Abstract
The incidence of acute kidney injury (AKI) has increased in the past decades. AKI complicates up to 15% of hospitalizations and can reach up to 50–60% in critically ill patients. Besides the short-term impact of AKI in patient outcomes, several studies report the association between AKI and adverse long-term outcomes, such as recurrent AKI episodes in 25–30% of cases, hospital re-admissions in up to 40% of patients, an increased risk of cardiovascular events, an increased risk of progression of chronic kidney disease (CKD) after AKI and a significantly increased long-term mortality. Despite the long-term impact of AKI, there are neither established guidelines on the follow-up care of AKI patients, nor treatment strategies to reduce the incidence of sequelae after AKI. Only a minority of patients have been referred to nephrology post-discharge care, despite the evidence of improved outcomes associated with nephrology referral by addressing cardiovascular risk and risk of progression to CKD. Indeed, AKI survivors should have specialized nephrology follow-up to assess kidney function after AKI, perform medication reconciliation, educate patients on nephrotoxic avoidance and implement strategies to prevent CKD progression. The authors provide a comprehensive review of the transition from AKI to CKD, analyse the current evidence on the long-term outcomes of AKI and describe predisposing risk factors, highlight the importance of follow-up care in these patients and describe the current therapeutic strategies which are being investigated on their impact in improving patient outcomes.
Keywords: AKI, cardiovascular, CKD, mortality, outcomes
INTRODUCTION
Acute kidney injury (AKI) is an acute decrease in kidney function defined by an increase in serum creatinine (SCr) or a decrease in urine output (UO) [1, 2]. The incidence of AKI has increased in the past decades, reflecting the increased recognition of this diagnosis, patient ageing and increase in AKI risk factors and co-morbidities [diabetes, hypertension, chronic kidney disease (CKD), cardiovascular disease (CVD), liver disease, lung disease, sepsis and surgery] and exposure to nephrotoxic drugs [1, 3–7]. AKI complicates 5.0 to 15.0% of hospitalizations and can reach up to 50–60% in critical care patients [1, 3–7]. Despite remaining significantly high, mortality rates have declined in the past decade reflecting improvements in patient care, namely by improvements in dialytic care, availability of less nephrotoxic drugs and a decrease in use of dopamine and diuretics [2, 7–9].
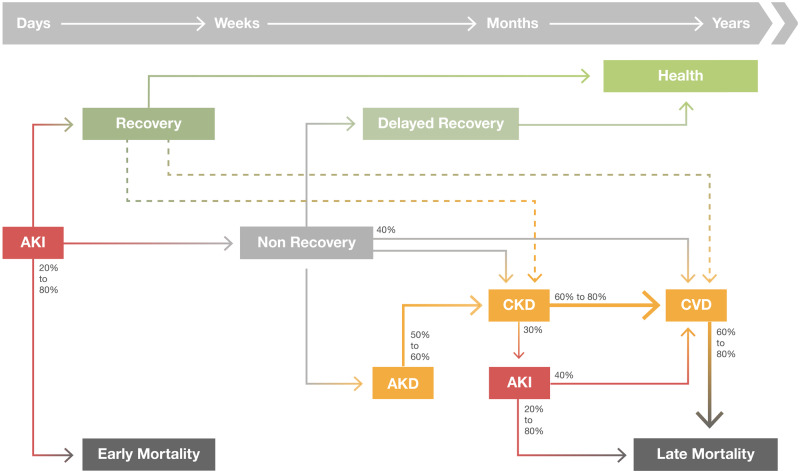
The increase in survival of AKI patients, has contributed to a significant increase in long-term outcomes associated with AKI, namely recurrence of AKI episodes, development or progression of CKD, characterized by the presence of kidney disease for >90 days, increasing risk of cardiovascular events, increasing hospital admissions, reduced quality of life and long-term mortality [10–15] (Figure 1).
FIGURE 1.
Long-term consequences after AKI. An AKI event can lead to renal recovery or development of Acute kidney disease (AKD). In the long-term, AKI and AKD are associated with development or progression of CKD, recurrent AKI episodes, risk of cardiovascular events and higher risk of long-term mortality. Even AKI recovery has been associated with increased long-term outcomes.
Despite the long-term impact of AKI, there are neither established guidelines on the follow-up care of AKI patients, nor treatment strategies to reduce the incidence of sequelae after AKI. Indeed, only a minority of patients received comprehensive nephrology post-discharge care, despite the evidence of improved outcomes associated with nephrology referral by addressing cardiovascular risk and risk of progression to CKD [16, 17].
The authors provide a comprehensive review of current evidence of the long-term outcomes of AKI and predisposing risk factors, and highlight the importance of follow-up care in improving patient outcomes.
MATERIALS AND METHODS
We conducted two literature searches in May 2020, using MEDLINE through the PubMed search engine with the MeSH terms: (i) AKI, prognosis and (ii) AKI, long term, outcomes.
We included articles published in English after the year 2010 up to 20 May 2020, of adult patients with AKI measuring mortality, dialysis dependence, CKD and cardiovascular events.
Diagnosis and pathophysiology of AKI
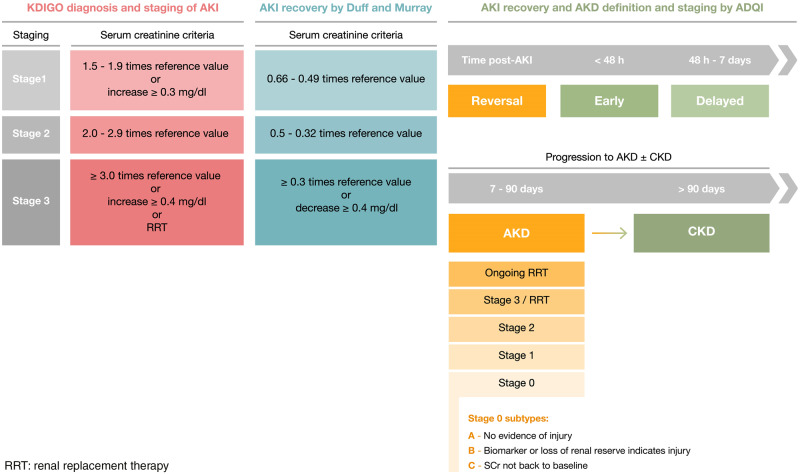
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines define AKI as an increase in SCr of at least 0.3 mg/dL within 48 h, or an increase in SCr >1.5 times baseline within the prior 7 days, or a decrease in UO to <0.5 mL/kg/h for 6 h [18]. This classification further stratifies AKI according to three stages of disease severity which correlate with worse degrees of prognosis [18] (Figure 2).
FIGURE 2.
AKI, AKI recovery and AKD definitions (adapted from KDIGO, Acute Disease Quality Initiative (ADQI) and from Duff and Murray [56]).
The kidney is highly susceptible to systemic imbalances and the causes of AKI range from pre-renal AKI, acute tubular necrosis, acute interstitial nephritis, acute glomerular diseases and acute obstructive nephropathy [19]. The most common causes of AKI in hospitalized patients are septic shock, post major surgery, cardiogenic shock and hypovolaemia [20].
The pathophysiology of AKI is a complex interplay of pathways triggered by an inciting event which leads to an imbalance of oxygen supply and demand [21–25]. These processes include haemodynamic instability, microcirculatory dysfunction, tubular cell injury, tubular obstruction, renal congestion, microvascular thrombi, endothelial dysfunction and inflammation [21–26].
Despite affecting all the segments of the nephron, proximal tubular cells are the most frequently injured, causing loss of polarity, apoptosis or necrosis, which depend on severity of injury [26, 27]. Damage to proximal tubular cells consequently results in afferent arteriolar vasoconstriction mediated by tubuloglomerular feedback, luminal obstruction and back leak of filtrate across injured proximal tubular cells, which leads to an abrupt decrease in glomerular filtration [21]. Additionally, kidney injury prompts the production and release of inflammatory and vasoactive mediators causing leucocyte adherence and interstitial infiltration and lead to microcirculatory flow disruption.
Indeed, inflammation is considered to play a critical role in the pathophysiology of AKI, namely in local kidney injury, in the multi-organ failure associated with AKI, in kidney recovery and also in the progression to CKD which can result if these immune mechanisms persist [26–30].
TIME COURSE OF AKI
It is postulated that transient AKI reflects a temporary reduction in renal function without structural damage, whereas persistent AKI reflects structural tubular damage [31–34]. The duration of AKI could also reflect the potential to recover the injured kidney [31, 33, 35]. Additionally, the degree of severity of the patient’s illness can also impact on AKI duration [31, 33]. Thus, non-recovery from AKI seems to be predisposed by several mechanisms resulting in the exposure of a reduced nephron mass to higher injury in association with impaired repair mechanisms [36].
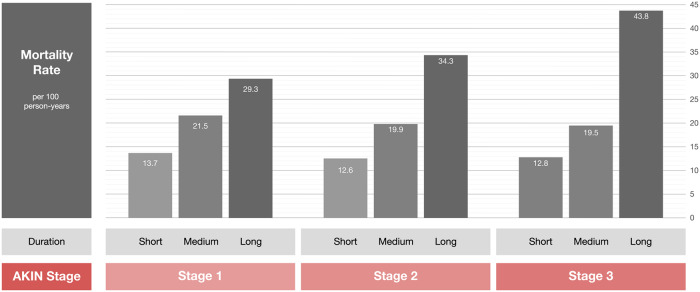
Several studies have reported an association of rapid recovery of kidney function and better short-term survival [37–42]. Coca et al. conducted the largest prospective study to date demonstrating the prognostic impact of AKI duration [31]. In their cohort of postoperative AKI diabetic patients, the mortality rate increased by AKI duration when stratified by AKIN stage [31]. They demonstrated that the mortality rate for patients with AKIN Stage 1 with a duration of >7 days was >2-fold higher than for patients with AKIN Stage 3 for <2 days [31] (Figure 3).
FIGURE 3.
Impact of AKI severity and duration on mortality (adapted from Coca et al. [31]).
The previous classifications systems of AKI do not take into account the duration of AKI, which is a significant aspect of AKI severity [37]. The Acute Disease Quality Initiative (ADQI) has recently defined transient AKI when baseline kidney function is recovered within 48 h, while persistent AKI is defined as kidney dysfunction which persists for longer than 48 h (Figure 2) [37].
AKI recovery
Renal recovery after AKI is a complex process which is not entirely understood though appears to be dependent on AKI severity, aetiology, duration and baseline renal function [43]. The timeline and trajectory of renal recovery will depend on reversal of the pathophysiological processes involved [44]. Renal repair may be the result of regeneration of cells and reestablishment of polarity [27].
Studies report that the incidence of renal recovery can range from 0% to 90% considering all stages of AKI severity, but from 0% to 40% in cases of dialysis requiring AKI [36]. The heterogeneity in populations studied and in AKI and reversibility definitions used has contributed to the difficulty in defining and quantifying renal recovery after AKI [36]. The most often used criteria to assess renal recovery is a decrease in SCr, which is associated with certain limitations, such as loss of muscle mass, changes in volume of distribution, changes in renal reserve and hyperfiltration [45]. This is supported by studies demonstrating the increased risk in CKD after AKI even when there is an apparent return of SCr to baseline [46, 47].
The presence of proteinuria has also been recognized as a marker of underlying kidney injury and has been associated with worse outcomes after AKI episodes [48]. Novel biomarkers for AKI are being researched to more accurately assess renal recovery, namely plasma neutrophil gelatinase-associated lipocalin (NGAL), tissue inhibitor metalloproteinase-2 and insulin-like growth factor binding protein-7 ([TIMP-2] × [IGFBP7]), urine concentrations of interleukin (IL)-18 and liver-type fatty acid-binding protein (L-FABP) [49–52].
The ideal definition of kidney recovery after AKI should accurately assess baseline kidney function to differentiate non-recovery from pre-existing CKD, current residual kidney function and reserve and be able to provide prognosis.
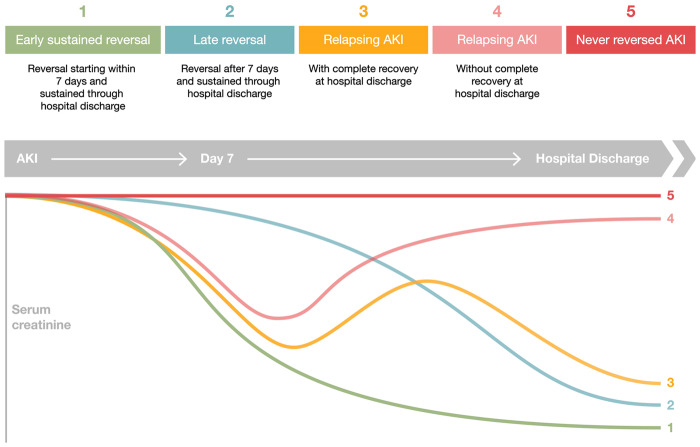
The trajectory of renal recovery can take many forms and is associated with long-term prognosis [36]. Recovery can be assessed as a relative or absolute change, or as a fixed threshold, and according to how persistent an episode of AKI is or to how sustained the recovery is [53]. Kellum et al. identified five phenotypes of renal recovery after AKI, namely early sustained AKI reversibility, late sustained AKI reversibility, relapse AKI and recovery, relapse AKI without recovery and never recovered AKI, which had distinct characteristics and correlated differently with prognosis [54]. In this study, non-recovery of renal function at hospital discharge was frequent and associated with an increase risk in mortality (Figure 4). Interestingly, late recovery of renal function was associated with better outcomes than non-recovery, and worse than early reversal of renal function [54]. Heung et al. also identified different patterns of renal recovery after AKI and reported an increasing risk of CKD according to AKI severity, duration of injury and time to recovery and that this risk was significant even in mildest forms of AKI with fast recovery [47]. Therefore, outcomes are not only associated with the degree of renal recovery but also with time to recovery.
FIGURE 4.
Time course of AKI (adapted from Kellum et al. [54]). Patients who develop AKI may experience (i) early sustained reversal of AKI (reversal before 7 days and sustained till hospital discharge), (ii) late reversal (reversal after 7 days and sustained till hospital discharge), (iii) relapsing AKI with complete recovery at hospital discharge, (iv) relapsing AKI without complete recovery at hospital discharge and (v) non-reversal of AKI.
In 2017, the ADQI defined AKI reversal as the absence of AKI by both SCr and UO criteria (according to the KDIGO classification) within 7 days after the AKI inciting event. The term acute kidney disease (AKD) was thus proposed to define a condition in which AKI Stage 1 or greater (KDIGO) is present for a duration between 7 and 90 days after AKI onset [55]. Thus, AKD follows on from AKI in patients who do not fully recover within 7 days and is stratified in four different stages of severity (Table 1).
Table 1.
AKD Classification according to the ADQI
| Stages | AKD |
|---|---|
| (7–90 days after AKI event) | |
| 0 | A: Absence of criteria for B or C |
| B: Continued evidence of ongoing injury, repair and/or regeneration or indicators of loss of renal glomerular or tubular reserve | |
| C: SCr < 1.5 times baseline but not back to baseline levels | |
| 1 | SCr = 1.5–1.9× baseline |
| 2 | SCr = 2–2.9× baseline |
| 3 | SCr = 3× baseline |
| SCr ≥ 4 mg/L | |
| Ongoing need for renal replacement therapy |
A recent perspective by Duff and Murray reflects on the lack of standardized definition of renal recovery in the literature and proposes that AKI recovery could be defined as a decrease of SCr of at least 33% from the reference SCr (value at admission or value which led to AKI diagnosis) within 7 days [56]. They also categorize AKI recovery into three stages inversely correlated with the KDIGO SCr criteria [56]. This approach would increase the recognition of AKI recovery and allow to assess its impact on long-term outcomes.
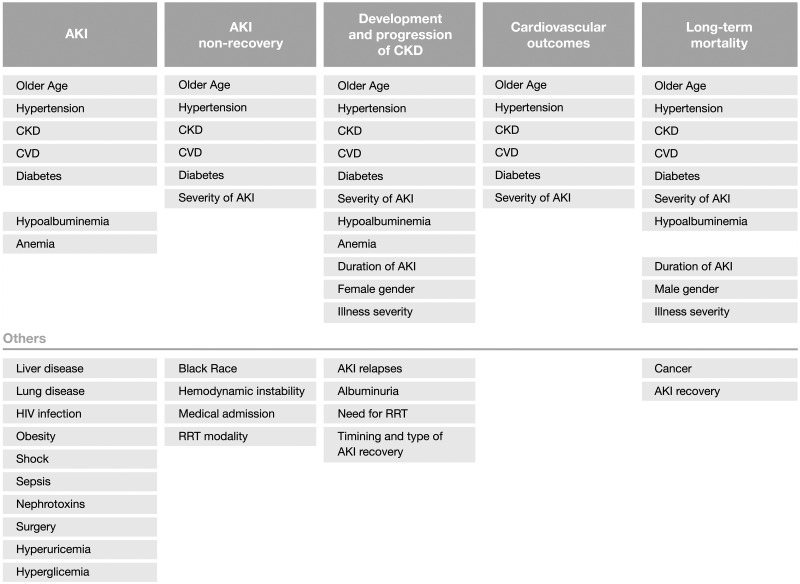
Important factors associated with non-recovery post-AKI include older age, presence of co-morbidities such as CKD, hypertension, diabetes mellitus and CVD, higher severity of the acute illness reflected by higher Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) or Simplified Acute Physiology Score (SAPS) scores, haemodynamic instability, medical admission, higher severity of AKI, requirement of renal replacement therapy (RRT) and potentially the intermittent modality of RRT, though this remains controversial [36, 54, 57–60] (Figure 5). Hence, non-recovery after AKI results from the exposure of a relatively decreased nephron mass or with impaired repair mechanisms to greater injury.
FIGURE 5.
Risk factors associated with AKI and outcomes post-AKI.
Pathophysiology of AKI to CKD transition
Under certain circumstances, the pro-fibrotic and pro-inflammatory pathways can result in maladaptive repair and transition to CKD after AKI [44]. Older age, lower baseline kidney function, longer duration of AKI and higher severity of AKI contribute to maladaptive repair [44]. Thus, severe and repeated injury superimposed on reduced renal reserve in an inflammatory environment can result in a maladaptive repair, characterized by a permanent reduction in kidney function associated with significant structural changes resulting from a complex interaction of injured renal tubular cells, endothelial cells, renin–angiotensin–aldosterone system (RAAS), immune system and interstitial fibroblasts [61, 62].
Persistent inflammation and RAAS activation are crucial mechanisms in AKI to CKD transition [62, 63]. Indeed, studies have demonstrated an association between RAAS inhibition and lower risk of CKD progression, though no randomized controlled trials have been performed with this goal [64]. The mechanisms of CKD progression after AKI include nephron loss and consequent hypertrophy of the remaining nephrons which leads to further tubulointerstitial fibrosis and further nephron loss; interstitial immune cell infiltration which leads to interstitial fibrosis; peritubular capillary loss resulting in renal hypoxia accelerating inflammation and fibrosis; injured renal tubular cells can adopt a profibrotic phenotype after cell cycle arrest, affecting other epithelial cells, pericytes and the immune system; and maladaptive repair which promotes the activation and growth of fibroblasts which contribute to the deposition of extracellular matrix and resulting fibrosis [44, 61, 65, 66].
The term AKD reflects the continuing pathological processes and adverse events developing after AKI, highlights the importance of renal recovery and reinforces the hypothesis that AKI and CKD are a continuum rather than separate entities [55].
Biomarkers of AKI to CKD transition
Biomarkers of these pathological processes, such as epithelial tubular injury, cell cycle arrest, systemic inflammatory pathways and glomerular filtration, would help identify patients at risk for CKD development [44]. Elevated levels of urinary and serum kidney injury molecule 1 (KIM-1), NGAL, L-FABP, IL-18 and TIMP-2 × IGFBP7 indicate persistent tubular injury, which could be used to identify risk of later CKD development [44, 48, 67, 68]. Indeed, in 692 patients, TIMP-2 × IGFBP7 > 2.0 at admission was associated with mortality or dialysis at 9 months (hazard ratio (HR) = 2.16, 95% CI 1.32–3.53) [69]. Also, urinary angiotensinogen has been associated with higher AKI severity and mortality and considering the role of RAAS activation in progression to CKD is considered a promising prognostic marker [44, 70]. Further investigations on anti-fibrotic interventions are required to develop strategies to preserve renal function and prevent the transition from AKI to CKD.
Long-term renal outcomes
Recent research described the association between AKI and long-term renal outcomes such as recurrent AKI episodes, development and progression to CKD. In recent systematic reviews, the pooled rate of CKD after AKI ranged from 10.17 to 25.8 cases per 100 person-years [71, 72].
Recurrent AKI
AKI has been associated with increased rates of hospital readmissions. The rate of readmission increases with AKI severity and can reach up to 40% in AKI requiring dialysis within the first month [73–76].
Several studies have focused on the incidence of recurrent episodes of AKI (Table 2). Liu et al. conducted one of the largest studies on AKI, including 38 659 patients with hospital-acquired AKI and identified a second AKI episode in 28.6% of patients [77]. Furthermore, recurrent AKI was associated with an increased mortality risk (HR = 1.66; 95% CI 1.57–1.77). Likewise, Siew et al. reported recurrent AKI in 25% cases in a population of 11 683 patients [78]. Furthermore, the 1-year mortality was higher in patients with recurrent AKI (35% versus 18%, P < 0.001) [78].
Table 2.
Summary of studies assessing AKI and AKI recurrence
| References | Design | Setting | N | AKI definition | Incidence (%) | Recurrent AKI (%) | CKD | Mortality |
|---|---|---|---|---|---|---|---|---|
| Liu et al. [77] | Retrospective | Hospitalized patients | 429 852 | KDIGO | 9 | 28.6 | Recurrent AKI = HR = 1.66, 95% CI 1.56–1.77 | |
| Siew et al. [78] | Retrospective | Hospitalized patients | 11 683 | KDIGO | 100 | 25 | ||
| Holmes et al. [79] | Case series | Hospitalized and community patients | 111 528 | KDIGO | 100 | 29.3 | Recurrent AKI = OR = 1.38, P < 0.001 | |
| Harris et al. [80] | Retrospective | Non-cardiac surgery ICU patients | 624 | RIFLE | 47 | 31 | Recurrent AKI = OR = 1.2, 95% CI 1.1–1.3 | |
| Rodrigo et al. [81] | Prospective | ICU septic patients | 400 | KDIGO | 82.8 | 19.8 | Recurrent AKI = HR = 1.97, 95% CI 1.36–2.84 | |
| Thakar et al. [82] | Prospective | Diabetic patients | 3679 | AKIN | 70 | 30 |
13.6% HR = 3.56, 95% CI 2.76– 4.61 |
|
| Rodriguez et al. [4] | Retrospective | Hospitalized patients | 359 | ADQI | 100 | 34 | HR = 2.2, 95% CI 1.09–4.3, P = 0.003 |
In a large population of hospitalized and community patients, Holmes et al. identified a recurrence rate of AKI of 30% and an association of recurrent AKI and higher 30-day mortality rate (OR = 1.33, 95% CI 1.28–1.38) [79]. Interestingly, with each episode of AKI, the probability of another episode also increased [79].
This has also been proven in intensive care unit (ICU) patients. Harris et al. demonstrated that AKI was associated with recurrent AKI episodes (OR = 1.9, 95% CI 1.0–3.6) in a cohort of 624 critically ill surgical patients. Moreover, recurrent AKI was associated with increased 1-year mortality (OR = 2.6, 95% CI 1.4–5.1) [80]. An increasing mortality risk associated with recurrent AKI was also demonstrated in a study of 400 critically ill septic patients in which the incidence of AKI was 20% (HR = 1.97, 95% CI 1.36–2.84) [81].
The impact of recurrent episodes of AKI on prognosis is also reflected in a cohort of 3679 diabetic patients with preserved renal function analysed by Thakar et al. In this study, 30% of patients experienced >2 AKI episodes which had a cumulative risk effect for developing CKD [82].
Furthermore, in a cohort of 359 patients in which recurrent AKI was observed in 34% of cases, recurrent AKI was associated with increased development of CKD (HR = 2.2, 95% CI 1.09–4.3), increased risk of cardiovascular events and increased mortality risk (HR = 4.5, 95% CI 2.7–7.5) [83].
AKI recurrence has a strong impact on renal, cardiovascular and patient outcomes. So, to prevent recurrent AKI, it is important to identify at-risk patients. Older age [77, 79, 81], black race and Hispanic ethnicity, [77] diabetes, [77, 83] CVD, [77, 78, 83] decreased baseline kidney function, [77, 79, 81, 83] liver disease, [77, 78] cancer, [78] higher illness severity, [77, 81] proteinuria, [77] anaemia [77] and longer AKI duration [78] have been associated with incomplete renal recovery after AKI [79]. Impaired renal reserve and the lingering effects of acute illness or its therapies might contribute to AKI recurrence and outcomes in these patients. Strategies to reduce the incidence of recurrent AKI might improve the long-term outcomes.
CKD development and/or progression
Several studies have demonstrated the association of CKD development following AKI, which have been summarized in previous systematic reviews [71, 72]. It is, therefore, crucial to identify risk factors associated with CKD development (Table 3).
Table 3.
Summary of studies assessing AKI and long-term outcomes
| References | Design | Setting | N | AKI definition | Incidence (%) | CKD | CVD | Mortality | Follow-up, years |
|---|---|---|---|---|---|---|---|---|---|
| Wald et al. [84] | Retrospective case- match | Hospitalized patients | 3769–13.598 | AKI requiring dialysis | HR = 3.26, 95% CI 2.73–3.89 | HR = 0.95, 95% CI 0.89–1.02 | 5 | ||
| Goldberg et al. [85] | Prospective | STEMI patients | 1957 | RIFLE | 17.7 |
Persistent mild AKI: HR = 2.3, 95% CI 1.4–3.7, P < 0.001 Transient moderate/severe AKI: HR = 2.0, 95% CI 1.2–3.2, P < 0.004 Persistent moderate/severe AKI: HR = 2.7, 95% CI 1.5–4.6, P < 0.001 |
3 | ||
| Wu et al. [86] | Observational | Surgical CKD patients | 9425 | RIFLE | 46.6 | HR = 2.62, 95% CI 1.92–3.57, P < 0.001 | 5 | ||
| Schiffl et al. [87] | Prospective | ICU patients | 425 | AKI requiring dialysis | 100 |
1 year 65% 5-year 75% 10-year 80% |
10 | ||
| Pannu et al. [88] | Observational | Hospitalized patients | 190 714 | KDIGO | 3.7 | AKI non-recovery HR = 4.13, 95% CI 3.38–5.04 |
AKI non-recovery HR = 1.26, 95% CI 1.10–1.43 |
6 | |
| Fuchs et al. [89] | Retrospective | Critical care pateints | 12 399 | AKIN | 54.3 |
AKIN 1: HR = 1.26, 95% CI 1.14–1.40, P < 0.001 AKIN 2: HR = 1.28, 95% CI 1.11–1.47, P = 0.001 AKIN 3: HR = 1.61, 95% CI 1.30–1.99, P < 0.001 |
2 | ||
| Horne et al. [90] | Prospective case control | Hospitalized patients | 300 | AKIN | 50 | 14% versus 0.7%, P < 0.001 | 1 | ||
| Chawla et al. [61] | Retrospective | Hospitalized patients | 36 980 | KDIGO | 22.8 | HR = 2.07, 95% CI 1.99–2.16 | AKI + MI: HR = 1.24, 95% CI 1.18–1.30 | HR = 1.85, 95% CI 1.76–1.94 | 1 |
| Ryden et al. [91] | Retrospective | Cardiac surgery patients | 29 330 | AKIN | 13 | HR = 6.24 (1.94–20.1) |
AKIN 1: 1.34, 95% CI 1.23–1.45 AKIN 2/3: 2.32, 95% CI 2.01–2.68 |
6 | |
| Wu et al. [92] | Case-control | Hospitalized patients | 4869–4869 | AKI requiring dialysis | 50 | HR = 1.67, 95% CI 1.36–2.04, P < 0.001 | HR = 1.67, P < 0.001 | 3 | |
| Rimes-Stigare et al. [93] | Retrospective | ICU patients | 97 782 | KDIGO | 5.4 | 6.0% versus 0.44%, IRR 7.6% | 61.8% versus 39.1% | 5 | |
| Xu et al. [94] | Prospective | Cardiac surgery patients | 3245 | KDIGO | 39.9 | HR = 20.32, 95% CI 4.55–97.31, P < 0.001 | HR = 1.74, 95% CI 1.27–2.37, P < 0.001 | 2 | |
| Hansen et al. [95] | Observational | Cardiac surgery patients | 4742 | KDIGO | 30.7 | HR 1.41, 95% CI 1.11–1.80 | HR = 1.37, 95% CI 1.05–1.80 | 5 | |
| Heung et al. [47] | Retrospective | Hospitalized patients | 104 764 | KDIGO | 16.3 | 31.8% (AKI) versus 15.5% (no AKI), P = 0.001 | 1 | ||
| Gameiro et al. [96] | Retrospective | Abdominal surgery patients | 390 | KDIGO | 18.5 | 47.2% versus 22.0%; HR 1.65, 95% CI 1.01–2.50, P = 0.046 | 44% versus 19.8%; HR = 1.4, 95% CI 1.10–2.00, P = 0.043 | 4 | |
| Soliman et al. [97] | Retrospective | Critical care pateints | 2420 | RIFLE | 34.4 |
Injury: HR = 1.35, 95% CI 1.10–1.65, P = 0.004 Failure: HR = 1.77, 95% CI 1.37–2.28, P < 0.001 |
1 | ||
| Parikh et al. [98] | Prospective | Cardiac surgery patients | 968 | AKIN | 36 | HR 1.99, 95% CI 1.46–2.71 | 4 | ||
| Ferreiro et al. [99] | Retrospective | Cardiac surgery patients | 7075 | KDIGO | 36.1 | Mortality in 3.5 years: HR = 1.330, 95% CI 1.123–1.750 | 15 | ||
| Kofman et al. [100] | Retrospective | STEMI patients | 225 | KDIGO | 100 | AKD: HR = 2.42, 95% CI 1.52–3.92, P < 0.001 | 4 | ||
| Arias-Cabrales et al. [101] | Retrospective | Hospitalized patients | 360 | ADQI | 100 | 6.5% |
CKD: OR = 1.03, 95% CI 1.03–1.06, P < 0.05 |
4 | |
| Pourafkari et al. [102] | Prospective | Non-cardiac surgery patients | 7564 | AKIN | 8.5 | OR = 34.95, 95% CI 20.88–58.52, P < 0.001 | OR = 6.37, 95% CI 5.27–7.70, P < 0.001 | OR = 2.26, 95% CI 1.97–2.60, P < 0.001 | 15 |
| Lee et al. [103] | Observational | Cardiac surgery patients | 45 108 | AKI requiring dialysis | 2.8 | HR = 15.59, 95% CI 13.89–18.33, P < 0.001 | HR = 1.97, 95% CI 1.75–2.23, P < 0.001 | HR = 2.72, 95% CI 2.44–3.02, P < 0.001 | 3 |
| Mizota et al. [104] | Retrospective | Abdominal surgery patients | 3751 | KDIGO | 6.9 |
Transient AKI: OR = 3.87, 95% CI 2.12–7.08, P < 0.001 Persistent AKI: OR = 23.70, 9.64–58.22, P < 0.001 |
Transient AKI: HR = 2.01, 95% CI 1.34–2.93, P = 0.001 Persistent AKI: HR = 6.20, 95% CI 3.00–11.43, P < 0.001 |
1 | |
| Bhatraju et al. [105] | Prospective | Hospitalized patients | 1538 | KDIGO | 31 | HR = 1.52, 95% CI 1.01–2.29, P = 0.04 | 5 | ||
| Gameiro et al. [41] | Retrospective | Septic ICU | KDIGO | 100 | HR = 2.87, 95% CI 2.0–4.1, P < 0.001 | HR = 1.51, 95% CI 1.0–2.2, P = 0.040 | 5 | ||
| Cheng et al. [106] | Retrospective | Post-constrast hospitalized patients | 34 709 |
European Society of Urogenital Radiology |
0.83 | Persistent renal dysfunction: OR = 3.685, 95% CI 1.628–8.340, P = 0.002 | 1 | ||
| Coca et al. [31] | Prospective | Non-cardiac surgery diabetic patients | 35 302 | AKIN | 17.8 |
AKIN 1: HR = 1.24, 95% CI 1.17–1.31 AKIN 2: HR = 1.64, 95% CI 1.43–1.88 AKIN 3: HR = 1.96, 95% CI 1.63–2.37 |
4 |
Coca et al. reported that AKI increased the risk of CKD by 8-fold (HR = 8.8, 95% CI 3.1–25.5) and the risk of end-stage kidney disease by 3-fold (HR = 3.1, 95% CI 1.9–5.0). Moreover, the risk was higher according to AKI severity (mild AKI HR = 2.3, 95% CI 1.7–3.3; severe AKI HR = 8.0, 95% CI 1.3–48.6) [72]. Similarly, See et al. demonstrated that the risk of CKD was 3-fold higher in AKI patients (HR = 2.67, 95% CI 1.99–3.58) [71].
The increased risk of development and/or progression of CKD as a long-term outcome associated with AKI have been demonstrated in multiple settings [47, 90]. Chawla et al. demonstrated an increased risk of adverse kidney events [long-term dialysis, 25% decrease in estimated glomerular filtration rate (eGFR) and death] in AKI patients in a cohort of 36 980 Veterans (HR = 2.07, 95% CI 1.99–2.16) [14]. A Swedish cohort of 97 782 patients critical care patients reported an increased rate of CKD [6.0% versus 0.44%, adjusted incidence rate ratio (IRR) 7.6] and end stage renal disease (ESRD) (3.9% versus 0.3%, adjusted IRR = 22.5) [93]. In 3245 cardiac surgery patients, the prevalence of CKD was significantly higher in AKI patients (6.8% versus 0.2%, P < 0.001; RR = 1.92, 95% CI 1.37–2.69) in the 2 years after surgery, even with complete recovery of renal function at discharge [94]. This was also reported in major abdominal surgery in a previous study of 390 patient in whom AKI was a risk factor for need for long-term dialysis and/or a 25% decrease in eGFR after hospital discharge (adjusted HR = 1.6, P = 0.046) [96].
The risk of CKD is also higher in relation to the severity of AKI as demonstrated in previous studies, and even higher in patients who require dialysis [84]. Ryden et al. studied 29 330 patients who underwent primary isolated coronary artery bypass grafting and demonstrated that the risk for ESRD increased with AKI severity, and was 2.92 (95% CI 1.87–4.55) for AKIN Stage 1 and 3.81 (95% CI 2.14–6.79) for AKIN Stages 2 and 3 [91]. In AKI requiring dialysis, the incidence rate of ESRD was 2.63/100 person-years in a study by Wald et al. (HR = 3.23, 95% CI 2.70–3.86) [84].
The risk of ESRD is higher in patients with previous CKD. Wu et al. reported an incidence rate of long-term dialysis of 17.8/100 person-years in patients with AKI-on-CKD when compared with patients without previous CKD [86].
Other than AKI severity, considering that even less severe stages of AKI are associated with long-term outcomes, the recovery of renal function after an AKI episode and its risk of developing CKD have also been studied.
Patients who recover renal function less often progress to CKD. Pannu et al. analysed long-term outcomes among 3231 survivors of hospitalization over a 6-year period, and reported an 2.1% incidence of ESRD and 9.8% of CKD in the AKI group, and demonstrated that patients who did not recover renal function after AKI had a higher risk of CKD and ESRD (HR = 4.13, 95% CI 3.38–5.04) [88]. On the contrary, in a retrospective cohort of 221 087 hospitalized patients by Heung et al. 31.8% of AKI patients progressed to CKD as compared to only 15.5% of non-AKI patients, on a 1-year follow-up (P < 0.001). In this study, even after recovery of AKI within 48 h, there was a relative risk of CKD progression which also increased associated with AKI severity [47]. Therefore, the duration of AKI might also impact on outcomes.
Indeed, a different threshold of AKI duration has been reported in the prospective study by Bhatraju et al. of 1538 participants in which non-recovery of renal function within the first 72 h after AKI was associated with a 51% greater risk of CKD development or dialysis requirement or death (95% CI 22–88%, P < 0.001) [105]. However, the risk of CKD development was still higher than non-AKI patients.
Accordingly, the presence of AKD has also been associated with CKD development. Among 225 patients undergoing PCI after myocardial infarction, 58.5% of AKD patients developed new or progressed CKD [100]. In 256 septic-AKI patients, AKD was also independently associated with adverse renal outcomes (HR = 2.87, 95% CI 2.0–4.1, P < 0.001) [107].
The majority of studies assessed the link between AKI and CKD based on eGFR decline, still more recent studies have identified new-onset albuminuria following AKI as a marker of CKD progression.
Proteinuria is a well-established and risk factor for CKD progression and cardiovascular events [108–110]. Interestingly, in a retrospective cohort of 657 840 patients, the risk of developing or worsening proteinuria was higher in AKI patients (OR = 1.39, 95% CI 1.33–1.46) and increased according to AKI severity [90]. Also, proteinuria and albuminuria have been more prevalent in AKI patients and associated with CKD progression [90]. Parr et al. also demonstrated that AKI patients had increased risk of developing proteinuria (OR range 1.20–1.39), which also increased according to AKI severity [111].
Factors associated with higher risk of CKD following AKI were previous increased baseline SCr, male gender, African American race, older age, diabetes, previous CVD, hypoalbuminaemia, lower haematocrit, AKI severity, duration and recovery pattern of AKI, recurrent AKI episodes (Figure 5) [14, 59, 80, 84, 87, 93, 96, 103, 112, 113].
Development of CKD after AKI is also a risk factor for the development of cardiovascular events and for long-term mortality. Cabrales et al. demonstrated that on a 4-year follow-up after an AKI episode, patients with hypertension (OR = 1.62, 95% CI 1.2–2.6) had a higher risk of developing CKD, patients with previous CKD had a higher risk of CKD progression. Furthermore, CKD patients had an increased risk of cardiovascular events (62.7 versus 21.7%, P < 0.05), and that mortality risk was increased by 4-fold (OR = 4.3, 95% CI 1.13–4.90) [101].
Furthermore, CKD after AKI has also been associated with increased mortality risk. For instance, in 634 AKI patients, Lai et al. found that the long-term mortality risk increased as kidney function declined during follow-up [114]. In 425 critically ill AKI patients, development of CKD was an independent predictor of mortality (OR = 4.3, 95% CI 2.9–6.2) [87].
Long-term cardiovascular outcomes
AKI is associated with increased long-term cardiovascular morbidity [115]. Odutayo et al. performed a systematic review and reported that AKI was associated with an 86% increased risk of cardiovascular mortality and a 38% increased risk of major cardiovascular events, namely development of chronic heart failure, acute myocardial infarction and stroke [116].
The relationship between CKD and CVD is well documented [115]. The term cardiorenal syndrome has been proposed to describe the complex bidirectional interactions between heart and kidneys [115, 117]. AKI may lead to direct and indirect effect on cardiac function and structure and thus increase the risk of cardiovascular events. The pathophysiological processes include activation of the sympathetic nervous system, activation of the RAAS, endothelial dysfunction, inflammation, cardiac fibrosis, volume expansion, hypertension, electrolyte disturbances, acidaemia and anaemia [115, 118].
AKI has been associated with development with blood pressure elevation. In a retrospective study of 43 611 hospitalized patients, AKI was associated with a 22% increased risk of hypertension within a 2-year follow-up, which greater according to AKI severity [119]. Indeed, in experimental studies, it has been reported that sodium-sensitive hypertension develops after an ischaemic renal event, due to impaired renal sodium excretion [120].
The association between AKI and CVD has been demonstrated in several studies (Table 3). In a retrospective study of US Veterans, Chawla et al. reported an increased risk of cardiovascular outcomes, namely myocardial infarction, stroke or heart failure (HR = 1.24, 95% CI 1.18–1.30), in AKI patients [14]. In a cohort of 4742 cardiac surgery patients the risk of myocardial infarction, heart failure and stroke was significantly increased in AKI patients on a 5-year follow-up (HR = 1.37, 95% CI 1.05–1.80). Additionally, AKI was associated with an increase in both short- and long-term mortality [95]. Wu et al. demonstrated an incidence rate of coronary events of 19.8/1000 person-years (HR = 1.67, 95% CI 1.36–2.04) independently of progression to CKD [92]. Similar results have also been reported in non-cardiovascular surgery patients [102]. Also, in a post hoc analysis of 9361 patients enrolled in the Systolic Blood Pressure Intervention Trial, AKI patients had a higher risk of cardiovascular events (HR = 1.52, 95% CI 1.05–2.20).
The risk of cardiovascular events increases according to AKI severity [98, 103]. Lee et al. reported that AKI patients who required dialysis more frequently developed cardiovascular events (HR = 1.97, 95% CI 1.75–2.23) and that this risk was high even patients who recovered renal function (HR = 1.73, 95% CI 1.54–1.95) [103]. Furthermore, even small transient and changes in renal function have been associated with increased risk of cardiovascular events [85].
Parikh conducted a study on 968 adults who underwent cardiac surgery which also demonstrated the association of AKI and AKI severity and an increased risk of cardiovascular events [98]. Interestingly, in this study, there was a significant association between peak postoperative cardiac injury biomarkers and CV outcomes while there was no association between peak postoperative urinary injury biomarkers and CV outcomes [98]. This further reinforces the systemic and haemodynamic effects of AKI on cardiac dysfunction.
Reported factors associated with higher risk of CVD following AKI were older age, CKD, diabetes and previous cardiovascular events (Figure 5) [14, 15, 98, 121].
Long-term mortality
The association between AKI and mortality in the long-term is well recognized (Table 3). Interestingly, this association is independent of pre-existing CKD. In a review by Coca et al., the pooled mortality was 8.9 deaths/100 person-years following AKI [122]. Similarly, a more recent review by See et al. reported a pooled mortality of 13.2 deaths/100 person-year after AKI [71].
Chawla et al. demonstrated that the risk of mortality after AKI (HR = 1.85, 95% CI 1.76–1.94, P < 0.001) was almost 2-fold higher than the risk of mortality after myocardial infarction [14]. This was an important finding which raised awareness to the detrimental impact of AKI on long-term outcomes.
In a longitudinal cohort of 7075 adult patients who were submitted to cardiac surgery, AKI was a predictor of mortality in the 5 years following surgery (30 days to 1 year: HR = 1.834, 95% CI 1.459–2.306; 1–3 years: HR = 1.285, 95% CI 1.023–1.610; and 3–5 years: HR = 1.330, 95% CI 1.123–1.750) [99].
The increased severity of AKI is also associated with increased mortality risk [71, 122]. Fuchs et al. analysed the long-term outcomes of 12 399 survivors from an ICU admission and reported that patients with AKI Stage 3 AKIN had a 61% higher mortality risk on a 2-year follow-up compared with patients without AKI (P = 0.001) [89]. Less severe changes in renal function were also associated with mortality risk, though this risk was progressively higher with the increasing severity of AKI (AKIN 1: HR = 1.12, AKIN 2: 1.19, AKIN 3: 1.24, compared with no AKI, P < 0.05) [89]. Soliman et al. also demonstrated that moderate or severe AKI was associated with higher mortality in 2420 patients, 1 year after ICU admission [Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) injury adjusted relative risk 1.14, 95% CI 1.01–1.29; P = 0.03; RIFLE failure adjusted relative risk 1.25, 95% CI 1.01, 1.55; P = 0.04] [97]. Another cohort demonstrated that patients who required RRT had the highest risk of long-term mortality (adjusted HR = 2.65, 95% CI 2.0–3.52) [31].
The duration of AKI has also been studied as a predictor of long-term mortality. Coca et al. prospectively studied 35 302 diabetic patients who underwent non-cardiac surgery and described that not only the severity but also the duration of AKI decreased long-term survival. Remarkably, patients with longer duration of AKI had higher mortality in every stage of AKIN (log-rank test P < 0.001) [31]. Similarly, Cheng et al. demonstrated that compared with transient renal dysfunction, patients with persistent AKI following contrast administration had higher mortality after 1 year (RR = 3.768, 95% CI 1.612–8.810; RR = 4.106, 95% CI 1.765–9.551) [106]. Still, transient changes in renal function are also associated with higher mortality when compared with patients without AKI. Mizota et al. reported that transient AKI was frequent in patients undergoing major abdominal surgery and that compared to patients without AKI, patients with transient (adjusted HR = 2.01, 95% CI 1.34–2.93, P = 0.001) and persistent AKI (adjusted HR = 6.20, 95% CI 3.00–11.43, P < 0.001) had increased mortality rates on a 1-year follow-up [104].
Importantly, recovery of renal function after AKI appears to be predictor of better long-term survival. In a prospective cohort of 425 critically ill patients the mortality rate patients who recovered renal function after AKI was lower than patients with CKD (46% versus 83%, P < 0.001) [87]. In a study of 1742 septic patients, it was reported that patients who recovered renal function after AKI had similar survival compared with patients without AKI (HR = 1.01, 95% CI 0.69–1.47, P = 0.96) over a median follow-up of 1.7 years [123]. Kofman et al. evaluated the incidence and prognosis of AKD after AKI and described that development of AKD was associated with increased long-term mortality in 225 patients with myocardial infarction (35% versus 17%, P < 0.001) [100].
As previously described, development of CKD and cardiovascular events after AKI is also associated with higher mortality rates.
Whether AKI directly contributes to mortality or serves as a surrogate marker of illness severity in higher-risk patients is still uncertain. The most frequently reported causes of mortality have been CVD, infections and cancer [87, 124].
Other than AKI severity, duration and renal recovery; lower baseline kidney function, male gender, older age, presence of comorbidities (diabetes, hypertension, CVD and cancer) and hypoalbuminaemia have also been associated with increased mortality risk following AKI (Figure 5) [14, 99, 123, 125–127].
Follow-up care after AKI
Despite consistent evidence that AKI has a negative impact on long-term outcomes, the reported rates of nephrology follow-up after AKI are low [128, 129]. Furthermore, to date, there is no standardized AKI or AKD follow-up care.
The US Renal Data System annual report of 2015 states that only 19% of patients had a nephrology follow-up at 12 months after an AKI hospitalization [130]. Siew et al. reported nephrology referral in only 4% of patients at 3 months and only 9% at 1 year, despite the fact that the mortality rate during this period was 22% [16]. Patients who were not referred to nephrology consult were slightly older, but had better kidney function and were less likely to have diabetes or heart failure [16]. An important limitation of this study is that the reasons for referral or non-referral are not reported, namely decisions to limit care.
The benefit of nephrology referral is uncertain, though, Harel et al. reported that on the 41% of 3877 AKI patients who were referred to nephrology follow-up within 90 days there was a 24% mortality reduction in 2 years of follow-up, in a retrospective analysis [131]. Interestingly, to reduce survival bias, patients were matched using a propensity score for co-morbidities and required a minimum survival period following hospital discharge of 90 days, to exclude patients with high disease burden in whom nephrology follow-up would be of less benefit.
The exact factors that contribute to this survival benefit are not completely clarified; however, recognition and treatment of cardiovascular risk factors and CKD complications are most likely implicated. Additionally, the nephrologist can be useful in patients with decision to limit care, as part of the palliative care team.
During the AKI episode the main goal should be the recovery to baseline kidney function in the shortest period of time in order to reduce the duration and disease severity [132–134]. After discharge it is crucial to preserve renal function and prevent further deterioration, by controlling hypertension, proteinuria, diabetes mellitus and CVD [135].
The current recommendations by the KDIGO and the ADQI state that patients should be followed by a nephrologist at least 3 months after an AKI episode in order to estimate kidney recovery and/or progression to CKD, or progressive CKD [18, 133]. The follow-up assessment should include kidney function and proteinuria, medication reconciliation, patient education to nephrotoxic avoidance and strategies to prevent CKD progression [133, 136].
Prescribing RAAS inhibitors after an AKI episode is promising as it might decrease the loss of kidney function, decrease CVD events and decrease mortality [64, 137, 138]. Chou et al. prospectively analysed 587 patients who recovered kidney function after cardiac-surgery associated AKI, and demonstrated that prescription of RAAS inhibitors after kidney function recovery was independently associated with lower risk of CKD development (26.6% versus 42.2%, HR = 0.46, P < 0.001) [64]. A retrospective cohort of 46 253 AKI survivours who were prescribed RAAS inhibitors within 6 months of the AKI episode, demonstrated a decreased mortality risk after 2 years (HR = 0.85, 95% CI 0.81–0.89). However, there was an increased risk of hospitalization for a renal cause [137]. Still, the risk of AKI recurrence associated with RAAS inhibitors has not been demonstrated in more recent studies [139].
Another promising therapeutic intervention is the use of statins. On a retrospective cohort of 19 707 patients with CKD after AKI, patients who were prescribed statins after AKI had lower rate of hospitalizations and mortality risk over a 2-year follow-up [75]. Still, timing of statin prescription was not assessed neither were some important covariates, namely blood pressure.
The use of RAAS inhibitors and statins is promising to reduce the cardiovascular risk after AKI, which has a significant prevalence in these patients. However, interventional and further observational studies are needed to establish the concrete benefit of RAAS inhibitors and/or statins after AKI and to define the adequate timing and dose to start these medications after AKI.
Several medications which can decrease renal function or are associated with adverse events due to drug accumulation in CKD patients must also be adjusted in high-risk patients. Non-steroidal anti-inflammatory drugs (NSAIDs) affect renal function due to prostaglandin inhibition and are associated with an increased risk of AKI and CKD [140]. AKI is predominantly haemodynamically mediated but can also be immune-mediated, and is higher in elderly patients and with concomitant use of RAAS inhibitors or diuretics [140]. The development of CKD appears to be associated with higher doses of NSAIDs [141]. Although the risk of adverse effects of NSAIDs following AKI has not been specifically evaluated it is important to recognize the increased risk of this population and to carefully prescribe these medications [141].
Given that the risk of hypoglycaemia after discharge following AKI has been reportedly higher in patients medicated with insulin and sulfonylurea, it is necessary to intervene and lower the risk of these episodes in patients medicated with glucose-lowering agents with renal excretion [142]. The use of sodium–glucose cotransporter 2 (SGLT2) inhibitors to improve glycaemic control after AKI is promising as these have been associated with decreasing the risk of cardiovascular events and CKD progression [143]. Still, further randomized trials are necessary to assess the safety of these drugs in patients with recent AKI or CKD patients.
Assessing which patients are at higher risk for CKD development, cardiovascular events and long-term mortality after AKI are therefore crucial (Figure 5). Prediction models applied at discharge could estimate the risk of adverse outcomes and target patients at risk with specialized interventions [144]. The use of biomarkers to estimate risk is also promising as higher levels of NGAL, IL-18, KIM-1, albuminuria, TIMP-2 × IGFBP7 have been associated with increased long-term adverse outcomes after AKI [69, 145]. To date, only NGAL and TIMP-2 × IGFBP7 have been approved, though their widespread use in clinical practice is still distant. These biomarkers might provide prognostic information during the AKI period, although no studies have analysed patient follow-up assessed with these biomarkers.
Currently, two randomized clinical trials are comparing standardized care to nephrology follow-up [146, 147]. The FUSION trial will compare patients with a referral to family physician after hospitalization and later local nephrology follow-up if considered appropriate by the physician versus patients who will be referred to an AKI follow-up clinic within 30 days after discharged and routine laboratory every 3 months, and assess patient outcomes on a 5-year follow-up [146]. This trial The AFTER-AKI trial will compare patients discharged with usual discharge protocols and patients who will receive specific follow-up protocols based on their risk of CKD, and assess outcomes after 1-year follow-up [147]. These are highly anticipated to clarify the importance of specialized care after AKI on improving patient outcomes.
Further research is essential to stratify high-risk patients, define timing for nephrology follow-up and to develop strategies to improve patient outcomes.
CONCLUSION
It is crucial to stop regarding AKI as a short-term reversible condition and to raise awareness on the long-term complications, such as progression to CKD, increased cardiovascular events and mortality. Whether AKI is a marker of patients with more co-morbidities and increased risk for poor prognosis or directly contributes to adverse outcomes is still unclear.
It is evident that AKI survivors should have specialized long-term follow-up. Nephrology follow-up is crucial to assess kidney function after AKI, perform medication reconciliation, educate patients on nephrotoxic avoidance and implement strategies to prevent CKD progression.
Still, further investigation is required on assessment of biomarkers of renal recovery and worse renal and vital prognosis, and on assessment of safety and dosing of specific therapeutic strategies (RAAS inhibitors, statins and SGLT2 inhibitors) to prevent CKD and CVD after AKI, and in defining optimal follow-up timing (Table 4).
Table 4.
List of research needs on the period after AKI
| Research requirements |
|---|
| Optimal timing for nephrology follow-up |
| Biomarkers of the transition from AKI to CKD |
| Benefits of RAAS inhibitors, statins, SGLT2 inhibitors |
| Benefits of other anti-fibrotic or anti-inflammatory drugs |
ACKNOWLEDGEMENTS
The authors would like to acknowledge Joana Paraíba for the significant contributions in designing the figures.
FUNDING
No funding was received for this study.
AUTHORS’ CONTRIBUTIONS
J.G. made substantial contributions to the study concept and was involved in drafting the manuscript, F.M. made substantial contributions to the study design and J.A.L. was involved in revising it critically for intellectual content.
CONFLICT OF INTEREST STATEMENT
The authors declare there is no conflict of interest.
REFERENCES
- 1. Susantitaphong P, Cruz DN, Cerda J. et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013; 8: 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu RK, McCulloch CE, Dudley RA. et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 2013; 24: 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lameire N, Van Biesen W, Vanholder R.. The changing epidemiology of acute renal failure. Nat Rev Nephrol 2006; 2: 364–377 [DOI] [PubMed] [Google Scholar]
- 4. Rodrigues FB, Bruetto RG, Torres US. et al. Incidence and mortality of acute kidney injury after myocardial infarction: a comparison between KDIGO and RIFLE criteria. PLoS One 2013; 8: e69998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo X, Jiang L, Du B. et al. ; The Beijing Acute Kidney Injury Trial (BAKIT) Workgroup. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 2014; 18: R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujii T, Uchino S, Takinami M. et al. Validation of the kidney disease improving global outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol 2014; 9: 848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagshaw SM, George C, Bellomo R.. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11: R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellomo R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care 2006; 12: 557–560 [DOI] [PubMed] [Google Scholar]
- 9. Cruz DN, Ronco C.. Acute kidney injury in the intensive care unit: current trends in incidence and outcome. Crit Care 2007; 11: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chertow GM, Burdick E, Honour M. et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16: 3365–3370 [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Kim YJ, Ryoo S. et al. One-year progression and risk factors for the development of chronic kidney disease in septic shock patients with acute kidney injury: a single-centre retrospective cohort study. J Clin Med 2018; 7: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin S, Orieux A, Clouzeau B. et al. The incidence of chronic kidney disease three years after non-severe acute kidney injury in critically ill patients: a single-center cohort study. J Clin Med 2019; 8: 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sawhney S, Mitchell M, Marks A. et al. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015; 5: e006497–e006497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chawla LS, Amdur RL, Shaw AD. et al. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 2014; 9: 448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Omotoso BA, Abdel-Rahman EM, Xin W. et al. Acute kidney injury (AKI) outcome, a predictor of long-term major adverse cardiovascular events (MACE). Clin Nephrol 2016; 85: 1–11 [DOI] [PubMed] [Google Scholar]
- 16. Siew ED, Peterson JF, Eden SK. et al. outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol 2012; 23: 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirwan CJ, Blunden MJ, Dobbie H. et al. critically ill patients requiring acute renal replacement therapy are at an increased risk of long-term renal dysfunction, but rarely receive specialist nephrology follow-up. Nephron 2015; 129: 164–170 [DOI] [PubMed] [Google Scholar]
- 18. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron 2012; 120: c179–c184 [DOI] [PubMed] [Google Scholar]
- 19. Kellum JA, Lameire N, for the KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uchino S. Acute renal failure in critically ill patients a multinational, multicenter study. JAMA 2005; 294: 813. [DOI] [PubMed] [Google Scholar]
- 21. Ostermann M, Liu K.. Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol 2017; 31: 305–314 [DOI] [PubMed] [Google Scholar]
- 22. Case J, Khan S, Khalid R. et al. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013; 2013: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akcay A, Nguyen Q, Edelstein CL.. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009; 2009: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basile DP, Anderson MD. Pathophysiology of acute kidney injury. Comprehensive physiology. Hoboken, NJ: John Wiley & Sons, Inc, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 2006; 17: 1503–1520 [DOI] [PubMed] [Google Scholar]
- 26. Sharfuddin AA, Molitoris BA.. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 2011; 7: 189–200 [DOI] [PubMed] [Google Scholar]
- 27. Liu KD, Brakeman PR.. Renal repair and recovery. Crit Care Med 2008; 36: S187–S192 [DOI] [PubMed] [Google Scholar]
- 28. Bonventre JV. Pathophysiology of acute kidney injury: roles of potential inhibitors of inflammation. Contrib Nephrol 2007; 156: 39–46 [DOI] [PubMed] [Google Scholar]
- 29. Lee DW, Faubel S, Edelstein CL.. Cytokines in acute kidney injury (AKI). Clin Nephrol 2011; 76: 165–173 [DOI] [PubMed] [Google Scholar]
- 30. Jang HR, Rabb H.. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 2015; 11: 88–101 [DOI] [PubMed] [Google Scholar]
- 31. Coca SG, King JT, Rosenthal RA. et al. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int 2010; 78: 926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanmassenhove J, Glorieux G, Hoste E. et al. AKI in early sepsis is a continuum from transient AKI without tubular damage over transient AKI with minor tubular damage to intrinsic AKI with severe tubular damage. Int Urol Nephrol 2014; 46: 2003–2008 [DOI] [PubMed] [Google Scholar]
- 33. Darmon M, Truche AS, Abdel-Nabey M. et al. Early recognition of persistent acute kidney injury. Semin Nephrol 2019; 39: 431–441 [DOI] [PubMed] [Google Scholar]
- 34. Ronco C, Kellum JA, Haase M.. Subclinical AKI is still AKI. Crit Care 2012; 16: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmitt R, Coca S, Kanbay M. et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis 2008; 52: 262–271 [DOI] [PubMed] [Google Scholar]
- 36. Forni LG, Darmon M, Ostermann M. et al. Renal recovery after acute kidney injury. Intensive Care Med 2017; 43: 855–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chawla LS, Bellomo R, Bihorac A. et al. ; on behalf of the Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 38. Sood MM, Shafer LA, Ho J. et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care 2014; 29: 711–717 [DOI] [PubMed] [Google Scholar]
- 39. Perinel S, Vincent F, Lautrette A. et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients. Crit Care Med 2015; 43: e269–e275 [DOI] [PubMed] [Google Scholar]
- 40. Fidalgo P, Ahmed M, Meyer SR. et al. Association between transient acute kidney injury and morbidity and mortality after lung transplantation: a retrospective cohort study. J Crit Care 2014; 29: 1028–1034 [DOI] [PubMed] [Google Scholar]
- 41. Gameiro J, Duarte I, Marques F. et al. Transient and persistent AKI and outcomes in patients undergoing major abdominal surgery. Nephron 2020; 144: 236–244 [DOI] [PubMed] [Google Scholar]
- 42. Coelho S, Fonseca JN, Gameiro J. et al. Transient and persistent acute kidney injury in acute liver failure. J Nephrol 2019; 32: 289–296 [DOI] [PubMed] [Google Scholar]
- 43. Bagshaw SM. Epidemiology of renal recovery after acute renal failure. Curr Opin Intern Med 2007; 6: 31–37 [DOI] [PubMed] [Google Scholar]
- 44. Yang L. How acute kidney injury contributes to renal fibrosis . Adv Exp Med Biol 2019; 1165: 117–142 [DOI] [PubMed] [Google Scholar]
- 45. Prowle JR, Kolic I, Purdell-Lewis J. et al. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol 2014; 9: 1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bucaloiu ID, Kirchner HL, Norfolk ER. et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012; 81: 477–485 [DOI] [PubMed] [Google Scholar]
- 47. Heung M, Steffick DE, Zivin K. et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans health administration data. Am J Kidney Dis 2016; 67: 742–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malhotra R, Siew ED.. Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 2017; 12: 149–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pike F, Murugan R, Keener C. et al. Biomarker enhanced risk prediction for adverse outcomes in critically ill patients receiving RRT. Clin J Am Soc Nephrol 2015; 10: 1332–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kashani K, Cheungpasitporn W, Ronco C.. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med 2017; 55: 1074–1089 [DOI] [PubMed] [Google Scholar]
- 51. Srisawat N, Wen X, Lee M. et al. Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol 2011; 6: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dewitte A, Joannès-Boyau O, Sidobre C. et al. Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol 2015; 10: 1900–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kellum JA. How can we define recovery after acute kidney injury? considerations from epidemiology and clinical trial design. Nephron Clin Pract 2014; 127: 81–88 [DOI] [PubMed] [Google Scholar]
- 54. Kellum JA, Sileanu FE, Bihorac A. et al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017; 195: 784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bagshaw SM, George C, Bellomo R. et al. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 2008; 12: R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duff S, Murray PT.. Defining early recovery of acute kidney injury. Clin J Am Soc Nephrol. 2020; Apr 1;CJN.13381019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ali T, Khan I, Simpson W. et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007; 18: 1292–1298 [DOI] [PubMed] [Google Scholar]
- 58. Liang KV, Sileanu FE, Clermont G. et al. Modality of RRT and recovery of kidney function after AKI in patients surviving to hospital discharge. Clin J Am Soc Nephrol 2016; 11: 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Macedo E, Zanetta DMT, Abdulkader RCRM.. Long-term follow-up of patients after acute kidney injury: patterns of renal functional recovery. PLoS One 2012; 7: e36388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wald R, Shariff SZ, Adhikari NKJ. et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury. Crit Care Med 2014; 42: 868–877 [DOI] [PubMed] [Google Scholar]
- 61. Chawla LS, Eggers PW, Star RA. et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014; 371: 58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fiorentino M, Grandaliano G, Gesualdo L. et al. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol 2018; 193: 45–54 [DOI] [PubMed] [Google Scholar]
- 63. Venkatachalam MA, Weinberg JM, Kriz W. et al. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 2015; 26: 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chou YH, Huang TM, Pan SY. et al. Renin-angiotensin system inhibitor is associated with lower risk of ensuing chronic kidney disease after functional recovery from. Sci Rep 2017; 7: 46518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferenbach DA, Bonventre JV.. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 2015; 11: 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chawla LS, Kimmel PL.. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012; 82: 516–524 [DOI] [PubMed] [Google Scholar]
- 67. Han WK, Bailly V, Abichandani R. et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62: 237–244 [DOI] [PubMed] [Google Scholar]
- 68. Hall IE, Yarlagadda SG, Coca SG. et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 2010; 21: 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Koyner JL, Shaw AD, Chawla LS. et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)⋅IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol 2015; 26: 1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alge JL, Arthur JM.. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 2015; 10: 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. See EJ, Jayasinghe K, Glassford N. et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 2019; 95: 160–172 [DOI] [PubMed] [Google Scholar]
- 72. Coca SG, Singanamala S, Parikh CR.. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012; 81: 442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Walther CP, Winkelmayer WC, Deswal A. et al. Readmissions after acute kidney injury during left ventricular assist device implantation hospitalization. Am J Nephrol 2020; 51: 172–181 [DOI] [PubMed] [Google Scholar]
- 74. Koulouridis I, Price LL, Madias NE, Jaber BL.. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am J Kidney Dis 2015; 65: 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brar S, Ye F, James M. et al. Statin use and survival after acute kidney injury. Kidney Int Reports 2016; 1: 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hirayama A, Goto T, Hasegawa K.. Association of acute kidney injury with readmissions after hospitalization for acute exacerbation of chronic obstructive pulmonary disease: a population-based study. BMC Nephrol 2020; 21: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu KD, Yang J, Tan TC. et al. Risk factors for recurrent acute kidney injury in a large population-based cohort. Am J Kidney Dis 2019; 73: 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Siew ED, Parr SK, Abdel-Kader K. et al. Predictors of recurrent AKI. J Am Soc Nephrol 2016; 27: 1190–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Holmes J, Geen J, Williams JD. et al. Recurrent acute kidney injury: predictors and impact in a large population-based cohort. Nephrol Dial Transplant 2019. Aug 3;gfz155 [DOI] [PubMed] [Google Scholar]
- 80. Harris DG, Koo G, McCrone MP. et al. Recurrent kidney injury in critically ill surgical patients is common and associated with worse outcomes. J Trauma Acute Care Surg 2014; 76: 1397–1401 [DOI] [PubMed] [Google Scholar]
- 81. Rodrigo E, Suberviola B, Santibáñez M. et al. Association between recurrence of acute kidney injury and mortality in intensive care unit patients with severe sepsis. J Intensive Care 2017; 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thakar CV, Christianson A, Himmelfarb J, Leonard AC.. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 2011; 6: 2567–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rodríguez E, Arias-Cabrales C, Bermejo S. et al. Impact of recurrent acute kidney injury on patient outcomes. Kidney Blood Press Res 2018; 43: 34–44 [DOI] [PubMed] [Google Scholar]
- 84. Wald R. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009; 302: 1179. [DOI] [PubMed] [Google Scholar]
- 85. Goldberg A, Kogan E, Hammerman H. et al. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int 2009; 76: 900–906 [DOI] [PubMed] [Google Scholar]
- 86. Wu VC, Huang TM, Lai CF. et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 2011; 80: 1222–1230 [DOI] [PubMed] [Google Scholar]
- 87. Schiffl H, Lang SM, Fischer R.. Long-term outcomes of survivors of ICU acute kidney injury requiring renal replacement therapy: a 10-year prospective cohort study. Clin Kidney J 2012; 5: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pannu N, James M, Hemmelgarn B. et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 2013; 8: 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fuchs L, Lee J, Novack V. et al. Severity of acute kidney injury and two-year outcomes in critically ill patients. Chest 2013; 144: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Horne KL, Packington R, Monaghan J. et al. The effects of acute kidney injury on long-term renal function and proteinuria in a general hospitalised population. Nephron Clin Pract 2014; 128: 192–200 [DOI] [PubMed] [Google Scholar]
- 91. Rydén L, Sartipy U, Evans M. et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130: 2005–2011 [DOI] [PubMed] [Google Scholar]
- 92. Wu VC, Wu CH, Huang TM. et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol 2014; 25: 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rimes-Stigare C, Frumento P, Bottai M. et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care 2015; 19: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu JR, Zhu JM, Jiang J. et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine 2015; 94: e2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hansen MK, Gammelager H, Jacobsen CJ. et al. Acute kidney injury and long-term risk of cardiovascular events after cardiac surgery: a population-based cohort study. J Cardiothorac Vasc Anesth 2015; 29: 617–625 [DOI] [PubMed] [Google Scholar]
- 96. Gameiro J, Neves JB, Rodrigues N. et al. Acute kidney injury, long-term renal function and mortality in patients undergoing major abdominal surgery: a cohort analysis. Clin Kidney J 2016; 9: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Soliman IW, Frencken JF, Peelen LM. et al. The predictive value of early acute kidney injury for long-term survival and quality of life of critically ill patients. Crit Care 2016; 20: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Parikh CR, Puthumana J, Shlipak MG. et al. Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. J Am Soc Nephrol 2017; 28: 3699–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ferreiro A, Lombardi R.. Acute kidney injury after cardiac surgery is associated with mid-term but not long-term mortality: a cohort-based study. PLoS One 2017; 12: e0181158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kofman N, Margolis G, Gal-Oz A. et al. Long-term renal outcomes and mortality following renal injury among myocardial infarction patients treated by primary percutaneous intervention. Coron Artery Dis 2019; 30: 87–92 [DOI] [PubMed] [Google Scholar]
- 101. Arias-Cabrales C, Rodríguez E, Bermejo S. et al. Short- and long-term outcomes after non-severe acute kidney injury. Clin Exp Nephrol 2018; 22: 61–67 [DOI] [PubMed] [Google Scholar]
- 102. Pourafkari L, Arora P, Porhomayon J. et al. Acute kidney injury after non-cardiovascular surgery: risk factors and impact on development of chronic kidney disease and long-term mortality. Curr Med Res Opin 2018; 34: 1829–1837 [DOI] [PubMed] [Google Scholar]
- 103. Lee S, Park S, Kang MW. et al. Postdischarge long-term cardiovascular outcomes of intensive care unit survivors who developed dialysis-requiring acute kidney injury after cardiac surgery. J Crit Care 2019; 50: 92–98 [DOI] [PubMed] [Google Scholar]
- 104. Mizota T, Dong L, Takeda C. et al. Transient acute kidney injury after major abdominal surgery increases chronic kidney disease risk and 1-year mortality. J Crit Care 2019; 50: 17–22 [DOI] [PubMed] [Google Scholar]
- 105. Bhatraju PK, Zelnick LR, Chinchilli VM. et al. Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw Open 2020; 3: e202682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cheng W, Wu X, Liu Q. et al. Post-contrast acute kidney injury in a hospitalized population: short-, mid-, and long-term outcome and risk factors for adverse events. Eur Radiol 2020; 30: 3516–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gameiro J, Carreiro C, Fonseca JA. et al. Acute kidney disease and long-term outcomes in critically ill acute kidney injury patients with sepsis: a cohort analysis. Clin Kidney J 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lea J. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease. Arch Intern Med 2005; 165: 947–53 [DOI] [PubMed] [Google Scholar]
- 109.Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wu VC, Huang TM, Wu PC. et al. Preoperative proteinuria is associated with long-term progression to chronic dialysis and mortality after coronary artery bypass grafting surgery. PLoS One 2012; 7: e27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Parr SK, Matheny ME, Abdel-Kader K. et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93: 460–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bihorac A, Yavas S, Subbiah S. et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 2009; 249: 851–858 [DOI] [PubMed] [Google Scholar]
- 113. Harel Z, Bell CM, Dixon SN. et al. Predictors of progression to chronic dialysis in survivors of severe acute kidney injury: a competing risk study. BMC Nephrol 2014; 15: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lai CF, Wu VC, Huang TM. et al. the National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF). Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care 2012; 16: R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Legrand M, Rossignol P.. Consequences of acute kidney injury. N Engl J Med 2020; 382: 2238–2247 [DOI] [PubMed] [Google Scholar]
- 116. Odutayo A, Wong CX, Farkouh M. et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017; 28: 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bagshaw SM, Hoste EA, Braam B. et al. Syndrome type 3: pathophysiologic and epidemiologic considerations. Cardiorenal In, 2013, 137–157. [DOI] [PubMed] [Google Scholar]
- 118. Di Lullo L, Reeves PB, Bellasi A. et al. Cardiorenal syndrome in acute kidney injury. Semin Nephrol 2019; 39: 31–40 [DOI] [PubMed] [Google Scholar]
- 119. Hsu C, Hsu RK, Yang J. et al. Elevated BP after AKI. J Am Soc Nephrol 2016; 27: 914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Spurgeon-Pechman KR, Donohoe DL, Mattson DL. et al. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Physiol 2007; 293: F269–F278 [DOI] [PubMed] [Google Scholar]
- 121. Ou SM, Chu H, Chao PW. et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors a nationwide population-based study. Am J Respir Crit Care Med 2016; 194: 209–217 [DOI] [PubMed] [Google Scholar]
- 122. Coca SG, Yusuf B, Shlipak MG. et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 53: 961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fiorentino M, Tohme FA, Wang S. et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One 2018; 13: e0198269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Silver SA, Harel Z, McArthur E. et al. Causes of death after a hospitalization with AKI. J Am Soc Nephrol 29: 1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Stads S, Fortrie G, van Bommel J. et al. Impaired kidney function at hospital discharge and long-term renal and overall survival in patients who received CRRT. Clin J Am Soc Nephrol 2013; 8: 1284–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rimes-Stigare C, Frumento P, Bottai M. et al. Long-term mortality and risk factors for development of end-stage renal disease in critically ill patients with and without chronic kidney disease. Crit Care 2015; 19: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fortrie G, Stads S, Aarnoudse AJH. et al. Long-term sequelae of severe acute kidney injury in the critically ill patient without comorbidity: a retrospective cohort study. PLoS One 2015; 10: e0121482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Karsanji DJ, Pannu N, Manns BJ. et al. Disparity between nephrologists’ opinions and contemporary practices for community follow-up after AKI hospitalization. Clin J Am Soc Nephrol 2017; 12: 1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Silver SA, Siew ED.. Follow-up care in acute kidney injury: lost in transition. Adv Chronic Kidney Dis 2017; 24: 246–252 [DOI] [PubMed] [Google Scholar]
- 130. Saran R, Li Y, Robinson B. et al. US renal data system 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2016; 67: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Harel Z, Wald R, Bargman JM. et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 2013; 83: 901–908 [DOI] [PubMed] [Google Scholar]
- 132. Moore PK, Hsu RK, Liu KD.. Management of acute kidney injury: core curriculum 2018. Am J Kidney Dis 2018; 72: 136–1418 [DOI] [PubMed] [Google Scholar]
- 133. Kashani K, Rosner MH, Haase M. et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol 2019; 14: 941–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hodgson LE, Selby N, Huang TM. et al. The role of risk prediction models in prevention and management of AKI. Semin Nephrol 2019; 39: 421–430 [DOI] [PubMed] [Google Scholar]
- 135. Chen TK, Knicely DH, Grams ME.. Chronic kidney disease diagnosis and management. JAMA 2019; 322: 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vanmassenhove J, Vanholder R, Lameire N.. Points of concern in post acute kidney injury management. Nephron 2018; 138: 92–103 [DOI] [PubMed] [Google Scholar]
- 137. Brar S, Ye F, James MT. et al. for the Interdisciplinary Chronic Disease Collaboration. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 2018; 178: 1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gayat E, Hollinger A, Cariou A. et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med 2018; 44: 598–605 [DOI] [PubMed] [Google Scholar]
- 139. Hsu C, Liu KD, Yang J. et al. Renin-angiotensin system blockade after acute kidney injury (AKI) and risk of recurrent AKI. Clin J Am Soc Nephrol 2020; 15: 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang X, Donnan PT, Bell S. et al. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol 2017; 18: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baker M, Perazella MA. NSAIDs in CKD: are they safe? Am J Kidney Dis. 2020 doi: 10.1053/j.ajkd.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 142. Hung AM, Siew ED, Wilson OD. et al. Risk of hypoglycemia following hospital discharge in patients with diabetes and acute kidney injury. Dia Care 2018; 41: 503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 144. Sawhney S, Beaulieu M, Black C. et al. Predicting kidney failure risk after acute kidney injury among people receiving nephrology clinic care. Nephrol Dial Transplant 2020; 35: 836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Coca SG, Garg AX, Thiessen-Philbrook H. et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 2014; 25: 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. ClinicalTrials.gov. Nephrologist follow-up versus usual care after an acute kidney injury hospitalization (FUSION). NCT02483039. [DOI] [PMC free article] [PubMed]
- 147. ClinicalTrials.gov. Improving post discharge care after acute kidney injury (AFTER AKI). NCT02915575.