Abstract
Background
Despite early referral of uraemic patients to nephrological care, suboptimal dialysis initiation (SDI) remains a common problem associated with increased morbimortality. We hypothesized that SDI is related to pre-dialysis care.
Methods
In the ‘Peridialysis’ study, time and reasons for dialysis initiation (DI), clinical and biochemical data and centre characteristics were registered during the pre- and peri-dialytic period for 1583 end-stage kidney disease patients starting dialysis over a 3-year period at 15 nephrology departments in the Nordic and Baltic countries to identify factors associated with SDI.
Results
SDI occurred in 42%. Risk factors for SDI were late referral, cachexia, comorbidity (particularly cardiovascular), hypoalbuminaemia and rapid uraemia progression. Patients with polycystic renal disease had a lower incidence of SDI. High urea and C-reactive protein levels, acidosis and other electrolyte disorders were markers of SDI, independently of estimated glomerular filtration rate (eGFR). SDI patients had higher eGFR than non-SDI patients during the pre-dialysis period, but lower eGFR at DI. eGFR as such did not predict SDI. Patients with comorbidities had higher eGFR at DI. Centre practice and policy did not associate with the incidence of SDI.
Conclusions
SDI occurred in 42% of all DIs. SDI was associated with hypoalbuminaemia, comorbidity and rate of eGFR loss, but not with the degree of renal failure as assessed by eGFR.
Keywords: chronic kidney disease, dialysis, glomerular filtration rate, pre-dialysis care, unplanned dialysis
INTRODUCTION
Optimal timing of dialysis initiation (DI) in patients with end-stage kidney disease (ESRD) is important for reducing the risk of increased morbimortality due to uraemic complications. Suboptimal dialysis initiations (SDIs)—using a temporary central dialysis catheter access for haemodialysis (HD) or peritoneal dialysis (PD) immediately after placement of a PD catheter—are common, involving as many as 40–50% of ESRD patients starting dialysis [1, 2], even in the absence of late referral to Nephrology care [3]. SDI is associated with increased need for hospitalizations [4–6], increased early mortality after DI [5–8], lower quality of life [9, 10] and high socioeconomic costs [1, 5]. Use of central dialysis catheters for HD is associated with markedly increased risk for fatal infections and cardiovascular mortality [11–15]. However, despite the awareness that SDI represents a major problem, there are relatively few studies on the causes of SDI, and previous studies on SDI have either been registry studies, or generally characterized by low patient numbers. These have recently been reviewed by Hassan et al. [16]. We have therefore initiated an observational multi-centre international study, the ‘Peridialysis’ study, in the Nordic and Baltic countries, in order to improve our understanding of why and when physicians prescribe dialysis [17].
We hypothesized that SDI is a consequence of multiple factors such as inadequate pre-dialysis care, deficient organization on the system- and provider-level and insufficient methods to determine optimal timing of DI. The aim of this study was therefore to describe characteristic features of SDI and to identify risk factors for SDI.
MATERIALS AND METHODS
Fifteen Nephrology departments from seven Nordic and Baltic countries took part in this observational prospective study of causes and timing of DI. They were publicly financed. They all had a well-developed multidisciplinary pre-dialysis care structure including specialist physicians and nurses; 13/15 centres also had a dietician and 5/15 a social worker.
The most common method of assessing residual renal function to guide clinical treatment was estimated glomerular filtration rate (eGFR) as measured by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [18]. The centres followed the European Renal Best Practice (ERBP) guidelines for the initiation of dialysis [19], i.e. dialysis is to be initiated to treat clinical uraemic symptoms, unless there are specific life-threatening biochemical problems such as hyperkalaemia.
Patients
All patients starting chronic dialysis therapy for ESRD at the participating centres between 1 January 2015 and 31 December 2017 were potentially includable. Five centres joined the study after 1 January 2015 and had a shorter recruiting period.
Methods
Centres supplied estimates of incidence rates of HD and PD. The number of nephrology specialists at the centre was noted. Centre policies concerning dialysis planning, access placement and DI were registered. Late referral to nephrological care was defined as <3 months before DI.
The following patient data were registered at DI: age, sex, height, weight, body mass index (BMI), renal diagnosis and comorbidity.
Dialysis access at first dialysis was registered. DI was classified as optimal if:
the vascular access for HD was an arteriovenous fistula (AVF) or arteriovenous graft (AVG);
the access for HD was a tunnelled catheter as the patient’s permanent access; and
the access was a PD catheter, and PD was started >6 days after placement.
DI was suboptimal (SDI) if:
the access for HD was a temporary vascular catheter;
the access for HD was a tunnelled catheter, but a later AVF/AVG was planned; and
the access was a PD catheter, and PD was started <6 days after placement, due to dialysis requirement before the catheter had completely healed.
As patients undergoing pre-emptive kidney transplantation were often assessed and treated at other departments, they were excluded from the study.
Clinical data for this population have previously been published [17]. For the purposes of this study, clinical symptoms were classified as being present or not present and representing a primary cause or not a primary cause of DI. Life-threatening conditions were defined as the presence of pulmonary stasis, dyspnoea, cardiac symptoms, pericarditis, acidosis or hyperkalaemia and similarly classified.
Patient biochemical data
The following biochemical data prior to or in conjunction with the first dialysis were registered: blood haemoglobin, plasma concentrations of urea, creatinine, potassium, hydrogen carbonate (bicarbonate), albumin, C-reactive protein (CRP), calcium and phosphate.
Whenever available, plasma creatinine concentration and date of measurement were registered at the following time points: referral to the nephrology department; ∼6 and 3 months before DI; information about dialysis (and associated modality choice); dialysis access prescription; dialysis access placement; first dialysis. eGFR was calculated using the CKD-EPI formula [18]. ‘Rapid eGFR loss’ was defined as a fall in eGFR (ΔeGFR) >1 mL/min/1.73 m2/month. A positive ΔeGFR means a falling eGFR.
Based on post hoc analysis showing that all variables except blood haemoglobin and plasma albumin correlated to the inverse of eGFR (CRP after logarithmic transformation), we defined the ‘excess’ variable concentration as the difference between the actual variable concentration and the value that would be expected from the correlation to eGFR. The ‘excess’ variable concentration was used as a supplementary measure of biochemical uraemia in addition to eGFR.
The study protocol was approved by the ethical review boards in centres located in countries where according to the country’s regulations such perusal was required. The study was approved by the Swedish Ethical Review Authority (Ref 2017/7). However, in Denmark, due to the observational non-interventional design of the study using anonymized patient data, the study protocol was not considered to be eligible for ethical review. Informed consent—either written or verbal depending on the regulations in the different countries—was obtained from participants in all centres including those in Denmark with the exception of Lithuania, where patient permission was waived by the ethics board (P2-BE-2-9/2014). The study is registered with ClinicalTrials.gov, identifier NCT02488200.
Statistics
Data are presented as mean (standard deviation) for normally distributed variables, median [interquartile range (IQR)] for non-normally distributed variables, or as numbers and percentage. Parametric variables were compared using the Students t-test and analysis of variance, and non-parametric variables using the Chi-squared, Mann–Whitney and Kruskal–Wallis tests. Odds ratios were calculated according to Altman. A probability level of <0.05 was considered significant. Significant values were expressed as P < 0.05, P < 0.01 or P < 0.001.
RESULTS
Centre details
A total of 1622 patients were included; 24 patients were excluded due to lack of basic data (age, sex and initial dialysis access) and 14 patients were excluded due to pre-emptive transplantation. The remaining 1583 patients were included in this study. The number of included patients was 67 (62–153)/centre, corresponding to an incidence of 35 (21–65) patients/centre/year. Included patients represented 81% of the estimated total incidence of 43 (25–77) patients/centre/year. Three centres had inclusion <70% of the estimated total incidence. The incidence of included patients varied according to centre size; five non-university centres reported an included incidence <30 new ESRD patients/year/centre.
Intervals for out-patient visits varied according to the severity of renal failure. Average visit intervals in weeks were: stable chronic kidney disease Stage 3 (CKD 3), 28 ± 10; unstable CKD 3, 18 ± 5; stable CKD 4, 17 ± 4; unstable CKD 4, 10 ± 4; stable CKD 5, 8 ± 4; and unstable CKD 5, 5 ± 3 weeks. eGFR was measured at every visit.
Four centres recommended starting dialysis information at an eGFR of 20–25 mL/min/1.73 m2, three at 15–19 mL/min/1.73 m2 and seven at 10–14 mL/min/1.73 m2, while one centre did not take eGFR into account. One centre stated that they also considered the rate of eGFR loss.
AVF placement was recommended at an eGFR of <15 mL/min/1.73 m2 in eight centres and at eGFR of 10–12 mL/min/1.73 m2 in one centre. Five centres used eGFR loss rate, and one centre had no eGFR recommendation for timing of AVF placements. AVF placement was performed at median (IQR) 3.8 (1.6–10.5) months before DI at an eGFR of 9.9 ± 3.8 mL/min/1.73 m2. Planned PD catheter placements were performed with a median of 0.9 (0.6–1.6) months before DI at an eGFR of 8.5 ± 3.7 mL/min/1.73 m2.
Five centres (33%) had no official guideline for the warranted level of eGFR at DI, six (40%) recommended eGFR of 9–14 mL/min/1.73 m2 and four (27%) centres stated that they accepted eGFR <9 mL/min/1.73 m2 at DI. However, in nine centres (60%), uraemic symptoms were the major indication for DI.
Patient characteristics
Clinical patient data for patients with optimal DI and SDI are shown in Table 1. There was no relationship of SDI to age, sex or diabetic status. Patients with SDI were characterized by high comorbidity, particularly cardiovascular, and were less likely to have polycystic renal disease. About 40.2% had life-threatening conditions, and these were the primary reason for DI in 19.6%. SDI was required in 52.5% of patients with life-threatening reasons, and this group comprised 339 (51.1%) patients out of all 664 SDI patients.
Table 1.
Clinical characteristics of patients with optimal DI and SDI
| Variable | All | Optimal DI | SDI |
|---|---|---|---|
| Patients, n (%) | 1583 | 919 (58.1) | 664 (41.9) |
| Age, years | 63.8 ± 15.3 | 63.7 ± 15.2 | 64.1 ± 15.4 |
| Female sex, % | 570 (36.0) | 342 (37.2) | 228 (34.3) |
| BMI, kg/m2 | 26.9 ± 6.2 | 27.1 ± 6.3 | 26.5 ± 6.1 |
| BMI excluding Type 2 diabetes mellitus | 26.1 ± 5.9 | 26.4 ± 5.9 | 25.8 ± 5.8 |
| Late referral,a % | 311 (21.0) | 59 (6.8)** | 252 (41.0)** |
| Renal diagnosis, n (%) | |||
| Glomerulonephritis | 284 (17.9) | 160 (17.4) | 124 (18.7) |
| Interstitial | 185 (11.7) | 111 (12.1) | 74 (11.1) |
| Polycystic | 106 (6.7) | 85 (9.2) | 21 (3.2)** |
| Diabetic | 387 (24.4) | 233 (25.3) | 154 (23.2) |
| Hypertensive | 302 (19.1) | 196 (21.3) | 106 (16.0)* |
| Other | 178 (11.2) | 57 (6.2) | 121 (18.2)** |
| Unknown | 141 (8.9) | 77 (8.4) | 64 (9.6) |
| Comorbidity | |||
| No comorbidity | 425 (26.9) | 265 (28.8) | 160 (24.1)* |
| AMI | 171 (10.8) | 86 (9.4) | 85 (12.8)* |
| Heart failure | 262 (16.6) | 128 (13.9) | 134 (20.2)** |
| Cerebrovascular | 189 (11.9) | 105 (11.4) | 84 (12.7) |
| Peripheral vascular (%) | 191 (12.1) | 99 (10.8) | 92 (13.9)P = 0.06 |
| Diabetes | 549 (34.7) | 315 (34.3) | 234 (35.2) |
| Cancer | 263 (16.6) | 137 (14.9) | 126 (19.0)* |
| Pulmonary | 151 (9.5) | 83 (9.0) | 68 (10.2) |
| Hepatic | 61 (3.8) | 27 (2.9) | 34 (5.1)* |
| Previous transplantation | 81 (5.1) | 51 (5.5) | 30 (4.5) |
| Psychiatric | 68 (4.3) | 38 (4.1) | 30 (4.5) |
| Clinical symptoms | 1546 pts. | ||
| Present | 1291(83.5) | 751 (83.7) | 540 (83.7) |
| Primary DI cause | 950 (61.4) | 574 (63.8) | 376 (58.2)* |
| Life-threatening, n (%) | |||
| Present | 621 (40.2) | 276 (30.7) | 339 (52.5)** |
| Primary DI cause | 303 (19.6) | 111 (12.3) | 192 (29.7)** |
Results stated as numbers (%), mean ± standard deviation or median (IQR).
One hundred and seven patients not stated. AMI, acute myocardial infarction.
P < 0.05 (versus optimal),
P < 0.001.
The primary causes of SDI according to the treating physician were: acute progression of chronic uraemia in 236 (35.5% of all SDI), acute uraemia 138 (20.8%), late referral 81 (12.2%), delayed planning 69 (10.4%), patient non-concordance 60 (9.0%) [of whom 18 (2.7%) initially refused of dialysis], access problems 28 (4.2%) and other/not stated reasons 29 (4.4%).
Late referrals
Referral times were available for 1478 patients: 495 (33.5% of all DI) were referred <12 months before DI and 311 (21.0% of all DI) were referred late (<3 months before DI). Of the latter, 252 (81.0% of all late referrals; 41.0% of all SDI) underwent SDI.
Dialysis access and access placement
The elapsed time between prescription of dialysis access and access placement did not seem to have any effect on SDI. Excluding patients with a very short interval (<2 weeks), there was no correlation between operational delay, even >3 months and SDI. The average incidence of SDI for patients with an interval >2 weeks was 16.3%.
Rate of eGFR decline prior to DI
The relationships of optimal DI and SDI to eGFR and ΔeGFR are shown in Table 2. For optimal DI patients, ΔeGFR was constant, around median 6–7 mL/min/1.73 m2/year during the 6 months prior to DI, while for SDI patients, it accelerated from 8.9 to 21.9 mL/min/1.73 m2/year. Patients with SDI had a significantly faster ΔeGFR during the intervals 6–3 months and 3–0 months before DI. As a corollary, their eGFR was higher at 6 and 3 months before DI, but their eGFR at dialysis information, access prescription, access placement and DI were all lower, even after excluding patients with late referral. Dialysis information to early referral SDI patients was given at median eGFR 9.8 (7.2–12.2) mL/min/1.73 m2, versus 11.2 (9.1–11.2) for non-SDI, and access was prescribed at median eGFR 7.4 (5.4–9.3) mL/min/1.73 m2 versus 8.8 (7.2–10.6) (all P < 0.001).
Table 2.
Time intervals, eGFR levels and eGFR progression rates at different time points prior to first dialysis in patients with optimal DI and SDI
| Variable | Patients included (n) | All patients | Optimal DI | SDI |
|---|---|---|---|---|
| Time interval, months | ||||
| Referral dialysis | 1320 | 29.0 (5.6–59.7) | 40.4 (18.5–67.4) | 7.5 (0.2–45.8) |
| Information dialysis | 1204 | 4.0 (0.5–10.1) | 6.7 (3.0–14.0) | 0.3 (0.0–3.8) |
| Access prescription dialysis | 1124 | 1.1 (0–2.9) | 2.3 (1.2–5.0) | 0.0 (0.0–0.3) |
| Access placement dialysis | 939 | 0.5 (0–1.6) | 1.2 (0.7–3.4) | 0.0 (0.0–0.0) |
| eGFR, mL/min/1.73 m2 | ||||
| Referral | 1414 | 20.7 (11.5–32.4) | 23.0 (15.0–34.0) | 15.9 (7.0–30.4) |
| 6 months prior | 1202 | 12.0 (9.6–16.5) | 11.1 (9.2–13.6) | 16.7 (11.7–26.2) |
| 3 months prior | 1225 | 10.0 (8.0–13.5) | 9.3 (7.6–11.4) | 13.5 (9.5–20.6) |
| Dialysis information | 1448 | 10.1 (7.4–12.8) | 10.9 (8.8–13.4) | 8.1 (5.4–11.5) |
| Access prescription | 1537 | 7.9 (6.0–10.0) | 8.6 (7.1–10.5) | 6.6 (4.7–8.7) |
| Access placement | 1499 | 7.5 (5.5–9.7) | 8.3 (6.7–10.4) | 6.2 (4.4–8.1) |
| First dialysis | 1571 | 6.6 (4.9–8.4) | 7.0 (5.5–8.9) | 6.0 (4.3–7.9) |
| ΔeGFR, mL/min/1.73 m2/year | ||||
| Referral dialysis | 1320 | 5.3 (2.8–10.7) | 4.8 (2.8–7.9) | 7.6 (3.1–21.2) |
| 6 months prior dialysis | 1194 | 8.5 (4.1–16.5) | 6.5 (3.2–10.9) | 16.7 (9.1–35.0) |
| 6–3 months prior | 1147 | 6.9 (1.8–14.6) | 6.1 (1.6–11.8) | 8.9 (2.7–22.3) |
| 3 months prior dialysis | 1219 | 9.6 (3.8–21.0) | 7.0 (2.3–13.4) | 21.9 (10.8–54.3) |
| Information dialysis | 1204 | 5.6 (2.0–12.1) | 4.8 (1.9–9.2) | 9.5 (2.8–25.7) |
| Access prescription dialysis | 1124 | 4.3 (−0.1 to 12.3) | 3.8 (−0.3 to 9.2) | 10.4 (0.0–37.5) |
| Access placement dialysis | 939 | 3.9 (−0.6 to 12.3) | 3.9 (−0.5 to 11.1) | 0.9 (−3.6 to 44.9) |
Median values (IQR). All P < 0.001. Δ, rate of change of eGFR. Positive values mean falling eGFR.
Biochemistry at DI
Biochemical values at DI are shown in Table 3. After correcting for eGFR, SDI patients were characterized by lower albumin, calcium, bicarbonate and higher urea, phosphate, CRP and potassium levels.
Table 3.
Biochemical variables at DI in patients with optimal DI and SDI
| Variable | Number | All | Optimal DI | SDI |
|---|---|---|---|---|
| Patients with any value, n | 1571 | 911 | 657 | |
| eGFR, mL/min/1.73 m2a | 1571 | 7.3 (3.6) | 7.7 (3.4) | 6.6 (3.8) |
| Haemoglobin, mM | 1568 | 6.4 (1.3) | 6.6 (1.1) | 6.1 (1.4) |
| Urea, mM | 1538 | 33.8 (12.3) | 30.2 (9.6) | 38.7 (13.8) |
| Potassium, mM | 1543 | 4.53 (0.9) | 4.40 (0.7) | 4.7 (1.0) |
| Bicarbonate, mM | 1182 | 21.0 (5.1) | 21.9 (4.3) | 19.6 (5.8) |
| Albumin, g/L | 1418 | 32.8 (6.7) | 34.6 (5.8) | 30.1 (7.0) |
| CRP, mg/L | 1445 | 10 (3–41) | 6 (3–16) | 28 (6–80) |
| Calcium ion, mM | 1502 | 1.14 (0.13) | 1.17 (0.11) | 1.11 (0.14) |
| Phosphate, mM | 1481 | 1.98 (0.62) | 1.85 (0.52) | 2.19 (0.68) |
| Variable excess compared with expected from eGFRb | ||||
| Urea, mM | 1532 | 0.0 (11.1) | −2.4 (9.1) | 3.5 (12.6) |
| Potassium, mM | 1536 | 0.0 (0.8) | −0.08 (0.7) | 0.11 (1.0) |
| Bicarbonate, mM | 1178 | 0.0 (4.8) | 0.6 (4.3) | −0.9 (5.4) |
| Log CRP, mg/L | 1440 | 0.0 (1.56) | −0.47 (1.36) | 0.64 (1.59) |
| Calcium ion, mM | 1495 | 0.0 (0.13) | 0.02 (0.12) | −0.03 (0.13) |
| Phosphate excess, mM | 1475 | 0.0 (0.55) | −0.08 (0.47) | 0.12 (0.64) |
Mean (standard deviation) or median (IQR). All P < 0.001.
For median values, see Table 2.
For method of calculation, see Materials and methods section.
Risk factors for SDI
Significant risk factors for SDI are shown in Table 4. In addition to the above-mentioned factors, a subgroup of cachectic patients (BMI <18 kg/m2) had a higher SDI incidence. These patients had a higher eGFR at DI than non-cachectic patients (9.9 ± 5.9 versus 7.2 ± 3.5 mL/min/1.73 m2; P < 0.001).
Table 4.
Significant risk factors for SDI results stated as n (%) and odds ratio (95% CI)
| Variable | Group | All patients (n; % of column) | SDI (n; % of row) | Odds ratio (95% CI) |
|---|---|---|---|---|
| All patients, n (%) | – | 1583 | 667 (42.2) | |
| Centre ESRD incidence | <30a | 264 (16.7) | 54 (20.8) | Reference |
| (n/year) | 30–70 | 474 (30.0) | 203 (42.8) | 2.91 (2.05–4.13)*** |
| >70 | 845 (53.4) | 407 (48.2) | 3.61 (2.60–5.00)*** | |
| BMIb<18 kg/m2 | – | 40 (2.9) | 23 (57.5) | 1.92 (1.01–3.60)* |
| Polycystic disease | – | 106 (6.7) | 21 (19.8) | 0.32 (0.20–0.52)*** |
| Comorbidity numberc | 1 | 342 (21.6) | 160 (46.8) | 1.38 (1.08–1.77)** |
| >1 | 155 (9.8) | 82 (52.9) | 1.77 (1.26–2.48)*** | |
| Previous myocardial infarction | – | 171 (10.8) | 85 (49.7) | 1.42 (1.03–1.95)* |
| Heart failure | – | 262 (16.6) | 134 (51.2) | 1.56 (1.20–2.03)*** |
| Hepatic disease | – | 61 (3.9) | 34 (55.7) | 1.78 (1.06–2.98)* |
| Peripheral atherosclerosis | – | 191 (12.1) | 92 (48.2) | 1.37 (0.97–1.78)P = 0.06 |
| Late referral (<3 months) | – | 311 (21.0) | 252 (81.0) | 9.49 (6.97–12.94)*** |
| Life-threatening problem | – | 621 (40.2) | 345 (55.6) | 2.58 (2.09–3.19)*** |
| 6–3 months ΔeGFR >1 mL/min/1.73 m2/month | – | 349 (30.4) | 156 (44.7) | 2.26 (1.74–2.94)*** |
| 3–0 months ΔeGFR >1 mL/min/1.73 m2/month | – | 526 (43.2) | 288 (54.8) | 6.01 (4.61–7.80)*** |
| Average eGFR at DI <7.0 mL/min/1.73 m2 | – | 845 (53.9) | 413 (48.9) | 1.89 (1.54–2.32)*** |
| Albumin, g/L | <30 | 489 (34.5) | 288 (58.9) | 4.72 (3.59–6.19)*** |
| 30–34.9 | 412 (29.1) | 156 (37.9) | 2.01 (1.51–2.67)*** | |
| >34.9 | 515 (36.4) | 120 (23.3) | Reference |
All non-university.
Type 2 diabetes mellitus excluded.
Sum of four identified significant risk factors.
CI, confidence interval. *P < 0.05 (versus optimal); **P < 0.01; ***P < 0.001.
In a post hoc analysis of 88 patients, plasma albumin at DI was found to be highly correlated to plasma albumin 3 months previously (r = 0.87), and the difference small (0.2 ± 2.7 g/L). Using three classification groups (plasma albumin <30, 30–34.9 and >34.9 g/L), there was agreement in 86% of patients. Plasma albumin at DI, which represented a surrogate measure of plasma albumin 3 months previously, was a highly significant predictor of SDI.
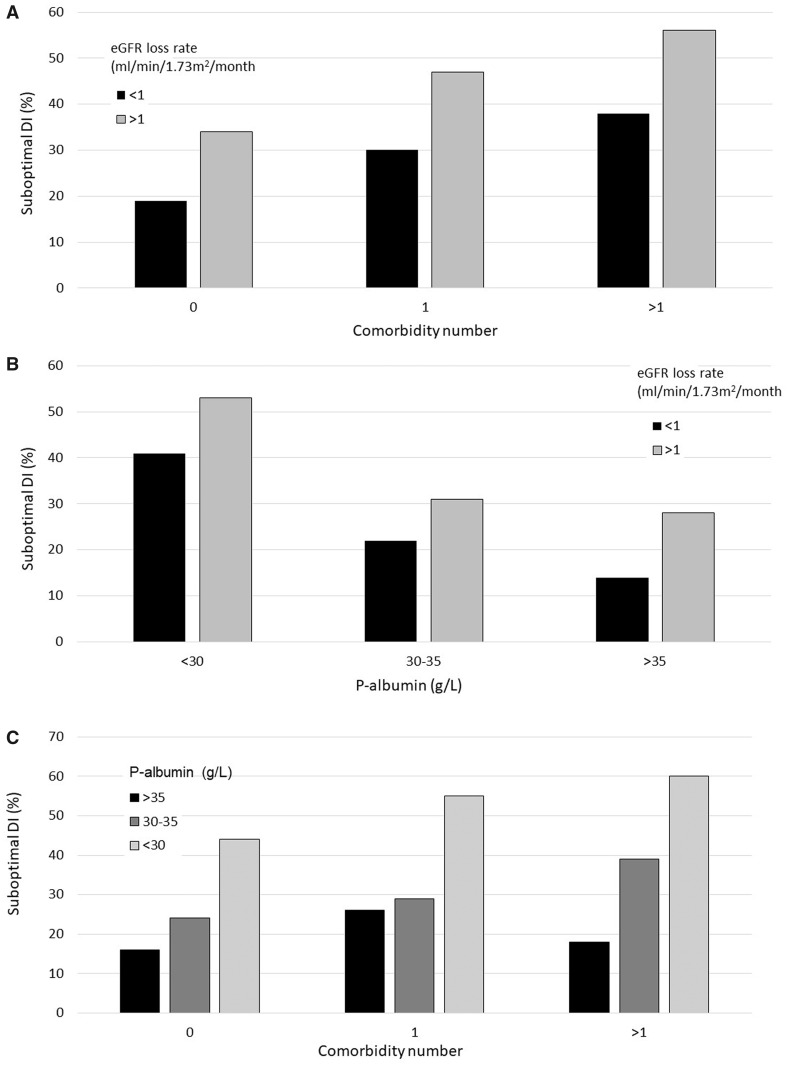
The most important factors for predicting SDI were late referral, patient comorbidity, plasma albumin and ΔeGFR (Figure 1). About 100% of 35 late referrals with a registered ΔeGFR 6–3 months of >1 mL/min/1.73 m2 experienced SDI. For all subgroups, eGFR 3 months before DI was higher for SDI compared with optimal DI. For patients with plasma albumin <35 g/L, median eGFR at this point was 15 (10–24) mL/min/1.73 m2.
FIGURE 1.
Relationship of comorbidity, plasma albumin and ΔeGFR 6–3 months before DI to SDI. (A) Comorbidity and uraemia progression; (B) plasma albumin and uraemia progression; and (C) comorbidity and plasma albumin. Late referrals are excluded. Results are stated as percentage of patients with SDI.
Centre factors related to SDI
Excluding late referrals, there was no relationship of SDI to length of pre-dialysis care. Small centres had a significantly lower SDI rate; these were all non-university centres. There was no difference in comorbidity between university and non-university centres, but more patients were referred late to university centres (23.2% versus 7.7%, P < 0.01), and patients at university centres had a significantly faster ΔeGFR 3 months before DI than patients in non-university centres [10.3 (4.1–22.5) versus 8.0 (2.6–15.0) mL/min/1.73 m2/year; P < 0.01]. There was no relationship between average centre eGFR at DI and incidence of SDI. The departmental policies for dialysis information, fistula placement and DI had no measurable effect on SDI. However, patients treated at centres with a lower eGFR target for DI (<9 mL/min/1.73 m2) started dialysis at a lower eGFR than those starting dialysis in centres with higher eGFR target (eGFR 7.2 ± 3.1 versus 7.9 ± 3.6 mL/min/1.73 m2; P < 0.01).
Recommended referral eGFR and average visit intervals were unrelated to SDI regardless of CKD stage or progression rate. Indeed, a tendency for higher SDI incidence was seen for centres with relatively short visit intervals.
Other factors related to SDI
Clinical data were available in 1546 patients and has already been published [17]. The relationship of presence and character of clinical symptoms to DI is shown in Table 1. The presence of life-threatening conditions was higher for all forms of SDI and was the primary cause of DI in 30% (versus 12% for optimal DI).
DISCUSSION
In our study, SDI accounted for 42% of all DIs, similar to that reported in several previous studies [1, 2], but lower than the proportion in a recently published report [20]. The high frequency of SDI at our centres may seem surprising, considering that ∼85% of the patients were already known to the Nephrology departments, and were subject to regular multidisciplinary pre-dialysis care.
This is probably the largest prospective multicentre study assessing patient and centre factors associated with SDI. A major advantage of this study compared with previous studies is that SDI was clearly defined, based on the use of catheters for dialysis access, thus permitting a more accurate estimate of SDI incidence. Furthermore, we distinguished prospectively between tunnelled catheters as the patient’s optimal access, and those due to delayed placement of an AVF.
We chose not to use in-patient status as part of the definition of SDI [1], since in our experience many in-patient DI patients will have received optimal pre-dialysis care, e.g. a patient with a functioning AVF, who starts dialysis in connection with acute pneumonia.
Some stated SDI causes were more or less unavoidable: patient refusal of starting dialysis, clinical indications necessitating DI despite acceptable biochemistry and doubts about reversibility. These comprised, however, only 2.6% of patients. Patient non-concordance and access problems are potentially avoidable, but comprised only 4.4% of patients, the main stated causes being an acute progression of chronic uraemia and delayed planning as assessed by a review of the notes. The latter comprised 10% of SDI, suggesting that regular audits of pre-dialysis treatment in SDI patients could reduce SDI.
A previous review [1] identified the following causes of SDI despite early referral: acute on CKD; suboptimal nephrology care, patient induced delays and indecision; barriers to surgical resources; and lack of dialysis resources to accommodate new patients. In this study, only the first two factors seemed to have a substantial effect on SDI incidence. This is in contrast to a previous study on the subject [21], where patient non-concordance and surgical delays were major causes of SDI. This may in part explain the high SDI incidence in that study (56%), emphasizing that SDI incidence is modifiable.
A major finding in this study was that eGFR per se had no power to predict SDI. Instead, the most important factors linked to SDI were late referral, rapid decline of eGFR (ΔeGFR), high comorbidity and hypoalbuminaemia. eGFR was stated as the most important factor guiding dialysis information timing by most departments; only one mentioned progression rate. While other centres will presumably also have delayed information given to patients with very slow progression rates, the results suggest that results would be improved if more attention is paid to progression rate rather than eGFR per se. Presence of cardiovascular disease, especially heart failure, was important. These findings are similar to those reported by Arulkumaran et al. in their study on causes and risk factors for acute DI among 825 consecutive patients initiating dialysis [20]. The relationship of SDI to hypoalbuminaemia has previously been noted [3], as has the relationship to heart disease [2, 3, 22]. The latter may well be causal, since the combination of heart failure and uraemia can lead to intractable oedema and pulmonary stasis requiring acute dialysis.
An increased rate of eGFR loss in patients with SDI has previously been noted [20, 23] and was already apparent in many of our patients 3 months before DI. The CKD-EPI formula for deriving eGFR may, however, distort the rate of eGFR loss in CKD Stage 5; further investigation of the validity of CKD-EPI is warranted. If patients are first referred to the pre-dialysis clinic once they reach CKD Stage 5 (eGFR <15 mL/min/1.73 m2), half of the patients with plasma albumin <35 g/L will be referred <3 months before DI, with consequent high risk of SDI. It is possible that earlier identification of patients at risk of rapid progression, allowing early dialysis information and accelerated preparation for start of dialysis, would help to reduce the number of patients undergoing SDI.
Delayed access placement after prescription did not seem to be a factor associated with SDI. This may be due to generally acceptable access to surgery, but it is also possible that surgical departments are flexible and are able to perform urgent access placement in patients at greater risk of DI at the time of access prescription. While access problems at DI could possibly be averted by earlier access placement, this was not a common issue. Delayed PD catheter placement is rarely a cause of SDI for most PD patients since PD can be initiated within 1 month after placement. On the other hand, too early AVF creation could potentially be a problem; however, recent studies suggest that the placement of AVF may, in fact, delay uraemia progression [24–26].
eGFR was used for measuring renal function in this study, being the most common method in the participating centres. However, eGFR is not an accurate method for assessment of renal function in CKD 5. Since these patients often suffer from muscle wasting, leading to low creatinine production and consequent overestimation of eGFR, eGFR is negatively correlated to muscle mass in patients with CKD 5 [27]. This may be the explanation for the common observation that mortality after DI is paradoxically positively correlated to eGFR at DI [27–30]. These limitations of eGFR could be one explanation as to why eGFR was of no value in predicting SDI; in our study, cachectic patients had a raised eGFR as had patients with high comorbidity who have an increased risk of malnutrition. It is possible that measurements of GFR based on urine collections could be a better tool to identify patients at risk of SDI. If instead of using eGFR, the mean of urea and creatinine clearance, measured for a 24-h urine collection is used, then the correlations of GFR to muscle mass and mortality disappear [27]. The ERBP guidelines, therefore, recommend this method [19].
In our study, even after correcting for eGFR, higher levels of urea, potassium, phosphate and CRP and lower levels of haemoglobin, bicarbonate and calcium, were associated with SDI. This suggests that SDI patients were more uraemic than indicated by eGFR, and that inflammation and acute infections also play a role. Physicians concentrating on eGFR for DI indication may miss these biochemical warning signs.
Other risk factors for SDI have previously been described including the negative impact of late referrals to nephrological care [23, 31–34]. In this study, 21% of referrals were late (<3 months before DI), and 81% of these suffered SDI. Educational programmes for primary and secondary health organizations may reduce this figure.
We found no overall difference in duration of pre-dialysis care between optimal DI and SDI, in opposition to previous findings in the literature [35]. This is perhaps not surprising as this study suggests that it mainly cares during the 6 months preceding DI that is important for preventing SDI. Attention to the ‘quality’ of pre-dialysis care is important. Integrated multidisciplinary care with detailed dialysis information given to patients is reported to be associated with reduced risk of SDI [23, 31, 35–38], whereas late dialysis information has been shown to be related to an increased risk of SDI [23, 31]. Frequent out-patient visits are especially important [35, 39, 40], and more than five out-patient visits per year seem warranted as frequent visits may increase the chance of identifying patients with rapid eGFR loss, a group with a high incidence of SDI. However, there was no discernible relationship between official departmental policy concerning visit intervals and SDI. The explanation may be that most centres permitted a wide variation in visit timing at the discretion of the treating physician. In our study, SDI patients received dialysis information at a lower eGFR (8.1 versus 10.9 mL/min/1.73 m2), and it is possible that many cases of SDI may have been due to delayed response of physicians—and failure to take action—to eGFR data showing rapid loss of GFR prior to dialysis information (Figure 1). While multidisciplinary pre-dialysis care was routine in the centres involved in this study, the quality and quantity of this care were not assessed. Nor was patient–doctor continuity, which has emerged as an important factor for better planning and lower mortality [41].
Other factors that were associated with an increased incidence of SDI included high age, in accordance with the literature [3, 22, 31, 35], but this was not a major factor. Cachexia was a cause of SDI in our study in agreement with other studies [23, 35], as was diabetic nephropathy. SDI was less common among patients with polycystic kidney disease, as reported previously [2, 22, 23].
The larger university centres participating in this study had a higher incidence of SDI. The most probable explanation is differences in case mix, since patients starting dialysis at university centres were more often referred late and had a higher rate of eGFR loss than patients at smaller non-university centres. However, it cannot be excluded that the presence of physicians undergoing specialist training at university centres was more prone to start dialysis and this could have contributed to a higher incidence of SDI.
Official departmental policies did not seem to have had much effect on physician practice, with the exception that centres with lower eGFR target for DI started dialysis at a lower eGFR.
There are several limitations to this study. As in all observational studies, correlations do not prove causality. As compared with the overall incidence of DI at the participating centres, an estimated 20% of patients were not included in the study. These patients were not quantified or characterized and may have differed from the included population. Whereas one might hypothesize that the proportion of patients with SDI was higher among non-included patients, the already high rate of SDI in the study group suggests that inclusion of these unreported patients would not have changed the results substantially. Patients with very slow eGFR loss may not have reached ESRD, or may have died during the study period, and will not have been included. The time interval between referral from the primary sector to first nephrological consultation, a potentially modifiable cause of late referral, was not recorded. While most patients were included at or before DI, a few will have been erroneously classified as having reversible uraemia and included retrospectively.
While treatment practices in the Nordic/Baltic region were fairly homogeneous, they may differ from clinical practice in other parts of the world. In particular, the mean eGFR at the time of dialysis information was relatively low, particularly when compared with North America. Similar studies from other regions with differing treatment practices are warranted.
In summary, SDI accounted for 42% of all DIs among 15 dialysis centres in the Nordic countries. Whereas SDI is often unavoidable, many were due to potentially avoidable causes. The level of eGFR as such was of no value in identifying patients at risk of SDI; instead, these patients were characterized by late referral, accelerated eGFR loss rate, hypoalbuminaemia and high comorbidity. Early identification of these patients, with subsequent close clinical control and accelerated preparation for dialysis, might be a strategy that could potentially reduce SDI incidence. Thus, many patients would probably benefit by earlier referral to a pre-dialysis clinic, e.g. at eGFR of 20 mL/min/1.73 m2, to address comorbidity and uraemia progression rate. Studies exploring if early identification of potential SDI patients will reduce SDI incidence are warranted. However, any nephrologist taking care of CKD patients that transition to ESRD can attest that even though optimal planning for DI had occurred, subsequent care processes and clinical events often waylay even the best-laid plans, thus contributing to the high incidence of SDI.
ACKNOWLEDGEMENTS
We thank all physicians and other staff members who participated in this study, and Sara Denguir for data collection assistance.
FUNDING
This study was funded in part by a grant from Baxter Healthcare, grant number 15002474. Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet.
CONFLICT OF INTEREST STATEMENT
B.L. is employed by Baxter Healthcare at Baxter Novum, Karolinska Institutet.
REFERENCES
- 1. Mendelssohn DC, Malmberg C, Hamandi B.. An integrated review of “unplanned” dialysis initiation: reframing the terminology to “suboptimal” initiation. BMC Nephrol 2009; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown PA, Akbari A, Molnar AO. et al. Factors associated with unplanned dialysis starts in patients followed by nephrologists: a retrospective cohort study. PLoS One 2015; 10: e0130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mendelssohn DC, Curtis B, Yeates K. et al.; for the STARRT Study investigators. Suboptimal initiation of dialysis with and without early referral to a nephrologist. Nephrol Dial Transplant 2011; 26: 2959–2965 [DOI] [PubMed] [Google Scholar]
- 4. Castellano I, Gallego S, Labrador PJ. et al. The start of renal replacement therapy in a Spanish department. Nefrologia 2006; 26: 445–451 [PubMed] [Google Scholar]
- 5. Gorriz JL, Sancho A, Pallardo LM. et al. Prognostic significance of programmed dialysis in patients who initiate renal substitutive treatment. Multicenter study in Spain. Nefrologia 2002; 22: 49–59 [PubMed] [Google Scholar]
- 6. Metcalfe W, Khan IH, Prescott GJ. et al. Can we improve early mortality in patients receiving renal replacement therapy? Kidney Int 2000; 57: 2539–2545 [DOI] [PubMed] [Google Scholar]
- 7. Couchoud C, Moranne O, Frimat L. et al. Associations between comorbidities, treatment choice and outcome in the elderly with end-stage renal disease. Nephrol Dial Transplant 2007; 22: 3246–3254 [DOI] [PubMed] [Google Scholar]
- 8. Chen YM, Wang YC, Hwang SJ. et al. Patterns of dialysis initiation affect outcomes of incident hemodialysis patients. Nephron 2016; 132: 33–42 [DOI] [PubMed] [Google Scholar]
- 9. Caskey FJ, Kramer A, Elliott RF. et al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011; 26: 2604–2610 [DOI] [PubMed] [Google Scholar]
- 10. Loos C, Briançon S, Frimat L. et al. Effect of end-stage renal disease on the quality of life of older patients. J Am Geriatr Soc 2003; 51: 229–233 [DOI] [PubMed] [Google Scholar]
- 11. Ravani P, Palmer SC, Oliver MJ. et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol 2013; 24: 465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhingra RK, Young EW, Hulbert-Shearon TE. et al. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 2001; 60: 1443–1451 [DOI] [PubMed] [Google Scholar]
- 13. Pastan S, Soucie JM, McClellan WM.. Vascular access and increased risk of death among hemodialysis patients. Kidney Int 2002; 62: 620–626 [DOI] [PubMed] [Google Scholar]
- 14. Nassar GM, Ayus JC.. Infectious complications of the hemodialysis access. Kidney Int 2001; 60: 1–13 [DOI] [PubMed] [Google Scholar]
- 15. Lorenzo V, Martín M, Rufino M. et al. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: an observational cohort study. Am J Kidney Dis 2004; 43: 999–1007 [DOI] [PubMed] [Google Scholar]
- 16. Hassan R, Akbari A, Brown PA. et al. Risk factors for unplanned dialysis initiation: a systematic review of the literature. Can J Kidney Health Dis 2019; 6: 205435811983168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heaf J, Petersons A, Vernere B. et al. Why do physicians prescribe dialysis? A prospective questionnaire study. PLoS One 2017; 12: e0188309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH. et al.; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tattersall J, Dekker F, Heimburger O. et al.; on behalf of the ERBP Advisory Board. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol Dial Transplant 2011; 26: 2082–208621551086 [Google Scholar]
- 20. Arulkumaran N, Navaratnarajah A, Pillay C. et al. Causes and risk factors for acute dialysis initiation among patients with end-stage kidney disease-a large retrospective observational cohort study. Clin Kidney J 2019; 12: 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hughes SA, Mendelssohn JG, Tobe SW. et al. Factors associated with suboptimal initiation of dialysis despite early nephrologist referral. Nephrol Dial Transplant 2013; 28: 392–397 [DOI] [PubMed] [Google Scholar]
- 22. Chiu K, Alam A, Iqbal S.. Predictors of suboptimal and crash initiation of dialysis at two tertiary care centers. Hemodial Int 2012; 16 (Suppl 1): S39–S46 [DOI] [PubMed] [Google Scholar]
- 23. Marron B, Ostrowski J, Torok M. et al. Type of referral, dialysis start and choice of renal replacement therapy modality in an international integrated care setting. PLoS One 2016; 11: e0155987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golper TA, Hartle PM, Bian A.. Arteriovenous fistula creation may slow estimated glomerular filtration rate trajectory. Nephrol Dial Transplant 2015; 30: 2014–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weekers L, Vanderweckene P, Pottel H. et al. The closure of arteriovenous fistula in kidney transplant recipients is associated with an acceleration of kidney function decline. Nephrol Dial Transplant 2017; 32: 196–200 [DOI] [PubMed] [Google Scholar]
- 26. Sumida K, Molnar MZ, Potukuchi PK. et al. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant 2017; 32: 1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grootendorst DC, Michels WM, Richardson JD. et al.; for the NECOSAD Study Group. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant 2011; 26: 1932–1937 [DOI] [PubMed] [Google Scholar]
- 28. Clark WF, Na Y, Rosansky SJ. et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ 2011; 183: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hwang SJ, Yang WC, Lin MY. et al.; Taiwan Society of Nephrology. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant 2010; 25: 2616–2624 [DOI] [PubMed] [Google Scholar]
- 30. Kazmi WH, Gilbertson DT, Obrador GT. et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis 2005; 46: 887–896 [DOI] [PubMed] [Google Scholar]
- 31. Buck J, Baker R, Cannaby AM. et al. Why do patients known to renal services still undergo urgent dialysis initiation? A cross-sectional survey. Nephrol Dial Transplant 2007; 22: 3240–3245 [DOI] [PubMed] [Google Scholar]
- 32. Goransson LG, Bergrem H.. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250: 154–159 [DOI] [PubMed] [Google Scholar]
- 33. Curtis BM, Barret BJ, Jindal K. et al.; CREDA (Canadian Renal Disease Alliance). Canadian survey of clinical status at dialysis initiation 1998-1999: a multicenter prospective survey. Clin Nephrol 2002; 58: 282–288 [PubMed] [Google Scholar]
- 34. Smart NA, Titus TT.. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 2011; 124: 1073–1080 [DOI] [PubMed] [Google Scholar]
- 35. Marron B, Ortiz A, de SP. et al. Impact of end-stage renal disease care in planned dialysis start and type of renal replacement therapy–a Spanish multicentre experience. Nephrol Dial Transplant 2006; 21 (Suppl 2): ii51–ii55 [DOI] [PubMed] [Google Scholar]
- 36. Chen YR, Yang Y, Wang SC. et al. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: a 3-year prospective cohort study. Nephrol Dial Transplant 2013; 28: 671–682 [DOI] [PubMed] [Google Scholar]
- 37. Chen YR, Yang Y, Wang SC. et al. Multidisciplinary care improves clinical outcome and reduces medical costs for pre-end-stage renal disease in Taiwan. Nephrology (Carlton) 2014; 19: 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lacson E Jr, Wang W, DeVries C. et al. Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis 2011; 58: 235–242 [DOI] [PubMed] [Google Scholar]
- 39. Avorn J, Bohn RL, Levy E. et al. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med 2002; 162: 2002–2006 [DOI] [PubMed] [Google Scholar]
- 40. Singhal R, Hux JE, Alibhai SM. et al. Inadequate predialysis care and mortality after initiation of renal replacement therapy. Kidney Int 2014; 86: 399–406 [DOI] [PubMed] [Google Scholar]
- 41. Pereira Gray DJ, Sidaway-Lee K, White E. et al. Continuity of care with doctors-a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open 2018; 8: e021161. [DOI] [PMC free article] [PubMed] [Google Scholar]