Abstract
Background
Systematic reviews have established the short‐term improvements of periodontal regenerative/reconstructive procedures compared to conventional surgical treatment in intrabony defects. However, a hierarchy of periodontal regenerative/reconstructive procedures regarding the medium‐ to long‐term results of treatment does not exist.
Aim
To systematically assess the literature to answer the focused question “In periodontitis patients with intrabony defects, what are the medium‐ and long‐term benefits of periodontal regenerative/reconstructive procedures compared with open flap debridement (OFD), in terms of clinical and/or radiographic outcome parameters and tooth retention?”.
Material & Methods
Randomized controlled clinical trials (RCTs), reporting on clinical and/or radiographic outcome parameters of periodontal regenerative/reconstructive procedures ≥3 years post‐operatively, were systematically assessed. Clinical [residual probing pocket depth (PD) and clinical attachment level (CAL) gain, tooth loss] and radiographic [residual defect depth (RDD), bone gain (RBL)] outcome parameters were assessed. Descriptive statistics were calculated, and Bayesian random‐effects network meta‐analyses (NMA) were performed where possible.
Results
Thirty RCTs, presenting data 3 to 20 years after treatment with grafting, GTR, EMD, as monotherapies, combinations thereof, and/or adjunctive use of blood‐derived growth factor constructs or with OFD only, were included. NMA based on 21 RCTs showed that OFD was clearly the least efficacious treatment; regenerative/reconstructive treatments resulted in significantly shallower residual PD in 4 out 8 comparisons [range of mean differences (MD): −2.37 to −0.60 mm] and larger CAL gain in 6 out 8 comparisons (range of MD: 1.26 to 2.66 mm), and combination approaches appeared as the most efficacious. Tooth loss after regenerative/reconstructive treatment was less frequent (0.4%) compared to OFD (2.8%), but the evidence was sparse. There were only sparse radiographic data not allowing any relevant comparisons.
Conclusion
Periodontal regenerative/reconstructive therapy in intrabony defects results, in general, in shallower residual PD and larger CAL gain compared with OFD, translating in high rates of tooth survival, on a medium (3–5 years) to long‐term basis (5–20 years). Combination approaches appear, in general, more efficacious compared to monotherapy in terms of shallower residual PD and larger CAL gain. A clear hierarchy could, however, not be established due to limited evidence.
Keywords: bone grafts, bone substitutes, EMD, enamel matrix proteins, GTR, long‐term, periodontal regeneration, systematic review
Clinical relevance.
Scientific rationale for the study: To systematically assess the literature on medium‐ to long‐term outcomes of periodontal regenerative/reconstructive procedures in intrabony defects and to provide a hierarchy of the procedures regarding various clinical and radiographic outcome parameters, by estimating their relative effectiveness on the basis of all possible comparisons among the procedures.
Principal findings: Periodontal regenerative/reconstructive therapy in intrabony defects results in better clinical (i.e. shallower residual probing depth and larger clinical attachment level gain) compared with OFD on a medium‐ to long‐term basis. Combination approaches (i.e. GTR + grafting, EMD + grafting) appeared, in general, as more effective compared to monotherapy but the evidence was overall weak, and thus, a clear hierarchy could not be established. Tooth loss after regenerative/reconstructive treatment was rare.
Practical implications: Periodontal regenerative/reconstructive therapy, especially combination approaches, is recommended for the treatment of intrabony defects, after critical assessment of the cost–benefit of treatment in the context of the overall treatment plan.
1. INTRODUCTION
Non‐surgical and conventional surgical periodontal therapy – including various types of access flaps and/or resective techniques – usually results in healthy periodontal tissues, with reduced probing pocket depths (PD) and gain in clinical attachment level (CAL) compared with pretreatment levels, for most patients and sites. Observations in animal and human histological studies (Caton & Zander, 1979; Caton et al., 1980; Wilson et al., 2008) have shown that healing after conventional periodontal therapy is predominantly characterized by repair, that is, a long epithelial attachment is formed along the major portion of the previously exposed and instrumented root surface, while in some instances limited amounts of periodontal regeneration may be observed at the apical aspects of the defects. In this context, periodontal regeneration implies that CAL gain is achieved through new cementum (NC) with functionally oriented inserting collagen fibres formed on the previously exposed/affected portion of the root, paralleled with alveolar bone (AB) formation and the establishment of a periodontal ligament (PDL) of physiologic width and composition; reformation of only a part of the periodontium (e.g. NC and PDL) is coined reconstruction.
Residual (deep) PD do, however, persist following non‐surgical and/or conventional surgical periodontal therapy, especially in sites/teeth harbouring deep intrabony defects and/or deep furcation involvements; resective surgical techniques can successfully eliminate deep defects, but are associated with undesirable substantial loss of attachment and soft tissue recession (Badersten et al., 1990; Claffey et al., 1990; Kaldahl et al., 1996). Various treatment protocols have been used during the years, aiming to enhance clinical treatment outcomes and to avoid the above‐mentioned shortcomings, but also with the intention to enhance periodontal regeneration. Despite the variability in the results observed after periodontal regenerative/reconstructive procedures, the clinical and/or histological outcomes obtained after such approaches have been in general significantly better compared to conventional surgical approaches (for review see: Kao et al., 2015; Sculean et al., 2015). In this context, considering the fact that the clinical conditions obtained after conventional periodontal therapy, including surgery, can be preserved for decades – provided the patient is maintaining adequate oral hygiene standards (Axelsson et al., 2004; Matuliene et al., 2010) – it is relevant that the improved clinical outcomes obtained after regenerative/reconstructive treatment can also be maintained long term. Several publications report on the outcomes of various regenerative/reconstructive periodontal procedures several years post‐operatively; however, a hierarchical assessment of the outcome of periodontal regenerative/reconstructive therapy on the medium (3–5 years) and long term (>5 years) is currently missing in the literature.
Thus, the aim of the current review was to a) systematically assess the literature to answer the focused question: “In periodontitis patients with deep intrabony defects, what is the medium‐ to long‐term outcome of periodontal regenerative/reconstructive procedures compared with open flap debridement (OFD) in terms of clinical and/or radiographic outcome parameters and tooth retention?” and b) to identify a hierarchy among the tested periodontal regenerative/reconstructive procedures regarding various clinical and radiographic outcome parameters.
2. MATERIALS AND METHODS
2.1. Information on the protocol, type of studies, participants
The present systematic review was performed according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA; Appendix S1) (Liberati et al., 2009; Moher et al., 2009). For details on the search process, data collection and extraction, and on the analysis, see Appendix S2.
During literature search for original studies, the following inclusion criteria were applied: (a) English or German language; (b) randomized controlled clinical trials (RCTs) on regenerative/reconstructive periodontal treatment; (c) ≥10 patients; (d) average follow‐up ≥36 months, but with minimum follow‐up ≥24 months; (f) reporting clearly or possible to calculate number of treated teeth/defects and clinical and/or radiographic treatment effect size; and (g) full text available. Studies were excluded if not meeting all inclusion criteria; or reporting on treatment of furcation defects, endodontic‐periodontal lesions, or peri‐implant defects.
2.2. Outcome measures
From the included RCTs, the following data (if available) were extracted and/or calculated: residual PD; CAL gain; PD reduction; gingival recession (REC) increase; residual radiographic defect depth (RDD); RDD reduction; radiographic bone level (RBL) gain; the sample size; the standard deviations of measures; and the observation period.
2.3. Search methods for identification of studies
Electronic search included MEDLINE (PubMed), EMBASE (Ovid) and CENTRAL (Ovid) (last search 17 April 2020; no date restriction used) and was complemented with manual search.
2.4. Data collection and extraction
Two authors (KB, AS) independently checked title, abstract and finally full text on the pre‐defined eligibility criteria. Abstracts with unclear methodology were included in full‐text assessment to avoid exclusion of potentially relevant articles. One author (KB) repeated the literature search. In case of ambiguity, consensus through discussion was achieved.
2.5. Assessment of risk of bias (RoB) of included studies
Two authors (AS, KB) independently evaluated RoB of the included studies applying the Cochrane Collaboration‘s Tool for assessing RoB (Higgins et al., 2011). For details, see Appendix S3.
2.6. Data synthesis
For details, see Appendix S2. Briefly, 3 primary outcome parameters (residual PD, CAL gain and tooth retention) and two secondary outcome parameters (residual RDD, RBL gain) were defined. Outcome parameters were often calculated, for example residual PD by subtracting PD reduction from baseline PD, CAL gain by subtracting CAL after treatment from baseline CAL, standard deviation from standard error of the mean, etc. Studies were arranged, mainly for reasons of clarity, in those reporting on medium‐ (3–5 years) and long‐term (> 5 years) outcomes.
Bayesian random‐effects pairwise meta‐analyses were initially conducted to account for the between‐study variance in the treatment effects across at least 2 studies that compared the same interventions (DerSimonian & Laird, 1986). The relative treatment effects of the compared interventions were estimated using the unstandardized mean difference (MD) assuming dissimilar population standard deviations (Borenstein et al., 2009).
To infer on the relative effectiveness of various interventions, a random‐effects network meta‐analysis (NMA) using Bayesian approaches was applied assuming the same between‐study variance for all comparisons and considering OFD as the reference treatment. For each intervention, the relative and cumulative ranking probabilities were estimated and illustrated using rankograms and surface under the cumulative ranking (SUCRA) plots, respectively (Salanti et al., 2011). In addition, using the SUCRA values, the interventions were ranked from the most effective (the largest SUCRA) to the least effective (the smallest SUCRA). Possible inconsistency was assessed locally with the node‐splitting approach and globally with the unrelated mean effects model together with the posterior deviance and the deviance information criterion (DIC). Further, possible sources of heterogeneity and/or inconsistency were investigated using hierarchical meta‐regression analysis for observation period (3–5 years versus >5 years), the year of publication, small‐study size, and RoB, and the variance of study‐specific intervention effects. Possible small‐study effects were also explored using the comparison‐adjusted funnel plot after ordering the interventions according to their SUCRA value.
The STATA routines were used to create the network plots, rankograms, SUCRA plots and the comparison‐specific funnel plots (Chaimani et al., 2013). All NMA models, as developed by Dias et al. (2013), were performed using the R package R2WinBUGS (Sturtz et al., 2005).
3. RESULTS
3.1. Study selection
The flow chart of the literature search is presented in Appendix S4. Out of 10461 identified studies, 110 full texts were reviewed; finally, 30 publications from moderate (3–5 years; 19 studies) and long‐term (> 5 years; 11 studies) RCTs were included. For list of excluded studies, and reason for exclusion, see Appendix S5.
3.2. ROB assessment
Among the included studies, only one publication was assessed as of low RoB, while 9 and 20 publications were of high and unclear RoB, respectively. For details, see Appendix S6.
3.3. Study characteristics
Table 1 presents the characteristics of all identified publications. Six main regenerative/reconstructive approaches were included the following: (a) grafting, (b) guided tissue regeneration (GTR), (c) enamel matrix derivatives (EMD), (d) GTR + grafting, (e) EMD + grafting and (f) various combinations, including those using different types of blood‐derived growth factor constructs (BC). GTR and EMD, as monotherapies, were the treatments most often used (i.e. 14 and 9 different groups each, respectively); mostly resorbable membranes were used for GTR (19 groups vs. 5 groups with non‐resorbable membranes). Further, alloplasts and xenografts (11 groups and 8 groups, respectively) were the most often used grafting materials. BC was used in 5 groups combined with GTR, EMD, and/or bone grafts.
Table 1.
Characteristics of the included studies arranged to those reporting on medium‐term (3–5 years) and long‐term (> 5 years) outcomes.
| Study | Study design |
a: No. of patients (BL/FE) b: No. of sites (BL/FE) |
a: Diagnosis b: Age range (mean) c: m/f d: Systemic condition e: No. of smokers/former smokers |
Intervention Group I Group II Group III Group IV |
a: Minimum defect depth b: No. of defect walls (n) c: Tooth type (n) d: FI |
Observation period (a) |
|---|---|---|---|---|---|---|
| 3–5 years | ||||||
| Bhutda & Deo, 2013 | RCT, SM |
a: 15/15 b: 30/30 |
a: chronic P b: 37–45 (41) c: NR d: healthy e: 0/0 |
I: OFD II: EMD |
a: CAL ≥ 6 mm b: I: 2 (4), 2–3 (3), 3 (8) walls II: 2(5), 2–3 (2), 3 (8) walls c: I: 15 mand. (3 PM, 12 M) II: 15 mand. (3 PM, 12 M) d: NR |
5 |
| Cetinkaya et al., 2014 | RCT, SM |
a: 15/11 b: 30/22 |
a: chronic P @FE b: 29–51 (39) c: 5/6 d: healthy e. 0/0 |
I: GTR (resorbable; Atrisorb) + platelet pellet II: GTR (resorbable; Atrisorb) + bioactive glass (PerioGlas) |
a: NR b: I: 2 (8), 3 (3) walls II: 2 (7), 3 (4) walls c: I: 6 max., 5 mand. (6 PM, 5 M) II: 6 max., 5 mand. (4 PM, 7 M) d: NR |
5 |
| Crea et al., 2008 | RCT, PG |
a: 40/39 b: 40/39 |
a: advanced chronic P @FE b: 35–66 (46) c: 18/21 d: healthy e: 0/0 |
I: GTR (non‐resorbable; Gore‐Tex) II: EMD |
a: intra (clinical) ≥ 4 mm, intra (radiographic) ≥ 3 mm b: 3 walls c: I: 10 posterior max., 7 anterior max., 3 posterior mand. II: 5 posterior max., 11 anterior max., 3 posterior mand. d: Excluded |
3 |
| Döri, Arweiler, Húszár et al., 2013 | RCT, PG |
a: 26/24 b: 26/24 |
a: generalized severe chronic P @FE b: 32–56 (NR) c: 11/13 d: healthy e. 0/0 |
I: EMD + NBM (BioOss) II: EMD + NBM (BioOss) + PRP |
a: PD ≥ 6 mm, intra ≥ 4 mm b: I: 1–2 (7), 2 (5) walls II: 1–2 (6), 2 (6) walls c: I: 7 max., 5 mand. (5 I/C, 4 PM, 3 M) II: 8 max., 4 mand. (6 I/C, 4 PM, 2 M) d: NR |
5 |
| Eickholz et al., 2004 b | RCT, SM |
a: 15/13 b: 30/26 |
a: moderate‐severe P. (6 aggressive, 9 chronic P) @BL b: 22–64 (42) c: 3/12 d: NR e: 3/1 |
I: GTR (resorbable; Guidor) II: GTR (resorbable; Mempol) |
a: NR b: 2 (10), 3 (16) walls c: 10 max. (6 PM, 4 M), 16 mand. (4 C, 2 PM, 10 M) d: NR |
5 |
| Flemmig et al., 1998 | RCT, SM |
a: 14/8 b: 28/16 |
a: NR @FE b: NR (47) c: 4/4 d: NR e: 1/0 |
I: OFD II: autolysed, antigen‐extracted, allogenic bone + neomycin sulphate + bacitracin |
a: CAL ≥ 6 mm b: I: 1 (1), 2 (2), 3 (5) walls II: 1 (3), 2 (1), 3 (4) walls c: NR d: NR |
3 |
| Gorski et al., 2020 |
RCT, SM |
a: 15/14 b: 30/27 |
a: AgP @BL b: 22–49 (38) c:5/10 d: healthy e: NR |
I: GTR (resorbable; BioGide) + NBM (BioOss) II: GTR (resorbable; BioGide) + NBM (BioOss) |
a: PD ≥ 6 mm, intra ≥ 3 mm b: I: 1 (2), 2 (4), 3 (9) walls II: 1 (3), 2 (4), 3 (8) walls c: I: 5 max., 10 mand. (4 I/C, 4 PM, 7 M) II: 7 max., 8 mand. (5 I/C, 3 PM, 7 M) d: excluded |
4 |
| Heijl et al., 1997 | RCT, SM |
a: 33/26 b: 68/54 |
a: NR @BL b: 33–68 (48) c: 7/26 d: regular medication intake (11) e: 16/13 |
I: OFD II: EMD |
a: PD ≥ 6 mm, intra ≥ 4 mm, width ≥ 2 mm b: I: 1 (11), 2 (23) walls II: 1 (17), 2 (17) walls c: NR d: NR |
3 |
| Hoffmann et al., 2015 | RCT, PG |
a: 73/30 b: 73/30 |
a: severe P @FE b: NR (47) c: 13/17 d: healthy e: 4/1 |
I: EMD II: EMD + biphasic calcium phosphate (BoneCeramic) |
a: intra ≥ 4 mm, width ≥ 2 mm b: I: 1 (2), 1–2 (6), 2 (2), circumferential (5) II: 1 (2), 1–2 (5), 2 (3), circumferential (5) c: NR d: excluded |
3 |
| Kim et al., 2002 b | RCT, SM |
a: 12/8 b: 24/16 |
a: advanced chronic P @FE b: 32–62 (46) c: 3/9 (@BL) d: diabetes (2) e: 3/0 |
I: GTR (non‐resorbable; Gore‐Tex) II: GTR (resorbable, polyglactin; Vicryl) |
a: NR b: NR c: I: 3 max., 5 mand. (1 C, 4 PM, 3 M) II: 2 max., 6 mand. (1 I, 2 C, 3 PM, 2 M) d: NR |
5 |
| Menezes & Rao, 2012 | RCT, SM |
a: 60/60 b: 120/120 |
a: chronic P b: NR (38) c: 30/30 d: healthy e: 0/0 |
I: HA (Periobone G) + saline II: HA (Periobone G) + PRP |
a: PD ≥ 6 mm, intra ≥ 3 mm b: I: 2–3 (10), 3 (50) walls II: 2–3 (13), 3 (47) walls c: I: 32 max. M, 28 mand. M II: 32 max. M, 28 mand. M d: FI not connected to the interproximal defect |
4 |
| Mengel et al., 2006 | (R)CT, PG |
a: 16/16 b: 42/42 |
a: generalized aggressive P b: 32–62 (45) c: 5/11 d: healthy e: 0/0 |
I: GTR (resorbable; Resolut) II: bioactive glass (PerioGlas) |
a: PD ≥ 7 mm, intra ≥ 4 mm b: 1–3 walls c: 9 I/C, 9 PM, 24 M d: excluded |
5 |
| Nevins et al., 2013 | RCT, PG |
a: 180/83 b: 180/83 |
a: NR b: 25–75 (NR) c: NR d: NR e: NR |
I: ß‐TCP + buffer II: ß‐TCP + rhPDGF‐BB (0.3 mg/ml) III: ß‐TCP + rhPDGF‐BB (1.0 mg/ml) |
a: PD ≥ 7 mm, intra ≥ 4 mm b: 1–3 walls c: NR d: NR |
3 |
| Ogihara & Tarnow, 2014 | RCT, PG |
a: 69/67 b: 69/67 |
a: chronic P @BL b: NR (53–56) c: 14/55 d: healthy e: 0/0 |
I: EMD II: EMD + FDBA + minocycline III: EMD + DFDBA + minocycline |
a: CAL ≥ 6 mm b: NR c: I: 12 max., 11 mand. (1 I, 1 C, 1 PM, 20 M) II: 10 max., 13 mand. (1 I, 1 C, 1 PM, 20 M) III: 11 max., 12 mand. (2 PM, 21 M) d: NR |
3 |
| Pietruska et al., 2012 | RCT, PG |
a: 24/24 b: 24/24 |
a: advanced chronic P b: 34–62 (NR) c: 10/14 d: healthy e: 0/0 |
I: EMD II: EMD + biphasic calcium phosphate (BoneCeramic) |
a: PD ≥ 6 mm, intra ≥ 4 mm b: I: 1–2 (1), 2 (8), 3 (3) walls II: 1–2 (1), 2 (9), 3 (2) walls c: I: 5 max., 7 mand. (5 I/C, 5 PM, 2 M) II: 6 max., 6 mand. (6 I/C, 4 PM, 2 M) d: NR |
4 |
| Sculean et al., 2001 b | (R)CT, SM |
a: 16/12 b: 32/24 |
a: NR @FE b: 37–55 (45) c: 6/6 d: healthy e: NR |
I: GTR (resorbable; Resolut) II: EMD |
a: PD ≥ 6 mm b: I: 1 (1), 2 (8), 3 (3) walls II: 1 (1), 2 (9), 3 (2) walls c: NR d: NR |
4 |
| Sculean et al., 2004 b | RCT, PG |
a: 56/42 b: 56/42 |
a: NR @FE b: NR (47) c: NR d: healthy e: NR |
I: OFD II: EMD III: GTR (resorbable; Resolut) IV: EMD + GTR (resorbable; Resolut) |
a: PD ≥ 6 mm, intra ≥ 3 mm b: I: 1–2 (3), 2 (5), 3 (2) walls II: 1–2 (3), 2 (7), 3 (1) walls III: 1–2 (3), 2 (6), 3 (2) walls IV: 1–2 (2), 2 (7), 3 (1) walls c: NR d: NR |
5 |
| Sculean, Pietruska, et al., 2007 | RCT, PG |
a: 30/25 b: 30/25 |
a: advanced chronic P @FE b: 38–55 (46) c: 11/14 d: healthy e: NR |
I: EMD II: EMD + bioactive glass (Emdogain Gel TS®) |
a: PD ≥ 6 mm, intra ≥ 3 mm b: I: 1–2 (7), 2 (5), 3 (1) walls II: 1–2 (6), 2 (5), 3 (1) walls c: NR d: NR |
4 |
| Sculean, Schwarz, et al., 2007 | RCT, PG |
a: 28/19 b: 28/19 |
a: advanced chronic P @BL b: NR (45) c: 9/10 d: healthy e: 0/0 |
I: OFD II: GTR (resorbable; BioGide) + NBM (BioOss) |
a: PD ≥ 6 mm, intra ≥ 3 mm b: I: 1–2 (1), 2 (7), 3 (1) walls II: 1–2 (2), 2 (7), 3 (1) walls c: NR d: NR |
5 |
| >5 years | ||||||
| Cortellini et al., 2017 | RCT, PG |
a: 45/41 b: 45/41 |
a: NR @BE b: 25–61 (43) c: 21/24 d: healthy e: 6 |
I: OFG II: GTR III: GTRt |
a: NR b: 1–3 walls c: 36 max., 9 mand., (17 I, 13 C, 7 PM, 8 M) d: excluded |
20 |
| Döri, Arweiler, Szántó, et al., 2013, b | RCT, PG |
a: 24/22 b: 24/22 |
a: generalized advanced chronic P @FE b: 34–67 (NR) c: 7/15 d: healthy e: 2/0 |
I: EMD + NBM (BioOss) II: EMD + ß‐TCP (Cerasorb) |
a: PD ≥ 6 mm, intra ≥ 4 mm b: I: 1–2 (1), 2 (8), 3 (2) walls II: 1–2 (1), 2 (7), 3 (3) walls c: I: 5 max., 6 mand. (5 I/C, 4 PM, 2 M) II: 4 max., 7 mand. (5 I/C, 5 PM, 1 M) d: NR |
10 |
| Nickles et al., 2009 a | (R)CT, SM, (PG) |
a: 10/10 b: 20/20 |
a: severe chronic P b: 41–73 (NR) c: NR d: NR e: 2/4 |
I: OFD II: GTR (resorbable; Guidor) |
a: PD ≥ 5 mm b: 2, 3 walls c: 6 max. (2 I/C, 4 PM), 14 mand. (2 I/C, 4 PM, 8 M) d: NR |
10 |
| Nygaard‐Østby et al., 2010 | RCT, PG |
a: 40/26 b: 40/26 |
a: chronic P @BL b: 42–67 (53) c: 20/20 d: healthy e: 0/0 |
I: autogenous bone II: GTR (resorbable; Atrisorb) + autogenous bone |
a: PD ≥ 6 mm, intra > 4 mm b: NR c: I: 8 max. (7 I/C, 1 PM), 5 mand. (4 I/C, 1 PM) II: 10 max (5 I/C, 5 PM), 3 mand I/C d: excluded |
10 |
| Orsini et al., 2008 | RCT, SM |
a: 12/12 b: 24/24 |
a: NR b: 29–62 (42) c: 7/5 d: healthy e: 0/0 |
I: GTR (resorbable; BioGide) + autogenous bone II: autogenous bone +calcium sulphate (Surgiplaster) |
a: NR b: 2, 3 walls c: NR d: NR |
6 |
| Petsos et al., 2019 a | R)CT, SM, (PG) |
a: 10/10 b: 20/20 |
a: severe chronic P b: 41–73 (NR) c: NR d: NR e: 2/4 |
I: OFD II: GTR (resorbable; Guidor) |
a: PD ≥ 5 mm b: 2, 3 walls c: 6 max. (2 I/C, 4 PM), 14 mand. (2 I/C, 4 PM, 8 M) d: NR |
20 (follow‐up of Nickles et al., 2009) |
| Pretzl et al., 2008 b | RCT, SM |
a: 12/8 b: 24/16 |
a: advanced P @BL b: 32–62 (46) c: 3/9 d: 2 DM e: 3/0 |
I: GTR (non‐resorbable; Gore‐Tex) II: GTR (resorbable; Vicryl) |
a: NR b: NR c: I: 3 max. (1 C, 2 PM), 5 mand. (3 PM, 2 M) II: 3 max. (1 C, 2 PM), 5 mand. (3 PM, 2 M) d: NR |
10 (follow‐up of Kim et al., 2002) |
| Pretzl et al., 2009 b | RCT, SM |
a: 15/9 b: 30/18 |
a: moderate‐severe P (6 aggressive, 9 chronic P) @BL b: 22–64 (42) c: 3/12 d: NR e: 3/1 |
I: GTR (resorbable; Guidor) II: GTR (resorbable; Mempol) |
a: NR b: 2, 3 walls c: 6 max. (2 PM, 4 M), 14 mand. (4 I/C, 4 PM, 6 M) d: NR |
10 (follow‐up of Eickholz et al., 2004) |
| Sculean et al., 2006 | (R)CT, SM |
a: 16/10 b: 32/20 |
a: NR @FE b: 38–55 (46) c: 4/6 d: healthy e: NR |
I: GTR (resorbable; Resolut) II: EMD |
a: PD ≥ 6 mm b: I: 1 (1), 2 (8), 3 (1) walls II: 1 (1), 2 (7), 3 (2) walls c: NR d: NR |
8 (follow‐up of Sculean et al., 2001) |
| Sculean, Kiss, et al., 2008 | RCT, PG |
a: 56/38 b: 56/38 |
a: NR @FE b: NR (52) c: NR d: healthy e: NR |
I: OFD II: EMD III: GTR (resorbable; Resolut) IV: EMD + GTR (resorbable; Resolut) |
a: PD ≥ 6 mm, intra ≥ 3 mm b: I: 1–2 (2), 2 (5), 3 (2) walls II: 1–2 (3), 2 (6), 3 (1) walls III: 1–2 (3), 2 (6), 3 (1) walls IV: 1–2 (2), 2 (7), 3 (1) walls c: NR d: NR |
10 (follow‐up of Sculean et al., 2004) |
| Stavropoulos & Karring, 2010 | RCT, PG |
a: 60/38 b: 60/38 |
a: advanced P @BL b: 26–62 (NR) c: 27/33 d: NR e: NR |
I: OFD II: GTR (resorbable; Resolut) III: GTR (resorbable; Resolut) + NBM (BioOss) + saline IV: GTR (resorbable; Resolut) + NBM (BioOss) + gentamicin sulphate |
a: PD ≥ 7 mm, intra ≥ 4 mm b: 1, 2 walls c: NR d: excluded |
6 |
Abbreviations: (R)CT, according to authors randomized, but randomization process not defined; BL, baseline; C, canine; CAL, clinical attachment level; CS, case series; CT, controlled trial; DFDBA, demineralized freeze‐dried bone allograft; EMD, enamel matrix derivatives; f, female; FDBA, freeze‐dried bone allograft; FE, final evaluation; FI, furcation involvement; GTR, guided tissue regeneration; HA, hydroxylapatite; I, incisor; intra, intrabony defect depth; m, male; M, molar; mand., mandibular teeth; max., maxillary teeth; NBM, natural bone mineral; NR, not reported; OFD, open flap debridement; P, periodontitis; PD, probing pocket depth; PG, parallel group; PM, premolar; PRP, platelet‐rich plasma; RCT, randomized controlled trial; rhPDGF, recombinant human platelet‐derived growth factor; RS, retrospective study; SM, split mouth; ß‐TCP, ß‐tricalcium phosphate; width, intrabony defect width.
Herein, data of the split mouth group are reported;
Studies not contributing with data in the network meta‐analyses.
In general, publications reported on treated tooth type and number of bone walls, and a minimum defect depth was most often required for inclusion; however, information on exact defect depth and presence or absence of furcation involvement in the treated teeth was often not explicitly reported. Twenty‐five publications reporting on 546 and 74 teeth treated with a regenerative/reconstructive approach or OFD, respectively, provided information on tooth loss. Nine publications reported 10 and 4 teeth extracted, treated regeneratively or with OFD, respectively. Periodontitis was reported as the reason for tooth loss for 2 teeth treated regeneratively and for 2 teeth treated with OFD; for 5 teeth treated regeneratively the reason for extraction was not periodontitis, while for the remaining 5 teeth, no specific reason was reported (Appendix S7). Considering only the teeth that were definitely lost due to periodontitis, the rate of tooth loss was 0.4% and 2.8% for the teeth treated regeneratively or with OFD, respectively, while all reason tooth loss was 1.9% and 5.4%, respectively. Noteworthy, the majority of losses were reported in publications reporting on >5 years from treatment.
3.4. Synthesis of results
Detailed clinical and radiographic data of the 30 identified studies, arranged per intervention, are presented in Table 2. Out of the 30 publications, 6 were not included in the NMA because they were comparing similar type of treatment (GTR vs. GTR with different type of membranes: Kim et al., 2002; Eickholz et al., 2004; Pretzl et al., 2008; Pretzl et al., 2009; GTR + grafting vs. GTR + grafting with different type of membranes: Górski et al., 2020; EMD + grafting with different type of grafts: Döri, Arweiler, Szántó, et al., 2013) and 3 because the same population was reported once more at a later time point (GTR vs. EMD: Sculean et al., 2001; EMD vs. GTR vs. EMD+GTR vs. OFD: Sculean et al., 2004; GTR vs. OFD: Nickles et al., 2009), that is, only the latest time point was used in the NMA. In studies with >2 arms, comparing similar type of treatments (e.g. GTR vs. GTR vs. OFD), only one of the arms was included (see Table 2); specifically, the group with the more modest outcome was included. The publications/groups included in the NMA, provided evidence on residual PD and CAL gain from 573 and 628 regeneratively treated sites, and from 94 sites treated with OFD.
Table 2.
Mean ±standard deviation (in mm) of clinical and radiographic parameters of individual studies, arranged per intervention. Studies within each intervention are arranged alphabetically by first authors’ name. When studies included >1 groups with the same intervention, groups are reported separately
| Intervention | No. Sites | Obs. period (years) |
Residual PD |
CAL gain | PD reduction | REC increase | Residual RDD | RDD reduction | RBL gain | PD change | CAL change | RDD change | RBL change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OFD | |||||||||||||
| Bhutda & Deo, 2013 | 15 | 3 – 5 | 4.9 ± 0.5 | 1.6 ± 0.5 | 1.9 ± 0.4 | 0.3 ± 0.5 | 2.8 ± 0.8 | 1.3 ± 0.7 | 0.3 ± 0.8 | 0.5 ± 1.2 | |||
| Flemmig et al., 1998 | 8 | 3.9 ± 1.5 | 0.8 ± 1.4 | 2.2 ± 1.6 | |||||||||
| Heijl et al., 1997 | 27 | 5.2 ± 1.5 | 1.7 ± 1.3 | 2.3 ± 1.0 | 0.0 ± 0.7 | ||||||||
| Sculean et al., 2004 a | 10 | 5.5 ± 1.2 | 1.3 ± 1.2 | 2.7 ± 1.2 | 1.7 ± 0.5 | 0.6 ± 1.2 | 0.3 ± 1.1 | ||||||
| Sculean, Schwarz et al., 2007 | 9 | 5.6 ± 1.1 | 1.4 ± 0.7 | 3.3 ± 1.4 | 2.0 ± 0.8 | 0.6 ± 0.7 | 0.3 ± 0.5 | ||||||
| Nickles et al., 2009 | 10 | >5 | 4.7 ± 1.3 | 3.7 ± 3.4 | 4.4 ± 2.8 | 0.7 ± 2.3 | −0.8 ± 2.5 | −0.1 ± 2.6 | |||||
| Petsos et al., 2019 | 10 | 4.4 ± 1.5 | 3.6 ± 2.6 | 4.4 ± 3.1 | 0.8 ± 2.1 | −0.9 ± 0.6 | −0.6 ± 0.4 | ||||||
| Sculean, Kiss, et al., 2008 | 9 | 5.1 ± 1.3 | 1.8 ± 1.1 | 3.5 ± 1.4 | 1.7 ± 1.2 | 0.2 ± 1.6 | 0.2 ± 1.1 | ||||||
| Stavropoulos & Karring, 2010 | 5 | 7.6 ± 2.1 | −1.2 ± 2.4 | 0.2 ± 1.9 | 1.4 ± 1.8 | 5.9 ± 1.6 | 0.2 ± 0.1 | −0.2 ± 0.6 | 1.4 ± 1.9 | 1 ± 2.6 | 0.6 ± 1.2 | 1.6 ± 0.8 | |
| Cortellini et al., 2017 b | 11 | 4.5 ± 1.8 | 1.2 ± 2.4 | 3.3 ± 1.9 | 2.1 ± 1.4 | 1.1 ± 0.6 | 1.3 ± 1.2 | ||||||
| Grafting | |||||||||||||
| Flemmig et al., 1998, e | 8 | 3 – 5 | 2.7 ± 2.0 | 2.0 ± 2.0 | 2.6 ± 2.2 | ||||||||
| Menezes & Rao, 2012_I | 60 | 3.7 ± 1.0 | 3.1 ± 1.1 | 4.0 ± 0.5 | 1.0 ± 1.0 | −2.2 ± 0.8 | −2.4 ± 0.9 | ||||||
| Mengel et al., 2006 | 20 | 4.2 ± 1.8 | 3.3 ± 2.1 | 3.5 ± 1.4 | 0.2 ± 1.7 | 0.7 ± 1.5 | 0.6 ± 2.6 | ||||||
| Nevins et al., 2013_I | 27 | 3.4 ± 0.4 | 4.2 ± 0.3 | 2.7 ± 0.4 | −0.1 ± 0.3 | 0.3 ± 0.3 | −1.3 ± 0.3 | ||||||
| Nygaard‐Østby et al., 2010 | 13 | >5 | 4.6 ± 1.8 | 2.2 ± 2.5 | 2.7 ± 1.8 | 0.6 ± 1.8 | |||||||
| Orsini et al., 2008 | 12 | 3.7 ± 1.1 | 2.4 ± 1.1 | 4.2 ± 1.2 | 1.9 ± 1.3 | ||||||||
| Grafting + BC | |||||||||||||
| Menezes & Rao, 2012_II c | 60 | 3 – 5 | 2.4 ± 0.9 | 5.4 ± 1.2 | 5.8 ± 0.5 | 0.4 ± 0.7 | −3.5 ± 0.8 | −4.5 ± 1.1 | |||||
| Nevins et al., 2013_II d a | 28 | 4.3 ± 0.4 | 4.6 ± 0.3 | 3.4 ± 0.3 | −0.1 ± 0.3 | −0.5 ± 0.3 | −0.5 ± 0.3 | ||||||
| Nevins et al., 2013_III d | 28 | 3.5 ± 0.4 | 4.0 ± 0.3 | 2.5 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.4 | −0.3 ± 0.3 | ||||||
| GTR | |||||||||||||
| Crea et al., 2008 | 20 | 3 – 5 | 4.0 ± 0.9 | 2.1 ± 1.1 | 3.3 ± 1.1 | 1.2 ± 1.2 | 2.2 ± 0.6 | 2.7 ± 1.2 | 0.4 ± 0.8 | 0.6 ± 1.6 | 0.2 ± 0.7 | ||
| Eickholz et al., 2004_I a | 13 | 3.4 ± 1.1 | 2.4 ± 1.0 | 4.0 ± 1.4 | 1.6 ± 1.7 | 1.0 ± 1.6 | 1.0 ± 1.2 | 1.6 ± 1.1 | −0.1 ± 3.0 | ||||
| Eickholz et al., 2004_II a | 13 | 3.7 ± 1.5 | 2.2 ± 1.8 | 3.6 ± 1.6 | 1.4 ± 1.7 | 1.6 ± 2.2 | 0.5 ± 1.7 | 1.2 ± 1.9 | −0.4 ± 3.0 | ||||
| Kim et al., 2002_I a | 8 | 5.3 ± 1.9 | 1.6 ± 1.5 | 2.6 ± 2.0 | 1.0 ± 2.0 | ||||||||
| Kim et al., 2002_II a | 8 | 4.2 ± 1.1 | 3.0 ± 0.7 | 3.6 ± 1.8 | 0.6 ± 1.8 | ||||||||
| Mengel et al., 2006 | 22 | 4.1 ± 1.6 | 3.0 ± 2.0 | 3.6 ± 2.0 | 0.6 ± 1.4 | 0.6 ± 1.6 | 0.6 ± 2.0 | ||||||
| Sculean et al., 2001 a | 12 | 4.7 ± 1.2 | 2.9 ± 2.1 | 3.4 ± 1.6 | 0.5 ± 1.4 | 1.1 ± 1.1 | 0.3 ± 1.8 | ||||||
| Sculean et al., 2004 a | 11 | 3.9 ± 1.4 | 2.7 ± 0.9 | 4.4 ± 1.4 | 1.2 ± 1.0 | 0.5 ± 1.5 | 0.5 ± 0.9 | ||||||
| Nickles et al., 2009 | 10 | >5 | 4.5 ± 1.4 | 2.9 ± 2.2 | 4.2 ± 2.5 | 1.3 ± 1.8 | −0.2 ± 2.0 | 0.4 ± 1.2 | |||||
| Petsos et al., 2019 | 10 | 4.9 ± 1.8 | 3.8 ± 2.7 | 4.8 ± 3.4 | 1.0 ± 2.1 | 0.5 ± 0.4 | 0.30 ± 0.2 | ||||||
| Pretzl et al., 2008_I a | 8 | 5.2 ± 1.9 | 1.5 ± 1.2 | 2.4 ± 1.6 | 0.7 ± 1.8 | −2.3 ± 1.6 | 1.7 ± 1.3 | ||||||
| Pretzl et al., 2008_II a | 8 | 3.6 ± 1.0 | 3.5 ± 2.5 | 4.2 ± 2.5 | 0.7 ± 1.7 | −0.2 ± 1.8 | −0.2 ± 2.0 | ||||||
| Pretzl et al., 2009_I a | 9 | 4.6 ± 2.5 | 2.4 ± 1.7 | 3.2 ± 1.3 | 0.7 ± 2.3 | 3.5 ± 1.6 | 2.2 ± 2.0 | 1.7 ± 1.5 | 2.0 ± 2.6 | 1.6 ± 2.5 | −0.1 ± 1.8 | −0.2 ± 1.6 | |
| Pretzl et al., 2009_II a | 9 | 4.3 ± 1.9 | 2.4 ± 1.8 | 3.2 ± 2.3 | 0.7 ± 1.8 | 3.7 ± 3.0 | 1.7 ± 2.8 | 1.2 ± 2.5 | 1.2 ± 2.1 | 1.4 ± 1.5 | 0.0 ± 2.5 | 0.5 ± 2.6 | |
| Sculean et al., 2006 | 10 | 4.5 ± 0.8 | 2.9 ± 1.3 | 3.7 ± 0.9 | 0.8 ± 1.5 | 0.9 ± 0.7 | 0.1 ± 1.1 | ||||||
| Sculean, Kiss, et al., 2008 | 10 | 5.0 ± 1.0 | 2.8 ± 1.2 | 3.4 ± 1.5 | 0.6 ± 1.4 | 0.8 ± 1.2 | 0.4 ± 1.2 | ||||||
| Stavropoulos & Karring, 2010 | 12 | 5.8 ± 1.9 | 2.4 ± 2.1 | 3.0 ± 1.8 | 0.6 ± 2.0 | 1.7 ± 1.8 | 4.2 ± 2.0 | 3.6 ± 2.4 | 1.0 ± 1.7 | 0.6 ± 2.1 | −0.7 ± 2.1 | −0.4 ± 2.3 | |
| Cortellini et al., 2017_Ia b | 13 | 2.9 ± 0.9 | 5.2 ± 2.6 | 5.2 ± 2.6 | 0.1 ± 1.2 | 0.9 ± 0.9 | 0.1 ± 1.0 | ||||||
| Cortellini et al., 2017_II b | 12 | 3.6 ± 1.0 | 3.6 ± 2.3 | 4.6 ± 2.3 | 1 ± 1.0 | 1.0 ± 0.8 | 0.5 ± 0.5 | ||||||
| GTR + BC | |||||||||||||
| Cetinkaya et al., 2014 c | 11 | 4.7 ± 0.8 | 2.4 ± 0.9 | 2.9 ± 0.9 | 0.5 ± 1.2 | 3.5 ± 2.1 | |||||||
| EMD | |||||||||||||
| Bhutda & Deo, 2013 | 15 | 3 – 5 | 3.4 ± 0.6 | 3.2 ± 0.9 | 3.8 ± 1.1 | 0.7 ± 0.0 | 1.6 ± 0.7 | 3.2 ± 0.6 | 0.3 ± 0.8 | 0.8 ± 1.1 | |||
| Crea et al., 2008 | 19 | 3.5 ± 0.8 | 2.5 ± 1.2 | 3.2 ± 1.3 | 0.6 ± 1.2 | 2.0 ± 0.7 | 2.7 ± 1.0 | 0.3 ± 0.8 | 0.4 ± 0.8 | −0.4 ± 0.8 | |||
| Heijl et al., 1997 | 27 | 4.6 ± 1.0 | 2.2 ± 1.1 | 3.1 ± 1.0 | 2.6 ± 1.7 | ||||||||
| Hoffmann et al., 2015 | 15 | 3.3 ± 1.9 | 3.8 ± 2.2 | 3.9 ± 2.3 | 0.1 ± 2.9 | −0.4 ± 1.8 | −1.8 ± 2.7 | ||||||
| Ogihara & Tarnow, 2014 | 23 | 3.6 ± 0.5 | 3.0 ± 1.3 | 3.1 ± 0.7 | −0.1 ± 0.8 | 3.3 ± 1.3 | 0.2 ± 0.5 | 0.0 ± 0.9 | 0.0 ± 0.8 | ||||
| Pietruska et al., 2012 | 12 | 4.4 ± 0.8 | 3.2 ± 1.3 | 4.4 ± 0.9 | 1.2 ± 0.9 | 0.3 ± 0.7 | 0.3 ± 1.1 | ||||||
| Sculean et al., 2001 a | 12 | 4.7 ± 1.2 | 3.0 ± 1.9 | 3.4 ± 1.6 | 0.4 ± 1.0 | 0.9 ± 1.2 | 0.4 ± 1.7 | ||||||
| Sculean et al., 2004 a | 11 | 3.9 ± 1.5 | 2.9 ± 1.6 | 4.3 ± 1.7 | 1.3 ± 0.7 | 0.3 ± 1.5 | 0.5 ± 1.4 | ||||||
| Sculean, Pietruska, et al., 2007 | 13 | 4.4 ± 0.6 | 3.4 ± 1.4 | 4.2 ± 0.8 | 0.9 ± 1.1 | 0.5 ± 0.6 | 0.3 ± 1.0 | ||||||
| Sculean et al., 2006 | 10 | 4.7 ± 1.2 | 2.8 ± 1.4 | 3.4 ± 1.0 | 0.6 ± 1.0 | 0.7 ± 1.1 | 0.4 ± 1.5 | ||||||
| Sculean, Kiss, et al., 2008 | 10 | 4.8 ± 1.1 | 2.9 ± 1.4 | 3.6 ± 1.7 | 0.7 ± 1.2 | 0.5 ± 1.2 | 0.5 ± 1.4 | ||||||
| GTR + Grafting | |||||||||||||
| Cetinkaya et al., 2014 | 11 | 3 – 5 | 4.1 ± 0.6 | 2.6 ± 1.1 | 3.5 ± 0.9 | 0.8 ± 0.8 | 3.0 ± 2.2 | ||||||
| Gorski et al., 2020_I | 14 | 3.6 ± 1.3 | 4.8 ± 1.7 | 3.9 ± 1.2 | 0.7 ± 0.7 | 0.6 ± 0.5 | 5.1 ± 1.3 | 5.1 ± 1.3 | 0.3 ± 1.3 | −0.07 ± 1.3 | 0.06 ± 0.3 | 0.01 ± 0.3 | |
| Gorski et al., 2020_II | 13 | 3.8 ± .1.0 | 4.0 ± 1.7 | 3.3 ± 1.7 | 0.6 ± 0.9 | 0.7 ± 0.4 | 4.3 ± .1.6 | 4.4 ± 1.6 | 0.1 ± 1.6 | 0.6 ± 0.9 | 0.1 ± 0.4 | −0.07 ± 0.5 | |
| Sculean, Schwarz, et al., 2007 | 10 | 4.3 ± 0.8 | 3.7 ± 1.1 | 4.8 ± 1.6 | 1.1 ± 1.2 | 0.6 ± 0.7 | 0.3 ± 0.6 | ||||||
| Nygaard‐Østby et al., 2010 | 13 | >5 | 3.4 ± 1.1 | 3.8 ± 1.8 | 4.2 ± 1.8 | 0.7 ± 1.1 | |||||||
| Orsini et al., 2008 | 12 | 4.4 ± 1.1 | 2.5 ± 1.2 | 3.3 ± 1.6 | 0.7 ± 1.2 | ||||||||
| Stavropoulos & Karring, 2010_III a | 8 | 4.9 ± 1.3 | 2.3 ± 2.1 | 3.6 ± 1.2 | 1.3 ± 1.6 | 1.2 ± 2.2 | 3.8 ± 2.1 | 3.7 ± 2.4 | 0.3 ± 1.2 | 0.2 ± 2.3 | −1.4 ± 1.8 | −0.9 ± 2.1 | |
| Stavropoulos & Karring, 2010_IV ¢ | 7 | 4.6 ± 1.2 | 4.1 ± 1.6 | 4.7 ± 1.2 | 0.6 ± 1.4 | 1.7 ± 2.4 | 3.9 ± 2.5 | 4.4 ± 2.4 | 0.4 ± 1.1 | 0.0 ± 1.7 | −0.4 ± 2.4 | −0.2 ± 2.3 | |
| EMD + Grafting | |||||||||||||
| Döri, Arweiler, Húszár, et al., 2013_I | 12 | 3 – 5 | 3.8 ± 1.3 | 4.3 ± 1.6 | 5.0 ± 1.8 | 0.7 ± 1.3 | 0.5 ± 1.2 | −0.3 ± 1.4 | |||||
| Hoffmann et al., 2015 | 15 | 3.3 ± 1.3 | 4.1 ± 3.6 | 3.9 ± 2.0 | −0.2 ± 2.3 | −0.5 ± 1.7 | −2.0 ± 2.8 | ||||||
| Ogihara & Tarnow, 2014_II e | 21 | 2.1 ± 0.4 | 4.2 ± 1.1 | 4.4 ± 0.9 | 0.3 ± 0.8 | 4.2 ± 2.4 | −0.1 ± 0.4 | 0.0 ± 0.7 | 0.0 ± 0.9 | ||||
| Ogihara & Tarnow, 2014_III a , e | 23 | 2.7 ± 0.5 | 3.6 ± 1.2 | 3.7 ± 0.7 | 0.1 ± 0.8 | 4.3 ± 2.3 | 0.0 ± 0.5 | −0.1 ± 0.6 | 0.0 ± 0.9 | ||||
| Pietruska et al., 2012 | 12 | 4.7 ± 0.8 | 3.2 ± 1.7 | 4.1 ± 1.3 | 0.9 ± 1.1 | 0.4 ± 0.9 | 0.2 ± 1.7 | ||||||
| Sculean, Pietruska, et al., 2007 | 12 | 4.5 ± 1.0 | 3.4 ± 1.4 | 4.1 ± 1.0 | 0.7 ± 0.9 | 0.4 ± 1.0 | 0.2 ± 1.1 | ||||||
| Döri, Arweiler, Szántó, et al., 2013_I a | 11 | >5 | 4.1 ± 0.9 | 3.1 ± 1.3 | 3.9 ± 1.1 | 0.8 ± 0.8 | 0.6 ± 0.9 | 0.5 ± 1.0 | |||||
| Döri, Arweiler, Szántó, et al., 2013_II a | 11 | 4.1 ± 0.9 | 3.0 ± 1.5 | 4.0 ± 1.2 | 1.0 ± 0.7 | 0.8 ± 0.8 | 0.7 ± 1.3 | ||||||
| EMD + GTR | |||||||||||||
| Sculean et al., 2004 a | 10 | 3–5 | 4.4 ± 1.0 | 4.0 ± 1.0 | 2.6 ± 0.7 | 1.5 ± 0.7 | 0.4 ± 0.9 | 0.4 ± 0.9 | |||||
| Sculean, Kiss, et al., 2008 | 9 | >5 | 5.1 ± 1.2 | 3.5 ± 1.4 | 2.9 ± 1.2 | 0.6 ± 1.1 | 1.0 ± 1.3 | 0.4 ± 1.2 | |||||
| EMD + Grafting + BC | |||||||||||||
| Döri, Arweiler, Húszár, et al., 2013_II c | 12 | 3 – 5 | 3.8 ± 1.1 | 4.3 ± 1.6 | 4.9 ± 1.5 | 0.6 ± 1.4 | 0.6 ± 1.1 | −0.4 ± 1.5 | |||||
Changes regard comparisons with 1 year data.
Abbreviations: BC, blood‐derived growth factor constructs; CAL, clinical attachment level; CI, confidence interval; EMD, enamel matrix derivatives; GTR, guided tissue regeneration; Obs., observation; OFD, open flap debridement; PD, probing pocket depth; RBL, radiographic bone level; RDD, radiographic defect depth; REC, gingival recession.
Studies/groups not contributing with data in the network meta‐analyses.
Data recalculated to exclude patients that were re‐operated during the observation time.
Blood construct as adjunct;
Recombinant growth factor as adjunct;
Local antibiotic as adjunct.
The results of the pairwise meta‐analyses for both clinical outcomes, when feasible, are presented in Appendix S8. Figure 1a and b shows forest plots of the various studies included in the NMA arranged per comparison of interventions and ordered by year of publication, for residual PD and CAL gain. Figure 2a and 2b shows the networks for both primary outcomes; overall, both networks show the same network geometry, appearing relatively poorly connected, with direct comparisons among the various interventions often including only 1 to 2 trials. Only about 33% of the comparisons among the various interventions were direct comparisons and GTR, EMD and OFD being the most connected nodes. The NMA results for residual PD and CAL gain are summarized in league Tables 3a and 3b, respectively. The majority of interventions, except for GTR + BC and EMD + grafting + BC, seemed to be significantly superior to OFD in terms of shallower residual PD (MD range: −2.37 to −0.60 mm) and larger CAL gain (MD range: 1.26 to 2.66 mm). Comparatively, among the regenerative/reconstructive interventions, combination procedures seemed to give the largest effect in terms of both residual PD and CAL gain; however, the differences were not statistically significant. Results on 95% predictive intervals for comparisons with OFD can be found in Appendix S9. The ranking probabilities (Figure 3a and b) and SUCRA values (Figure 4a and b) indicate that combination approaches are comparatively better than monotherapies whereas OFD is the inferior treatment regarding both primary outcomes; however, the hierarchy of the various procedures is characterized by great uncertainty regarding both outcomes, due to the fact that their ranking probabilities appear dispersed below 50%.
Figure 1.

a and b. Forest plots of the various studies included in the NMA arranged per comparison of interventions and order by year of publication for residual PD and CAL gain
Figure 2.
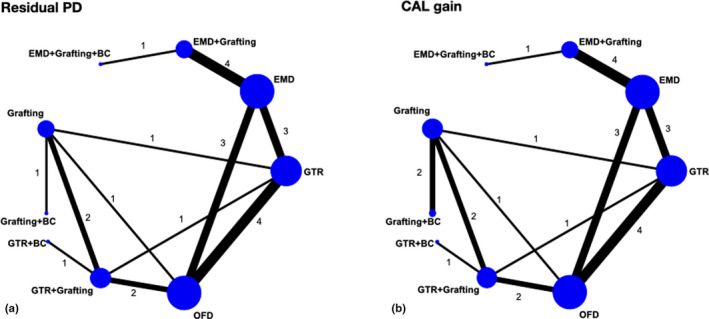
a and b. Networks for residual PD and CAL gain. Only about 33% of the comparisons among the various interventions were direct comparisons
Table 3a.
League table for residual PD. Mean differences (in mm) with 95% credible intervals (read as row versus column) among interventions, ordered from best to worst according to the mean SUCRA values provided in the diagonal
| Grafting + BC | GTR + Grafting | EMD + Grafting | EMD + Grafting + BC | Grafting | EMD | GTR + BC | GTR | OFD | |
|---|---|---|---|---|---|---|---|---|---|
| Grafting + BC | 92% | −1.03 (−2.77, 0.77) | −1.16 (−3.26, 0.89) | −1.16 (−3.89, 1.52) | −1.31 (−2.78, 0.15) | −1.51 (−3.44, 0.39) | −1.68 (−3.98, 0.67) | −1.77 (−3.61, 0.08) | −2.37 (−4.20, −0.55) |
| GTR + Grafting | 68% | −0.13 (−1.64, 1.28) | −0.14 (−2.44, 2.09) | −0.29 (−1.31, 0.68) | −0.49 (−1.74, 0.70) | −0.66 (−2.16, 0.84) | −0.74 (−1.86, 0.34) | −1.34 (−2.42, −0.32) | |
| EMD + Grafting | 63% | −0.002 (−1.73, 1.73) | −0.16 (−1.60, 1.33) | −0.35 (−1.13, 0.46) | −0.52 (−2.56, 1.62) | −0.61 (−1.70, 0.53) | −1.21 (−2.30, −0.07) | ||
| EMD + Grafting + BC | 58% | −0.15 (−2.40, 2.15) | −0.35 (−2.24, 1.56) | −0.52 (−3.20, 2.24) | −0.61 (−2.64, 1.47) | −1.21 (−3.25, 0.86) | |||
| Grafting | 53% | −0.20 (−1.43, 1.03) | −0.37 (−2.14, 1.47) | −0.45 (−1.56, 0.66) | −1.06 (−2.14, 0.03) | ||||
| EMD | 43% | −0.17 (−2.06, 1.80) | −0.25 (−1.03, 0.54) | −0.86 (−1.62, −0.08) | |||||
| GTR + BC | 37% | −0.08 (−1.97, 1.75) | −0.69 (−2.55, 1.12) | ||||||
| GTR | 30% | −0.60 (−1.35, 0.14) | |||||||
| OFD | 6% |
Interventions are reported in order of their ranking, that is, the larger the mean SUCRA value, the better the treatment. Comparisons between interventions should be read from left to right and the estimate in the cell refers to the row‐defining intervention against the column‐defining intervention. Mean differences (MD) lower than 0 favours the row‐defining intervention. To obtain MD for comparisons in the opposite direction, negative values should be converted into positive values, and vice versa. Results that indicate strong evidence in favour of the row‐defining intervention (i.e. the respective 95% credible interval does not include the zero value of no difference) are underlined.
Abbreviations: BC, blood‐derived growth factor constructs; EMD, enamel matrix derivatives; GTR, guided tissue regeneration; OFD, open flap debridement; PD, probing pocket depth; SUCRA, surface under the cumulative ranking.
Table 3b.
League table for CAL gain. Mean differences in mm with 95% credible intervals (read as row versus column) among interventions, ordered from best to worst according to the mean SUCRA values provided in the diagonal
| Grafting + BC | GTR + Grafting | GTR + BC | EMD + Grafting | EMD + Grafting + BC | Grafting | GTR | EMD | OFD | |
|---|---|---|---|---|---|---|---|---|---|
| Grafting BC | 87% | 0.53 (−1.09, 2.12) | 0.81 (−1.55, 3.14) | 0.96 (−1.09, 3.02) | 0.96 (−1.91, 3.83) | 1.19 (0.05, 2.34) | 1.31 (−0.40, 2.99) | 1.40 (−0.40, 3.18) | 2.66 (1.00, 4.33) |
| GTR + Grafting | 73% | 0.28 (−1.42, 2.00) | 0.43 (−1.24, 2.15) | 0.42 (−2.19, 3.09) | 0.65 (−0.46, 1.81) | 0.78 (−0.48, 2.04) | 0.86 (−0.49, 2.24) | 2.13 (0.97, 3.31) | |
| GTR + BC | 59% | 0.15 (−2.23, 2.58) | 0.14 (−2.96, 3.31) | 0.37 (−1.65, 2.44) | 0.49 (−1.62, 2.61) | 0.58 (−1.57, 2.78) | 1.84 (−0.19, 3.93) | ||
| EMD + Grafting | 57% | −0.003 (−2.02, 2.01) | 0.23 (−1.48, 1.93) | 0.35 (−1.02, 1.68) | 0.44 (−0.58, 1.42) | 1.70 (0.37, 3.02) | |||
| EMD + Grafting + BC | 54% | 0.23 (−2.42, 2.86) | 0.35 (−2.10, 2.75) | 0.44 (−1.83, 2.68) | 1.70 (−0.71, 4.11) | ||||
| Grafting | 44% | 0.12 (−1.15, 1.36) | 0.21 (−1.18, 1.58) | 1.47 (0.26, 2.67) | |||||
| GTR | 39% | 0.09 (−0.80, 0.99) | 1.35 (0.48, 2.25) | ||||||
| EMD | 35% | 1.26 (0.41, 2.14) | |||||||
| OFD | 2% |
Interventions are reported in order of their ranking, that is the larger the mean SUCRA value, the better the treatment. Comparisons between interventions should be read from left to right and the estimate in the cell refers to the row‐defining intervention against the column‐defining intervention. Mean differences (MDs) larger than 0 favour the row‐defining intervention. To obtain MDs for comparisons in the opposite direction, negative values should be converted into positive values, and vice versa. Results that indicate strong evidence in favour of the row‐defining intervention (i.e. the respective 95% credible interval does not include the zero value of no difference) are underlined.
Abbreviations: BC, blood‐derived growth factor constructs; CAL, clinical attachment level; EMD, enamel matrix derivatives; GTR, guided tissue regeneration; OFD, open flap debridement; CAL, clinical attachment level; SUCRA, surface under the cumulative ranking.
Figure 3.
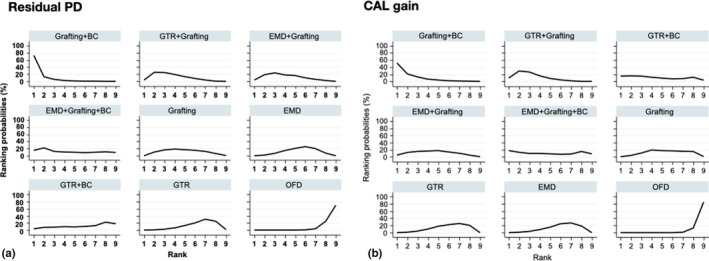
Rankograms illustrating the relative ranking probability of the various interventions for residual PD and CAL gain. Interventions are arranged from the best intervention (upper left corner) to the worst (lower right corner)
Figure 4.
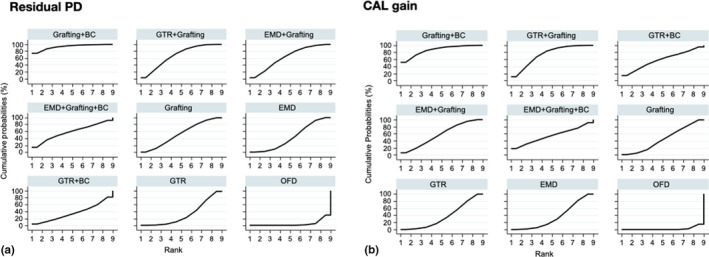
a and b. SUCRA plots illustrating the cumulative ranking probability of the various interventions for residual PD and CAL. Interventions are arranged from the best intervention (upper left corner) to the worst (lower right corner)
The network for residual RDD included only singleton studies whereas the network for RBL gain comprised from disconnected pieces of evidence (Appendix S10); hence, NMA was not feasible. Based on only few pairwise comparisons, regenerative/reconstructive treatment appeared more effective compared with OFD in terms of shallower residual RDD and larger RBL gain (MD ranges: −4.74 to −1.20 mm and 3.79 to 3.88 mm, respectively).
The results regarding possible local and global inconsistency for both primary outcomes, and the assessment for possible effect modification due to publication year, small‐study size, observation period (3–5 years versus >5 years) or RoB are presented inAppendix S11. Shortly, there seems to be no evidence of statistical inconsistency in both networks. Τhe year of publication appeared to have a negligible effect, whereas investigation of small‐study size, observation period, and RoB indicated a slight, statistically insignificant, effect exaggerating the results of OFD regarding residual PD and CAL gain. Overall, sensitivity analyses indicate robustness of the results (Appendix S11).
4. DISCUSSION
The results of the present systematic review and NMA showed that, periodontal regenerative/reconstructive therapy in intrabony defects results, in general, in significantly better clinical outcomes compared with OFD on a medium‐ to long‐term basis (i.e. from at least 3 to up to 20 years). In particular, the NMA showed that intrabony defects treated with a regenerative/reconstructive approach presented significantly shallower residual PD [range of MD: −2.37 to −0.60 mm] and larger CAL gain (MD: 1.26 to 2.66 mm) compared with what was achieved with OFD. Importantly, residual PD after most of regenerative/reconstructive modalities was at a level considered maintainable (i.e. with low risk of progression) by supportive treatment; indeed, in 33 out of 35 groups (94%), representing various regenerative/reconstructive approaches in the studies included in the NMA, average residual PD was <5 mm, while this was the case in only 3 out of 8 (33%) OFD groups (Table 2). Deep pockets after periodontal therapy are indeed associated with an increased risk for disease progression and tooth loss; specifically, a dose‐dependent association between deep residual PD and periodontitis progression, together with a multifold higher risk for tooth loss, has been reported compared to teeth with residual PD of ≤3 mm (Matuliene et al., 2008). Herein, out of 25 publications reporting on tooth loss, only 9 reported ≥1 tooth lost; 0.4% and 2.8% of the teeth treated with a regenerative/reconstructive approach or with OFD, respectively, were lost due to periodontitis. Since there were only few studies directly comparing regenerative treatment with OFD and reporting on tooth loss, no assumptions on the superiority of regenerative/reconstructive therapy over OFD should be made regarding tooth retention; nevertheless, it appears that the impact of the above‐mentioned medium‐ to long‐term clinical improvements achieved with regenerative/reconstructive treatment can be translated in decreased tooth mortality, a major goal of periodontal treatment.
Several of the studies reported that some of the CAL gain achieved post‐operatively was lost during the years; in general, the extent of CAL loss was relatively limited and regarded only a fraction of the treated teeth. Partial loss of the CAL gain obtained 1 year after GTR treatment has been previously associated with smoking, poor oral hygiene and lack of compliance with a supportive periodontal programme (Cortellini et al., 1994, 1996; Weigel et al., 1995; Cortellini & Tonetti, 2004). In perspective, disease recurrence and tooth loss following periodontal therapy are, in general, not solely associated with the treatment approach, but are also related to patient compliance, including supportive periodontal therapy and/or general dental care (Löe et al., 1978; Axelsson et al., 2004; Matuliene et al., 2010); the majority of studies herein report that the patients received long‐term maintenance at specialist public/private centres, and thus, generalizability of the results may not be applicable to all clinical settings.
NMA allows for assumptions on the relative effectiveness of various treatments, even in the absence of direct comparisons. According to the NMA, combination approaches (e.g. GTR + grafting, EMD + grafting) appeared more efficacious comparing to monotherapies, that is, combination approaches presented with relatively shallower residual PD and larger CAL gain. Indeed, the only groups—out of the various regenerative/reconstructive approaches herein—with average residual PD ≥5 mm, represented a monotherapy (i.e. GTR); in contrast, in none of the 13 groups representing various combination approaches was that the case (Table 2). Comparatively, grafting + BC appeared to be the most effective treatment; however, this finding should be interpreted with much caution, since it is based on only two groups contributing with data [i.e. hydroxyapatite + platelet‐rich plasma (PRP)—(Menezes & Rao, 2012); ß‐tricalcium phosphate (ß‐TCP) in combination with recombinant human platelet‐derived growth factor (rhPDGF‐B)—(Nevins et al., 2013)]. In context, a recent systematic review with meta‐analysis revealed that grafting with adjunct use of PRP provided an additional, yet small, benefit over grafting alone 6 to 12 months after treatment of intrabony defects, while only half of the included studies showed a positive effect of PRP (Hou et al., 2016). Similarly, only slightly larger CAL gain (0.3 mm) than that achieved with only use of ß‐TCP has been reported in a meta‐analysis of the very few available short‐term studies involving ß‐TCP/rhPDGF‐B (Khoshkam et al., 2015). Further, proof‐of‐principle human histological studies (Ridgway et al., 2008) have not been convincing that periodontal regeneration can indeed be achieved with ß‐TCP/rhPDGF‐B at a magnitude over than the limited extent achieved with sole ß‐TCP implantation (Stavropoulos, Windisch, et al., 2011; Stavropoulos et al., 2010).
The finding that combination approaches yield better results are corroborated by results in preclinical in vivo and human histological studies, collectively presented in systematic reviews (Stavropoulos & Wikesjö, 2012; Ivanovic et al., 2014; Sculean et al., 2015). Specifically, combination of GTR or EMD with grafting results, in general, in larger amounts of periodontal regeneration and more predictable outcomes comparing to monotherapies alone, whereas sole implantation of bone grafts/substitutes in periodontal defects does not predictably lead in substantial amounts of periodontal regeneration and a portion of the bone graft/substitute particles often remains encapsulated within gingival connective tissue. In this context, preclinical in vivo studies on periodontal wound healing/regeneration, conducted in the 1980 s‐1990 s‐2000 s, have clearly demonstrated that periodontal regeneration is a function of post‐operative wound stability, uncompromised wound maturation and space provision (Wikesjo et al., 2010); apparently this triad of preconditions can be better facilitated by combination approaches, compared with monotherapies, especially in defects with large and/or non‐supportive anatomy (Ivanovic et al., 2014). It has to be stressed out, however, that purely clinical studies have clearly demonstrated that in narrow and/or well‐contained defects, monotherapies are equally successful with combination approaches (Cortellini & Tonetti, 2011).
Herein, only a quite small number of studies reported on radiographic outcomes, and thus, no NMA was possible for residual RDD and RBL gain. In general, regenerative/reconstructive treatment appeared more effective compared with OFD, presenting shallower residual RDD and larger RBL gains. In the only study reporting on a combination approach (GTR + grafting) vs. OFD, and presenting radiographic data, a MD of −4.74 mm and 3.88 mm in residual RDD and RBL gain, respectively, was observed (Stavropoulos & Karring, 2010). Indeed, in a study in dogs, treatment of large box‐type 1‐wall periodontal defects with GTR in combination with the same type of bone substitute material as in the above‐mentioned clinical study (i.e. deproteinized bovine bone, DBB), resulted in complete reconstitution of the periodontium (Stavropoulos & Wikesjö, 2010). Nevertheless, no assumptions on the magnitude of regeneration should, in general, be made when interpreting radiographic results in the presence of low/barely resorbable and radiopaque bone substitute materials. Indeed, in human histological reports, the magnitude of regeneration has been much less compared to the observed CAL gain (Stavropoulos et al., 2010; Stavropoulos, Chiantella, et al., 2011; Stavropoulos, Windisch, et al., 2011), and biomaterial particles occupied a major portion of the newly formed tissues even after a long period of time (Sculean, Chiantella, et al., 2008). On the other hand, the findings herein, showing stable periodontal conditions after use of several types of grafting materials, also imply that mere presence of bone graft/substitute particles within the regenerated/reconstructed periodontal tissues does not have per se any negative consequence for periodontal stability on the medium to long term.
In perspective, the present study is limited by the relatively low number of available studies and the amount/type of information reported in the studies. Consequently, the networks for both residual PD and CAL gain were sparse, due to the low number of direct comparisons and the low number of associated studies; only 12 out of the 36 possible comparisons were direct comparisons, and around half of the observed comparisons were based only in one trial. Thus, estimates for most of the comparisons were quite imprecise, which in turn reduces confidence in the observed hierarchy of interventions regarding both outcomes, except of the fact that OFD was the worse treatment in all performed analyses. A more distinct hierarchical allocation of the various treatment approaches, for example, which of the combination treatment is best, second best, etc., could also not be established herein. Further, the limited number of trials prohibited the investigation of possible “treatment by covariate interactions” for publication year, small‐study effects, observation period and RoB, as statistical significance could not be demonstrated. Further, it was not possible to investigate any impact of study design (parallel vs. split mouth) on the primary outcomes due to insufficient number of trials with both designs for the various comparisons. The low number of studies in each comparison also yielded low power in detecting any possible statistical inconsistency for both residual PD and CAL gain. For a more extensive discussion of the methodological challenges of the current NMA, please see Appendix S12. Finally, lack of detailed information, for example, on the frequency distribution of pocket and/or intrabony defect depths (deep vs. very deep) prior to treatment, did not allow a more sophisticated analysis on possibly relevant factors.
5. CONCLUSIONS
Implications for practice
a) periodontal regenerative/reconstructive therapy in intrabony defects results, in general, in shallower residual PD and larger CAL gain compared with OFD, translating in high rates of tooth survival, on a medium‐ to long‐term basis; b) combination approaches appeared, in general, as more efficacious comparing to monotherapy, but a clear hierarchy could not be established.
Future research
The present study is limited due to the quantity and quality of available evidence; for example, missing information on defect depth not allowing stratified analysis in moderate vs deep defects needs to combine in NMA medium‐ and long‐term studies, high or unclear risk of bias of the included studies, etc. Thus, large‐scale RCTs and more detailed/complete reporting of relevant clinical and radiographic data are necessary for the future. Further, future studies should investigate the cost–benefit of regenerative treatment vs. OFD on the long term.
CONFLICT OF INTEREST
Dr. Stavropoulos has received over the years research support, mostly in terms of biomaterials free of charge, from W.L. Gore and Associates, Geistlich AG and Straumann AG, in conjunction with his work on periodontal regeneration. Dr. Bertl and Dr. Spineli declare no conflict of interest. Dr. Sculean has received over the years research support and lecture fees from Geistlich AG, Straumann AG and Regedent AG in conjunction with his work on periodontal regeneration. Dr. Tonetti and Dr. Cortellini have received over the years research support and lecture fees from W.L. Gore and Associates, Guidor AB, Sunstar Ltd, Geistlich AG, Biora AB, Procter and Gamble, 3i, and Straumann AG in conjunction with their work on periodontal regeneration.
AUTHOR CONTRIBUTION
Dr. Stavropoulos and Dr. Bertl contributed to the conception and design of the study and to the collection and interpretation of the data; Dr. Spineli contributed to the statistical analysis and interpretation of the data; Dr. Sculean, Dr. Cortellini and Dr. Tonetti contributed to the interpretation of the data; all authors contributed to drafting and/or critically revising the manuscript.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
Appendix S10
Appendix S11
Appendix S12
ACKNOWLEDGEMENTS
Dr. Stavropoulos costs associated with this review were covered from ASTI, a research consortium financed partly from the Danish Innovation Foundation. No other external funding was obtained for performing the current review. This work is attributed to the memory of Prof. Thorkild Karring, Aarhus University, Denmark, for his pivotal contribution to the field of periodontal regeneration.
Data availability statement
Data available upon reasonable request.
REFERENCES
- Axelsson, P. , Nyström, B. , & Lindhe, J. (2004). The long‐term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. Journal of Clinical Periodontology, 31, 749–757. [DOI] [PubMed] [Google Scholar]
- Badersten, A. , Nilvéus, R. , & Egelberg, J. (1990). Scores of plaque, bleeding, suppuration and probing depth to predict probing attachment loss. 5 years of observation following nonsurgical periodontal therapy. Journal of Clinical Periodontology, 17, 102–107. [DOI] [PubMed] [Google Scholar]
- Bhutda, G. , & Deo, V. (2013). Five years clinical results following treatment of human intra‐bony defects with an enamel matrix derivative: A randomized controlled trial. Acta Odontologica Scandinavica, 71, 764–770. [DOI] [PubMed] [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. T. , & Rothstein, H. R. 2009. Effect Sizes Based on Means. Pages 21–32 editors. Introduction to Meta‐Analysis. John Wiley & Sons Ltd, . [Google Scholar]
- Caton, J. , Nyman, S. , & Zander, H. (1980). Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. Journal of Clinical Periodontology, 7, 224–231. [DOI] [PubMed] [Google Scholar]
- Caton, J. G. , & Zander, H. A. (1979). The attachment between tooth and gingival tissues after periodic root planing and soft tissue curettage. Journal of Periodontology, 50, 462–466. [DOI] [PubMed] [Google Scholar]
- Cetinkaya, B. O. , Keles, G. C. , Pamuk, F. , Balli, U. , & Keles, Z. P. (2014). Long‐term clinical results on the use of platelet concentrate in the treatment of intrabony periodontal defects. Acta Odontologica Scandinavica, 72, 92–98. [DOI] [PubMed] [Google Scholar]
- Chaimani, A. , Higgins, J. P. , Mavridis, D. , Spyridonos, P. , & Salanti, G. (2013). Graphical tools for network meta‐analysis in STATA. PLoS One, 8, e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claffey, N. , Nylund, K. , Kiger, R. , Garrett, S. , & Egelberg, J. (1990). Diagnostic predictability of scores of plaque, bleeding, suppuration and probing depth for probing attachment loss. 3 1/2 years of observation following initial periodontal therapy. Journal of Clinical Periodontology, 17, 108–114. [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Buti, J. , Pini Prato, G. P. , & Tonetti, M. S. (2017). Periodontal regeneration compared with access flap surgery in human intra‐bony defects 20‐year follow‐up of a randomized clinical trial: tooth retention, periodontitis recurrence and costs. Journal of Clinical Periodontology, 44, 58–66. [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Paolo, G. , Prato, P. , & Tonetti, M. S. (1996). Long‐term stability of clinical attachment following guided tissue regeneration and conventional therapy. Journal of Clinical Periodontology, 23, 106–111. [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Pini‐Prato, G. , & Tonetti, M. (1994). Periodontal regeneration of human infrabony defects (V). Effect of oral hygiene on long‐term stability. Journal of Clinical Periodontology, 21, 606–610. [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , & Tonetti, M. S. (2004). Long‐term tooth survival following regenerative treatment of intrabony defects. Journal of Periodontology, 75, 672–678. [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , & Tonetti, M. S. (2011). Clinical and radiographic outcomes of the modified minimally invasive surgical technique with and without regenerative materials: a randomized‐controlled trial in intra‐bony defects. Journal of Clinical Periodontology, 38, 365–373. [DOI] [PubMed] [Google Scholar]
- Crea, A. , Dassatti, L. , Hoffmann, O. , Zafiropoulos, G. G. , & Deli, G. (2008). Treatment of intrabony defects using guided tissue regeneration or enamel matrix derivative: a 3‐year prospective randomized clinical study. Journal of Periodontology, 79, 2281–2289. [DOI] [PubMed] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Dias, S. , Sutton, A. J. , Ades, A. E. , & Welton, N. J. (2013). Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Medical Decision Making, 33, 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döri, F. , Arweiler, N. , Húszár, T. , Gera, I. , Miron, R. , & Sculean, A. (2013). Five year results evaluating the effects of platelet‐rich plasma on the healing of intrabony defects treated with an enamel matrix derivative and a natural bone mineral. Journal of Periodontology, 84, 1546–1555. [DOI] [PubMed] [Google Scholar]
- Döri, F. , Arweiler, N. B. , Szántó, E. , Agics, A. , Gera, I. , & Sculean, A. (2013). Ten‐year results following treatment of intrabony defects with an enamel matrix protein derivative combined with either a natural bone mineral or a β‐tricalcium phosphate. Journal of Periodontology, 84, 749–757. [DOI] [PubMed] [Google Scholar]
- Eickholz, P. , Krigar, D. M. , Pretzl, B. , Steinbrenner, H. , Dörfer, C. , & Kim, T. S. (2004). Guided tissue regeneration with bioabsorbable barriers. II. Long‐term results in infrabony defects. Journal of Periodontology, 75, 957–965. [DOI] [PubMed] [Google Scholar]
- Flemmig, T. F. , Ehmke, B. , Bolz, K. , Kübler, N. R. , Karch, H. , Reuther, J. F. , & Klaiber, B. (1998). Long‐term maintenance of alveolar bone gain after implantation of autolyzed, antigen‐extracted, allogenic bone in periodontal intraosseous defects. Journal of Periodontology, 69, 47–53. [DOI] [PubMed] [Google Scholar]
- Górski, B. , Jalowski, S. , Górska, R. , & Zaremba, M. (2020). Treatment of intrabony defects with modified perforated membranes in aggressive periodontitis: a 4‐year follow‐up of a randomized controlled trial. Clinical Oral Investigations, 24, 1183–1196. [DOI] [PubMed] [Google Scholar]
- Heijl, L. , Heden, G. , Svärdström, G. , & Ostgren, A. (1997). Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. Journal of Clinical Periodontology, 24, 705–714. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Altman, D. G. , Gotzsche, P. C. , Juni, P. , Moher, D. , Oxman, A. D. , Savovic, J. , Schulz, K. F. , Weeks, L. , & Sterne, J. A. C. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, T. , Al‐Machot, E. , Meyle, J. , Jervøe‐Storm, P. M. , & Jepsen, S. (2015). Three‐year results following regenerative periodontal surgery of advanced intrabony defects with enamel matrix derivative alone or combined with a synthetic bone graft. Clinical Oral Investigations, 20, 357–364. [DOI] [PubMed] [Google Scholar]
- Hou, X. , Yuan, J. , Aisaiti, A. , Liu, Y. , & Zhao, J. (2016). The effect of platelet‐rich plasma on clinical outcomes of the surgical treatment of periodontal intrabony defects: A systematic review and meta‐analysis. BMC Oral Health, 16, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic, A. , Nikou, G. , Miron, R. J. , Nikolidakis, D. , & Sculean, A. (2014). Which biomaterials may promote periodontal regeneration in intrabony periodontal defects? A systematic review of preclinical studies. Quintessence International, 45, 385–395. [DOI] [PubMed] [Google Scholar]
- Kaldahl, W. B. , Kalkwarf, K. L. , Patil, K. D. , Molvar, M. P. , & Dyer, J. K. (1996). Long‐term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. Journal of Periodontology, 67, 93–102. [DOI] [PubMed] [Google Scholar]
- Kao, R. T. , Nares, S. , & Reynolds, M. A. (2015). Periodontal regeneration ‐ intrabony defects: a systematic review from the AAP Regeneration Workshop. Journal of Periodontology, 86, S77–104. [DOI] [PubMed] [Google Scholar]
- Khoshkam, V. , Chan, H.‐L. , Lin, G.‐H. , Mailoa, J. , Giannobile, W. V. , Wang, H.‐L. , & Oh, T.‐J. (2015). Outcomes of regenerative treatment with rhPDGF‐BB and rhFGF‐2 for periodontal intra‐bony defects: a systematic review and meta‐analysis. Journal of Clinical Periodontology, 42, 272–280. [DOI] [PubMed] [Google Scholar]
- Kim, T. S. , Holle, R. , Hausmann, E. , & Eickholz, P. (2002). Long‐term results of guided tissue regeneration therapy with non‐resorbable and bioabsorbable barriers. II. A case series of infrabony defects. Journal of Periodontology, 73, 450–459. [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. et al (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology, 62, e1–34. [DOI] [PubMed] [Google Scholar]
- Löe, H. , Anerud, A. , Boysen, H. , & Smith, M. (1978). The natural history of periodontal disease in man. Tooth mortality rates before 40 years of age. Journal of Periodontal Research, 13, 563–572. [DOI] [PubMed] [Google Scholar]
- Matuliene, G. , Pjetursson, B. E. , Salvi, G. E. , Schmidlin, K. , Brägger, U. , Zwahlen, M. , & Lang, N. P. (2008). Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. Journal of Clinical Periodontology, 35, 685–695. [DOI] [PubMed] [Google Scholar]
- Matuliene, G. , Studer, R. , Lang, N. P. , Schmidlin, K. , Pjetursson, B. E. , Salvi, G. E. , Brägger, U. , & Zwahlen, M. (2010). Significance of Periodontal Risk Assessment in the recurrence of periodontitis and tooth loss. Journal of Clinical Periodontology, 37, 191–199. [DOI] [PubMed] [Google Scholar]
- Menezes, L. M. , & Rao, J. (2012). Long‐term clinical evaluation of platelet‐rich plasma in the treatment of human periodontal intraosseous defects: A comparative clinical trial. Quintessence International, 43, 571–582. [PubMed] [Google Scholar]
- Mengel, R. , Schreiber, D. , & Flores‐de‐Jacoby, L. (2006). Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: results of a 5‐year clinical and radiological study. Journal of Periodontology, 77, 1781–1787. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med, 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins, M. , Kao, R. T. , McGuire, M. K. , McClain, P. K. , Hinrichs, J. E. , McAllister, B. S. , Reddy, M. S. , Nevins, M. L. , Genco, R. J. , Lynch, S. E. , & Giannobile, W. V. (2013). Platelet‐derived growth factor promotes periodontal regeneration in localized osseous defects: 36‐month extension results from a randomized, controlled, double‐masked clinical trial. Journal of Periodontology, 84, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickles, K. , Ratka‐Krüger, P. , Neukranz, E. , Raetzke, P. , & Eickholz, P. (2009). Open flap debridement and guided tissue regeneration after 10 years in infrabony defects. Journal of Clinical Periodontology, 36, 976–983. [DOI] [PubMed] [Google Scholar]
- Nygaard‐Østby, P. , Bakke, V. , Nesdal, O. , Susin, C. , & Wikesjö, U. M. E. (2010). Periodontal healing following reconstructive surgery: effect of guided tissue regeneration using a bioresorbable barrier device when combined with autogenous bone grafting. A randomized‐controlled trial 10‐year follow‐up. Journal of Clinical Periodontology, 37, 366–373. [DOI] [PubMed] [Google Scholar]
- Ogihara, S. , & Tarnow, D. P. (2014). Efficacy of enamel matrix derivative with freeze‐dried bone allograft or demineralized freeze‐dried bone allograft in intrabony defects: a randomized trial. Journal of Periodontology, 85, 1351–1360. [DOI] [PubMed] [Google Scholar]
- Orsini, M. , Orsini, G. , Benlloch, D. , Aranda, J. J. , & Sanz, M. (2008). Long‐term clinical results on the use of bone‐replacement grafts in the treatment of intrabony periodontal defects. comparison of the use of autogenous bone graft plus calcium sulfate to autogenous bone graft covered with a bioabsorbable membrane. Journal of Periodontology, 79, 1630–1637. [DOI] [PubMed] [Google Scholar]
- Petsos, H. , Ratka‐Krüger, P. , Neukranz, E. , Raetzke, P. , Eickholz, P. , & Nickles, K. (2019). Infrabony defects 20 years after open flap debridement and guided tissue regeneration. Journal of Clinical Periodontology, 46, 552–563. [DOI] [PubMed] [Google Scholar]
- Pietruska, M. , Pietruski, J. , Nagy, K. , Brecx, M. , Arweiler, N. B. , & Sculean, A. (2012). Four‐year results following treatment of intrabony periodontal defects with an enamel matrix derivative alone or combined with a biphasic calcium phosphate. Clinical Oral Investigations, 16, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Pretzl, B. , Kim, T. S. , Holle, R. , & Eickholz, P. (2008). Long‐term results of guided tissue regeneration therapy with non‐resorbable and bioabsorbable barriers. IV. A case series of infrabony defects after 10 years. Journal of Periodontology, 79, 1491–1499. [DOI] [PubMed] [Google Scholar]
- Pretzl, B. , Kim, T. S. , Steinbrenner, H. , Dörfer, C. , Himmer, K. , & Eickholz, P. (2009). Guided tissue regeneration with bioabsorbable barriers III 10‐year results in infrabony defects. Journal of Clinical Periodontology, 36, 349–356. [DOI] [PubMed] [Google Scholar]
- Ridgway, H. K. , Mellonig, J. T. , & Cochran, D. L. (2008). Human histologic and clinical evaluation of recombinant human platelet‐derived growth factor and beta‐tricalcium phosphate for the treatment of periodontal intraosseous defects. International Journal of Periodontics and Restorative Dentistry, 28, 171–179. [PubMed] [Google Scholar]
- Salanti, G. , Ades, A. E. , & Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology, 64, 163–171. [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Chiantella, G. C. , Arweiler, N. B. , Becker, J. , Schwarz, F. , & Stavropoulos, A. (2008) Five‐year clinical and histologic results following treatment of human intrabony defects with an enamel matrix derivative combined with a natural bone mineral. International Journal of Periodontics & Restorative Dentistry 28, 153–161. [PubMed] [Google Scholar]
- Sculean, A. , Donos, N. , Miliauskaite, A. , Arweiler, N. , & Brecx, M. (2001). Treatment of intrabony defects with enamel matrix proteins or bioabsorbable membranes. A 4‐year follow‐up split‐mouth study. Journal of Periodontology, 72, 1695–1701. [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Donos, N. , Schwarz, F. , Becker, J. , Brecx, M. , & Arweiler, N. B. (2004). Five‐year results following treatment of intrabony defects with enamel matrix proteins and guided tissue regeneration. Journal of Clinical Periodontology, 31, 545–549. [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Kiss, A. , Miliauskaite, A. , Schwarz, F. , Arweiler, N. B. , & Hannig, M. (2008). Ten‐year results following treatment of intra‐bony defects with enamel matrix proteins and guided tissue regeneration. Journal of Clinical Periodontology, 35, 817–824. [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Nikolidakis, D. , Nikou, G. , Ivanovic, A. , Chapple, I. L. , & Stavropoulos, A. (2015). Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontology 2000, 68, 182–216. [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Pietruska, M. , Arweiler, N. B. , Auschill, T. M. , & Nemcovsky, C. (2007). Four‐year results of a prospective‐controlled clinical study evaluating healing of intra‐bony defects following treatment with an enamel matrix protein derivative alone or combined with a bioactive glass. Journal of Clinical Periodontology, 34, 507–513. [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Schwarz, F. , Chiantella, G. C. , Donos, N. , Arweiler, N. B. , Brecx, M. , & Becker, J. (2007). Five‐year results of a prospective, randomized, controlled study evaluating treatment of intra‐bony defects with a natural bone mineral and GTR. Journal of Clinical Periodontology, 34, 72–77. [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Schwarz, F. , Miliauskaite, A. , Kiss, A. , Arweiler, N. , Becker, J. , & Brecx, M. (2006). Treatment of Intrabony Defects With an Enamel Matrix Protein Derivative or Bioabsorbable Membrane: An 8‐Year Follow‐Up Split‐Mouth Study. Journal of Periodontology, 77, 1879–1886. [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , Chiantella, G. , Costa, D. , Steigmann, M. , Windisch, P. , & Sculean, A. (2011). Clinical and histologic evaluation of a granular bovine bone biomaterial used as an adjunct to GTR with a bioresorbable bovine pericardium collagen membrane in the treatment of intrabony defects. J Periodont, 82, 462–470. [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , & Karring, T. (2010). Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio‐Oss) in the treatment of intrabony periodontal defects: 6‐year results from a randomized‐controlled clinical trial. Journal of Clinical Periodontology, 37, 200–210. [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , & Wikesjö, U. M. (2010). Influence of defect dimensions on periodontal wound healing/regeneration in intrabony defects following implantation of a bovine bone biomaterial and provisions for guided tissue regeneration: an experimental study in the dog. Journal of Clinical Periodontology, 37, 534–543. [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , & Wikesjö, U. M. E. (2012). Growth and differentiation factors for periodontal regeneration: A review on factors with clinical testing. Journal of Periodontal Research, 47, 545–553. [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , Windisch, P. , Gera, I. , Capsius, B. , Sculean, A. , & Wikesjö, U. M. E. (2011). A phase IIa randomized controlled clinical and histological pilot study evaluating rhGDF‐5/β‐TCP for periodontal regeneration. J Clin Periodont 38, 1044–1054. [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , Windisch, P. , Szendröi‐Kiss, D. , Peter, R. , Gera, I. , & Sculean, A. (2010). Clinical and histologic evaluation of granular Beta‐tricalcium phosphate for the treatment of human intrabony periodontal defects: a report on five cases. Journal of Periodontology, 81, 325–334. [DOI] [PubMed] [Google Scholar]
- Sturtz, S. , Ligges, U. , & Gelman, A. (2005). R2WinBUGS: A Package for Running WinBUGS from R. Journal of Statistical Software, 12, 1–16. [Google Scholar]
- Weigel, C. , Brägger, U. , Hämmerle, C. H. , Mombelli, A. , & Lang, N. P. (1995). Maintenance of new attachment 1 and 4 years following guided tissue regeneration (GTR). Journal of Clinical Periodontology, 22, 661–669. [DOI] [PubMed] [Google Scholar]
- Wikesjo, U. M. , Polimeni, G. , Xiropaidis, A. V. , & Stavropoulos, A. (2010). Periodontal wound healing/regeneration. In Sculean A. (Ed.), Periodontal Regenerative Therapy (pp. 25–45). Quintessence Publishing. [Google Scholar]
- Wilson, T. G. , Carnio, J. , Schenk, R. , & Myers, G. (2008). Absence of histologic signs of chronic inflammation following closed subgingival scaling and root planing using the dental endoscope: human biopsies ‐ a pilot study. Journal of Periodontology, 79, 2036–2041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
Appendix S10
Appendix S11
Appendix S12
Data Availability Statement
Data available upon reasonable request.


