Abstract
Background
In dialysis patients, non-adherence to oral cinacalcet adds complexity to the control of secondary hyperparathyroidism. The present study aims to evaluate the use of intravenous calcimimetic, etelcalcetide, in the control of secondary hyperparathyroidism in patients adherent and non-adherent to oral calcimimetics.
Method
The Simplified Medication Adherence Questionnaire was used to identify non-adherence. Almost half of the patients were non-adherent to the treatment with cinacalcet. Twenty-five patients (15 non-adherent) were switched from cinacalcet to etelcalcetide and were followed-up monthly for 8 months.
Results
Cinacalcet was discontinued for 1 week before the initiation of etelcalcetide. After this period, the serum PTH levels increased by2-fold in adherent patients, whereas it did not change in non-adherent patients suggesting that they were not taking the medication. Etelcalcetide progressively reduced serum parathyroid hormone (PTH) (mean ± standard deviation) from 818 ± 395 to 367 ± 289 pg/mL (P < 0.001) in non-adherents, and from 496 ± 172 to 228 ± 111 pg/mL (P < 0.01) in adherent patients with a mean dose of 7.0 ± 2.3 and 5.1 ± 1.2 mg in non-adherent and in adherent patients, respectively. Etelcalcetide increased the percentage of patients with PTH on target from 28% to 58%. Patients with serum calcium <8.4 mg/dL increased from 8% to 40%, although they remained asymptomatic. The percent of patients with serum phosphate on target increased from 40% to 65%.
Conclusion
The lack of adherence to cinacalcet is a possible cause of the apparent lack of response to oral calcimimetic. The use of etelcalcetide ensures compliance and control of secondary hyperparathyroidism in both non-adherent and adherent patients.
Keywords: adherence, calcium, cinacalcet, etelcalcetide, PTH
INTRODUCTION
Treatment of abnormalities of mineral metabolism in haemodialysis (HD) patients is complex, and the lack of adherence complicates the daily clinical practice. According to the World Health Organization, adherence to treatments in patients with chronic diseases is around 50% [1]. The activation of calcium-sensing receptors (CaSR) by calcimimetics reduces serum parathyroid hormone (PTH) levels in HD patients with uncontrolled hyperparathyroidism. The oral calcimimetic, cinacalcet, has been available for >14 years [2–4]. Etelcalcetide, a new calcimimetic given intravenously, has demonstrated to be effective and non-inferior to cinacalcet in the control of secondary hyperparathyroidism [5–7].
In some patients with severe hyperparathyroidism, the administration of cinacalcet fails to reduce PTH levels [8]. These may be patients with tertiary hyperparathyroidism, with nodular parathyroid hyperplasia, containing cells with low expression of CaSR unable to respond to calcimimetics [9, 10]. It is also possible that the poor control of PTH is caused by non-adherence to the treatment [10–12]. The use of IV calcimimetics will determine if uncontrolled hyperparathyroidism is due to a real resistance to the action of calcimimetics or the lack of adherence to the prescribed medication.
The use of intravenous (IV) calcimimetics should be appropriate for patients that are not adherent to oral calcimimetics. However, there are also data suggesting that IV calcimimetics (etelcalcetide) are more effective than the oral medication [13], but it is not clear if, in patients adherent to oral calcimimetics, the change to IV calcimimetics improves the control of secondary hyperparathyroidism.
The present study aims to evaluate the use of the IV calcimimetic, etelcalcetide, in the control of secondary hyperparathyroidism in patients adherent and non-adherent to oral calcimimetics.
MATERIALS AND METHODS
Study population
Patients were informed about the study, and those who signed the consent form were included. The study was conducted in two outpatient HD facilities of Vithas: Hospital Perpetuo Internacional from Alicante and Elche (Spain).
Identification of adherence to treatment with cinacalcet
Self-reported non-adherence to cinacalcet was estimated using the Simplified Medication Adherence Questionnaire (SMAQ) shown in Table 1, together with data collected relative to the use of the prescribed medication. SMAQ has been validated in the Spanish population with AIDS [12, 13] and has shown sufficient internal consistency in HD patients [14]. Patients were considered non-adherent when answering the questions as follows: 1: No; 2: Yes; 3: Yes; 4: Yes; 5: C, D or E; 6: more than 2 days.
Table 1.
SMAQ
| 1. Do you always take your medication at the appropriate time? | □ Yes □ No |
| 2. When you feel bad, have you ever discontinued taking your medication? | □ Yes □ No |
| 3. Have you ever forgotten to take your medication? | □ Yes □ No |
| 4. Have you ever forgotten to take your medications during the weekend? | □ Yes □ No |
| 5. In the last week, how many times did you fail to take your prescribed dose? |
|
| 6. Since your last visit how many whole days have gone by in which you did not take your medication? | Days: …. |
Patients were classified as (i) adherent, those that were not identified as being non-adherent in the SMAQ questionnaire, and collected the prescribed medication from the pharmacy, or (ii) non-adherent, those with occasional or persistent non-compliance or those that failed to collect medication regularly. Tolerability to cinacalcet was assessed in a different specific questionnaire.
Changes in PTH, 1 week after discontinuation of oral calcimimetic in non-adherent and adherent patients
In non-adherent patients, the discontinuation of the medication should not have a significant effect on PTH levels. Twenty-five patients maintained on cinacalcet were switched to etelcalcetide, and 15 of them were classified as non-adherent. Following the instructions for the administration of etelcalcetide, cinacalcet was discontinued for 1 week before the initiation of etelcalcetide. Serum PTH, calcium and phosphate were measured just before and 1 week after the discontinuation of cinacalcet.
Efficacy of etelcalcetide in the control of PTH in non-adherent and adherent patients to oral calcimimetics
Non-adherent (N = 15) and adherent (N = 10) patients received etelcalcetide and were followed monthly for 8 months. Blood for biochemistry was obtained monthly before the midweek dialysis.
Protocol for the use of etelcalcetide
The initial dose of etelcalcetide was 2.5 mg at the end of the HD session. A low dose of paricalcitol (maximum of 2 mg post-HD) was administered to prevent hypocalcaemia. Dose titration of etelcalcetide and paricalcitol was as follows: if PTH levels were >300 pg/mL and serum calcium <7.5 mg/dL with phosphate <5 mg/dL, the dose of paricalcitol was increased; if serum calcium was >7.5 mg/dL and serum phosphate >5 mg/dL, the dose of etelcalcetide was increased. In the case of PTH <150 pg/mL and serum calcium <8.3 mg/dL, the dose of etelcalcetide was reduced. If PTH was <150 pg/mL and phosphate >5 mg/dL, the dose of paricalcitol was reduced. The prescription of phosphate binders was not changed. Dialysate calcium concentration was 2.5 mEq/L in all patients during 8 months of follow-up. The rest of the treatment was unchanged.
Statistical analysis
Data are expressed as mean [standard deviation (SD)] or median [interquartile range (IQR)] as appropriate. Categorical data are expressed as a percentage. Differences between means were analysed using the Wilcoxon signed-rank or the Mann–Whitney test. Statistics were performed using R 3.6.1 (R Development Core Team, 2019), the tableOne package (v0.10.0; Kazuki and Bhon, 2019) and the GraphPad Prism 6.0c (GraphPad Software, La Jolla, CA, USA). Two-sided P-values <0.05 were considered statistically significant.
RESULTS
Characteristics of non-adherent and adherent patients
There were 25 patients included in the analysis. Results from the SMAQ questionnaire revealed that 15 out of 25 (60.0%) recognized non-adherence to cinacalcet treatment. Seven patients (13.5%) admitted intentional non-adherence (they decided not to take the medication), 12 patients (23.1%) forgot to take the medication and 10 (19.2%) admitted both reasons. In only three patients, gastric intolerance was the cause of non-adherence to cinacalcet (5.8%). The main clinical and biochemical characteristics of the overall cohort, and adherent and non-adherent patients at baseline are shown in Table 2. The mean PTH value throughout the previous 6 months was slightly higher in non-adherent (761 ± 377 pg/mL) than in adherent patients (579 ± 228 pg/mL), although this difference did not reach statistical significance (P = 0.184). As compared with adherent patients, the non-adherent patients showed a higher concentration of phosphate (P = 0.070) and comparable values of serum calcium (P = 0.201). The prescribed dose of paricalcitol and oral calcimimetic was superior in non-adherent to adherent patients; however, the difference did not reach statistical significance.
Table 2.
Characteristics of the patients and the comparison between adherent and non-adherent patients at baseline
| Variable | Overall | Adherent | Non-adherent | P |
|---|---|---|---|---|
| N = 25 | N = 10 | N = 15 | ||
| Agea (years) | 61.8 (14.3) | 61.4 (15.6) | 62.1 (13.9) | 0.903 |
| Gender (male, %) | 10 (40.0) | 5 (50.0) | 5 (33.3) | 0.442 |
| Dialysis vintageb (years) | 10.0 (3.0–13.0) | 8.4 (1.7–12.3) | 10.0 (4.7–15.9) | 0.487 |
| Time on cinacalcetb (years) | 5.0 (1.4–9.0) | 4.4 (0.6–9.4) | 5.0 (2.1–9.0) | 0.739 |
| Dose of cinacalceta (mg/week) | 33.0 (21.6) | 24.0 (14.4) | 39.0 (23.9) | 0.090 |
| Number of phosphate bindersa (pills/day) | 4.6 (2.6) | 3.6 (1.0) | 5.2 (3.1) | 0.178 |
| Paricalcitol dosea (μg/week) | 4.7 (1.9) | 5.0 (2.4) | 4.5 (1.6) | 0.652 |
| PTHb (pg/mL) | 643.0 (385.1–869.1) | 566.6 (380.5–694.5) | 857.3 (423.0–987.7) | 0.222 |
| Calciuma,c (mg/dL) | 8.5 (0.8) | 8.8 (0.4) | 8.3 (0.9) | 0.201 |
| Phosphatea,c (mg/dL) | 4.4 (1.7) | 3.7 (1.3) | 5.0 (1.8) | 0.069 |
Mean ± SD.
Median (IQR).
Average levels during the 6 months prior to inclusion.
Changes in PTH, 1 week after discontinuation of oral calcimimetic in non-adherent and adherent patients
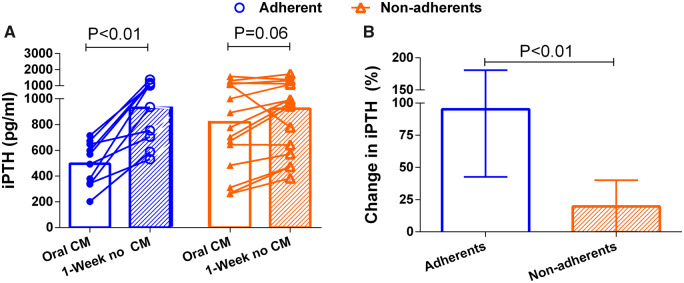
Twenty-five patients (15 non-adherent and 10 adherent) were switched from cinacalcet to etelcalcetide. Cinacalcet was discontinued for 1 week before starting IV calcimimetics. One week after the discontinuation of oral calcimimetics, the serum PTH level increased by 2-fold in adherent patients (Figure 1A). By contrast, in non-adherent patients, the level of PTH did not increase significantly after the discontinuation of oral calcimimetic (Figure 1A). Figure 1B shows the percent increase in PTH 1 week after discontinuation of cinacalcet in adherent versus non-adherent patients.
FIGURE 1:
Comparison of PTH before and 1 week after the discontinuation of cinacalcet in adherent and non-adherent patients. (A) Individual changes. (B) Median percent change in PTH. Bars represent IQR.
The efficacy of the IV calcimimetic, etelcalcetide, in the control of PTH in patients non-adherent and adherent to oral calcimimetics
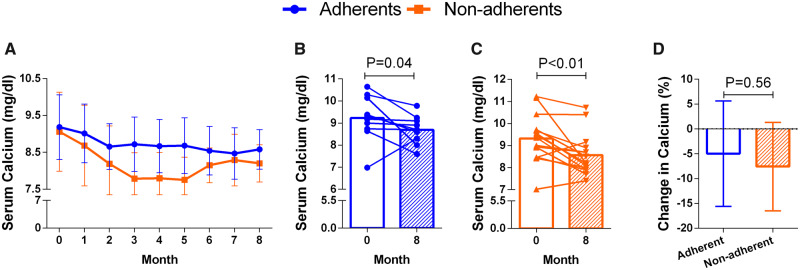
The change in PTH after 8 months of treatment with etelcalcetide in non-adherent and adherent patients is shown in Figure 2. The serum concentration of PTH progressively decreased in non-adherent patients, from 740 ± 504 pg/mL to 330 ± 306 pg/mL (P < 0.001). In adherent patients, serum PTH dropped from 362 ± 209 pg/mL to 187 ± 137 pg/mL (P < 0.01; Figure 2A–C). The percent reduction of PTH (Figure 2D) and the final absolute concentration of PTH were comparable in non-adherent and adherent patients. The dose of etelcalcetide was modified in each patient to achieve a target PTH of 200–400 pg/mL (Figure 3). The mean doses per session required to achieve target PTH were 7.0 ± 2.3 and 5.1 ± 1.2 mg in non-adherent and adherent patients, respectively (Figure 3). Thus, the dose required to control PTH in non-adherent patients was superior than in adherent patients, although these differences tended to decrease at the end of the follow-up period.
FIGURE 2:
Comparison of serum PTH concentration before and after 8 months of etelcalcetide in non-adherent and adherent patients. Bars represent IQR.
FIGURE 3:
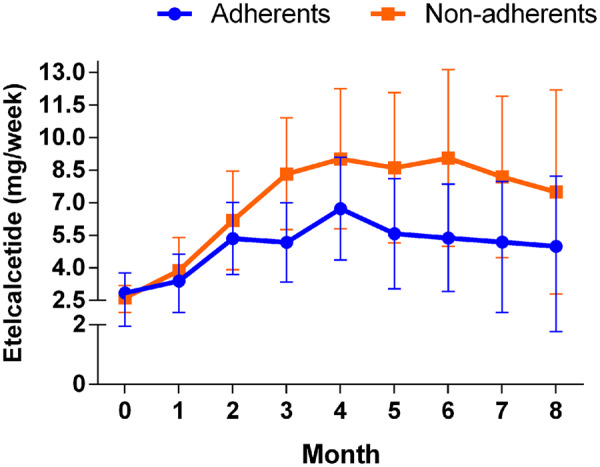
Comparison of etelcalcetide dose (mg post HD) in adherent and non-adherent patients. Bars represent SD.
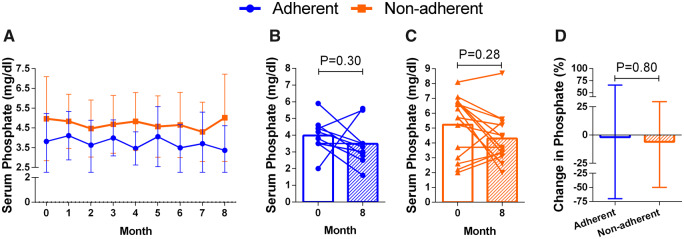
Changes in serum calcium and phosphate after treatment with the IV calcimimetic are shown in Figures 4 and 5. Serum calcium concentration decreased in the same proportion in both adherent and non-adherent patients, and serum phosphate concentration did not change in any of the two groups (Figure 5).
FIGURE 4:
Changes in serum calcium concentration before and after 8 months of etelcalcetide in non-adherent and adherent patients. Bars represent SD.
FIGURE 5:
Changes in serum phosphate concentration before and after 8 months of etelcalcetide in non-adherent and adherent patients. Bars represent SD.
Considering all patients (adherents and non-adherents), after 8 months on etelcalcetide, the percentage of patients with PTH values within the target range increased from 28% to 58% and the percentage of patients with PTH >400 pg/mL decreased from 76% to 20%. The percentage of patients with serum calcium <8.4 mg/dL increased from 8% to 40%, but symptomatic hypocalcaemia was not observed. Finally, the percentage of patients with serum phosphate within the target range increased from 40% to 65% at the end of the study (Figure 6).
FIGURE 6:
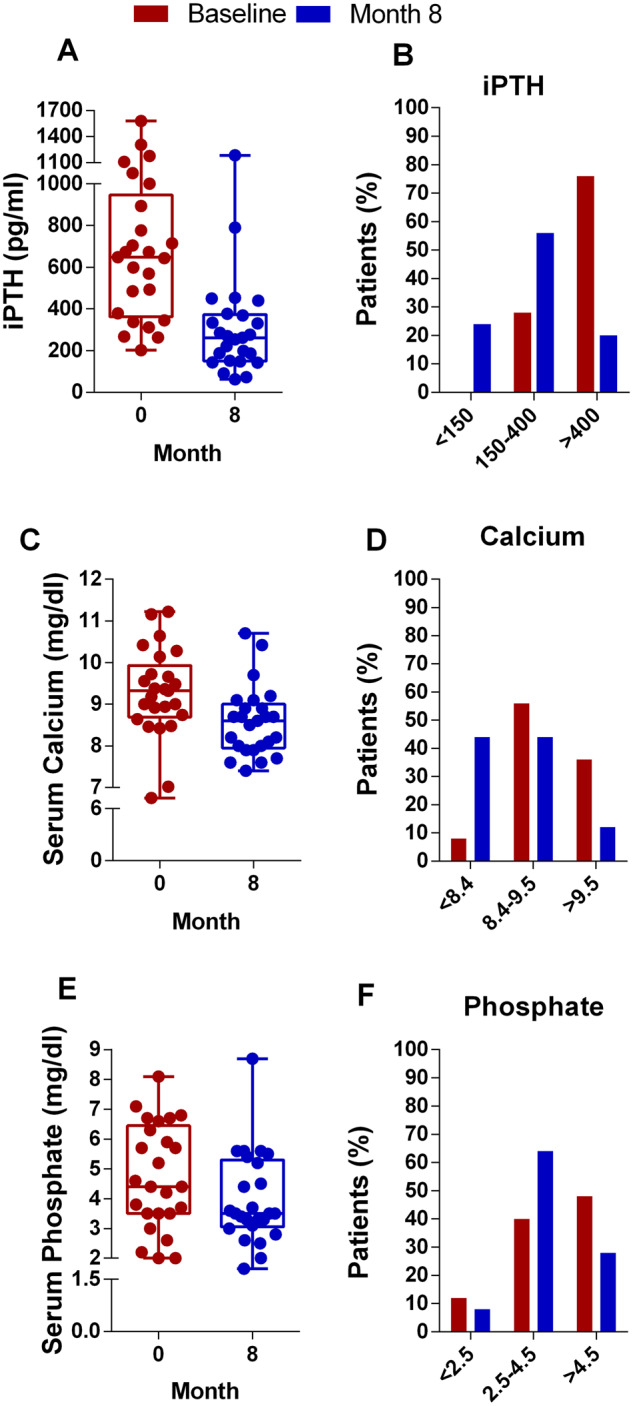
Number of subjects and percent of patients achieving the target of intact PTH (iPTH) (A and B), calcium (C and D) and phosphate (E and F).
DISCUSSION
Our report shows that half of the patients on oral calcimimetics were not fully compliant with the treatment. Non-adherence was evaluated by a questionnaire and by demonstrating that PTH did not change significantly after discontinuation of the oral calcimimetic. The change from oral to IV calcimimetic improved the control of PTH in both adherent and non-adherent patients without clinically relevant adverse effects.
Patients were suspected to be non-adherent when serum PTH remained elevated despite high prescribed doses of cinacalcet. A relatively simple and validated questionnaire helped to identify non-compliance. Furthermore, an easy test to assess patients' compliance is to discontinue the administration of cinacalcet for 7 days and determine whether there is a change in PTH levels. Non-adherent patients showed no perceptible change in PTH concentration after discontinuation of cinacalcet, whereas a significant increase in serum PTH levels was observed in adherent patients after suspension of the calcimimetic. Most often, the change in PTH could be observed in less than 7 days.
The lack of adherence to cinacalcet could be interpreted as a lack of response to the treatment. It is unknown the number of non-adherent patients that may have undergone parathyroidectomy due to an apparent resistance to calcimimetics. It is also essential to recognize that in HD patients the frequency of non-compliance is very high [10–13]. Assessment of compliance is essential to discriminate between drug resistance and lack of adherence; this is particularly important in the case of oral calcimimetics, which now can be switched to IV administration.
In daily clinical practice, assessment of the patient’s adherence is frequently based on non-objective methods; however, there are more specific tools that can be used to identify non-adherence. As shown by Burnier et al. [14], adherence questionnaires require few resources, and are affordable and adaptable to the characteristics of each centre. Furthermore, self-report adherence scales as SMAQ have the potential to measure both medication-taking behaviours and also to identify the causes of non-adherence. Traditionally, it has been considered that the complexity of the therapeutic regimen, intolerance and side effects of the drugs are the leading causes of the lack of adherence [15]. In the case of cinacalcet, the therapeutic regimen is simple (only one daily tablet), and still, non-adherence was high. Gastrointestinal intolerance was the cause of non-compliance in only 5.8% of our patients.
The recent approval of a calcimimetic for IV administration, etelcalcetide [11], with similar or superior efficacy as compared with cinacalcet [7], offers the possibility of ensuring adherence with better control of the treatment. In our study, in both adherent and non-adherent patients, PTH levels were better controlled after 8 months of treatment with modest doses of IV calcimimetic, suggesting that the use of IV calcimimetic should not be restricted to non-adherent patients.
The reduction of PTH by the administration of IV calcimimetics was associated with a decrease in serum calcium concentration, which was similar in adherent and non-adherent patients but never decreased below 7.5 mg/dL. Serum phosphate concentration did not change significantly, which may be due in part to the use of paricalcitol that, even in moderate doses, may prevent a significant reduction in serum phosphate concentration [16].
Regarding the dose of IV calcimimetics, the non-adherent group had poor control of PTH, and they required higher doses of IV calcimimetics. At 8 months, the dose requirements were stable and somewhat superior in non-adherent than in adherent patients, but the difference was not statistically significant. The proportion of patients with PTH on target was 80%, and the proportions with calcium and phosphate on target were 44% and 64%, respectively. Thus, our results are in line with those reported by Bushinsky et al. [17].
Our study has some limitations. There are some other methods to evaluate adherence, but there is not a gold standard. It seems that new approaches such as Medication Event Monitoring System (MEMS) are likely more objective compared with SMAQ. MEMS is currently considered a good and reliable measure of medication-taking behaviour. Nevertheless, medication-taking and the possibility of intentional dose dumping remain. Furthermore, MEMS implementation is expensive, not readily available for some dose forms and time-consuming. SMAQ is the largest implemented and validated adherence scale worldwide [18]. Studies that have compared different scales to evaluate adherence have shown that SMAQ shows high sensitivity and specificity, with an excellent positive likelihood ratio compared with MEMS. Indeed, both scales identified similar rates of non-adherence . Therefore, SMAQ is a valid instrument to identify non-adherence [13].
In conclusion, the lack of adherence to cinacalcet should always be considered as a cause of poor control of hyperparathyroidism. The use of the IV route ensures compliance and improves the control of hyperparathyroidism in all patients, adherents and non-adherents.
FUNDING
M.V.P.-R.d.M. is the recipient of a research contract supported by the Rio Hortega Programme from the National Institute of Health Carlos III.
AUTHORS’ CONTRIBUTIONS
Each co-author has contributed to the creation of this manuscript and has approved its submission to Clinical Kidney Journal. M.D.A. and M.R. contributed to the study design; M.D.A., C.R.-H. and M.V.P.-R.d.M. contributed to data collection; M.D.A., C.R.-H., M.V.P.-R.d.M. and M.R. contributed to data analysis/interpretation; M.D.A., C.R.-H., M.V.P.-R.d.M. and M.R. contributed to the statistical analysis; M.D.A., C.R.-H., M.V.P.-R.d.M. and M.R. contributed to drafting the manuscript; and M.D.A., C.R.-H., M.V.P.-R.d.M. and M.R. contributed to approving the final version of manuscript. M.D.A., C.R.-H., M.V.P.-R.d.M. and M.R. take responsibility for the integrity of the data analysis.
CONFLICT OF INTEREST STATEMENT
M.R. has received research grants from Amgen and Fresenius Medical Care and lecture fees from the following companies: Amgen, Abbott, Shire and Fresenius Medical Care. M.D.A. has received lecture fees from the following companies: Amgen, Abbott, Shire and Vifor Fresenius. The companies highlighted in the financial disclosures had no role in the study design, collection, analysis or interpretation of data. The rest of the authors have no conflict of interest to declare. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. De Geest S, Sabaté E.. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs 2003; 2: 323. [DOI] [PubMed] [Google Scholar]
- 2. Block GA, Martin KJ, de Francisco ALM. et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004; 350: 1516–1525 [DOI] [PubMed] [Google Scholar]
- 3. Lindberg JS, Culleton B, Wong G. et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol 2005; 16: 800–807 [DOI] [PubMed] [Google Scholar]
- 4. Wei M, Taskapan H, Esbaei K. et al. K/DOQI guideline requirements for calcium, phosphate, calcium phosphate product, and parathyroid hormone control in dialysis patients: can we achieve them? Int Urol Nephrol 2006; 38: 739–743 [DOI] [PubMed] [Google Scholar]
- 5. Li X, Yu L, Asuncion F. et al. Etelcalcetide (AMG 416), a peptide agonist of the calcium-sensing receptor, preserved cortical bone structure and bone strength in subtotal nephrectomized rats with established secondary hyperparathyroidism. Bone 2017; 105: 163–172 [DOI] [PubMed] [Google Scholar]
- 6. Hirai T, Nakashima A, Takasugi N. et al. Association of nodular hyperplasia with resistance to cinacalcet therapy for secondary hyperparathyroidism in hemodialysis patients. Ther Apher Dial 2010; 14: 577–582 [DOI] [PubMed] [Google Scholar]
- 7. Block GA, Bushinsky DA, Cheng S. et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017; 317: 156–164 [DOI] [PubMed] [Google Scholar]
- 8. Lee A, Song X, Khan I. et al. Association of cinacalcet adherence and costs in patients on dialysis. J Med Econ 2011; 14: 798–804 [DOI] [PubMed] [Google Scholar]
- 9. Forni Ogna V, Pruijm M, Zweiacker C et al.. Clinical benefits of an adherence monitoring program in the management of secondary hyperparathyroidism with cinacalcet: results of a prospective randomized controlled study. BioMed Res Int 2013; 2013: 104892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gincherman Y, Moloney K, McKee C et al.. Assessment of adherence to cinacalcet by prescription refill rates in hemodialysis patients. Hemodial Int 2010; 14: 68–72 [DOI] [PubMed] [Google Scholar]
- 11. Block GA, Bushinsky DA, Cunningham J. et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017; 317: 146–155 [DOI] [PubMed] [Google Scholar]
- 12. Ghimire S, Castelino RL, Lioufas NM et al.. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PloS One 2015; 10: e0144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knobel H, Alonso J, Casado JL. et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS 2002; 16: 605–613 [DOI] [PubMed] [Google Scholar]
- 14. Burnier M, Pruijm M, Wuerzner G. et al. Drug adherence in chronic kidney diseases and dialysis. Nephrol Dial Transplant 2015; 30: 39–44 [DOI] [PubMed] [Google Scholar]
- 15. Horne R, Weinman J.. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health 2002; 17: 17–32 [Google Scholar]
- 16. Wetmore JB, Gurevich K, Sprague S. et al. A randomized trial of cinacalcet versus vitamin D Analogs as monotherapy in secondary hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol 2015; 10: 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bushinsky DA, Chertow GM, Cheng S. et al. One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism. Nephrol Dial Transplant 2019; doi: 10.1093/ndt/gfz039 [DOI] [PMC free article] [PubMed]
- 18. Nguyen T-M-U, La Caze A, Cottrell N.. What are validated self-report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol 2014; 77: 427–445 [DOI] [PMC free article] [PubMed] [Google Scholar]