Abstract
The present article evaluates the results of the treatment with adjuvant hyperbaric oxygen therapy (HBOT) of patients with nonhealing, chronic wounds. In the period 2013 to 2016, 248 patients were referred from various hospitals because of chronic wounds that were recalcitrant in healing despite standard wound care as described in national and international guidelines. After inclusion, all patients were treated with HBOT and subjected to a weekly standard wound care treatment. During each HBOT session, 100% O2 was administered for 75 minutes under increased pressure of 2.4 ATA. Wounds and quality of life were assessed before and after the total treatment period. A total of 248 patients have been evaluated. Diabetic foot ulcers were present in 134 patients, the remainder (114 patients) showed a variety of wound locations and etiologies. The number of HBOT treatments amounted to an average of 48 (range 20‐68) sessions. Before referral to our clinic, 31% of all wounds had existed for at least 18 months (72 patients). After HBOT, 81% of all wounds were near complete healing or completely healed, in 13% of the cases the wound was stable, and in 2% minor or major amputation had to be carried out. The mean treatment time for wounds pre‐existing fewer than 6 weeks (“early referrals”) was 67 days, and 119 days for wounds pre‐existing more than 18 months (“late referrals”). A majority of the patients in our study referred with nonhealing wounds clinically improved when adjuvant HBOT was added to standard wound care protocols. No differences in success rate were seen between diabetic and nondiabetic wounds. It showed that HBOT is a well‐tolerated treatment.
1. INTRODUCTION
Chronic nonhealing wounds are a major health problem resulting in increasing health care costs. 1 According to Lazarus and colleagues, a chronic wound can be defined as healing failing to proceed through an orderly and timely process to produce anatomic and functional integrity, or to proceed through a repair process without establishing a sustained anatomic and functional result. 2 In the Netherlands, the prevalence of chronic wounds amounts to circa 5.4% for clients in nursing homes and 3.7% in the homecare setting. 3 Wounds frequently become infected, due to great morbidity, and are frequently the first step to lower extremity amputation. 4 Moreover, chronic wounds are usually the cause of a decreased quality of life as a result of pain, insomnia, and immobility. 5
According to Mustoe and collegues, more than 90% of all wounds can be classified in one of the following three categories: diabetic foot ulcers, venous ulcers and pressure ulcers. Despite these different etiologies they identified four common contributing factors that increase the likelihood of wounds becoming chronic: ischemia, older age of patients, ischemia/reperfusion, and bacterial contamination. 6
Based on the above theory, hyperbaric oxygen therapy (HBOT) may have a positive effect on healing of, all wounds, not merely diabetic wounds. 7 , 8 , 9 In addition to the evident hyperoxia, the effect of HBOT can be summarized as follows: reduction of edema, 10 phagocytosis activation, 11 anti‐inflammatory effects, 10 neovascularization, 12 osteoneogenesis, 11 stimulation of collagen formation by fibroblasts, 10 , 11 reduction of ulcer hypoxia, 12 increased erythrocyte deformability, 12 antimicrobial effects, and a significant increase in mobilization of stem cells from the bone marrow. 13 An abstract by Sorice and colleagues concluded that HBOT has an immediate effect on the microcirculation, both on arterial and venous level, and this appears to be cumulative and sustained. 14 Significant angiogenesis could already be measured after eight HBOT sessions. 15 Altogether, these factors accelerate the process of wound healing, irrespective of the origin of the wound. 16 , 17 , 18 HBOT has already proven to be effective in healing diabetic ulcers, 6 late radiation‐induced tissue toxicity, 19 , 20 necrotic soft tissues, 6 chronic therapy refractory osteomyelitis, 6 and ischemia‐reperfusion injury. 21
Patients referred to our center for treatment with hyperbaric oxygen, had been fully examined and treated in the referral hospital. Despite this full treatment regimen, patients showed no healing and therefore we may consider the period during which the patients were treated in the referral hospital as a benchmark or control group. 22 The results of patients with (chronic) wounds treated with HBOT will be evaluated in this article together with an analysis of possible differences in healing rates between patients with and without diabetes.
2. METHODS
All patients with chronic nonhealing wounds referred to our clinic from 2013 to 2016 who underwent a minimum of 20 HBOT sessions were prospectively evaluated. Patients were referred from various specialisms, including vascular surgery, plastic surgery and orthopedic surgery. Prior to referral, a thorough vascular examination had been conducted and if arterial or venous vascular disease was found revascularization was performed, if possible. Patients were only referred if no sufficient healing (a period of at least 3 months taken as the standard) was observed after optimal wound care in accordance with the national guidelines (www.wcs.nl), or if a wound deteriorated dramatically and the principle of stepped care was no longer an option.
No ethical approval or patient consent applied because nonhealing wounds are a treatment indication for hyperbaric oxygen treatment and the questionnaires (EQ‐5D) were distributed as part of the regular treatment evaluation.
The primary study criterion was healing of the wound (100% wound surface healing percentage), assessed at the moment of discharge from our clinic. Patient Related Outcome Measures (PROMs) were also assessed by means of the EQ‐5D questionnaire (www.euroqol.org).
Wounds were classified by the duration of their existence prior to referral to our clinic, in weeks/months (Table 1). In addition, diabetic wounds were classified according to the Texas classification 23 at intake. Finally, due to a lack of an international recognized classification, a self‐composed outcome classification was used. This 6‐point classification (Table 2) consists of the following items: 1: wound is completely healed, 2: wound near complete healing, 3: wound is stable, 4: wound has deteriorated, 5: minor amputation, and 6: major amputation. Amputation was carried out in cases of fast deterioration of the wound at the discretion of the referral vascular surgeon. This makes for a uniform outcome evaluation for diabetic and nondiabetic wounds. The outcome classification category 2 (wound is near complete healing) applies when the following conditions are met: wound surface healing percentage >80%, depth wound maximal 0.5 cm, 100% tissue granulation, no clinical infection, and epithelization of all wound edges. After HBOT treatment, patients soon return to their referring physician for further healing of the wound. The category conditions of the outcome classification were chosen in line with Wicke and colleagues: the reduction in wound surface over time can be used as a prognostic tool for final wound closure. Their study showed a sensitivity of at least 90% when the wound surface showed a >80% decrease within the first 12 weeks of treatment. 24 A study by Sheehan, Cardinal, and collegues also indicated a reduction of final wound healing using early healing rates. 25 , 26
TABLE 1.
Total duration (days) in our clinic per wound category (duration of the wound prior to referral to our clinic)
| Diabetic foot (n) | Non‐DM (n) | Average (n) | |
|---|---|---|---|
| Acute wound, 0‐3 wk | 84 | 43 | 67 |
| Subacute wound, 3‐6 wk | 80 | 72 | 79 |
| Complex wound, 1.5‐3 mo | 118 | 95 | 108 |
| Complicated wound, 3‐18 mo | 108 | 95 | 103 |
| Highly complicated wound, >18 mo | 124 | 115 | 119 |
TABLE 2.
Percentage of positive wound outcomes per wound category (defined as duration of the wound prior to referral to our clinic)
| N | Wound healed/near complete healing | |
|---|---|---|
| Cat 1: Acute wound, 0‐3 wk | 7 | 86% |
| Cat 2: Subacute wound, 3‐6 wk | 22 | 91% |
| Cat 3: Complex wound, 1,5–3 mo | 43 | 91% |
| Cat 4: Complicated wound, 3–18 mo | 100 | 75% |
| Cat 5: Highly complicated wound, >18 mo | 76 (31%) | 79% |
The correlation between the wound evaluation score and mobility, self‐care, activity, pain, and anxiety in the statistical analysis (SPSS, version 26) was calculated by means of the Fisher exact test. Statistical differences in wound evaluation between diabetic and nondiabetic wounds were also calculated. A P‐value less than .05 is considered as statistically significant.
2.1. Hyperbaric oxygen therapy
HBOT treatment consisted of an average of 48 sessions (range 20‐68) (1 session a day/5 days a week) in a multiplace (20‐person) hyperbaric chamber. In total, 75 min of 100% oxygen was administered to patients under increased pressure of 2.4 atmospheres absolute during a 110‐minutes hyperbaric session. At this pressure, 100% oxygen was delivered via an oronasal mask in three episodes of 20 minutes, each interrupted by 5 minutes of air breathing. The fourth period takes 15 minutes and connects into the ascent when patients also get 100% oxygen for the first 7 m. During pressure changes, great care was taken to avoid barotraumas, particularly of the middle ear, which is the most common complication of a hyperbaric treatment. 27 If pressure equalization failed frequently, myringotomy with tympanostomy tubes were placed by the ENT surgeon.
2.2. Quality of life questionnaires
Patients completed the EQ‐5D health status questionnaire. Questionnaires were supplied at the start of the treatment, in the last week of HBOT and at the end of the treatment in the outpatient wound clinic. Patients who did not indicate any complaints at the start of treatment were not analyzed.
3. RESULTS
A total of 248 patients were available for evaluation. Table 3 presents the baseline characteristics of the study population. Table 4 shows the referring specialties; the vast majority of all treated patients were referred by the surgery department. The number included 134 patients with diabetic foot ulcers and 114 nondiabetic patients with a variety of wounds. Overall, at the moment of discharge from our center, 81% of the wounds were healed completely or nearly completely (Figure 1A/B). In 13%, the wound remained stable. A minor or major foot amputation was required in about 2% of all cases (Table 5). Looking at the duration of the treated wounds, 31% existed for over 18 months prior to referral to our center (Table 3). The average total treatment time in the clinic was 107 days, ranging from 67 days for patients with acute wounds to 119 days for highly complicated wounds existing for more than 18 months prior to referral (Table 4). On average, 48 hyperbaric oxygen treatment sessions were administered. The total treatment time was longer than the number of HBOT sessions would presume, because many patients were not able to accomplish the full series of HBOT without an interruption (eg, illness, social obligations, and holidays). Furthermore, wound healing was monitored for a maximum of 4 weeks after finishing HBOT to assure that wound progression was sufficient (near‐complete healing). Only then were the patients sent back to the referring specialist.
TABLE 3.
Patient characteristics (n = 248)
| Total | Diabetic wounds, N | Nondiabetic wounds, N | ||
|---|---|---|---|---|
| Male/female | 153/95 | 99/35 | 54/60 | |
| Mean age (years) | 66 | |||
| Nondiabetic wounds | 114 |
Arterial: 19 Venous: 10 Combined AV: 4 Pressure ulcer: 3 Preoxygenation (before graft transplant): 4 Radiation wounds: 13 Osteomyelitis: 25 Surgical wounds: 16 Traumatic wounds: 14 Other wounds: 16 |
||
| Texas classification | 134 | |||
| 1 | 27 | |||
| 2 | 41 | |||
| 3 | 66 (49%) | |||
| A | 30 | |||
| B | 31 | |||
| C | 34 | |||
| D | 26 | |||
TABLE 4.
Wound patient characteristics (n = 248): referring specialties
| n | % | |
|---|---|---|
| Vascular surgery | 215 | 86.7% |
| Plastic surgery | 9 | 3.6% |
| Orthopedics | 4 | 1.6% |
| Internal medicine/oncology | 2 | 0.8% |
| ENT (ear, nose, throat) | 3 | 1.2% |
| Family practice | 14 | 5.7% |
| Radiation‐oncologist | 1 | 0.4% |
FIGURE 1.
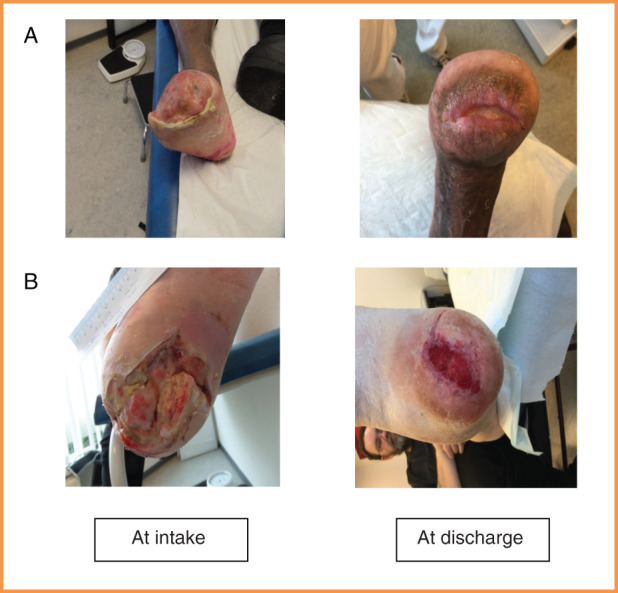
Wound examples from the outcome classification category 2 of the 6‐point classification
TABLE 5.
Wound outcomes 2013 to 2016
| Wound evaluation | DM | Non‐DM | P | |||
|---|---|---|---|---|---|---|
| 1 | Wound healed completely | 84/248 | 33.9% | 31.3% | 36.8% | .52 |
| 2 | Wound condition near complete healing | 116/248 | 46.8% | 52.2% | 40.6% | |
| 3 | Wound is stable (little to no improvement) | 32/248 | 12.9% | 7.5% | 19.3% | |
| 4 | Wound condition deteriorated | 6/248 | 2.4% | 2.2% | 2.6% | |
| 5 | Minor amputation | 6/248 | 2.4% | 3.7% | 0.9% | |
| 6 | Major amputation | 5/248 | 2.0% | 3.0% | 0% |
With respect to the EQ‐5D, 147 to 150 completed questionnaires could be analyzed before and at the end of the wound treatment in the outpatient clinic, with the exception of the patients without complaints at the start of treatment. Mobility, self‐care, activity, pain, and anxiety were stable or improved at least by 1 point (on a scale of 1‐5) in 98%, 92%, 91%, 95%, and 98%, respectively. Statistical testing showed no significance in the correlation of wound evaluation score with mobility, self‐care, pain, and anxiety. There were no statistical variants in wound evaluation between diabetic and nondiabetic wounds.
Side effects of HBOT amounted to 11% in total (incidences out of the total number of treatments administered), mostly concerning nonsevere reversible barotraumas of the ear.
4. DISCUSSION
The present study evaluates the results of the treatment of patients referred for hyperbaric oxygen treatment because of chronic wounds that proved to be resistant to healing for a period longer than 3 months, despite optimal standard wound care in the referral hospital. Among this selection of patients, an improvement in wound healing after HBOT was observed for 81% of all wounds that resulted in nearly complete or fully completed healing. Given the fact that there is scarcely any literature on the effects of HBOT on nondiabetic wounds, the presented results have at least shown similar positive effect of HBOT compared to diabetic wounds in the treatment results. This is promising, as we have hypothesized, based on the contributing factors for wound healing discussed by Mustoe and collegues discussed. 6 In the literature there is still controversial evidence of hyperbaric medicine in woundcare. 28 , 29 , 30 , 31 , 32 , 33
The present study did not make use of a placebo group, and it may be argued that observed changes were due to factors other than hyperbaric oxygen treatment, which is a limitation of the current study. However, the inclusion criteria were chronic wounds that proved to be resistant to healing for a period longer than 3 months despite optimal standard wound care, and as such this functions as a benchmark or control group.
A randomized clinical trial at Lund university examined 94 patients with wounds that pre‐existed for at least 3 months. Complete healing of the index ulcer in 37 patients at 1‐year follow‐up amounted to 52% in the hyperbaric oxygen treatment group and to 29% in the placebo group (P = .03). 34 We have obtained 81% of the wounds being near complete healing or completely healed.
Impaired wound healing in the diabetic foot is multifactorial. HBOT accelerates wound healing, ultimately leading to a higher quality of life, 35 , 36 , 37 which also bears out in the EQ‐5D results presented, even though it lacks significance. Improved healing and reduced amputation rates were the most common outcomes observed in the review by Bishop and Mudge, 38 including studies by Duzgun 39 and Kessler. 40 Up to 12% of all diabetic foot ulcers may lead to amputation. 25 The present study reports a total of 4% amputations (both minor and major). HBOT has been associated with a significant risk reduction of above‐the‐ankle amputations. 39 , 41 Faglia et al concluded from their application of a multidisciplinary therapeutic protocol including HBOT that it is effective in decreasing major amputations in diabetic patients with severe ischemic foot ulcers. 41 The present research added dedicated wound care consisting of frequent wound debridement, using adequate wound dressings and anticipating an improvement in wound healing. Recently, a promising animal study was published by André‐Lévigne in which the effects of HBOT were examined in four different wound conditions. 42 Forty‐four animals received HBOT for five times a week until complete wound closure, compared with 44 rats as a control group receiving standard dressings only. The researchers found an increased blood flow, accelerated wound closure, wound contraction, and re‐epithelialization. Besides, the study showed a significant increase in collagen deposition in ischemic wounds and in hyperglycemic due to early application of HBOT. The authors stated that this early application of HBOT might be crucial to its efficacy. Our results confirm this hypothesis, because wounds existing up to 3 months showed increased healing compared to wounds existing for more than 3 months. Included were wounds existing up to 3 months, when they were in such a deteriorated condition that the principle of stepped care does not apply and the treating doctor had referred these patients to our clinic. Nevertheless, there is still a positive effect to be achieved with these patients. With patients with wounds existing for more than 3 months, the wound healed or was near complete healing in 75% to 79% in this study.
Hammarlund et al evaluated the effect of hyperbaric oxygen in a randomized double‐blind study of 16 nondiabetic, chronic leg ulcers with no large vessel disease. They found a significant decrease in the wound area. 43 Another randomized double‐blind study by Thistlethwaite et al studied hyperbaric oxygen for nonhealing venous leg ulcers. The researchers found a significant difference in ulcer area reduction after 12 weeks compared to a placebo group, 95% versus 54%. 44 The results are compliant with the results we presented in nondiabetic wounds, although up to this date hardly any literature is available on the effects of HBOT on nondiabetic wounds.
Side effects of HBOT were comparable to results published by Plafki et al, who reported an incidence of 17% for ear pain or discomfort, while oxygen toxicity of the central nervous system affected 4 patients out of 782 patients during the 11 376 hyperbaric oxygen sessions evaluated. 45 Most of the side‐effects are minor and self‐limiting.
Future research should examine the criteria of wounds who potentially benefits the most from HBOT and determine the optimal number of HBO treatments for chronic nonhealing wounds.
5. CONCLUSIONS
The present study has shown a positive effect on wound healing in a mixed population of diabetic and nondiabetic wounds with hyperbaric oxygen: 81% of all patients referred showed that the wound was healed/or was near complete healing. First, in all patients, no difference was seen between diabetic and nondiabetic wounds, irrespective of the causative mechanism. Second, even highly complicated wounds that had existed for more than 18 months showed accelerated wound healing (79%). Last, HBOT may decrease the need for minor or major amputations in the case of diabetic foot ulcers in particular. Further research is needed to distinguish which type of wounds benefits most from HBOT and to determine the optimum number of treatments.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
The authors thank Ida Akkerman, Rosalinde Arnoldus, Stephanie van der Does, Marco Huigen, Patrick Just, Pieter‐Jan van Ooij, Marieke Schouten, Saskia Stolk, Shirley van den Toorn, Stephanie Troquay, Dirk Vellinga, and Kirsten Welbers, for their helpful contribution to the logistics, data collection, statistics, and/or editing of the manuscript. Open access funding enabled and organized by Projekt DEAL.
Teguh DN, Bol Raap R, Koole A, et al. Hyperbaric oxygen therapy for nonhealing wounds: Treatment results of a single center. Wound Rep Reg. 2021;29:254–260. 10.1111/wrr.12884
REFERENCES
- 1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2(3):165‐170. [DOI] [PubMed] [Google Scholar]
- 3. Halfens RJG, Meesterberends E, Neyens JCL, Rondas AALM, Rijcken S, Wolters S, Schols JMGA. Landelijke Prevalentiemeting Zorgproblemen: rapportage resultaten 2015. Maastricht: Universiteit Maastricht; CAPHRI. 2015. [Google Scholar]
- 4. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217‐228. [DOI] [PubMed] [Google Scholar]
- 5. Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health Qual Life Outcomes. 2007;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117(7 Suppl:35S‐41S. [DOI] [PubMed] [Google Scholar]
- 7. Lalieu RC, Brouwer RJ, Ubbink DT, Hoencamp R, Bol Raap R, van Hulst RA. Hyperbaric oxygen therapy for nonischemic diabetic ulcers: a systematic review. Wound Repair Regen. 2020;28(2):266‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahman N, Mohamad W, Bajuri M, Shafee R, Flemming J. Use of hyperbaric oxygen therapy (HBOT) in chronic diabetic wound—a randomised trial. Med J Malaysia. 2019;74(5):418‐424. [PubMed] [Google Scholar]
- 9. Bajuri M. The physiological, biochemical and quality of life changes in chronic diabetic foot ulcer after hyperbaric oxygen therapy. Med Health. 2017;12(2):210‐219. [Google Scholar]
- 10. Feldmeier J, Carl U, Hartmann K, Sminia P. Hyperbaric oxygen: does it promote growth or recurrence of malignancy? Undersea Hyperb Med. 2003;30(1):1‐18. [PubMed] [Google Scholar]
- 11. Roberts GP, Qu B, Harding KG. The effects of hyperbaric oxygen on cultured fibroblasts. J Wound Care. 1994;3(4):189‐193. [DOI] [PubMed] [Google Scholar]
- 12. Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 1990;160(5):519‐524. [DOI] [PubMed] [Google Scholar]
- 13. Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290(4):H1378‐H1386. [DOI] [PubMed] [Google Scholar]
- 14. Sorice SC, Lundh T, Gurtner GC, et al. Hyperbaric oxygen acutely increases wound circulation as assessed by fluorescent angiography. J Vasc Surg. 2016;63(6):100s‐101s. [Google Scholar]
- 15. Marx RE, Johnson RP, Kline SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. J Am Dent Assoc. 1985;111(1):49‐54. [DOI] [PubMed] [Google Scholar]
- 16. Mayer R, Hamilton‐Farrell MR, van der Kleij AJ, et al. Hyperbaric oxygen and radiotherapy. Strahlenther Onkol. 2005;181(2):113‐123. [DOI] [PubMed] [Google Scholar]
- 17. Pasquier D, Hoelscher T, Schmutz J, et al. Hyperbaric oxygen therapy in the treatment of radio‐induced lesions in normal tissues: a literature review. Radiother Oncol. 2004;72(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 18. van Merkesteyn R, Bakker DJ. Comment on "Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo‐controlled, double‐blind trial from the ORN96 Study Group". J Clin Oncol. 2005;23(19):4465‐4466. author reply 4466–4468. [DOI] [PubMed] [Google Scholar]
- 19. Teguh DN, Bol Raap R, Struikmans H, et al. Hyperbaric oxygen therapy for late radiation‐induced tissue toxicity: prospectively patient‐reported outcome measures in breast cancer patients. Radiat Oncol. 2016;11(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennett MH, Feldmeier J, Hampson NB, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016;4:CD005005. [DOI] [PubMed] [Google Scholar]
- 21. Buras J. Basic mechanisms of hyperbaric oxygen in the treatment of ischemia‐reperfusion injury. Int Anesthesiol Clin. 2000;38(1):91‐109. [DOI] [PubMed] [Google Scholar]
- 22. Sadler GM, Wallace HJ, Stacey MC. Oral doxycycline for the treatment of chronic leg ulceration. Arch Dermatol Res. 2012;304(6):487‐493. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong DG, Lavery LA, Harkless LB. Treatment‐based classification system for assessment and care of diabetic feet. J Am Podiatr Med Assoc. 1996;86(7):311‐316. [DOI] [PubMed] [Google Scholar]
- 24. Wicke C, Bachinger A, Coerper S, Beckert S, Witte MB, Konigsrainer A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized Wound Care Center. Wound Repair Regen. 2009;17(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 25. Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008;16(1):19‐22. [DOI] [PubMed] [Google Scholar]
- 26. Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Plast Reconstr Surg. 2006;117(7 Suppl):239S‐244S. [DOI] [PubMed] [Google Scholar]
- 27. Tahir AR, Westhuyzen J, Dass J, et al. Hyperbaric oxygen therapy for chronic radiation‐induced tissue injuries: Australasia's largest study. Asia Pac J Clin Oncol. 2015;11(1):68‐77. [DOI] [PubMed] [Google Scholar]
- 28. Fedorko L, Bowen JM, Jones W, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double‐blind, randomized controlled clinical trial. Diabetes Care. 2016;39(3):392‐399. [DOI] [PubMed] [Google Scholar]
- 29. Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care. 2013;36(7):1961‐1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santema KTB, Stoekenbroek RM, Koelemay MJW, et al. Hyperbaric oxygen therapy in the treatment of ischemic lower‐ extremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial. Diabetes Care. 2018;41(1):112‐119. [DOI] [PubMed] [Google Scholar]
- 31. Huang ET, Mansouri J, Murad MH, et al. A clinical practice guideline for the use of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers undersea & hyperbaric medicine. J Undersea Hyperbar Med Soc. 2015;42(3):205‐247. [PubMed] [Google Scholar]
- 32. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American podiatric medical association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2 Suppl):3S‐21S. [DOI] [PubMed] [Google Scholar]
- 33. Londahl M, Boulton AJM. Hyperbaric oxygen therapy in diabetic foot ulceration: useless or useful? A battle. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3233. [DOI] [PubMed] [Google Scholar]
- 34. Londahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33(5):998‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double‐blind randomised‐controlled trial. Eur J Vasc Endovasc Surg. 2003;25(6):513‐518. [DOI] [PubMed] [Google Scholar]
- 36. Londahl M, Landin‐Olsson M, Katzman P. Hyperbaric oxygen therapy improves health‐related quality of life in patients with diabetes and chronic foot ulcer. Diabet Med. 2011;28(2):186‐190. [DOI] [PubMed] [Google Scholar]
- 37. Londahl M. Hyperbaric oxygen therapy as adjunctive treatment of diabetic foot ulcers. Med Clin North Am. 2013;97(5):957‐980. [DOI] [PubMed] [Google Scholar]
- 38. Bishop AJ, Mudge E. Diabetic foot ulcers treated with hyperbaric oxygen therapy: a review of the literature. Int Wound J. 2014;11(1):28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duzgun AP, Satir HZ, Ozozan O, Saylam B, Kulah B, Coskun F. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J Foot Ankle Surg. 2008;47(6):515‐519. [DOI] [PubMed] [Google Scholar]
- 40. Kessler L, Bilbault P, Ortega F, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26(8):2378‐2382. [DOI] [PubMed] [Google Scholar]
- 41. Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19(12):1338‐1343. [DOI] [PubMed] [Google Scholar]
- 42. Andre‐Levigne D, Modarressi A, Pignel R, Bochaton‐Piallat ML, Pittet‐Cuenod B. Hyperbaric oxygen therapy promotes wound repair in ischemic and hyperglycemic conditions, increasing tissue perfusion and collagen deposition. Wound Repair Regen. 2016;24:954‐965. [DOI] [PubMed] [Google Scholar]
- 43. Hammarlund C, Sundberg T. Hyperbaric oxygen reduced size of chronic leg ulcers: a randomized double‐blind study. Plast Reconstr Surg. 1994;93(4):829‐833; discussion 834. [PubMed] [Google Scholar]
- 44. Thistlethwaite KR, Finlayson KJ, Cooper PD, et al. The effectiveness of hyperbaric oxygen therapy for healing chronic venous leg ulcers: a randomized, double‐blind, placebo‐controlled trial. Wound Repair Regen. 2018;26(4):324‐331. [DOI] [PubMed] [Google Scholar]
- 45. Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med. 2000;71(2):119‐124. [PubMed] [Google Scholar]


