Abstract
Background
A subset of patients with hypertrophic cardiomyopathy (HCM) is at high risk of sudden cardiac death (SCD). Practice guidelines endorse use of a risk calculator, which requires entry of left atrial (LA) diameter. However, American Society of Echocardiography (ASE) guidelines recommend the use of LA volume index (LAVI) for routine quantification of LA size. The aims of this study were to (a) develop a model to estimate LA diameter from LAVI and (b) evaluate whether substitution of measured LA diameter by estimated LA diameter derived from LAVI reclassifies HCM‐SCD risk.
Methods
The study cohort was comprised of 500 randomly selected HCM patients who underwent transthoracic echocardiography (TTE). LA diameter and LAVI were measured offline using digital clips from TTE. Linear regression models were developed to estimate LA diameter from LAVI. A European Society of Cardiology endorsed equation estimated SCD risk, which was measured using LA diameter and estimated LA diameter derived from LAVI.
Results
The mean LAVI was 48.5 ± 18.8 mL/m2. The derived LA diameter was 45.1 mm (SD: 5.5 mm), similar to the measured LA diameter (45.1 mm, SD: 7.1 mm). Median SCD risk at 5 years estimated by measured LA diameter was 2.22% (interquartile range (IQR): 1.39, 3.56), while median risk calculated by estimated LA diameter was 2.18% (IQR: 1.44, 3.52). 476/500 (95%) patients maintained the same risk classification regardless of whether the measured or estimated LA diameter was used.
Conclusions
Substitution of measured LA diameter by estimated LA diameter in the HCM‐SCD calculator did not reclassify risk.
Keywords: hypertrophic cardiomyopathy, left atrial diameter, left atrial volume index, sudden cardiac death risk
1. INTRODUCTION
A subset of patients with hypertrophic cardiomyopathy (HCM) are at high risk of sudden cardiac death (SCD). 1 Guidelines recommend early SCD risk assessment to identify candidates for prophylactic insertion of automated implantable cardioverter defibrillators. 2 , 3 To support decision‐making, a prognostic model and corresponding equation for estimation of individualized risk (HCM‐SCD risk) have been developed and validated. 4 , 5
Risk factors for the HCM‐SCD risk stratification model include left atrial (LA) diameter, age, maximal left ventricular (LV) wall thickness, maximal left ventricular outflow tract (LVOT) gradient, family history of SCD, nonsustained ventricular tachycardia (NSVT) by Holter monitoring, and unexplained syncope. 3 , 4 However, the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging 6 recommend LA size be quantified by volume (left atrial volume index (LAVI) and LA diameter is therefore not routinely assessed at our institution in favor of reporting LAVI.
For HCM‐SCD risk estimation, the European Society of Cardiology (ESC) endorses the use of an online calculator. 3 In institutions such as ours, LAVI is routinely available in echocardiography reports. However, LA diameter is not routinely measured. This study developed a method for estimation of LA diameter from LAVI data reported in the EHR. Accordingly, the study aims were to a) develop a model to estimate LA diameter from LAVI for automated calculators and b) evaluate whether substitution of the measured LA diameter by estimated LA diameter from LAVI for the HCM‐SCD risk calculator would reclassify HCM patients at risk of SCD.
2. METHODS
This study was approved by the Mayo Institutional Review Board. The study cohort was comprised of randomly selected patients with HCM evaluated at the Mayo Clinic in Rochester, Minnesota, who underwent transthoracic echocardiography (TTE) from 2003 to 2012. Digital images were prospectively acquired for clinical indications and subsequently stored, retrieved, and screened for offline analysis; 523 TTE were screened to obtain 500 cases with adequate images for measurement.
Patient age, standardized echocardiography measurements, and NSVT by Holter were retrieved from the Mayo Clinic EHR data warehouse. Echocardiographic measurements included maximal LV wall thickness, maximal instantaneous LVOT gradient by continuous‐wave Doppler, and left ventricular ejection fraction. Family history of SCD and unexplained syncope were manually abstracted from clinical notes in the EHR.
Variables used to estimate the HCM‐SCD risk by the ESC endorsed equation included age, family history of SCD, maximal LV wall thickness, maximal instantaneous LVOT gradient by continuous‐wave Doppler, unexplained syncope, NSVT on Holter monitoring, and LA diameter. 3 , 4 The equation used to estimate the probability of SCD at 5 years was 1 − 0.998exp(prognostic index), where prognostic index = 0.15939858 × maximal wall thickness (mm) − 0.00294271 × maximal wall thickness2 (mm) + 0.0259082 × LA diameter (mm) + 0.00446131 × maximal LVOT gradient (mmHg) + 0.4583082 × family history of SCD + 0.82639195 × NSVT + 0.71650361 × unexplained syncope − 0.01799934 × age at clinical evaluation (years). 3 , 4 We did not use the HCM‐SCD risk calculator available via the Internet but instead recreated the HCM‐SCD risk calculator in SAS software using the validated equation. 3 , 4
2.1. Left atrial measurements
LA measurements were made offline from digital images. All measurements were performed by one sonographer, blinded to clinical information including echocardiography reports. Measurements included LA linear diameter from anteroposterior measurement in parasternal long‐axis view and LA volume by biplane method of disks using apical 4‐chamber and apical 2‐chamber views (Figure 1). 6 LAVI was obtained by dividing LA volume by body surface area (BSA). 6
FIGURE 1.
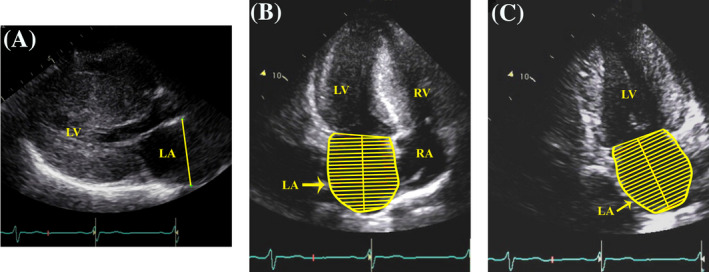
Left atrial measurements. LA linear diameter from anteroposterior measurement in parasternal long‐axis view (A). LA volume by biplane method of disks using apical 4‐chamber (B) and apical 2‐chamber views (C). LA = left atrium, LV = left ventricle, RA = right atrium. RV = right ventricle
2.2. Statistical analysis
Continuous variables were expressed as mean ± SD or median with interquartile range (IQR) according to the pattern of data distribution. Categorical variables were summarized as counts and percentages. LA diameter was derived from LAVI by linear regression. A multiplicative interaction between BSA and LAVI was tested in this model and reported (p int). To visualize associations, plots were created using BSA quartiles. BSA values as continuous variable were used in the regression model. A Bland–Altman plot was generated to compare the measured LA diameter and estimated LA diameter. Lin's concordance correlation coefficients were used to summarize the agreement between measured LA and estimated LA diameter from LAVI. After categorizing HCM‐SCD risk of measured and estimated LA diameters, the weighted kappa statistic was used to summarize the agreement. Intra‐observer variability was estimated from a random sample of 25 subjects using intra‐class correlation coefficients (ICC). All statistical analyses were performed using SAS version 9.4 (Cary, NC), and two‐sided P‐values < .05 were considered to be statistically significant.
3. RESULTS
The baseline clinical characteristics are shown in Table 1. HCM patients (age 53 ± 15 years) had maximal LV wall thickness of 17 mm (IQR 14, 21). There was high intra‐observer agreement for the measurements of LA diameter (ICC: 0.96 (95% confidence interval (CI): 0.90, 0.98)) and LAVI (ICC: 0.95 (CI: 0.86, 0.98)). The measured LA diameter was 45.1 mm (SD: 7.1) and LAVI 48.5 mL/m2 (SD: 18.8). Figure 2 shows the correlation of measured LA diameter and LAVI by BSA quartiles.
TABLE 1.
Baseline clinical characteristics
| Variables | HCM patients (n = 500) |
|---|---|
| Age, y | 53 (15) |
| Male sex, n (%) | 270 (54) |
| LV maximal wall thickness, mm, median (IQR) | 17(14, 21) |
| Left atrial diameter, mm, mean (SD) | 45.1 (7.1) |
| Maximal LVOT gradient, mmHg, median (IQR) | 4 (4, 61) |
| Family history of SCD, n (%) | 107 (21) |
| NSVT (by Holter), n (%) | 12 (2) |
| History of unexplained syncope, n (%) | 100 (22) |
| Prior myectomy, n (%) | 16 (3) |
| Implanted AICD, n (%) | 66 (13%) |
| Body surface area, m2 | 2.02 (0.26) |
| Left atrial volume index (biplane), mL/m2 | 48.5 (18.8) |
| LV ejection fraction, %, mean (SD) | 69 (7.8) |
Abbreviations: AICD = automated implantable cardioverter–defibrillator; IQR = interquartile range; LV = left ventricle; LVOT = left ventricular outflow tract; NSVT = nonsustained ventricular tachycardia; SCD = sudden cardiac death.
FIGURE 2.
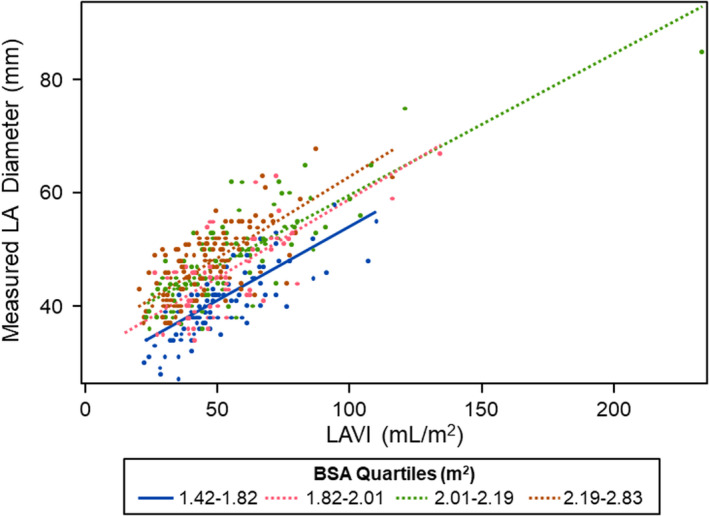
Correlation of measured LA diameter and LAVI by BSA quartiles of HCM patients. Patients in the highest BSA quartiles had larger LA diameters and LAVI, compared with patients in the lowest BSA quartiles. BSA = body surface area, LA = left atrial, LAVI = left atrial volume index
3.1. Estimating LA diameter
Regression models for LA diameter were fit using LAVI and BSA. The slope of association did not vary by BSA (p int = 0.31) but model fit improved with BSA (P < .001). LA diameter was estimated by the equation LA diameter = 10.7 + 0.27 * LAVI + 10.6 * BSA. This model generated an R‐square = 0.62. Figure 3 shows the correlation between measured and estimated LA diameter. A Bland–Altman plot comparing measured LA and estimated LA diameter is shown in Figure 4. The estimated LA diameter was 45.1 mm (SD: 5.5 mm) which was similar to the measured LA diameter 45.1 mm (SD: 7.1 mm). An alternative model including the nonindexed left atrial volume had a lower R‐square of 0.59.
FIGURE 3.
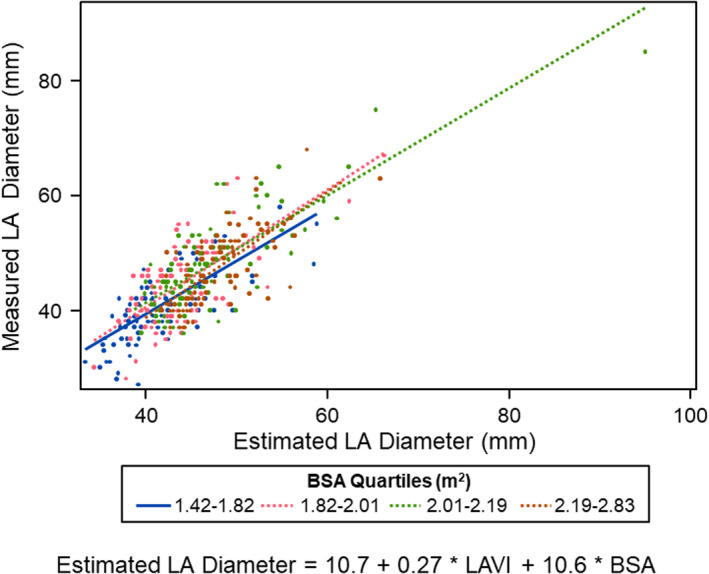
Correlation of measured and estimated LA diameter by BSA quartiles of HCM patients. This model generated an R 2 = 0.62. LA diameter was estimated by the following equation: LA diameter = 10.7 + 0.27 * LAVI + 10.6 * BSA. BSA = body surface area, LA = left atrial, LAVI = left atrial volume index
FIGURE 4.
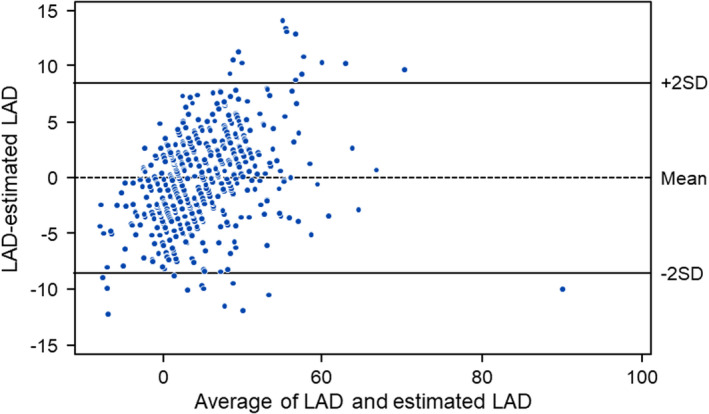
A Bland–Altman plot comparing measured and estimated left atrial diameter. LAD = Left atrial diameter
3.2. Estimated LA diameter and SCD risk
Figure 5 shows the correlation between risk estimated by the HCM‐SCD risk equation comparing measured and estimated LA diameter. Lin's correlation coefficient applied to these results was excellent 7 at 0.98 (95% CI: 0.98, 0.99). Median risk of SCD calculated by measured LA diameter was 2.22 (IQR: 1.39, 3.56) compared with estimated LA diameter 2.18 (IQR: 1.44, 3.52). The absolute risk difference was 0.15 (0.07, 0.31); there were 13 patients (3%) with ≥1% difference in calculated SCD risk.
FIGURE 5.
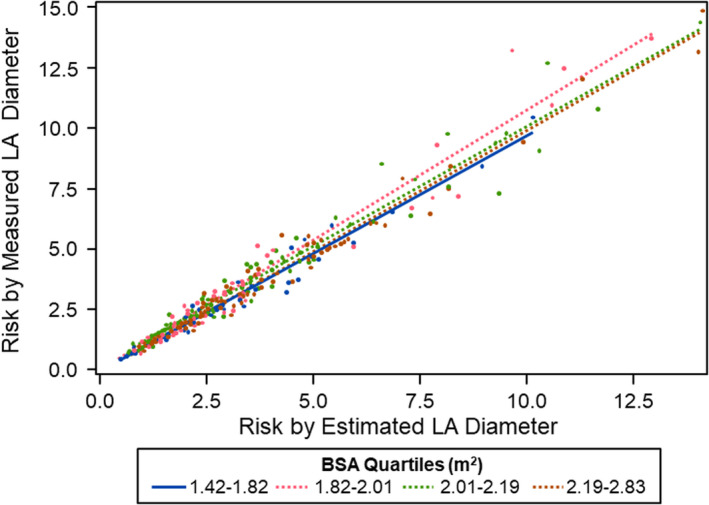
Correlation between risk estimated by the HCM‐SCD risk equation comparing measured and estimated LA diameter by BSA quartiles of HCM patients. Lin's correlation coefficient applied to these results was 0.98 (95% CI: 0.98, 0.99). BSA = body surface area, LA = left atrial
3.3. Risk classification
The majority of patients (476/500 [95%]) maintained the risk classification regardless of whether the measured or estimated LA diameter was used for risk estimation. The kappa correlation coefficient for risk classification was high (0.90 (95% confidence interval: 0.86, 0.94)). Of 395 patients classified as low risk (<4%) by measured LA diameter, 386 (98%) remained in the low‐risk group, while 9 (2%) moved to intermediate risk (4%–6%) by estimated LA diameter (Figure 6). Of 61 patients in the intermediate (4%–6%) SCD risk category, 51 (84%) were also classified as intermediate by the estimated LA diameter and the remaining 10 patients (16%) were reclassified in the low (<4%) SCD risk category by estimated LA diameter. There were 44 patients in the high‐risk group (>6%) by measured LA diameter, 39 (89%) remained in the high‐risk group, while 5 (11%) moved to intermediate risk (4%–6%) by estimated LA diameter. When the LA diameter was estimated using the model with nonindexed left atrial volume, a slightly worse performance was obtained, as 474 patients maintained the same risk classification with a kappa correlation classification of 0.89.
FIGURE 6.
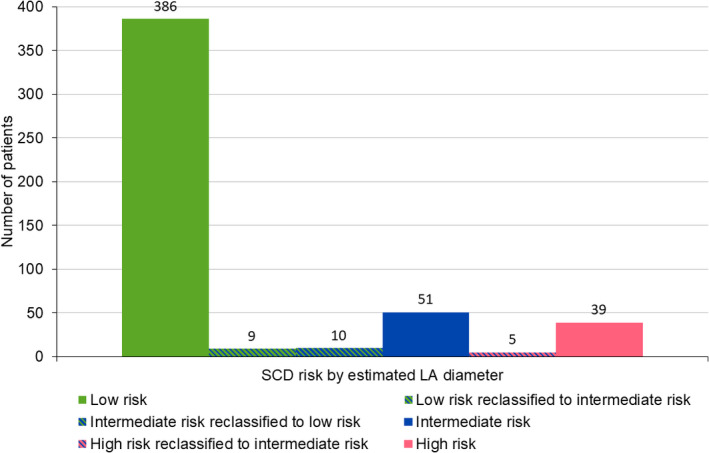
Number of patients reclassified in each SCD risk category using estimated LA diameter. 95% of patients (476/500) maintained the risk classification regardless of whether the measured or estimated LA diameter was used for calculation. LA = Left atrial
3.4. Follow‐up
Among the 500 patients, follow‐up information was available in 458 (92%). Of these, twenty‐four patients had anti‐tachycardia pacing or discharge events at a median duration of follow‐up of 1.1 years (IQR: 0.1, 3.8). Risk was reclassified in only 2 of these patients, and both had discharge events. Patient #1 had risk based on measured LA diameter of 3.5% (low‐risk category) and increased to 4.0 (intermediate‐risk category) using estimated LA diameter. This event occurred at 11.5 years of follow‐up. For patient #2, the risk based on measured LA diameter was 5.1% (intermediate‐risk category) and decreased to 3.7% (low‐risk category) using the estimated LA diameter. This event occurred at 105 days of follow‐up.
4. DISCUSSION
The novel observation of this study of 500 consecutive HCM patients was that SCD risk estimated using measured LA diameter compared with estimated diameter from LAVI was not significantly different. ASE practice guidelines recommend LA dimension quantification by volumetric methods. 6 Hence, most echocardiography laboratories in the United States have adopted the measurement of LAVI in clinical practice. LA volume provides the closest agreement to conventional tridimensional reconstruction of the left atrium and a more accurate and reproducible estimate of LA size. 6 , 8 LA volume is also a robust marker of cardiovascular events. 9 Furthermore, LAVI has been used to predict the adverse outcomes in patients with HCM. 10 , 11 However, available risk calculators for HCM‐SCD require entry of LA diameter, which is no longer measured at our institution during routine echocardiography in favor of reporting of LAVI.
In this study, the majority of patients maintained the risk classification regardless of whether the measured or estimated LA diameter was used for risk estimation. It is important to note that the LA diameter has a very small contribution to the risk score, and this may be the reason that minor differences in the variable are unlikely to produce a clinical difference. Currently, the calculation of HCM‐SCD risk requires manual entry to an ESC website. 12 Providers at the point‐of‐care or investigators for HCM clinical research review medical records for SCD risk factors and subsequently manually enter the relevant information at the website. 12 This inefficient process is time‐consuming, error‐prone, and potentially disruptive to provider workflow. We are developing an automated HCM‐SCD risk calculator populated by EHR data to calculate HCM‐SCD risk automatically for use by clinicians at the point‐of‐care. We have been developing methodologies to automatically extract these risk factors from EHR data. 13 However, in our institution the LA diameter is not available from EHR data. Conducting offline measurements of the LA diameter for the risk calculation of all HCM patients for clinical practice and for large EHR‐based cohort studies would be impractical, time‐consuming, and costly. Importantly, the study herein demonstrated that estimated LA diameter can be used to generate input for automated HCM‐SCD risk calculators without requirement for manual data entry.
It is not advisable to enter LA volume into the SCD risk equation as the volume measurement has a different scale and units compared with LA diameter. In the study herein, the mean LA diameter was 45.1 mm (SD = 7.1), while mean LA volume was 97 mL (SD = 39 mL). If volume is entered, this greatly alters results estimated by the HCM‐SCD risk equation. With these caveats, the median risk of SCD estimated using LA diameter was 2.2 (IQR: 1.4, 3.6). However, when LA volume was used in the equation a much higher value of 7.5 (3.8, 15.5) was obtained. This emphasizes the importance of first estimating LA diameter using the equation reported herein so that we use an estimated measurement value on the same scale as measured LA diameter. In future studies, the risk equation could be recreated based on LA volume instead of LA diameter. In addition, future studies could test the hypothesis that LA volume may be strongly and independently associated with higher risk of SCD in HCM patients. Subsequent to such studies, consideration could be given to replacement of LA diameter with LA volume in calculating SCD risk for HCM patients.
4.1. Limitations
The present study included data of 500 HCM patients from a single center. In the future, independent validation in other centers may also assess risk using estimated LA diameter in the HCM‐SCD risk equation. Even though the model reported herein performed well, the regression model is inherently limited by the fact that there is no uniform change between the LA diameter and LA volume and that LAVI takes BSA into account. Future studies with larger samples could further evaluate outcomes of HCM patients classified using the estimated LA diameter. Patients younger than 18 years old were excluded in our study, and the use of estimated LA diameter in the pediatric population also warrants future investigation.
5. CONCLUSIONS
Substitution of measured LA diameter by estimated LA diameter from LAVI in the HCM‐SCD calculator did not reclassify risk which suggests automated calculation of HCM‐SCD risk for cohort studies, and generation of input for clinical decision support systems can be performed from volumetric data.
ACKNOWLEDGMENTS
The authors thank Katie M. Nagel for secretarial support.
Bhopalwala H, Dewaswala N, Liu S, et al. Conversion of left atrial volume to diameter for automated estimation of sudden cardiac death risk in hypertrophic cardiomyopathy. Echocardiography.2021;38:183–188. 10.1111/echo.14943
Huzefa Bhopalwala and Nakeya Dewaswala contributed equally to the study and should be considered joint first authors.
Funding information
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute of National Institutes of Health (K01HL124045), Mayo Clinic K2R, and the Tsai Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DATA AVAILABILITY STATEMENT
The research data are confidential because participants of this study did not consent for their data to be shared publicly.
REFERENCES
- 1. Geske JB, Ommen SR, Gersh BJ. Hypertrophic cardiomyopathy: clinical update. JACC Heart Fail. 2018;6(5):364–375. [DOI] [PubMed] [Google Scholar]
- 2. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58(25):e212–e260. [DOI] [PubMed] [Google Scholar]
- 3. Authors/Task Force members , Ponikowski P, Voors AA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779. [DOI] [PubMed] [Google Scholar]
- 4. O'Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk‐SCD). Eur Heart J. 2014;35(30):2010–2020. [DOI] [PubMed] [Google Scholar]
- 5. O'Mahony C, Jichi F, Ommen SR, et al. International External Validation Study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE‐HCM). Circulation. 2018;137(10):1015–1023. [DOI] [PubMed] [Google Scholar]
- 6. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39e14. [DOI] [PubMed] [Google Scholar]
- 7. Akoglu H. User's guide to correlation coefficients. Turkish J Emerg Med. 2018;18(3):91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khankirawatana B, Khankirawatana S, Porter T. How should left atrial size be reported? Comparative assessment with use of multiple echocardiographic methods. Am Heart J. 2004;147(2):369–374. [DOI] [PubMed] [Google Scholar]
- 9. Tsang TSM, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size. Is volume superior to area or diameter?. J Am Coll Cardiol. 2006;47(5):1018–1023. [DOI] [PubMed] [Google Scholar]
- 10. Yang WI, Shim CY, Kim YJ, et al. Left atrial volume index: a predictor of adverse outcome in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22(12):1338–1343. [DOI] [PubMed] [Google Scholar]
- 11. Tani T, Yagi T, Kitai T, et al. Left atrial volume predicts adverse cardiac and cerebrovascular events in patients with hypertrophic cardiomyopathy. Cardiovasc Ultrasound. 2011;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cardiology, T.E.S.o . HCM Risk‐SCD Calculator. 2014. Available fromhttps://doc2do.com/hcm/webHCM.html
- 13. Moon S, Liu S, Scott CG, et al. Automated extraction of sudden cardiac death risk factors in hypertrophic cardiomyopathy patients by natural language processing. Int J Med Inform. 2019;128:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data are confidential because participants of this study did not consent for their data to be shared publicly.


