Abstract
Background
Pancreatic trauma is reportedly associated with high morbidity and mortality. Main pancreatic duct (MPD) injury is critical for treatment.
Methods
As a study project of the Japanese Society for Abdominal Emergency Medicine (JSAEM), we collected the data of 163 patients with pancreatic trauma who were diagnosed and treated at JSAEM board‐certified hospitals from 2006 to 2016. Clinical backgrounds, diagnostic approaches, management strategies, and outcomes were evaluated.
Results
Sixty‐four patients (39%) were diagnosed as having pancreatic trauma with MPD injury that resulted in 3% mortality. Blunt trauma and isolated pancreatic injury were independent factors predicting MPD injury. Nine of 11 patients with MPD injury who were initially treated nonoperatively had serious clinical sequelae and five (45%) required surgery as a secondary treatment. Among all cases, the detectability of MPD injury of endoscopic retrograde pancreatography (ERP) was superior to that of other imaging modalities (CT or MRI), with higher sensitivity and specificity (sensitivity = 0.96; specificity = 1.0).
Conclusions
Acceptable outcomes were observed in pancreatic trauma patients with MPD injury. Nonoperative management should be carefully selected for MPD injury. ERP is recommended to be performed in patients with suspected MPD injury and stable hemodynamics.
Keywords: endoscopic retrograde pancreatography, main pancreatic duct injury, management, nationwide survey, pancreatic trauma
Highlight
Ando and colleagues analyzed data from 163 patients with pancreatic trauma in a nationwide multicenter study. They found that serum amylase and C‐reactive protein levels were significantly higher in patients with main pancreatic duct injuries, indicating that endoscopic retrograde pancreatography was the most accurate imaging modality for assessing pancreatic duct injury.
![]()
1. INTRODUCTION
Pancreatic trauma is relatively rare, with an overall frequency of only 0.2%‐0.3% among all trauma but a high mortality rate of 17%‐46%. 1 , 2 , 3 , 4 Pancreatic fistula is one of the major complications of pancreatic trauma. This can contribute to prolonged hospital stays and fatal outcomes. Some reports stated that delayed diagnosis and inappropriate treatment of main pancreatic duct (MPD) injury could increase both complications and mortality rates. 4 , 5 , 6 However, because of limited cases at a single institution, experienced specialists and high‐quality evidence are scarce. Consequently, a consensus regarding diagnostic and treatment algorithms as well as optimal surgical selection has not been established.
Operative management (OM) is often selected for deep injury, especially severe pancreatic trauma with MPD injury. Pancreatic‐sparing surgery may be selected depending on the patient's age and general condition, the extent of injury, and the skill of the surgeon. In recent years, some reports have recommended conservative pancreatic drainage for pancreatic trauma with MPD injury, but the short‐ and long‐term outcomes remain unclear. 6 , 7 , 8 Because accurate evaluation of MPD injury by abdominal computed tomography (CT) scan alone is often difficult, some reports recommended emergency endoscopic retrograde pancreatography (ERP) if the patient's general condition is stable. 6 , 7 , 8 In some cases with MPD injury, nonoperative management (NOM) with endoscopic pancreatic stenting (EPS) is possible after diagnosis by ERP; however, comprehensive evaluation of ERP for pancreatic trauma has not yet been performed.
This study aimed to evaluate the current status and optimal management strategy, including the diagnostic role of ERP, for pancreatic trauma by conducting a multicenter nationwide survey in Japan. In particular, we focused more on cases with MPD injuries that are difficult to diagnose and treat.
2. METHODS
2.1. Patients and data collection
We conducted a questionnaire survey at abdominal emergency specialist institutions as a project study of the Japanese Society for Abdominal Emergency Medicine (JSAEM). Clinical data were collected from 173 patients who were diagnosed and treated for pancreatic trauma at the 31 board‐certified hospitals in JSAEM between January 2006 and December 2016. Furthermore, 10 patients with cardiopulmonary arrest before or immediately after arrival who died within 24 hours after examination or were transferred to another hospital for treatment were excluded. We retrospectively analyzed patients’ demographics, physical findings, and laboratory data within 48 hours after injury (n = 144), along with existence of the MPD injury, imaging modalities, treatment strategies (NOM/OM), perioperative variables, and outcomes in the finally enrolled 163 patients.
2.2. Groups and definitions
All pancreatic trauma cases were divided into the groups of MPD intact and MPD injured according to the final diagnosis made in each center, being judged by one or some imaging modalities combined with clinical, intraoperative and/or pathological findings. Major complications associated with treatment were defined as those with a Clavien–Dindo classification 9 of ≥IIIa. A postoperative pancreatic fistula (POPF) was defined according to the criteria of the International Study Group on Pancreatic Fistula (ISGPF). 10
2.3. Statistical analysis
Descriptive statistics are reported as mean (standard deviation) for normally distributed continuous variables, median (range) for other continuous variables, and frequency (percent) for categorical variables. Chi‐squared test or Fischer exact test was used to analyze categorical variables of two groups, whereas unpaired t‐test or Mann–Whitney U‐test was used to compare continuous variables. Additionally, analysis of covariance was used to adjust for confounding factors. The level of significance was set at P < .05. JMP Pro version 14 (SAS Institute Inc.) was used to conduct all statistical analyses.
2.4. Ethical statements
The protocol for this research project has been approved by the Committee of JSAEM (Approval No. 18‐1‐R1), and the Committee of Kagawa University (Approval No. 29‐200). It conforms to the provisions of the Declaration of Helsinki. Consent was obtained from the subjects in an informative or disclosure document.
3. RESULTS
3.1. Patient characteristics
A total of 163 cases were classified into two groups, “MPD intact” (n = 99) and “MPD injured” (n = 64). Table 1 shows the clinical characteristics of patients divided into these groups. There were 109 (67%) males and 54 (33%) females, with a mean age of 42.7 years. As indicated in Figure S1, male patients showed a bimodal distribution and tended to be in their 10s and 60s. In contrast, female patients tended to be young, especially in their teens. As presented in Table 1, there were 140 patients (86%) with blunt trauma and 90 (55%) with injury caused by traffic accidents. Fifty‐five patients (34%) had isolated pancreatic injury, and 108 (66%) were associated with coexisting severe abdominal injury. Blunt trauma and isolated pancreatic injury were revealed as the independent clinical factors predicting MPD injury when adjusted by multivariate analysis (P = .007 and P = .013, respectively). Concurrent liver damage was most frequently observed in 24 patients (15%). Major vascular damage, considered fatal, occurred in 13 patients (8%). Anatomical injury sites included the head of the pancreas in 66 patients (40%) and the pancreatic body/tail in 97 (60%). The mean injury severity score (ISS) was 15.8. The mean serum amylase level determined within 48 hours after injury was 332.4 U/L. Figure 1 shows laboratory values within 48 hours of injury between the groups with and without MPD injury. Serum amylase levels as well as CRP were significantly higher in patients with MPD injury compared to those without MPD injury (506.1 ± 64 vs 220.0 ± 51, P < .001).
TABLE 1.
Demographics and clinical characteristics of patients with or without main pancreatic duct (MPD) injury (number [%] or value)
| Variables |
Overall n = 163 |
MPD intact n = 99 |
MPD injured n = 64 |
Univariate analysis | Multivariate analysis |
|---|---|---|---|---|---|
| Gender | |||||
| Male/Female | 109(67)/54 (33) | 62 (63)/37 (37) | 47 (73)/17 (27) | 0.152 | |
| Age (y) | 42.7 ± 1.8 | 45.6 ± 2.3 | 38.3 ± 2.8 | 0.045 | 0.388 |
| Cause of trauma | |||||
| Traffic accident | 90 (55) | 55 (56) | 35 (55) | 0.913 | |
| Non–traffic accident | 73 (45) | 44 (44) | 29 (45) | ||
| Injury type | |||||
| Blunt | 140 (86) | 78 (79) | 62 (97) | 0.001 | 0.007 |
| Penetrating | 23 (14) | 21 (21) | 2 (3) | ||
| Intraabdominal injury | |||||
| Pancreas only | 55 (34) | 23 (23) | 32 (50) | <0.001 | 0.013 |
| Pancreas injury location | |||||
| Head | 66 (40) | 47 (47) | 19 (30) | 0.033 | |
| Body or tail | 97 (60) | 52 (53) | 45 (70) | ||
| Injury Severity Score (ISS) | 15.8 ± 0.9 | 16.2 ± 1.1 | 15.3 ± 1.4 | 0.613 | 0.998 |
| Revised Trauma Score (RTS) | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.4 ± 0.1 | 0.655 | |
Frequency (%) for categorical variables.
Mean (standard deviation [SD]) for continuous variables.
FIGURE 1.
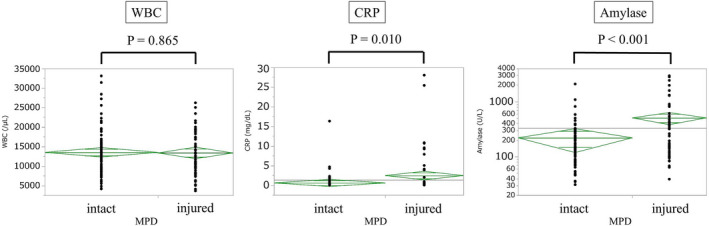
Laboratory values within 48 h of injury between the groups with and without MPD injury (n = 144). Serum amylase levels in the vertical axis are displayed using a logarithmic scale. WBC (/μL; 13 491 ± 609 vs 13 328 ± 749, P = .865). CRP (mg/dL; 0.56 ± 0.5 vs 2.44 ± 51, P = .010). Amylase (U/L; 220.0 ± 51 vs 506.1 ± 64, P < .001). CRP, C‐reactive protein; MPD, main pancreatic duct; WBC, white blood cell. One‐way analysis of variance
3.2. Treatment and outcomes
Treatment and outcomes of the patients are shown in Table 2. Forty‐one (25%) patients underwent ERP; significantly more patients with MPD injury underwent ERP than those with MPD intact (14 [14%] vs 27[42%]). Furthermore, 58 (36%) patients were nonoperatively treated, whereas 105 (64%) were surgically treated. In addition, 53 (83%) patients with MPD injury and 52 (53%) with MPD intact had OM (P < .001). Clinical sequelae were observed significantly more frequently in patients with MPD injury, particularly in the NOM group. Moreover, 17 (29%) and 49 (47%) patients had one or more sequential problems in NOM and OM groups, respectively. The average length of stay in hospital was 43.2 days (37.6 and 51.8 days in MPD intact and MPD injured cases, respectively, P = .055). There were eight (5%) hospital deaths, and all but one patient died of multiple organ injury.
TABLE 2.
Treatment and outcomes of patients with or without main pancreatic duct (MPD) injury (number (%) or value)
| Variables | Overall n = 163 | MPD intact n = 99 | MPD injured n = 64 | P‐value |
|---|---|---|---|---|
| Treatment | ||||
| Rate of ERP | 41 (25) | 14 (14) | 27 (42) | <.001 |
| Initial treatment strategy | ||||
| Non‐operative management (NOM) | 58 (36) | 47 (47) | 11 (17) | <.001 |
| Operative management (OM) | 105 (64) | 52 (53) | 53 (83) | |
| Outcomes | ||||
| Complications a | 66 (40) | 28 (28) | 38 (59) | <.001 |
| NOM | 17 (29) | 8 (17) | 9 (82) | <.001 |
| OM | 49 (47) | 20 (38) | 29 (55) | .095 |
| Hospital stay (d) | 43.2 ± 3.6 | 37.6 ± 4.6 | 51.8 ± 5.7 | .055 |
| Mortality | 8 (5) | 6 (6) | 2 (3) | .397 |
Frequency (%) for categorical variables.
Mean (standard deviation [SD]) for continuous variables.
Abbreviation: ERP, endoscopic retrograde pancreatography.
Clavien–Dindo classification of grade IIIa or higher.
3.3. Diagnostic imaging of MPD injury
Table 3 shows the imaging modalities performed to detect MPD injury in all patients. The rates of performance of CT, ERP, and magnetic resonance perfusion imaging (MRP) were 94%, 25%, and 12%, respectively. CT was performed in the majority of patients with pancreatic injury, whereas MRP was performed at a much lower rate. The sensitivities/specificities of MPD injury detection by CT, ERP, and MRP were 0.81/0.99, 0.96/1.0, and 0.8/0.89, respectively. The diagnostic accuracy of ERP was superior to that of other imaging modalities (CT and MRP) for the diagnosis of MPD injury.
TABLE 3.
Accuracy of imaging modalities to detect MPD injury and diagnose the type of injury in grade III patients
| Imaging modality | n (%) | CT | ERP | MRP | |||
|---|---|---|---|---|---|---|---|
| Number of patients | n = 84 | 80 (95) | 35 (42) | 11 (13) | |||
| MPD injury | |||||||
| Grade | Positive | Negative | Positive | Negative | Positive | Negative | |
| IIIa | 1 | 17 | 0 | 8 | 0 | 1 | |
| IIIb | 50 | 12 | 26 | 1 | 8 | 2 | |
| Sensitivity | 0.81 | 0.96 | 0.8 | ||||
| Specificity | 0.99 | 1.0 | 0.89 | ||||
Abbreviations: MPD, Main pancreatic duct; CT, Computed tomography; ERP, Endoscopic retrograde pancreatography; MRP, Magnetic resonance pancreatography.
3.4. Management of MPD injury
Table 4 focuses on 64 patients with MPD injury and compares backgrounds and outcomes between their initial treatment strategies. There were 11 patients in the NOM group and 53 in the OM group. There were no significant differences in clinical factors such as age, morphology of pancreatic injury, ISS, or blood test results at the time of visit. However, the length of hospital stay was significantly extended in the NOM group (77.3 and 47.2 days in NOM and OM groups, respectively, P = .038).
TABLE 4.
Demographics and clinical characteristics of main pancreatic duct injured patients initially treated with operative and nonoperative management
| Variables |
Nonoperative management n = 11 |
Operative management n = 53 |
P value |
|---|---|---|---|
| Background | |||
| Age (y) | 49.5 ± 6.9 | 35.9 ± 3.1 | .079 |
| Injury type | |||
| Blunt | 11 (100) | 51 (96) | .513 |
| Penetrating | 0 (0) | 2 (4) | |
| Intraabdominal injury | |||
| Pancreas only | 5 (45) | 27 (51) | .740 |
| Pancreas injury location | |||
| Head | 4 (36) | 15 (28) | .594 |
| Body or tail | 7 (64) | 38 (72) | |
| Rate of comorbidities | 5 (45) | 10 (23) | .058 |
| Injury Severity Score (ISS) | 14.7 ± 2.5 | 15.4 ± 1.1 | .814 |
| Laboratory values | |||
| WBC (/μL) | 12,825 ± 1634 | 13,577 ± 744 | .677 |
| CRP (mg/dL) | 2.9 ± 2.2 | 3.2 ± 1.0 | .895 |
| Amylase (U/L) | 562.8 ± 205.7 | 482.6 ± 91.1 | .723 |
| Treatment | |||
| Rate of ERP | 6 (55) | 22 (42) | .428 |
| Outcomes | |||
| Complications a | 9 (82) | 29 (55) | .096 |
| Pseudocyst b | 4 (36) | 6 (11) | |
| Pancreatic fistula b | 4 (36) | 22 (42) | |
| Abscess b | 2 (18) | 10 (19) | |
| Peritonitis b | 2 (18) | 2 (4) | |
| Conversion to surgery | 5 (45) | ||
| Hospital stay (d) | 77.3 ± 13.0 | 47.2 ± 5.7 | .038 |
| Mortality | 0 (0) | 2 (4) | .513 |
Frequency (%) for categorical variables.
Mean (standard deviation [SD]) for continuous variables.
Abbreviations: CRP, C‐reactive protein; ERP, endoscopic retrograde pancreatography; WBC, white blood cell.
Clavien–Dindo classification of grade IIIa or higher.
Overlapping distribution.
Table 5 lists individual data of 11 patients with MPD injury who were initially treated nonoperatively. The initial treatment included drainage in five patients, EPS in five, and octreotide injection in one. Only two (18%) patients successfully recovered after the initial treatment. In contrast, nine (82%) patients had clinical sequelae. Furthermore, five (45%) of these nine patients required surgery as a secondary treatment. Four patients (36%) required long‐term hospitalization for 90 days or more, although there was no hospital death.
TABLE 5.
Individual characteristics and outcomes of main pancreatic duct (MPD) injured patients initially treated with nonoperative management
| Case | Age/Gender | Pancreas injury (location) | Type of MPD injury (CT/ERP/MRP) | ERP | Initial treatment | Second treatment | Hospital stay (days) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment modality | Post‐traumatic hours | Outcome and complications | Conversion modality | Post‐traumatic days | Outcome and complications | ||||||
| 1 | 77/F | Body/Tail | Partial (CT) | No | Drainage | 2 | Recovered | None | None | None | 235 |
| 2 | 61/M | Body | Complete (CT/ERP) | Yes | Drainage | 1 | Pseudocyst | Operation (DP) | 18 | Pancreatic fistula | 119 |
| 3 | 86/M | Body | Partial (CT/MRP) | No | Drainage | 792 | Pseudocyst | ENPD | unknown | Recovered | 20 |
| 4 | 73/M | Tail | Partial (CT) | No | Drainage | 1 | Abscess | ENPD | >30 | Recovered | 185 |
| Pancreatic fistula | |||||||||||
| 5 | 60/M | Body | Partial (CT) | No | Drainage | 144 | Pancreatic fistula | Operation (DP) | 32 | Pancreatic fistula | 87 |
| 6 | 24/M | Head | Complete (CT/ERP) | Yes | EPS | 10 | Recovered | None | None | None | 34 |
| 7 | 8/M | Head / Body | Complete (CT/ERP) | Yes | EPS | 5 | Peritonitis | Operation (PD) | 2 | Bile leakage | 26 |
| 8 | 71/M | Head | Partial (CT/ERP) | Yes | EPS | 4 | Peritonitis | Operation (Operative drainage) | 2.5 | Intestinal perforation | 34 |
| 9 | 57/M | Tail | Partial (CT/ERP) | Yes | EPS | 1.5 | Pseudocyst | Drainage | 4 | Recovered | 105 |
| Pancreatic fistula | |||||||||||
| Abscess | |||||||||||
| 10 | 11/M | Head | Complete (CT/ERP/MRP) | Yes | EPS | 30 | Pseudocyst | None | None | None | 82 |
| 11 | 16/M | Body | Complete (CT/ERP/MRP) | No | Octreotide | 1 | Pancreatic fistula | Operation (L&W) | 24 | Recovered | 31 |
Abbreviations: CT, computed tomography; DP, distal pancreatectomy; Drainage, percutaneous drainage; ENPD, endoscopic nasopancreatic drainage; EPS, endoscopic pancreatic stenting; ERP, endoscopic retrograde pancreatography; L&W, Letton–Wilson's procedure; MRP, magnetic resonance pancreatography; PD, pancreatoduodenectomy.
Conversely, in a series of 53 patients with MPD injury who were treated with OM, 48 (90%) received pancreatic resection and five (10%) received drainage alone and/or pancreatic suture repair without pancreatic resection (Table 6). When comparing pancreatic resection and no pancreatic resection, the length of hospital stay was significantly longer in patients without pancreatic resection (P = .003). Patients who underwent the Letton–Wilson procedure were significantly younger (P = .038) and had more blood loss (P = .010) than those who underwent pancreaticoduodenectomy (PD). Moreover, regarding deep pancreatic body/tail injury, the Warshaw procedure was performed at a younger age (P = .018) than distal pancreatectomy. There were no statistically significant differences in postoperative morbidity, grade B and C POPF, and mortality rates among surgical procedures. Two patients (4%) had death outcomes secondary to hemorrhagic shock.
TABLE 6.
Clinical characteristics and outcomes of 53 main pancreatic duct injured patients initially treated with operative management
| Operation | Patient number (%) | Age (year) | Operation time (min) | Amount of bleeding (mL) | POPF ≧ grade B n (%) | Postoperative complication a | Hospital stay (days) | Mortality n (%) |
|---|---|---|---|---|---|---|---|---|
| Pancreatic resection | 48 (90) | 29 [4‐83] | 258 [85‐690] | 1,090 [0‐5831] | 19 (40) | 30 (63) | 37 [2‐130] | 2 (4) |
| Pancreaticoduodenectomy | 10 (19) | 29.5 [16‐77] | 347.5 [85‐550] | 2,525.5 [563‐4300] | 4 (40) | 5 (50) | 43.5 [25‐126] | 0 (0) |
| Distal pancreatectomy | 14 (26) | 42[11‐78] | 176.5 [85‐360] | 1908[100‐5831] | 6 (43) | 9 (64) | 35[2‐97] | 2 (4) |
| Warshaw procedure | 9 (17) | 22 e [4‐65] | 180 [120‐348] | 555.5 [70‐1340] | 5 (56) | 7 (78) | 30 [7‐126] | 0 (0) |
| Pancreas‐sparing surgery | ||||||||
| Letton–Wilson's procedure | 11 (20) | 18 c [9‐75] | 280 [181‐690] | 514 d [0‐2575] | 3 (27) | 7 (64) | 31 [7‐55] | 0 (0) |
| Bracy procedure | 4 (8) | 31 [24‐83] | 398.5 [300‐526] | 1584.5 [400‐2400] | 1 (25) | 2 (50) | 37 [32‐52] | 0 (0) |
| No pancreatic resection | 5 (10) | 32 [19‐57] | 240 [190‐418] | 850 [520‐11673] | 4 (80) | 5 (100) | 65 b [30‐175] | 0 (0) |
| Drainage | 2 (4) | 34.5 [19‐50] | 304 [190‐418] | 1,075 [850‐1300] | 1 (50) | 2 (100) | 119.5 [64‐175] | 0 (0) |
| Pancreatic suture repair | 3 (6) | 32 [28‐57] | 240 [221‐265] | 700 [520‐11673] | 3 (100) | 3 (100) | 65 [30‐117] | 0 (0) |
Frequency (%) for categorical variables.
Median (range) for other continuous variables.
Clavien–Dindo classification of grade IIIa or higher.
P = .003 vs Pancreatic resection.
P = .038 vs Pancreaticoduodenectomy.
P = .010 vs Pancreaticoduodenectomy.
P = .018 vs Distal pancreatectomy.
4. DISCUSSION
Traumatic pancreatic injury is a relatively rare trauma but is associated with a high mortality rate. 1 , 2 , 3 , 4 The Japanese trauma data bank previously reported that this injury accounted for 0.25% of all trauma, 2.4% of abdominal trauma, and 4.3% of mortality. 4 In Japan, more than 90% of these injuries are caused by blunt trauma resulting from traffic accidents. 4 , 11 MPD involvement is the most important factor of patient outcomes, and delays in diagnosis are associated with high postoperative morbidity and mortality rates, particularly in cases of MPD injury. 12 Because the pancreas is surrounded by the liver, biliary tract, spleen, stomach, duodenum, colon, and large blood vessels, the frequency of concomitant other organ injury is also high. The mortality rate is relatively high in such cases. 4 , 13 The frequency of single pancreatic injury is less than 10%. The number of concurrently damaged organs averages 3.5‐4.1, with mortality increasing with the number of damaged organs. 3 , 4 This multicenter nationwide survey conducted over the last decade revealed that mortality was 5% for all cases, 1.8% for patients with isolated pancreatic injury, and 3% for patients with MPD injury. This mortality rate was similar to that reported in previous studies, which found that the mortality rate is 0%‐10%. 2 , 4 , 14 , 15
Age distribution was analyzed in male and female patients. Young patients were more common regardless of sex. However, male patients showed bimodal distribution with another peak at 60s. Additionally, a similar result was previously reported from Japan. 4 This might be explained by the fact that males in middle‐aged or older populations work outside and drive more frequently compared to the same generation females in this country. In addition, skeletal muscle mass forming visceral protective layer tends to be more vulnerable with aging.
Herman et al. reported that there was no association between elevated serum amylase levels and severity of pancreatic injury according to the American Association for the Surgery of Trauma (AAST) grades, although this indicates that elevated serum amylase levels may be diagnostic indicators. 16 , 17 , 18 Alternatively, our analysis was performed between the two groups, focusing on the existence of the MPD injury. Patients with MPD injury had significantly higher serum amylase and CRP levels early after the injury occurred. In this regard, Nadler et al. 19 have also reported that high amylase levels were more consistent with MPD injury. The MPD injury likely contributes to early elevation of serum amylase and CRP. Rapid serum amylase and CRP elevation might be important markers for predicting MPD injury.
Blunt trauma and isolated pancreatic injury were independent factors predicting MPD injury. It is difficult to speculate clear mechanisms for these results. Blunt injury to the pancreas results in the organ being compressed over the vertebra. Localized deep compressive forces may result in an isolated pancreatic laceration depending on the magnitude of the force. 20 However, the influence of selection bias in a retrospective setting should be considered.
The diagnostic accuracy of ERP was superior to that of other imaging modalities (CT and MRP) in this survey for the diagnosis of MPD injury. In 1978, Belohravek et al. first reported ERP experience to identify MPD injury in trauma cases. 21 ERP allows visualization of the MPD and determines injury severity, facilitating optimal treatment, such as drainage using EPS. 7 Many other reports also described the value of ERP in patients with pancreatic trauma. 6 , 7 , 8 , 11 , 22 multi‐detector‐row CT, with sensitivity and specificity of up to 80%, is the best noninvasive imaging modality to detect pancreatic injury. 23 In contrast, although MRP imaging has the advantage of being minimally invasive and can evaluate upstream of the injured area, the rate of implementation is low in Japan because of the lack of examination agility. In patients with suspected deep pancreatic injury on CT examination and stable vital signs, ERP should be performed to evaluate MPD injury more accurately for developing more appropriate treatment strategy.
Complications of the initial treatment were more likely to occur in the MPD injured group, that is, the presence or absence of MPD injury was found to affect short‐term outcomes. Regarding which treatment strategy, OM or NOM, should be taken for patients with the MPD injury, OM has been generally recommended. 24 Recently published evidence, however, suggested that NOM might be an acceptable and safe option even in patients with MPD injury. 4 Previous studies have shown that endoscopic management, such as EPS, reduced the need of surgery for pancreatic injury by blocking the pancreatic leak and draining the pancreatic fluid into the duodenum. 6 , 7 , 8 Nevertheless, in this survey, NOM was associated with a longer hospital stay than OM. Frequent sequential problems observed in the NOM group most likely resulted in prolonged hospital stay. It is also noteworthy that in this study, five of 11 patients initially treated with NOM subsequently underwent surgery as a secondary treatment although there was no mortality with timely and appropriate additional treatments. In contrast, in a total of 64 patients with MPD injury, six patients eventually received successful NOM and no subsequent surgical intervention. Among them, five patients were treated with endoscopic pancreatic stenting or endoscopic nasopancreatic drainage. NOM including endoscopic stenting procedures may be a less invasive and effective treatment option for selected patients. Because the number of NOM for MPD injury is very small in this series, the actual role of NOM for MPD injury is still unclear and should be explored further in large scale studies.
Our study included a large series of patients with MPD injury who were initially treated with surgery. Pancreatic resection was the more frequently performed procedure in this series. It has been previously reported that emergency PD for pancreatic injury was associated with a very high mortality rate (>30%) because of the patient's poor condition and surgical complexity. 14 , 25 However, compared with the outcomes of these previous reports, the outcome of PD in this recent series was excellent with zero mortality. This is presumably because the attending centers are all JSAEM board‐certified hospitals that have abundant experiences in abdominal emergencies. Appropriate initial diagnosis, meticulous perioperative management, prompt decision of surgical intervention based on each patient condition, and recent improvement in perioperative intensive care would be related to the excellent postoperative outcomes. On the other hand, there were only five patients who underwent surgery without pancreatic resection. The length of hospital stay was significantly longer in these five patients than in those who underwent pancreatic resection. Otherwise, pancreatic‐conserving surgery including Letton‐Wilson and Bracy procedures is preferable to preserve the pancreatic function if the following condition is met: (a) if the patient is young, (b), if the patient's vital signs are stable, and (c) if there is a highly qualified pancreatic surgeon. 5 In Japan, a surgical qualification system has been implemented by the Japanese Society of Hepatobiliary Pancreatic Surgery.
This study has some limitations as follows. First, the sample size was limited and questionnaires were used to obtain data. Second, because this was a retrospective observational study, selection bias was intrinsic. Third, only board‐certified abdominal emergency specialist hospitals of the JSAEM were registered for participation in this survey rather than a large national study. There was an inherent bias in participating institutes. Finally, we were unable to evaluate the long‐term outcomes of patients with MPD injury.
In conclusion, serum amylase levels were significantly higher in patients with MPD injury. ERP is the most accurate imaging modality for assessing pancreatic duct injury and is recommended to be performed in patients with stable hemodynamics. Although mortality from pancreatic trauma, including MPD injury, was relatively low regardless of treatment strategy in this series, serious sequelae occurred more frequently and length of hospital stay was significantly longer in patients initially treated nonoperatively.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
Funding information
Project study funds of Japanese Society for Abdominal Emergency Medicine (2016‐P‐02).
Supporting information
Fig S1
ACKNOWLEDGMENTS
We are deeply grateful to the Japanese Society for Abdominal Emergency Medicine and the facilities for completing the survey. We also would like to thank Enago (www.enago.com) for English language editing.
Ando Y, Okano K, Yasumatsu H, et al. Current status and management of pancreatic trauma with main pancreatic duct injury: A multicenter nationwide survey in Japan. J Hepatobiliary Pancreat Sci. 2021;28:183–191. 10.1002/jhbp.877
Yasuhisa Ando and Keiichi Okano contributed equally to this work.
REFERENCES
- 1. Antonacci N, Di Saverio S, Ciaroni V, Biscardi A, Giugni A, Cancellieri F, et al. Prognosis and treatment of pancreaticoduodenal traumatic injuries: which factors are predictors of outcome? J Hepatobiliary Pancreat Sci. 2011;18:195–201. [DOI] [PubMed] [Google Scholar]
- 2. O'Reilly DA, Bouamra O, Kausar A, Malde DJ, Dickson EJ, Lecky F. The epidemiology of and outcome from pancreatoduodenal trauma in the UK, 1989–2013. Ann R Coll Surg Engl. 2015;97:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scollay JM, Yip VS, Garden OJ, Parks RW. A population‐based study of pancreatic trauma in Scotland. World J Surg. 2006;30:2136–41. [DOI] [PubMed] [Google Scholar]
- 4. Shibahashi K, Sugiyama K, Kuwahara Y, Ishida T, Okura Y, Hamabe Y. Epidemiological state, predictive model for mortality, and optimal management strategy for pancreatic injury: a multicentre nationwide cohort study. Injury. 2020;51:59–65. [DOI] [PubMed] [Google Scholar]
- 5. Iacono C, Zicari M, Conci S, Valdegamberi A, De Angelis M, Pedrazzani C, et al. Management of pancreatic trauma: a pancreatic surgeon's point of view. Pancreatology. 2016;16:302–8. [DOI] [PubMed] [Google Scholar]
- 6. Kim S, Kim JW, Jung PY, Kwon HY, Shim H, Jang JY, et al. Diagnostic and therapeutic role of endoscopic retrograde pancreatography in the management of traumatic pancreatic duct injury patients: single center experience for 34 years. Int J Surg. 2017;42:152–7. [DOI] [PubMed] [Google Scholar]
- 7. Kim HS, Lee DK, Kim IW, Baik SK, Kwon SO, Park JW, et al. The role of endoscopic retrograde pancreatography in the treatment of traumatic pancreatic duct injury. Gastrointest Endosc. 2001;54:49–55. [DOI] [PubMed] [Google Scholar]
- 8. Lin BC, Liu NJ, Fang JF, Kao YC. Long‐term results of endoscopic stent in the management of blunt major pancreatic duct injury. Surg Endosc. 2006;20:1551–5. [DOI] [PubMed] [Google Scholar]
- 9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 11. Gupta A, Stuhlfaut JW, Fleming KW, Lucey BC, Soto JA. Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics. 2004;24:1381–95. [DOI] [PubMed] [Google Scholar]
- 12. Oláh A, Issekutz A, Haulik L, Makay R. Pancreatic transection from blunt abdominal trauma: early versus delayed diagnosis and surgical management. Dig Surg. 2003;20:408–14. [DOI] [PubMed] [Google Scholar]
- 13. Potoka DA, Gaines BA, Leppäniemi A, Peitzman AB. Management of blunt pancreatic trauma: what's new? Eur J Trauma Emerg Surg. 2015;41:239–50. [DOI] [PubMed] [Google Scholar]
- 14. Kang WS, Park YC, Jo YG, Kim JC. Pancreatic fistula and mortality after surgical management of pancreatic trauma: analysis of 81 consecutive patients during 11 years at a Korean trauma center. Ann Surg Treat Res. 2018;95:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siboni S, Kwon E, Benjamin E, Inaba K, Demetriades D. Isolated blunt pancreatic trauma: a benign injury? J Trauma Acute Care Surg. 2016;81:855–9. [DOI] [PubMed] [Google Scholar]
- 16. Moore EE, Cogbill TH, Malangoni MA, Jurkovich GJ, Champion HR, Gennarelli TA, et al. Organ injury scaling, II: pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990;30:1427–9. [PubMed] [Google Scholar]
- 17. Herman R, Guire KE, Burd RS, Mooney DP, Ehlrich PF. Utility of amylase and lipase as predictors of grade of injury or outcomes in pediatric patients with pancreatic trauma. J Pediatr Surg. 2011;46:923–6. [DOI] [PubMed] [Google Scholar]
- 18. Mahajan A, Kadavigere R, Sripathi S, Rodrigues GS, Rao VR, Koteshwar P. Utility of serum pancreatic enzyme levels in diagnosing blunt trauma to the pancreas: a prospective study with systematic review. Injury. 2014;45:1384–93. [DOI] [PubMed] [Google Scholar]
- 19. Nadler EP, Gardner M, Schall LC, Lynch JM, Ford HR. Management of blunt pancreatic injury in children. J Trauma. 1999;47:1098–103. [DOI] [PubMed] [Google Scholar]
- 20. Antonsen I, Berle V, Søreide K. Blunt pancreatic injury in children. Tidsskr Nor Laegeforen. 2017;137:17–28. [DOI] [PubMed] [Google Scholar]
- 21. Belohlavek D, Merkle P, Probst M. Identification of traumatic rupture of the pancreatic duct by endoscopic retrograde pancreatography. Gastrointest Endosc. 1978;24:255–6. [DOI] [PubMed] [Google Scholar]
- 22. Kumar A, Panda A, Gamanagatti S. Blunt pancreatic trauma: a persistent diagnostic conundrum? World J Radiol. 2016;8:159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Degiannis E, Glapa M, Loukogeorgakis SP, Smith MD. Management of pancreatic trauma. Injury. 2008;39:21–9. [DOI] [PubMed] [Google Scholar]
- 24. Lin BC, Wong YC, Chen RJ, Liu NJ, Wu CH, Hwang TL, et al. Major pancreatic duct continuity is the crucial determinant in the management of blunt pancreatic injury: a pancreatographic classification. Surg Endosc. 2017;31:4201–10. [DOI] [PubMed] [Google Scholar]
- 25. van der Wilden GM, Yeh D, Hwabejire JO, Klein EN, Fagenholz PJ, King DR, et al. Trauma Whipple: do or don't after severe pancreaticoduodenal injuries? An analysis of the National Trauma Data Bank (NTDB). World J Surg. 2014;38:335–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


