregnancy (EURAP) registry noted marked changes in the use of different ASMs over a 14‐year period (2000–2013), with increased use of lamotrigine and levetiracetam and a decrease in the use of valproate and carbamazepine. 11 A 27% decrease in the prevalence of major congenital malformations was observed in parallel with this shift in ASM utilization, and there was no indication of an increase in the proportion of pregnancies with poor seizure control. 11 An analysis of data from the Australian Register of Antiepileptic Drugs in Pregnancy revealed that approximately half of women with epilepsy who received ASMs throughout pregnancy still experienced at least one seizure during their pregnancy. 12
Perampanel is a once‐daily ASM that has been licensed for clinical use in the United States since 2012 for focal‐onset seizures (previously known as partial‐onset seizures) with or without focal to bilateral tonic–clonic seizures (previously known as secondarily generalized seizures), and generalized tonic–clonic seizures (previously known as primary generalized tonic–clonic seizures). 13 , 14 Perampanel is eliminated primarily via cytochrome P450 3A (CYP3A) metabolism, and concomitant enzyme‐inducing ASMs reduce exposure by ~50%–67%. 14 In vitro studies have shown that at clinically relevant concentrations (20–2000 ng/ml), perampanel is ~95%–96% bound to plasma proteins. 14
There are no adequate studies specifically in pregnant women treated with perampanel to assess outcomes of pregnancies that are exposed to perampanel. 14 Additionally, unknown effects of pregnancy on perampanel PK limit our understanding of whether dose adjustments may be necessary to maintain seizure control during pregnancy and/or reduce the likelihood of fetal adverse events (AEs). The aim of the analyses presented here is to summarize pregnancy and fetal/postnatal outcomes following maternal exposure to perampanel using preclinical and clinical data, and to gain a greater understanding of how perampanel PK may be affected by pregnancy through the use of physiologically based PK (PBPK) modeling.
2. MATERIALS AND METHODS
2.1. Preclinical data in pregnancy
Preclinical studies were performed in accordance with Japanese guidelines in place at the time of study initiation. 15 , 16
Developmental studies of perampanel were conducted in pregnant animals in line with licensing requirements. Dose‐ranging (0, 10, 30, and 60 mg/kg/day) and pivotal (0, 1, 3, and 10 mg/kg/day) embryo‐fetal development studies were performed in Sprague–Dawley rats (n = 7 and n = 20 per group, respectively) and New Zealand white rabbits (n = 5 and n = 20 per group, respectively), with perampanel administered orally on gestation Days 6–17 and Days 6–18 for rats and rabbits, respectively.
Dose‐ranging and pivotal pre‐ and postnatal development studies were performed in Sprague–Dawley rats (0, 1, 3, and 10 mg/kg/day). Perampanel was administered orally from gestation Day 6 to postnatal Day 6 in the dose‐ranging study (n = 8 per group) and from gestation Day 6 to postnatal Day 20 (weaning) in the pivotal study (n = 20 per group).
2.2. Safety review in pregnancy
A search of the Eisai global safety database for perampanel was performed for events coded to the Medical Dictionary for Regulatory Activities (MedDRA) high‐level group terms of Pregnancy, Labor, Delivery, and Postpartum Conditions, and/or the Standardized MedDRA Query terms of Congenital, Familiar, and Genetic Disorders. This analysis included all reports of exposure to perampanel during pregnancy from spontaneous sources (routine clinical settings) as well as solicited reports from interventional clinical studies and non‐interventional post‐marketing studies, up until the cut‐off date of August 31, 2018. Pregnancies from interventional clinical studies where the woman was reported to be exposed to placebo rather than perampanel, or where exposure only occurred post‐partum (e.g., during breastfeeding), and unpublished reports received from EURAP were excluded (no other registry data were included in the database). Pregnancies where perampanel exposure occurred via the father were evaluated separately from those with maternal perampanel exposure. Reported pregnancies were followed up every trimester and with a form requesting details of the final pregnancy outcome (including selection of category from the following: normal, abnormal baby, congenital abnormality, and died during perinatal period). There was no specific protocol for classification of congenital malformations, nor was there a requirement for assessment by a teratologist in cases of suspected abnormal outcomes. Any additional investigation was at the discretion of the treating physician, who was also responsible for reporting the pregnancy outcome. Outcomes for pregnancies reported before August 31, 2018 were updated in line with any follow‐up reports received by May 27, 2019 (additional information related to exposure in one woman during breastfeeding was received on June 17, 2019). In cases where no outcome was recorded by May 27, 2019, these pregnancies were either considered ongoing (if last reported as ongoing any time from January 2018 onwards) or lost to follow‐up (if last reported as ongoing any time prior to January 2018, or where dates of the pregnancy were unknown).
The clinical studies with perampanel in the global safety database included data from patients with or without epilepsy (focal‐onset or generalized tonic–clonic seizures), and healthy volunteers from Phase 1 studies. Pregnancy was an exclusion criterion for all these studies. The protocols for each study required patients to be abstinent or to use at least one medically acceptable method of contraception starting at Visit 1, throughout the entire study period, and for 2 months after the last dose of study drug. Pregnancy was captured as a treatment‐emergent AE, and confirmed with urine and serum pregnancy tests.
2.3. PK modeling predictions in pregnancy
A PBPK model for perampanel was developed using Simcyp® version 15.1. A previous model developed with Simcyp® version 11 17 was used as the starting point. The previous model predicted a slightly longer half‐life for perampanel than that observed clinically; thus, the following modifications were made to correct this discrepancy: (1) a middle‐out approach was used, where clinical total drug clearance values from clinical trial data (Study 005 17 ) were escalated to intrinsic hepatic drug clearance values using the retrograde calculator, (2) the scalar for tissue‐to‐plasma partition coefficients (Kps scalar) was manually adjusted from 1.0 to 0.75 to improve fit to the clinical data, (3) active uptake into hepatocytes was changed from 1.0 to 1.5, and (4) the absorption model was changed from the advanced dissolution absorption and metabolism model 18 to a first‐order absorption model with predicted inputs. The effective permeability in vivo (Peff = 7.73 × 10−4 cm/s), the absorption rate constant (ka = 3.38 h−1), and fraction absorbed (F = 0.999) were predicted by Simcyp® with the mechanistic permeability model (MechPeff 19 , 20 ) based on perampanel's partition co‐efficient, logPoctanol:water, of 2.86. 17 The model was evaluated by simulating clinical PK of single and multiple doses of perampanel in 100 healthy volunteers (10 trials each with 10 virtual subjects aged 20–50 years; 1:1 ratio of males to females). These predictions were compared with PK profiles for single oral doses of perampanel administered at 1, 2, 4, 6, or 8 mg and once‐daily doses of oral perampanel administered at 1, 2, 4, or 6 mg for 14 days that were observed in several clinical studies.
Simcyp® supports modeling and simulations to assess potential PK‐related effects of pregnancy. The in‐built Simcyp® Pregnancy population file, with an updated CYP3A4 ontogeny (Figure S1), 21 was used to predict clinical PK in 25 pregnant women (five trials each with five virtual subjects aged 20–45 years) for single oral doses of perampanel 8 mg administered during pregnancy at Weeks 0, 10, 19, 28, and 36, and once‐daily doses of oral perampanel 8 mg administered over a 270‐day pregnancy.
3. RESULTS
3.1. Preclinical data of perampanel in pregnancy
In reproductive toxicology and developmental preclinical studies, perampanel induced developmental toxicity in pregnant rats and rabbits at clinically relevant doses, where 1 mg/kg/day is similar to 8 mg/day in humans. 14 No drug‐related effects of perampanel on fertility and early embryonic development were noted with doses of 1, 10, or 30 mg/kg/day. Dose‐dependent increases in rates of post‐implantation loss were observed with perampanel exposure following 30 and 60 mg/kg in rats and 10, 30, and 60 mg/kg in rabbits. In the pivotal embryo‐fetal development study in rats, perampanel was associated with increased rates of diverticulum of the intestine at all doses tested (1, 3, or 10 mg/kg/day).
In the dose‐ranging pre‐ and postnatal development study in rats, no developmental toxicity was observed. In the pivotal study, stillbirths were increased and the viability index was decreased for the mid‐ and high‐level doses (3 and 10 mg/kg/day). Behavioral and reproductive development of the offspring were not affected, but some parameters of physical development showed some delay (preputial separation in males and vaginal opening in females) in offspring born to mothers who received 10/mg/kg/day, which are probably secondary to the pharmacology‐based central nervous system effects of perampanel.
3.2. Clinical data of pregnancy events and outcomes with perampanel
At the data cutoff of August 31, 2018, 96 pregnancies had been reported in 90 women aged 17–48 (age was unknown in 21 women) exposed to perampanel globally, including six women who had two pregnancies each. These pregnancies included 33 from patients enrolled in clinical studies or from other solicited reports, and 63 spontaneous reports from routine clinical settings. Perampanel dosing was discontinued or modified according to clinical need during the pregnancy in some women. The demographic characteristics and sources of clinical data for the women included in this analysis are shown in Table 1. Excluding three instances of perampanel exposure via a partner, perampanel was reported to have been taken by 26 of 90 (28.9%) women with no concomitant ASM use recorded (due to limited information provided in most cases, these women cannot be confirmed as receiving monotherapy). Perampanel administered with one concomitant ASM was reported for 24 of 90 (26.7%) women, and with ≥2 concomitant ASMs for 38 of 90 (42.2%) women; concomitant ASM use was unknown in two (2.2%) women, as only the neonatal records were available. The majority of patients from clinical trials included in this analysis were from trials that enrolled patients with focal‐onset seizures. For spontaneously reported pregnancies, the seizure types that patients had were not usually recorded. An additional three pregnancies were identified following paternal exposure to perampanel.
TABLE 1.
Demographic characteristics and sources of clinical data for women exposed to perampanel during pregnancy
| Characteristic | From clinical studies or solicited reports, n = 30 | From spontaneous reports, n = 60 a | Total, n = 90 |
|---|---|---|---|
| Country of origin, n (%) | |||
| Japan | 3 (10.0) | 9 (15.0) | 12 (13.3) |
| Spain | 0 (0) | 12 (20.0) | 12 (13.3) |
| France | 0 (0) | 8 (13.3) | 8 (8.9) |
| United Kingdom | 1 (3.3) | 7 (11.7) | 8 (8.9) |
| Russia | 1 (3.3) | 6 (10.0) | 7 (7.8) |
| United States of America | 4 (13.3) | 3 (5.0) | 7 (7.8) |
| China | 4 (13.3) | 0 (0) | 4 (4.4) |
| Germany | 0 (0) | 4 (6.7) | 4 (4.4) |
| Australia | 2 (6.7) | 1 (1.7) | 3 (3.3) |
| Austria | 0 (0) | 3 (5.0) | 3 (3.3) |
| South Korea | 3 (10.0) | 0 (0) | 3 (3.3) |
| Latvia | 3 (10.0) | 0 (0) | 3 (3.3) |
| Other b | 9 (30.0) | 7 (11.7) | 16 (17.8) |
| Age group at time of pregnancy, n (%) c | |||
| <20 years | 2 (6.7) | 5 (8.3) | 7 (7.8) |
| 20–24 years | 7 (23.3) | 6 (10.0) | 13 (14.4) |
| 25–29 years | 5 (16.7) | 12 (20.0) | 17 (18.9) |
| 30–34 years | 5 (16.7) | 8 (13.3) | 13 (14.4) |
| 35–39 years | 6 (20.0) | 10 (16.7) | 16 (17.8) |
| ≥40 years | 3 (10.0) | 0 (0) | 3 (3.3) |
| Unknown | 2 (6.7) | 19 (31.7) | 21 (23.3) |
| Number of reported concomitant ASMs, n (%) | |||
| 0 | 5 (16.7) | 21 (35.0) d | 26 (28.9) |
| 1 | 6 (20.0) | 18 (30.0) | 24 (26.7) |
| 2 | 9 (30.0) e | 11 (18.3) | 20 (22.2) |
| ≥3 | 10 (33.3) | 8 (13.3) | 18 (20.0) |
| Unknown | 0 (0) | 2 (3.3) | 2 (2.2) |
| Clinical data source, n (%) | |||
| Clinical studies f | 30 (100.0) | 0 (0) | 30 (33.3) |
| Spontaneous reports | 0 (0) | 60 (100.0) | 60 (66.7) |
Abbreviations: ASM, anti‐seizure medication; n, number of women with pregnancies exposed to perampanel.
Spontaneous reports of pregnancies in routine clinical practice.
Fewer than three patients each were from the following countries: Argentina, Belgium, Canada, Denmark, Estonia, India, Ireland, Israel, Lithuania, Mexico, and Slovakia.
For women with multiple pregnancies, age is recorded as age during the first perampanel‐exposed pregnancy.
No concomitant ASMs were reported; however, due to limited information being provided in most cases, monotherapy cannot be confirmed.
Includes one woman who received two ASMs during her first pregnancy and one ASM during a second pregnancy.
Studies 048 (NCT02279485), 207 (NCT00368472), 210 (NCT00154063), 304 (NCT00699972), 307 (NCT00735397), 332 (NCT01393743), 335 (NCT01618695), 342 (NCT03201900), 401 (NCT01871233), 402 (NCT02033902), 502 (NCT03059329), 505 (NCT02722590), and others.
Of the 96 pregnancies reported in women receiving perampanel, 43 reached full term, 28 did not go to term, 18 were lost to follow‐up, and seven were ongoing at data cutoff (Table 2). Of the pregnancies that went to full term, all 43 resulted in normal live births. Of the pregnancies that did not go to term, 18 cases were induced abortions, six cases were spontaneous miscarriages, two cases were incomplete spontaneous miscarriages, one case resulted in premature delivery, and one resulted in stillbirth due to Fallot’s tetralogy (Table 2). The reasons for the decision to have an induced abortion were not usually known, but most were considered by the investigator or treating physician to be not related to perampanel; causality in two spontaneous reports was not reported by the treating physician, and the cases were therefore assumed to be possibly related to perampanel. Of the six women who had two perampanel‐exposed pregnancies, one woman had two full‐term normal births. Three women had one full‐term birth each, plus a spontaneous abortion, incomplete spontaneous abortion, or an unknown outcome, and two women had both an induced abortion and a spontaneous abortion. Of the three pregnancies with paternal exposure to perampanel, two resulted in normal live births; the outcome of the third pregnancy was unknown (Table 2).
TABLE 2.
Pregnancy outcomes in the global safety database
| Outcome | From clinical studies or solicited reports | From spontaneous reports a | Total |
|---|---|---|---|
| Maternal exposure to perampanel | n = 33 | n = 63 | n = 96 |
| Reached full term | 8 | 35 | 43 |
| Normal live birth | 8 | 35 b | 43 |
| Did not reach full term | 22 | 6 | 28 |
| Induced abortion | 15 c | 3 | 18 |
| Spontaneous miscarriage | 5 | 1 | 6 |
| Incomplete spontaneous miscarriage | 2 | 0 | 2 |
| Premature delivery | 0 | 1 | 1 |
| Stillbirth (Fallot’s tetralogy) | 0 | 1 | 1 |
| Lost to follow‐up d | 2 | 16 | 18 |
| Ongoing pregnancy e | 1 | 6 | 7 |
| Paternal exposure to perampanel | n = 2 | n = 1 | n = 3 |
| Reached full term | 2 | 0 | 2 |
| Normal live birth | 2 | 0 | 2 |
| Lost to follow‐up e | 0 | 1 | 1 |
Spontaneous reports of pregnancies in routine clinical practice.
Includes two cases where babies were exposed to perampanel during pregnancy and via breastfeeding.
Includes one case of benign hydatidiform mole and one case of ectopic pregnancy.
Cases that were last reported as ongoing any time prior to January 2018, and where no subsequent updates have been received, or where dates of pregnancy were unknown.
Cases that were last reported as ongoing any time from January 2018 onward, and where no subsequent updates have been received.
AEs were reported in five of the 43 babies whose mothers received perampanel that reached full term: low Apgar score in two babies, fatal neonatal aspiration in one baby, cystic fibrosis and congenital deafness in one baby, and poor sucking reflex and shallow breathing in one baby (Table 3). In the cases where the relationship of AEs to perampanel was reported, poor sucking reflex and shallow breathing (reported in one baby who was also exposed to perampanel via breastfeeding) were considered possibly related to perampanel by the treating physician, whereas fatal neonatal aspiration, congenital deafness, and cystic fibrosis were not considered by the treating physician to be related to perampanel. In the case of fatal neonatal aspiration, the mother discontinued perampanel at ~2 months of gestation following the positive pregnancy test; the baby was born by Caesarian section at 39 of weeks' gestation, and the investigator considered that “the death was probably due to aspiration of fluid during birth." The baby with cystic fibrosis and congenital deafness was born to parents with congenital deafness who were carriers of the gene for cystic fibrosis. The relationship between perampanel exposure and low Apgar score in both babies was not reported, and therefore assumed to be possibly related to perampanel. Concomitant ASM use was reported for the mothers of the babies with fatal neonatal aspiration (carbamazepine and clobazam), poor sucking reflex and shallow breathing (clonazepam), and cystic fibrosis and congenital deafness (two unspecified ASMs). No concomitant ASMs were reported for the mothers of either baby with low Apgar score. No other birth defects or fetal malformations were reported in babies who reached full term.
TABLE 3.
Adverse events in babies from pregnancies with maternal exposure to perampanel that reached full term (n = 43)
| Baby | Outcome | Causality a | Perampanel dose | Source b | Concomitant ASMs taken by mother |
|---|---|---|---|---|---|
| 1 | Low Apgar score | Not reported c | 6 mg daily | Spontaneous | None reported |
| 2 | Low Apgar score | Not reported c | 8 mg daily | Spontaneous | None reported |
| 3 | Neonatal aspiration (fatal) | Not related to perampanel | 12 mg daily d | Clinical study | Carbamazepine, clobazam |
| 4 e | Cystic fibrosis | Not related to perampanel | Unknown | Spontaneous | Two unspecified ASMs |
| Congenital deafness | Not related to perampanel | ||||
| 5 f | Poor sucking reflex | Possibly related to perampanel | 2 to >12 mg daily g | Spontaneous | Clonazepam |
| Shallow breathing | Possibly related to perampanel |
Abbreviation: ASM, antiseizure medication.
As considered by the investigator/reporting physician.
Solicited reports of pregnancies from clinical studies/other solicited sources or spontaneous reports of pregnancies in routine clinical practice.
Not reported and therefore assumed to be possibly related.
Perampanel was discontinued at the time of positive pregnancy test (7 months prior to full‐term delivery by Caesarian section).
Both parents had congenital deafness and were carriers of the gene for cystic fibrosis.
This baby was also exposed to perampanel via breastfeeding.
Perampanel dose was increased to 6 mg/day following positive pregnancy test, to 10 mg/day approximately 1 month before birth, and at the mother's own discretion exceeded 12 mg/day prior to labor before reducing to 10 mg/day after the birth. Maternal perampanel plasma concentration was increased at the time of birth (2510 ng/ml).
The AEs related to pregnancies that did not reach full term were benign hydatidiform mole and ectopic pregnancy (one woman each, both of whom had an induced abortion; perampanel 6 mg daily and 2–4 mg daily, respectively), and stillbirth with Fallot’s tetralogy at the 30th week of pregnancy in one baby (perampanel 2–6 mg daily until the 11th week of pregnancy). None of these AEs, nor their associated induced abortions, was considered by the investigator or reporting physician to be related to perampanel, although no further explanation was provided. Use of concomitant ASM(s) was reported for all three of these mothers (benign hydatidiform mole: carbamazepine, clobazam, lamotrigine; ectopic pregnancy: lorazepam, levetiracetam, lacosamide, clonazepam; Fallot’s tetralogy: levetiracetam).
There were two cases of babies who were exposed to perampanel via breastfeeding in addition to in utero exposure to perampanel. As mentioned, poor sucking reflex and shallow breathing were reported in one of these babies. This baby was suspected to have withdrawal syndrome of perampanel and had a perampanel plasma concentration of 264 ng/ml 4 h after birth; the perampanel plasma concentration in the mother was 2510 ng/ml. Maternal concomitant clonazepam use was also reported for this pregnancy, and the baby had a clonazepam concentration of 2 mg/ml 4 h after birth. The plasma perampanel concentration in the baby decreased to 224 ng/ml 7 days after birth and to 124 ng/ml 14 days after birth; maternal plasma perampanel concentration had decreased to 845 ng/ml within approximately 2 months of the birth. No AEs were reported for the other baby who was exposed to perampanel via breastfeeding, and no information was recorded on the plasma concentrations of perampanel in that baby or its mother. No concomitant medication use was reported for the mother of this baby.
3.3. PK modeling of perampanel in pregnancy
During model evaluation, the PBPK model for perampanel generally predicted the PK profiles of perampanel well in single‐ and multiple‐dose studies in healthy volunteers (Figure S2). Single‐dose simulations of total and unbound plasma perampanel concentrations at timepoints throughout pregnancy are shown in Figure 1. These simulations predicted that total perampanel exposure is 2.5‐fold lower at the end of pregnancy (Week 36) compared with non‐pregnant women, whereas exposure for unbound (free) perampanel was predicted to be two‐fold lower (Table 4). Multiple‐dose simulations predicted that total perampanel exposure decreases over time during pregnancy, and is up to four‐fold lower toward the end of pregnancy (Week 36) compared with non‐pregnant women (Table 4 and Figure 2). Exposure for unbound perampanel was predicted to be three‐fold lower (Table 4).
FIGURE 1.
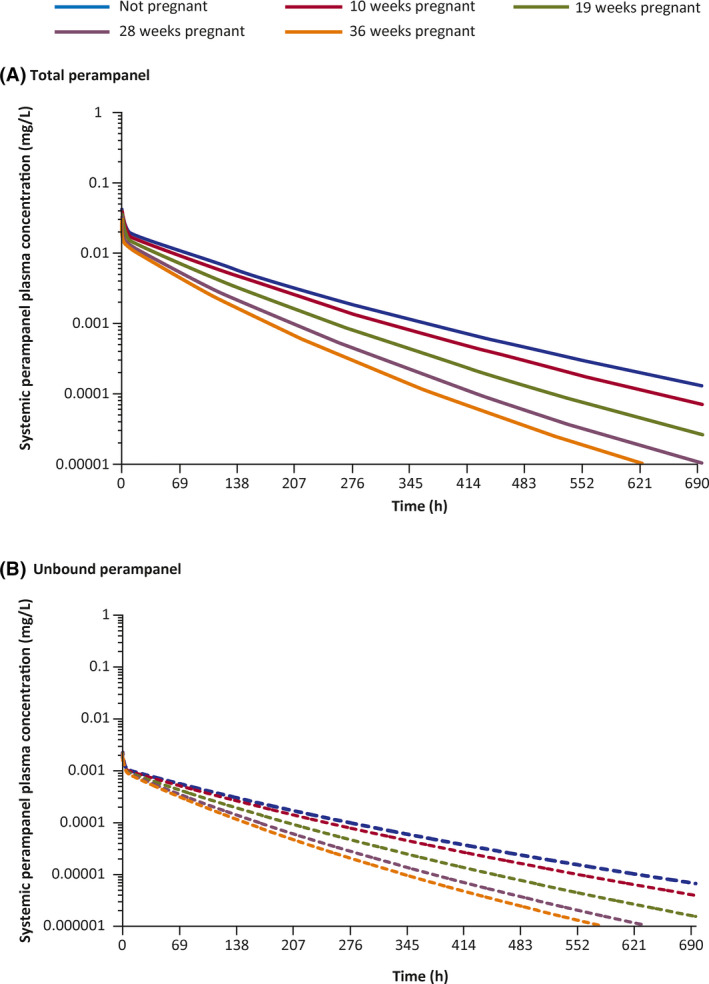
Single oral dose simulations of perampanel 8 mg administered during pregnancy at Weeks 0, 10, 19, 28, and 36. (A) Mean predicted total plasma concentrations of perampanel. (B) Mean predicted unbound plasma concentrations of perampanel
TABLE 4.
Total and free exposure parameters in physiologically based pharmacokinetic simulations in pregnant and non‐pregnant women
| Gestational age at dosing | Cmax, mg/L | AUC, mg/L/h | Cmax ratio | AUC ratio |
|---|---|---|---|---|
| Total perampanel exposure for single 8‐mg doses | ||||
| Not pregnant | 0.180 | 12.83 | 1.00 | 1.00 |
| 10 weeks | 0.166 | 10.78 | 0.92 | 0.84 |
| 19 weeks | 0.152 | 8.12 | 0.84 | 0.63 |
| 28 weeks | 0.139 | 6.16 | 0.77 | 0.48 |
| 36 weeks | 0.128 | 5.07 | 0.71 | 0.40 |
| Free perampanel exposure for single 8‐mg doses | ||||
| Not pregnant | 0.0090 | 0.657 | 1.00 | 1.00 |
| 10 weeks | 0.0089 | 0.577 | 0.99 | 0.88 |
| 19 weeks | 0.0087 | 0.459 | 0.97 | 0.70 |
| 28 weeks | 0.0085 | 0.374 | 0.94 | 0.57 |
| 36 weeks | .0084 | 0.333 | 0.94 | 0.51 |
| Pregnancy status | Cmax, mg/L | AUC, mg/L/h | Cmax ratio | AUC ratio |
|---|---|---|---|---|
| Total perampanel exposure for multiple 8‐mg doses after 270 days of simulations | ||||
| Not pregnant | 0.53 | 55.51 | 1.00 | 1.00 |
| Pregnant | 0.26 | 14.01 | 0.49 | 0.25 |
| Free perampanel exposure for multiple 8‐mg doses after 270 days of simulations | ||||
| Not pregnant | 0.027 | 2.84 | 1.00 | 1.00 |
| Pregnant | 0.017 | 0.94 | 0.63 | 0.33 |
Abbreviations: AUC, area under the curve; Cmax, maximum concentration.
FIGURE 2.
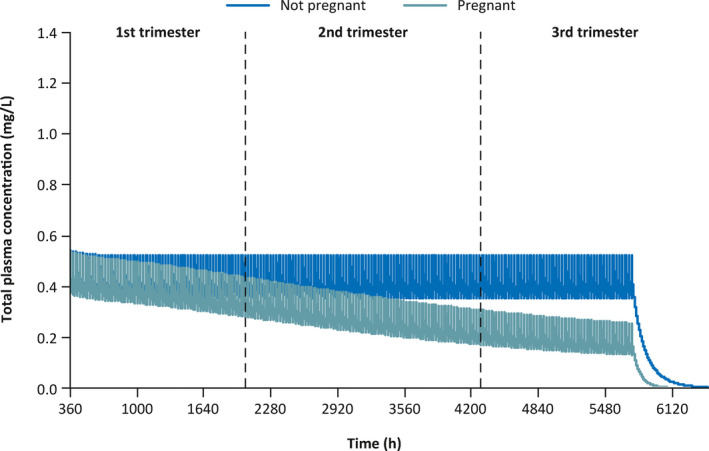
Predicted steady‐state pharmacokinetic profiles of perampanel 8 mg administered once daily for 240 days (5760 h) plus post‐dosing follow‐up period in pregnant and non‐pregnant women
4. DISCUSSION
In the absence of any previously published systematic summary of pregnancy outcomes with perampanel, 14 the preclinical and clinical data presented here may offer some valuable information on the effects of in utero exposure to perampanel, supplemented by PK simulations of perampanel during pregnancy.
The preclinical studies indicate that perampanel may be linked with post‐implantation loss in pregnant rats and rabbits, and with diverticulum of the intestine and specific delays in physical development when administered to pregnant rats. Preclinical data for carbamazepine, phenytoin, topiramate, and valproate, which are ASMs associated with an increased risk of congenital malformations, reveal similar types of teratogenic effects to those observed clinically, 2 , 22 , 23 , 24 , 25 highlighting the possible relevance of preclinical data for determining potential adverse pregnancy outcomes in humans. Although the risks of congenital malformations are generally considered lower in lamotrigine, levetiracetam, and oxcarbazepine (vs. carbamazepine, phenytoin, topiramate, and valproate), 2 , 26 teratogenic effects have still been observed in preclinical data for these ASMs. 27 , 28 , 29 Thus, there is an unmet need for clinical data to confirm any teratogenic effects of perampanel in human babies.
Of 71 pregnancies with a known outcome following maternal exposure to perampanel, there was one report of stillbirth with Fallot’s tetralogy and one report of fatal neonatal aspiration. Other AEs in babies included low Apgar scores in two babies, and poor sucking reflex and shallow breathing in one baby (all full‐term births). In the case of the baby with poor sucking reflex and shallow breathing, both mother and baby had elevated plasma perampanel concentrations following increased dosing of perampanel (>12 mg/day) at the mother's discretion prior to labor (concomitant clonazepam was also being taken by the mother). Additional AEs related to pregnancies that did not reach full term were benign hydatidiform mole and ectopic pregnancy (one woman each).
The conclusions that can be drawn from this safety review are limited by the low number of pregnancies included in the analysis. For example, there was a lack of information available in many of the pregnancy reports despite follow‐up at every trimester and until delivery or other outcome; this was particularly true for the reasons for induced abortions and in general for spontaneously reported pregnancies. In addition, causality with perampanel was as noted by the reporting physician, meaning that some AEs such as Fallot’s tetralogy were not attributed as related to perampanel although no alternate explanation was provided. Conversely, some AEs may have been incorrectly attributed as possibly related to perampanel due to expected effects of the drug (observer bias). A sizeable proportion of reported pregnancies (18.8%) were lost to follow‐up, which further reduces the number of pregnancy outcomes collated in this analysis. This highlights a need for the systematic collection of safety data related to epilepsy drug use during pregnancy via pregnancy registries. Finally, the mixed nature of the clinical trial and real‐world settings of perampanel administration for the patients included in this analysis meant that many patients received adjunctive perampanel rather than perampanel monotherapy. As a result, concomitant medications were administered in many, but not all, patients and some of the concomitant ASMs may have reduced perampanel exposure due to PK interactions.
The PK modeling of perampanel in pregnancy predicted that perampanel exposure may decline by two‐ to four‐fold during pregnancy, not taking into consideration any known effects of concomitant enzyme‐inducing ASMs. These differences predicted in pregnancy are a reflection of several physiological changes that are expected to occur during pregnancy, namely the two‐fold increase in CYP3A activity and an approximate 30% reduction in plasma albumin, which lead to higher free fractions in plasma and higher drug clearance. 5 , 30 Because perampanel is a low‐extraction drug, steady‐state unbound drug concentrations are independent of changes in protein binding, 31 which could explain why the predicted decrease in free perampanel exposure was smaller than that of total perampanel exposure during pregnancy.
Pregnancy can result in considerable changes to the PK of ASMs, which may have implications for seizure control and/or exposure of the fetus to ASMs. 7 Decreased serum concentrations for several ASMs, including carbamazepine, lamotrigine, levetiracetam, oxcarbazepine, phenytoin, phenobarbital, and zonisamide, have been observed during pregnancy. 7 For example, total concentration of carbamazepine, which is ~75% protein bound and metabolized similarly to perampanel (mainly via CYP3A4), 32 is reported to decrease over the course of a pregnancy, with a more limited decrease of up to 28% in unbound carbamazepine. However, reported decreases have varied widely between studies (0%–42%), 7 and some studies have reported limited or no clinically significant change in total and free carbamazepine concentrations during pregnancy. 32 , 33 Lamotrigine serum concentration is suppressed during pregnancy by 50%–60% on average, although inter‐patient variability may result in greater decreases. 7 Although total valproate concentrations are reported to fall by up to 40% during late pregnancy compared with pre‐pregnancy, the PK of valproate, an ASM that is highly protein bound (~90%), are somewhat variable, 7 , 34 and data are limited due to the known teratogenic and possible cognitive effects of valproate. 35 , 36 Based on the information presented for the PK modeling of perampanel in pregnancy, the predicted changes appear to exceed those of other ASMs. However, it should be noted that the effect of perampanel in pregnancy has not been investigated in a clinical trial and PK modeling has not been validated against observed perampanel concentrations in pregnant women. PBPK modeling has been used previously to predict the PK of other types of drugs, including dexamethasone, betamethasone, caffeine, midazolam, metoprolol, and rilpivirine, during pregnancy, and in general, the values were found to be comparable with observed PK. 21 , 37 , 38
5. CONCLUSIONS
Understanding the implications of ASM use during pregnancy is important due to an increased use in pregnant women. Although the perampanel data reported here are limited, they summarize available information on perampanel use during pregnancy and provide some insights regarding the effects of in utero exposure to perampanel. These preliminary data should be interpreted with caution, as the sample size is limited, and 18.8% of the pregnancies were lost to follow‐up. More outcome data are required to estimate the prevalence of adverse pregnancy outcomes with perampanel exposure. Physicians are encouraged to enroll pregnant women taking perampanel in a pregnancy registry, such as the North American Antiepileptic Drug Pregnancy Registry, EURAP, the UK and Irish Epilepsy & Pregnancy Registers, or the Australian Pregnancy Register, to allow more data to be collected on perampanel exposure during pregnancy. Pregnant women should also be carefully monitored, including drug‐level monitoring, where available, whilst taking perampanel as dose adjustments may be needed in order to maintain a similar level of exposure relative to a non‐pregnancy condition and to prevent the occurrence of breakthrough seizures.
CONFLICT OF INTEREST
B.V. has received research support from Biogen MA Inc., Cavion LLC, Engage Therapeutics Inc., Neurelis, Ovid Therapeutics, SK Life Science Inc., UCB Biopharma SPRL, and UCB Biosciences Inc. T.T. has received speaker's honoraria to his institution from Eisai, Sandoz, Sanofi, Sun Pharmaceutical Industries Ltd, and UCB, and research support from Bial, CURE, Eisai, the European Union (ESBACE), GlaxoSmithKline, Stockholm County Council, Teva, and UCB. C.D. and E.S. are employees of Eisai Inc. T.J.O. has received research and speaker honoraria from Eisai and UCB Pharma.
AUTHOR CONTRIBUTIONS
C.D. was involved in collating and reviewing the data relating to pregnancy events with exposure to perampanel. E.S. was responsible for developing the PBPK model for perampanel and running the pregnancy simulations. All authors were involved in the interpretation of the results, the reviewing and approval of the manuscript, and the decision to submit the article for publication. All authors also confirm accountability for the accuracy and integrity of the work.
Supporting information
Fig S1‐S2
ACKNOWLEDGMENTS
Funding for these analyses was provided by Eisai Inc. Rick Dabagian is thanked for assistance with review of the data. Medical writing support, under the direction of the authors, was provided by Rebekah Waters, PhD, and Kirsty Muirhead, PhD, of CMC AFFINITY, McCann Health Medical Communications, funded by, Eisai Inc., in accordance with Good Publication Practice 3 (GPP3) guidelines.
REFERENCES
- 1. Wang M, Li W, Tao Y, Zhao L. Emerging trends and knowledge structure of epilepsy during pregnancy research for 2000–2018: a bibliometric analysis. PeerJ. 2019;7:e7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veroniki AA, Cogo E, Rios P, Straus SE, Finkelstein Y, Kealey R, et al. Comparative safety of anti‐epileptic drugs during pregnancy: a systematic review and network meta‐analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacDonald SC, Bateman BT, McElrath TF, Hernandez‐Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. [DOI] [PubMed] [Google Scholar]
- 5. Pariente G, Leibson T, Carls A, Adams‐Webber T, Ito S, Koren G. Pregnancy‐associated changes in pharmacokinetics: a systematic review. PLoS Med. 2016;13:e1002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bobo WV, Davis RL, Toh S, Li D‐K, Andrade SE, Cheetham TC, et al. Trends in the use of antiepileptic drugs among pregnant women in the US, 2001–2007: a medication exposure in pregnancy risk evaluation program study. Paediatr Perinat Epidemiol. 2012;26:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomson T, Landmark CJ, Battino D. Antiepileptic drug treatment in pregnancy: changes in drug disposition and their clinical implications. Epilepsia. 2013;54:405–14. [DOI] [PubMed] [Google Scholar]
- 8. Reimers A. New antiepileptic drugs and women. Seizure. 2014;23:585–91. [DOI] [PubMed] [Google Scholar]
- 9. Bialer M. Why are antiepileptic drugs used for nonepileptic conditions? Epilepsia. 2012;53(Suppl 7):26–33. [DOI] [PubMed] [Google Scholar]
- 10. Leong C, Mamdani MM, Gomes T, Juurlink DN, Macdonald EM, Yogendran M. Antiepileptic use for epilepsy and nonepilepsy disorders: a population‐based study (1998–2013). Neurology. 2016;86:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Declining malformation rates with changed antiepileptic drug prescribing: an observational study. Neurology. 2019;93:e831–40. [DOI] [PubMed] [Google Scholar]
- 12. Vajda FJ, Hitchcock A, Graham J, O'Brien T, Lander C, Eadie M. Seizure control in antiepileptic drug‐treated pregnancy. Epilepsia. 2008;49:172–6. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency . Fycompa®. Annex I: Summary of Product Characteristics, 2017. https://www.ema.europa.eu/en/documents/product‐information/fycompa‐epar‐product‐information_en.pdf. Accessed 1 Oct 2020.
- 14. Food and Drug Administration . FYCOMPA®. Prescribing information. September 2020. https://www.fycompa.com/‐/media/Files/Fycompa/Fycompa_Prescribing_Information.pdf. Accessed 1 Oct 2020.
- 15. Ministry of the Environment (Japan) . Standards relating to the care and keeping and reducing pain of laboratory animals. https://www.env.go.jp/nature/dobutsu/aigo/2_data/laws/nt_h25_84_en.pdf. Accessed 2 July 2020.
- 16. Ministry of the Environment (Japan ). Act on Welfare and Management of Animals (Act No. 105 of October 1, 1973). https://www.env.go.jp/nature/dobutsu/aigo/1_law/files/aigo_kanri_1973_105_en.pdf. Accessed 2 July 2020.
- 17. Gidal BE, Maganti R, Laurenza A, Yang H, Verbel DA, Schuck E, et al. Effect of enzyme inhibition on perampanel pharmacokinetics: why study design matters. Epilepsy Res. 2017;134:41–8. [DOI] [PubMed] [Google Scholar]
- 18. Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami‐Hodjegan A, et al. Population‐based mechanistic prediction of oral drug absorption. AAPS J. 2009;11:225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pade D, Jamei M, Rostami‐Hodjegan A, Turner DB. Application of the MechPeff model to predict passive effective intestinal permeability in the different regions of the rodent small intestine and colon. Biopharm Drug Dispos. 2017;38:94–114. [DOI] [PubMed] [Google Scholar]
- 20. Food and Drug Administration . Certara®. FDA workshop. https://www.fda.gov/downloads/drugs/newsevents/ucm503765.pdf. Accessed 19 May 2020.
- 21. Ke AB, Milad MA. Evaluation of maternal drug exposure following the administration of antenatal corticosteroids during late pregnancy using physiologically‐based pharmacokinetic modeling. Clin Pharmacol Ther. 2019;106:164–73. [DOI] [PubMed] [Google Scholar]
- 22. Food and Drug Administration . Tegretol® and Tegretol®‐XR. Prescribing information. March 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/016608s115_018281_s058_018927s055_020234_s047.pdf. Accessed 19 May 2020.
- 23. Food and Drug Administration .Dilantin®. Prescribing information. October 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/084349s085lbl.pdf. Accessed 19 May 2020.
- 24. Food and Drug Administration . Topamax®. Prescribing information. May 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020505s060,020844s051lbl.pdf. Accessed 19 May 2020.
- 25. Food and Drug Administration . Depakene®. Prescribing information. February 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018081s069,018082s052lbl.pdf. Accessed 19 May 2020.
- 26. Tomson T, Battino D, Perucca E. Teratogenicity of antiepileptic drugs. Curr Opin Neurol. 2019;32:246–52. [DOI] [PubMed] [Google Scholar]
- 27. Food and Drug Administration . Lamictal®. Prescribing information. July 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020241s056,020764s049,022251s020lbl.pdf. Accessed 19 May 2020.
- 28. Food and Drug Administration . KEPPRA®. Prescribing information. October 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021035s100,021505s040lbl.pdf. Accessed 19 May 2020.
- 29. Food and Drug Administration . Trileptal®. Prescribing information. January 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021014s043lbl.pdf. Accessed 19 May 2020.
- 30. Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, et al. Effects of pregnancy on CYP3A and P‐glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84:248–53. [DOI] [PubMed] [Google Scholar]
- 31. Gibaldi M, Perrier D. Pharmacokinetics. New York, NY: Marcel Dekker; 1982. [Google Scholar]
- 32. Johnson EL, Stowe ZN, Ritchie JC, Newport DJ, Newman ML, Knight B, et al. Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav. 2014;33:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Epilepsy and pregnancy: a prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35:122–30. [DOI] [PubMed] [Google Scholar]
- 34. Johannessen Landmark C, Farmen AH, Burns ML, Baftiu A, Lossius MI, Johannessen SI, et al. Pharmacokinetic variability of valproate during pregnancy—implications for the use of therapeutic drug monitoring. Epilepsy Res. 2018;141:31–7. [DOI] [PubMed] [Google Scholar]
- 35. Tomson T, Marson A, Boon P, Canevini MP, Covanis A, Gaily E, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 2015;56:1006–19. [DOI] [PubMed] [Google Scholar]
- 36. European Medicines Agency . PRAC recommends strengthening the restrictions on the use of valproate in women and girls. https://www.ema.europa.eu/en/documents/press‐release/prac‐recommends‐strengthening‐restrictions‐use‐valproate‐women‐girls_en.pdf. Accessed 10 Oct 2014.
- 37. Gaohua L, Abduljalil K, Jamei M, Johnson TN, Rostami‐Hodjegan A. A pregnancy physiologically based pharmacokinetic (p‐PBPK) model for disposition of drugs metabolized by CYP1A2, CYP2D6 and CYP3A4. Br J Clin Pharmacol. 2012;74:873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gockenbach M. Physiologically‐based pharmacokinetic modeling of rilpivirine during pregnancy. 2019. http://regist2.virology-education.com/presentations/2019/20AntiviralPK/33_Gockenbach.pdf. Accessed 27 Jan 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2


