Abstract
Background
The incidence of acute tubulointerstitial nephritis (ATIN) related to drugs has dramatically increased over recent years. A new subtype of ATIN, apparently different from classical drug-related ATIN, has emerged that has been related to the administration of immune checkpoint inhibitors (ICIs). We investigated these differences between ICI-related ATIN (ICI ATIN) and non-ICI-related ATIN in terms of clinical features, response to treatment with steroids and the evolution of kidney function.
Methods
A total of 47 patients diagnosed with ATIN from two centres were recruited. Of these, 13 patients presented with ATIN during ICI treatment and 34 were diagnosed with ATIN attributed to other drugs. The main demographic, clinical and analytical variables such as gender, age and current medication were recorded. The type of malignancy, oncological treatment, ICI dose and presence of extrarenal immune-related adverse events were also reviewed. Renal biopsy diagnosis, time to drug withdrawal and ATIN-specific treatment, as well as laboratory data during follow-up, were also studied.
Results
Patients diagnosed with ICI ATIN presented with lower creatinine (ICI ATIN 3.8 ± 1.03 versus classical ATIN 5.98 ± 4.15 mg/dL, P = 0.007) at diagnosis and higher urinary leucocyte counts (ICI ATIN 263.2 ± 418.04 versus classical ATIN 133.55 ± 284.62, P = 0.048) compared with patients with non-ICI-related ATIN. Time from initiation of the culprit drug to ATIN diagnosis was longer in patients with ICI ATIN than in those with classical ATIN (197.07 ± 184.99 versus 114.4 ± 352.16 days, P = 0.006). In addition, during follow-up, the slope of decreasing creatinine over time was lower for ICI ATIN compared with non-ICI-related ATIN.
Conclusions
In this study, we analysed differences between ICI ATIN and classical ATIN. We found that patients with ICI ATIN presented with a larger latency period after culprit drug initiation, milder acute kidney injury and slower creatinine amelioration compared with those with classical ATIN. These results may, in part, be ascribed to potential differences in the pathological mechanisms involved in ATIN development, suggesting that ICI and classical ATIN may be different diseases with similar renal histologies.
Keywords: acute kidney injury, acute tubular nephritis, immune checkpoint inhibitors, kidney biopsy, steroid therapy
INTRODUCTION
The incidence of acute tubulointerstitial nephritis (ATIN) related to drugs has dramatically increased over recent decades. Data from the Spanish Registry of Glomerulonephritis have demonstrated that the diagnosis of biopsy-proven ATIN has increased from 3% to 20% over the last 20 years [1]. ATIN has become the third most common cause of acute kidney injury (AKI) in hospitalized patients [2]. The main trigger associated with ATIN development is the use of certain nephritogenic drugs that are able to induce antigen-initiated cell-mediated injury in kidney tubule cells. The remaining cases are attributable to autoimmune conditions and infections, and a few cases are classified as idiopathic. Diagnosis is based on histological findings, mainly tubulointerstitial lymphocytic infiltrates without immune deposits [3]. Early withdrawal of the offending drug and early treatment with corticosteroids constitute the basis of treatment of the disease [4].
Over the last decade, a new subtype of ATIN has emerged that is different from classical drug-related ATIN, which has been related to the administration of immune checkpoint inhibitors (ICIs). This group of drugs has shown promising results in the treatment of tumours with limited responses to conventional chemotherapeutic treatment such as melanoma, non-small-cell lung carcinoma, renal cell carcinoma and Hodgkin lymphoma. ICIs are antibodies directed against programmed cell death-1 (PD-1), PD-1 ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA4). The PD-1/PD-L1 and CTLA4 pathways control the differentiation of autoreactive T lymphocytes in order to maintain tolerance to self-antigens [5]. The blockade of these pathways stimulates the immune response toward cancer cells that express negative T cell surface ligands, but the immune response triggered by ICIs may result in immune-mediated adverse events [6]. The incidence of ICI-induced AKI varies between 2.2% and 29% [7, 8]. ATIN is the dominant pattern of ICI-induced AKI [7] and is histologically indistinguishable from ATIN secondary to other drugs. The exact mechanisms of ICI-related ATIN (ICI ATIN) are not completely understood, but there are some clues in the literature. ICI may promote T cell migration to the kidney, reducing tolerance to endogenous antigens and initiating an inflammatory response that could lead to ATIN. ICI could also reactivate exhausted drug-specific T cells and induce loss of tolerance to other medications [9]. Interestingly, many cases of ICI ATIN occur in patients receiving drugs independently associated with ATIN, such as non-steroidal anti-inflammatory drugs (NSAIDs) or proton pump inhibitors (PPIs), preceding ICI treatment [10].
Although the clinical characteristics seem to be similar, the pathogenic mechanisms of ICI ATIN and classical ATIN do not appear to be the same, which suggests that they may require different clinical management and confer different prognoses. To the best of our knowledge, there is no study comparing ICI ATIN and classical ATIN in terms of clinical presentation, and response to treatment with steroids and follow-up. Moreover, in both cases, available data are almost limited to case series. Thus, the aim of this study is to evaluate differences between ICI ATIN and non-ICI-related ATIN, to examine clinical features, response to treatment with steroids and the evolution of kidney function.
MATERIALS AND METHODS
Patients
All cases of biopsy-proven ATIN between January 2015 and May 2019 at Bellvitge University Hospital and Vall d’Hebron University Hospital were eligible for the study. Exclusion criteria were as follows: age <18 years old, ATIN related to autoimmune conditions and presence of urinary tract infections at the time of inclusion. All patients were prospectively followed by expert clinicians in the outpatient clinics of both hospitals. The Ethical Committee of Bellvitge University Hospital approved the study protocol (PR143/19).
Biopsies
Two kidney cores were obtained with ultrasound guidance using a 16-gauge spring-loaded biopsy gun: one for optical microscopy (haematoxylin and eosin and saffron, periodic acid–Schiff and Masson trichrome) and one for immunofluorescence (IF). Biopsies contained at least one glomerular and one arterial profile, and sufficient tubulointerstitial tissue to grade interstitial inflammation, tubulitis, interstitial fibrosis and tubular atrophy. All ATIN diagnoses were confirmed by kidney biopsy reviewed by the local pathologist. ATIN histopathological diagnosis was defined by the presence of diffuse interstitial oedema and inflammatory cell infiltrate mainly composed of lymphocytes and eosinophils, tubulitis and fibrosis, with negative IF. The definition of severity of the tubulointerstitial lesions was made according to the percentage of tissue affected. Cell infiltration and atrophy were considered severe when >75% of the tubular surface was affected. Severe fibrosis was defined when it affected >75% of the renal parenchyma.
Clinical variables
We recorded key demographic, clinical and analytical variables such as gender, age, ethnicity, comorbidities and current medication with special emphasis on ICIs, NSAIDs, PPIs, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and antibiotics. Medical records regarding the type of malignancy, oncological treatment, first and last dose of ICI, and presence of extrarenal immune-related adverse events were also reviewed. At the time of ATIN onset, serum creatinine level, sediment findings, proteinuria, urine cytology, serological information, renal biopsy diagnosis, time to withdrawal of the offending drug and specific treatment of ATIN were registered, as well as laboratory data during the follow-up period. Baseline kidney function was defined as the value of serum creatinine 6 months before ATIN. The evolution of clinical and analytical parameters was registered during the prospective follow-up of the patients. Relapse was defined as worsening of kidney function not attributable to other causes together with the presence of compatible urinalysis and inflammation parameters. Normalization of kidney function was defined as glomerular filtration rate (GFR) >60 mL/min/1.73 m2 or GFR prior to the ATIN episode.
Statistical analysis
Data were analysed using GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA, USA) and IBM SPSS Statistics Version 20.0 (IBM Corp., Armonk, NY, USA). Results were expressed as frequencies for categorical variables or as the mean ± standard deviation for continuous variables. The Kolmogorov–Smirnov test was applied to determine whether quantitative variables were normally distributed. For comparison of means of two groups, a Student’s t-test or Mann–Whitney U test was used depending on the distribution of the variable. The Chi-square test was used for qualitative variables. Actuarial survival analysis was performed by Kaplan–Meier curves. P < 0.05 was considered significant.
RESULTS
Baseline characteristics
Forty-seven patients diagnosed with ATIN were recruited according to the inclusion and exclusion criteria of the study. Of these, 13 patients presented with ATIN during treatment with an ICI and 34 patients were diagnosed with ATIN attributed to other drugs. Mean time since diagnosis to end of follow-up was 27.04 ± 20.75 months (range 0.5–69).
Table 1 shows the main baseline characteristics of both cohorts. Patients diagnosed with ICI ATIN presented with lower creatinine levels and higher urinary leucocyte counts compared with patients with non-ICI-related ATIN at diagnosis. We did not find differences in the prevalence of systemic symptoms at presentation such as fever, arthralgias or rash. We found a tendency towards a more severe tubulointerstitial infiltrate and tubular atrophy, together with lesser fibrosis, in the diagnostic kidney biopsies of ICI ATIN patients compared with non-ICI-related ATIN cases, but differences did not reach statistical significance (see Table 1). The length of treatment with ICIs for patients who presented with ICI ATIN was 197.07 ± 184.99 days. Regarding neoplasia, malignancies found were: lung (nine patients), melanoma (three patients) and pancreas (one patient). Five patients did not receive any oncospecific treatment before ICI treatment. Three patients received cisplatin before ICI treatment: one of these three received cisplatin only, one in combination with docetaxel, and one in combination with etoposide, chimeric human/mouse immunoglobulin G1 monoclonal antibody and paclitaxel. Five patients received carboplatin before ICI treatment: four of these five received carboplatin only and the other in combination with folate antagonist/antineoplastic agent. Finally, one patient received five lines of treatment before initiating ICI treatment, one of which was with anti-PD-L1 plus an anti-CTLA4.
Table 1.
Baseline characteristics of the population
| Clinical data | ICI ATIN | Non-ICI-related ATIN | P-value |
|---|---|---|---|
| n = 13 | n = 34 | ||
| Age (years) | 71 ± 8.48 | 63.09 ± 16.12 | 0.101 |
| Sex: male/female (% patients) | 69.24/30.76 | 50/50 | 0.453 |
| Hypertension (% patients) | 53.84 | 64.7 | 0.52 |
| Diabetes (% patients) | 23.08 | 23.53 | 1 |
| Creatinine prior to ATIN (mg/dL) | 1.01 ± 0.32 | 0.96 ± 0.37 | 0.69 |
| Creatinine at diagnosis (mg/dL) | 3.8 ± 1.033 | 5.98 ± 4.15 | 0.007 |
| C-reactive protein at diagnosis (mg/mL) | 59.95 ± 95.74 | 37.1 ± 67.22 | 0.408 |
| Blood eosinophil count at diagnosis (× 10e9/L) | 0.2531 | 0.3424 | 0.394 |
| Proteinuria at diagnosis (mg/g creatinine) | 467.12 ± 183.73 | 1086 ± 1558.95 | 0.808 |
| Haematuria at diagnosis (% patients) | 66.7 | 37.5 | 0.084 |
| Urinary leucocyte count at diagnosis (count/µL) | 263.20 ± 418.04 | 133.55 ± 284.62 | 0.048 |
| Systemic symptoms at presentation (% patients) | |||
| Rash | 0 | 11.8 | 0.564 |
| Fever | 30.8 | 22.1 | 0.7 |
| Arthralgia | 15.4 | 5.9 | 0.304 |
| Histologic features in diagnostic kidney biopsy (% patients) | |||
| Severe inflammatory infiltrate | 46.2 | 35.3 | 0.516 |
| Severe tubular atrophy | 15.4 | 11.8 | 0.086 |
| Severe interstitial fibrosis | 0 | 8.8 | 0.544 |
| Presence of granuloma | 8.3 | 8.8 | 1 |
| Presence of eosinophilic infiltrate | 69.2 | 88.2 | 0.35 |
| Presence of plasma cells infiltrate | 33.3 | 41.2 | 0.739 |
Data are presented as mean ± standard deviation unless otherwise indicated.
Among patients treated with ICIs, the most frequently involved drugs were antibodies against PD-1 (61.5%). Nine out of 13 patients in the ICI ATIN group were chronically treated with PPIs (omeprazole). Three of those nine patients were also treated with NSAIDs (ibuprofen), while the remaining four patients did not receive any other drugs that have been classically associated with ATIN. Regarding patients presenting with ATIN attributed to other drugs, NSAIDs (29.4%) and fluoroquinolones (20.6%) were the most frequent aetiologies. Table 2 summarizes the more probable aetiologies of ATIN in both cohorts.
Table 2.
Aetiology of ATIN in the cohorts of ICI ATIN and non-ICI-related patients
| ICI related ATIN (n = 13) |
Non-ICI related ATIN (n = 34) |
||
|---|---|---|---|
| Drug | % cases | Drug | % cases |
| Anti-PD-1 | 61.5 | NSAIDs | 29.4 |
| Anti-PD-L1 | 23.1 | Fluoroquinolones | 20.6 |
| Anti-PD-1 + anti-CTLA4 | 7.7 | β-Lactams | 8.8 |
| Anti-PD-L1 + anti-CTLA4 | 7.7 | PPIs | 2.9 |
| Others | 38.2 | ||
The non-ICI-related ATIN cases were attributed to: sitagliptin, rifampicin, repaglinide, fosfomycin, lenalidomide, cocaine, vandetanib and cobimetinib.
Follow-up and evolution of ATIN
Creatinine
AKI was less severe in ICI ATIN patients compared with non-ICI-related ATIN patients. At the time of diagnosis, creatinine levels were significantly lower in ICI ATIN patients (3.8 ± 1.03 versus 5.98 ± 4.15 mg/dL, P = 0.007). Peak creatinine levels reached during AKI were also lower in ICI ATIN compared with non-ICI-related ATIN patients (4.19 ± 1.43 versus 6.22 ± 4.63 mg/dL, P = 0.034). Time from initiation of the culprit drug to the ATIN diagnostic biopsy was longer in ICI ATIN compared with non-ICI-related ATIN patients (197.07 ± 184.99 versus 114.4 ± 352.16 days, P = 0.006).
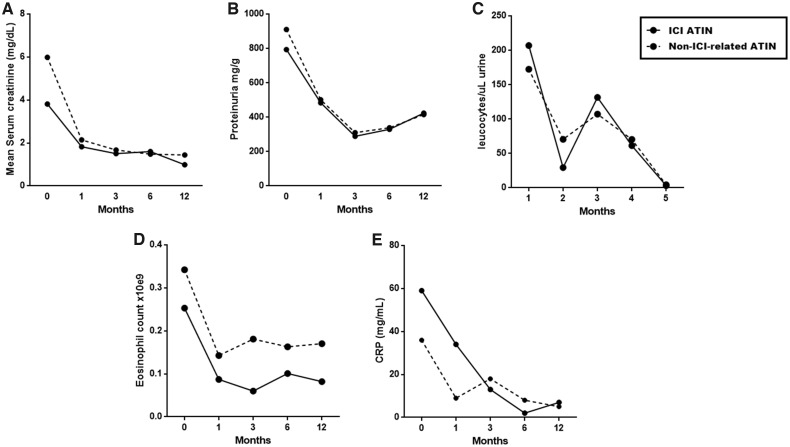
During follow-up, the slope of decreasing creatinine over time was lower in ICI ATIN compared with non-ICI-related patients. Creatinine decrease (Δ creatinine) was lower from baseline to Month 1, from baseline to Month 3, and from baseline to Month 6 in ICI ATIN patients compared with non-ICI-related ATIN patients. (P = 0.023, 0.014 and 0.004, respectively). Figure 1A shows mean serum creatinine evolution during the first year after diagnosis of ATIN in both cohorts.
FIGURE 1.
Evolution of creatinine (A), proteinuria (B), leucocyturia (C), eosinophil count (D) and C-reactive protein (E) after 5 months to 1 year of follow-up.
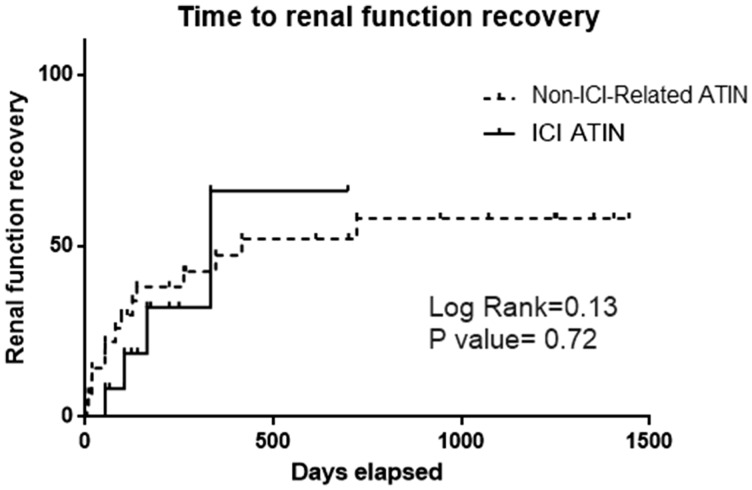
We registered the time elapsed since the initiation of ATIN treatment with prednisone to the normalization of kidney function (defined as GFR >60 mL/min/1.73 m2 or GFR prior to the ATIN episode). We noticed that there tended to be slower renal function recovery in ICI ATIN compared with non-ICI-related ATIN patients, but differences did not reach statistical significance. A similar percentage of patients achieved normal renal function at the end of follow-up (44.1% versus 30.8% of patients in the ICI ATIN versus the non-ICI-related ATIN cohort of patients, P = 0.4). Of note, a year after prednisone initiation, the percentage of patients who showed kidney function recovery remained stable during the rest of the follow-up period (see Figure 2).
FIGURE 2.
Time from ATIN diagnosis to renal function recovery in both studied ATIN groups.
Proteinuria
We did not find differences in proteinuria at diagnosis between ICI ATIN and non-ICI-related ATIN patients (P = 0.808). Despite this, the amelioration of proteinuria (Δ proteinuria) was lower in ICI ATIN compared with non-ICI-related ATIN patients. Decrease in proteinuria was lower in ICI ATIN patients between baseline and Month 1, and between baseline and Month 3, compared with non-ICI related patients (P = 0.015 and 0.033, respectively) (see Figure 1B).
Urinary leucocyte count
Urinary leucocyte count was higher in ICI ATIN compared with non-ICI-related ATIN patients at diagnosis (P = 0.048). Both cohorts presented similar outcomes of this parameter (see Figure 1C).
Blood eosinophil count
Mean blood eosinophil count remained lower in ICI ATIN patients compared with non-ICI-related ATIN patients during the follow-up period, but differences did not reach statistical significance (see Figure 1D).
Serum C-reactive protein levels
Mean serum C-reactive protein levels were higher in ICI ATIN patients compared with non-ICI-related ATIN patients from Month 3, although differences did not reach statistical significance at the time points evaluated (see Figure 1E).
Treatment of the ATIN episode
The offending drug was withdrawn in all cases. All patients were treated with prednisone. Three out of 13 patients in the ICI ATIN cohort and 3/34 patients in the non-ICI-related ATIN cohort received intravenous methylprednisolone pulses at diagnosis. Three out of 34 patients in the non-ICI-related cohort received mycophenolate mofetil compared with 0/13 patients in the ICI ATIN cohort.
Time to initiation of treatment with steroids was inferior in ICI ATIN compared with non-ICI-related ATIN cases (P = 0.027). The dosing and schedule of prednisone was similar for ICI ATIN and non-ICI-related ATIN patients. The prednisone cumulative dose was similar between patients in the two cohorts (P = 0.883), as was the duration of the treatment (P = 0.932). There were no differences in the prednisone cumulative dose during the first 3 months of treatment. Time since diagnosis to prednisone initiation was similar in both cohorts (P = 0.387). Table 3 compares the dosing of prednisone in the two cohorts.
Table 4.
Cases of patients with ATIN relapse, treatment and evolution
| Sex/age (years)/ATIN cause | Time to relapse since diagnosis (months) | Prednisone cumulative dose at the moment of relapse (mg) | Relapse cause | Serum creatinine at relapse (mg/dL) | Relapse treatment | Evolution |
|---|---|---|---|---|---|---|
| M/71/anti-PD-1 | 8 | 4075 | Offending drug re-initiation | 2.07 | Prednisone 30 mg/day | Normalization of serum creatinine |
| M/81/anti-PD-1 | 4 | 2425 | Ciprofloxacin initiation plus decrease in prednisone dose | 3.02 | Prednisone 30 mg/day | Normalization of serum creatinine |
| F/83/anti-PD-1 | 3 | 2572.5 | Prednisone withdrawal | 10.59 | Methylprednisolone 750 mg (bolus) + prednisone 40 mg/day | Stabilization—creatinine 1.4 mg/dL |
| M/77/NSAIDs | 15 | 3750 | Prednisone withdrawal | 2.51 | Prednisone 40 mg/day | Stabilization—creatinine 1.5 mg/dL |
| M/74/amoxicillin | 5 | 4125 | Unknown | 2.49 | Prednisone 30 mg/day | Stabilization—creatinine 2.5 mg/dL |
| M/66/ciprofloxacin | 5 | 2060 | Unknown | 2.03 | Prednisone 15 mg/day | Stabilization—creatinine 1.5 mg/dL |
Relapses
Relapse rate did not differ between the two cohorts of ATIN patients (P = 0.334). Three cases of relapse were identified during follow-up in each cohort. The main characteristics of patients who relapsed are displayed in Table 4.
Table 3.
Treatment comparison between patients with ICI ATIN and non-ICI-related ATIN patients
| Steroid treatment | ICI ATIN | Non-ICI-related ATIN | P-value |
|---|---|---|---|
| Time since ATIN diagnosis to steroid initiation (days) | −9.92 ± 17.57 | 2.52 ± 6.15 | 0.027 |
| Initial methylprednisolone bolus administration (% patients) | 25 | 8.82 | 0.178 |
| Prednisone cumulative dose (g) | 9.39 ± 21.92 | 3.59 ± 2.78 | 0.883 |
| Months of prednisone treatment | 5 ± 3.32 | 9 ± 9.87 | 0.932 |
| Prednisone cumulative dose per month of treatment (g/month) | 1.31 ± 1.96 | 0.62 ± 0.407 | 0.132 |
| Prednisone cumulative dose at Month 1 (mg) | 1564.38 ± 511.28 | 1456 ± 637 | 0.597 |
| Prednisone cumulative dose at Month 2 (mg) | 2270.92 ± 693.67 | 2115.5 ± 741.56 | 0.537 |
| Prednisone cumulative dose at Month 3 (mg) | 2735.73 ± 1026.31 | 2454.30 ± 1070.19 | 0.959 |
DISCUSSION
Herein, we present 13 biopsy-proven cases of ATIN in the setting of treatment with ICIs, and we compare their baseline characteristics and evolution with 34 cases of ATIN related to other drugs. To the best of our knowledge, this is the largest case series of ICI-related ATIN reported to date and the only published clinical comparison that has attempted to find differences in features compared with other forms of nephritis.
ICI ATIN patients in our cohort presented with milder forms of AKI compared with non-ICI-related cases. In agreement with this finding, other case series in the literature have also reported slight increases in creatinine in patients with ICI AKI. In the case series published by Cortazar et al. [7], 12 patients treated with ICIs presenting with ATIN showed a mean peak creatinine level of 4.5 mg/dL, similar to the peak creatinine noticed in our cohort (4.15 mg/dL). Cassol et al. [11] published a report of nine cases of ICI ATIN with peak creatinine levels between 1.4 and 4.3 mg/dL. In concordance, Shirali et al. reported six cases of ICI ATIN with a peak creatinine ranging between 1.8 and 2.3 mg/dL in five cases, and a peak creatinine of 5.5 mg/dL in the remaining one [10]. Only in one case series of five patients with ATIN related to ICI treatment [12] did the authors observe higher peak creatinine levels between 4.8 and 7.8 mg/dL. Urinary leucocyte counts at diagnosis were significantly higher in ICI ATIN compared with non-ICI-related ATIN patients.
Although a similar proportion of patients recovered baseline kidney function at the end of follow-up, renal function recovery was slower in ICI ATIN compared with non-ICI-related ATIN patients. This is reflected by the differences in Δ creatinine between diagnosis and Months 1, 3 and 6, which were statistically lower in ICI ATIN compared with non-ICI-related ATIN patients. The same pattern was followed by Δ proteinuria followed during the first 3 months. Although differences did not reach statistical significance, slower recovery of the kidney function of ICI-treated patients was observed via Kaplan–Meier survival analysis curves. One could hypothesize that slower renal recovery after ICI discontinuation may be ascribed to the longer half-life of ICIs, ranging between 20 and 25 days [13], and the fact that this class of drugs can remain bound to lymphocytes for up to 57 days [14]. Other case series have described variable kidney function normalization rates ranging from as few as 2/12 patients [7] to almost all patients in a case series (5/6 patients) [10]. These differences were not related to ATIN treatment. All patients were treated with steroids and there were no differences in prednisone cumulative doses and the duration of treatment. Time to steroid initiation has been proposed to be one of the main factors that determines the outcome of classical ATIN [15]. In our study, the initiation of steroid treatment after ATIN was delayed in non-ICI-related cases. However, this differential delay did not influence the outcome: we did not find differences in renal function at the end of the follow-up period.
The period of time between offending drug initiation and ATIN diagnosis was longer in ICI ATIN compared with non-ICI-related cases. Long latency periods until ATIN development in patients treated with ICIs have been previously reported [7, 14]. This may, in part, be explained by differential pathological mechanisms of ATIN development. It has been demonstrated previously that classical drug-induced ATIN is an allergic drug hypersensitivity reaction mediated by T lymphocytes. However the pathological mechanisms of ICI ATIN are unknown. It has been hypothesized that the use of an ICI breaks down immune tolerance to antigens that would not otherwise be nephritogenic [10]. In agreement, Koda et al. [16] performed a lymphocyte stimulation test in a nivolumab-associated ATIN patient and confirmed drug-induced (lansoprazole) lymphocyte stimulation, although it had been used safely for a long time before the initiation of anti-PD-1 therapy. Further research focusing on the implicated molecular mechanisms is necessary to confirm this hypothesis. Longer latency periods together with strict monitoring of patients who undergo ICI therapy may explain the lower creatinine levels of these patients at the moment of diagnosis compared with the faster development of ATIN in the setting of other drugs in individuals who are not monitored.
Although differences did not reach statistical significance, ICI ATIN patients showed more inflammatory infiltrates and less fibrosis. We hypothesize that this may be in line with the finding of lower peak creatinine levels and, possibly, the early diagnosis of such patients compared with those with non-ICI-related ATIN. Another difference between ICI and non-ICI-related ATIN reported in the literature is the occurrence of a flare after drug re-exposure. Recurrence of classical drug-induced ATIN upon re-exposure to the offending drug has been widely described [3]. In contrast, Cortazar et al. [17] recently reported a low prevalence of recurrence of ATIN upon ICI re-challenge after successful treatment of the first episode.
The observational, retrospective nature of the study constitutes its main limitation. Although differences in treatment of ATIN episodes were not noticed between the two cohorts, standardization of the prednisone schedule would allow stronger conclusions about differences in ATIN evolution between ICI and non-ICI-related ATIN.
CONCLUSION
In this study, we present the largest case series of ICI ATIN to date and, to our knowledge, the first published comparison with non-ICI-related ATIN cases. We observe that ICI ATIN patients manifest a longer latency period after offending drug initiation, milder AKI and slower creatinine amelioration compared with ATIN in the setting of another drug treatments. This differential evolution may be ascribed to potential differences in the pathological mechanisms behind ATIN development. Further investigation and cumulative experience are required to determine whether the differences between classical ATIN and ICI ATIN are enough for them to be considered as two different diseases.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of FIS/Fondos FEDER (REDinREN RD016/0009).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Goicoechea M, Rivera F, Lopez-Gomez JM; Spanish Registry of Glomerulonephritis. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2013; 28: 112–115 [DOI] [PubMed] [Google Scholar]
- 2. Raghavan R, Shawar S.. Mechanisms of drug-induced interstitial nephritis. Adv Chronic Kidney Dis 2017; 24: 64–71 [DOI] [PubMed] [Google Scholar]
- 3. Raghavan R, Eknoyan G.. Acute interstitial nephritis-a reappraisal and update. Clin Nephrol 2014; 82: 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez-Juarez G, Perez JV, Caravaca-Fontán F. et al.; on behalf of the Spanish Group for the Study of Glomerular Diseases (GLOSEN). Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol 2018; 13: 1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uchida A, Watanabe M, Nawata A. et al. Tubulointerstitial nephritis as adverse effect of programmed cell death 1 inhibitor, nivolumab, showed distinct histological findings. CEN Case Rep 2017; 6: 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sury K, Perazella MA.. The nephrotoxicity of new immunotherapies. Expert Rev Clin Pharmacol 2019; 12: 513–521 [DOI] [PubMed] [Google Scholar]
- 7. Cortazar FB, Marrone KA, Troxell ML. et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016; 90: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Izzedine H, Gueutin V, Gharbi C. et al. Kidney injuries related to ipilimumab. Invest New Drugs 2014; 32: 769–773 [DOI] [PubMed] [Google Scholar]
- 9. Izzedine H, Mathian A, Champiat S. et al. Renal toxicities associated with pembrolizumab. Clin Kidney J 2019; 12: 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shirali AC, Perazella MA, Gettinger S.. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 2016; 68: 287–291 [DOI] [PubMed] [Google Scholar]
- 11. Cassol C, Satoskar A, Lozanski G. et al. Anti-PD-1 immunotherapy may induce interstitial nephritis with increased tubular epithelial expression of PD-L1. Kidney Int Rep 2019; 4: 1152–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mamlouk O, Selamet U, Machado S. et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer 2019; 7: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim K-P, Jung H.. Clinical pharmacologic aspects of immune checkpoint inhibitors in cancer therapy. Transl Clin Pharmacol 2016; 24: 7–12 [Google Scholar]
- 14. Shingarev R, Glezerman IG.. Kidney complications of immune checkpoint inhibitors: a review. Am J Kidney Dis 2019; 74: 529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. González E, Gutiérrez E, Galeano C. et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008; 73: 940–946 [DOI] [PubMed] [Google Scholar]
- 16. Koda R, Watanabe H, Tsuchida M. et al. Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: a case report. BMC Nephrol 2018; 19: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cortazar FB, Kibbelaar ZA, Glezerman IG. et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol 2020; 31: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]