Abstract
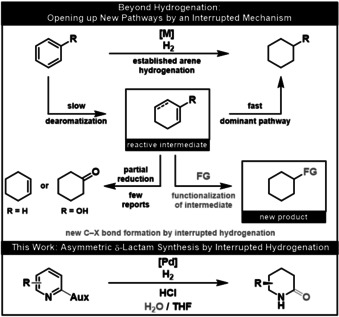
Metal‐catalyzed hydrogenation is an effective method to transform readily available arenes into saturated motifs, however, current hydrogenation strategies are limited to the formation of C−H and N−H bonds. The stepwise addition of hydrogen yields reactive unsaturated intermediates that are rapidly reduced. In contrast, the interruption of complete hydrogenation by further functionalization of unsaturated intermediates offers great potential for increasing chemical complexity in a single reaction step. Overcoming the tenet of full reduction in arene hydrogenation has been seldom demonstrated. In this work we report the synthesis of sought‐after, enantioenriched δ‐lactams from oxazolidinone‐substituted pyridines and water by an interrupted hydrogenation mechanism.
Keywords: asymmetric catalysis, heterogeneous catalysis, hydrogenation, lactams, nitrogen heterocycles
The metal‐catalyzed hydrogenation of arenes is a well‐known and powerful transformation to yield saturated carbo‐ and heterocycles. Yet, this transformation is limited to the sole formation of C−H and N−H bonds. Herein, we present a method to synthesize enantioenriched δ‐lactams from readily available oxazolidinone‐substituted pyridines. Crucial for this reaction is the interruption of the arene reduction by nucleophilic substitution.
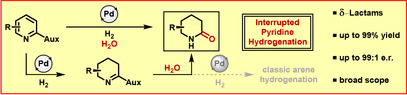
Metal‐catalyzed hydrogenation is known to be a simple and powerful method to increase molecular complexity. [1] In particular, the hydrogenation of easily accessible N‐heteroarenes such as pyridines offers access to important saturated azacycles. [2] This established reaction is limited solely to the formation of new C−H and N−H bonds; additional synthetic manipulations are required to introduce further chemical functionality. [3] The stepwise transfer of molecular hydrogen in arene hydrogenation yields intermediates with double bonds remaining, which in principle offer the possibility of further functionalization (Figure 1). [4] Nevertheless, harnessing unsaturated intermediates in arene hydrogenation for other C−X bond forming events is unknown in the literature, although the partial hydrogenation of certain substrates like benzene or phenol has been achieved. [5] Recently, Donohoe and co‐workers reported a pioneering combination of C−H and C−C bond formation in a transfer hydrogenation of pyridinium salts. [6] Protected tetrahydropyridines were thus synthesized using iridium‐catalyzed hydride transfer followed by hydroxymethylation. This precedent underlines the huge potential of an interrupted hydrogenation strategy to rapidly build up chemical complexity, yet, further method development is necessary to elevate this approach to a general synthetic strategy. We now report a pyridine hydrogenation interrupted by nucleophilic substitution of an unsaturated intermediate. Our procedure enables the synthesis of valuable δ‐lactams by Pd‐catalyzed hydrogenation of oxazolidinone‐substituted pyridines in the presence of water. [7]
Figure 1.
Mechanistic pathways of unsaturated intermediates in arene hydrogenation and this work. Aux=chiral auxiliary.
Lactams, such as 2‐piperidones, are important building blocks and represent core motifs in many pharmaceuticals and natural products. [8] Despite interest in this moiety, synthetic access to enantioenriched δ‐lactams remains challenging. [9] In this regard, we envision metal‐catalyzed hydrogenation as a powerful strategy to address this problem. However, the synthesis of 2‐piperidones via direct asymmetric hydrogenation [10] of pyridone precursors is hampered by amide resonance and, to the best of our knowledge, has never been achieved. [11] In contrast, our procedure starts with oxazolidinone‐substituted pyridines, which are readily available from cheap and abundant 2‐halogenated pyridines in a single step. [12] The oxazolidinone is cleaved within the reaction, thus acting as a traceless chiral auxiliary which can be fully recycled.
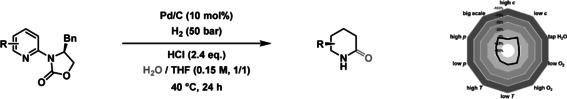
After our initial discovery, we studied the influence of the reaction parameters in more detail (see Supporting Information for details). The presence of strong Brønsted acids like hydrochloric acid is essential. [13] Using weak Brønsted acids like formic acid resulted in the exclusive formation of piperidine product (Table S1, Entry 7). Furthermore, different solvents were tested, with a combination of THF and H2O giving the best result (Table S1, Entries 1–6). The nature of the catalyst is also decisive for the reaction outcome.While various Pd catalysts generally gave the desired δ‐lactam in high yields and enantioselectivities, other catalysts such as PtO2, Ru/C or Rh/C resulted in little or no yield (Table S2, Entries 2–9). Various oxazolidinones from the chiral pool provided the product in a high enantiomeric ratio while a benzyl substituent gave the best result (Table S2, Entries 10–12). [14] Lowering the hydrogen pressure to 25 bar results in a decrease of the enantiomeric ratio (Table S2, Entry 13). To investigate the reproducibility of our new method, we performed a reaction‐condition‐based sensitivity screen (Table 1; see Supporting Information for details). [15]
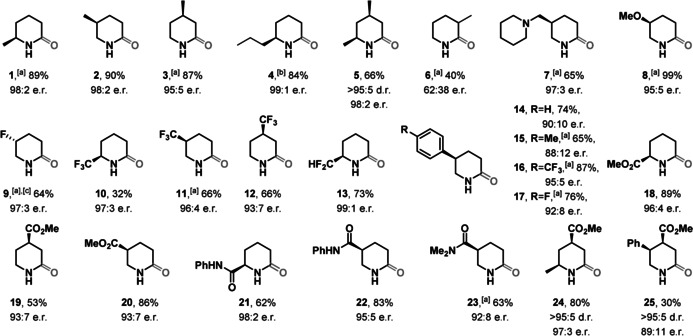
Table 1.
Reaction scope for the palladium‐catalyzed synthesis of 2‐piperidones. See Supporting Information for full experimental details.
|
|
[a] 4‐Isopropyloxazolidinone as chiral auxiliary. [b] 4‐tert‐Butyloxazolidinone as chiral auxiliary. [c] Epimeric (R)‐isopropyloxazolidinone as chiral auxiliary.
With optimized conditions in hand, we investigated the reaction scope of this new transformation. A series of alkylated δ‐lactams (1–4) was synthesized in high yields and excellent enantiomeric ratios. Furthermore, our method was capable of forming multiple stereocenters in a highly selective fashion. Dimethylated lactam 5 was isolated in 66 % yield with a 98:2 enantiomeric ratio and only one diastereomer observed. Additional functional groups such as basic azacycles (7) or alkyl ethers (8) were tolerated without significant decrease in yield or enantiomeric ratio. Although hydrogenation of fluorinated arenes is challenging, [16] several fluorinated δ‐lactams (9–13) were prepared containing CF, CF3, and CF2H groups in moderate to good yields. Interestingly, a chemoselective hydrogenation of the pyridine core over a phenyl substituent was observed for our catalytic system to give various substituted phenyl‐piperidones (14–17). Moreover, δ‐lactams carrying important carbonyl functionalities such as esters and amides (18–23) were prepared in moderate to high yields and high enantiomeric ratios. Disubstituted δ‐lactams 24 and 25 were synthesized highly selectively with only one diastereomer observed in 97:3 and 89:11 enantiomeric ratios, respectively. Substitution in the pyridine 3‐position however decreased product formation and resulted in an almost racemic mixture (6, see Supporting Information for details).
The absolute configurations of δ‐lactams 1 and 10 were unambiguously determined by X‐ray analysis and are in accordance with our previous observations (Figure 2, see Supporting Information for details).[ 7a , 7b , 17 ]Based on the Horiuti–Polanyi mechanism [18] for heterogeneous hydrogenation, the absolute configuration of the products with substituents in 4‐ and 5‐ position are assigned analogously (see Scheme S1). In agreement with this model, a deuteration experiment confirms the addition of deuterium to one diastereotopic face of the pyridine exclusively (see Supporting Information for details).
Figure 2.
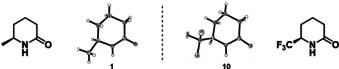
Absolute configuration of δ‐lactams 1 and 10.
Subsequently, we investigated the scalability of our procedure and the possibility of recycling the auxiliary. The hydrogenation was performed on a 2 g scale without significant loss in yield or enantiomeric ratio (Scheme 1, a). Lactam ent ‐4 was obtained in 78 % yield and 97:3 enantiomeric ratio and oxazolidinone 26 was recovered in 92 % yield and >99:1 e.r. thus outlining its good recyclability.
Scheme 1.
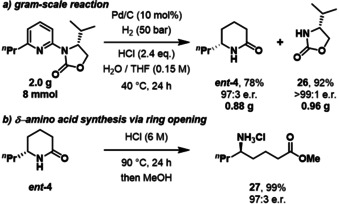
Gram‐scale reaction and ring opening.
As further product application, δ‐lactam ent ‐4 was hydrolyzed to give the δ‐amino acid methyl ester 27 in 99 % yield and 97:3 e.r. after heating in hydrochloric acid for 24 h and treatment with methanol (Scheme 1, b).
Intrigued by these observations, we investigated the reaction mechanism. To identify potential intermediates, the reaction was stopped after 2 h and the mass of an intermediate with one remaining double bond was detected by GC‐MS (Scheme 2, a). Although unsaturated intermediates are very short‐lived in arene hydrogenation and are rapidly further hydrogenated, imine 28 was isolated in 5 % yield (Scheme 2, b). Conversion of imine 28 in water/ THF with hydrochloric acid in the absence of Pd catalyst and H2 provided the δ‐lactam product in almost quantitative yield (Scheme 2, c). Thus, imine 28 is an intermediate species in our procedure and can undergo nucleophilic substitution with water. It is worth mentioning that only imine 28 as one of six potential intermediates in this reaction is suitable to hydrolyze to yield the desired 2‐piperidone (Scheme 2, a). Based on these experiments we postulate the following reaction mechanism (Scheme 2, d): By addition of a Brønsted acid, the basic nitrogen of the oxazilidinone‐substituted pyridine is protonated and forms a stable conformer through hydrogen bonding to the oxazolidinone. Owing to the steric repulsion of the oxazolidinone substituent of this rigid conformation, a diastereoselective hydrogenation of the unhindered face of the pyridine proceeds to set the new stereocenter. After the addition of two equivalents of H2, oxazolidinone‐substituted imine I is formed (see Scheme S1). In contrast to a classic arene hydrogenation, this intermediate, following our new protocol, does not undergo another catalytic reduction (see product III) but reacts with water in a nucleophilic substitution. In this substitution, the oxazolidinone is released and therefore serves as a recyclable, traceless auxiliary. The resulting iminium II tautomerizes rapidly to yield the final δ‐lactam product.
Scheme 2.
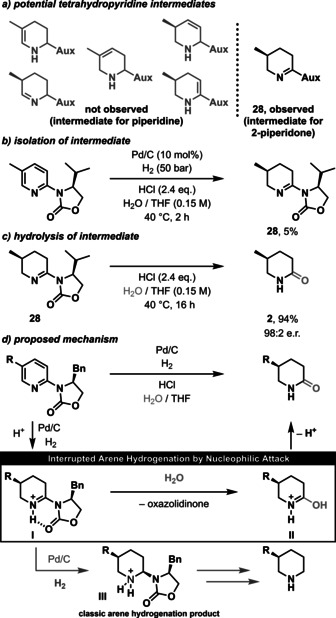
Mechanistic investigation and proposal.
In summary, we have disclosed a heteroarene hydrogenation interrupted by nucleophilic substitution. Hydrogenation of oxazolidinone‐substituted pyridines gives access to enantioenriched δ‐lactams by applying oxazolidinones as traceless chiral auxiliaries. Our procedure was used to access a plethora of sought‐after δ‐lactams in high yields and excellent enantiomeric ratios. Harnessing unsaturated intermediates in arene hydrogenation is an underexplored research area and overcomes the inherent limitation of forming solely C−H and N−H bonds. We see huge potential in this research direction since it tremendously increases molecular complexity in a single step.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
Generous financial support by the European Research Council (ERC Advanced Grant Agreement No. 788558), the Deutsche Forschungsgemeinschaft (Leibniz Award), and the Alfried Krupp von Bohlen und Halbach Foundation are gratefully acknowledged. The authors thank Cornelia Weitkamp and Lisa Grabinski for experimental support as well as Toryn Dalton, Arne Heusler, Daniel Moock, Dr. J. Luca Schwarz, and Marco Wollenburg for many helpful discussions. Open access funding enabled and organized by Projekt DEAL.
T. Wagener, L. Lückemeier, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2021, 60, 6425.
Dedicated to Professor David A. Evans on the occasion of his 80th birthday
References
- 1.
- 1a. Wiesenfeldt M. P., Nairoukh Z., Dalton T., Glorius F., Angew. Chem. Int. Ed. 2019, 58, 10460; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 10570; [Google Scholar]
- 1b. Wertjes W. C., Southgate E. H., Sarlah D., Chem. Soc. Rev. 2018, 47, 7996; [DOI] [PubMed] [Google Scholar]
- 1c. Qi S.-C., Wei X.-Y., Zong Z.-M., Wang Y.-K., RSC Adv. 2013, 3, 14219; [Google Scholar]
- 1d. Lovering F., MedChemComm 2013, 4, 515; [Google Scholar]
- 1e. Lovering F., Bikker J., Humblet C., J. Med. Chem. 2009, 52, 6752; [DOI] [PubMed] [Google Scholar]
- 1f. The Handbook of Homogeneous Hydrogenation (Eds.: de Vries H. G., Elsevier C. J.), Wiley-VCH, Weinheim, 2006; [Google Scholar]
- 1g. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (Ed.: Nishimura S.), Wiley, New York, 2001. [Google Scholar]
- 2.For selected examples, see:
- 2a. Kim S., Loose F., Bezdek M. J., Wang X., Chirik P. J., J. Am. Chem. Soc. 2019, 141, 17900; [DOI] [PubMed] [Google Scholar]
- 2b. Duan Y.-N., Du X., Cui Z., Zeng Y., Liu Y., Yang T., Wen J., Zhang X., J. Am. Chem. Soc. 2019, 141, 20424; [DOI] [PubMed] [Google Scholar]
- 2c. Adam R., Cabrero-Antonino J. R., Spannenberg A., Junge K., Jackstell R., Beller M., Angew. Chem. Int. Ed. 2017, 56, 3216; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 3264; [Google Scholar]
- 2d. Huang W.-X., Wu B., Gao X., Chen M.-W., Wang B., Zhou Y.-G., Org. Lett. 2015, 17, 1640; [DOI] [PubMed] [Google Scholar]
- 2e. Liu Y., Du H., J. Am. Chem. Soc. 2013, 135, 12968; [DOI] [PubMed] [Google Scholar]
- 2f. Dobereiner G. E., Nova A., Schley N. D., Hazari N., Miller S. J., Eisenstein O., Crabtree R. H., J. Am. Chem. Soc. 2011, 133, 7547; [DOI] [PubMed] [Google Scholar]
- 2g. Farrell J. M., Hatnean J. A., Stephan D. W., J. Am. Chem. Soc. 2012, 134, 15728; [DOI] [PubMed] [Google Scholar]
- 2h. Wang X.-B., Zeng W., Zhou Y.-G., Tetrahedron Lett. 2008, 49, 4922; [Google Scholar]
- 2i. Legault C. Y., Charette A. B., J. Am. Chem. Soc. 2005, 127, 8966; [DOI] [PubMed] [Google Scholar]
- 2j. Douja N., Besson M., Gallezot P., Pinel C., J. Mol. Catal. A: Chem. 2002, 186, 145; [Google Scholar]
- 2k. Hegedűs L., Háda V., Tungler A., Máthé T., Szepesy L., Appl. Catal. A 2000, 201, 107. [Google Scholar]
- 3.For selected examples of chemoselective hydrogenations, see:
- 3a. Moock D., Wiesenfeldt M. P., Freitag M., Muratsugu S., Ikemoto S., Knitsch R., Schneidewind J., Baumann W., Schäfer A. H., Timmer A., Tada M., Hansen M. R., Glorius F., ACS Catal. 2020, 10, 6309; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Wollenburg M., Moock D., Glorius F., Angew. Chem. Int. Ed. 2019, 58, 6549; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6621; [Google Scholar]
- 3c. Ling L., He Y., Zhang X., Luo M., Zeng X., Angew. Chem. Int. Ed. 2019, 58, 6554; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6626; [Google Scholar]
- 3d. Wiesenfeldt M. P., Knecht T., Schlepphorst C., Glorius F., Angew. Chem. Int. Ed. 2018, 57, 8297; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 8429; [Google Scholar]
- 3e. Tran B. L., Fulton J. L., Linehan J. C., Lercher J. A., Bullock R. M., ACS Catal. 2018, 8, 8441; [Google Scholar]
- 3f. Wei Y., Rao B., Cong X., Zeng X., J. Am. Chem. Soc. 2015, 137, 9250. [DOI] [PubMed] [Google Scholar]
- 4.For a general combination of hydrogenation and further functionalization, see:
- 4a. Brechmann L. T., Teichert J. F., Synthesis 2020, 52, 2483; [Google Scholar]
- 4b. Kim S. W., Zhang W., Krische M. J., Acc. Chem. Res. 2017, 50, 2371; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Miao T., Tian Z.-Y., He Y.-M., Chen F., Chen Y., Yu Z.-X., Fan Q.-H., Angew. Chem. Int. Ed. 2017, 56, 4135; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 4199; [Google Scholar]
- 4d. Zbieg J. R., Yamaguchi E., McInturff E. L., Krische M. J., Science 2012, 336, 324; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4e. Breit B., Acc. Chem. Res. 2003, 36, 264; [DOI] [PubMed] [Google Scholar]
- 4f. Jang H.-Y., Huddleston R. R., Krische M. J., J. Am. Chem. Soc. 2002, 124, 15156. [DOI] [PubMed] [Google Scholar]
- 5.For selected examples, see:
- 5a. Wollenburg M., Heusler A., Bergander K., Glorius F., ACS Catal. 2020, 10, 11365; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Foppa L., Dupont J., Chem. Soc. Rev. 2015, 44, 1886; [DOI] [PubMed] [Google Scholar]
- 5c. Wang Y., Yao J., Li H., Su D., Antonietti M., J. Am. Chem. Soc. 2011, 133, 2362; [DOI] [PubMed] [Google Scholar]
- 5d. Liu H., Jiang T., Han B., Liang S., Zhou Y., Science 2009, 326, 1250; [DOI] [PubMed] [Google Scholar]
- 5e. Nagahara H., Ono M., Konishi M., Fukuoka Y., Appl. Surf. Sci. 1997, 121/122, 448. [Google Scholar]
- 6. Grozavu A., Hepburn H. B., Smith P. J., Potukuchi H. K., Lindsay-Scott P. J., Donohoe T. J., Nat. Chem. 2019, 11, 242. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Heitbaum M., Fröhlich R., Glorius F., Adv. Synth. Catal. 2010, 352, 357; [Google Scholar]
- 7b. Glorius F., Spielkamp N., Holle S., Goddard R., Lehmann C. W., Angew. Chem. Int. Ed. 2004, 43, 2850; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 2910. For the use of achiral oxazolidinones in asymmetric hydrogenation, see: [Google Scholar]
- 7c. Fan D., Zhang J., Hu Y., Zhang Z., Gridnev I. D., Zhang W., ACS Catal. 2020, 10, 3232. [Google Scholar]
- 8.
- 8a. Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem. 2014, 57, 10257; [DOI] [PubMed] [Google Scholar]
- 8b. Taylor R. D., MacCoss M., Lawson A. D. G., J. Med. Chem. 2014, 57, 5845; [DOI] [PubMed] [Google Scholar]
- 8c. O'Hagan D., Nat. Prod. Rep. 2000, 17, 435. [DOI] [PubMed] [Google Scholar]
- 9.For selected examples, see:
- 9a. Yin C., Yang T., Pan Y., Wen J., Zhang X., Org. Lett. 2020, 22, 920; [DOI] [PubMed] [Google Scholar]
- 9b. Hassan I. S., Ta A. N., Danneman M. W., Semakul N., Burns M., Basch C. H., Dippon V. N., McNaughton B. R., Rovis T., J. Am. Chem. Soc. 2019, 141, 4815; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Jayakumar S., Louven K., Strohmann C., Kumar K., Angew. Chem. Int. Ed. 2017, 56, 15945; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16161; [Google Scholar]
- 9d. Campello H. R., Parker J., Perry M., Ryberg P., Gallagher T., Org. Lett. 2016, 18, 4124; [DOI] [PubMed] [Google Scholar]
- 9e. Khong D. T., Pamarthy V. S., Gallagher T., Judeh Z. M. A., Eur. J. Org. Chem. 2016, 3084; [Google Scholar]
- 9f. He Z.-T., Wei Y.-B., Yu H.-J., Sun C.-Y., Feng C.-G., Tian P., Lin G.-Q., Tetrahedron 2012, 68, 9186; [Google Scholar]
- 9g. Zhang F., Liu Z.-J., Liu J.-T., Tetrahedron 2010, 66, 6864; [Google Scholar]
- 9h. Lee H. K., Chun J. S., Pak C. S., J. Org. Chem. 2003, 68, 2471; [DOI] [PubMed] [Google Scholar]
- 9i. Koulocheri S. D., Magiatis P., Haroutounian S. A., J. Org. Chem. 2001, 66, 7915. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Shi L., Zhou Y.-G. Catalytic, Enantioselective Hydrogenation of Heteroaromatic Compounds in Organic Reactions, Wiley, New York, 2018, pp. 1–228; [Google Scholar]
- 10b. Mashima K., Nagae H., Iimuro A., Higashida K., Heterocycles 2017, 95, 63; [Google Scholar]
- 10c. Zhang Z., Butt N. A., Zhang W., Chem. Rev. 2016, 116, 14769; [DOI] [PubMed] [Google Scholar]
- 10d. Zhao D., Candish L., Paul D., Glorius F., ACS Catal. 2016, 6, 5978; [Google Scholar]
- 10e. Chen Z.-P., Zhou Y.-G., Synthesis 2016, 48, 1769; [Google Scholar]
- 10f. He Y.-M., Song F.-T., Fan Q.-H., Top. Curr. Chem. 2013, 343, 145; [DOI] [PubMed] [Google Scholar]
- 10g. Wang D.-S., Chen Q.-A., Lu S.-M., Zhou Y.-G., Chem. Rev. 2012, 112, 2557; [DOI] [PubMed] [Google Scholar]
- 10h. Glorius F., Org. Biomol. Chem. 2005, 3, 4171. [DOI] [PubMed] [Google Scholar]
- 11.For an asymmetric hydrogenation of N-methylated pyridones, see: Wysocki J., Schlepphorst C., Glorius F., Synlett 2015, 26, 1557. [Google Scholar]
- 12. Klapars A., Huang X., Buchwald S. L., J. Am. Chem. Soc. 2002, 124, 7421. [DOI] [PubMed] [Google Scholar]
- 13. Yu Z., Jin W., Jiang Q., Angew. Chem. Int. Ed. 2012, 51, 6060; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 6164. [Google Scholar]
- 14.
- 14a. Diaz-Muñoz G., Miranda I. L., Sartori S. K., de Rezende D. C., Nogueira Diaz M. A., Chirality 2019, 31, 776; [DOI] [PubMed] [Google Scholar]
- 14b. Evans D. A., Helmchen G., Rüping M. Chiral Auxiliaries in Asymmetric Synthesis in Asymmetric Synthesis—The Essentials (Eds.: Christmann M., Bräse S.), Wiley-VCH, Weinheim, 2007, pp. 3–9; [Google Scholar]
- 14c. Gnas Y., Glorius F., Synthesis 2006, 1899. [Google Scholar]
- 15.
- 15a. Pitzer L., Schäfers F., Glorius F., Angew. Chem. Int. Ed. 2019, 58, 8572; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 8660; [Google Scholar]
- 15b. Gensch T., Glorius F., Science 2016, 352, 294–295. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Wagener T., Heusler A., Nairoukh Z., Bergander K., Daniliuc C. G., Glorius F., ACS Catal. 2020, 10, 12052; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Nairoukh Z., Wollenburg M., Schlepphorst C., Bergander K., Glorius F., Nat. Chem. 2019, 11, 264; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16c. Zhang X., Ling L., Luo M., Zeng X., Angew. Chem. Int. Ed. 2019, 58, 16785; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 16941; [Google Scholar]
- 16d. Wiesenfeldt M. P., Nairoukh Z., Li W., Glorius F., Science 2017, 357, 908; [DOI] [PubMed] [Google Scholar]
- 16e. Chen M.-W., Ji Y., Wang J., Chen Q.-A., Shi L., Zhou Y.-G., Org. Lett. 2017, 19, 4988; [DOI] [PubMed] [Google Scholar]
- 16f. Guo R.-N., Cai X.-F., Shi L., Ye Z.-S., Chen M.-W., Zhou Y.-G., Chem. Commun. 2013, 49, 8537. [DOI] [PubMed] [Google Scholar]
- 17.Deposition numbers 2043968 (1) and 2043969 (10) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 18.
- 18a. Mattson B., Foster W., Greimann J., Hoette T., Le N., Mirich A., Wankum S., Cabri A., Reichenbacher C., Schwanke E., J. Chem. Educ. 2013, 90, 613; [Google Scholar]
- 18b. Horiuti I., Polanyi M., Trans. Faraday Soc. 1934, 30, 1164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary