Abstract
BACKGROUND
Lack of fitness costs has been reported for multiple herbicide resistance traits, but the underlying evolutionary mechanisms are not well understood. Compensatory evolution that ameliorates resistance costs, has been documented in bacteria and insects but rarely studied in weeds. Dicamba resistant IAA16 (G73N) mutated kochia was previously found to have high fecundity in the absence of competition, regardless of significant vegetative growth defects. To understand if costs of dicamba resistance can be compensated through traits promoting reproductive success in kochia, we thoroughly characterized the reproductive growth and development of different G73N kochia biotypes. Flowering phenology, seed production and reproductive allocation were quantified through greenhouse studies, floral (stigma‐anthers distance) and seed morphology, as well as resulting mating and seed dispersal systems were studied through time‐course microcopy images.
RESULTS
G73N covaried with multiple phenological, morphological and ecological traits that improve reproductive fitness: (i) 16–60% higher reproductive allocation; (ii) longer reproduction phase through early flowering (2–7 days); (iii) smaller stigma‐anthers separation (up to 60% reduction of herkogamy and dichogamy) that can potentially promote selfing and reproductive assurance; (iv) ‘winged’ seeds with 30–70% longer sepals that facilitate long‐distance seed dispersal.
CONCLUSION
The current study demonstrates that costs of herbicide resistance can be ameliorated through coevolution of other fitness penalty alleviating traits. As illustrated in a hypothetical model, the evolution of herbicide resistance is an ongoing fitness maximization process, which poses challenges to contain the spread of resistance. © 2020 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: dicamba resistance, kochia (Bassia scoparia), stigma‐anther distance, reproductive allocation, seed dispersal, compensatory evolution
Characterization of the effects of a dicamba resistant IAA16 mutated kochia indicated the presence of compensatory evolution. The mutation covaried with multiple reproductive fitness promoted traits such as smaller stigma‐anther separation promoting selfing, higher reproductive allocation and altered seed morphology facilitating long distance dispersal.
© 2020 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
1. INTRODUCTION
A widely accepted ecological theory for plant defensive traits, is they usually entail a concomitant fitness cost, due to resource restrictions and the trade‐offs between growth and defense. 1 , 2 , 3 However, fitness costs of resistance against either herbivores, pathogens or weeds, was only detected in 25–50% of the studies summarized by Bergelson and Purrington. 4 A general conclusion for the fitness costs of herbicide resistance, is that costs are not ubiquitous, and depend on resistance traits, weed species and the environment. 5 , 6 , 7 There are both methodological and biological factors contributing to the absence of detectable fitness costs. First, without the knowledge of the resistance genes or studied species with appropriately controlled genetic background, it is challenging to unequivocally attribute the fitness effects to herbicide resistance traits. 8 , 9 Another relatively less studied factor, is that compensatory evolution may be involved to ameliorate the fitness costs of resistance endowing mutations. 10 , 11
Compensatory evolution for resistance has been well documented in bacteria and insects but rarely studied in higher plants especially herbicide resistant weeds. 9 , 12 , 13 According to Baucom, 14 there are three evolutionary paths for fitness costs: (i) replacement of resistance alleles of high costs with ones with low costs; (ii) optimized genetic background of the selecting population might help offset the fitness costs; (iii) interaction with a fitness modifier loci might ameliorate the fitness costs. 10 , 15 , 16 A pioneer study by Paris et al. 17 used axr1‐3 2,4‐D resistant Arabidopsis thaliana suggested that resistance costs can be potentially compensated through the genetic diversity present within a species. Another study by Darmency et al. 18 also found that genetic backgrounds of weed populations might evolve to counterbalance the fitness costs of certain acetyl‐coenzyme A carboxylase (ACCase) inhibitors resistance endowing mutations. However, other strategies (e.g. replacement theories and fitness modifiers) agricultural weeds adopt to compensate for resistance costs are largely unknown for many herbicide resistance traits including synthetic auxins.
Synthetic auxins are important herbicides that have been in the market for more than 70 years. 19 It is also the first herbicide mode of action (MOA) to which a weed species evolved resistance. 20 Kochia (Bassia scoparia) is a key weed species that first developed resistance to synthetic auxins in 1994. 21 , 22 Due to lack of knowledge on the MOA of synthetic auxins, the genetic basis of resistance was not known for decades. Recently, the first resistance endowing mutation was documented in kochia to be a Gly‐73‐Asn substitution at one of the auxin co‐receptor AUX/IAA16 proteins. 23 This target‐site based mechanism provides a unique and highly relevant model to investigate compensatory evolution in naturally occurring synthetic auxins resistant weeds. Previously, our group has reported that G73N can cause significant vegetative growth defects and impaired competitiveness in kochia. Strikingly, even with significantly diminished plant sizes, resistant plants were able to produce similar amounts of seeds under low intra‐specific competition or in the absence of competition. 23 , 24
The cause of high fecundity of G73N mutant kochia even in the presence of vegetative growth defects is not clear. Previous research suggested that herbicide resistance traits could shift the plant mating system through changes in stigma‐anther distance, which promotes selfing and reproductive assurance. 25 Few studies have characterized the impact of specific herbicide resistance endowing mutations on the mating system, reproductive phenology and morphology of herbicide‐resistant weed biotypes. Furthermore, it is possible that resistant kochia might be associated with altered resource allocation pattern, such as investing more energy in reproduction than vegetative growth. 26 , 27 However, quantitative data for resources allocation in resistant weeds is scarce.
In this study, we used G73N mutant kochia as a model to understand how weeds can overcome the fitness costs of synthetic auxin resistance and investigate if compensatory evolution is present. Our hypothesis is that due to the high standing genetic diversity of kochia, there are reproductive and developmental traits that might potentially be co‐selected with the resistance mutation and act as fitness modifiers to offset the fitness costs of G73N. To test this hypothesis, we characterized the effect of G73N on multiple reproductive traits in kochia including flowering time, floral and seed morphology and reproductive allocation, as well as outcrossing propensity. Knowledge of compensatory evolution might shed light on how synthetic auxins resistance rapidly spread and thus is topical. Crops engineered with synthetic auxin resistance are being rapidly and widely adopted 28 , 29 , 30 , increasing the selection pressure that could potentially lead to spread of weed resistance. Results from the current study will provide insights to design novel proactive weed management practices by leveraging the deleterious effects of G73N mutation and counter the benefits of resistance to synthetic auxins.
2. MATERIALS AND METHODS
2.1. Plant materials, genotyping method and greenhouse experimental settings
Kochia seeds from a segregating parental line 9425 and a susceptible WT population used in the current study were described previously. 23 , 24 Three F2 lines were generated from self‐pollinating F1 plants from crosses of a male RR plant from the 9425 line with a female WT plant from Herbiseed (www.herbiseed.com/). The genotypes of F1 progenies were confirmed through molecular marker and spray test (Fig. 1(a)). All kochia seeds were first sown onto plastic insert trays filled with commercial potting media (Premier Pro‐Mix BX mycorrhizae, Premier Horticultural Services, PA, USA). Seedlings were grown in the growth chamber set at the following conditions: 16 h photoperiod, 26 °C/20 °C day/night temperature; and light intensity at 550 μmol m−2 s−1. When seedlings were 3–4 cm high, one cotyledon was sampled and each plant was genotyped through the allelic specific Taqman® assay as described in LeClere et al., 23 and labeled as homozygous‐, heterozygous‐resistant (RR, RS) or homozygous susceptible (SS) or WT. Uniform size of genotyped seedlings were then transplanted into bigger pots for downstream studies.
Figure 1.

Schematic illustration of the generation of the study populations and experimental layouts. (a) Segregating 9425 parental line was derived from a previously characterized dicamba resistant line maintained through a single seed descent (SSD) method. Three F2 lines were generated through introgression of G73N from 9425 RR plants into female WT plants, followed by self‐pollinating different F1 crossed progenies with confirmed genotypes. (b) F2 lines and 9425 parental line were subjected to greenhouse studies with or without intra‐specific competition; (c) Two runs of greenhouse experiments were conducted during different time of the year for both parental and F2 lines.
Two glasshouse fitness studies were conducted in parental line (9425) in May and July 2019 respectively, with detailed glasshouse growth conditions listed in Table S1 and timing listed in Fig. 1(b). Genotyped 9425‐RR, ‐RS ‐SS and WT kochia plants of 5 to 7‐cm tall were transplanted into 4.5 in plastic pots (10.2 cm width × 10.2 cm length × 17.8 cm height, one plant per pot). Pots were prefilled with the above‐mentioned commercial potting media, which were incorporated with slow release granular fertilizer (Osmocote [14‐14‐14], Scotts Company LLC, OH, US) at the rate of 3.6 g L−1. In total, 72 plants (18 per genotype) were included in Exp‐1 and 48 plants (12 per genotype) were included in Exp‐2. Plants were watered as needed and fertilized biweekly. Pots were evenly spaced to avoid any interplant shading and re‐randomized weekly.
Two runs of replacement series studies were conducted on three F2 lines in July and September 2016 respectively, with detailed glasshouse conditions listed in Table S1 and timing listed in Fig. 1(b). Genotyped F2‐RR, ‐RS ‐SS kochia plants were transplanted into 20 L plastic pots filled with a commercial potting media fertilized with 3.6 g L−1 of osmocote 14–14‐14. Each pot contained eight plants either as pure stands or in mixtures at ratios of 0:8, 2:6, 4:4, 6:2 and 8:0 (RR:SS or RS:SS). The corresponding frequencies of resistant biotypes were: 0%, 25%, 50%, 75% and 100% (monoculture), respectively. In Exp‐1, 480 plants from three F2 lines (160 per line) were included; in Exp‐2, 320 plants from two F2 lines (160 per line, one line was dropped due to poor germination) were included. All the pots were randomized weekly and the plants were only watered as needed. More details of the settings of replacement series studies are provided in Fig. 1(c).
2.2. Plant biomass, seed production and reproductive allocation
Plants were harvested individually at maturity, dried (9425 line: at 40 °C for 72 h; F2 lines: air dried for 6 weeks) and weighed for biomass. Harvest time of 9425 plants were recorded for each individual plant and used to calculate seed maturation time (days after flowering initiation). Both parental and F2 plants were cleaned through U.S. standard brass sieves (2 mm fb 0.5 mm, Dual Manufacturing Co., Inc. IL, US) and a column seed blower (Hoffman Manufacturing, Inc, OR, US) for total seed production measurement. Harvest index (HI), a measurement of reproductive allocation, was calculated using the following formula 31
| (1) |
2.3. Flowering phenology, floral and seed morphology
The flowering initiation time was recorded every 2 days for all the plants from both parental and F2 lines. Flowering initiation was defined as noticeable elongation of the filaments that separates stigma and anthers. A subset of 9425 kochia plants were used to study reproductive morphology through microscopy. Three plants per genotype were selected for non‐destructive time‐course images over a 45 day period (5‐12WAP), through a standard brightfield microscope (Leica, model: M205 FA, Wetzlar, DEU) fitted with a digital camera (Leica, model: M205 FA, DFC310 FX) through Leica application suite X (LAS X) platform. Images were taken daily on the same spikelet from the 7th node from the meristem with the above described microscope, to study the dichogamy (temporal separation of anthers and stigma) behavior in flowers. In our study, dichogamy was characterized by rapid pollen shedding and limited coexistence of mature anthers and receptive stigmas. Presence of dichogamy was checked on all the studied plants beyond the ones that were imaged.
As a measurement of physical distance between stigma and anthers, degree of herkogamy were estimated by the length difference between the stamens (mean of three stamens) and the pistil of the same flower. Stamen and pistil (ovary + style + stigma) length, and seed sepal length were measured on eight plants per genotype, two to three flowers/seeds per plant. All floral and seed sepal quantifications were done with Fiji, 32 using digital photographs taken from cross‐sections of matured flowers or buds. To compare two different progeny types potentially resulting from different mating behaviors (herein we defined as selfed vs outcrossed progenies), 30 seeds were collected from similar positions of four plants from each genotype, dried at 40 °C for 72 h, weighed and 1000 seed weights were calculated.
2.4. Germination of F1 progenies and outcrossing propensity of different genotypes
To confirm the viability of resulting F1 progenies of different genotypes, seeds from all the harvested plant were subjected to a germination test in a growth chamber set at the following conditions: 22 °C with 16‐h day/8‐h night light cycle with a light intensity of 200 μmol m−2 s−1 and relative humidity maintained at 50%. 10 seeds per plant were placed onto six‐well microplates (Corning™ Costar™ six‐well cell culture plate, CLS3506) prefilled with one layer of filter paper (GE Healthcare Life Sciences, Whatman™ Grade 1, 85MM, Cat. No. 1001–085) and 1 mL of deionized water. Germinated seedlings were counted daily for 7 days, followed by a simple seed crush test to determine the viability of non‐germinated seeds. 33 The whole germination test was repeated once.
Outcrossing rates were estimated for RR, SS and WT as the proportion of heterozygous F1 plants resulting from crosses between RR and SS/WT plants (RR♂ X SS♀ or SS♀ X RR♂). We subjected F1 seeds harvested from each individual plant of 9425‐RR, ‐RS, ‐SS, and WT from two runs of the greenhouse studies (Exp‐1, Exp‐2) to molecular marker assay (four to six parental plants per experiment, four F1 seeds per parental plant). The seeds were sowed and seedlings were genotyped as described above. In total, 48 F1 seedlings per parental genotype (9425‐RR, ‐RS, SS or WT) were genotyped. To compare the estimates from molecular marker with herbicide response, we also sprayed 24 more 3‐5 cm high F1 seedlings per parental plant (N = 8 in exp‐1, N = 7 in Exp‐2) with 1X rate of dicamba (560 g, XtendiMax, BayerCrop Science, St Louis, MO, USA) using the method described in Wu et al. 24 Mortality rates were scored 28 days after herbicide application.
2.5. Statistical analysis
For the comparison of different genotypes of 9425 and WT lines on different plants fitness traits, as well as the germination of F1 progenies, ANOVA (Analysis of Variance) was conducted according to the following model.
| (2) |
in which Yi is the fitness measurement for genotype i, μ is the overall mean, Mi is the fixed effect of genotype i, εi is the residual error. For the comparison of different genotypes of F2 lines on different plants fitness traits in monoculture or at different mixture ratios, ANOVA was conducted according to the following model.
| (3) |
in which Yij is the fitness measurement for genotype i in F2 line j, μ is the overall mean, Mi is the fixed effect of genotype i, Sj is the random effect of F2 line j, εij is the residual error. All pairwise comparisons between the three genotypes for each fitness trait and competition level were defined within the ANOVA and tested using t‐tests. Studentized residual of the range (−6,6) and quantile box plot was explored for outlier detection.
To visualize the flowering pattern of both F2 lines and parental lines, daily flowering plants were plotted to Julian date of year using a 3‐parameter log‐logistic regression model in the DRC package in RStudio (version 3.5.1, The R Foundation for Statistical Computing, Vienna, Austria). For the germination of F1 progenies, violin plots showing the distribution of final accumulated germination rates of different genotypes, and line charts showing the time course of the mean accumulated germination rates of each genotype were generated using the GGPLOT2 package in RStudio. Boxplots were generated for the mortality rates of F1 progenies.
3. RESULTS
3.1. G73N is associated with early flowering and more synchronous flowering pattern
The G73N mutation had profound effects on kochia's reproductive phenology. Flowering initiation timing and synchrony patterns were documented in both parental line 9425 and F2 lines in two glasshouse experiments (Fig. 2). In Exp‐1 (14‐15 h photoperiod, Table S1), 9425‐RR plants flowered 38 days after planting, which was 2, 4, 8 days earlier than ‐RS, ‐SS and WT plants, respectively (Table 1, Fig. 2(a)). At the population level, it took about 9 days for 80% of the 9425‐RR plants to flower while 13, 15 and 22 days for RS, SS and WT plants, respectively (Fig. 2(b), N = 72). However, no difference on the flowering initiation time was observed in Exp‐2 when daylength was shorter (11‐12 h, Table S1), since all the genotypes transitioned into the reproductive phases within 42 days after planting (Table 1, Fig. 2(a)). Similar flowering phenology patterns were observed on F2 lines when a larger number of plants were studied (Fig. 2; N = 400; LeClere et al. 23 ). All 9425 kochia genotypes took similar amount of time for seed maturation, except for the WT plants, which had a slightly shorter reproductive phase as observed in Exp‐1 (Table 1). Our data suggests that kochia is a facultative short‐day species with plasticity in adjusting the time required for reproductive growth stages to maximize their reproductive success.
Figure 2.
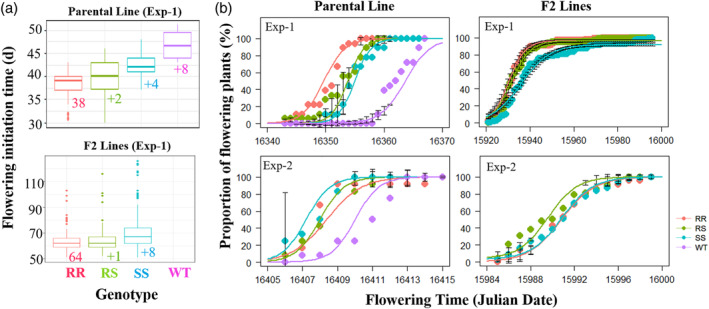
Flowering phenology of different G73N genotypes of both F2 and parental (9425) kochia (Bassia scoparia) line. RR, RS, SS/WT stand for homozygous‐, heterozygous‐resistant and susceptible plants (with or without the dicamba resistance endowing mutation G73N), respectively. (a) Boxplots showing different flowering initiation time for different genotypes of parental and F2 lines in Exp‐1. (b) Flowering synchronous patterns as measured by the accumulated flowering plants (%) over Julian date.
Table 1.
Measurement of reproductive traits of different G73N genotypes from the 9425 and WT B. scoparia line
| Fitness measurement | Timing | Genotype † | ||||
|---|---|---|---|---|---|---|
| 9425‐RR | 9425‐RS | 9425‐SS | WT | ANOVA ‡ | ||
| Flowering time (d) | Exp‐1 | 37.8 ± 0.8 (a § ) | 40.3 ± 0.9 (b) | 42.3 ± 0.7 (b) | 46.6 ± 0.7 (c) | <0.0001 |
| Exp‐2 | 41.6 ± 0.6 (a) | 41.7 ± 0.5 (a) | 41.1 ± 0.4 (a) | 42.1 ± 0.5 (a) | 0.6164 | |
| Seed maturation time (d) | Exp‐1 | 54.4 ± 0.9 (a) | 54.1 ± 1.0 (a) | 54.6 ± 1.0 (a) | 46.7 ± 0.8 (b) | <0.0001 |
| Exp‐2 | 45.3 ± 0.5 (a) | 48.9 ± 1.7 (b) | 45.6 ± 1.1 (ab) | 43.5 ± 1.3 (a) | 0.0345 | |
| Biomass (g) | Exp‐1 | 33.1 ± 1.2 (a) | 44.2 ± 1.3 (b) | 46.7 ± 1.9 (b) | 58.8 ± 1.9 (c) | <0.0001 |
| Exp‐2 | 26.8 ± 0.8 (a) | 28.7 ± 1.3 (a) | 34.6 ± 1.1 (b) | 25.9 ± 2.5 (a) | 0.0021 | |
| Seed production (g) | Exp‐1 | 13.2 ± 0.7 (ab) | 15.2 ± 1.0 (a) | 12.8 ± 0.8 (b) | 14.4 ± 0.7 (ab) | 0.1426 |
| Exp‐2 | 5.7 ± 0.5 (ab) | 5.6 ± 0.5 (b) | 7.5 ± 0.7 (a) | 5.3 ± 0.7 (b) | 0.0694 | |
| Harvest index (%) | Exp‐1 | 39.8 ± 1.6 (a) | 34.2 ± 1.8 (b) | 28.0 ± 2.0 (c) | 24.8 ± 1.5 (c) | <0.0001 |
| Exp‐2 | 21.2 ± 1.5 (a) | 20.0 ± 2.4 (a) | 21.8 ± 2.1 (a) | 20.3 ± 1.4 (a) | 0.9103 | |
| F1 germination rate (%) | Exp‐1 | 89.3 ± 3.1 (a) | 92.1 ± 3.0 (a) | 88.0 ± 3.9 (a) | 87.0 ± 4.3 (a) | 0.7776 |
| Exp‐2 | 100.0 ± 0.0 (a) | 99.4 ± 0.6 (ab) | 96.3 ± 2.2 (b) | 98.8 ± 0.9 (ab) | 0.0157 | |
| Outcrossing rates (%) | Exp‐1 | 33.3 ± 13.9 (a) | — | 8.3 ± 5.5 (a) | 14.3 ± 9.2 (a) | 0.1893 |
| Exp‐2 | 36.1 ± 11.7 (a) | — | 18.8 ± 12.0 (a) | 10.0 ± 6.1 (a) | 0.2114 | |
RR, RS, SS stand for homozygous‐, heterozygous‐resistant and susceptible plants (with or without the dicamba resistance endowing mutation G73N), respectively.
P‐values for ANOVA for the effects of genotype on different fitness traits are listed in the far‐right column.
Different letters represent significant difference among the means at p = 0.05 level.
3.2. G73N is associated with simultaneous decrease in both herkogamy and dichogamy that might promote selfing
Besides flowering phenology, G73N is also associated with altered floral morphology and mating system of kochia. Flowers of 9425‐RR plants displayed smaller stigma‐anthers physical distance (decreased herkogamy) than other genotypes (Fig. 3, Table 2). At bud stages, all genotypes had similar stamen (♂) length. However, the pistil (♀) of WT and SS plants elongated faster and were 10–15% taller than that of the resistant plants, resulting in a higher level of herkogamy (Table 2, Fig. 3(a),(b)). In open flowers, the pistils were largely similar among the genotypes while RR plants had 10–25% shorter filaments (Table 2, Figs 3(a), (b) and S1a,b), resulting in 60% and 67% lower herkogamy compared with RS and SS plants (Table 2; Fig. 3(a), (b)). Though the herkogamy level for RR plants was similar to that of WT plants (Table 2), the stigma of WT plants appeared to mature earlier (Figs 3(b) and S1c,d), indicating higher temporal separation of stigma and anthers (dichogamy).
Figure 3.
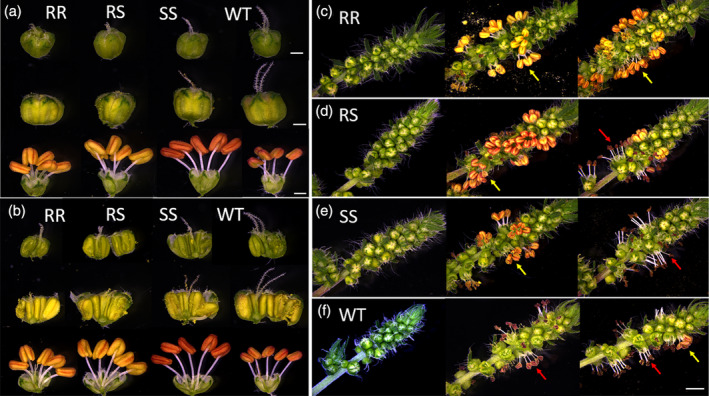
Floral morphology and stigma‐anther distance of different G73N endowing resistant or susceptible genotypes. (a), (b) intact and dissected young and mature flower buds and open flowers of different genotypes, showing the physical distance between the anthers and stigma (herkogamy). (c)‐(f)mages of a single flowering spikelet that was taken from the seventh node below the meristem for different genotypes 9425‐RR (c); ‐RS (d); ‐SS (e), WT plants (f). Yellow arrows indicate flowers with intact anthers, and synchronous male and female phases that promote selfing; red arrows indicate flowers with rapidly dehiscent anthers and separated male and female phases (dichogamy). Scale bar = 2 mm for spikelet (a–d), 500 μm for flower bud and 1 mm for mature flower (e,f).
Table 2.
Anatomical observations and quantifications of seed and flower morphology of different G73N genotypes from the 9425 and WT B. scoparia line
| Fitness measurement | Timing | Genotype † | ||||
|---|---|---|---|---|---|---|
| 9425‐RR | 9425‐RS | 9425‐SS | WT | ANOVA ‡ | ||
| Stamen length (mm) | Young bud | 0.88 ± 0.04 (a § ) | 0.96 ± 0.06 (a) | 0.91 ± 0.04 (a) | 0.84 ± 0.05 (a) | 0.3666 |
| Mature bud | 1.21 ± 0.06 (a) | 1.23 ± 0.05 (a) | 1.24 ± 0.04 (a) | 1.18 ± 0.07 (a) | 0.8748 | |
| Flower | 2.90 ± 0.07 (a) | 3.43 ± 0.11 (b) | 3.89 ± 0.08 (c) | 3.19 ± 0.10 (b) | <0.0001 | |
| Pistil length (mm) | Young bud | 1.63 ± 0.09 (a) | 1.57 ± 0.07 (a) | 1.84 ± 0.07 (ab) | 1.95 ± 0.16 (b) | 0.044 |
| Mature bud | 1.76 ± 0.08 (ab) | 1.60 ± 0.10 (a) | 1.97 ± 0.08 (b) | 2.08 ± 0.16 (b) | 0.0258 | |
| Flower | 2.37 ± 0.06 (b) | 2.05 ± 0.09 (a) | 2.34 ± 0.11 (ab) | 2.70 ± 0.16 (c) | 0.0013 | |
| Herkogamy (mm) | Young bud | 0.75 ± 0.06 (ab) | 0.60 ± 0.05 (a) | 0.93 ± 0.06 (bc) | 1.11 ± 0.12 (c) | 0.0003 |
| Mature bud | 0.50 ± 0.10 (ab) | 0.37 ± 0.07 (a) | 0.73 ± 0.09 (bc) | 0.90 ± 0.14 (c) | 0.0005 | |
| Flower | 0.55 ± 0.08 (a) | 1.38 ± 0.18 (b) | 1.69 ± 0.20 (b) | 0.49 ± 0.09 (a) | <0.0001 | |
| 1000 Seed weight (g) | Self | 1.01 ± 0.08 (a) | 0.96 ± 0.15 (a) | 0.98 ± 0.05 (a) | 0.84 ± 0.10 (a) | 0.6269 |
| Outcross | 1.40 ± 0.06 (a) | 0.74 ± 0.09 (bc) | 0.90 ± 0.10 (b) | 0.63 ± 0.03 (c) | <0.0001 | |
| Sepal length (mm) | Self | 0.92 ± 0.03 (a) | 0.65 ± 0.02 (b) | 0.68 ± 0.02 (b) | 0.53 ± 0.02 (c) | <0.0001 |
RR, RS, SS stand for homozygous‐, heterozygous‐resistant and susceptible plants (with or without the dicamba resistance endowing mutation G73N), respectively.
P‐values for ANOVA for the effects of genotype on different fitness traits are listed in the far‐right column.
Different letters represent significant difference among the means at P = 0.05 level.
In addition to decreased herkogamy, the anthers and stigma of RR plants also appeared to mature in a more synchronous pattern (reduced dichogamy, Figs 3(c) and S2a). We observed dichogamy exclusively in almost all WT plants and in varied proportions of RS and SS plants, but not in any of the RR plants (Figs 3(c)–(f) and S2c,d). RS and SS plants consistently displayed more dehiscent anthers due to rapid pollen shedding overnight or early morning before imaging (Figs 3(c)‐(f) and S2(b)–(d); black/brownish stigma tips in SS and WT flowers before their own pollen shedding). Decreased dichogamy and longer longevity of the anthers might potentially enable G73N kochia mutant plants to constantly pollinate their own flowers or flowers of nearby plants, facilitating the spread of resistance alleles (Figs 3(c)–(f) and S2a).
3.3. G73N is associated with altered seed morphology and higher reproductive allocation
Our data also revealed that kochia is a self‐compatible, mixed‐mating species which can produce both selfed and outcrossed progenies (Figs 4, S3, and S4e–f). Although different progenies can co‐exist on the same branch (Figs 4(a), (c) and S4a–d), the selfed progenies were more commonly observed at the bottom portion of the plants, tips of late flowering branches, as well as at the nodes of lateral branches attaching directly to plant stems (Fig. 4(b)). In both cases, those flowers exhibited delayed development and thus less exposure to mature pollen either from other flowers of the same plant, or flowers from other plants. The selfed progenies were flat, slightly bigger in size and with longer and greenish sepals (Fig. 4(e)), while outcrossed progenies were featured with a succulent type of sepals (thickened, fleshy and engorged) (Fig. 4(d)). Selfed seeds of RR plants had 30–70% longer sepals and potentially lower shattering propensity than other genotypes (Fig. 4(a), (c), (e) and S3, S5), which might serve as an efficient seed dispersal mechanism allowing ‘winged’ seeds to spread across the landscape through tumbling in winds (Table 2, Fig. 4(e)).
Figure 4.

Seed morphology of different G73N endowing resistant or susceptible genotypes. (a), (c) Seed spikelet (one per genotype) showing the co‐existence of different types of progenies. Genotypes from left to right are: 9425‐RR, ‐RS, ‐SS and WT, respectively. Red, yellow, and white arrows in (a) indicate outcrossed, selfed and aborted seeds, respectively. (b) seed spikelet (two per genotype) taken from upper (U) and lower portion (L) of a plant; (d), (e) fresh and dried seeds of different genotypes: outcross‐progenies (d), self‐progenies (e); Scale bars = 2 mm in (a), 5 mm in (b), (c) and 1 mm in (d), (e), respectively.
Even though 9425 mutant plants produced 30–50% less biomass, they produced a similar amount of seeds as SS or WT plants (Table 1). As a result, 9425‐RR plants had up to 60% higher reproductive allocation (Table 1). More biomass reduction was observed (up to 80–90%) in F2 lines when mutant plants were grown in mixture (25–75% of resistance) with susceptible genotypes in the replacement series study (Fig. 5(a), (b)). Interestingly, when grown in monoculture or without competition, both F2 and 9425‐resistant plants produced similar amounts of seeds as SS or WT plants, although there were some variations among the two experiments (Fig. 5(c), (d), Table 1). F2‐resistant plants also had slightly higher reproductive allocation than SS plants at monoculture though the difference was not statistically significant (Fig. 5(e), (f)).
Figure 5.
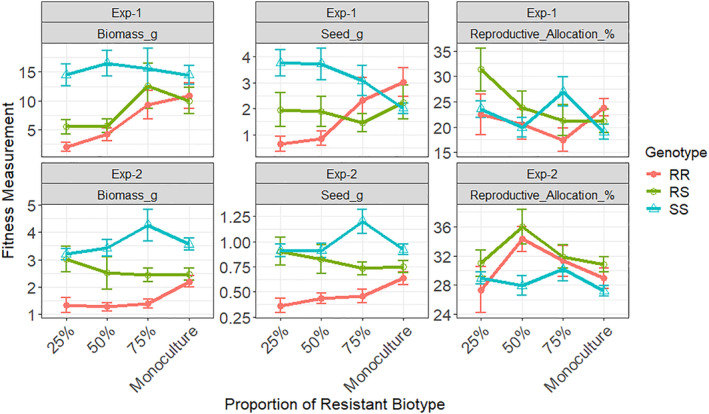
Biomass, seed production and reproductive allocation of different G73N genotypes of F2 kochia (Bassia scoparia) lines across different competition levels in the replacement series studies. RR, RS, SS stand for homozygous‐, heterozygous‐resistant and susceptible plants (with or without the dicamba resistance endowing mutation G73N), respectively. Means of each fitness trait of F2‐RR, ‐RS, and SS plants were plotted across different competition levels. Reproductive Allocation or Harvest Index = (Seed Production)/(Total Biomass) × 100%.
3.4. G73N mutant plants did not suffer from low F1 fitness or outcrossing propensity
No difference was observed on germination ability or viability of seeds produced by different G73N genotypes (Table 1, and Fig. S6). The slightly lower germination rates (~90%) for seeds from Exp‐1, is likely because seeds have started to decay when the germination tests were conducted. Seed crush tests indicated the non‐germinated seeds were close to 100% viable. The high fecundity of mutant plants might be due to higher seed densities per spikelet and less aborted seeds than the SS and WT plants (Figs 4(a)–(c) and S5). The 1000 seed weight was similar or even slightly heavier (Table 2; LeClere et al. 21 ), indicating seed quality was also similar. Interestingly, outcrossing rates as measured by the frequency of heterozygous plants were similar among the F1 progenies of 9425‐RR and ‐SS and WT plants (Table 1), indicates the highly inbred 9425 line might still maintain high outcrossing propensity. Results from the molecular marker assay generally agreed with the mortality rates observed in the spray test (10–20%, Fig. S7), except that the 1X rate of dicamba killed some mutant plants likely with slower growth and smaller sizes.
4. DISCUSSION
Auxin plays an essential role in regulating plant reproductive process. 34 , 35 , 36 , 37 Therefore, auxin deficient mutants are often associated with impaired reproductive traits such as anther filament elongation, embryogenesis, and seed development. 37 , 38 , 39 In an extreme example Arabidopsis (Arabidopsis thaliana) iaa16‐1 homozygote is incapable of seed production, because their stamens fail to reach the stigma before dehiscence. 40 Since the 9425 line was maintained through a single seed descent method, it is possible that inbreeding depression might be a confounding effect resulting in lower fitness in the resistant kochia. 24 , 41 , 42 Strikingly, though G73N can cause significant vegetative growth defects, it does not appear to reduce plant fecundity in the absence of competition. Similar to what was previously reported in F2 lines, 23 resistant plants (9425‐RR, 9425‐RS) from the parental 9425 line produced a similar amount of seeds as 9425‐SS or WT plants regardless of the drastic difference in plant sizes. Our result is consistent with previous reports in kochia, 23 , 43 as well as other studies showing that resistant plants had increased fecundity when grown in monoculture. 44 , 45
One possible reason for the limited fitness costs of G73N at the highly conserved degron region (GWPPV/I) of IAA16 in kochia, 23 might be the high redundancy of the AUX/IAA protein family. There are 29 AUX/IAA proteins in Arabidopsis, and some of which might show no visible developmental defects. 46 , 47 , 48 Furthermore, different AUX/IAA proteins have different interaction partners (e.g. auxin response factors (ARF)), which might lead to varied phenotypic effects for different AUX/IAA mutants. 49 From an evolutionary point of view, plants might manage to mitigate the growth‐dense trade‐offs, through co‐evolution of other fitness enhancing genes/ecological strategies. 50 In the current study, the deleterious effects of G73N on kochia's vegetative growth and competitiveness appeared to be compensated by multiple highly effective reproduction strategies.
First, G73N is associated with alteration of kochia reproductive phenology such as flowering initiation time, leading to longer reproduction phase. In both parental and F2 lines, kochia mutants consistently flowered earlier but took a similar amount of time to finish reproductive development. Early flowering can be evolutionarily advantageous since it allows plants to rapidly divert resources from vegetative growth to seed maturation. 51 This finding, however, contradicts with Kumar & Jha 52 and Mai et al., 53 who observed delayed flowering associated with auxin resistance. The difference might be due to different auxin deficient mutants that are involved, as well as other confounding environmental and geographical factors on flowering phenology. 54 Results from Exp‐1 also showed that G73N mutant plants allocated significantly higher proportion of energy captured from photosynthesis to reproduction. Research has suggested that plants might allocate more resources to reproduction when they are under stress. 55 These plasticity and strategic differences allow resistant kochia to maximize its reproductive output and adapt to unpredictable and stressful field environments.
Second, G73N covaried with multiple floral and seed morphological changes, which shifted kochia's mating systems. Flowers of G73N mutant plants are linked with decreased herkogamy and dichogamy (smaller spatial and temporal separation of stigma‐anther), both are mechanisms that promote selfing and limit outcrossing. 56 , 57 , 58 , 59 , 60 Self‐pollination is often linked with a higher risk of inbreeding depression 61 , 62 and lower levels of heterozygosity/recombination rates. As a result, self‐pollinating species might have lower standing genetic variation on which the selection may act. 63 , 64 However, self‐pollination can also, serve as a mechanism of reproductive assurance to maximize seed production, especially in pollen‐limited conditions. 57 When conditions are not favorable for outcrossing (e.g. no pollinators around or limited number of plants are in the reproductive stage), autonomous within‐flower selfing allows ovules that otherwise would have gone unfertilized to be fertilized and develop into seeds. 65 Indeed, we observed more aborted flowers in SS and WT plants which failed to develop into seeds, while RR and RS plants had higher seed density per spikelet. Besides serving as a mechanism to maximize seed production, higher self‐fertility of resistant plants compared with the susceptible plants, might also be a mechanism to reduce the influx of susceptible alleles in the field, also known as the ‘prevention of gene flow’ hypothesis. 14 , 66 To our surprise, the outcross rates of 9425‐RR plants we estimated were similar to that of 9425‐SS and WT plants, suggesting the high reproductive success of mutant kochia might be more complicated with other unknown factors involved. For example, though we sampled representative floral and seeds in our dichogamy and herkogamy measurements, we did observe variations among samples from different positions of a single plant. Nevertheless, more thorough studies are needed to fully elucidate the impact of G73N on the mating behavior of dicamba resistant kochia.
Lastly, G73N mutant seeds had longer sepals (‘winged’ seeds) and seemingly lower shattering rate, which might facilitate long‐distance seed dispersal and the spread of resistance over the landscape. Previous research found that Dipterocarpaceae species produce multiple‐winged seeds which can autorotate to maximize descent time. 67 , 68 , 69 It is not known if kochia seeds are able to autorotate while tumbling, but they clearly adopt a similar multiple‐winged approach which can influence dispersal distances and germination locations. We do have to point out that the pleiotropic effects of the G73N mutation need to be tested in more diverse genetic backgrounds or in transgenic plants, to fully establish a causal relationship between the mutation and the reproductive traits we observed.
To put our fragmentary understanding of the evolution of herbicide resistance costs into a framework, we propose a 4‐phase hypothetic model (Fig. 6(a)). Phase I denotes the pre‐ or early selection stage, during which most of the plants are sensitive. Genetic variations for adaptations/tolerance can be selected and enriched as the magnitudes of selection pressure fluctuate in the field. 70 , 71 Phase II features a period driven by increased selection pressure, along with a surge of fitness costs (we might fail to detect such highly costly and thus rare resistance endowing mutations). When overall resistance costs reach a plateau, the evolution transits from a defense‐focused into a growth‐defense balanced phase. During Phase III, plants can evolve secondary compensatory mutations or combine multiple mechanisms that mitigate the fitness costs while maintaining a high level of resistance. 13 , 72 Eventually, resistance traits without fitness costs get fixed in a homogeneous population at phase IV. During the evolution, the energy plants fixed from photosynthesis can be allocated to defense, growth, and reproduction with different prioritization levels. What is worth mentioning, it is possible a population might have to have optimized genetic backgrounds for the successful integration of the resistance genes without suffering a fitness cost. As a result, the compensatory evolution (phase III) might overlap with the evolutionary rescue stage (phase II) as illustrated in Fig. 6(b).
Figure 6.
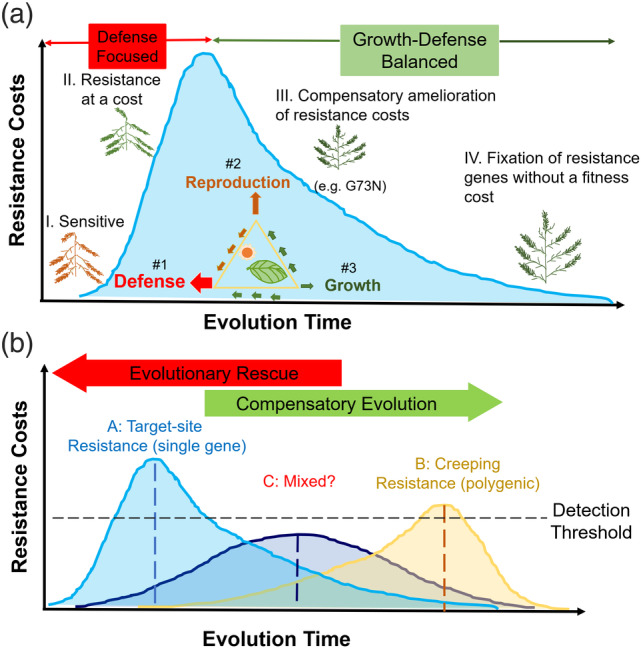
Schematic illustration of evolutionary trajectories of fitness costs of herbicide resistance traits. (a) A hypothetical four‐phase model depicting the evolutionary trajectory of target‐site based synthetic auxins resistance. The energy plants fixed from photosynthesis can be allocated to defense, growth and reproduction with different prioritization levels. (b) Bell‐shaped curves of different colors represent the evolution trajectories of fitness costs of resistance with different genetic basis. (A) Target‐site resistance controlled by a single gene with major effects; (B) Creeping resistance controlled by multiple genes of small effects and (C) The mixture of the two types of resistance. Detection threshold indicates the sensitivity level of the fitness studies to detect the fitness costs.
We also believe that the genetic basis of resistance might transform this evolutionary trajectory into different forms and scales (Fig. 6(b)). For example, resistance endowed by a single mutation will likely incur an instant and significant fitness cost initially, resulting in a positively‐skewed curve (Type A). Whereas fitness costs might be gradually accumulated for resistance endowed by multiple genes of smaller effects (‘creeping resistance’), 73 resulting in a negatively‐skewed curve with a lower peak (Type B). There might also be species that follow an evolutionary trajectory that is intermediate to the above two models (Type C) due to a more confounding and overlapping genetic basis that is involved. These models might also help explain how fitness studies with different detection sensitivities might affect how often we see a cost for herbicide resistance traits. Nevertheless, more empirical data are required to validate these hypotheses on the intertwining relationship of evolutionary rescue and compensatory evolution. 64
5. CONCLUSIONS
Overall, the current study demonstrated that though G73N can lead to significantly diminish plant size and impaired vegetative growth, its deleterious effects can be compensated through co‐evolution of other fitness traits that promote reproductive fitness. We do have to point out that since the 9425 parental line was maintained through a modified single seed descent method, some of the reproductive traits might be the result of the inbreeding depression and limited to the genetic background of the 9425 line. However, as one of the most important fitness indices, no reduction in fecundity and high outcrossing propensity of mutant plants, are both strong evidence that inbreeding depression was not severe in our kochia populations. Nevertheless, future studies that characterize the effects of G73N in more diverse genetic backgrounds are needed to fully rule out the confounding effects of inbreeding depression.
From an evolutionary point of view, it is possible that G73N might only be an initial step during the synthetic auxin resistance evolutionary trajectory, which might later be replaced by other mutations or non‐target site resistance (NTR) mechanisms with lower or negligible resistance cost, as seen in the case of atrazine resistance in common waterhemp (Amaranthus tuberculatus). 74 In fact, NTR mechanisms such as decreased translocation have already been reported in other dicamba or 2,4‐D resistant weeds. 75 , 76 , 77 , 78 Future research could focus on using the newly available kochia genome to identify the genetic basis for compensatory evolution. 79 For example, identifying specific secondary mutations or genes that are involved in the co‐selected weediness traits. 13 , 25 , 72 , 80 These genes might also be a valuable source for engineering crops with certain desirable traits.
Our findings re‐enforced the importance of controlling resistant weeds when they are still in low frequency, since the competitiveness of resistant plants can increase during selection, especially when they become the dominant biotypes within a population. From a weed management point of view, the altered flowering phenology, floral and seed morphology for RR plants, might enable novel management tactics that target the mating systems and reproductive strategies of resistant plants. 81 , 82 Considering the extent of selection pressure that might be imposed by the widely adopted synthetic auxins resistant cropping system, it is highly recommended that farmers adopt diverse weed control approaches that harness evolutionary thinking, 83 to delay the resistance evolution and sustain the utility of the limited resistant weed management technologies we still have.
AUTHOR CONTRIBUTIONS
CW, SL and RS conceived the project; CW and MP performed the research with assistance from SL and AP; KL and CW conducted statistical analysis; CW, PW and RS wrote the manuscript with contributions from all other authors.
CONFLICT OF INTEREST
CW, MP, SL, KL, AP are employed by Bayer CropScience.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
The authors want to thank Cindy Trembly, Jeff Haines, Megan Gilley, and Jenny Krebel for plant propagation and herbicide application; Drs. Chandra Aradhya and Jeff Stain for proof reading and editing the manuscript.
†The paper was given in part, at the annual meeting of Weed Science Society of America (WSSA) in March 2020.
REFERENCES
- 1. Cipollini D, Walters D, Voelckel C. Costs of resistance in plants: from theory to evidence. Ann Plant Rev. 47:263–307 (2014). [Google Scholar]
- 2. Fineblum WL and Rausher MD, Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature 377:517–520 (1995). [Google Scholar]
- 3. Huot B, Yao J, Montgomery BL and He SY, Growth‐defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant 7:1267–1287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergelson J and Purrington CB, Surveying patterns in the cost of resistance in plants. Am Nat 148:536–558 (1996). [Google Scholar]
- 5. Vila‐Aiub MM, Neve P and Powles SB, Fitness costs associated with evolved herbicide resistance in plants. New Phytol 184:751–767 (2009). [DOI] [PubMed] [Google Scholar]
- 6. Vila‐Aiub MM, Yu Q and Powles SB, Do plants pay a fitness cost to be resistant to glyphosate. New Phytol 223:532–547 (2019). [DOI] [PubMed] [Google Scholar]
- 7. Frenkel E, Matzrafi M, Rubin B and Peleg Z, Effects of environmental conditions on the fitness penalty in Brachypodium hybridum . Front Plant Sci 8:94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coustau C, Chevillon C and ffrench‐constant R, resistance to xenobiotics and parasites: can we count the cost? Trends Ecol Evol 15:378–383 (2000). [DOI] [PubMed] [Google Scholar]
- 9. ffrench‐Constant RH and Bass C , Does resistance really carry a fitness cost? Curr Opin Insect Sci 21:39–46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maisnier‐Patin S and Andersson DI, Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res Microbiol 155:360–369 (2004). [DOI] [PubMed] [Google Scholar]
- 11. Andersson DI and Hughes D, Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol 8:260–271 (2010). [DOI] [PubMed] [Google Scholar]
- 12. Guillemaud T, Lenormand T, Bourguet D, Chevillon C, Pasteur N and Raymond M, Evolution of resistance in Culex pipiens: allele replacement and changing environment. Evolution 52:443–453 (1998). [DOI] [PubMed] [Google Scholar]
- 13. Händel N, Schuurmans JM, Brul S and ter Kuile BH, Compensation of the metabolic costs of antibiotic resistance by physiological adaptation in Escherichia coli . Antimicrob Agents Chemother 57:3752–3762 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baucom RS, Evolutionary and ecological insights from herbicide‐resistant weeds: what have we learned about plant adaptation, and what is left to uncover? New Phytol 223:68–82 (2019). [DOI] [PubMed] [Google Scholar]
- 15. Lenski RE, Experimental studies of pleiotropy and epistasis in escherichiacoli.2. Compensation for maladaptive effects associated with resistance to virusT4. Evolution 42:433–440 (1988). [DOI] [PubMed] [Google Scholar]
- 16. McKenzie JA and Clarke GM, Diazinon resistance, fluctuating asymmetry and fitness in the Australian sheep blowfly. Lucilia cuprina Genetics 120:213–220 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paris M, Roux F, Berard A and Reboud X, The effects of the genetic background on herbicide resistance fitness cost and its associated dominance in Arabidopsis thaliana . Heredity 101:499–506 (2008). [DOI] [PubMed] [Google Scholar]
- 18. Darmency H, Menchari Y, Le Corre V and Délye C, 2015. Fitness cost due to herbicide resistance may trigger genetic background evolution. Evolution 69:271–278 (2015). [DOI] [PubMed] [Google Scholar]
- 19. Grossmann K, Auxin herbicides: current status of mechanism and mode of action. Pest Manage Sci 66:113–120 (2010). [DOI] [PubMed] [Google Scholar]
- 20. Whitehead CW and Switzer CM, The differential response of strains of wild carrot to 2,4‐D and related herbicides. Can J Plant Sci 43:255–262 (1963). [Google Scholar]
- 21. Heap I, The International survey of herbicide resistant weeds: weeds resistant to synthetic auxins (O/4). http://www.weedscience.org/Summary/MOA.aspx. [5 July 2020].
- 22. Preston C, Belles DS, Westra PH, Nissen SJ and Ward SM, Inheritance of resistance to the auxinic herbicide dicamba in Kochia (Bassia scoparia). Weed Sci 57:43–47 (2009). [Google Scholar]
- 23. LeClere S, Wu C, Westra P and Sammons RD, Cross‐resistance to dicamba, 2,4‐D, and fluroxypyr in Bassia scoparia is endowed by a mutation in an AUX/IAA gene. Proc Natl Acad Sci U S A 115:2911–2920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu C, LeClere S, Liu K, Paciorek M, Perez‐jones A, Westra P et al., A dicamba resistance‐endowing IAA16 mutation leads to significant vegetative growth defects and impaired competitiveness in kochia (Bassia scoparia). Pest Manag Sci (2020). 10.1002/ps.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuester A, Fall E, Chang SM and Baucom RS, Shifts in outcrossing rates and changes to floral traits are associated with the evolution of herbicide resistance in the common morning glory. Ecol Lett 20:41–49 (2017). [DOI] [PubMed] [Google Scholar]
- 26. Stearns SC. Trade‐Offs in Life‐History Evolution. Funct Ecol. 3:259–268 (1989). [Google Scholar]
- 27. Wenk EH and Falster DS, Quantifying and understanding reproductive allocation schedules in plants: a lifetime of decisions. New Phytol 5:5521–5538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behrens MR, Mutlu N, Chakaborty S, Dumitru R, Wen ZJ, LaVallee BJ et al., Dicamba resistance: enlarging and preserving biotechnology‐based weed management strategies. Science 316:1185–1188 (2007). [DOI] [PubMed] [Google Scholar]
- 29. Chekan JR, Ongpipattanakul C, Wright TR, Zhang B, Bollinger JM, Jr. Rajakovich LJ et al., Molecular basis for enantioselective herbicide degradation imparted by aryloxyalkanoate dioxygenases in transgenic plants. Proc Natl Acad Sci U S A 116:13299–13304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. L Dodson, Recent trends in GE adoption. https://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/recent-trends-in-ge-adoption/ [9 December 2020].
- 31. Sadras VO, Bange MP and Milroy SP, Reproductive allocation of cotton in response to plant and environmental factors. Ann Bot 80:75–81 (1997). [Google Scholar]
- 32. Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al., Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9:676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sawma JT and Möhler CL, Evaluating seed viability by an unimbibed seed crush test in comparison with the tetrazolium test. Weed Technol 16:781–786 (2002). [Google Scholar]
- 34. Cheng Y, Dai X and Zhao Y, Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng Y, Dai X and Zhao Y, Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19:2430–2439 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sundberg E and Østergaard L, Distinct and dynamic auxin activities during reproductive development. Cold Spring Harb Perspect Biol 1:a001628 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tenorio‐Berrío R, Pérez‐Alonso MM, Vicente‐Carbajosa J, Martín‐Torres L, Dreyer I and Pollmann S, Identification of two auxin‐regulated potassium transporters involved in seed maturation. Int J Mol Sci 19:2132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, Yan DW, Yuan TT, Gao X and Lu YT, A gain‐of‐function mutation in IAA8 alters Arabidopsis floral organ development by change of jasmonic acid level. Plant Mol Biol 82:71–83 (2013). [DOI] [PubMed] [Google Scholar]
- 39. Tabata R, Ikezaki M, Fujibe T, Aida M, Tian CE, Ueno Y et al., Arabidopsis auxin response factor6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol 51:164–175 (2009). [DOI] [PubMed] [Google Scholar]
- 40. Rinaldi MA, Liu J, Enders TA, Bartel B and Strader LC, A gain‐of‐function mutation in IAA16 confers reduced responses to auxin and abscisic acid and impedes plant growth and fertility. Plant Mol Biol 79:359–373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hedrick PW and Garcia‐Dorado A, Understanding inbreeding depression, purging, and genetic rescue. Trends Ecol Evol 31:940–952 (2016). [DOI] [PubMed] [Google Scholar]
- 42. Roff DA, Inbreeding depression: tests of the overdominance and partial dominance hypotheses. Evolution 56:768–775 (2002). [DOI] [PubMed] [Google Scholar]
- 43. Menalled FD and Smith RG, Competitiveness of herbicide‐resistant and herbicide susceptible kochia (Bassia scoparia [L.] Schrad.) under contrasting management practices. Weed Biol Manage 7:115–119 (2007). [Google Scholar]
- 44. Tardif FJ, Rajcan I and Costea M, A mutation in the herbicide target site acetohydroxyacid synthase produces morphological and structural alterations and reduces fitness in Amaranthus powellii . New Phytol 169:251–264 (2006). [DOI] [PubMed] [Google Scholar]
- 45. Liu Y, Ge F, Liang Y, Wu G and Li J, Characterization of competitive interactions in the coexistence of Bt‐transgenic and conventional rice. BMC Biotechnol 15:27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reed JW, Roles and activities of aux/IAA proteins in Arabidopsis. Trends Plant Sci 6:420–425 (2001). [DOI] [PubMed] [Google Scholar]
- 47. Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, Ecker JR et al., Functional genomic analysis of the AUXIN/INDOLE‐3‐ACETIC ACID gene family members in Arabidopsis thaliana . Plant Cell 17:3282–3300 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luo J, Zhou JJ and Zhang JZ, Aux/IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci 19:259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liscum E and Reed JW, Genetics of aux/IAA and ARF action in plant growth and development. Plant Mol Biol 49:387–400 (2002). [PubMed] [Google Scholar]
- 50. Karaso TL, Chae E, Herman JJ and Bergelson J, Mechanisms to mitigate the trade‐off between growth and defense. Plant Cell 29:666–680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Houle G, The advantages of early flowering in the spring ephemeral annual plant Floerkea proserpinacoides . New Phytol 154:689–694 (2002). [DOI] [PubMed] [Google Scholar]
- 52. Kumar V and Jha P, Differences in germination, growth, and fecundity characteristics of dicamba‐fluroxypyr‐resistant and susceptible Bassia scoparia . PLoS One 11:e0161533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mai YX, Wang L and Yang HQ, A gain‐of‐function mutation in IAA7/AXR2 confers late flowering under short‐day light in Arabidopsis. J Integr Plant Biol 53:480–492 (2011). [DOI] [PubMed] [Google Scholar]
- 54. Bell AR, Nalewaja JD and Schooler AB, Light period, temperature, and kochia flowering. Weed Sci 20:462–464 (1972). [Google Scholar]
- 55. Aronson J, Kigel J and Shmida A, Reproductive allocation strategies in desert and Mediterranean populations of annual plants grown with and without water stress. Oecologia 93:336–342 (1993). [DOI] [PubMed] [Google Scholar]
- 56. Barrett SCH, Mating strategies in flowering plants: the outcrossing–selfing paradigm and beyond. Philos Trans R Soc B Biol Sci 358:991–1004 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brys R, Geens B, Beeckman T and Jacquemyn H, Differences in dichogamy and herkogamy contribute to higher selfing in contrasting environments in the annual Blackstonia perfoliata (Gentianaceae). Ann Bot 111:651–661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luo Y and Widmer A, Herkogamy and its effects on mating patterns in Arabidopsis thaliana . PLoS One 8:e57902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kalisz S, Randle AM, Chaiffetz D, Faigeles M, Butera A and Beight C, Dichogamy correlates with outcrossing rate and defines the selfing syndrome in the mixed mating genus Collinsia. Ann Bot 109:571–582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Opedal OH, Herkogamy, a principal functional trait of plant reproductive biology. Int J Plant Sci 179:677–687 (2018). [Google Scholar]
- 61. Dudash MR, Relative fitness of selfed and outcrossed progeny in a selfcompatible. Protandrous species, Sabatia angularis L. (Gentianaceae): a comparison of three environments. Evolution 44:1129–1139 (1990). [DOI] [PubMed] [Google Scholar]
- 62. Husband BC and Schemske DW, Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50:54–70 (1996). [DOI] [PubMed] [Google Scholar]
- 63. Nordborg M, Linkage disequilibrium, gene trees and selfing: an ancestral recombination graph with partial self‐fertilization. Genetics 154:923–929 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kreiner JM, Stinchcombe JR and Wright SI, Population genomics of herbicide resistance: adaptation via evolutionary rescue. Annu Rev Plant Biol 69:611–635 (2018). [DOI] [PubMed] [Google Scholar]
- 65. Morgan MT and Wilson WG, Self‐fertilization and the escape from pollen limitation in variable pollination environments. Evolution 59:1143–1148 (2005). [PubMed] [Google Scholar]
- 66. Antonovics J, Evolution in closely adjacent plant populations V Evolution of self‐fertility. Heredity 23:219–238 (1968). [DOI] [PubMed] [Google Scholar]
- 67. Lentink D, Dickson WB, van Leeuwen JL and Dickinson MH, Leading‐edge vortices elevate lift of autorotating plant seeds. Science 324:1438–1440 (2009). [DOI] [PubMed] [Google Scholar]
- 68. Fauli RA, Rabault J and Carlson A, Effect of wing fold angles on the terminal descent velocity of double‐winged autorotating seeds, fruits, and other diaspores. Phys Rev E 100:e013108 (2019). [DOI] [PubMed] [Google Scholar]
- 69. Seale M and Nakayama N, From passive to informed: mechanical mechanisms of seed dispersal. New Phytol 225:653–658 (2019). [DOI] [PubMed] [Google Scholar]
- 70. Baucom RS and Mauricio R, Fitness costs and benefits of novel herbicide tolerance in a noxious weed. Proc Natl Acad Sci U S A 101:13386–13390 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hartley CJ, Newcomb RD, Russell RJ, Yong CG, Stevens JR, Yeates DK et al., Amplification of DNA from preserved specimens shows blowflies were preadapted for the rapid evolution of insecticide resistance. Proc Natl Acad Sci U S A 103:8757–8762 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ma C, Tran J, Li C, Ganesan L, Wood D and Morrissette N, Secondary mutations correct fitness defects in Toxoplasma gondii with dinitroaniline resistance mutations. Genetics 180:845–856 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gressel J, Evolving understanding of the evolution of herbicide resistance. Pest Manage Sci 65:1164–1173 (2009). [DOI] [PubMed] [Google Scholar]
- 74. Wu C, Davis AS and Tranel PJ, Limited fitness costs of herbicide‐resistance traits in Amaranthus tuberculatus facilitate resistance evolution. Pest Manage Sci 74:293–301 (2017). [DOI] [PubMed] [Google Scholar]
- 75. Goggin DE, Cawthray GR and Powles SB, 2,4‐D resistance in wild radish: reduced herbicide translocation via inhibition of cellular transport. J Exp Bot 67:3223–3235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ou J, Stahlman PW and Jugulam M, Reduced absorption of glyphosate and decreased translocation of dicamba contribute to poor control of kochia (Bassia scoparia) at high temperature. Pest Manage Sci 74:1134–1142 (2017). [DOI] [PubMed] [Google Scholar]
- 77. Pettinga DJ, Ou J, Patterson EL, Jugulam M, Westra P and Gaines TA, Increased chalcone synthase (chs) expression is associated with dicamba resistance in Kochia scoparia . Pest Manage Sci 74:2306–2315 (2018). [DOI] [PubMed] [Google Scholar]
- 78. Shyam C, Jhala AJ, Kruger G and Jugulam M, Rapid metabolism increases the level of 2,4‐D resistance at high temperature in common waterhemp (Amaranthus tuberculatus). Sci Rep 9:16695 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Patterson EL, Gaines TA, Saski CA, Westra P and Sparks CD, The genome of Kochia scoparia: a story of evolution in action; Proceedings of the 60th Weed Science Society of America conference; Mar 2–5; HI, US. 60:340 (2020).
- 80. Wang W, Xia H, Yang X, Xu T, Si HJ, Cai XX et al., A novel 5‐enolpyruvoylshikimate‐3‐phosphate (EPSP) synthase transgene for glyphosate resistance stimulates growth and fecundity in weedy rice (Oryza sativa) without herbicide. New Phytol 202:679–688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Guttieri MJ and Eberlein CV, Inbreeding coefficients of field populations of Kochia scoparia using chlorsulfuron resistance as a phenotypic marker. Weed Sci 46:521–525 (1998). [Google Scholar]
- 82. Nili EL, Shwartz I, Huet H, Aminia M, Owen MD, Gressel J and Noivirt‐Brik O, Inhibiting herbicide resistant amaranthus by suppressing reproduction. Proceedings of the 60th Weed Science Society of America Conference proceedings; Mar 2–5; HI, US. 60: 531 (2020).
- 83. Neve P, Vila‐Aiub M and Roux F, Evolutionary‐thinking in agricultural weed management. New Phytol 184:783–793 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


