Abstract
What is known and objectives
Coronary artery disease (CAD) is the leading cause of death in the United States. For patients on whom guideline‐driven measures have been tried, enhanced external counterpulsation (EECP) is the only truly noninvasive and safe intervention for which a reduction of angina symptoms and nitrate use, increased exercise tolerance, and improvement in myocardial ischaemia have been shown. The objective of this study was to demonstrate, by way of literature review, the efficacy of EECP as a treatment modality for the relief of refractory angina and improvement in quality of life in CAD patients.
Methods
This article reviewed the safety and efficacy of EECP in patients with refractory angina, by conducting a sweeping search and analysis of existing published literature.
Results and Discussion
Critical review of a multitude of studies revealed that EECP consistently reduces angina pectoris, extends time to exercise‐induced ischaemia, decreases dependency on nitroglycerine for frequent chest pain, increases maximum workload, and improves the quality of life in patients with symptomatic stable angina. The literature reviewed also indicated that EECP is well‐tolerated by the vast majority of patients, with relatively few adverse events reported.
Conclusion
The present study suggests that EECP is a safe and likely best available method of treatment for patients presenting with symptomatic CAD not amenable to further revascularization.
Keywords: angina pectoris, coronary artery disease, EECP
A growing data pool suggests that EECP is safe, noninvasive, unique, and cost‐effective treatment strategy, providing relief of angina and improving the quality of life in the majority of patients treated.
1. WHAT IS KNOWN AND OBJECTIVES
Coronary artery disease (CAD) is the leading cause of death in the United States. 1 A debilitating indication of its presence affecting the quality of life of millions of Americans is “angina pectoris.” Patients who present with this symptom are typically offered optimal medical therapy and revascularization procedures, such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) surgery, to alleviate angina symptoms and to improve survival. 2 With the decline of mortality and increase of life expectancy from these measures, an increasing number of patients consequently develop advanced CAD with recurrent angina pectoris not amenable to revascularization. In the United States alone, more than 2.5 million patients with symptomatic coronary artery disease are not amenable to such interventions because of unsuitable coronary anatomy, multiple previous revascularization attempts, age, additional comorbid conditions or patient preference. 3 , 4 Even among patients who were given intervention, about one third had significant lifestyle limitations and were not able to return to work after a year. 5 , 6 This certain growing population who are not suitable for revascularization procedures, with failure to reduce anginal pain despite aggressive and/or tolerable guideline‐directed medical therapy (GDMT) and persistence of angina despite revascularization, are classified as patients with refractory angina pectoris (RAP).
The prevalence of refractory angina pectoris in the United States has been estimated to be 1.8 million, with an incidence of approximately 50,000 to 100,000 each year. 7 , 8 , 9 Although early mortality following diagnosis was previously assumed, a recent study of 1,200 patients from a dedicated refractory angina clinic reported a low annual mortality rate of 3–4 per cent. 10 A major focus of care for this population has therefore become quality of life in the face of debilitating symptoms and increased healthcare utilization. The treatment of patients with RAP is further complicated by the presence of comorbid conditions that overlap with myocardial perfusion abnormalities and coronary anatomy, leading to more elaborate and complex challenging situations, which require an individualized treatment approach.
This review summarizes the current evidence for the use of enhanced external counterpulsation therapy in patients with refractory angina pectoris.
2. DEFINITION
Enhanced external counterpulsation (EECP) is an outpatient nonpharmacologic and noninvasive therapy, involving the use of electrocardiogram‐synchronized inflatable cuffs wrapped around the lower extremities to produce cyclic inflation and deflation. 11 This technique increases venous return and augments diastolic blood pressure, 12 , 13 producing an acute hemodynamic effect that is presumed to be similar to that produced by the invasive intra‐aortic balloon pump. 14 , 15 It also causes shear stress on the vascular endothelium, 11 allowing promotion of angiogenesis and vasculogenesis, and a peripheral training effect. 2
Enhanced external counterpulsation was approved by the US Food and Drug Administration in 1995 and is currently considered an alternative therapy for relief of symptoms in patients with refractory angina who fail to respond to standard revascularization procedures and aggressive pharmacotherapy, with a class IIb recommendation from the American College of Cardiology and the American Heart Association. 2
3. EQUIPMENT AND TECHNIQUE
The EECP system consists of a compressor, a console, a treatment table and three sets of cuffs. Prior to treatment, the cuffs are wrapped around the patient's calves and the lower and upper thighs, including the buttocks. The inflation and deflation of pneumatic cuffs are timed to the patient's electrocardiogram, while the arterial pressure waveform is monitored by finger plethysmography. A microprocessor‐controlled system detects an R‐wave, calculates the heart rate and delivers triggering signals to engage the valves. This allows the air cuffs to be filled and then emptied. The system must detect three consecutive R‐waves before it can predict the next R‐wave. Once this pattern is established, the system begins the inflation and deflation sequence. This ensures that inflation of the cuffs occurs only during ventricular diastole.
The inflation cycle begins at the start of diastole, not earlier than 100 milliseconds (ms) after the R‐wave. The cuffs begin to inflate sequentially at 50 ms intervals from calves to thighs to buttocks, applying 4–6 pounds per square inch (psi) of pressure at 206 to 310 mm Hg. In the venous system, the compression of the vascular beds augments venous return, delivering a larger volume of blood to the heart, which consequently increases cardiac preload. In the arterial system, the vascular compression creates a rise in peak arterial diastolic pressure—“diastolic augmentation.” As a result, there is an increase in coronary blood flow and coronary perfusion pressure (see Figures 1 and 2).
FIGURE 1.
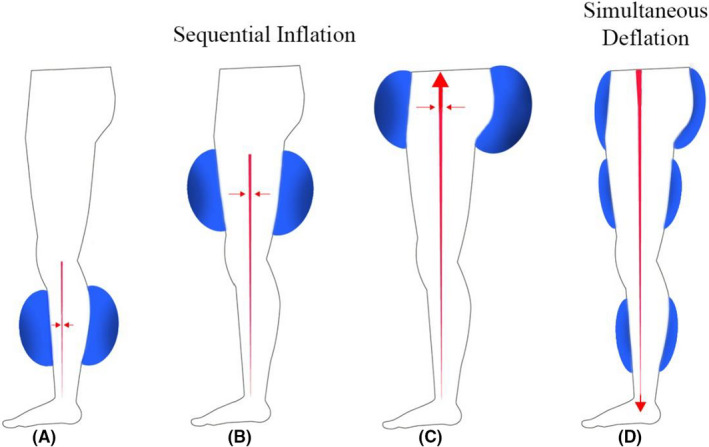
Inflation of the pneumatic cuffs, at the start of the diastole, initiates a retrograde pulse wave at the calves (A). Fifty milliseconds (ms) later, the cuffs at the thighs are inflated (B), followed by inflation of the buttocks 50 ms later (C). Simultaneous deflation begins at the end of diastole, for approximately 150 ms, which facilitates systolic unloading (D)
FIGURE 2.
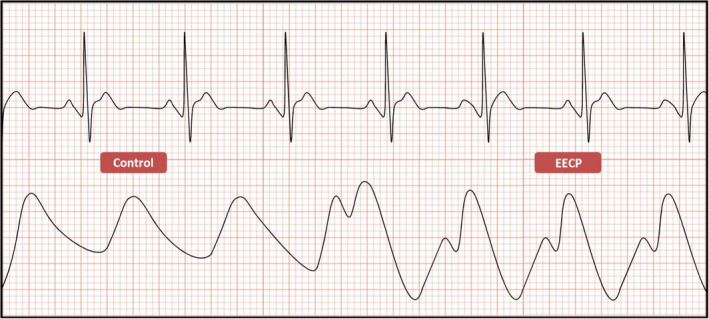
The first three cardiac cycles depict a normal finger plethysmographic recording in the absence of counterpulsation (control). In the presence of counterpulsation (EECP), as seen in the last three cycles, there is a decrease in systolic pressure—“systolic unloading” (first hump)—and a marked rise in peak arterial diastolic pressure—“diastolic augmentation” (second hump)—resulting in an increase of coronary blood flow and coronary perfusion pressure. EECP, Enhanced External Counterpulsation
The deflation begins at the end of diastole, approximately 150 ms prior to the next R‐wave. The cuffs simultaneously deflate for approximately 150 ms Since the vascular beds of the lower extremities were effectively compressed during cuff inflation, the rapid cuff deflation promotes lower extremity arterial “runoff” and leads to a decrease in systolic pressure—“systolic unloading”—in the aorta and the coronary arteries. The effect reduces cardiac afterload, thereby decreasing myocardial oxygen demand (see Figures 1 and 2).
The standard EECP treatment protocol consists of daily 1‐h sessions, five days a week for seven consecutive weeks, for a total of 35 treatment sessions. A modified treatment protocol consists of daily 2‐h sessions, 6 days a week for three consecutive weeks. EECP is generally effective, safe and well‐tolerated. 14 , 15 , 16
4. MECHANISM
Although the pathophysiological mechanisms underlying the effects of EECP have not been completely elucidated, central and peripheral effects, as well as hemodynamic outcomes, have been implicated.
4.1. Central mechanism
The central hemodynamic effects of EECP have been theorized to simulate the clinical use of the intra‐aortic balloon pump, thereby enhancing cardiac output, stroke volume and retrograde aortic diastolic flow. The cardiac output and stroke volume are best evaluated objectively through changes in ejection fraction (%), exemplified by the clinical studies done in patients with symptomatic CAD (see Table 1).
TABLE 1.
Effect of EECP on ejection fraction (EF)
| Study | Design | Sample size |
Pre‐EECP EF (%) |
Post‐EECP EF (%) |
p‐value | Data collection time points |
|---|---|---|---|---|---|---|
| Sardari et al, 2018 16 | Cross‐sectional study | 34 | 42.65 ± 11.82 | 44.26 ± 11.86 | <0.001 | Within 1 week before and after 35 sessions |
| Subramanian et al, 2016 11 | Open‐label study with 2 subgroups: (a) systolic > 100 and (b) <100 mm Hg | 72 |
(a) 46.40 ± 15.88 (b) 33.93 ± 12.39 |
(a) 50.05 ± 13.20 (b) 45.71 ± 13.43 |
<0.001 | Within 1 week before and after 35 sessions |
Abbreviation: EECP, Enhanced External Counterpulsation.
Enhanced external counterpulsation produces a diastolic retrograde aortic flow that enhances coronary artery mean pressure and peak diastolic pressure. This retrograde aortic flow acts to improve coronary vasodilation by directly vasodilating existent myocardial vessels. The aortic pressure gradient is also believed to stimulate recruitment of collateral vessels, and studies have demonstrated improved microcirculation in areas of myocardial infarction, increasing capillary density in infarcted regions by 30%. 17 , 18 In a meta‐analysis done by Qin et al (2016), 19 the following studies significantly increased myocardial perfusion in CAD patients after a standard EECP therapy (p < 0.01) (see Table 2).
TABLE 2.
Effect of EECP on myocardial perfusion
| Study | Design | Sample size | Outcome measure | Data collection time points |
|---|---|---|---|---|
| Tartaglia et al, 2003 17 | Prospective, observational study | 25 | Single‐photon emission computed tomography (SPECT) | Immediately before and after EECP |
| Michaels et al, 2002 34 | Prospective, randomized, blinded study | 10 | Doppler velocity guidewire positioned in the mid‐to‐distal portion of an unobstructed coronary artery under fluoroscopic guidance | Immediately before and during EECP |
| Masuda et al, 2001 35 | Prospective, observational blinded study | 11 | Dynamic 13N positron emission tomography (PET) | Immediately before and after EECP |
Abbreviation: EECP, Enhanced External Counterpulsation.
4.2. Peripheral mechanism
Enhanced external counterpulsation has been postulated to improve endothelial function by increased shear stress, which promotes the release of angiogenic and vasoactive factors, such as nitric oxide, prostacyclin, α‐actin, von Willebrand factor (vWF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (BFGF) and hepatocyte growth factor (HGF). 13 , 19 , 20 The elaboration of these substances from the arterial bed improves endothelial function and vascular remodelling. In addition, nitric oxide plays an anti‐inflammatory and anti‐atherosclerotic role, by inhibiting and reducing the expression of monocyte chemoattractant protein‐1 (MCP‐1) and vascular cell adhesion molecule‐1 (VCAM‐1), respectively. 21 , 22 The effects of EECP on cytokines and inflammatory biomarkers in patients with CAD and RAP are summarized in Table 3
TABLE 3.
Effect of EECP on Biomarkers of Inflammation
| Biomarkers | Effect of EECP | Study | Sample size | Pre‐EECP | Post‐EECP | p‐value |
|---|---|---|---|---|---|---|
| Anti‐inflammatory biomarkers | ||||||
| Nitric oxide | Increase | Braith et al 2010 3 | 42 | 22.8 ± 9.8 | 31.9 ± 11.1 | <0.01 |
| Nitric Oxide | Increase | Akhtar et al 2006 13 | 13 | 27.1 ± 2.6 | 43.6 ± 4.3 | <0.0001 |
| Pro‐inflammatory biomarkers | ||||||
| Endothelin‐1 | Decrease | Braith et al 2010 3 | 42 | 2.2 ± 0.8 pg/mL | 1.5 ± 1.3 pg/mL | <0.05 |
| Endothelin‐1 | Decrease | Akhtar et al 2006 13 | 13 | 119.5 ± 8.5 pg/L | 76.0.7 ± 9.5 pg/L | <0.0001 |
| Tumour necrosis factor‐a | Decrease | Casey et al 2008 21 | 21 | 6.9 ± 2.7 pg/mL | 4.9 ± 2.5 pg/mL | <0.01 |
| Monocyte chemoattractant protein‐1 | Decrease | Casey et al 2008 21 | 21 | 255 ± 56 pg/mL | 190 ± 48 pg/mL | <0.01 |
Abbreviation: EECP, Enhanced External Counterpulsation
With the increase in plasma nitric oxide (NO), a potent vasodilator, and a decrease in plasma endothelin‐1 (ET‐1) concentration and a potent vasoconstrictor, EECP is able to provide diastolic augmentation, increase coronary perfusion pressure and decrease peripheral resistance. The therapy is able to decrease the stiffness of both elastic and muscular arteries 11 and significantly improve brachial and femoral artery flow‐mediated dilation. 3 Consequently, a decrease in the resting blood pressure after EECP therapy has been observed (see Table 4).
TABLE 4.
Systolic blood pressure (mm Hg)
| Study | Design | Sample size | Pre‐EECP | Post‐EECP | p‐value | Data collection time points |
|---|---|---|---|---|---|---|
| Subramanian et al, 2016 11 | Open‐label study | 72 | 121.10 ± 11.84 | 114.66 ± 13.63 | 0.001 | Before and after 36 sessions |
| Briath et al, 2010 3 | Sham‐controlled randomized trial | 42 | 135 ± 20.5 | 127 ± 17.4 | 0.004 | Before and after 35 sessions |
| Michaels et al, 2002 34 | Prospective, randomized, blinded study | 10 | 116 ± 20 | 99 ± 26 | 0.002 | At baseline and during EECP therapy |
In brief, it may be postulated that EECP acts by a concert of different contributing mechanisms, both central and peripheral (see Figure 3). Centrally, the diastolic inflation leads to two major outcomes: augmenting the diastole to increase the coronary perfusion and increasing the venous return to improve the cardiac output. Systolic deflation synergistically improves the cardiac output by systolic unloading, leading to decreased workload of the heart. Peripherally, the cyclic inflation and deflation of EECP lead to increased shear stress on the endothelium, triggering the release and regulation of endothelial‐derived substances affecting vasoactivity, angiogenesis and inflammation as follows: (a) The interplay of vasoactive substances leads to an overall vasodilation and decrease in arterial stiffness, eventually leading to a decrease in resting blood pressure; (b) The release of certain angiogenic and growth factors, such as VEGF, BFGF and HGF eventually promote angiogenesis and improve microcirculation; and (c) The increase in anti‐inflammatory cytokines along with the decrease in pro‐inflammatory cytokines consequently leads to an overall decrease in inflammation. These effects from the production and regulation of endothelial‐derived substances help explain the long‐term sustained benefits of EECP even after discontinuation of treatment.
FIGURE 3.
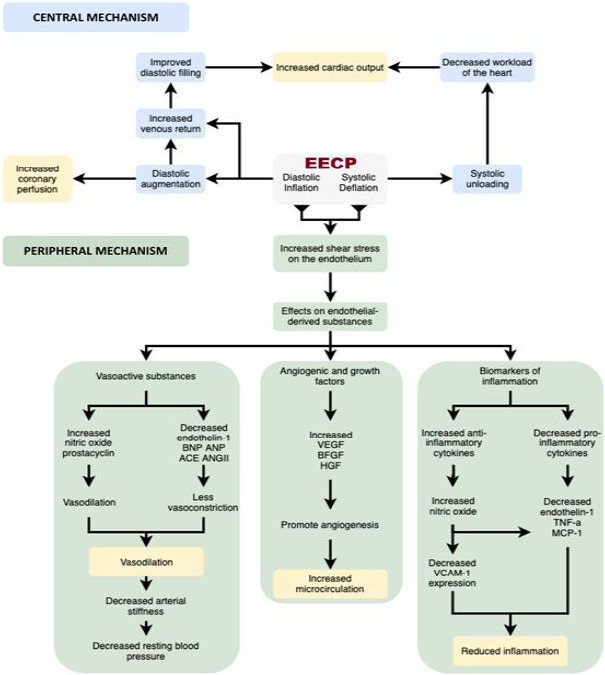
Central and peripheral mechanisms of EECP. VEGF‐Vascular endothelial growth factor; BFGF‐Basic fibroblast growth factor; HGF‐Hepatocyte growth factor; TNF‐a‐Tumour necrosis factor‐alpha; MCP‐1‐Monocyte chemoattractant protein‐1; VCAM‐1‐Vascular cell adhesion molecule‐1; BNP‐Brain natriuretic peptide; ANP‐Atrial natriuretic peptide; ACE‐Angiotensin‐converting enzyme; ANG II‐Angiotensin II; EECP, Enhanced External Counterpulsation
5. RESULTS
Multiple studies have demonstrated that EECP reduces angina pectoris, extends time to exercise‐induced ischaemia, decreases dependency on nitroglycerine for frequent chest pain, increases maximum workload and improves the quality of life in patients with symptomatic stable angina. 11 , 16 Its efficacy has been evaluated in several randomized controlled trials and observational registry studies.
The Multicenter Study of Enhanced External Counterpulsation (MUST‐EECP) was the first randomized, blinded, controlled trial conducted in seven university hospitals of the United States to assess the efficacy and safety of the therapy. One hundred thirty‐nine patients with angina, documented CAD and evidence of ischaemia on exercise testing were randomized to 35 h of either active or inactive counterpulsation. 20 Results showed a percentage reduction in the frequency of anginal episodes and a significant increase in the time to ST segment depression on stress testing (337 ± 18–379 ± 18 s, p < 0.05). An additional prospective, randomized sham‐controlled study conducted in 42 symptomatic CAD patients demonstrated an improvement in the Canadian Cardiovascular Society (CCS) angina class (3.16 ± 0.47–1.20 ± 0.40, p < 0.05) and a decrease in anginal episodes (1.8 ± 1.47–0.5 ± 0.70 times per day, p < 0.05) after 7 weeks of EECP. 3 A meta‐analysis of 13 observational studies also showed the impact of EECP on CCS angina class of 949 patients with chronic stable ischaemic heart disease. Even with a high degree of heterogeneity among the studies, results showed that angina class was reduced by at least one CCS score in 86% of patients (CI 82%‐90%, Qstat p = 0.008) with no publication bias (p = 0.97). 5
In patients taking nitroglycerine for recurrent and refractory angina, a significant decrease in nitroglycerine usage has also been noted: 1.1 ± 1.44 to 0.2 ± 0.41 times per day, p < 0.05 3 ; and 7 (2–16) times to 0 (0–2) times per day, p < 0.05. 23
After the first randomized trial (MUST‐EECP) showed a significant increase in the time to ST segment depression on stress testing (337 ± 18–379 ± 18 s, p < 0.05), 20 other studies focused on the effect of EECP on this outcome measure proved to be statistically significant (266 ± 106–320 ± 95 s, p < 0.05 24 and 229.1–315.8 s, p < 0.05 17 ).
Enhanced external counterpulsation therapy has also been shown to increase the exercise tolerance of patients with RAP. Thirty‐four patients in a recent study in 2018 showed marked improvement after a standard EECP protocol (6.48 ± 2.76–9.20 ± 2.71 min, p < 0.05). 16 This is supported by several other publications since 2001, which showed statistical and clinical improvement in exercise tolerance: 6.61 ± 1.88–7.41 ± 2.03 min, p < 0.05, 25 9.1 ± 3.7–10.2 ± 3.6 min, p < 0.05 26 and 5.7 ± 1.9–6.9 ± 2.1 min, p < 0.05. 27 Clinically, these outcome measures correlate well with decreasing anginal episodes, increasing anginal threshold and increasing tolerance to physical exertion.
With the primary goal of alleviating anginal symptoms and improving quality of life, the long‐term beneficial effects of EECP have also been elucidated 20 , 24 :
Akhtar et al found that nitric oxide remained significantly above baseline (+12%) and endothelin‐1 remained significantly below baseline (−11%) at 3 months after completion of EECP therapy in CAD patients. 3 This strengthens the effect of EECP on decreasing the inflammatory process and arterial stiffness of the blood vessels for a prolonged period of time.
Moreover, a follow‐up analysis of patients in the MUST‐EECP trial at 1 year showed greater improvement in the health‐related quality of life measures (performing activities of daily living, ability to work, bodily pain, confidence in health, energy, ability to engage in social activities with family and friends, anxiety and depression, and quality of life issues from the effects of angina on health and functioning) in the active treatment group. Objectively, an improvement of the CCS angina class by at least 2 classes at 1‐year follow‐up was demonstrated using univariate and multivariate analyses (OR 3.9; CI 1.2–12.7, p < 0.05). 23
At 2‐year follow‐up, 70% of class III‐IV patients continued to demonstrate a durable CCS class improvement over baseline and remained free of major adverse cardiovascular events (MACE). 28 One thousand and ninety‐seven patients from the International EECP Patient Registry also showed a significant improvement in quality of life parameters after 2 years. 29 Approximately 41% of patients also remained event‐free during this 2‐year period, and nitroglycerine use remained significantly decreased compared with baseline. 26 , 29
The benefits have also been sustained from 3–5 years in approximately 75% of patients who were responders to EECP treatment. 8 , 15 In addition, the majority of patients (64%) with CAD remained alive without MACE and without the need for revascularization 5 years after EECP treatment, indicating that EECP treatment may be an effective long‐term therapy for patients with coronary artery disease and refractory angina pectoris. 24 , 30
6. INDICATIONS
At present, EECP is indicated in patients with symptomatic coronary artery disease (CCS angina class III and IV), 15 , 31 guideline‐directed medical therapy (GDMT) and/or coronary revascularization, who are inoperable or at high risk for operative complications or postoperative failure, who are poor candidates for PCI or CABG, or continue to have symptoms despite PCI or CABG surgery.
The American Heart Association and American College of Cardiology recommend it as a Class IIb (Level of Evidence: B) intervention for treatment of refractory angina pectoris, among other nonpharmacologic approaches, such as neurostimulation and transmyocardial laser revascularization. 2
A repeat course of EECP is recommended to patients with RAP, as above, when the prior EECP has resulted in a sustained improvement in symptoms with a greater than 25% reduction of anginal symptoms, or improvement by 1 or more anginal classes, and three or more months have elapsed from the prior EECP treatment. 32
7. SAFETY
Multiple studies have shown that EECP is safe and well tolerated by most patients. Leg discomfort was the most frequent device‐related adverse effect from the MUST‐EECP RCT done in 1999. Other reported adverse events included leg back pain, skin abrasions, bruising, blistering, oedema and paresthesias. 20 Throughout the years, the numbers of adverse events have declined as elastic pants and padding have come into use. Some patients develop minor skin eruptions (eg contact dermatitis) which resolve with a topical steroid cream. 24 Other non‐device‐related adverse events are minor and generally tolerated. 15
8. MAIN CONTRAINDICATIONS
The following are contraindications to the use of EECP 20 :
Acute decompensated heart failure
Recent myocardial infarction within the last 3 months 27
Unstable angina pectoris 27
Severe hypertension > 180/110 mm Hg
Coagulopathy with international normalized ratio of prothrombin time > 2.0
Moderate to severe aortic regurgitation
Abdominal aortic aneurysm (>5 mm) or dissection
Arrhythmias that may interfere with triggering of EECP system (uncontrolled atrial fibrillation, flutter and very frequent premature ventricular contractions)
Heart rate of <35 or >125 beats per minute
Any surgical intervention within 6 weeks before EECP
Recent cardiac catheterization (1–2 weeks) or arterial femoral puncture
Severe peripheral arterial disease
Severe venous disease (thrombophlebitis, prior or current deep vein thrombosis or pulmonary embolism)
Severe chronic obstructive pulmonary disease
Pregnancy or women of childbearing age who do not have a negative pregnancy test
9. EECP AS AN APPLIED THERAPY
Enhanced external counterpulsation was implemented in the United States after the MUST‐EECP clinical trial was published in 1999; however, there remains a lack of hands‐on direct exposure to EECP during general adult cardiology fellowship training in the majority of programs. Therefore, many practicing cardiologists today are not familiar with this noninvasive therapy. As a result of this lack of exposure to EECP and the interrupted follow‐up of patients with angina pectoris and stable ischaemic heart disease, knowledge of the efficacy of EECP as a treatment modality for relief of symptoms and improved quality of life is limited.
The American College of Cardiology and the American Heart Association have recommended EECP for the relief of RAP patients with stable ischaemic heart disease, as class IIb, since 2002. 33 Nonetheless, most patients with angina have never heard of the treatment, as it has not entered the mainstream of cardiology practice, owing in part to the time required to achieve appreciable results, as compared to the immediate effects achieved with revascularization procedures, such as PCI.
The Centers for Medicare and Medicaid Services (CMS) began compensating for EECP therapy in 1999. Most private health insurances compensate for this therapy as well, following the same CMS guidelines as stated under “Indications.” Select third‐party payers consider EECP “experimental” and will refuse coverage, even when a letter of medical necessity is submitted.
As shown by Lawson WE and colleagues, 28 treatment of RAP with EECP results in improvement in angina, functional class and quality of life associated with a sustained reduction in healthcare cost over 1 year of follow‐up. EECP improved CCS angina class in patients with RAP and decreased hospitalization rates in the 6 months post‐treatment. 8 Full compensation for a 35‐session EECP treatment through Medicare is roughly $4,880, which accounts for 15% and 7% of the hospital reimbursement for PCI and CABG surgery, respectively, without including the physician compensation for both revascularization procedures.
The access to EECP centres in the United States is another unappreciated challenge, since these centres are not always located in the city or even the county of residency of the RAP patient. Under these circumstances, the necessity for special arrangements as well as travelling time may interfere with daily attendance and commitment to the therapy, as each EECP session usually lasts 1 h, and a complete course of therapy is 35 sessions.
10. CONCLUSION
Enhanced external counterpulsation is a valuable therapy which provides significant acute and long‐term relief of angina symptoms and improves quality of life of patients with stable ischaemic heart disease and RAP. EECP is a unique and effective treatment option for selected patients with GDMT and RAP, who are inoperable, or continue to have angina due to residual ischaemia despite PCI and/or CABG surgery. EECP represents a low‐cost strategy with high patient acceptability, but continues to be unused by most cardiologists, as they remain unfamiliar with this treatment.
Enhanced external counterpulsation is not intended to replace other forms of pharmaceutical or invasive therapies, including PCI and/or CABG surgery. Rather, a growing data pool supporting the effectiveness of the treatment suggest its viability as a potent and cost‐effective treatment strategy, providing relief of angina and improving the quality of life in the majority of patients treated.
CONFLICT OF INTEREST
The authors have no financial disclosures or conflicts of interest to report.
Caceres J, Atal P, Arora R, Yee D. Enhanced external counterpulsation: A unique treatment for the “No‐Option” refractory angina patient. J Clin Pharm Ther. 2021;46:295–303. 10.1111/jcpt.13330
Contributor Information
Jose Caceres, Email: joecaceresmd@gmail.com.
Derek Yee, Email: dytutor@gmail.com.
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics ‐ 2019 update: a report from the American Heart Association. Circulation. 2019;139:e56‐e528. [DOI] [PubMed] [Google Scholar]
- 2. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130:1749‐1767. [DOI] [PubMed] [Google Scholar]
- 3. Braith RW, Conti CR, Nichols WW, et al. Enhanced external counterpulsation improves peripheral artery flow‐mediated dilation in patients with chronic angina. Circulation. 2010;122:1612‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holmes DR Jr. Treatment options for angina pectoris and the future role of enhanced external counterpulsation. Clin Cardiol. 2002;25:I122‐I125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah SA, Shapiro RJ, Mehta R, et al. Impact of enhanced external counterpulsation on Canadian Cardiovascular Society angina class in patients with chronic stable angina: a meta‐analysis. Pharmacotherapy. 2010;30(7):639‐645. [DOI] [PubMed] [Google Scholar]
- 6. Parker JO. Angina pectoris: a review of current and emerging therapies. Am J Manag Care. 2004;10:S332‐S338. [PubMed] [Google Scholar]
- 7. Gowdak LHW. Prevalence of refractory angina in clinical practice. Heart Metab. 2017;72:9‐12. [Google Scholar]
- 8. Lawson WE, Hui JC, Kennard ED, et al. Enhanced external counterpulsation is cost‐effective in reducing hospital costs in refractory angina patients. Clin Cardiol. 2015;38(6):344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGillion M, Arthur HM, Cook A, et al. Management of patients with refractory angina: Canadian Cardiovascular Society/Canadian Pain Society joint guidelines. Can J Cardiol. 2012;28(2):S20‐S41. [DOI] [PubMed] [Google Scholar]
- 10. Henry T. Options for the “No‐Option” Refractory Angina Patient. Cedars‐Sinai Advances in Heart & Heart Surgery 2014 Spring.
- 11. Subramanian R, Nayar S, Meyyappan C, et al. Effect of enhanced external counter pulsation treatment on aortic blood pressure, arterial stiffness and ejection fraction in patients with coronary artery disease. J Clin Diagn Res. 2016;10(10):30‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manchanda A, Soran O. Enhanced external counterpulsation and future directions: step beyond medical management for patients with angina and heart failure. J AM Coll Cardiol. 2007;50(16):1523‐1531. [DOI] [PubMed] [Google Scholar]
- 13. Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin‐1 levels. Am J Cardiol. 2006;98(1):28‐30. [DOI] [PubMed] [Google Scholar]
- 14. Yavari M, Montazeri HR. Effects of enhanced external counterpulsation on anginal symptoms and improvements in objective measures of myocardial ischaemia. Cardiovasc J South Afr. 2007;18(3):154‐156. [PMC free article] [PubMed] [Google Scholar]
- 15. Sinvhal RM, Gowda RM, Khan IA. Enhanced external counterpulsation for refractory angina pectoris. Heart. 2003;89:830‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sardari A, Hosseini SK, Bozorgi A, et al. Effects of enhanced external counterpulsation on heart rate recovery in patients with coronary artery disease. J Teh Univ Heart Ctr. 2018;13(1):13‐17. [PMC free article] [PubMed] [Google Scholar]
- 17. Tartaglia J, Stenerson J, Charney R, et al. Exercise capability and myocardial perfusion in chronic angina patients treated with enhanced external counterpulsation. Clin Cardiol. 2003;26:287‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang W, Chen YC, Zheng ZS, et al. External counterpulsation increases capillary density during experimental myocardial infarction (abstract). XXIst Congress of the European Society of Cardiology, Barcelona, Spain. Eur Heart J. 1999;20:168. [Google Scholar]
- 19. Qin X, Deng Y, Wu D, et al. Does enhanced external counterpulsation (EECP) significantly affect myocardial perfusion?: A systematic review & meta‐analysis. PLoS ONE. 2016;11(4):e0151822. 10.1371/journal.pone.0151822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST‐EECP): effect of EECP on exercise‐induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833‐1840. [DOI] [PubMed] [Google Scholar]
- 21. Casey DP, Conti R, Nichols WW, et al. Effect of enhanced external counterpulsation on inflammatory cytokines and adhesion molecules in patients with angina pectoris and angiographic coronary artery disease. Am J Cardiol. 2008;101:300‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351‐363. [DOI] [PubMed] [Google Scholar]
- 23. Loh PH, Louis AA, Windram J, et al. The immediate and long‐term outcome of enhanced external counterpulsation in treatment of chronic stable refractory angina. J Intern Med. 2006;259:276‐284. [DOI] [PubMed] [Google Scholar]
- 24. Urano H, Ikeda H, Ueno T, et al. Enhanced external counterpulsation improves exercise tolerance, reduces exercise‐induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. J Am Coll Cardiol. 2001;37:93‐99. [DOI] [PubMed] [Google Scholar]
- 25. Stys TP, Lawson WE, Hui JC, et al. Effects of enhanced external counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. Am J Cardiol. 2002;89(7):822‐824. [DOI] [PubMed] [Google Scholar]
- 26. Michaels AD, Raisinghani A, Soran O, et al. The effects of enhanced external counterpulsation on myocardial perfusion in patients with stable angina: a multicenter radionuclide study. Am Heart J. 2005;150(5):1066‐1073. [DOI] [PubMed] [Google Scholar]
- 27. Novo G, Bagger JP, Carta R, et al. Enhanced external counterpulsation for treatment of refractory angina pectoris. J Cardiovasc Med. 2006;7:335‐339. [DOI] [PubMed] [Google Scholar]
- 28. Lawson WE, Hui JC, Kennard ED, Kelsey SF, Michaels AD, Soran O. Two‐year outcomes in patients with mild refractory angina treated with enhanced external counterpulsation. Clin Cardiol. 2006;29(2):69‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arora RR, Shah AG. The role of enhanced external counterpulsation in the treatment of angina and heart failure. Can J Cardiol. 2007;23(10):779‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawson WE, Hui JC, Cohn PF. Long‐term prognosis of patients with angina treated with enhanced external counterpulsation: five‐year follow‐up study. Clin Cardiol. 2000;23:254‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braith RW, Casey DP, Beck DT. Enhanced external counterpulsation for ischemic heart disease: a look behind the curtain. Exerc Sport Sci Rev. 2012;40(3):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Center for Medicare and Medicaid Services (CMS) . External counterpulsation therapy. Medicare Coverage Issues Manual Section 35‐74, Issue #CAG‐00003. Baltimore, MD: CMS; February 5, 1999. [Google Scholar]
- 33. Gibbons RJ, Abrams J, Chatterjee K, et al. American College of Cardiology (ACC); American Heart Association (AHA) Task Force on Practice Guidelines (Committee on the Management of Patients with Chronic Stable Angina). ACC/AHA 2002 guideline update for the management of patients with chronic stable angina‐ summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2003;41(1):159‐168. [DOI] [PubMed] [Google Scholar]
- 34. Michaels AD, Accad M, Ports TA, et al. Left ventricular systolic unloading and augmentation of intracoronary pressure and doppler flow during enhanced external counterpulsation. Circulation. 2002;106:1237‐1242. [DOI] [PubMed] [Google Scholar]
- 35. Masuda D, Nohara R, Hirai T, et al. Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina; evaluation by 13N‐ammonia positron emission tomography. Eur Heart J. 2001;22:1451‐1458. [DOI] [PubMed] [Google Scholar]


