Abstract
Iron deficiency is a frequent comorbidity of cardiovascular (CV) diseases and nearly 50% of patients with heart failure (HF) with or without anaemia have low levels of available iron. There is a strong association between anaemia and the increase in mortality and hospitalizations in patients with CV disease and HF. Moreover, anaemia and chronic kidney disease (CKD) often coexist in patients with HF, with anaemia increasing the risk of death in these subjects and with a further increased risk in CKD population. The evidence that the treatment of iron deficiency and the increase in haemoglobin are associated with a better prognosis in HF patients has elicited new interest in the utilization of iron in HF and CKD patients. One of the central players in CV disease is vascular calcification (VC), which has been recognized as a major independent risk factor for incident CV disease and overall mortality in chronic disease patients. In this review, we summarize the evidences generated by clinical trials aimed to study the effect of iron deficiency correction, the effect of iron-based phosphate binder in in vivo models of kidney failure and the effect of iron in in vitro models of VC, trying to give an overview of the present knowledge on iron effect and its mechanisms of action.
Keywords: chronic kidney disease, iron, vascular calcification
IRON DEFICIENCY AND CARDIOVASCULAR DISEASE
Anaemia and iron deficiency are common comorbidities in patients with cardiovascular (CV) disease and are often associated with poor clinical status and worse outcomes. In heart failure (HF), this comorbidity is regardless of gender, race and left ventricular hypertrophy [1]. In patients with CV disease and HF, iron deficiency can be absolute (reduced total body iron) or functional (normal or increased but inadequate total body iron). Clearly, iron replacement is appropriate in patients with anaemia resulting from absolute iron deficiency, but it has been unclear whether functional iron deficiency should be treated in non-anaemic patients with CV disease. Remarkable advances in better understanding the pathogenesis of HF have led to treatments with improved patient outcomes [2]. Although data from recent trials suggest that treating iron deficiency itself may be of benefit, significant knowledge gaps exist in the understanding of when, how and for how long it should be treated in HF and the mechanisms underlying the observed effects of treatment (Figure 1).
FIGURE 1.
Complex interplay between iron body balance, CV disease and CKD.
The prevalence of anaemia in patients with HF (haemoglobin <13 g/dL in men and <12 g/dL in women) [3] is ∼30–50%, compared with <10% in the general population [4]. Usually, anaemic patients with HF are older, with diabetes and chronic kidney disease (CKD). The severity of HF is associated with lower exercise capacity, worse health-related quality of life (QoL), oedema, lower blood pressure and increased dosage of diuretics [5].
Anaemia is strongly associated with increased mortality and hospitalizations in patients with CV disease and HF [6]. In addition, the increase in haemoglobin is associated with a better prognosis [7].
Although the role of iron deficiency in HF pathogenesis has been recently clarified, several investigators have been testing the safety and efficacy of intravenous iron in patients with HF and iron deficiency. The primary objectives of these studies were to investigate the safety and efficacy of intravenous iron on muscular exercise capacity, New York Heart Association (NYHA) class and QoL. Five studies (n = 103 patients) used intravenous iron sucrose and three studies (n = 504) used ferric carboxymaltose (FCM). Interestingly, the highest level of evidence for the safety and efficacy of intravenous iron therapy in patients with HF and iron deficiency was with FCM [8].
The first randomized study was a double-blind, placebo-controlled trial in 40 anaemic patients with HF [9]. Twenty control subjects received intravenous saline and 20 received 200 mg intravenous iron sucrose weekly for 5 weeks. After 6 months, haemoglobin increased by a mean of 1.4 g/dL (P < 0.01), and there was improvement in creatinine clearance, a decrease in C-reactive protein and N-terminal prohormone of brain natriuretic peptide, and an increase in left ventricular ejection fraction (LVEF) only in the intravenous iron group. Ferric Iron Sucrose in HF [10] was the first trial to use an inclusion criterion of iron deficiency, defined as ferritin <100 µg/L or 100–300 µg/L, with transferrin saturation (TSAT) <20%. Eighteen anaemic (haemoglobin, <12.5 g/dL) and 17 non-anaemic (haemoglobin, 12.5–14.5 g/dL) patients with iron deficiency were randomized to open-label, observer-blinded treatment with placebo or intravenous iron sucrose 200 mg/week for 4 weeks during the initial iron deficiency correction phase, and additional iron sucrose 200 mg/month, as required during the maintenance phase or to no treatment for the next 3 months. Iron treatment enhanced serum ferritin and improved NYHA class, but haemoglobin did not increase.
Ferinject Assessment in Patients with Iron Deficiency and Chronic HF is a large randomized study [11]. About 459 patients with HF and iron deficiency (ferritin <100 μg/L or 100–300 μg/L with TSAT < 20%), with anaemia (haemoglobin 9.5–12.0 g/dL) or without anaemia (haemoglobin 12.0–13.5 g/dL) were randomly assigned 2:1 to intravenous FCM (n = 304) or saline (n = 155). FCM increased ferritin levels in all patients with a modest increase in haemoglobin only in anaemic patients (0.9 g/dL; P < 0.001 versus controls), but not in those without anaemia (0.2 g/dL; P = 0.21). FCM improved NYHA class (P < 0.001). The beneficial effect of iron was similar in patients with and without baseline anaemia. However, there were no significant effects on all-cause mortality (3.4% versus 5.5%, FCM versus control) or rate of hospitalization (17.7% versus 24.8%). FCM was generally well tolerated, with minimal side effects.
The design of A Study to Compare the Use of Ferric Carboxymaltose with Placebo in Patients with Chronic HF and Iron Deficiency [12] was similar, with higher doses of FCM given for a longer duration (52 weeks). FCM therapy was also associated with a significant reduction in the risk of hospitalizations for worsening HF (hazard ratio = 0.39; 95% confidence interval 0.19–0.82; P = 0.009), without any difference in all-cause mortality.
Anaemia is a major consequence of CKD and they often coexist in patients with HF. In fact, anaemia increases the risk of death in patients with HF, with a further risk of death increased by 1.5-fold in CKD population [13].
Several mechanisms contribute to reduced outcomes in these patients: (i) reduced oxygen delivery to tissues with haemodynamic, neurohormonal and renal alterations [14]; (ii) increased myocardial workload, adverse left ventricular remodelling and left ventricular hypertrophy [15]; and (iii) CKD, cardiac cachexia-associated poor nutritional status and low albumin.
One of the most recent studies, the Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) study [16], is a multicentre, open-label trial with blinded end point evaluation, in randomly assigned adults undergoing maintenance haemodialysis (HD) to receive either high-dose intravenous iron sucrose (400 mg monthly, unless the ferritin concentration was >700 μg/L or the TSAT was ≥40%), or low-dose iv iron sucrose (0–400 mg monthly, with a ferritin concentration of <200 μg/L or a TSAT of <20% being a trigger for iron administration). The primary endpoint was the composite of nonfatal myocardial infarction, non-fatal stroke, hospitalization for HF or death. Among HD patients, a high-dose intravenous iron regimen was superior to a low-dose regimen and resulted in lower doses of erythropoiesis-stimulating agent being administered.
Since the prevalence of iron deficiency is high in CKD patients and increases with the severity of HF, in recent years, studies with intravenous iron agents in those patients with iron deficiency and HF showed new insights into the treatment with iron therapy.
VASCULAR CALCIFICATION IN CKD
Vascular calcification (VC) has been recognized as one of the main causes of arterial wall stiffness leading to significant mechanical changes that alter vessel extensibility inducing an increased pulse wave velocity and pressure. The structural changes induced by VC, in turn, led to hypertension, left ventricular hypertrophy and HF, accounting for arterial calcification as a major independent risk factor for incident CV disease and overall mortality in CKD patients [17].
VC has been defined as the pathological deposition of calcium crystals in the vasculature and, in CKD patients, several are the circulating factors responsible for VC, usually defined as the uraemic milieu [18]. Due to the impairment of the renal function, the uraemic milieu is characterized by the presence of a plethora of uraemic toxins and by high levels of phosphate (Pi), both inducers of VC. Therefore, uraemic patients have several circulating pro-calcifying factors that ultimately are responsible for the deposition of calcium and Pi in the tunica media of arteries. VC in uraemia is a complex multi-factorial process with a central role played by the trans-differentiation of vascular smooth muscle cells (VSMCs) and apoptosis. Indeed, VSMCs respond to the uraemic milieu with a transformation in simil-osteoblast that acquire the skills to deposit hydroxyapatite crystals in the extracellular matrix (ECM) and, due to the uraemic milieu toxicity, VSMCs could become apoptotic and act as a nidus for calcification initiation.
Among the VC inducers, a central role is played by high Pi load. Pi levels are maintained in the physiological range by the balance between intestinal absorption and renal Pi handling that in uremic patients, with the impairment of renal function, is altered with following high Pi circulating levels. At the cellular level, Pi uptake is controlled by the type III sodium-dependent Pi co-transporters, PiT-1 and PiT-2, the former a VC promoter in VSMCs and the latter that may act as an inhibitor in presence of high-Pi levels [19]. Interestingly, a Pi exporter has been discovered in metazoans, XPR1, a transmembrane protein initially identified as the cell-surface receptor for xenotropic and polytropic murine leukaemia retroviruses [20]. The mutations of the gene encoding this exporter have been involved in primary familial brain calcification, a complex neurodegenerative disorder which pathogenic mechanisms are still unclear but might be related to a compromised neurovascular physiology in these patients [21]. Whether there is an involvement of this Pi exporter and an eventual role in Pi cellular balance during VC in CKD might be investigated in the future. In response to Pi influx in the cell, VSMCs trans-differentiate and start to synthetize both the master genes of osteoblastic cascade RUNX2, MSX2, osterix and the chondrogenic transcription factors SOX9. Thus, VSMCs start to express several proteins, both VC inducers and inhibitors, typical of osteoblasts and chondrocytes such as BMP2, osteocalcin, osteopontin, osteonectin, ALP and MGP. The simil-osteoblastic transformation induces also VSMCs to lose smooth muscle cells specific markers, such as alpha-smooth muscle actin and smooth muscle protein 22-alpha, deeply changing their muscular phenotypes. The deposition of calcium-Pi crystals ultimately causes deep alteration in the mechanical fundamental proprieties of vessels, due to a modification in the balance between collagen IaI and elastin biosynthesis and degradation, with a deep alteration of the ECM elastic and muscular characteristics [22]. Recently, interesting studies have been focused on cell-to-cell communication during VC and especially on the role of exosomes [23] and microRNA (miRNA) [24]. Exosomes transport a cargo with proteins and RNAs that mediate cell-to-cell communication and, during VC, they have very few differences compared with matrix vesicles in terms of morphology, size and content. Interestingly, it has been demonstrated that exosomes, secreted by calcifying VSMCs, are able to promote calcium deposition in control VSMCs, supporting a crucial role for the transfer of information from cell to cell as a way of propagation of calcification [25]. Exosomes transport also miRNAs, which can affect osteogenic VSMCs trans-differentiation by different mechanisms, such as modulating anti-calcific proteins and muscular contractile markers or by targeting osteogenic transcription factors [26]. Modulating the miRNA composition of exosome cargo could be an interesting pharmacological approach to limit the propagation and development of calcification in the vasculature [22].
Due to the toxicity of the uraemic milieu, VSMCs can become apoptotic or necrotic and both processes are able to further trigger and induce VC. Apoptotic cells are thought to secrete apoptotic bodies that mimic the action of matrix vesicles in bone, probably the machinery that actively deposit hydroxyapatite crystals in the vasculature. Apoptotic bodies are loaded with high concentration of calcium that is deposited in the ECM thus inducing calcification. In high-Pi-induced apoptosis, a key role is played by the pro-survival pathway GAS6/Axl. In fact, Pi, inducing a downregulation of the GAS6/Axl pathway, causes an inactivation of Bcl2, with the following Bad and caspase-3 activation resulting in apoptosis [27]. Calcium has a central role in calcification, not only because it is deposited in ECM in hydroxyapatite crystals, but also for its intracellular role that can be pro-apoptotic. In fact, calcium–Pi crystals after entering into VSMC can be degraded by lysosomes, thus increasing intracellular calcium concentration and cause apoptosis [28]. Moreover, it has been demonstrated that Pi can increase store-operated Ca2+-entry by up-regulating the Ca2+ channel ORAI1-inducing osteoblastic marker expression [29]. Another apoptosis player in VC seems to be endoplasmic reticulum stress (ERS). In fact, in two different models of VC some ERS markers were up-regulated contextually to apoptosis in the vasculature and it has been demonstrated that there is a link between ATF4, an osteoblastogenesis transcription factor and ERS-induced apoptosis [30, 31]. Apoptosis is strictly linked to autophagy and can be considered as the dramatic consequence of the failure of autophagy to re-establish a positive balance for the cell evolving in survival. Autophagy in VC is activated as a response to high-Pi levels in models of calcification both in vitro in VSMCs and in vivo [32, 33]. Autophagy has a protective role in VC probably because it is a mechanism to degrade or excrete damaged structures induced by Pi toxicity as for example calcified mitochondria, found in autophagosomes and in the extracellular compartment [34]. Mitochondria are the principle source of oxidative stress in cells and high-Pi levels, targeting mitochondria, increase free radical release. One of the main effectors induced by Pi challenge is probably intracellular calcium increase that causes intracellular oxidative phosphorylation. The link between oxidative stress and VC has been proven finding that reactive oxygen species accumulation is able to induce RUNX2 expression sustaining the VSMCs osteoblastic trans-differentiation [35]. Pi causes an imbalance between antioxidants and reactive oxygen species in VSMCs and another evidence of oxidative stress involvement in VC is demonstrated by the effect of antioxidants that are protective in VSMCs calcification [36, 37].
IRON AND VC
Recently, two iron salts, iron citrate and sucroferric oxyhydroxide, can be used to manage hyperphosphataemia in CKD [38]. These salts are prescribed in clinical practice as Pi binders in the attempt to adequately control hyperphosphataemia in HD patients. Pi binders are active in chelating dietary Pi, thus ultimately limiting the quantity absorbed at intestinal level. One major advantage of the administration of iron based-Pi binders is that they are calcium-free. In fact, meta-analyses have shown a lower rate of VC and mortality in uraemic patients treated with non-calcium Pi binders compared with treatment with calcium-based ones [39–41]. Another relevant advantage to using the iron-based Pi-binder ferric citrate in CKD patients is its ability to simultaneously correct iron deficiency by enhancing availability of iron for erythropoiesis, thus improving anaemia. In vivo studies on animals show that an adequate control of hyperphosphataemia by iron salts together with the eventual anaemia management is able to improve cardiac and renal function. Ferric citrate added to the diet of 5/6 nephrectomized rats induced a reduction in serum Pi and an increase in serum iron and haemoglobin levels. These changes were associated with an improvement in renal function, in glomerulosclerosis and in tubulointerstitial lesions. The mechanisms involved in these positive effects of ferric citrate are a partial inhibition of the CKD-induced increase in oxidative stress, inflammation and fibrosis in the remnant kidney of CKD rats [42]. In a model of progressive CKD, the Col4a3 knockout mouse model, ferric citrate, besides reducing Pi intestinal absorption, increasing iron stores and correcting anaemia, was able to reduce fibroblast growth factor 23 (FGF23) levels. These effects preserved renal function, with a mitigated development of albuminuria and tubulointerstitial fibrosis, with also an effect on HF and an improved cardiac function. This combined effect on kidney and on heart resulted in a significant extended life span of Col4a3 knockout mouse [43].
In addition, a recent research showed the effect of sucroferric oxyhydroxide in the remnant kidney model finding a protective effect on renal injury due to Pi binding. Interestingly, the authors, despite an absence of ectopic calcification in the kidney, found a decrease in calciprotein particles (CPPs) [44]. CPPs are mineral binding proteins which sequester calcium Pi circulating nanoparticles by binding them, thus preventing the growth of the crystals. Their decrease might be linked with the improvement of kidney function since it has been demonstrated that their accumulation can induce apoptosis and inflammation. Moreover, being CPPs able to be uptaken by VSMCs to promote osteoblastic differentiation with following VC, their decrease might be a positive contributor in preventing calcification [45].
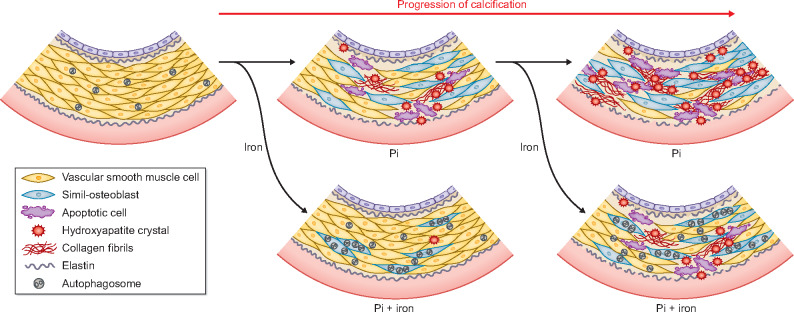
A more direct link between iron-based Pi binders and VC has been demonstrated for the first time in 2013 by Iida et al. [46]. They found that ferric citrate hydrate was effective in a model of chronic renal failure induced by adenine in rats. In fact, there was a reduction in calcium content in the aorta after 21 days of iron Pi binder dietary treatment, showing that beside reducing Pi absorption, ferric citrate hydrate could prevent secondary hyperparathyroidism, bone abnormalities and VC development [46]. In the same model, iron dextran suppressed the medial VC induced by the uraemic condition by affecting VSMC simil-osteoblastic differentiation, reducing RUNX2 and Pit-1 expression [47]. Other studies conducted with sucroferric oxyhydroxide (SFOH) showed its efficacy in decreasing VC in the adenine model of chronic renal failure. An interesting comparison between SFOH and a calcium-containing Pi binder (CaCO3) showed that the two treatments had similar effect in reducing circulating Pi and parathyroid hormone but PA21 had a superior efficacy in decreasing FGF23 and in preventing VC development [48]. It has been also demonstrated that PA21 was also as effective as lanthanum carbonate and sevelamer, two other non-calcium containing Pi binders, in controlling VC [49, 50]. The in vivo preclinical rat models of chronic kidney disease support an efficacy of iron-based Pi binders on VC linked to their ability to reduce diet Pi intestinal absorption. Data from pre-clinical and clinical studies show differences in iron absorption from sucroferric oxyhydroxyde and ferric citrate. Very recently, Floege et al. [51] compared iron uptake and tissue accumulation in an experimental model of rats treated with during treatment with SFOH or ferric citrate, demonstrating different properties and suggesting separate mechanisms of iron absorption for these two Pi binders. Nevertheless, ferric citrate increases iron plasmatic levels and the in vivo models do not allow to discriminate between a hypothetical direct effect of iron on vasculature and the systemic effect of controlling hyperphosphataemia. With the in vitro studies, it is possible to evaluate the direct effect of iron salts, independently from any Pi chelating action, on the mechanisms of calcium deposition in models of high-Pi-induced calcification in VSMCs. Some of these studies were conducted by our group. We showed that iron citrate was able to completely prevent the calcium–Pi crystal deposition in the ECM concentration dependently. This effect is to ascribe to the iron ion independently by citrate . The prevention of calcium deposition was associated to a modulating positive effect on some osteoblastic markers [Bone Morphogenic Protein-2 (BMP2) and Matrix Gla Protein (MGP)] and to an anti-apoptotic action, achieved by preventing the high-Pi-induced downregulation of the pro-survival pathway GAS6/AXL. Iron citrate was also able to induce autophagy, a protective mechanism in VC [52]. Another direct effect of iron citrate on VSMCs and vasculature was the modulation of the ECM osteo-chondrogenic shift. Iron prevents the shift in the nature of granules that maintain their glycogen nature, typical of muscular cells, and prevents the extracellular acid mucin deposition, typical of chondrocytes tissue. On aortic rings, iron citrate prevented the high-Pi-induced elastolysis and collagen deposition in ECM (Figure 2) [22]. The effect of iron in inhibiting calcification was also ascribed to ferritin induction and heavy chain ferroxidase activity [53], an effect supported also by the dose-dependent inhibition of osteoblastic transition by the ferritin inducer D3T [54, 55]. More interestingly, iron was able not only to prevent calcium deposition but also to completely block its progression when calcification was already established both in vitro and ex vivo. The mechanisms involved in the block of calcification are both an effect on apoptosis, autophagy and on the osteochondrogenic shift of ECM. But if the iron final action on calcification is the blocking of calcium crystal formation, the mechanisms targeted are not only inhibited but also in part reverted. Iron citrate added when calcification was already established was able to delay and block the progression of apoptosis, even if the process was already started and the apoptosis-inducing agent was present, and interestingly, this was achieved, at least in part, by reverting high-Pi downregulation of the pro-survival pathway GAS6/AXL with the induction of the proteins re-synthesis. There was also a reversion of autophagy downregulation achieved by iron addition, with a reactivation of the autophagic flux [56]. Regarding the ECM osteochondrogenic shift, iron was able to revert the production of calcifying granules, re-inducing the synthesis of glycogenic ones. Moreover, on aortic rings, there was a collagen fibrils reorganization in a structure similar to control samples and a reversion of elastic fibres fragmentation with the restoration of the elastic lamellae structure [22]. Therefore, iron targets some pathways of VC independently to the grade of high-Pi-induced simil-osteoblastic transformation of VSMCs, since it is effective both in prevention and when calcification was established (Figure 2).
FIGURE 2.
Schematic representation of the complex correlations between cellular biological phenomena during VC. Effect of iron is showed based on published data [22, 51, 55].
VSMCs are cells provided with some plasticity and share with osteoblasts the same mesenchymal origin. This plasticity was probably responsible for the delayed calcification obtained by suspending for a short period the pro-calcifying stimulus [34]. Supporting VSMCs plasticity in the calcification process, iron in vitro action demonstrates that if adequately targeted calcification mechanisms could be reverted and calcium deposition blocked. Increase in fibrosis and in arterial stiffness is relevant for CV complication, morbidity and mortality in CKD patients. These studies suggest that iron citrate might have several effects in uraemic patients: as dietary Pi binder, as therapy for iron deficiency and as an agent able to directly target VC mechanisms even if calcification is already established during hyperphosphataemia. In fact, in the in vitro and ex vivo studies, the iron effects were achieved at concentrations comparable to or lower than that obtained by an intravenous administration of iron [52, 57]. Furthermore, it would be interesting to investigate if at the same degree of Pi control, there is greater control of VC with ferric citrate and SFOH.
CONCLUSIONS AND PERSPECTIVES
A new clinical trial (EPISODE) comparing sucroferric oxyhydroxide versus lanthanum carbonate in dialysis patients is ongoing (Japan) to evaluate the effect of Pi control on VC as primary endpoint calculated as percent change in coronary artery calcification score, and the secondary endpoints will be change from baseline of serum Pi and calcium levels and variations in renal anaemia-related factors [58]. It will be interesting to investigate patients receiving iron and the effects on HF, to demonstrate the potential protective role of iron on the CV system of dialysis patients. Further investigation and new randomized controlled trials are necessary to confirm this fascinating hypothesis.
Finally, greater understanding needs to be gained of the effects on iron treatment on cardiac function and arterial stiffness, and the impact of iron deficiency on CV outcome of CKD patients.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this review have not been published previously in whole or part.
REFERENCES
- 1. Martens P, Nijst P, Verbrugge FH. et al. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol 2017; 73: 1–9 [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B. et al. 2017. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161 [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 4. Goodnough LT, Schrier SL.. Evaluation and management of anemia in the elderly. Am J Hematol 2014; 89: 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anand IS, Kuskowski MA, Rector TS. et al. Anemia and change in haemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from ValHeFT. Circulation 2005; 112: 1121–1127 [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Yang J, Ackerson LM. et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: outcomes and resource utilization (ANCHOR) study. Circulation 2006; 113: 2713–2723 [DOI] [PubMed] [Google Scholar]
- 7. Tang WH, Tong W, Jain A. et al. Evaluation and long-term prognosis of new-onset, transient, and persistent anemia in ambulatory patiens with chronic heart failure. J Am Coll Cardiol 2008; 51: 569–576 [DOI] [PubMed] [Google Scholar]
- 8. Anker SD, Kirwan BA, van Veldhuisen DJ. et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20: 125–133 [DOI] [PubMed] [Google Scholar]
- 9. Toblli JE, Lombrana A, Duarte P. et al. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665 [DOI] [PubMed] [Google Scholar]
- 10. Okonko DO, Grzeslo A, Witkowski T. et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008; 51: 103–112 [DOI] [PubMed] [Google Scholar]
- 11. Anker SD, Comin Colet J, Filippatos G. et al. ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448 [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, van Veldhuisen DJ, Comin-Colet J. et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herzog CA, Muster HA, Li S. et al. Impact of congestive heart failure, chronic kidney deisese, and anemia on survaival in the medicare population. J Card Fail 2004; 10: 467–472 [DOI] [PubMed] [Google Scholar]
- 14. Anand IS, Chandrashekhar Y, Wander GS. et al. Endothelium-derived relaxing factor is important in mediating the high outout state in chronic severe anemia. J Am Coll Cardiol 1995; 25: 1402–1407 [DOI] [PubMed] [Google Scholar]
- 15. Kao DP, Kreso E, Fonarow GC. et al. Characteristics and outcomes among heart failure patients with anemia and renal insufficiency with and without blood transfusions (public discharge data from California 2000–2006). Am J Cardiol 2011; 107: 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macdougall IC, White C, Anker SD. et al. Intravenous iron in patients undergoing maintenance hemidialysis. N Engl J Med 2019; 380: 447–458 [DOI] [PubMed] [Google Scholar]
- 17. Covic A, Vervloet M, Massy ZA. et al. Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and aging in the general population. Lancet Diabetes Endocrinol 2018; 6: 319–331 [DOI] [PubMed] [Google Scholar]
- 18. Demer LL, Tintut Y.. calcification: pathobiology of a multifaceted disease. Circulation 2008; 117: 2938–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larsen FT, Jensen N, Autzen JK. et al. Primary brain calcification causal PiT2 transport-knockout variants can exert dominant negative effects on wild-type PiT2 transport function in mammalian cells. J Mol Neurosci 2017; 61: 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giovannini D, Touhami J, Charnet P. et al. Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep 2013; 3: 1866–1873 [DOI] [PubMed] [Google Scholar]
- 21. Westenberger A, Balck A, Klein C.. Primary familial brain calcifications: genetic and clinical update. Curr Opin Neurol 2019; 32: 571–578 [DOI] [PubMed] [Google Scholar]
- 22. Ciceri P, Falleni M, Tosi D. et al. High-phosphate induced vascular calcification is reduced by iron citrate through inhibition of extracellular matrix osteo-chondrogenic shift in VSMCs. Int J Cardiol 2019; 297: 94–103 [DOI] [PubMed] [Google Scholar]
- 23. Kapustin AN, Chatrou MLL, Drozdov I. et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res 2015; 116: 1312–1323 [DOI] [PubMed] [Google Scholar]
- 24. Boulanger CM, Loyer X, Rautou PE. et al. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017; 14: 259–272 [DOI] [PubMed] [Google Scholar]
- 25. Chen NX, O’Neill KD, Moe SM.. Matrix vesicles induce calcification of recipient vascular smooth muscle cells through multiple signaling pathways. Kidney Int 2018; 93: 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang C, Zhang K, Huang F. et al. Exosomes, the message transporters in vascular calcification. J Cell Mol Med 2018; 22: 4024–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu C, Zheng H, Tao H. et al. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol Cell Biochem 2017; 433: 149–159 [DOI] [PubMed] [Google Scholar]
- 28. Ewence AE, Bootman M, Roderick HL. et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res 2008; 103: e28–e34 [DOI] [PubMed] [Google Scholar]
- 29. Ma K, Liu P, Al-Maghout T. et al. Phosphate-induced ORAI1 expression and store-operated Ca(2+) entry in aortic smooth muscle cells. J Mol Med 2019; 97: 1465–1475 [DOI] [PubMed] [Google Scholar]
- 30. Duan X, Zhou Y, Teng X. et al. reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem Biophys Res Commun 2009; 387: 694–699 [DOI] [PubMed] [Google Scholar]
- 31. Duan XH, Chang JR, Zhang J. et al. Activating transcription factor 4 is involved in endoplasmic reticulum stress-mediated apoptosis contributing to vascular calcification. Apoptosis 2013; 18: 1132–1144 [DOI] [PubMed] [Google Scholar]
- 32. Dai XY, Zhao MM, Cai Y. et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int 2013; 83: 1042–1051 [DOI] [PubMed] [Google Scholar]
- 33. Frauscher B, Kirsch AH, Schabhüttl C. et al. Autophagy protects from uremic vascular media calcification. Front Immunol 2018; 9: 1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ciceri P, Elli F, Cappelletti L. et al. A new in vitro model to delay high phosphate-induced vascular calcification progression. Mol Cell Biochem 2015; 410: 197–206 [DOI] [PubMed] [Google Scholar]
- 35. Byon CH, Javed A, Dai Q. et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 2008; 283: 15319–15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicoll R, Howard JM, Henein MY.. A review of the effect of diet on cardiovascular calcification. Int J Mol Sci 2015; 16: 8861–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada S, Taniguchi M, Tokumoto M. et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res 2012; 27: 474–485 [DOI] [PubMed] [Google Scholar]
- 38. Negri AL, Ureña Torres PA.. Iron-based phosphate binders: do they offer advantages over currently available phosphate binders? Clin Kidney J 2015; 8: 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jamal SA, Vandermeer B, Raggi P. et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet 2013; 382: 1268–1277 [DOI] [PubMed] [Google Scholar]
- 40. Liu L, Wang Y, Chen H. et al. The effects of non-calcium-based phosphate binders versus calcium-based phosphate binders on cardiovascular calcification and bone remodeling among dialysis patients: a meta-analysis of randomized trials. Ren Fail 2014; 36: 1244–1252 [DOI] [PubMed] [Google Scholar]
- 41. Block GA, Block MS, Smits G. et al. Pilot randomized trial of ferric citrate coordination complex for the treatment of advanced CKD. J Am Soc Nephrol 2019; 30: 1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jing W, Nunes ACF, Farzaneh T. et al. Phosphate binder, ferric citrate, attenuates anemia, renal dysfunction, oxidative stress, inflammation, and fibrosis in 5/6 nephrectomized CKD rats. J Pharmacol Exp Ther 2018; 367: 129–137 [DOI] [PubMed] [Google Scholar]
- 43. Francis C, Courbon G, Gerber C. et al. Ferric citrate reduces fibroblast growth factor 23 levels and improves renal and cardiac function in a mouse model of chronic kidney disease. Kidney Int 2019; 96: 1346–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nemoto Y, Kumagai T, Ishizawa K. et al. Phosphate binding by sucroferric oxyhydroxide ameliorates renal injury in the remnant kidney model. Sci Rep 2019; 9: 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Viegas CSB, Santos L, Macedo AL. et al. Chronic kidney disease circulating calciprotein particles and extracellular vesicles promote vascular calcification: a role for GRP (gla-rich protein). Arterioscler Thromb Vasc Biol 2018; 38: 575–587 [DOI] [PubMed] [Google Scholar]
- 46. Iida A, Kemmochi Y, Kakimoto K. et al. Ferric citrate hydrate, a new phosphate binder, prevents the complications of secondary hyperparathyroidism and vascular calcification. Am J Nephrol 2013; 37: 346–358 [DOI] [PubMed] [Google Scholar]
- 47. Seto T, Hamada C, Tomino Y.. Suppressive effects of iron overloading on vascular calcification in uremic rats. J Nephrol 2014; 27: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phan O, Maillard M, Peregaux C. et al. PA21, a new iron-based noncalcium phosphate binder, prevents vascular calcification in chronic renal failure rats. J Pharmacol Exp Ther 2013; 346: 281–289 [DOI] [PubMed] [Google Scholar]
- 49. Phan O, Maillard M, Malluche HH. et al. Effects of sucroferric oxyhydroxide compared to lanthanum carbonate and sevelamer carbonate on phosphate homeostasis and vascular calcifications in a rat model of chronic kidney failure. Biomed Res Int 2015; 2015: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cozzolino M, Funk F, Rakov V. et al. Preclinical pharmacokinetics, pharmacodynamics and safety of sucroferric oxyhydroxide. Curr Drug Metab 2014; 158109: 935–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Floege J, Funk F, Ketteler M. et al. Iron kinetics following treatment with sucroferric oxyhydroxide or ferric citrate in healthy rats and models of anaemia, iron overload or inflammation. Nephrol Dial Transplant 2020; 35: 946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ciceri P, Elli F, Braidotti P. et al. Iron citrate reduces high phosphate-induced vascular calcification by inhibiting apoptosis. Atherosclerosis 2016; 254: 93–101 [DOI] [PubMed] [Google Scholar]
- 53. Zarjou A, Jeney V, Arosio P. et al. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J Am Soc Nephrol 2009; 20: 1254–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Becs G, Zarjou A, Agarwal A. et al. Pharmacological induction of ferritin prevents osteoblastic transformation of smooth muscle cells. J Cell Mol Med 2016; 20: 217–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sikura KÉ, Potor L, Szerafin T. et al. Potential role of H-ferritin in mitigating valvular mineralization. Arterioscler Thromb Vasc Biol 2019; 39: 413–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ciceri P, Falleni M, Tosi D. et al. Therapeutic effect of iron citrate in blocking calcium deposition in high pi-calcified VSMC: role of autophagy and apoptosis. Int J Mol Sci 2019; 20: 5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Geisser P, Burckhardt S.. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011; 3: 12–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Isaka Y, Fujii H, Tsujimoto Y. et al. Rationale, design, and characteristics os a trial to evaluate the new phosphate iron-based binder sucroferric oxyhydroxide in dialysis patients with the goal of advancing the practice of E.B.M. (EPISODE). Clin Exp Nephrol 2018; 22: 967–972 [DOI] [PubMed] [Google Scholar]