Summary
The intimate association of host and fungus in arbuscular mycorrhizal (AM) symbiosis can potentially trigger induction of host defence mechanisms against the fungus, implying that successful symbiosis requires suppression of defence.
We addressed this phenomenon by using AM‐defective vapyrin (vpy) mutants in Petunia hybrida, including a new allele (vpy‐3) with a transposon insertion close to the ATG start codon. We explore whether abortion of fungal infection in vpy mutants is associated with the induction of defence markers, such as cell wall alterations, accumulation of reactive oxygen species (ROS), defence hormones and induction of pathogenesis‐related (PR) genes.
We show that vpy mutants exhibit a strong resistance against intracellular colonization, which is associated with the generation of cell wall appositions (papillae) with lignin impregnation at fungal entry sites, while no accumulation of defence hormones, ROS or callose was observed. Systematic analysis of PR gene expression revealed that several PR genes are induced in mycorrhizal roots of the wild‐type, and even more in vpy plants. Some PR genes are induced exclusively in vpy mutants.
Our results suggest that VPY is involved in avoiding or suppressing the induction of a cellular defence syndrome that involves localized lignin deposition and PR gene induction.
Keywords: arbuscular mycorrhiza, defence, lignin, pathogenesis‐related protein, Petunia hybrida, Rhizophagus irregularis, symbiosis, VAPYRIN
Introduction
Arbuscular mycorrhiza (AM) is a mutualistic association of the majority of land plants with fungi of the subphylum Glomeromycotina (Smith & Read, 2008; Spatafora et al., 2016), which confers various benefits to the plant host (Chen, M et al., 2018). Although AM require mutual recognition of the partners to establish intracellular compatibility, the interaction is characterized by a very low degree of host specificity (Smith & Read, 2008). For example, the AM fungal model species Rhizophagus irregularis can colonize all angiosperms that were tested with few exceptions, represented by plant taxa that are generally incapable of engaging in AM (e.g. the Brassicaceae, including oilseed rape and Arabidopsis thaliana).
A striking aspect of the strong compatibility in AM is the massive intracellular colonization of the root cortex by hyphae, arbuscules and vesicles, which can result in > 90% colonization of the entire root system of the host. AM fungi share typical fungal cell wall components (e.g. chitin) with fungal pathogens, and plants have very sensitive detection mechanisms for such general microbial molecules, which are known as pathogen‐associated molecular patterns (PAMPs) or, more generally, as microbe‐associated molecular patterns (MAMPs) (Boller & Felix, 2009). Hence, the abundance of fungal material in the root, and the intimate interaction between the two symbiotic partners imply that disease resistance mechanisms in the host must be under tight control to avoid defence reactions to be triggered against AM fungi (Gianinazzi‐Pearson, 1996; Gianinazzi‐Pearson et al., 1996; Marsh & Schultze, 2001; García‐Garrido & Ocampo, 2002; Zipfel & Oldroyd, 2017).
Several studies have reported induction of defence mechanisms at early stages of AM interactions (Gianinazzi‐Pearson et al., 1996; Kapulnik et al., 1996; Campos‐Soriano et al., 2010; Marcel et al., 2010). After initial induction, these defence responses usually become repressed to, or below, the responses in noninoculated control roots. Interestingly, in simultaneous inoculations, AM fungi can reduce the induction of defence responses elicited by a pathogen (Guenoune et al., 2001) or by a chemical inducer of defence (David et al., 1998). This indicates that infection by AM fungi is associated with active suppression of defence. Indeed, an AM fungal effector protein that promotes biotrophic compatibility in host plants by suppressing defence has been identified in R. irregularis (Kloppholz et al., 2011). On the other hand, it was shown in many cases that AM fungi mediate increased disease resistance in roots as well as in the aerial parts of colonized plants, a phenomenon known as mycorrhiza‐induced resistance (MIR), involving a mechanism that resembles induced systemic resistance (ISR) (Jung et al., 2012; Pieterse et al., 2014).
Characteristic markers of defence include the stress hormones salicylic acid (SA; Loake & Grant, 2007), jasmonic acid (JA; Browse, 2009; Wasternack & Hause, 2013) and ethylene (van Loon et al., 2006a), cell wall reinforcements such as callose and lignin (Millet et al., 2010; Miedes et al., 2014; Chowdhury et al., 2016; Liu et al., 2018), induction of reactive oxygen species (ROS) (Jones & Dangl, 2006), and the induction of pathogenesis‐related (PR) proteins that are thought to contribute to disease resistance (van Loon et al., 2006b). Indeed, several of these defence markers are induced in mycorrhizal roots, and the observation that AM fungi express ROS scavenging enzymes during symbiosis (Lanfranco et al., 2005) is compatible with the view that they face defence mechanisms, in particular in mutants with compromised symbiosis competencies (Gianinazzi‐Pearson et al., 1996; Marsh & Schultze, 2001). However, in this context it is important to note that most of our knowledge on plant defence mechanisms was gained in the shoot (mainly from leaves), while defence mechanisms in the roots are considerably different (Chuberre et al., 2018), and have been explored to a lesser extent.
We have previously described two allelic mutants in petunia (Petunia hybrida), penetration and arbuscule morphogenesis1‐1 (pam1‐1) and pam1‐2, which carry transposon insertions in the VAPYRIN (VPY) gene (hereinafter referred to as vpy‐1 and vpy‐2, respectively). Detailed phenotypic analysis showed that vpy mutants are defective in infection of hypodermal cells and in arbuscule formation, indicating that VPY is required for intracellular accommodation of AM fungi during symbiosis. VPY function is conserved between petunia and Medicago truncatula (Pumplin et al., 2010), and acts downstream of calcium spiking, the central element in symbiotic signalling (Murray et al., 2011). Interestingly, VPY protein is localized to small mobile compartments (VPY bodies), which are thought to be involved in cellular trafficking during symbiosis (Feddermann et al., 2010; Pumplin et al., 2010; Zhang et al., 2015; Bapaume et al., 2019; Liu et al., 2019), and during protonema development in the moss Physcomitrella patens (Rathgeb et al., 2020). The fact that the AM fungus in vpy mutants exhibits conspicuous deformations upon cell penetration, and forms hyphal septa (a sign of stress) (Sekhara Reddy et al., 2007; Feddermann et al., 2010), suggests that this trafficking pathway may be involved, directly or indirectly, in modulating defence during intracellular stages of AM.
Here, we describe a new mutant allele, vpy‐3, which is a null allele, as a result of a dTph1 transposon insertion after only eight codons from the start codon. Vpy‐3 exhibits similar defects as the two other vpy alleles, indicating that these also represent functional null alleles. Microscopic and molecular analysis using a range of defence markers indicates that the abortion of the AM fungus in vpy mutants involves a cellular defence response that is independent of callose deposition and of the classical stress hormones SA, JA and ethylene, but is associated with induction of several PR genes, and with cell wall lignification during intracellular invasion.
Materials and Methods
Plant lines, fungal material and growth conditions
Seeds of the petunia transposon line W138 or wild‐type W115 were germinated on seedling substrate (Klasmann‐Deilmann Europe GmbH, Geeste, Germany; http://www.klasmann‐deilmann.com). For the mutant screen, plantlets were transferred to a sterilized mixture of 75% sand with 25% unfertilized soil (also referred to as sand substrate), inoculated with c. 10 g of pot culture inoculum of Rhizophagus irregularis (MUCL 43204), and cultured as previously described (Nouri et al., 2014). For all experiments involving mutants, the plants were inoculated with nurse plants by co‐culturing in the same pot petunia plants with chive plants (Allium schoenoprasum) that had been inoculated at least 4 wk before with R. irregularis. Plants were grown in growth chambers under 12 h : 12 h, 25°C : 20°C, light : dark conditions.
Isolation of the vpy‐3 mutant allele
The vpy‐3 allele was isolated as previously described (Sekhara Reddy et al., 2007; Rich et al., 2015). Briefly, eight individuals per segregating family were assessed for mycorrhizal colonization after 5 wk of colonization with R. irregularis (primary screen). Root samples were taken from inoculated plants, stained with trypan blue and screened visually for the presence of AM fungal structures. Families with AM‐defective individuals were further grown for seed production, and additional seeds of the respective family were sown for phenotypic analysis and assessment of the segregation pattern (secondary screen). Homozygous mutant individuals of the new mutant line were crossed with pam1‐1 (vpy‐1) and pam1‐2 (vpy‐2) (Feddermann et al., 2010). One hundred per cent of the F1 progeny showed the mutant phenotype of the parents, indicating that vpy‐3 is allelic to the previous vpy mutants. Isolation of the vpy‐3 locus by PCR, cloning into pGEMT, and Sanger sequencing revealed an insertion of a dTph1 copy after 25 nucleotides from the start codon (ATG). For detailed phenotypic analysis, the vpy‐3 allele was stabilized by segregating out the active translocator locus ACT1 after crossing with the stabilizer line W5 (Stuurman & Kuhlemeier, 2005).
Assessing AM fungal colonization and papilla formation
For primary mutant screening, roots were harvested, washed and stained with trypan blue (0.01% w/v) in 0.5% (v/v) acetic acid for 10 min at 95°C, and washed with water for visual inspection. For secondary screening, roots were cleared in 10% KOH (30 min at 95°C), washed twice with water, stained for 10 min with trypan blue staining solution at 95°C (20% glycerol, 30% lactic acid and 0.01% Trypan blue) and rinsed twice with 10% lactic acid.
For initial assessment of fungal hyphae and cell wall papillae in vpy‐3, plants were inoculated with R. irregularis in nurse plant chambers for 4 wk. Roots were fixed for 2 h at room temperature in 4% (v/v) paraformaldehyde. After several washes, roots were stained overnight at 4°C in the dark with 5 µg ml−1 wheat germ agglutinin (WGA) coupled to fluorescein isothiocyanate (ThermoFisher Life Technologies, Carlsbad, CA, USA) in Soerensen's phosphate buffer (0.133 M, pH = 7.2). Before mounting, samples were incubated for 10 min in 50 µg ml−1 propidium iodide at room temperature. In a second experiment for quantification of papilla formation in all vpy alleles, plants were inoculated with R. irregularis in nurse plant chambers for 4 wk, then roots were harvested and cleared in 10% KOH for 20 min. After four washes with deionized water, roots were stained overnight with WGA‐Alexa488 (ThermoFisher Life Technologies) in Soerensen's phosphate buffer (0.133 M, pH = 7.2), followed by counterstaining with 0.2% basic fuchsin (857343; Sigma). For microscopy, the roots were immersed in a modified version of ClearSee (Kurihara et al., 2015) containing 10% (w/v) xylitol, 25% (w/v) urea and 2% (w/v) sodium dodcyl sulphate. Images were acquired on a Leica SP5 confocal microscope.
Callose and lignin staining
For callose detection, roots inoculated by nurse plants were fixed overnight with 4% (v/v) paraformaldehyde (Supporting Information Fig. S6, see later), or with 1 : 3 acetic acid : ethanol (Table S1), washed five times with water, followed by staining with 0.01% (w/v) aniline blue in 150 mM KH2PO4 (pH = 9.5) for 48 h. Lignin was stained with phloroglucinol solution (100 ml 95% EtOH, 16 ml concentrated HCl, 0.1 g phloroglucinol) for 30 min and analysed directly. Callose epifluorescence images and lignin accumulation were analysed with a Leica DMR microscope (Leica Microsystems, Heerbruck, Switzerland), equipped with an Axiocam (Zeiss, Oberkochen, Germany).
Detection of ROS
For detection of hydrogen peroxide (H2O2), roots inoculated from nurse plants were harvested and treated with a freshly prepared solution of 1 mg ml−1 3,3′‐diaminobenzidine (DAB) in 10 mM MES‐NaOH (pH 5.6) (Salzer et al., 1999). Preparing the solution requires intense stirring and careful acidification with HCl to around pH = 3 (to increase DAB solubility) followed by buffering with MES‐NaOH. After overnight incubation at room temperature in the dark, roots were washed four times with water and incubated in ClearSeeD for microscopic analysis. For detection of O2 –, roots were treated for 1 h at room temperature in the dark with a fresh solution of 1mg ml−1 nitroblue tetrazolium (NBT) in 10 mM phosphate buffer, pH = 7.8. After four washes with water, roots were incubated in ClearSeeD for microscopic analysis.
Electron microscopy and preparation of semithin sections
Roots inoculated from nurse plants were fixed for 2 h at room temperature in 4% (v/v) glutaraldehyde and postfixed with 1% (w/v) OsO4 at 4°C overnight. Further processing of the samples and embedding in Spurr's resin was carried out as previously described (Spurr, 1969). For immunocytochemical analysis, tissues were embedded in Lowicryl K4 (Sigma‐Aldrich) as described (Altman et al., 1984) (Methods S1). Semithin sections (1 µm) were stained with 1 % (w/v) toluidine blue in 1% (w/v) borax (Na2B4O7). Images were acquired in the bright field mode on a Leica DMR microscope equipped with an Axiocam (Zeiss). For transmission electron microscopy (TEM) analysis, ultrathin sections (70 nm) were prepared on a Reichert‐Jung Ultracut E (Leica Microsystems). Contrasting was performed with 2% (w/v) uranyl acetate (UO2(CH3COO)2) and lead citrate solution prepared according to Reynolds (1963). Images were acquired on a Philips Biotwin CM100 (FEI Inc., Hillsboro, OR, USA).
Identification of lignin biosynthetic genes and PR genes
Lignin biosynthetic genes with a potential role in roots (Vanholme et al., 2010) were identified from a list of genes annotated as lignin‐related in a previous expressed sequence tag sequencing project (Breuillin et al., 2010). The full‐length gene sequences were then identified from the predicted transcriptome of P. axillaris at the SolGenomics database (https://solgenomics.net). PR gene homologues were identified by searching the Petunia axillaris predicted transcriptome at the SolGenomics database (https://solgenomics.net) using tobacco PR proteins (van Loon et al., 2006b) by tblastn. Primers for quantitative real‐time reverse‐transcriptase polymerase chain reaction (qRT‐PCR) were designed by the Primer3 tool (https://bioinfo.ut.ee/primer3‐0.4.0/) (see Tables S2, S7). Preliminary analysis of gene expression was performed in mycorrhizal and nonmycorrhizal roots of wild‐type and vpy mutant roots to identify genes that were expressed in any of the tested conditions, and these were used for further qRT‐PCR analysis.
Determination of SA and JA
Concentrations of JA and JA‐isoleucine (JA‐Ile) were determined by ultrahigh performance liquid chromatography‐tandem MS (UHPLC‐MS/MS) according to Glauser et al. (2014). SA and conjugated SA were separated by two‐phase extraction, followed by acid hydrolysis of conjugated SA, and quantification as previously described (Fragniere et al., 2011).
Supplementary materials
M. truncatula growth conditions and experimental procedures (Broughton & Dillworth, 1971), as well as immunostaining procedures, manipulation of RNA, qRT‐PCR (Pfaffl, 2001) and statistical analyses, are described in Notes S1 and Methods S1.
Results
Isolation of a new vpy allele
In a forward genetic screen for AM‐defective mutants, we isolated a mutant candidate with severely decreased colonization of the AM fungus Rhizophagus irregularis. Colonization of the root surface resulted in the production of deformed hyphopodia, and penetration of the epidermis and hypodermis was often arrested. Based on the phenotypic similarity with the previously isolated mutants pam1‐1 and pam1‐2 (Sekhara Reddy et al., 2007; Feddermann et al., 2010), we crossed the new mutant with these pam1 alleles to test for allelism. One hundred per cent of the F1 progeny showed the same AM‐resistant phenotype as the two parents (data not shown), confirming that the new mutant is allelic to pam1‐1 and pam1‐2. As the encoded protein has since been named VAPYRIN (VPY) as a result of its domain structure (Feddermann et al., 2010; Pumplin et al., 2010; Feddermann & Reinhardt, 2011; Murray et al., 2011), we further refer to the new allele as vapyrin‐3 (vpy‐3), and the previously isolated alleles as vpy‐1 and vpy‐2, respectively. The coding region of the VPY gene in the vpy‐3 mutant was amplified by PCR and cloned in order to identify the nature of the mutation. Indeed, a dTph1 insertion was detected at position 25 from the predicted start codon (ATG), leaving only eight of the 535 amino acids of the predicted protein (Feddermann et al., 2010) (Fig. 1a), suggesting that vpy‐3 represents a null allele.
Fig. 1.

Arbuscular mycorrhizal (AM)‐defective phenotype and molecular characterization of vpy‐3 in Petunia hybrida. (a) Molecular aspects of the new vpy‐3 allele. The petunia VAPYRIN coding sequence is shown from the ATG start codon to the insertion site of a dTPh1 transposon (triangle). The 8 bp target site duplication is in bold and underlined. Because of stop codons in all three reading frames of the dTPh1 sequence, the truncated protein can be predicted to contain only eight residual amino acids of VAPYRIN. (b) The colonization pattern in the wild‐type (wt) revealed by trypan blue staining. Infection by hyphal coils in hypodermal cells, and arbuscules in the cortex. (c–e) Colonization pattern in vpy mutants revealed by trypan blue staining. Infection by enlarged penetration pegs, and subsequent profusely branched hyphal colonization (arrows) without arbuscules in vpy‐3 (c), and for comparison, vpy‐1 (d) and vpy‐2 (e). a, arbuscules; c, hyphal coils; p, penetration peg; arrows indicate aberrant hyphal colonization in the cortex. Bars, 50 µm.
Initial phenotypic analysis showed that all three vpy mutants showed very low amounts of overall root colonization (< 10%) after inoculation with pot inoculum (spores and fragmented mycorrhizal root), as shown before for vpy‐1 and vpy‐2 (Sekhara Reddy et al., 2007). Instead of forming a hyphal coil as in the wild‐type (Fig. 1b), mutants penetrated with thickened infection pegs. Subsequent colonization of the cortex resulted in the formation of arbuscules in the wild‐type (Fig. 1b), whereas all three mutants showed similar unstructured, profusely growing hyphal material (Fig. 1c–e).
To quantitatively assess the mutant phenotype we performed nurse plant inoculation (nearby growing mycorrhizal wild‐type plants), which results in efficient colonization of vpy mutants, while the characteristic mutant phenotype is maintained (Sekhara Reddy et al., 2007). All three mutants exhibited comparable amounts of colonization by extraradical hyphae, hyphopodia and intraradical hyphae, while the formation of infection coils and arbuscules was severely inhibited (Fig. S1). Instead of arbuscules, either aborted structures or abnormally retarded arbuscules were formed, and vesicle formation was reduced (Fig. S1). The similarity of the mutant phenotypes of the three vpy alleles suggest that they all represent functional null alleles.
vpy mutants exhibit cell wall alterations at sites of mycorrhizal infection
As all three alleles exhibited a similar mutant phenotype, we selected one (vpy‐3) for detailed microscopic characterization. Semithin sections of resin‐embedded mycorrhizal vpy‐3 mutants revealed that fungal hyphae were mostly confined to the apoplast at all stages of infection from the rhizodermis to the cortex (Fig. 2a, arrowheads), whereas the wild‐type showed extensive intracellular colonization by hyphal coils and arbuscules (Fig. 2b). Interestingly, hypodermal cells adjacent to fungal penetration hyphae exhibited thickened cell walls in vpy‐3 (Fig. 2a, arrows). Confocal microscopic analysis showed that in wild‐type plants, the fungus formed hyphopodia on the root surface, from which it invaded hypodermal cells. Intracellular hyphae were surrounded by a thin layer of interfacial material (Fig. 2c, arrowheads). By contrast, hyphopodia on vpy‐3 mutants were enlarged and septate (Fig. 2d,e). When fungal infection hyphae in the mutant produced projections into hypodermal cells, they remained short and were surrounded by thick cell wall appositions from the host (Fig. 2d,e, arrows). Occasionally, penetration proceeded between hypodermal cells in the apoplastic space (Fig. 2d, blue arrow; cf. Fig. 2a).
Fig. 2.
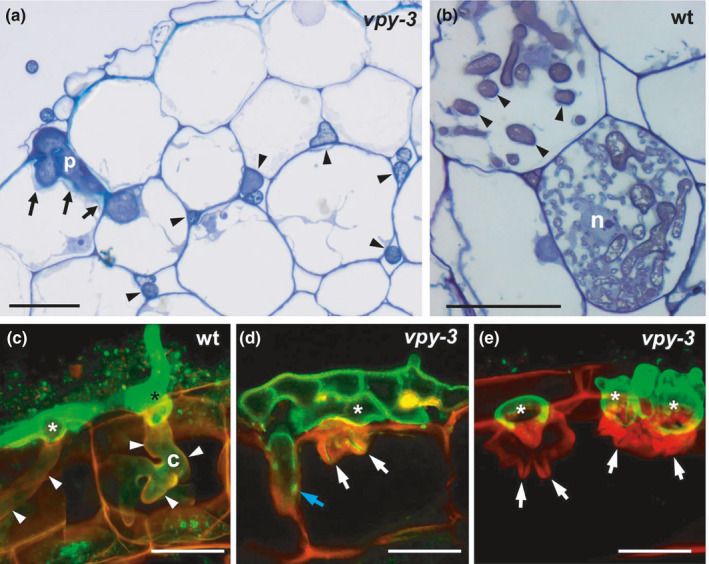
Cell wall appositions in vpy‐3 mutant of Petunia hybrida infected with Rhizophagus irregularis. (a) Colonization pattern in vpy‐3 mutant revealed by semithin sections of resin‐embedded material stained with toluidine blue. A hyphopodium with an intercellular infection peg (p), and cell wall appositions (arrows). Fungal colonization is mostly restricted to the intercellular spaces (arrowheads). (b) Colonization of the wild‐type (wt). Cortical cells contain abundant intracellular hyphae (arrowheads) and finely branched arbuscules, with the host nucleus (n) at a central position. (c–e) Confocal scanning micrographs of inoculated root hypodermal cells with the fungus stained by fluorescein isothiocyanate‐labeled wheat germ‐agglutinin (FITC‐WGA, green), and the plant cell walls by propidium iodide (PI, red). (c) In a wild‐type root, two fungal hyphopodia (asterisks) successfully invaded two adjacent hypodermal cells with hyphal coils. The infection hyphae are surrounded by a thin layer of plant extracellular matrix material (arrowheads). (d) On a vpy‐3 mutant plant, the fungus has formed a highly septate complex hyphopodium (asterisk) from which it has attempted to penetrate a hypodermal cell. Thick cell wall appositions surround the fungal penetration hyphae (white arrows). A penetration hypha has inserted itself between two adjacent cells (blue arrow). (e) Extreme case of an inoculated vpy‐3 mutant as in (d) with bloated hyphopodia (asterisks) and massive cell wall appositions (arrows) that have completely blocked fungal penetration. c, hyphal coil; p, penetration peg; n, nucleus; Bars, 40 µm.
A second experiment confirmed that in all three vpy alleles, similar cell wall appositions were induced by R. irregularis. Formation of local cell wall appositions that resembled papillae (Chowdhury et al., 2014) was particularly strong in hypodermal cells (Fig. S2b–d), whereas the wild‐type hypodermal cells allowed invasion and growth of infection hyphae without restriction (Fig. S2a). Quantification showed that the relative number of papillae was similar in all three vpy alleles, whereas papillae were almost never observed in the wild‐type (Fig. S2e).
Fungal passage through the hypodermis is blocked in vpy‐3
To assess in more detail the cellular aspects of the early stages of infection, we performed TEM on mycorrhizal wild‐type and vpy‐3. In the wild‐type, R. irregularis hyphae grew along the furrows between adjacent epidermal cells (Fig. 3a), followed by formation of hyphal swellings between adjacent epidermal cells (Fig. 3b). These represent the hyphopodia, from which the hypodermal cells were invaded, This process involved the breaching of external layers of the wall (Fig. 3c, white arrowheads), while the interior layers of the cell wall extended along the infection hypha to produce a continuous apoplastic sleeve that contained the fungal infection hypha (Fig. 3c, black arrowheads) (Rich et al., 2014). On vpy‐3 mutant roots, R. irregularis also attempted to insert between adjacent epidermal cells (Fig. 3d), and produced structures that resembled hyphopodia; however, they appeared more irregular than hyphopodia in the wild‐type, consistent with the previously observed fungal structures revealed by light microscopy (Fig. 1,2). After forcing their way to the surface of hypodermal cells, fungal hyphae penetrated the hypodermal cell wall in a fashion that superficially resembled the invasion of wild‐type cells (compare Fig. 3c and Fig. 3e), as, in both cases, the outer layer of the cell wall (slightly more electron‐translucent) was breached, and the hyphae entered the cellular lumen. However, in contrast to the wild‐type, the mutant roots produced thick wall appositions that surrounded the inserting hyphae (Fig. 3e, arrowheads). Subsequent stages of cell invasion appeared to face resistance, as the fungal hyphae grew rather irregularly, instead of forming a typical hyphal coil (Fig. 3f). Progressive deposition of electron‐dense cell wall material around the fungus continued (Fig. 3f, arrowheads), and occasionally resulted in extreme cell wall masses (Fig. 3g, arrowheads), while the fungal structures appeared collapsed and devoid of cytoplasm, indicating that the fungus was dead (Fig. 3g, asterisks).
Fig. 3.

Ultrastructural analysis of root penetration in Petunia hybrida wild‐type and vpy‐3. Transmission electron micrographs of transverse sections of wildtype (wt) (a–c) and vpy‐3 mutants (d–g) inoculated with Rhizophagus irregularis. All fungal structures are characterized by an electron‐dense cell wall that appears almost black. (a) Fungal hypha growing in the cleft between adjacent epidermal root cells. (b) A thick‐walled hyphopodium formed above a hypodermal cell (h) between two adjacent epidermal cells. (c) Hyphal entry point into a hypodermal cell (h). Electron translucent layers of the original cell wall were breached by the arbuscular mycorrhizal fungus (white arrowheads). The infection hypha is surrounded by a plant‐derived layer of cell wall matrix material (black arrowheads). (d) Fungal hypha growing on the root surface of vpy‐3 and attempting to insert between adjacent epidermal cells. (e) Extraradical hypha inserted between two adjacent epidermal cells of vpy‐3 (top), and hyphopodium‐like structure infecting a subtending hypodermal cell (h). At the entry points, the host has deposited thick layers of cell wall material (arrowheads). (f) Attempted entry into a hypodermal cell results in a distorted hyphal clump that is surrounded by an electron‐dense layer of cell wall with thickened regions (arrowheads). (g) Aborted entry in a vpy‐3 mutant with an extremely thick cell wall papilla at the site of attempted penetration (arrowheads). The fungal hyphopodium (asterisks) has collapsed and lost most of its content. e, epidermal cell; h, hypodermal cell; Bars, 2 µm.
AM fungal penetration triggers papilla‐like cell wall appositions in the cortex of vpy‐3
As vpy mutants have a distinct arbuscule phenotype (Figs 1c–e, 2a) (Sekhara Reddy et al., 2007), we assessed the AM fungal colonization pattern in the cortex of vpy‐3 by TEM analysis. In wild‐type plants, penetration hyphae entered cortical cells by breaching the cell wall as in the case of hypodermal cells (Fig. S3a, white arrowheads), and a thin layer of interfacial cell wall material from the host was deposited on the fungal cell walls (Fig. S3a, black arrowheads). Intracellular structures of arbuscules, such as trunk hypha (TH) and fine branches (asterisks), were surrounded by a periarbuscular membrane, but hardly any interfacial cell wall material was observed between the membrane and the fungal cell wall (Fig. S3a,b). In vpy‐3 mutants, by contrast, attempted cell penetration elicited the formation of local papillae that prevented hyphal entry (Fig. S3c). Confocal microscopy confirmed that vpy‐3 mutants formed papillae around fungal structures, in contrast to wild‐type cells with arbuscules (Fig. S3d,e).
Infection of vpy mutants by R. irregularis does not trigger accumulation of ROS
In order to visualize H2O2 accumulation, we used 3,3′‐diaminobenzidine (DAB) staining (Daudi & O’Brien, 2016). As confocal and TEM analysis had revealed the strongest defence reactions in hypodermal cells, we focused on this cell type. Wild‐type hypodermal cells with an infection hypha showed strongest DAB signal in the hyphae, rather than in the host cytoplasm (Fig. 4a). Similarly, penetrated vpy mutants exhibited the strongest DAB staining inside the fungal hyphae (Fig. 4b–d), and cell wall appositions of the host did not exhibit elevated signal (Fig. 4b–d, arrowheads), suggesting that the cellular defence response in vpy mutants does not involve H2O2 accumulation. Further examination of cortical colonization revealed that also in the later stages of AM development, strongest DAB signal was associated with fungal structures, including arbuscules (Fig. S4b,d,e) and vesicles (Fig. S4c,f). While the staining was comparable in vesicles of vpy‐2 and wild‐type roots (compare Figs. S4c and S4f), it was consistently weaker in cells with abnormal arbuscules in mutants vs fully developed arbuscules in the wild‐type (compare Figs S4b and S4d). This staining pattern was consistent in all three vpy alleles.
Fig. 4.

Cytochemical detection of H2O2 during infection of vpy mutants in Petunia hybrida. Mycorrhizal infection of hypodermal cells in the wild‐type (a), vpy‐1 (b), vpy‐2 (c) and vpy‐3 (d) were evaluated after staining with diaminobenzidine (DAB). Strongest staining was observed in the fungal cytoplasm (arrows) of hyphal coils and infection pegs. By contrast, host cell walls and cell wall appositions adjacent to penetration hyphae (arrowheads) exhibited only weak background signal. c, hyphal coil; p, penetration peg. Bars, 25 µm.
Next, we performed NBT staining (Kumar et al., 2014) to reveal O2 – accumulation. In general, NBT staining produced very strong signal in root tips of wild‐type as well as mutant roots, irrespective of their mycorrhizal status (Fig. S5a–e). Considering infection sites in colonized wild‐type roots, NBT staining was essentially restricted to fungal structures (Fig. S5f). Similarly, infected hypodermal and cortical cells in vpy mutants showed NBT staining almost exclusively in fungal hyphae (Fig. S5g–j). Taken together, it appears that ROS concentrations are higher in the fungal cytoplasm than in the host (except for the root tips), and that infected cells of vpy mutants did not accumulate higher ROS concentrations compared with the corresponding wild‐type cells.
Abortion of fungal entry in vpy mutants does not correlate with callose deposition
We next tested whether papillae in vpy mutants contain callose, which is often associated with cellular defence (Chowdhury et al., 2016). Inoculated wild‐type plants did not show any sign of local callose deposition (Fig. S6a,b). Likewise, colonized vpy‐3 mutants in general showed no callose (71%, n = 17) (Fig. S6c,d). However, in 12% of vpy‐3 plants, weak callose accumulation was observed in hypodermal cells with infection hyphae (Fig. S6e,f), and in 17% of the cases, strong callose accumulation was associated with fungal structures (Fig. S6g,h). In order to further examine whether callose deposition is related to particular AM fungal fates in the mutants, we performed a second experiment in which all three mutant alleles and the wild‐type were scored for callose deposition. Wild‐type plants exhibited weak background staining (4.1%; Table S1). Similarly, vpy mutants showed weak spurious callose signals in 1.8–10.1% of the examined infected cells (Table S1), and a strong callose signal was observed in only three cases (Table S1). These cases did not correlate with particular deformations in the fungus (data not shown), suggesting that there is no correlation between callose formation and fungal abortion.
Abortion of fungal penetration in petunia vpy mutants correlates with local lignin accumulation and induction of lignin biosynthetic genes
We further tested by phloroglucinol staining whether the papillae triggered by AM fungal penetration in vpy mutants contained lignin. In noninoculated wild‐type and mutant roots, a weak general signal in all cell types was observed, except for the stele that showed higher lignin staining (Fig. 5a). Penetrated wild‐type cells occasionally had a weak general signal, but in most cases, no accumulation over background levels was observed (Fig. 5b). By contrast, aborted infections in vpy‐1, vpy‐2, and vpy‐3 showed strong local accumulation of lignin in cell wall appositions of the host next to the fungal entry site (Fig. 5c–e, arrowheads). Quantification revealed that all three mutant alleles exhibited a significant increase in local lignin accumulation at fungal penetration sites (Fig. 5f). For comparison, we evaluated whether the ram1 mutant also accumulated lignin upon AM fungal infection (Fig. S7). Apart from the lignin signal in the vasculature of the stele (Fig. S7a,b), infection of the wild‐type did not lead to appreciable accumulation of lignin around fungal infection coils (Fig. S7c,d) or in cells with arbuscules (Fig. S7e,f). Similarly, the infection coils and the defective arbuscules in ram1 were not accompanied by accumulation of lignin (Fig. S7g,h; > 30 infection and colonization sites assessed, respectively).
Fig. 5.
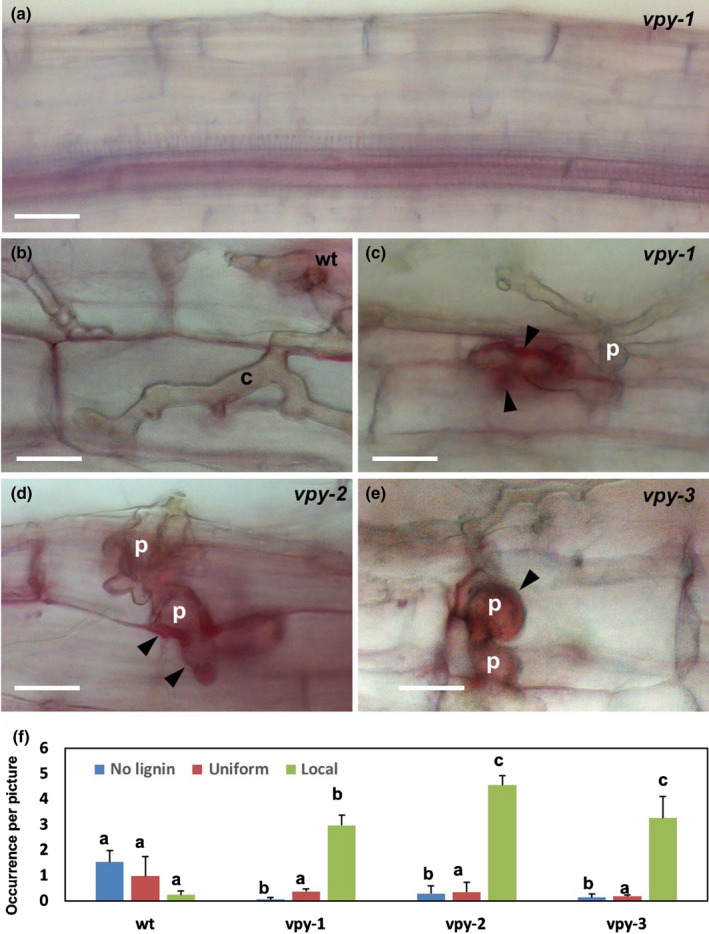
Lignin accumulation in infected hypodermal cells of vpy mutants in Petunia hybrida. Mycorrhizal roots were stained with phloroglucinol‐HCl, and entry points in hypodermal cells of wild‐type (b), vpy‐1 (c), vpy‐2 (d) and vpy‐3 (e) were evaluated for lignin accumulation. (a) Nonmycorrhizal vpy‐1 mutant root exhibits weak general signal in all cell types, and elevated signal in the central stele with the vasculature. (b–e) Infected cells of wild‐type (b), vpy‐1 (c), vpy‐2 (d) and vpy‐3 (e) exhibit weak background signal in the wild‐type (b) and strong lignin accumulation (arrowheads) in cell wall appositions of vpy mutants (c–e). (f) Quantification of lignin accumulation reveals a correlation of strong local lignin accumulation with abortion of arbuscular mycorrhizal fungal penetration in hypodermal cells of vpy mutants. Data are means + SD. Different letters indicate significant differences (one‐way ANOVA, n = 3). c, hyphal coil; p, penetration peg. Bars, 50 µm in (a), 25 µm in (b‐e).
Lignin biosynthesis involves a well characterized biosynthetic pathway (Vanholme et al., 2019). Thus, we identified the respective petunia homologues for all core biosynthetic genes (Table S2), and determined their expression during AM development in the wild‐type and the vpy mutants by qRT‐PCR in all three vpy alleles. As expected from lignin stainings (Fig. 5), mycorrhizal vpy mutants showed a concerted induction of all lignin biosynthetic genes (Tables 1, S3–S5; Fig. S8).
Table 1.
Expression ratios of lignin‐related genes in Petunia hybrida wild‐type (wt) and vpy mutants relative to the respective nonmycorrhizal controls (c).
| AM (wt)/c (wt) | AM (mutant)/c (mutant) | ||||
|---|---|---|---|---|---|
| wt* | wt | vpy1* | vpy2 | vpy3 | |
| PAL‐1 | 1.03 | 1.19 | 2.00 | 5.77 | 6.80 |
| PAL‐2 | 2.55 | 2.07 | 5.11 | 8.01 | 12.09 |
| PAL‐3 | 1.27 | 1.34 | 4.59 | 8.39 | 5.38 |
| 4CL‐1 | 2.87 | 1.85 | 15.72 | 7.59 | 11.39 |
| 4CL‐2 | 1.81 | 1.40 | 9.42 | 4.73 | 7.58 |
| 4CL‐3 | 0.94 | 1.09 | 14.97 | 20.22 | 33.55 |
| 4CL‐4 | 0.81 | 0.72 | 5.49 | 15.64 | 11.57 |
| CA4H | 0.96 | 1.05 | 4.20 | 5.98 | 4.08 |
| C3H‐1 | 1.52 | 1.20 | 7.61 | 6.75 | 7.50 |
| C3H‐2 | 1.83 | 1.03 | 6.24 | 3.26 | 5.27 |
| COMT | 1.08 | 1.92 | 3.52 | 13.44 | 15.71 |
| CCoAOMT‐1 | 1.02 | 1.54 | 6.95 | 6.74 | 6.52 |
| CCoAOMT‐2 | 1.19 | 1.20 | 3.63 | 5.86 | 8.63 |
| CCoAOMT‐3 | 1.65 | 1.06 | 5.29 | 18.68 | 40.17 |
| HCT | 2.38 | 3.39 | 5.76 | 19.76 | 41.14 |
| CAD1 | 1.23 | 2.25 | 6.87 | 14.84 | 14.03 |
| CAD2 | 1.05 | 1.11 | 8.63 | 16.76 | 29.45 |
| CCR1 | 1.17 | 1.49 | 7.22 | 31.70 | 44.90 |
| CCR2 | 0.80 | 1.22 | 4.97 | 8.10 | 7.08 |
| F5H2 | 1.02 | 1.04 | 4.39 | 4.98 | 14.98 |
Induction of lignin biosynthetic genes was determined by quantitative real‐time reverse‐transcriptase polymerase chain reaction with actin and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) as reference genes. Values represent induction ratios (‐fold) derived by dividing the expression values of mycorrhizal plants (AM) by values of nonmycorrhizal controls (both normalized with the two reference genes) in wt, vpy‐1, vpy‐2 and vpy‐3 plants. All expression values were derived from six biological replicates. Color shading represents induction > two‐fold (yellow), > four‐fold (orange), and > eight‐fold (red). Data represent two independent experiments, one with only vpy‐1 vs wild‐type (asterisks), and one with vpy‐2 and vpy‐3 vs wild‐type. Significant induction ratios are indicated in bold font (one‐way ANOVA, n = 6). Two‐way ANOVA revealed significant interactions between plant genotype and mycorrhizal treatments (see Supporting Information Table S13).
In order to test how general the lignin response in petunia vpy mutants is, we performed lignin staining in sym mutants of M. truncatula (Table S6; Figs S9–S11; Notes S1) (Endre et al., 2002; Lévy et al., 2004; Mitra et al., 2004; Kalo et al., 2005; Maillet et al., 2011; Gobbato et al., 2012). However, neither vpy‐2 nor any other mutant (ram1‐1, dmi2‐1, dmi3‐1, nsp2‐2) showed lignin accumulation (Fig. S11; Notes S1), suggesting that lignin does not play a role in the repression of AM fungi in these Medicago mutants.
Resistance of vpy‐3 does not correlate with accumulation of SA, JA or ethylene
We next tested whether papilla formation in mycorrhizal vpy mutants is accompanied by accumulation of the defence hormones SA, JA or ethylene. In order to assess early infection events, as well as fully established mycorrhizal colonization, we tested vpy‐3 mutant roots at 10 and 35 d after nurse plant inoculation. In general, SA concentrations were low and did not significantly change during AM infection either in the wild‐type or in the mutants (Fig. S12a). Next, we compared the concentrations of free and conjugated SA. Conjugated SA occurred in almost 10‐fold higher concentrations than free SA, but its concentrations were not induced in the mutant, independent of its mycorrhizal status (Fig. S12b). Ethylene was produced only in trace amounts in wild‐type and vpy mutants, and its concentrations did not change in mutants or in the wild‐type upon AM inoculation (data not shown). JA and JA‐Ile concentrations were slightly induced in wild‐type plants at the second time point, while the concentrations in mutants were not significantly changed (Fig. S13). As in the case of SA, the JA (Fig. S13a) and JA‐Ile (Fig. S13b) concentrations were generally low. Taken together, these results suggest that the cellular resistance reaction in vpy‐3 does not involve the accumulation of SA, ethylene or JA.
Induction of PR gene homologues in mycorrhizal roots of wild‐type and vpy mutants
In order to systematically assess the expression of PR genes during mycorrhizal development, we identified PR genes in petunia (Table S7) by protein blasts using established PR protein sequences (mostly from tobacco) as queries (van Loon et al., 2006b), and assessed their expression patterns by qRT‐PCR analysis in all three vpy alleles. Consistent with previous findings (Salzer et al., 2000; Breuillin et al., 2010; Campos‐Soriano et al., 2010), several PR gene homologues were induced in mycorrhizal roots of wild‐type plants (Tables 2, S10, S11), in particular β‐1,3‐glucanase (PR2a), chitinase (PR4b and PR4d), a thaumatin‐like gene (PR5a), a proteinase inhibitor (PR6a), a proteinase (PR7), a peroxidase (PR9) and a lipid transfer protein (PR14). In vpy mutants, expression of several genes was further induced (Table 2), while some were induced only in the mutants, namely PR2b (β‐1,3‐glucanase), PR3 (chitinase), PR4c (chitinase) and PR17 (unknown function). Comparing mycorrhizal mutants with mycorrhizal wild‐type plants directly showed which genes are particularly induced in the mutants (PR2a, PR2b, PR4c; Table S8, left), and comparison of nonmycorrhizal mutants and wild‐type showed that the mutants had constitutively higher levels of PR4d and PR6a expression (Table S8, right).
Table 2.
Expression ratios of pathogenesis‐related (PR) genes in Petunia hybrida wild‐type (wt) and vpy mutants relative to the respective nonmycorrhizal controls (c).
| AM (wt)/c (wt) | AM (mutant)/c (mutant) | ||||
|---|---|---|---|---|---|
| wt* | wt | vpy1* | vpy2 | vpy3 | |
| PR2a | 1.27 | 2.53 | 18.30 | 30.47 | 13.20 |
| PR2b | 2.02 | 1.42 | 8.42 | 17.47 | 24.69 |
| PR2c | 1.48 | 1.26 | 1.87 | 1.09 | 0.88 |
| PR3 | 1.17 | 1.39 | 6.58 | 5.02 | 3.11 |
| PR4b | 2.04 | 5.45 | 9.64 | 41.73 | 13.02 |
| PR4c | 0.92 | 1.10 | 8.71 | 10.76 | 13.82 |
| PR4d | 5.57 | 5.51 | 2.39 | 2.68 | 1.62 |
| PR5a | 6.67 | 3.48 | 1.14 | 1.02 | 1.13 |
| PR6a | 1.80 | 13.24 | 6.11 | 33.76 | 1.57 |
| PR7 | 4.28 | 5.21 | 26.91 | 33.59 | 7.05 |
| PR9 | 6.93 | 5.23 | 24.39 | 6.40 | 26.00 |
| PR14 | 2.53 | 3.02 | 14.91 | 21.59 | 19.79 |
| PR17 | 1.35 | 1.40 | 9.02 | 8.65 | 2.47 |
Induction of PR genes was determined by quantitative real‐time reverse‐transcriptase polymerase chain reaction with actin and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) as reference genes. Values represent induction ratios (‐fold) derived by dividing the expression values of mycorrhizal plants (AM) by values of nonmycorrhizal controls (both normalized with the two reference genes) in wt, vpy‐1, vpy‐2 and vpy‐3. All expression values were derived from six biological replicates. Color shading represents induction> two‐fold (yellow), > four‐fold (orange) and> eight‐fold (red). Data represent two independent experiments, one with only vpy‐1 vs wild‐type (asterisks), and one with vpy‐2 and vpy‐3 vs wild‐type. Significant induction ratios are indicated in bold (one‐way ANOVA, n = 6). Two‐way ANOVA revealed significant interactions between plant genotype and mycorrhizal treatments (see Supporting Information Table S13).
In order to identify PR genes that are responsive to MAMPs, we treated nonmycorrhizal wild‐type plants with a chitin hydrolysate that contains N‐acetyl‐glucosamine oligosaccharides (Chit), and an aqueous extract from Penicillium chrysogenum (Pen). Both preparations had previously been shown to induce a strong defence response and disease resistance in Arabidopsis thaliana (Thuerig et al., 2005). Several PR genes were induced by Pen elicitor, and only two of them were responsive to chitin (Tables S9, S12). The strongest induction was found for PR4d, one of the two genes that is constitutively induced in vpy mutants (Table S8).
Taken together, these results indicate that: wild‐type mycorrhizal roots express several PR genes at induced levels; mycorrhizal vpy mutants are overresponsive with regard to AM‐inducible PR genes, and they induce additional PR genes; and several PR genes are responsive to MAMPS in elicitor preparations.
Cell wall appositions in vpy‐3 contain β‐1,3‐glucanase
Pathogenesis‐related proteins may accumulate locally at sites of microbial infection, or they could be generally induced without a particular pattern of accumulation. As β‐1,3‐glucanase was induced in vpy mutants (PR2a and PR2b in Table 2, S8), we investigated whether papillae in vpy mutants contain β‐1,3‐glucanase by employing a polyclonal antiserum raised against tobacco β‐1,3‐glucanase (Beffa et al., 1993). Immunocytochemical detection with gold‐coupled secondary antibodies revealed a low general signal in the cell walls of noninoculated control roots in the wild‐type, as in vpy‐3 mutants (Fig. S14a,c,e,g). Inoculated wild‐type plants did not show an obvious increase (Fig. S14b,f), consistent with the weak induction of PR2 in mycorrhizal wild‐type plants (Table 2). Inoculated vpy‐3 mutants exhibited a general distribution of gold particles in a similar manner as the other treatments (Fig. S14d,h). However, papillae in the vicinity of aborted fungal hyphae exhibited a more prominent signal (Figs S15, S16). Hence, accumulation of β‐1,3‐glucanase appears to increase in papillae of vpy‐3. Quantification of immunogold signal confirmed the local accumulation of β‐1,3‐glucanase in papillae (Fig. S17).
Discussion
Signalling in symbiosis and defence
Transcript profiling of AM in various host species has shown that besides the induction of many AM‐related genes with presumed functions in symbiosis (e.g. phosphate and ammonium transporters), many AM‐induced genes are related to established defence genes (e.g. PR genes) (Gao et al., 2004; Grunwald et al., 2004; Deguchi et al., 2007; Liu et al., 2007; Siciliano et al., 2007; Fiorilli et al., 2009; Breuillin et al., 2010; Campos‐Soriano et al., 2010; Gaude et al., 2012; Handa et al., 2015; Fiorilli et al., 2018). In addition, the complex signalling mechanisms in symbiosis include, besides dedicated symbiosis signals (myc factors), molecular species that are known also to play a role in disease resistance (e.g. chitin oligomers; Zipfel & Oldroyd, 2017). Consistent with these findings, some receptors for chitinous signalling molecules have a dual role in disease resistance and symbiosis (Zipfel & Oldroyd, 2017). These findings point to overlapping mechanisms in the signalling pathways in symbiosis and defence, including for example the production of ROS (Scheler et al., 2013; Damiani et al., 2016), the induction of JA (Wasternack & Hause, 2013) and calcium‐related signals (Aldon et al., 2018), which all have been observed in both symbiosis and defence. Moreover, the outcome of AM symbiosis for the host plant is highly context‐dependent (Klironomos, 2003; Smith et al., 2009; Lanfranco et al., 2018). This implies that some additional mechanisms may be required to specify and determine whether roots engage in defence or in symbiosis. It is conceivable that the common symbiosis signaling pathway (CSSP) is one of the central elements to ensure that symbiosis‐related signalling overrides defence signalling during mycorrhizal infection. In addition to specific symbiosis signals, AM fungi use effectors to help prevent the induction of defence responses or to dampen their amplitude (Kloppholz et al., 2011; Sedzielewska Toro & Brachmann, 2016; Tang et al., 2016; Kamel et al., 2017; Chen, ECH et al., 2018; Voss et al., 2018; Morin et al., 2019).
Symbiosis mutants show symptoms of a defence response
Consistent with the assumption that commitment to symbiosis requires repression of defence reactions, many mutants that are defective in symbiosis signalling (sym mutants) show various symptoms of a cellular defence response (Gollotte et al., 1993; Gianinazzi‐Pearson, 1996; Wegel et al., 1998; Ruiz‐Lozano et al., 1999; Bonfante et al., 2000; Marsh & Schultze, 2001; Novero et al., 2002; Demchenko et al., 2004). In such mutants, AM fungus colonization is hampered or fully blocked, cells accumulate secondary metabolites that are known (or suspected) to act as defence agents, cell walls are altered or reinforced, and defence‐related transcripts are induced. This is strong evidence that one function of symbiotic signalling is to avoid, or repress, defence during AM interactions.
In vpy mutants, defence‐like aspects of the AM‐defective phenotype are particularly prominent. Here, we show that this syndrome resembles a bona‐fide cellular defence response, involving the formation of cell wall papillae (Figs 2, 3, S2, S3), the local accumulation of lignin (Fig. 5), the induction of lignin‐biosynthetic genes (Table 1), and the induction of a range of PR genes (Table 2). The fact that vpy mutants in petunia show these symptoms of defence suggests that one function of VPY is to suppress, directly or indirectly, the induction of defence. This appears to be a prerequisite for intracellular accommodation of AM fungi during infection at the root surface, and during arbuscule development in the cortex (Ercolin & Reinhardt, 2011; Gutjahr & Parniske, 2013). An interesting aspect of the colonization phenotype of vpy mutants in both petunia and Medicago is that intercellular hyphal colonization is not inhibited (Figs 2a, S10g) (Sekhara Reddy et al., 2007), suggesting that the interaction at the extracellular level is compatible. A similar phenotype was observed in della mutants that were essentially devoid of intracellular structures yet exhibited high colonization levels by profusely growing intercellular hyphae (Floss et al., 2013).
Defence response in petunia vpy mutants involves local lignin accumulation
Papilla formation is considered a highly effective defence mechanism of plants against fungal pathogens (Hückelhoven, 2007). Papillae inhibit fungal penetration in aerial plant tissues and often result in complete abortion of the pathogen. In the root, the formation of cell wall reinforcements as defence mechanism has been less explored. The cell wall appositions in mycorrhizal vpy mutants colonized by R. irregularis were either locally restricted to the site of cell penetration, or they surrounded intracellular hyphae, thereby encapsulating them (Figs 2, 3, 5, S2, S3, S15).
Interestingly, the papillae of vpy mutants did not, in most cases, contain callose, and they were only weakly autofluorescent, in contrast to previous reports in pea symbiosis mutant P2 (Gianinazzi‐Pearson et al., 1996), indicating that some hallmarks of defence are absent in mycorrhizal vpy mutants. However, papillae were impregnated by local accumulation of lignin (Fig. 5). Consistent with this observation, the lignin biosynthetic pathway was induced in a concerted fashion, in inoculated vpy mutants, involving all biosynthetic genes (Fig. S8) (Vanholme et al., 2019). Cell wall lignification is among the most effective cellular defence responses of plants. In general, high lignin content correlates with increased disease resistance in various plant species (Miedes et al., 2014). For example, lignin contributes to penetration resistance of wheat against powdery mildew (Bhuiyan et al., 2009). In tomato, roots of a cultivar resistant to the pathogen Ralstonia solanacearum accumulate higher lignin concentrations than a susceptible cultivar (Mandal et al., 2011). Whether the lignin was confined to papillae was not addressed in this case. It is difficult to attribute effective resistance to particular cell wall components (cellulose, hemicellulose, callose, lignin, cell wall proteins etc.), as papillae often have a complex composition. Callose is generally accepted as an important element that significantly reinforces papillae against pathogen penetration (Hückelhoven, 2014) and that is thought to contribute to MIR (Cordier et al., 1998); however, lignin impregnation is a rather unusual feature of papillae and has not been reported earlier in the context of resistance against AM fungi in sym mutants. Given the well‐documented role of lignin in reinforcing cell walls (Miedes et al., 2014), it is plausible to assume that lignin contributes to the abortion of AM fungal cell penetration in petunia vpy mutants.
In addition, the concerted induction of enzymes in the phenylpropanoid pathway (Table 1; Fig. S8) could potentially lead to the production of other defence‐related compounds (Dixon et al., 2002; Vogt, 2010). Interestingly, the ram1 mutant which exhibits a later defect in AM development during arbuscule formation, and which does not display markers of a cellular defence response (Park et al., 2015; Rich et al., 2015; Pimprikar et al., 2016), did not accumulate lignin (Fig. S7). Strikingly, in M. truncatula vpy‐2 mutants (as well as in dmi2‐1, dmi3‐1, nsp2‐2 and ram1‐1 mutants), no accumulation of lignin was detected, suggesting that in M. truncatula roots, abortion of AM fungal infection does not involve lignin. This points to taxon‐specific aspects in root defence, and possibly in AM‐related pathways between petunia and M. truncatula, and perhaps, more generally, between Solanaceae and legumes.
Induction of PR genes in mycorrhizal wild‐type and vpy mutants
A characteristic symptom of defence in plants is the accumulation of PR proteins (van Loon et al., 2006b). Several PR proteins have been shown to have antimicrobial activity, and some of them contribute to disease resistance; hence they are regarded as markers of defence. We assessed the expression of all PR gene homologues that could be identified in the petunia genome (Bombarely et al., 2016), and 13 were found to be expressed in roots (Table 2). PR genes have previously been reported to be induced in many mycorrhizal associations (reviewed in García‐Garrido & Ocampo, 2002). In most cases, the induction was characterized as early, weak and transient (Gianinazzi‐Pearson et al., 1996; Kapulnik et al., 1996). However, in some cases, PR genes were induced in a sustained fashion and at high levels (Salzer et al., 2000; Breuillin et al., 2010), indicating that they may have an important role in AM symbiosis. In addition, the phylogenomic signature of some AM‐induced PR proteins (e.g. chitinase III) indicates that they have been under positive selection in AM‐competent plant species (Favre et al., 2014; Rich et al., 2014), suggesting that they have specific functions in AM.
We observed that several PR genes were induced in mycorrhizal wild‐type roots (Table 2), possibly reflecting an elevated defence status in mycorrhizal roots (García‐Garrido & Ocampo, 2002; Pozo & Azcon‐Aguilar, 2007). In mycorrhizal vpy mutant roots, several PR genes (PR2a, PR2b, PR4c, PR7, PR9, PR14 and PR17) were expressed at even higher levels than in the wild‐type, consistent with our findings that mycorrhizal vpy mutants mount a defence response (Figs 2, 3, 4, 5). The strong induction of two PR2 genes in vpy mutants, and the local accumulation in papillae of PR2 protein (Figs S15, S17) are of particular interest as transgenic tobacco‐overexpressing PR2 exhibited significantly delayed mycorrhizal colonization of the roots (Vierheilig et al., 1995). Hence, PR2, and perhaps other PR proteins, could be involved in restricting mycorrhizal colonization in vpy mutants.
Defence response in vpy mutants does not affect concentrations of SA, JA, ethylene or ROS
Initiation of a defence response in plants is often associated with the accumulation of the stress and defence hormones SA, JA or ethylene (Browse, 2009; Vlot et al., 2009; Broekgaarden et al., 2015). In mycorrhizal roots, the picture is more complex, as SA and JA can be either up‐ or downregulated during symbiosis (Fernandez et al., 2014). We found no significant differences in the concentrations of free or conjugated SA (Fig. S12), consistent with the general lack of expression of the SA‐marker PR1 in roots. JA and JA‐Ile concentrations were not affected at the early time point of the interaction (10 d), but were later slightly induced in the wild‐type, in contrast to vpy‐3 mutants (Fig. S13). JA concentrations are known to be induced in mycorrhizal roots in some cases; however, the role of JA in symbiosis is controversial (Wasternack & Hause, 2013).
A commonly observed phenomenon associated with plant defence is the production of ROS in the host (Torres et al., 2006). In AM, ROS may also play a role, although in this case, it is not clear which of the symbiotic partners is the main source of ROS (Fester & Hause, 2005). We used two established staining procedures to detect H2O2 (DAB) and O2 – (NBT), respectively. Interestingly, both methods revealed high ROS concentrations in the fungus, irrespective of the host genotype (Figs 4, S4,S5). As described in M. truncatula (Salzer et al., 1999), highest H2O2 concentrations were associated with clumped arbuscules that appeared to undergo senescence (Fig. S4). NBT staining in the host was strongest in root tips (Fig. S5a–e), as has been shown for Arabidopsis and Medicago (Dunand et al., 2007; Chen et al., 2015). In infected areas of the cortex, NBT signal was confined to fungal hyphae (Fig. S5f–j) and arbuscules (in the wild‐type). Taken together, these results show that none of the classical defence hormones (SA, JA, ethylene) were induced during the defence response in vpy‐3, and ROS accumulation patterns did not reveal a correlation with defence in vpy mutants.
VAPYRIN is involved in repression of cellular defence
How could VPY interfere with defence? The localization of VPY to mobile subcellular compartments (Feddermann et al., 2010; Pumplin et al., 2010), and its interaction with EXO70I and EXO70H4 (Zhang et al., 2015; Liu et al., 2019) suggest that VPY could be involved in subcellular trafficking towards fungal hyphae. Hence, the mobile compartments could transport a cargo or a membrane component that interferes with the induction of defence. Alternatively, VPY could be a target of an AM fungal effector which represses defence in the host. Future research should identify the interaction partners of VPY and potential downstream components to explain how it acts on defence mechanisms.
Author contributions
MC, SB, LB, GD, MS and DR planned and conducted the experiments and were involved in data collection. GG performed hormone analytics. SB, MC, and DR were involved in data analysis. DR wrote the paper with assistance from SB and MC.
Supporting information
Fig. S1 Quantitative phenotypic analysis of vpy mutants.
Fig. S2 Confocal analysis of papilla formation in vpy mutants.
Fig. S3 Infection phenotype in vpy‐3 root cortex cells.
Fig. S4 Cytochemical detection of H2O2 in the cortex of vpy mutants.
Fig. S5 Cytochemical detection of O2 – in vpy mutants.
Fig. S6 Callose accumulation associated with aborted fungal infection of vpy‐3.
Fig. S7 Lack of lignin accumulation in mycorrhizal ram1 mutants.
Fig. S8 Concerted induction of lignin biosynthetic genes in mycorrhizal vpy mutants.
Fig. S9 Mycorrhizal colonization pattern in M. truncatula sym mutants.
Fig. S10 Mycorrhizal colonization pattern in M. truncatula sym mutants.
Fig. S11 Lack of lignin accumulation at fungal entry points in M. truncatula sym mutants.
Fig. S12 Salicylic acid concentrations in mycorrhizal wild‐type and vpy‐3 roots.
Fig. S13 Jasmonic acid concentrations in mycorrhizal wild‐type and vpy‐3 roots.
Fig. S14 Immunocytochemical analysis of β‐1,3‐glucanase in mycorrhizal roots.
Fig. S15 Accumulation of β‐1,3‐glucanase in a cell wall apposition of vpy‐3.
Fig. S16 Low‐magnification overview picture of the sample shown in Fig. S15.
Fig. S17 Quantification of β‐1,3‐glucanase immunogold signal.
Methods S1 Methods relating to experiments on Medicago truncatula, β‐1,3‐glucanase immunostaining, RNA extraction and qPCR, and statistical analysis.
Notes S1 Characterization of lignin accumulation in Medicago truncatula wild‐type and symbiosis mutants.
Table S1 Quantification of callose accumulation in vpy mutants.
Table S2 Primers used for qRT‐PCR of lignin‐related genes.
Table S3 Induction of lignin‐related genes in vpy mutants vs wild‐type in either the mycorrhizal or nonmycorrhizal context.
Table S4 Expression values for lignin‐related genes for the experiment on vpy‐1 from the genes for which induction ratios were calculated in Tables 1 and S3.
Table S5 Expression values for lignin‐related genes for the experiment on vpy‐2 and vpy‐3 from the genes for which induction ratios were calculated in Tables 1 and S3.
Table S6 M. truncatula mutants used in this study.
Table S7 Primers used for qRT‐PCR of PR genes.
Table S8 Induction of pathogenesis‐related (PR) genes in mutants vs wild‐type in either the mycorrhizal or nonmycorrhizal context.
Table S9 Induction of pathogenesis‐related (PR) genes in wild‐type plants treated with fungal elicitor preparations.
Table S10 Expression values for the experiment on vpy‐1 from the genes for which induction ratios were calculated in Tables 2 and S8.
Table S11 Expression values for the experiment on vpy‐2 and vpy‐3 from the genes for which induction ratios were calculated in Tables 2 and S8.
Table S12 Expression values from the experiment with wild‐type plants treated with elicitors from genes for which induction ratios were calculated in Table S9.
Table S13 P‐values from statistical analyses and interaction report from two‐way ANOVA tests.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank Jeremy Murray for providing seeds of M. truncatula mutants, Jürg Felix for providing chitin and Penicillium MAMP preparations, Rudolf Rohr for assistance with statistical analysis, and Christine Lang and Eliane Abou‐Mansour for assistance with salicylic acid determination. This work was funded by a grant of the Swiss National Science Foundation to DR (grant no. 31003A_169732).
References
- Aldon D, Mbengue M, Mazars C, Galaud JP. 2018. Calcium signalling in plant biotic interactions. International Journal of Molecular Sciences 19: 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman LG, Schneider BG, Papermaster DS. 1984. Rapid embedding of tissues in Lowicryl K4M for immunoelectron microscopy. Journal of Histochemistry and Cytochemistry 32: 1217–1223. [DOI] [PubMed] [Google Scholar]
- Bapaume L, Laukamm S, Darbon G, Monney C, Meyenhofer F, Feddermann N, Chen M, Reinhardt D. 2019. VAPYRIN marks an endosomal trafficking compartment involved in arbuscular mycorrhizal symbiosis. Frontiers in Plant Science 10: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffa RS, Neuhaus JM, Meins F. 1993. Physiological compensation in antisense transformants: specific induction of an "Ersatz"‐glucan endo‐1,3‐ß‐glucosidase in plants infected with necrotizing viruses. Proceedings of the National Academy of Sciences, USA 90: 8792–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan NH, Selvaraj G, Wei Y, King J. 2009. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. Journal of Experimental Botany 60: 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: Perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annual Review of Plant Biology 60: 379–406. [DOI] [PubMed] [Google Scholar]
- Bombarely A, Moser M, Amrad A, Bapaume L, Barry CS, Bliek M, Boersma MR, Borghi L, Bruggmann R, Bucher M et al. 2016. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida . Nature Plants 2: 16074–16082. [DOI] [PubMed] [Google Scholar]
- Bonfante P, Genre A, Faccio A, Martini I, Schauser L, Stougaard J, Webb J, Parniske M. 2000. The Lotus japonicus LjSym4 gene is required for the successful symbiotic infection of root epidermal cells. Molecular Plant–Microbe Interactions 13: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, Hause B, Bucher M, Kretzschmar T, Bossolini E, et al. 2010. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. The Plant Journal 64: 1002–1017. [DOI] [PubMed] [Google Scholar]
- Broekgaarden C, Caarls L, Vos IA, Pieterse CMJ, Van Wees SCM. 2015. Ethylene: traffic controller on hormonal crossroads to defense. Plant Physiology 169: 2371–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MJ. 1971. Control of leghaemoglobin synthesis in snake beans. Biochemical Journal 125: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. 2009. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology 60: 183–205. [DOI] [PubMed] [Google Scholar]
- Campos‐Soriano L, Garcıa‐Garrido M, San Segundo B. 2010. Activation of basal defense mechanisms of rice plants by Glomus intraradices does not affect the arbuscular mycorrhizal symbiosis. New Phytologist 188: 597–614. [DOI] [PubMed] [Google Scholar]
- Chen DS, Liu CW, Roy S, Cousins D, Stacey N, Murray JD. 2015. Identification of a core set of rhizobial infection genes using data from single cell‐types. Frontiers in Plant Science 6: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ECH, Morin E, Beaudet D, Noel J, Yildirir G, Ndikumana S, Charron P, St‐Onge C, Giorgi J, Krüger M et al. 2018. High intraspecific genome diversity in the model arbuscular mycorrhizal symbiont Rhizophagus irregularis . New Phytologist 220: 1161–1171. [DOI] [PubMed] [Google Scholar]
- Chen M, Arato M, Borghi L, Nouri E, Reinhardt D. 2018. Beneficial services of arbuscular mycorrhizal fungi ‐ From ecology to application. Frontiers in Plant Science 9: 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury J, Henderson M, Schweizer P, Burton RA, Fincher GB, Little A. 2014. Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp hordei . New Phytologist 204: 650–660. [DOI] [PubMed] [Google Scholar]
- Chowdhury J, Schober MS, Shirley NJ, Singh RR, Jacobs AK, Douchkov D, Schweizer P, Fincher GB, Burton RA, Little A. 2016. Down‐regulation of the Glucan synthase‐like 6 gene (HvGsl6) in barley leads to decreased callose accumulation and increased cell wall penetration by Blumeria graminis f. sp hordei . New Phytologist 212: 434–443. [DOI] [PubMed] [Google Scholar]
- Chuberre C, Plancot B, Driouich A, Moore JP, Bardor M, Gugi B, Vicre M. 2018. Plant immunity is compartmentalized and specialized in roots. Frontiers in Plant Science 9: 1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier C, Pozo MJ, Barea JM, Gianinazzi S, Gianinazzi‐Pearson V. 1998. Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Molecular Plant–Microbe Interactions 11: 1017–1028. [Google Scholar]
- Damiani I, Pauly N, Puppo A, Brouquisse R, Boscari A. 2016. Reactive oxygen species and nitric oxide control early steps of the legume–rhizobium symbiotic interaction. Frontiers in Plant Science 7: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi A, O’Brien JA. 2016. Detection of hydrogen peroxide by DAB staining in arabidopsis leaves. Bio Protocols 2012: 18. [PMC free article] [PubMed] [Google Scholar]
- David R, Itzhaki H, Ginzberg I, Gafni Y, Galili G, Kapulnik Y. 1998. Suppression of tobacco basic chitinase gene expression in response to colonization by the arbuscular mycorrhizal fungus Glomus intraradices . Molecular Plant–Microbe Interactions 11: 489–497. [DOI] [PubMed] [Google Scholar]
- Deguchi Y, Banba M, Shimoda Y, Chechetka SA, Suzuri R, Okusako Y, Ooki Y, Toyokura K, Suzuki A, Uchiumi T et al. 2007. Transcriptome profiling of Lotus japonicus roots during arbuscular mycorrhiza development and comparison with that of nodulation. DNA Research 14: 117–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchenko K, Winzer T, Stougaard J, Parniske M, Pawlowski K. 2004. Distinct roles of Lotus japonicus SYMRK and SYM15 in root colonization and arbuscule formation. New Phytologist 163: 381–392. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MSS, Wang LJ. 2002. The phenylpropanoid pathway and plant defence ‐ a genomics perspective. Molecular Plant Pathology 3: 371–390. [DOI] [PubMed] [Google Scholar]
- Dunand C, Crevecoeur M, Penel C. 2007. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytologist 174: 332–341. [DOI] [PubMed] [Google Scholar]
- Endre G, Kereszt A, Kevei Z, Mihacea S, Kaló P, Kiss GB. 2002. A receptor kinase gene regulating symbiotic nodule development. Nature 417: 962–966. [DOI] [PubMed] [Google Scholar]
- Ercolin F, Reinhardt D. 2011. Successful joint ventures of plants: arbuscular mycorrhiza and beyond. Trends in Plant Science 16: 356–362. [DOI] [PubMed] [Google Scholar]
- Favre P, Bapaume L, Bossolini E, Delorenzi M, Falquet L, Reinhardt D. 2014. A novel bioinformatics pipeline to discover genes related to arbuscular mycorrhizal symbiosis based on their evolutionary conservation pattern among higher plants. BMC Plant Biology 14: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddermann N, Duvvuru Muni RR, Zeier T, Stuurman J, Ercolin F, Schorderet M, Reinhardt D. 2010. The PAM1 gene of petunia, required for intracellular accommodation and morphogenesis of arbuscular mycorrhizal fungi, encodes a homologue of VAPYRIN. The Plant Journal 64: 470–481. [DOI] [PubMed] [Google Scholar]
- Feddermann N, Reinhardt D. 2011. Conserved residues in the ankyrin domain of VAPYRIN indicate potential protein‐protein interaction surfaces. Plant Signaling and Behavior 6: 680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez I, Merlos M, Lopez‐Raez JA, Martinez‐Medina A, Ferrol N, Azcon C, Bonfante P, Flors V, Pozo MJ. 2014. Defense related phytohormones regulation in arbuscular mycorrhizal symbioses depends on the partner genotypes. Journal of Chemical Ecology 40: 791–803. [DOI] [PubMed] [Google Scholar]
- Fester T, Hause G. 2005. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15: 373–379. [DOI] [PubMed] [Google Scholar]
- Fiorilli V, Catoni M, Miozzi L, Novero M, Accotto GP, Lanfranco L. 2009. Global and cell‐type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytologist 184: 975–987. [DOI] [PubMed] [Google Scholar]
- Fiorilli V, Vannini C, Ortolani F, Garcia‐Seco D, Chiapello M, Novero M, Domingo G, Terzi V, Morcia C, Bagnaresi P et al. 2018. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Scientific Reports 8: 9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss DS, Levy JG, Levesque‐Tremblay V, Pumplin N, Harrison MJ. 2013. DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences, USA 110: E5025–E5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragniere C, Serrano M, Abou‐Mansour E, Metraux JP, L'Haridon F. 2011. Salicylic acid and its location in response to biotic and abiotic stress. Febs Letters 585: 1847–1852. [DOI] [PubMed] [Google Scholar]
- Gao LL, Knogge W, Delp G, Smith FA, Smith SE. 2004. Expression patterns of defense‐related genes in different types of arbuscular mycorrhizal development in wild‐type and mycorrhiza‐defective mutant tomato. Molecular Plant–Microbe Interactions 17: 1103–1113. [DOI] [PubMed] [Google Scholar]
- García‐Garrido JM, Ocampo JA. 2002. Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. Journal of Experimental Botany 53: 1377–1386. [PubMed] [Google Scholar]
- Gaude N, Schulze WX, Franken P, Krajinski F. 2012. Cell type‐specific protein and transcription profiles implicate periarbuscular membrane synthesis as an important carbon sink in the mycorrhizal symbiosis. Plant Signaling and Behavior 7: 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi‐Pearson V. 1996. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. The Plant Cell 8: 1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi‐Pearson V, Dumas‐Gaudot E, Gollotte A, Tahiri‐Alaoui A, Gianinazzi S. 1996. Cellular and molecular defence‐related root responses to invasion by arbuscular mycorrhizal fungi. New Phytologist 133: 45–57. [Google Scholar]
- Glauser G, Vallat A, Balmer D. 2014. Hormone profiling. Methods in Molecular Biology 1062: 597–608. [DOI] [PubMed] [Google Scholar]
- Gobbato E, Marsh JF, Vernié T, Wang E, Maillet F, Kim J, Miller JB, Sun J, Bano SA, Ratet P et al. 2012. A GRAS‐type transcription factor with a specific function in mycorrhizal signaling. Current Biology 22: 2236–2241. [DOI] [PubMed] [Google Scholar]
- Gollotte A, Gianinazzi‐Pearson V, Giovannetti M, Sbrana C, Avio L, Gianinazzi S. 1993. Cellular localization and cytochemical probing of resistance reactions to arbuscular mycorrhizal fungi in a locus a myc‐mutant of Pisum sativum L. Planta 191: 112–122. [Google Scholar]
- Grunwald U, Nyamsuren O, Tamasloukht M, Lapopin L, Becker A, Mann P, Gianinazzi‐Pearson V, Krajinski F, Franken P. 2004. Identification of mycorrhiza‐regulated genes with arbuscule development‐related expression profile. Plant Molecular Biology 55: 553–566. [DOI] [PubMed] [Google Scholar]
- Guenoune D, Galili S, Phillips DA, Volpin H, Chet I, Okon Y, Kapulnik Y. 2001. The defense response elicited by the pathogen Rhizoctonia solani is suppressed by colonization of the AM‐fungus Glomus intraradices . Plant Science 160: 925–932. [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Parniske M. 2013. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annual Review of Cell and Developmental Biology 29: 593–617. [DOI] [PubMed] [Google Scholar]
- Handa Y, Nishide H, Takeda N, Suzuki Y, Kawaguchi M, Saito K. 2015. RNA‐seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis . Plant and Cell Physiology 56: 1490–1511. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R. 2007. Cell wall‐associated mechanisms of disease resistance and susceptibility. Annual Review of Phytopathology 45: 101–127. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R. 2014. The effective papilla hypothesis. New Phytologist 204: 438–440. [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Jung SC, Martinez‐Medina A, Lopez‐Raez JA, Pozo MJ. 2012. Mycorrhiza‐induced resistance and priming of plant defenses. Journal of Chemical Ecology 38: 651–664. [DOI] [PubMed] [Google Scholar]
- Kalo P, Gleason C, Edwards A, Marsh J, Mitra RM, Hirsch S, Jakab J, Sims S, Long SR, Rogers J et al. 2005. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308: 1786–1789. [DOI] [PubMed] [Google Scholar]
- Kamel L, Tang NW, Malbreil M, San Clemente H, Le Marquer M, Roux C, Frey NFD. 2017. The comparison of expressed candidate secreted proteins from two arbuscular mycorrhizal fungi unravels common and specific molecular tools to invade different host plants. Frontiers in Plant Science 8: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Volpin H, Itzhaki H, Ganon D, Galili S, David R, Shaul O, Elad Y, Chet I, Okon Y. 1996. Suppression of defence responses in mycorrhizal alfalfa and tobacco roots. New Phytologist 133: 59–64. [Google Scholar]
- Klironomos JN. 2003. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84: 2292–2301. [Google Scholar]
- Kloppholz S, Kuhn H, Requena N. 2011. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Current Biology 21: 1204–1209. [DOI] [PubMed] [Google Scholar]
- Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB. 2014. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protocols 4: 1–7. [Google Scholar]
- Kurihara D, Mizuta Y, Sato Y, Higashiyama T. 2015. ClearSee: a rapid optical clearing reagent for whole‐plant fluorescence imaging. Development 142: 4168–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco L, Fiorilli V, Gutjahr C. 2018. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytologist 220: 1031–1046. [DOI] [PubMed] [Google Scholar]
- Lanfranco L, Novero M, Bonfante P. 2005. The mycorrhizal fungus Gigaspora margarita possesses a CuZn superoxide dismutase that is up‐regulated during symbiosis with legume hosts. Plant Physiology 137: 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, Journet EP, Ané JM, Lauber E, Bisseling T et al. 2004. A putative Ca2+ and calmodulin‐dependent protein kinase required for bacterial and fungal symbioses. Science 303: 1361–1364. [DOI] [PubMed] [Google Scholar]
- Liu C‐W, Breakspear A, Stacey N, Findlay K, Nakashima J, Ramakrishnan K, Liu M, Xie F, Endre G, de Carvalho‐Niebel F et al. 2019. A protein complex required for polar growth of rhizobial infection threads. Nature Communications 10: 2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Maldonado‐Mendoza I, Lopez‐Meyer M, Cheung F, Town CD, Harrison MJ. 2007. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. The Plant Journal 50: 529–544. [DOI] [PubMed] [Google Scholar]
- Liu QQ, Luo L, Zheng LQ. 2018. Lignins: Biosynthesis and biological functions in plants. International Journal of Molecular Sciences 19: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loake G, Grant M. 2007. Salicylic acid in plant defence‐the players and protagonists. Current Opinion in Plant Biology 10: 466–472. [DOI] [PubMed] [Google Scholar]
- van Loon LC, Geraats BPJ, Linthorst HJM. 2006a. Ethylene as a modulator of disease resistance in plants. Trends in Plant Science 11: 184–191. [DOI] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CMJ. 2006b. Significance of inducible defense‐related proteins in infected plants. Annual Review of Phytopathology 44: 135–162. [DOI] [PubMed] [Google Scholar]
- Maillet F, Poinsot V, André O, Puech‐Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A et al. 2011. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469: 58–64. [DOI] [PubMed] [Google Scholar]
- Mandal S, Das RK, Mishra S. 2011. Differential occurrence of oxidative burst and antioxidative mechanisms in compatible and incompatible interactions of tomato and Ralstonia solanacearum . Plant Physiology and Biochemistry 49: 117–123. [DOI] [PubMed] [Google Scholar]
- Marcel S, Sawers R, Oakeley E, Angliker H, Paszkowski U. 2010. Tissue‐adapted invasion strategies of the rice blast fungus Magnaporthe oryzae . The Plant Cell 22: 3177–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JF, Schultze M. 2001. Analysis of arbuscular mycorrhizas using symbiosis‐defective plant mutants. New Phytologist 150: 525–532. [Google Scholar]
- Miedes E, Van Holme R, Boerjan W, Molina A. 2014. The role of the secondary cell wall in plant resistance to pathogens. Frontiers in Plant Science 5: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck‐Reichhart D, Ausubel FM. 2010. Innate immune responses activated in Arabidopsis roots by microbe‐associated molecular patterns. The Plant Cell 22: 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GED, Long SR. 2004. A Ca2+ calmodulin‐dependent protein kinase required for symbiotic nodule development: gene identification by transcript‐based cloning. Proceedings of the National Academy of Sciences, USA 101: 4701–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin E, Miyauchi S, San Clemente H, Chen ECH, Pelin A, de la Providencia I, Ndikumana S, Beaudet D, Hainaut M, Drula E et al. 2019. Comparative genomics of Rhizophagus irregularis, R. cerebriforme, R. diaphanus and Gigaspora rosea highlights specific genetic features in Glomeromycotina. New Phytologist 222: 1584–1598. [DOI] [PubMed] [Google Scholar]
- Murray JD, Duvvuru Muni R, Torres‐Jerez I, Tang Y, Allen S, Andriankaja M, Li G, Laxmi A, Cheng X, Wen J et al. 2011. Vapyrin, a gene essential for intracellular progression of arbuscular mycorrhizal symbiosis, is also essential for infection by rhizobia in the nodule symbiosis of Medicago truncatula . The Plant Journal 65: 244–252. [DOI] [PubMed] [Google Scholar]
- Nouri E, Breuillin‐Sessoms F, Feller U, Reinhardt D. 2014. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida . PLoS ONE 9: e90841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novero M, Faccio A, Genre A, Stougaard J, Webb KJ, Mulder L, Parniske M, Bonfante P. 2002. Dual requirement of the LjSym4 gene for mycorrhizal development in epidermal and cortical cells of Lotus japonicus roots. New Phytologist 154: 741–749. [DOI] [PubMed] [Google Scholar]
- Park H‐J, Floss DS, Levesque‐Tremblay V, Bravo A, Harrison MJ. 2015. Hyphal branching during arbuscule development requires Reduced Arbuscular Mycorrhiza1 . Plant Physiology 169: 2774–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Research 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker P. 2014. Induced systemic resistance by beneficial microbes. Annual Review of Phytopathology, 52: 347–375. [DOI] [PubMed] [Google Scholar]
- Pimprikar P, Carbonnel S, Paries M, Katzer K, Klingl V, Bohmer MJ, Karl L, Floss DS, Harrison MJ, Parniske M et al. 2016. A CCaMK‐CYCLOPS‐DELLA complex activates transcription of RAM1 to regulate arbuscule branching. Current Biology 26: 987–998. [DOI] [PubMed] [Google Scholar]
- Pozo MJ, Azcon‐Aguilar C. 2007. Unraveling mycorrhiza‐induced resistance. Current Opinion in Plant Biology 10: 393–398. [DOI] [PubMed] [Google Scholar]
- Pumplin N, Mondo SJ, Topp S, Starker CG, Gantt JS, Harrison MJ. 2010. Medicago truncatula Vapyrin is a novel protein required for arbuscular mycorrhizal symbiosis. The Plant Journal 61: 482–494. [DOI] [PubMed] [Google Scholar]
- Rathgeb U, Chen M, Buron F, Feddermann N, Schorderet M, Raisin A, Häberli GY, Marc‐Martin S, Keller J, Delaux PM et al. 2020. VAPYRIN‐like is required for development of the moss Physcomitrella patens . Development 147: 184762. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. 1963. The use of lead citrate at high pH as an electron‐opaque stain in electron microscopy. Journal of Cell Biology 17: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MK, Schorderet M, Bapaume L, Falquet L, Morel P, Vandenbussche M, Reinhardt D. 2015. A petunia GRAS transcription factor controls symbiotic gene expression and fungal morphogenesis in arbuscular mycorrhiza. Plant Physiology 168: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MK, Schorderet M, Reinhardt D. 2014. The role of the cell wall compartment in mutualistic symbioses of plants. Frontiers in Plant Science 5: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Lozano JM, Roussel H, Gianinazzi S, Gianinazzi‐Pearson V. 1999. Defense genes are differentially induced by a mycorrhizal fungus and Rhizobium sp in wild‐type and symbiosis‐defective pea genotypes. Molecular Plant–Microbe Interactions 12: 976–984. [Google Scholar]
- Salzer P, Bonanomi A, Beyer K, Vögeli‐Lange R, Aeschbacher RA, Lange J, Wiemken A, Kim D, Cook DR, Boller T. 2000. Differential expression of eight chitinase genes in Medicago truncatula roots during mycorrhiza formation, nodulation, and pathogen infection. Molecular Plant–Microbe Interactions 13: 763–777. [DOI] [PubMed] [Google Scholar]
- Salzer P, Corbière H, Boller T. 1999. Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza‐forming fungus Glomus intraradices . Planta 208: 319–325. [Google Scholar]
- Scheler C, Durner J, Astier J. 2013. Nitric oxide and reactive oxygen species in plant biotic interactions. Current Opinion in Plant Biology 16: 534–539. [DOI] [PubMed] [Google Scholar]
- Sedzielewska Toro K, Brachmann A. 2016. The effector candidate repertoire of the arbuscular mycorrhizal fungus Rhizophagus clarus . BMC Genomics 17: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhara Reddy DMR, Schorderet M, Feller U, Reinhardt D. 2007. A petunia mutant affected in intracellular accommodation and morphogenesis of arbuscular mycorrhizal fungi. The Plant Journal 51: 739–750. [DOI] [PubMed] [Google Scholar]
- Siciliano V, Genre A, Balestrini R, Cappellazzo G, deWit P, Bonfante P. 2007. Transcriptome analysis of arbuscular mycorrhizal roots during development of the prepenetration apparatus. Plant Physiology 144: 1455–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FA, Grace EJ, Smith SE. 2009. More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytologist 182: 347–358. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. New York, NY, USA: Academic Press. [Google Scholar]
- Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A et al. 2016. A phylum‐level phylogenetic classification of zygomycete fungi based on genome‐scale data. Mycologia 108: 1028–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr AR. 1969. A low‐viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Stuurman J, Kuhlemeier C. 2005. Stable two‐element control of dTph1 transposition in mutator strains of Petunia by an inactive ACT1 introgression from a wild species. The Plant Journal 41: 945–955. [DOI] [PubMed] [Google Scholar]
- Tang N, San Clemente H, Roy S, Bécard G, Zhao B, Roux C. 2016. A survey of the gene repertoire of Gigaspora rosea unravels conserved features among Glomeromycota for obligate biotrophy. Frontiers in Microbiology 7: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuerig B, Felix G, Binder A, Boller T, Tamm L. 2005. An extract of Penicillium chrysogenum elicits early defense‐related responses and induces resistance in Arabidopsis thaliana independently of known signalling pathways. Physiological and Molecular Plant Pathology 67: 180–193. [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiology 141: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, De Meester B, Ralph J, Boerjan W. 2019. Lignin biosynthesis and its integration into metabolism. Current Opinion in Biotechnology 56: 230–239. [DOI] [PubMed] [Google Scholar]
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. 2010. Lignin biosynthesis and structure. Plant Physiology 153: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierheilig H, Alt M, Lange J, Gutrella M, Wiemken A, Boller T. 1995. Colonization of transgenic tobacco constitutively expressing pathogenesis‐related proteins by the vesicular‐arbuscular mycorrhizal fungus Glomus mosseae . Applied and Environmental Microbiology 61: 3031–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. 2009. Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology 47: 177–206. [DOI] [PubMed] [Google Scholar]
- Vogt T. 2010. Phenylpropanoid biosynthesis. Molecular Plant 3: 2–20. [DOI] [PubMed] [Google Scholar]
- Voss S, Betz R, Heidt S, Corradi N, Requena N. 2018. RiCRN1, a crinkler effector from the arbuscular mycorrhizal fungus Rhizophagus irregularis, functions in arbuscule development. Frontiers in Microbiology 9: 2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111: 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegel E, Schauser L, Sandal N, Stougaard J, Parniske M. 1998. Mycorrhiza mutants of Lotus japonicus define genetically independent steps during symbiotic infection. Molecular Plant‐Microbe Interactions 11: 933–936. [Google Scholar]
- Zhang XC, Pumplin N, Ivanov S, Harrison MJ. 2015. EXO70I is required for development of a sub‐domain of the periarbuscular membrane during arbuscular mycorrhizal symbiosis. Current Biology 25: 2189–2195. [DOI] [PubMed] [Google Scholar]
- Zipfel C, Oldroyd GED. 2017. Plant signalling in symbiosis and immunity. Nature 543: 328–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Quantitative phenotypic analysis of vpy mutants.
Fig. S2 Confocal analysis of papilla formation in vpy mutants.
Fig. S3 Infection phenotype in vpy‐3 root cortex cells.
Fig. S4 Cytochemical detection of H2O2 in the cortex of vpy mutants.
Fig. S5 Cytochemical detection of O2 – in vpy mutants.
Fig. S6 Callose accumulation associated with aborted fungal infection of vpy‐3.
Fig. S7 Lack of lignin accumulation in mycorrhizal ram1 mutants.
Fig. S8 Concerted induction of lignin biosynthetic genes in mycorrhizal vpy mutants.
Fig. S9 Mycorrhizal colonization pattern in M. truncatula sym mutants.
Fig. S10 Mycorrhizal colonization pattern in M. truncatula sym mutants.
Fig. S11 Lack of lignin accumulation at fungal entry points in M. truncatula sym mutants.
Fig. S12 Salicylic acid concentrations in mycorrhizal wild‐type and vpy‐3 roots.
Fig. S13 Jasmonic acid concentrations in mycorrhizal wild‐type and vpy‐3 roots.
Fig. S14 Immunocytochemical analysis of β‐1,3‐glucanase in mycorrhizal roots.
Fig. S15 Accumulation of β‐1,3‐glucanase in a cell wall apposition of vpy‐3.
Fig. S16 Low‐magnification overview picture of the sample shown in Fig. S15.
Fig. S17 Quantification of β‐1,3‐glucanase immunogold signal.
Methods S1 Methods relating to experiments on Medicago truncatula, β‐1,3‐glucanase immunostaining, RNA extraction and qPCR, and statistical analysis.
Notes S1 Characterization of lignin accumulation in Medicago truncatula wild‐type and symbiosis mutants.
Table S1 Quantification of callose accumulation in vpy mutants.
Table S2 Primers used for qRT‐PCR of lignin‐related genes.
Table S3 Induction of lignin‐related genes in vpy mutants vs wild‐type in either the mycorrhizal or nonmycorrhizal context.
Table S4 Expression values for lignin‐related genes for the experiment on vpy‐1 from the genes for which induction ratios were calculated in Tables 1 and S3.
Table S5 Expression values for lignin‐related genes for the experiment on vpy‐2 and vpy‐3 from the genes for which induction ratios were calculated in Tables 1 and S3.
Table S6 M. truncatula mutants used in this study.
Table S7 Primers used for qRT‐PCR of PR genes.
Table S8 Induction of pathogenesis‐related (PR) genes in mutants vs wild‐type in either the mycorrhizal or nonmycorrhizal context.
Table S9 Induction of pathogenesis‐related (PR) genes in wild‐type plants treated with fungal elicitor preparations.
Table S10 Expression values for the experiment on vpy‐1 from the genes for which induction ratios were calculated in Tables 2 and S8.
Table S11 Expression values for the experiment on vpy‐2 and vpy‐3 from the genes for which induction ratios were calculated in Tables 2 and S8.
Table S12 Expression values from the experiment with wild‐type plants treated with elicitors from genes for which induction ratios were calculated in Table S9.
Table S13 P‐values from statistical analyses and interaction report from two‐way ANOVA tests.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


