Abstract
Background
The uncertainty about COVID-19 outcomes in angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) users continues with contradictory findings. This study aimed to determine the effect of ACEI/ARB use in patients with severe COVID-19.
Methods
This retrospective cohort study was done in two Saudi public specialty hospitals designated as COVID-19 referral facilities. We included 354 patients with a confirmed diagnosis of COVID-19 between April and June 2020, of which 146 were ACEI/ARB users and 208 were non-ACEI/ARB users. Controlling for confounders, we conducted multivariate logistic regression and sensitivity analyses using propensity score matching (PSM) and Inverse propensity score weighting (IPSW) for high-risk patient subsets.
Results
Compared to non-ACEI/ARB users, ACEI/ARB users had an eight-fold higher risk of developing critical or severe COVID-19 (OR = 8.25, 95%CI = 3.32–20.53); a nearly 7-fold higher risk of intensive care unit (ICU) admission (OR = 6.76, 95%CI = 2.88–15.89) and a nearly 5-fold higher risk of requiring noninvasive ventilation (OR = 4.77,95%CI = 2.15–10.55). Patients with diabetes, hypertension, and/or renal disease had a five-fold higher risk of severe COVID-19 disease (OR = 5.40,95%CI = 2.0−14.54]. These results were confirmed in the PSM and IPSW analyses.
Conclusion
In general, but especially among patients with hypertension, diabetes, and/or renal disease, ACEI/ARB use is associated with a significantly higher risk of severe or critical COVID-19 disease, and ICU care.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; RAAS, renin-angiotensin-aldosterone system; COVID-19, Coronavirus disease,2019; IPSW, inverse propensity score weighting; PSM, propensity score matching; ICU, intensive care unit; OR, odds ratio; WHO, World Health Organization; SARS-CoV-2, severe acute respiratory syndrome coronavirus; MOH, Ministry of Health; NIV, non-invasive ventilation; MV, mechanical ventilation; RT-PCR, real-time polymerase chain reaction (RT-PCR); LOVD, Leiden open variation database
Keywords: Angiotensin-converting enzyme inhibitor, Angiotensin II receptor blocker, COVID-19, Mortality, Disease severity, Hospital admission, Saudi Arabia
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. It emerged in Wuhan, the largest city in Hubei Province, China, and rapidly spread to become a worldwide pandemic [2]. The World Health Organization (WHO) reported as of 31 August 2020 more than 25 million confirmed cases of COVID-19 and more than 800,000 death cases globally [3]. The virus is transmitted through respiratory droplets or aerosols and direct contact. The disease is characterized by a wide range of symptoms, and patients may experience mild, severe, or critical illness [4]. The overall mortality rate in various countries ranges from 2% to 13% [5].
Risk factors associated with the clinical severity of COVID-19 include age (especially >75 years old), obesity, selected medications, and comorbidities such as hypertension, diabetes, and cardiovascular disease [[6], [7], [8], [9], [10]]. Noteworthy is the frequent presence of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II type-I receptor blockers (ARB) in these patients’ pharmacological treatment. It has been hypothesized that exposing patients to ACEI/ARB may increase the expression of ACE2 by a negative feedback mechanism, thus facilitating the binding process of coronavirus to epithelial cells of the kidney, intestine, lung, and blood vessels [11] and increasing the risk of developing severe and fatal COVID-19 disease [[12], [13], [14]]. Clinical research evidence to date has been equivocal, especially in terms of identifying differentiating factors that may mediate the ACEI/ARB and COVID-19 severity relationship [[15], [16], [17]].
In the case of Saudi Arabia, we have a young developing country with a median age of 30.8 years burdened with comorbid diseases [18]. Saudi Arabia's healthcare system is mainly funded by the public sector through the Ministry of Health (MOH) which serves 23 million people. Though at different levels, critical care services are provided in primary, secondary, and tertiary/specialty hospitals; with complex cases being referred to tertiary/specialty hospitals that are well equipped with specialized intensive care units (ICU) and highly trained intensivists [19]. Regarding comorbid disease in the Saudi general population and despite extensive healthcare resources, the WHO ranks Saudi Arabia 7th in the world in terms of rates of diabetes with an estimated 7 million people affected, followed by hypertension (26.1%), and rapidly emerging because of its association with both conditions, chronic kidney disease (6%) [[20], [21], [22]]. Studies link the development of these diseases to ACE insertion/deletion (I/D) polymorphism in certain populations including Saudis [[23], [24], [25], [26]].
The uncertainty about COVID-19 outcomes in ACE/ARB users may be a function of how patients with diabetes, hypertension, and/or chronic renal disease are clinically compromised and therefore more susceptible to severe COVID-19 disease. A systematic review concluded that patients with diabetes, hypertension, and chronic renal disease had an increased mortality risk in the setting of Middle Eastern respiratory syndrome (MERS) coronavirus infection (a coronavirus closely related to SARS-CoV-2 that originated in Saudi Arabia in 2012) [27]. We report here on a retrospective real-world clinical study in two public tertiary/specialty hospitals to which severe COVID-19 cases were referred to assess the association of ACEI/ARB and clinical severity of COVID-19. The severity of COVID-19 disease in ACE/ARB users was based on the following variables: ICU admission, non-invasive and invasive mechanical ventilation, in-hospital mortality, and hospital length of stay. In addition to outcomes observed in the overall population of referred patients, we also evaluated severity outcomes for high-risk patients with diabetes, hypertension, and/or renal disease.
Methods
Study design and study setting
This retrospective study was conducted in the tertiary specialty referral hospitals known as King Fahad Medical City (KFMC; 1200 beds) and Prince Mohammed Bin Abdulaziz hospital (PMAH; 500 beds), both in Riyadh, Saudi Arabia. The Institutional Review Boards at KFMC and PMAH (IRB 20-200) approved the study. All methods were performed following the relevant guidelines and regulations. Institutional review boards of both KFMC and PMAH waived informed consent since it was an exempt study conducting a retrospective analysis.
Participant selection
Lists of discharged or dead COVID-19 patients were obtained from the health informatics officer and categorized into ACEI/ARB users and ACEI/ARB non-users based on their medication history. Inclusion criteria were age >18 years admitted at KFMC or PMAH from April to June 2020; being treated with ACE/ARB inhibitors 6 months before and continued during hospital admission and after discharge for any indications such as hypertension, stroke, heart failure, myocardial infarction, diabetes mellitus, chronic kidney disease or nephrotic syndrome (criteria only for ACEI/ARB users); and SARS-CoV-2 infection confirmed by real-time polymerase chain reaction (RT-PCR) from nasopharyngeal swab for inclusion. Patients were excluded if pregnant, incomplete medical records due to patients being transferred recently from other hospitals or unknown medications history.
Data collection
Data were extracted manually from electronic health records (Cortex system and HIM system in KFMC, and Cerner Systems in PMAH) by a trained team and included demographic and anthropometric variables such as age, height, weight, body mass index (BMI), class of obesity; a medical history of hypertension, diabetes, asthma, cardiovascular disease (including coronary artery disease, heart failure), renal disease (chronic kidney disease or nephrotic syndrome); COVID-19 treatment regimen; use of ACE/ARB; the spectrum of illness severity; the need for ICU admission; the need for non-invasive ventilation (NIV) (e.g., face mask, nasal cannula, nasal mask, or helmet); need for invasive mechanical ventilation (MV); in hospital-death; and length of hospitalization.
COVID-19 Spectrum of severity definitions
We defined the spectrum of disease severity according to the WHO [28]. The mild illness was defined as uncomplicated upper respiratory tract viral infection and may have non-specific symptoms such as fever, fatigue, cough, anorexia, malaise, muscle pain, sore throat, dyspnea, nasal congestion, or headache. The moderate illness was the development of non-severe pneumonia that does not require supplemental oxygen. Severe pneumonia was defined as fever plus symptoms ≥1 of the following: respiratory rate ≥30/min, dyspnea, respiratory distress, SpO2 ≤ 93% on room air, PaO2/FiO2 ratio <300 or lung infiltrate >50% of lung field within 24−48 h. Critical illness manifested by symptoms ≥1 of the following: acute respiratory distress syndrome (ARDS), septic shock, altered consciousness, or multi-organ failure.
Study outcomes
The primary outcome was COVID-19 severity classified as mild, moderate, severe, or critical according to the WHO classification in ACEI/ARB users as compared to non-ACEI/ARB users. Secondarily, we evaluated the need for ICU admission, NIV and MV, in-hospital death, and length of hospital stay in patients on ACEI/ARB as compared to non-ACEI/ARB users. We also assessed COVID-19 severity (severe or critical) in patients with (1) one of the three comorbidities of interest (diabetes, hypertension, or renal disease); (2) both diabetes and hypertension; (3) diabetes only; and (4) hypertension only.
Statistical analysis
Using R Core Team (2020) software (R Foundation for Statistical Computing, Version 4.0.1, Vienna, Austria), continuous data were expressed as mean with standard deviation (±SD) or median with interquartile range (IQR). If the normality assumption was met using a normal Q—Q plot and the Shapiro–Wilk test, we used the Student’s t-test for group comparisons; if not met, we used the Mann-Whitney. Categorical data were reported as frequencies and percentages and analyzed either using the Chi-square test for nxm tables or Fisher's exact test for 2 × 2 tables group comparisons. To obtain odds ratios, we performed a multivariable logistic regression model adjusting for the confounders (either P < 0.3 in a univariable logistic regression model or clinically important confounders) of age, sex, BMI, diabetes, hypertension, renal disease, number of comorbidities (diabetes, hypertension, cardiovascular disease [heart failure/coronary artery disease], stroke, renal disease, asthma, and obesity) and COVID-19 treatment regimen. All statistical inferences were drawn with 95% confidence intervals with P < 0.05. Bonferroni adjustments were applied to control for multiplicity [29].
Sensitivity analyses
Propensity score-matched analysis
The propensity score-matched (PSM) procedure was implemented to the dataset. Using the MatchIt package in R [30], the treatment assignment was modeled with a multivariable logistic regression with the following specifications: nearest neighbor 1:3 with replacement and a caliper of 0.1. Multiple models were fitted and compared using the “cobalt” package to plot propensity score distributional mirror diagrams and standardized mean difference (SMD) before and after matching. The SMD check for data balance is an indicated statistical procedure to judge the quality of matched data rather than the means or standard deviations of the matched subjects [31]. In our case, we considered values of SMD < 0.1 to be indicative of adequate balance and fruitful matching. We carefully examined models in which we evaluated different interaction terms. The final model was then selected based on the above criterion. The following covariates were included in the final model: age, BMI, diabetes, hypertension, renal disease, and several comorbidities. We illustrated the propensity score distribution (prior matching was also evaluated using mirror diagram and the love plot for SMD distribution) (see Supplementary Figs. S1 and S2).
Inverse propensity score weighting (IPSW)
In addition to PSM, we considered a secondary sensitivity analysis for high-risk patients (diabetes, hypertension, or renal disease). We limited the analysis to the severity outcome to avoid a multiplicity problem. In this technique, we used “Weightit” and “ipw” packages in R to calculate inverse propensity weights [32,33]. The following covariates were considered in the IPSW analysis: age, gender, diabetes, hypertension, renal disease, cardiovascular disease, body mass index, and asthma, and several comorbidities. For specifics, see Fig. S3 on covariates selected and balance distribution. Again, various models were fitted and toughly tested before selecting the best model for each data subsets. Following the recommendation from Desai et al. [34], the estimand was the average treatment effect. Stabilized weights were utilized in our analysis and then we examined weights with plots and summary statistics to prevent variance inflation from extreme weights. Love plots for SMD distribution presented in supplementary. Lastly, we fitted a structural causal model using “Survey” package to obtain robust variance estimation (sandwich type).
Results
General description
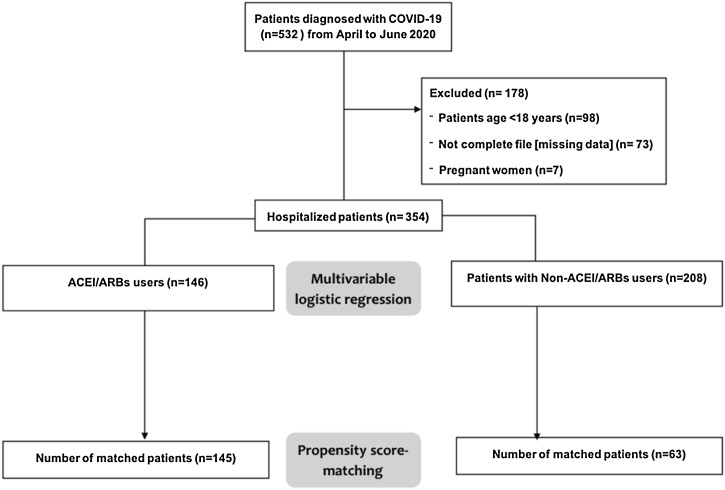
From April to June 2020, a total of 532 confirmed cases of COVID-19 disease were admitted to the KFMC and PMAH hospitals. Of these, 178 patients were excluded from analysis as they did not meet eligibility criteria. A total of 146 patients on ACEI/ARB and 208 were non-ACEI/ARB users included in multivariable logistic regression analysis. Propensity score matching yielded 145 ACEI/ARB users and 63 patients' non-ACEI/ARB users (Fig. 1 ).
Fig. 1.
Study flow diagram. Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; COVID-19, coronavirus disease 2019.
The mean (±SD) age of the ACEI/ARB group was 56.3 (11.9), BMI was 28.8 kg/m2 (6.3) and 78.1% were men, compared with 40.2 years (11.5), BMI of 27.4 kg/m2 (7.4) and 81.7% were men in non-ACEI/ARB group (Table 1 ). Rates of diabetes of 41.6%, hypertension of 34.5%, and renal disease of 7.3% were observed in the total sample. The propensity score matching controlling for the selected covariates was done and presented in supplementary table (Table S1). Supplementary Fig. S1 for propensity scores distribution and Fig. S2 (love plot) shows the balance of covariates. Covariate balance for IPSW/PSM analysis presented in supplementary (Fig. S3). In the PSM analyses, the balance of these covariates variation was checked using standardized mean difference (SMD) and found to be acceptable. Using SMD, we judged the quality of age balance between the two groups and found it to be acceptable (−0.083) in the PSM analysis. In addition to this, SMD for diabetes and hypertension were 0.124 and 0.066, respectively.
Table 1.
Patients' baseline characteristics.
| Baseline | ACEI/ARB (intervention) n = 146 | Non-ACEI/ARB (control) n = 208 | Total n = 354 | P value |
|---|---|---|---|---|
| Age, mean (±SD) | 56.36 (11.9) | 40.29 (11.6) | 46.9(14.11) | <0.001 |
| Male, n (%) | 114 (78.1) | 170 (81.7) | 284 (80.2) | 0.396 |
| BMI, mean (±SD) | 28.8 (6.3) | 27.4 (7.4) | 28(6.9) | 0.059 |
| Obesity, n (%) | 41 (28.1) | 40 (19.2) | 81 (22.9) | 0.051 |
| Hypertension, n (%) | 123 (84.2) | 22 (10.6) | 145 (41.0) | <0.001 |
| Diabetes, n (%) | 98 (67.1) | 42 (20.2) | 140 (39.5) | <0.001 |
| Cardiovascular disease (HF,CAD), n (%) | 42 (28.8) | 5 (2.4) | 47 (13.3) | <0.001 |
| Stroke, n (%) | 3 (2.1%) | 1 (0.5) | 4 (1.1) | 0.168 |
| Renal disease, n (%) | 17 (11.6) | 9 (4.3) | 26 (7.3) | 0.009 |
| Asthma, n (%) | 24 (11.5) | 15 (10.3) | 39 (11.01) | 0.708 |
| Current use of NSAIDs, n (%) | 24 (16.4) | 27 (13.0) | 51 (14.4) | 0.362 |
| Number of comorbiditiesa, Median (IQR) | 3.322 (1.050) 2 (0-2) | 1.688 (0.990) 0 (0−1) | 2.362 (1.295) 1 (0-2) | <0.001 |
| COVID-19 regimen | ||||
| Supportive Care | 105 (71.4) | 159 (76.4) | 264 (74.6) | 0.402 |
| Lopinavir/ritonavir | 2 (1.4) | 0(0) | 2 (0.6) | 0.00 |
| Favipravir | 2 (1.4) | 0(0) | 2 (0.6) | 0.00 |
| Hydroxychloroquine + Azithromycin | 29 (19.9) | 39(18.8) | 68 (19.2) | 0.357 |
| Hydroxychoroquine | 8 (5.5) | 10(4.8) | 18 (5.1) | 0.332 |
IQR: interquartile range. SD: standard deviations. ACEI: angiotensin-converting-enzyme inhibitors. ARBs: angiotensin II receptor blockers. COVID-19: coronavirus disease 2019. HF: heart failure. CAD: coronary artery disease.
Comorbidities include diabetes, hypertension, cardiovascular disease (heart failure/coronary artery disease), stroke, renal disease, asthma, and obesity.
Samples
From crude counts and percentages (Table 2 ), the two multivariable logistic regression models estimated the adjusted odds ratios for the unmatched and matched data for the entire sample. Compared to non-ACEI/ARB users, patients treated with ACEI/ARB were at significantly higher risk of developing severe/critical COVID-19 compared to non-users (OR = 8.25, 95%CI = 3.32–20.53, P < 0.001); being admitted to the ICU (OR = 6.76, 95%CI = 2.88–15.89, P < 0.001); and requiring noninvasive ventilation (OR = 4.77, 95%CI = 2.15–10.55, P < 0.001); but at statistically equal risk of requiring mechanical ventilation or in-hospital death. The results remained significant after adjusting for multiplicity and were confirmed in the PSM sensitivity analysis. Cases classified as a mild-moderate category were higher among non-ACEI/ARB users (P < 0.001; Supplementary Table S2). All other comparisons between ACEI/ARB users and non-users on the outcomes of interest were statistically non-significant. Of note, age was independently associated with an increased need for non-invasive ventilation in the multivariable logistic regression (OR = 1.03, 95%CI = 1.00−1.06, P = 0.036].
Table 2.
Adjusted odds ratio for clinical outcomes among users and non-users of ACEI/ARB.
| Variable | ACEI/ARB (unmatched) n = 146 | Non-ACEI/ARBs (unmatched) n = 208 | Adjusted odds ratiob (95% CI) | P value | Bonferroni adjustment* | ACEI/ARB (matched) n = 145 | Non-ACEI/ARB (matched) n = 63 | Adjusted odds ratioc (95% CI) | P value | Bonferroni adjustment* |
|---|---|---|---|---|---|---|---|---|---|---|
| Severe or criticala, n (%) | 126 (86.3) | 67 (32.2) | 8.25 (3.32–20.53) | <0.001 | <0.001 | 125 (86.2) | 30 (47.6) | 5.55 (2.41–12.80) | <0.001 | <0.001 |
| ICU admission, n (%) |
74 (50.7) | 23 (11.1) | 6.76 (2.88–15.89) | <0.001 | <0.001 | 73 (50.3) | 11 (17.5) | 4.98 (2.02−12.21) | <0.001 | 0.002 |
| Noninvasive Ventilation, n (%) | 103 (70.5) | 54 (26.0) | 4.77 (2.15–10.55) | <0.001 | <0.001 | 103 (71.0) | 25 (39.7) | 3.57 (1.68–8.80) | 0.001 | 0.005 |
| Mechanical ventilation, n (%) | 32 (21.9) | 13 (6.2) | 1.68 (0.58–4.83) | 0.337 | 1.000 | 31 (21.4) | 6 (9.5) | 1.63 (0.53−4.96) | 0.358 | 1.000 |
| In-hospital death, n (%) | 3 (2.1) | 2 (1.0) | 0.35 (0.03–4.63) | 0.427 | 1.000 | 3 (2.1) | 2 (3.2) | 0.48 (0.05–4.49) | 0.521 | 1.000 |
Severe or critical defined according to WHO severity definition. ICU: Intensive care unit.
Odds ratio adjusted using logistic regression model with the following variables: age, sex, BMI, diabetes, hypertension, renal disease, number of comorbidities (that includes diabetes, hypertension, cardiovascular disease (heart failure/coronary artery disease), stroke, renal disease, asthma and obesity) and inpatient COVID-19 regimen.
Odds ratios adjusted using propensity score matching model.
For multiplicity correction, Bonferroni method was used to adjust the P values.
Diabetes, hypertension or renal disease
Patients with diabetes, hypertension, or renal disease on ACEI/ARB therapy were found to have significantly higher COVID-19 severity as compared to non-ACEI/ARB users (72.2% vs 27.8%) with OR 5.34[95% CI = 1.87–15.30] (Table 3 ). Patients with diabetes and hypertension, diabetes alone, or hypertension on ACEI/ARB had a higher severity when compared to non-ACEI/ARB users (Table 3. After adjusting for multiplicity, results remained significant except for the presence of diabetes alone. The PSM sensitivity analyses confirmed these results except for the presence of hypertension and diabetes together. IPSW agrees with the PSM results in which we found significant severity outcome in the following subset of patients: the presence of diabetes, hypertension or renal disease (OR 1.44[95% CI = 1.18–1.76]), diabetes alone (OR 1.33[95% CI = 1.04–1.71]) and hypertension alone (OR 1.47[1.13–1.91]). IPSW results shown in Table 4 .
Table 3.
Clinical severity by comorbidity.
| Population | ACEI/ARB (unmatched) n, (%) | Non-ACEI/ARB (unmatched) n, (%) | Adjusted odds ratiob (95% CI) | P value | Adjustment* | ACEI/ARB (matched) n, (%) | Non-ACEI/ARB (matched) n, (%) | Adjusted odds ratio‡ (95% CI) | P value | Adjustment* |
|---|---|---|---|---|---|---|---|---|---|---|
| DM or HTN or Renal disease | 135(72.2) | 52(27.8) | – | – | – | 134(77.5) | 39(22.5) | – | – | – |
| unmatched (n = 187) | ||||||||||
| Matched (n = 173) | ||||||||||
| Severe or criticala, n (%) | 117(86.7) | 28(53.8) | 5.34(1.87–15.30) | 0.002 | 0.004 | 116(86.6) | 19(48.7) | 5.40(2.01−14.54) | <0.001 | 0.003 |
| DM + HTN | 87(87) | 13(13.0) | – | – | – | 86(86.9) | 13(13.1) | – | – | – |
| Unmatched (n = 100) | ||||||||||
| Matched (n = 99) | ||||||||||
| Severe or criticala, n (%) | 81(93.1) | 10(76.9) | 5.54(2.00−15.34) | 0.001 | 0.004 | 80(93) | 10(76.9) | 5.01(0.80 31.43) | 0.085 | 0.339 |
| DM alone | 98(70) | 42(30.0) | – | – | – | 97(77) | 29(23) | – | – | – |
| Unmatched (n = 140) | ||||||||||
| Matched (n = 126) | ||||||||||
| Severe or criticala, n (%) | 89(90.8) | 24(57.1) | 5.19(1.29−20.87) | 0.020 | 0.081 | 88(51.7) | 15(90.7) | 5.32(1.45−19.56) | 0.012 | 0.047 |
| HTN alone | 123(84.8) | 22(15.2) | – | – | – | 122(84.7) | 22(15.3) | – | – | – |
| Unmatched (n = 145) | ||||||||||
| Matched (n = 144) | ||||||||||
| Severe or criticala, n (%) | 108(87.8) | 13(59.1) | 5.72(1.69−19.32) | 0.005 | 0.020 | 107(87.7) | 13(59.1) | 5.279(1.60–17.47) | 0.006 | 0.025 |
DM: diabetes; HTN: hypertension; CI: confidence interval.
‡Note: Odds ratios adjusted using propensity score matching model.
Severe or critical defined according to WHO severity definition.
Odds ratio adjusted using logistic regression model with the following variables: age, sex, BMI, diabetes, hypertension, renal disease, number of comorbidities (that includes diabetes, hypertension, cardiovascular disease (heart failure/coronary artery disease), stroke, renal disease, asthma and obesity) and inpatient COVID-19 regimen.
For multiplicity correction, Bonferroni method was used to adjust the P values.
Table 4.
Inverse propensity score weighting (IPSW) results for clinical severity/critical outcomes.
| Populationa | Odds ratio (95% CI)b | P value |
|---|---|---|
| DM or HTN or renal disease (n = 187) | 1.44(1.18–1.76) | <0.001 |
| DM + HTN (n = 100) | 1.37(0.90−2.10) | 0.138 |
| DM alone (n = 140) | 1.33(1.04–1.71) | 0.023 |
| HTN alone (n = 145) | 1.47(1.13–1.91) | 0.004 |
DM: diabetes; HTN: hypertension; CI: confidence interval.
Severe or critical defined according to WHO severity definition.
Odds ratios were obtained after fitting a marginal structural model for average treatment effect using weights calculated from propensity scores. Robust sandwich type estimator was used to estimate confidence intervals. The following covariates were considered in the IPSW analysis: age, gender, diabetes, hypertension, renal disease, cardiovascular disease, body mass index, and asthma, and the number of comorbidities. For specifics, see Fig. S3 on covariate selected and balance distribution.
Discussion
Our findings suggested that ACEI/ARB use can adversely affect the severity of COVID-19 illness as evident by the need for ICU admission, and non-invasive ventilation (suggesting more oxygen demand) in ACEI/ARB users. However, similar to the majority of reported studies, there was no association found in the increased need for mechanical ventilation and in-hospital death [15,35,36]. Whether this is a general trend among ACEI/ARB users with COVID-19 or limited to some high-risk substrata is unclear and here our findings may prove relevant.
Many of the patients in our study had comorbid hypertension, diabetes, and renal disease and therefore were being treated with ACEI/ARB agents. It is in these high-risk patients that we observed proportionately more of the severe COVID-19-associated outcomes, which is physiologically plausible. The renin-angiotensin-aldosterone system (RAAS) is reported to play a major role in the pathogenesis of type 2 diabetes and hypertension [37]. Over 30 different variants of the ACE2 gene reported in the Leiden open variation database (LOVD) [38]. Several studies showed a potential link between certain ACE2 variants and the development of cardiovascular diseases such as hypertension [39,40], while other reports find the evidence is inconclusive [[41], [42], [43]]. The high rates of comorbidities in our population may be linked to the existence of ACE2 polymorphism. In particular, the association between ACE insertion/deletion (I/D) polymorphism and the development of type 2 diabetes and hypertension was reported by Alsaikhan et al. in 2017 in Saudi Arabian population [23]. Moreover, studies in Malaysian, Taiwanese, Iranian, and Turkish populations reported similar findings [[24], [25], [26],42]. The evidence that ACEI/ARB use in COVID-19 patients is associated with more severe COVID-19 disease is growing but still insufficient to be conclusive yet biologically plausible. One hypothesis is that SARS-CoV-2 uses ACE2 receptors for cellular entry and that, therefore, ACEI/ARB use can block the receptor and thus prevent viral attachment, entry, and multiplication. In this hypothesis, ACEI/ARB use would ultimately improve patients' outcomes. Conversely, and the hypothesis being evaluated more intensively, that continuous use of these medications may lead to over-expression of ACE2 receptors by a negative feedback mechanism and thus increase coronaviruses binding to target cells [11,43]. Whether the existence of genetic susceptibility to ACE2 polymorphism and ACEI/ARB use has an effect on COVID-19 severity or not is yet to be determined [44].
Although the definitions of the severity of COVID-19 disease need to be harmonized, the contradictory findings reported in different populations with regards to the severity of COVID-19 may suggest population differences [16,17,[45], [46], [47], [48], [49]]. For example, in a US study, Mehta et al. showed no significant association between ACEI/ARB use and mortality, though it reported potential harmful effects with ACEI/ARB use including, higher hospital and ICU admissions [45]. Another study in France by Liabeuf et al. and two studies in the United States by Rentsch et al. and Richardson et al. reported similar findings with regards to greater COVID-19 disease severity [46,49]. In contrast, a large multicenter retrospective study in the United Kingdom (UK) and a single-center retrospective study in Italy observed significantly lower severity of COVID-19 illness among ACEI/ARB users [16,48]. Most of the above-mentioned studies defined the severity of COVID-19 illness as ICU admission rates [16,48]. Further, there could be an ethnicity signal certain populations are more susceptible to severe COVID-19 disease in association with ACEI/ARB use. In addition to our study in a high-risk Saudi population treated in tertiary referral hospitals, a large UK cohort study found that Black Africans on ACEI/ARB had a higher risk of COVID-19 disease [50]. Our population may be considered similar to a study conducted in Turkey [51] that found that ACEI/ARB use in hypertensive patients was independently associated with ICU admission, mechanical ventilation, and in-hospital mortality.
Our study has several strengths and provides additional insights into the association of ACEI/ARB use and severity of COVID-19 disease as potentially mediated by ethnicity. First, to our knowledge, this is the first retrospective study conducted in two public specialty hospitals in the Gulf Cooperation Council (GCC) region designated specifically as national referral hospitals for complex COVID-19 patients that other hospitals in the country cannot manage. The setting lends external validity to the study as these hospitals accommodate patients from all social backgrounds in Saudi Arabia. Secondly, we used multiple definitions to capture the severity of the disease, all of which were significant except for the need for mechanical ventilation, length of stay, and in-hospital death. Thirdly, unlike other studies, we conducted three models to adjust for potential confounders with adjustment for multiplicity problems [47,[52], [53], [54], [55]]. The three analytical approaches confirm the severity outcome. It is noteworthy, the research question of ACEI/ARB versus non-ACEI/ARB users and COVID-19 severity presents a statistical challenge because ACEI/ARB medications are indicated for a particular set of patients that differs from control subjects. This will eventually lead to non-overlapping propensity score distributions. The PSM model will try to find close matches of the control and the treatment based on our specifications and propensity scores, but in this process, many cases from the control/or treatment will be discarded leading to decrease sample size and imprecise estimates. The IPSW circumvents this problem by utilizing all the available sample by assigning weights to observations. The balance can be achieved by creating a pseudo-population in which treatment allocation is independent of observed covariates. In this analysis, extreme weights can be problematic, and running weighted logistic regression may lead to variance inflation because pseudo-population size may be inflated or deflated. In our analysis, we used stabilized weights and weights trimming was not necessary. The robust variance estimator from the “Survey” package gave us precise confidence intervals. We limited the IPSW analysis to a subset of the high population to which we think it's safe to assume that this population will have an indication for ACEI/ARB use. All of the three analyses point towards higher odds of severity among ACEI/ARB users; especially in high risk patients. Due to the small sample size, the COVID-19 severity was not found in the diabetes and hypertension subset of patients. Both PSM and IPSW are very powerful tools for observational studies as means to reduce bias.
Clinically, a temporary ACEI/ARB withdrawal in COVID-19 patients with frequent blood pressure monitoring and management with other appropriate therapies may be a prudent action especially for ICU patients where the risk is higher than the (short-term) benefit in the short term until randomized controlled trials evaluate the impact of interim ACEI/ARB cessation in COVID-19 patients in the hospital setting. This may be viewed by some as a strong statement that goes against the mainstream recommendation to continue these medications [56]; however, our clinical experience is that a short-term ACEI/ARB withdrawal (≤2 weeks) strategy does not seem to increase the risk of cardiovascular disease deterioration in low-risk patients. Indirect evidence may be inferred from a randomized trial comparing ACEI (quinapril) continuation or withdrawal (by giving placebo) for 16 weeks in heart failure patients, which showed that heart failure symptoms worsened only after 4–6 weeks following ACEI withdrawal [57]. The two weeks strategy we propose as an interim clinical approach, though subject to formal evaluation in a clinical trial, might reduce the severity of COVID-19 disease, the need for ICU admission, and the need for oxygen through non-invasive ventilation. Admittedly, this debated question can be answered by well-designed RCTs. Until such trials as REPLACECOVID in the US, the BRACE-CORONA trial in Brazil, and the ACEI-COVID trial in Austria, that compare the effects of ACEI/ARB continuation or withdrawal in hospitalized COVID19 patients [[58], [59], [60]].
Limitations
We recognize that the retrospective nature of this study is a limitation, though due largely because it was virtually impossible to mount a study when the hospitals were being overwhelmed with complex and severe cases of COVID-19. We could not stratify patients according to their chronicity of ACEI/ARB use because this information was not available. Notwithstanding the multiple models to adjust for confounding variables, we may have overlooked other variables that may explain the severity of COVID-19 disease. Some confidence intervals are wide and may limit the interpretability of some of the results. We could not assess the duration of non-invasive ventilation in patients on ACEI/ARB as compared to non-ACEI/ARB users. Lastly, our study did not differentiate between the two classes of antihypertensive agents (ACEI or ARB).
Conclusions
ACEI/ARB use was found to be associated with adverse effects on the severity of COVID-19 disease in Saudi Arabian patients, most of them with the comorbidities, singularly or concomitantly, of hypertension, diabetes, and renal disease. As compared to non-ACE/ARB users, ACEI/ARB users had higher disease severity and ICU admission, and non-invasive ventilation. Notably, patients on ACEI/ARB with diabetes, hypertension and renal disease were at higher risk of developing severe COVID-19 disease compared to non-ACEI/ARB.
Funding
The authors would like to thank the Research Center at King Fahd Medical City, Riyadh, Saudi Arabia, for their financial support provided for the manuscript.
Competing interests
None declared.
Ethics approval
The Institutional Review Boards at KFMC and PMAH (IRB 20-200) approved the study. All methods were performed following the relevant guidelines and regulations. Informed consent was waived since it was an exempt study conducting a retrospective analysis.
Acknowledgments
The authors would like to thank the staff at the Research Center of King Fahad Medical City and Prince Mohammed Bin Abdulaziz Hospital for their valuable contribution. The authors are also thankful to AlMaarefa University, Riyadh for providing support to do this research.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.03.004.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.W.H. Organization . 2020. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 4.Lotfi M., Hamblin M.R., Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Tawfiq J.A., Leonardi R., Fasoli G., Rigamonti D. Prevalence and fatality rates of COVID-19: What are the reasons for the wide variations worldwide? Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H., Wang S., Zhong F., Bao W., Li Y., Liu L. Age-dependent risks of incidence and mortality of COVID-19 in Hubei Province and other parts of China. Front Med. 2020;7:190. doi: 10.3389/fmed.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caussy C., Wallet F., Laville M., Disse E. Obesity is associated with severe forms of COVID-19. Obesity. 2020;28(7):1175. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Xiang J. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38(5):781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Tanja H., Sandra E. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover A., Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blocker. Eur Heart J Cardiovasc Pharmacother. 2021;7(2):148–157. doi: 10.1093/ehjcvp/pvaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felice C., Nardin C., Di Tanna G.L., Grossi U., Bernardi E., Scaldaferri L. Use of RAAS Inhibitors and Risk of Clinical Deterioration in COVID-19: Results From an Italian Cohort of 133 Hypertensives. Am J Hypertens. 2020;33(10):944–948. doi: 10.1093/ajh/hpaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liabeuf S., Moragny J., Benni Y., Batteux B., Brochot E., Schmit L. Association between renin-angiotensin system inhibitors and COVID-19 complications. Eur Heart J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.H. Plecher, Saudi Arabia: Average age of the population from 1950 to 2050, Statista. https://www.statista.com/statistics/262482/median-age-of-the-population-in-saudi-arabia/ (accessed Sep. 01, 2020).
- 19.Al-Khaldi Y.M., Al-Ghamdi E.A., Al-Mogbil T.I., Al-Khashan H.I. Family medicine practice in Saudi Arabia: the current situation and proposed strategic directions plan 2020. J Family Commun Med. 2017;24(3):156. doi: 10.4103/jfcm.JFCM_41_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham I., Kurdi S., MacDonald K. The hypertension, diabetes and chronic kidney disease triangle in Arab countries. J Hum Hypertens. 2017;31(6):373. doi: 10.1038/jhh.2017.16. [DOI] [PubMed] [Google Scholar]
- 21.Naeem Z. Burden of diabetes mellitus in Saudi Arabia. Int J Health Sci (Qassim) 2015;9(3):V. doi: 10.12816/0024690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alwin Robert A., Abdulaziz Al Dawish M., Braham R., Ali Musallam M., Abdullah Al Hayek A., Hazza Al Kahtany N. Type 2 diabetes mellitus in Saudi Arabia: major challenges and possible solutions. Curr Diabetes Rev. 2017;13(1):59–64. doi: 10.2174/1573399812666160126142605. [DOI] [PubMed] [Google Scholar]
- 23.Al-Saikhan F.I., Abd-Elaziz M.A., Ashour R.H. Association between risk of type 2 diabetes mellitus and angiotensin-converting enzyme insertion/deletion gene polymorphisms in a Saudi Arabian population. Biomed Rep. 2017;7(1):56–60. doi: 10.3892/br.2017.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghazali D.M., Rehman A., Rahman A.R.A. Candidate gene polymorphisms and their association with hypertension in Malays. Clin Chim Acta. 2008;388(1–2):46–50. doi: 10.1016/j.cca.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Nikzamir A.R., Nakhjavani M., Golmohamadi T., Dibai L. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with metabolic syndrome in Iranians with type 2 diabetes mellitus. Arch Iran Med. 2008;11(1):3–9. [PubMed] [Google Scholar]
- 26.Agachan B., Isbir T., Yilmaz H., Akoglu E. Angiotensin converting enzyme I/D, angiotensinogen T174M-M235T and angiotensin II type 1 receptor A1166C gene polymorphisms in Turkish hypertensive patients. Exp Mol Med. 2003;35(6):545–549. doi: 10.1038/emm.2003.71. [DOI] [PubMed] [Google Scholar]
- 27.Morra M.E. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-analysis. Rev Med Virol. 2018;28(3):e1977. doi: 10.1002/rmv.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.W.H. Organization . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. 13 March 2020. [Google Scholar]
- 29.Lee S., Lee D.K. What is the proper way to apply the multiple comparison test? Korean J Anesthesiol. 2018;71(5):353. doi: 10.4097/kja.d.18.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuart E.A., King G., Imai K., Ho D. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011 [Google Scholar]
- 31.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Comput. 2009;38(6):1228–1234. [Google Scholar]
- 32.Greifer Noah. 2020. WeightIt: weighting for covariate balance in observational studies.https://cran.r-project.org/web/packages/WeightIt/index.html [Google Scholar]
- 33.van der Wal W.M., Geskus R.B. Ipw: an R package for inverse probability weighting. J Stat Softw. 2011;43(13):1–23. [Google Scholar]
- 34.Desai R.J., Franklin J.M. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657. doi: 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Yu J., Pan L., Jiang H. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. 2020:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alamer A., Abraham I. Mortality in COVID-19 patients treated with ACEIs/ARBs: re-estimated meta-analysis results following the Mehra et al. Retraction. Pharmacol Res. 2020;160:105053. doi: 10.1016/j.phrs.2020.105053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jandeleit-Dahm K.A.M., Tikellis C., Reid C.M., Johnston C.I., Cooper M.E. Why blockade of the renin--angiotensin system reduces the incidence of new-onset diabetes. J Hypertens. 2005;23(3):463–473. doi: 10.1097/01.hjh.0000160198.05416.72. [DOI] [PubMed] [Google Scholar]
- 38.Global Variome shared LOVD . 2008. Unique variants in gene ACE2.https://databases.lovd.nl/shared/variants/ACE2/unique (Accessed 27 July 2020) [Google Scholar]
- 39.Pinheiro D.S. The combination of ACE I/D and ACE2 G8790A polymorphisms revels susceptibility to hypertension: a genetic association study in Brazilian patients. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0221248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duru K., Farrow S., Wang J.-M., Lockette W., Kurtz T. Frequency of a deletion polymorphism in the gene for angiotensin converting enzyme is increased in African-Americans with hypertension. Am J Hypertens. 1994;7(8):759–762. doi: 10.1093/ajh/7.8.759. [DOI] [PubMed] [Google Scholar]
- 41.Yang M., Zhao J., Xing L., Shi L. The association between angiotensin-converting enzyme 2 polymorphisms and essential hypertension risk: A meta-analysis involving 14,122 patients. J Renin-Angiotensin-Aldosterone Syst. 2015;16(4):1240–1244. doi: 10.1177/1470320314549221. [DOI] [PubMed] [Google Scholar]
- 42.Tseng C.H., Tseng C.P., Chong C.K., Sheu J.J., Cheng J.C. Angiotensin-converting enzyme gene polymorphism and stroke in type 2 diabetic patients in Taiwan. Eur J Clin Invest. 2007;37(6):483–491. doi: 10.1111/j.1365-2362.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- 43.Khan S.H., Zaidi S.K. Review of evidence on using ACEi and ARBs in patients with hypertension and COVID-19. Drugs Ther Perspect. 2020:1. doi: 10.1007/s40267-020-00750-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippi G., Lavie C.J., Henry B.M., Sanchis-Gomar F. Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin Chem Lab Med. 2020;58(9):1415–1422. doi: 10.1515/cclm-2020-0727. [DOI] [PubMed] [Google Scholar]
- 45.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson S., Hirsch J.S., Narasimha M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bean D., Kraljevic Z., Searle T., Bendayan R., Kevin O., Pickles A. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur J Heart Fail. 2020;22(6):967–974. doi: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rentsch C.T., Kidwai-Khan F., Tate J.P., Park L.S., King J.T., Skanderson M. Covid-19 testing, hospital admission, and intensive care among 2,026,227 united states veterans aged 54–75 years. MedRxiv. 2020 doi: 10.1101/2020.04.09.20059964. [DOI] [Google Scholar]
- 50.Hippisley-Cox J., Young D., Coupland C., Channon K.M., Tan P.S., Harrison D.A. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selçuk M., Çınar T., Keskin M., Çiçek V., Kılıç S., Kenan B. Is the use of ACE inb/ARBs associated with higher in-hospital mortality in Covid-19 pneumonia patients? Clin Exp Hypertens. 2020;42(8):738–742. doi: 10.1080/10641963.2020.1783549. [DOI] [PubMed] [Google Scholar]
- 52.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang G., Tan Z., Zhou L., Yang M., Peng L., Liu J. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension. 2020;76(1):51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 54.Huang Z., Cao J., Yao Y., Jin X., Luo Z., Xue Y. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020;8(7):430. doi: 10.21037/atm.2020.03.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Huang F., Xu Y., Yang P., Qin Y., Cao M. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.03.20.20039586. [DOI] [Google Scholar]
- 56.Krahn A., Bewick S.J., Chow C.M., Clarke B., Cowan S. Guidance from the CCS COVID-19 rapid response team. Can Cardiovasc Soc. 2020 https://ccs.ca/guidance-from-the-ccs-covid-19-rapid-response-team-and-ccs-affiliate-organizations/. (Accessed 27 July 2020) [Google Scholar]
- 57.Pflugfelder P.W., Baird G., Tonkon M.J., DiBianco R., Pitt B. Clinical consequences of angiotensin-converting enzyme inhibitor withdrawl in chronic heart failure: a double-blind, placebo-controlled study of quinapril. J Am Coll Cardiol. 1993;22(6):1557–1563. doi: 10.1016/0735-1097(93)90578-o. [DOI] [PubMed] [Google Scholar]
- 58.Clinicaltrial.gov . 2020. Elimination or prolongation of ACE inhibitors and ARB in coronavirus disease 2019 (REPLACECOVID)https://clinicaltrials.gov/ct2/show/NCT04338009 [Google Scholar]
- 59.Clinicaltrial.gov Angiotensin receptor blockers and angiotensin-converting enzyme inhibitors and adverse outcomes in patients with COVID19 (BRACE-CORONA) https://clinicaltrials.gov/ct2/show/NCT04364893
- 60.Clinicaltrial.gov . 2020. Stopping ACE-inhibitors in COVID-19 (ACEI-COVID)https://clinicaltrials.gov/ct2/show/NCT04353596 (Accessed 27 July 2020) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.