Abstract
Background
Membranous nephropathy (MN) can be associated with hepatitis infection and less commonly with human immunodeficiency virus (HIV) infection. The significance of anti-phospholipase A2 receptor (PLA2R) and anti-thrombospondin type 1 domain-containing 7A (THSD7A) antibodies in this setting is unclear.
Methods
We describe the clinical, histopathological and outcome data of 19 patients with MN and hepatitis B virus (HBV), hepatitis C virus (HCV) or HIV infection identified through our renal biopsy database and the association with anti-PLA2R antibodies and anti-THSD7A antibodies.
Results
The cohort consisted of 19 patients, 8 male and 11 female, with a median age of 42 years (range 23–74). HBV infection was found in six cases, HCV in four and HIV in nine (two HIV patients had HBV co-infection and one HCV co-infection). PLA2R staining on biopsy was positive in 10/19 patients: 4 with HBV-MN, 3 with HCV-MN and 3 with HIV-MN and circulating anti-PLA2R antibodies were detected in 7/10 cases. THSD7A staining on biopsy was positive in three PLA2R-negative cases, one with HBV-MN and two with HIV-MN. Mean proteinuria was higher in the PLA2R-positive group and the median urinary protein:creatinine ratio (uPCR) was 963 mg/mmol (range 22–2406) compared with the PLA2R-negative group [median uPCR 548 mg/mmol (range 65–1898); P = 0.18 Mann–Whitney]. Spontaneous remission occurred in 6/19 patients and after-treatment remission occurred in 7/11 patients. Renal function was preserved in all but two patients who required haemodialysis 2 and 11 years from diagnosis.
Conclusions
We describe a cohort of patients with MN associated with viral infection, including rare cases of HIV-MN with PLA2R and THSD7A positivity. The mechanism of coincidental or viral-related MN needs to be investigated further.
Keywords: hepatitis B, hepatitis C, HIV, membranous nephropathy, PLA2R, THSD7A
INTRODUCTION
Membranous nephropathy (MN) is a common cause of nephrotic syndrome in adults. Discovery of the phospholipase A2 receptor (PLA2R) as the major target antigen in MN [1] significantly contributed to our understanding of the condition. Autoantibodies against PLA2R are most commonly found in up to 70% of primary (idiopathic) cases of MN. It has been suggested that the differentiation between primary and secondary MN should be based on the presence or absence of PLA2R in the glomerular immune deposits [2] and that this is more sensitive than circulating anti-PLA2R antibodies [3]. However, anti-PLA2R antibodies can be found in cases considered secondary due to association with malignancy or infection [4–6]. More recently, the thrombospondin type 1 domain-containing 7A (THSD7A) was identified as a target antigen in PLA2R-negative cases of MN, although its incidence is much lower, accounting for 3–9% of cases [7–10].
Hepatitis B virus (HBV) infection has a high worldwide incidence and can cause HBV-MN [11–14]. Viral components can be found in the immune deposits in MN [11, 14], although this is not always the case [15]. The role of anti-PLA2R antibodies in this setting is unclear; however, it is likely that HBV infection can induce autoimmunity and PLA2R positivity. Viraemia can be associated with the severity of nephrotic syndrome and antiviral treatment appears to be beneficial in reducing proteinuria and achieving remission [16–18].
Hepatitis C can often be associated with immune-complex glomerulonephritis (GN), mostly in the context of membranoproliferative GN or cryoglobulinaemic GN. Little is known about the role of anti-PLA2R antibodies in hepatitis C virus (HCV) infection in the context of nephrotic syndrome, with only a few cases described with PLA2R-positive HCV-MN [19].
MN has been infrequently described in the context of human immunodeficiency virus (HIV) infection, with a few published case reports [20, 21]. However, to our knowledge, no PLA2R-positive cases of HIV-MN have been reported.
Here we report the clinical, histopathological and outcome data of 19 patients with MN associated with HBV, HCV and HIV and association with PLA2R positivity.
MATERIALS AND METHODS
Study patients
From January 2008 to January 2019, 4853 native renal biopsies were performed in our centre and 466 received a diagnosis of MN (primary or secondary). We identified 21 cases of MN with a previous or active viral infection with HBV, HCV or HIV. Clinical, laboratory and histopathological data were available for 19 cases. A diagnosis of viral infection was based on the following criteria: (i) for HBV infection: evidence of serum positivity for HBV surface antigen (HbSAg), HBV e antigen (HBeAg), HBV core antibody (HBcAb), HBV e antibody (HBeAb) or detectable HBV DNA; (ii) for HCV infection: serum positivity for HCV antibody (HCVAb) or detectable HCV RNA and (iii) for HIV infection: serum positivity for anti-HIV1/HIV2 antibody or detectable HIV1/2 RNA.
Patients’ medical records were reviewed for demographics, clinical findings, treatment and clinical outcomes. Laboratory results were reviewed for viral titres, anti-PLA2R antibodies (tested by enzyme-linked immunosorbent assay, Euroimmun, Lubeck, Germany) and parameters of renal function.
Histopathology
All renal biopsies were processed according to standard techniques for light microscopy (LM), immunofluorescence (IF), immunoperoxidase (IP) and electron microscopy (EM). For each patient, glass slides were prepared and stained with haematoxylin and eosin, periodic acid–Schiff, trichrome and Jones methenamine silver. IF was performed on 4-μm cryostat sections using polyclonal fluorescein isothiocyanate (FITC)-conjugated antibodies to immunoglobulin G (IgG), IgM, IgA and complement components 3 (C3) and 1q (C1q), kappa and lambda chains. IF was scored by the pathologist on a scale of 0 to 3+. When frozen sections were not available for IF, IgG and C3 were tested by IP on 4-μm paraffin-embedded sections. IgG subtype 4 (IgG4) staining was performed on paraffin-embedded sections. EM was performed as per clinical routine.
Biopsies were stained for PLA2R by IF on proteinase-digested paraffin sections using an anti-PLA2R1 primary antibody (Sigma-Aldrich, St. Louis, MO, USA) and an FITC-conjugated anti-IgG (Life Technologies, Waltham, MA, USA) secondary antibody. For THSD7A staining on PLA2R-negative biopsies, the heat antigen retrieval method (pH 9, 95°C) was performed followed by anti-THSD7A primary antibody (Atlas). HBV-MN biopsies were also stained for HbSAg by immunohistochemistry after standard heat antigen retrieval method (pH 9, 95°C) with anti-HbSAg (Biorbyt, Cambridge, UK); HBV-positive liver sections were used as controls. Staining was visualized using a DAKO (Santa Clara, CA, USA) EnVision kit as per the manufacturer’s instructions.
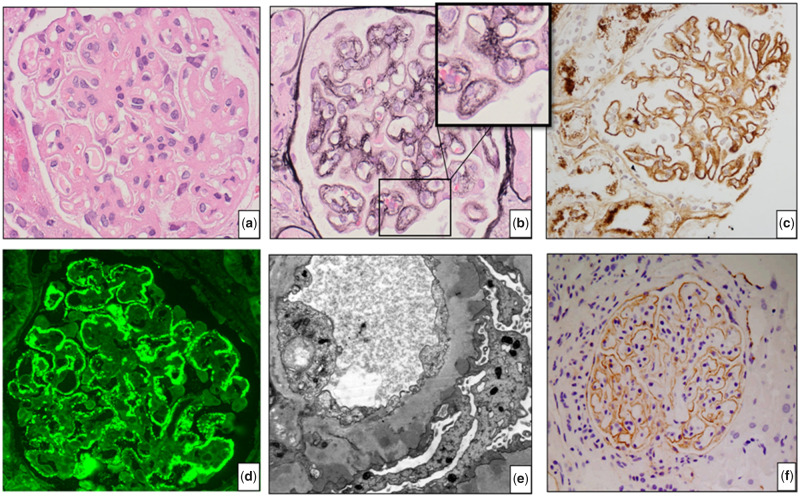
A diagnosis of MN was based on the characteristic histopathological findings on renal biopsy. These were subepithelial deposits evidenced as ‘spikes’ and ‘holes’ on silver stain, granular capillary loop staining for IgG and C3 on IF or IP and subepithelial and/or intramembranous electron dense deposits (EDDs) on EM [22]. A characteristic renal biopsy is illustrated in Figure 1.
FIGURE 1.
Renal biopsy of Case 15 (MN, HIV and HBV): (A) haematoxylin and eosin staining shows thickened glomerular capillary walls; (B) silver staining demonstrates characteristic spikes and holes along the capillary wall, shown in the inset; (C) IgG staining shows granular staining along the capillary wall; (D) IF for PLA2R shows granular staining along the capillary walls; (E) EM shows typical EDD in the subepithelial area and (F) THSD7A staining of Case 14 (HIN-MN) shows granular staining along the capillary walls.
Statistical analysis
Statistical analysis was performed using GraphPad PRISM (GraphPad Software, San Diego, CA, USA). Continuous variables are reported as median with range. Analysis was performed using non-parametric tests including the Fisher’s exact test as appropriate.
RESULTS
Clinical and laboratory features
Our cohort consisted of 19 patients, 8 males and 11 females with a median age of 42 years (range 23–74). Five patients were white, seven South Asian and seven Afro-Caribbean. Indication for biopsy was nephrotic syndrome or abnormal renal function in the context of known HBV, HCV or HIV infection.
Based on current or historical serological positivity for viral infection, six patients had a diagnosis of HBV, four had HCV and six had HIV. Two patients had HIV–HBV co-infection and one had HIV–HCV co-infection (Table 1).
Table 1.
PLA2R and THSD7A biopsy staining results in MN associated with HBV, HCV, HIV
| Virology | Total | PLA2R pos | THSD7A pos | PLA2R/THSD7A neg |
|---|---|---|---|---|
| HBV | 6 | 4 | 1 | 1 |
| HCV | 4 | 3 | 0 | 1 |
| HIV | 6 | 2 | 2 | 2 |
| HIV + HBV | 2 | 1 | 0 | 1 |
| HIV + HCV | 1 | 0 | 0 | 1 |
pos: positive; neg: negative.
PLA2R was tested by renal biopsy staining and was positive in four HBV-MN cases, three HCV-MN cases and two HIV-MN cases. In the HIV co-infection group, one HIV-HBV case was PLA2R positive but the single HIV–HCV co-infection case was PLA2R negative.
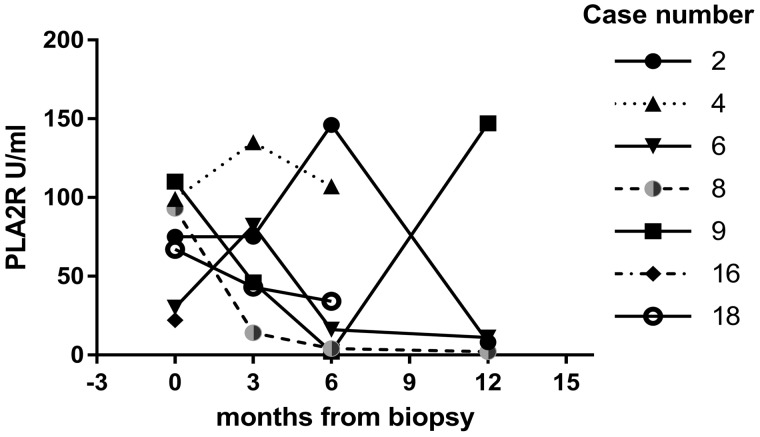
Serum anti-PLA2R antibody levels at the time of biopsy were available in 7/10 PLA2R-positive cases: 3 with HBV-MN, 2 HCV-MN and 2 HIV-MN. Serial results were available in 6/10 patients and are shown in Figure 2.
FIGURE 2.
Serum anti-PLA2R antibody levels in seven PLA2R-positive patients at the time of biopsy and 3-, 6- and 12-months post-biopsy. The increase in antibody titre in Case 9 occurred at the time of relapse.
THSD7A testing was performed by renal biopsy staining of the PLA2R-negative cases and was positive in one HBV-MN case and two HIV-MN cases.
In the HBV-MN group, the two patients with detectable HBV viral load at the time of diagnosis with MN received entecavir or tenofovir to suppress the viral load prior to the introduction of immunosuppression. Others received lamivudine or tenofovir prophylaxis to avoid viral replication while on immunosuppression for MN.
Of the four patients with HCV infection, two had received treatment with pegylated interferon at 2 and 3 years, respectively, prior to the diagnosis of PLA2R-positive MN and two had spontaneously cleared the virus. The exact time of HCV viraemia was unknown in both these cases. At the time of renal biopsy, all patients in the HCV group had a positive anti-HCVAb and none had a detectable viral load. One patient with HCV and HIV co-infection was treated with Viekirax/Exviera/Ribavirin for hepatitis C.
Nine patients had HIV including those with HBV and HCV co-infection. Most patients were on antiretroviral treatment at the time of biopsy. HIV viral loads and CD4 counts at the time of biopsy are detailed in Table 2.
Table 2.
Demographic, serological and clinical findings in patients with MN and viral infection
| Treatment |
Last follow-up |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no | MN age | Sex | Ethnicity | Virology | PLA2R biopsy | THSD7A | Lupus serology | Viral load | CD4 cells/uL | GFR | uPCR | anti-PLA2R serum | MN | Anti-viral | FU months | GFR | uPCR | anti-PLA2R serum | Complications |
| 1 | 23 | F | Asian | HBV | Neg | No tissue | Neg | HBV 83 210 copies/mL | NA | >60 | 590 | NA | Tacrolimus | Tenofovir | 24 | >60 | 0 | NA | Nephrotic in pregnancy |
| 2 | 43 | F | Asian | HBV | Pos | NA | Neg | HBV 1190 iu/mL | NA | >90 | 1030 | 75 | Tacrolimus | Entecavir | 48 | 48 | 229 | 2 | Crescentic transformation, treated with RTX and CyP |
| 3 | 39 | M | Asian | HBV | Pos | NA | Neg | HBV <100 copies/mL | NA | 55 | 170 | NA | Tacrolimus | Lamivudine | 48 | 50 | 0 | NA | None |
| 4 | 65 | F | Caucasian | HBV | Pos | NA | Neg | HBV <20 copies/mL | NA | 89 | 1337 | 99 | Supportive | None | 7 | 81 | 542 | 28 | None |
| 5 | 39 | F | Asian | HBV | Neg | Pos | Neg | HBV<20 copies/mL | NA | >90 | 675 | Neg | Supportive | None | 32 | >90 | 240 | NA | None |
| 6 | 26 | M | Asian | HBV | Pos | NA | Neg | HBV <20 copies/mL | NA | >90 | 896 | 30 | Tacrolimus | Tenofovir | 15 | >90 | 57 | 9 | None |
| 7 | 50 | M | Caucasian | HCV | Pos | NA | Neg | HCV undetectable | NA | >60 | 1253 | NA | Tacrolimus | Interferon and ribavirin | 132 | HD | HD | 3 | Relapse, treated with CyP and steroids, ESRD |
| 8 | 68 | M | Asian | HCV | Pos | NA | Neg | HCV undetectable | NA | >90 | 801 | 93 | Tacrolimus | Interferon | 46 | 80 | 425 | 2 | Guillain-Barre pre-MN, flare MN treated with RTX |
| 9 | 41 | M | Caucasian | HCV | Pos | NA | ANA+ 1:160 | HCV undetectable | NA | >90 | 1800 | 110 | Tacrolimus | None | 39 | >90 | 570 | 3.5 | Alc liver disease, non- compliant, changed to RTX |
| 10 | 33 | F | Asian | HCV | Neg | Neg | Neg | HCV undetectable | NA | >90 | 1.989 | Neg | Tacrolimus | None | 15 | >90 | 51 | NA | None |
| 11 | 31 | M | Afro-Caribbean | HIV | Neg | NA | Neg | HIV 1489 copies/mL | 800 | 67 | 65 | NA | Supportive | None | 64 | 75 | 53 | NA | None |
| 12 | 46 | M | Caucasian | HIV | Neg | Pos | Neg | HIV 81 copies/mL | 630 | >90 | 854 | Neg | Tacrolimus | Atazanavir, emtricitabine, ritonavir and tenofovir | 64 | 47 | 47 | NA | None |
| 13 | 26 | M | Afro-Caribbean | HIV | Neg | Neg | Neg | HIV <20 copies/mL | 477 | >90 | 77 | NA | Supportive | Abacavir, darunavir, lamividine and ritonavir | 16 | >90 | 50 | NA | None |
| 14 | 47 | F | Afro-Caribbean | HIV | Pos | NA | Neg | HIV <20 copies/mL | 558 | >90 | 207 | NA | Supportive | Abacavir, lamivudine and rilpivirine | 42 | >90 | 220 | NA | None |
| 15 | 44 | F | Afro-Caribbean | HIV | Neg | Pos | Neg | HIV <20 copies/mL | 600 | 16 | 548 | NA | Supportive | Abacavie, darunavir, lamivudine and ritonavir | 27 | HD | HD | NA | ESRD |
| 16 | 37 | F | Afro-Caribbean | HIV | Pos | NA | Neg | HIV <20 copies/mL | 480 | 82 | 22 | 22 | RTX | Abacavir, efavirenz and lamivudine | 6 | 82 | 22 | NA | Relapse at 5 years—retreated with RTX |
| 17 | 25 | F | Afro-Caribbean | HIV HBV | Neg | Neg | Neg | HBV 102 copies/mL HIV <20 copies/mL | NA | >60 | 286 | NA | Supportive | Abacavir, entacavir, lamivudine, lopinavir and ritonavir | 6 | >60 | 150 | NA | None |
| 18 | 74 | M | Afro-Caribbean | HIV HBV | Pos | NA | Neg | HBV undetectable HIV 21 copies/mL | 505 | 61 | 2.406 | 67 | Tacrolimus | Efavirenz, emtricitabine and tenofovir | 6 | 61 | 626 | 34 | Prostate cancer |
| 19 | 73 | M | Caucasian | HIV HCV | Neg | Neg | Neg | HCV undetectable HIV <20 copies/mL | 571 | 44 | 133 | Neg | Supportive | Emtricitabine, rilpivirine and tenofovir | 10 | 57 | 52 | NA | None |
Lupus serology: antinuclear antibody, double-stranded DNA and endonuclear antibody; GFR: mL/min/1.73 m2; UPCR: mg/mmol; anti-PLA2R antibodies: IU/mL; FU: follow-up; HD: haemodialysis; ESRD: end-stage renal disease; Pos: positive; Neg: negative; NA: not available.
Demographic and clinical findings are presented in Table 2.
Most patients had preserved renal function at the time of diagnosis with MN and only one patient had a glomerular filtration rate (GFR) <40 mL/min/1.73 m2. The median GFR at presentation was 89 mL/min/1.73 m2 (range 16–90) and the median urinary protein:creatinine ratio (uPCR) was 632 mg/mmol (range 22–2406). In the PLA2R-positive group, the median uPCR was 963 mg/mmol (range 22–2406) compared with 543 mg/mmol (range 65–1989) in the PLA2R-negative group (P = 0.18, Mann–Whitney test).
Pathologic features
LM showed a median of 17 glomeruli per biopsy (range 5–35) and 10% of total glomeruli were globally sclerosed with a median of two per biopsy (range 0–7). Interstitial fibrosis and tubular atrophy were present in 14 cases with a median of 10% (range 5–60%). Typical ‘holes’ and ‘spikes’ on silver stain were seen in 12/19 biopsies.
Immunostaining (IP or IF) revealed granular glomerular capillary wall positivity for IgG in all but one patient in whom staining was not performed. C3, was positive in all 15 cases with tissue available for staining.
Three patients with HIV infection showed positive ‘full house’ IF for IgG, IgA, IgM, C1q, C3, kappa and lambda. Mesangial EDDs were also noted in these cases, but lupus serology was negative.
PLA2R testing with IF was performed in all 19 cases and was positive in 4/6 HBV-MN, 3/4 HCV-MN and 3/9 HIV-MN cases. THSD7A was positive in 1/6 HBV-MN and 2/9 HIV-MN cases (Table 1).
IgG4 staining was performed in 15/19 cases with tissue available for staining and was positive in 8/9 PLA2R-positive and 2/6 PLA2R-negative cases. IgG4 positivity on biopsy in the PLA2R-positive and -negative groups was not statistically significantly different (P = 0.088, Fisher’s exact test).
HbSAg was not detected in any of the HBV-MN renal biopsies.
EM was performed in all patients and showed features of MN with subepithelial EDDs. In 12/19 cases, these were mostly Stage II and the remaining cases showed deposits of all stages I–IV. Tubuloreticular inclusions were noted in five cases: three with HBV-MN and two with HCV-MN. Mesangial deposits were seen in 11 cases.
None of the biopsies demonstrated segmental or global collapse of the glomerular tuft characteristic of HIV-associated nephropathy.
The renal biopsy findings are illustrated in Figure 1 and detailed in Table 3.
Table 3.
Renal biopsy findings in patients with MN and viral infection
| Deposits |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Virology | PLA2R staining | THSD7A staining | HbS Ag staining | Total glomeruli | Globally sclerotic glomeruli | IFTA (%) | IF, IP | IgG4 | EM stage | Sub epithelial | Intra membranous | Sub endothelial | Mesangial | TRIs |
| 1 | HBV | Neg | NA | Neg | 8 | 0 | 0 | IgG++, IgM+++ | NA | I–II | Yes | No | No | No | No |
| 2 | HBV | Pos | NA | Neg | 16 | 0 | 10 | Not done | Pos++ | I–II | Yes | Yes | No | No | No |
| 3 | HBV | Pos | NA | Neg | 17 | 7 | 30 | IgG+, C3+,mesangial IgM+ | Pos+++ | I–IV | Yes | Yes | No | Yes | No |
| 4 | HBV | Pos | NA | Neg | 15 | 2 | 10 | IgG+ IgM+/−, C3 trace, IgA and C1q: negative | Pos+++ | II | Yes | No | Yes | Yes | Yes |
| 5 | HBV | Neg | Pos | Neg | 21 | 1 | 0 | IgG++ IgA+/− IgM+/− C3+/− C1q negative | Neg | I | Yes | No | Yes | Yes | Yes |
| 6 | HBV | Pos | NA | Neg | 25 | 0 | 0 | IgG++ IgM+/− C3+/− IgA and C1q: negative | Pos+++ | II | Yes | Yes | No | Yes | Yes |
| 7 | HCV | Pos | NA | NA | 18 | 1 | 15 | IgG++, mesangial IgM+, C1q++, C3+ | Pos+ | II | Yes | Yes | No | No | Yes |
| 8 | HCV | Pos | NA | NA | 16 | 2 | 5 | IgG++, IgM+/−,C3+ | Pos++ | I–II | Yes | Yes | No | Yes | No |
| 9 | HCV | Pos | NA | NA | 16 | 1 | 10 | IgG+++, C3++ | Pos++ | II | Yes | No | No | No | Yes |
| 10 | HCV | Neg | Neg | NA | 28 | 0 | 0 | IgG+ | Pos+ | II | Yes | Yes | No | Yes | No |
| 11 | HIV | Neg | NA | NA | 27 | 5 | 10 | IgG+, IgA+, IgM++, C3++ | NA | I–IV | Yes | Yes | Yes | Yes | No |
| 12 | HIV | Neg | Pos | NA | 23 | 2 | 5 | IgG+, IgM++, IgA Neg | NA | I–II | Yes | Yes | No | No | No |
| 13 | HIV | Neg | Neg | NA | 35 | 2 | 15 | IgG+, IgA+,IgM++ C1q+, C3++ | Neg | I–IV | Yes | Yes | No | Yes | No |
| 14 | HIV | Pos | NA | NA | 15 | 1 | 0 | IgG+, C3+ | Neg | I–IV | Yes | Yes | No | No | No |
| 15 | HIV | Neg | Pos | NA | 15 | 5 | 60 | IgG++, IgM++, C1q++, C3++ | Neg | II | Yes | Yes | No | Yes | No |
| 16 | HIV | Pos | NA | NA | 12 | 1 | 10 | IgA+ (segmental), IgG+++, IgM++, Mesangial IgA+, IgM+ | Pos ++ | III–IV | Yes | Yes | No | Yes | No |
| 17 | HIV HBV | Neg | Neg | Neg | 19 | 4 | 5 | IgG+, IgA++, C3+, C1q++, IgM+ mesangial IgM+ and C1q++ | Neg | II | Yes | No | Yes | No | No |
| 18 | HIV HBV | Pos | NA | Neg | 5 | 2 | 5 | IgG++, IgM+, segmental IgA+, C3+, C1q+ | NA | II | Yes | Yes | No | Yes | No |
| 19 | HIV HCV | Neg | Neg | NA | 24 | 3 | 5 | IgG+, scanty mesangial IgA+/−, IgM+/− C3+/− | Pos + | II | Yes | No | No | No | No |
IFTA: interstitial fibrosis and tubular atrophy; TRIs: tubuloreticular inclusions; NA: not available.
Clinical follow-up and treatment
Follow-up was available for 19 patients. The median follow-up was 32 months (range 6–132). Complete remission (CR) or partial remission (PR) occurred in 13/19 cases; CR was defined as a uPCR <50 mg/mmol and PR was defined as a reduction of uPCR by 50% and <300 mg/mmol. Spontaneous remission occurred in 6/19 cases; 1 with THSD7A-positive HBV-MN and 5 with HIV-MN, 4 with PLA2R-negative HIN-MN and 1 with PLA2R-positive HIV-MN. These patients received supportive care for nephrotic syndrome with angiotensin enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), diuretics, cholesterol-lowering agents, anticoagulation and antiviral treatment for the viral infection as required.
Immunosuppressive treatment for MN was administered to 11/19 cases, with tacrolimus (n = 10) or rituximab (RTX;n = 1) as the first-line treatment (Table 2). In the tacrolimus group, 6/10 cases achieved remission (Table 4). Three cases did not respond to or relapsed after discontinuation of tacrolimus and were treated with RTX (Cases 8 and 9) or with cyclophosphamide (CyP) and steroids (Case 7) (Table 2). One patient remains under active monitoring on tacrolimus with a treatment duration of 6 months and a response has not yet been established (Table 2). The patient treated with RTX as first line achieved remission (Table 4).
Table 4.
Response to treatment in relation to viral infection and antibody status based on biopsy staining
| Virology | MN antibody | Treatment | Outcome |
|---|---|---|---|
| HBV | PLA2R | Tacrolimus | CR |
| HBV | Neg | Tacrolimus | CR |
| HBV | PLA2R | Tacrolimus | CR |
| HBV | PLA2R | Tacrolimus | PR |
| HCV | Neg | Tacrolimus | CR |
| HCV | PLA2R | Tacrolimus | Relapse/HD |
| HCV | PLA2R | Tacrolimus | Relapse |
| HCV | PLA2R | Tacrolimus | NR |
| HIV | THSD7A | Tacrolimus | CR |
| HIV + HBV | PLA2R | Tacrolimus | NA |
| HIV | PLA2R | RTX | CR |
HD: haemodialysis; NR: no response; NA: not available.
Interestingly, Case 2 with PLA2R-positive HBV-MN was treated with tacrolimus and developed crescentic transformation 1 year from the original presentation in the absence of circulating anti-neutrophil cytoplasm antibodies (ANCA) or anti-glomerular basement (anti-GBM) antibodies. Tacrolimus was then discontinued and treatment for crescentic GN with CyP and RTX was administered with a good response.
Anti-PLA2R antibodies were detected in the serum of seven PLA2R biopsy-positive cases and decreased or disappeared after treatment with tacrolimus. In cases that relapsed, a return of the circulating antibody was noted. Case 4, with HBV-MN and high antibody titre at presentation, was treated with supportive treatment alone at the patient’s choice and 7 months later proteinuria and antibody titres had markedly decreased, although the patient remained nephrotic.
Two THSD7A-positive cases had HIV-MN; both achieved remission of nephrotic syndrome, one with supportive treatment and one with tacrolimus. The third case with THSD7A positivity had HBV-MN and achieved spontaneous remission with supportive treatment alone (Table 2).
Renal function was preserved in all but two patients who required dialysis 2 and 11 years from diagnosis of MN (Table 2). The former (Case 15) with HIV-MN presented late, with significant renal dysfunction and atrophy on biopsy, and the latter (Case 7) with HCV-MN received treatment initially with tacrolimus and then with CyP and steroids (Table 2).
The duration of the viral infection was not clear in any of the patients.
DISCUSSION
MN can be associated with the viral infection; however, the role of PLA2R in this setting is unclear. The first case of membranous GN associated with HBV was described in 1971, with a patient developing MN after transfusion-related HBV infection and detection of the Australian antigen in the immune complexes [13]. Since then, more cases have been described [11, 14]. Recently, in a large series of Chinese patients with MN and HBV infection, 25 of 39 (64%) patients were found to be PLA2R positive on renal biopsies and the PLA2R co-localized with HbSAg along the capillary walls [5]. Other studies have found a smaller proportion of PLA2R positivity [23], and HBV antigens are not always found in renal biopsies [15].
In our cohort, 6/19 cases had HBV-MN and 4 were PLA2R positive on biopsy with detectable circulating anti-PLA2R antibody in 3/4 cases tested. The presence of PLA2R suggests primary MN, and although the coexistence of viral infection could be a coincidence, it is difficult to ignore the possibility of the viral infection acting as a trigger for the production of anti-PLA2R antibodies. The histopathological findings in some cases in our series extended beyond the typical positivity for IgG, C3 and subepithelial EDDs. Interestingly, mesangial deposits were seen in 11 cases and 8 of these were PLA2R positive. Although typically mesangial deposits are seen in lupus MN, there was no clinical or serological evidence of lupus in these cases.
One patient with PLA2R-positive HBV-MN (Case 2) developed crescentic transformation on biopsy almost 2 years from the original diagnosis. This patient initially presented with nephrotic syndrome, a high HBV viral load and elevated anti-PLA2R titres. Despite treatment with entecavir and suppression of the viral load, the anti-PLA2R titre remained high and treatment with tacrolimus was commenced. Although there was an initial response to tacrolimus with an improvement of proteinuria, renal function deteriorated and a renal biopsy showed crescents in the absence of circulating ANCA or anti-GBM antibody. The patient was subsequently treated with steroids, RTX and CyP with good results. RTX has been proven to be safe in HBV-associated MN once the viral load has been controlled [15]; however, it is unclear what triggered this crescentic transformation at the time the HBV viral load was undetectable and the patient was established on anti-HBV therapy.
In the hepatitis C cohort, we found no evidence of the proliferative changes usually associated with HCV nephritis but the typical ‘spikes’ and ‘holes’ of the capillary walls found in MN. Two of the three PLA2R-positive HCV-MN cases were previously treated with interferon, which raises the question about the potential link between interferon and PLA2R positivity. Our cases are consistent with the theory that interferon triggers an immunological response leading to PLA2R-positive MN [24, 25]. One of these cases also had tubuloreticular inclusions on biopsy and the other had mesangial deposits. The two cases that spontaneously cleared the virus had a mixture of deposits, including the typical subepithelial deposits seen in MN, suggesting immune complex deposition during the active HCV infection. In our cohort, 3/4 cases with HCV-MN were PLA2R positive on biopsy with circulating anti-PLA2R antibodies detected in 2/3 cases tested. Hepatitis C-associated MN has been described as PLA2R positive in 24% of HCV-MN cases in a recent study [19], which suggested a possible immunological trigger during the viral infection that leads to autoimmunity, although it is impossible to rule out a coincidence.
MN has been described in the context of HIV; however, to our knowledge, no reports of PLA2R-positive HIV-related MN are available. Here we present three cases with PLA2R-positive HIV-MN and two with THSD7A-positive HIV-MN. The first PLA2R-positive case had been on highly active antiretroviral treatment with undetectable viral load at the time of presentation with nephrotic syndrome and went into spontaneous remission with ACEi/ARB therapy. The second case presented with nephrotic syndrome, preserved renal function and detectable circulating anti-PLA2R antibodies. Biopsy showed a ‘full house’ IF pattern, suggesting a possible lupus-like GN; however, there was no clinical or serological evidence of lupus and the patient was treated with RTX with good results. Little is known about lupus-like GN in the context of HIV, but it is recognized that HIV infection is associated with polyclonal B-cell activation, potentially explaining the positivity for PLA2R antibodies found in our cases. Although circulating immune complexes are seen in patients infected with HIV, it is believed that in MN, the immune complexes are formed in situ at the podocyte, suggesting a different mechanism of disease in HIV-MN than in the lupus-like GN of HIV. The full house IF in some HIV cases has been described previously [26], and we report three cases with full house IF in our cohort. It is recognized that the HIV viral load can correlate negatively with renal function in cases of HIV-associated nephropathy and one study failed to show a significant effect of antiretroviral therapy on renal survival in HIV-infected patients [27]. In our cohort, however, all but one patient had well-controlled HIV with undetectable viral load at the time of presentation, suggesting a different mechanism of disease. The third patient with HIV and PLA2R positivity had HIV–HBV coinfection and presented with nephrotic syndrome and a high serum anti-PLA2R antibody titre on a background of prostatic cancer and undetectable viral load for both HIV and HBV. The presence of malignancy in the background possibly argues towards a ‘secondary’ MN. However, it is difficult to determine with certainty if the PLA2R-positive HIV-MN in the setting of both malignancy and viral infection is in fact primary or secondary to any of the underlying conditions or a chance occurrence.
The significance of THSD7A positivity in the three cases in our cohort is not fully understood. THSD7A is thought to be more common in cases of MN associated with malignancy [9], although the overall incidence is still unclear. In our series, 3/19 (15%) cases with viral MN were THSD7A positive, and this incidence is higher than described; it is possible that the THSD7A-positive MN is more common in the context of a viral infection, and this should be investigated. The viral infection could act as an immunological stimulus for the production of anti-THSD7A antibodies in these cases of MN, just like in PLA2R-related MN. Two of the THSD7A-positive cases had HIV-MN; both achieved remission of nephrotic syndrome: one with supportive treatment and one with tacrolimus. The third case with THSD7A positivity had HBV-MN and achieved spontaneous remission with supportive treatment alone.
Spontaneous remission was more likely in the HIV-MN antibody-negative group. Of the 6/19 patients that achieved spontaneous remission within 1 year from diagnosis 4 (67%) where HIV-MN antibody negative, one was HIV-MN, PLA2R positive and one was HBV-MN, THSD7A positive. Of the patients that received treatment with tacrolimus or RTX (n = 11), response to treatment (CR or PR) was observed in 64% (n = 7) of cases (4 HBV-MN, 1 HCV-MN and 2 HIV-MN; Table 4). Serum anti-PLA2R antibodies decreased or disappeared in the cases that went into remission and returned in cases that relapsed. Antiviral therapy with tenofovir or entecavir was administered to patients with HBcAb positivity to prevent reactivation while on immunosuppression and this was tolerated well with no side effects.
In conclusion, we present a cohort of 19 patients with MN and viral infection with HBV, HCV and HIV, 10 cases with anti-PLA2R positivity and 3 with anti-THSD7A positivity. We present the first patients with HIV and PLA2R or THSD7A positivity on biopsy. Spontaneous remission was common in the HIV-MN antibody group and 31% of patients in our cohort achieved remission with supportive treatment alone. It is likely that the presence of PLA2R or THSD7A is associated with an immunological trigger related to the viral infection; however, the development of MN in these cases could be a coincidence and it is difficult to determine whether MN in this setting is primary or secondary. These results provide a valuable addition to our knowledge of PLA2R and THSD7A associated MN, but further research is needed to clarify the role of these antibodies in the setting of viral infection and MN.
ACKNOWLEDGEMENTS
Infrastructure support for this research was provided by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre. C.R. is supported by the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
FUNDING
Infrastructure support for this research was provided by the NIHR Imperial Biomedical Research Centre.
AUTHORS’ CONTRIBUTIONS
A.N. conceived the study, collected data, performed the biopsy staining, designed the analysis and wrote the article. C.T. collected data. H.T.C. and C.R. contributed histopathology data and critically revised the manuscript. J.B.L. contributed data and revised the manuscript. T.H.D.C. and M.E.G. contributed data. M.E.G. and C.D.P., joint senior authors, oversaw the analysis and final approval.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Beck LH Jr, Bonegio RG, Lambeau G. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoxha E, Kneißler U, Stege G. et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 2012; 82: 797–804 [DOI] [PubMed] [Google Scholar]
- 3. Debiec H, Ronco P.. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 2011; 364: 689–690 [DOI] [PubMed] [Google Scholar]
- 4. Ponticelli C, Glassock RJ.. Glomerular diseases: membranous nephropathy–a modern view. Clin J Am Soc Nephrol 2014; 9: 609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie Q, Li Y, Xue J. et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol 2015; 41: 345–353 [DOI] [PubMed] [Google Scholar]
- 6. Qin W, Beck LH Jr, Zeng C. et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 2011; 22: 1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomas NM, Beck LH Jr, Meyer-Schwesinger C. et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014; 371: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoxha E, Beck LH, Wiech T. et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol 2017; 28: 520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang C, Zhang M, Chen D. et al. Features of phospholipase A2 receptor and thrombospondin type-1 domain-containing 7A in malignancy-associated membranous nephropathy. J Clin Pathol 2019; 72: 705–711 [DOI] [PubMed] [Google Scholar]
- 10. Tian C, Li L, Liu T. et al. Circulating antibodies against M-type phospholipase A2 receptor and thrombospondin type-1 domain-containing 7A in Chinese patients with membranous nephropathy. Int Urol Nephrol 2019; 51: 1371–1377 [DOI] [PubMed] [Google Scholar]
- 11. Lai KN, Li PKT, Lui SF. et al. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med 1991; 324: 1457–1463 [DOI] [PubMed] [Google Scholar]
- 12. Levy M, Kleinknecht C.. Membranous glomerulonephritis and hepatitis B virus infection. Nephron 1980; 26: 259–265 [DOI] [PubMed] [Google Scholar]
- 13. Combes B, Shorey J, Barrera A. et al. Glomerulonephritis with deposition of Australia antigen-antibody complexes in glomerular basement membrane. Lancet 1971; 2: 234–237 [DOI] [PubMed] [Google Scholar]
- 14. Bhimma R, Coovadia HM.. Hepatitis B virus-associated nephropathy. Am J Nephrol 2004; 24: 198–211 [DOI] [PubMed] [Google Scholar]
- 15. Berchtold L, Zanetta G, Dahan K. et al. Efficacy and safety of rituximab in hepatitis B virus-associated PLA2R-positive membranous nephropathy. Kidney Int Rep 2018; 3: 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung DR, Yang WS, Kim SB. et al. Treatment of hepatitis B virus associated glomerulonephritis with recombinant human alpha interferon. Am J Nephrol 1997; 17: 112–117 [DOI] [PubMed] [Google Scholar]
- 17. Gan SI, Devlin SM, Scott-Douglas NW. et al. Lamivudine for the treatment of membranous glomerulopathy secondary to chronic hepatitis B infection. Can J Gastroenterol 2005; 19: 625–629 [DOI] [PubMed] [Google Scholar]
- 18. Kanaan N, Horsmans Y, Goffin E.. Lamivudine for nephrotic syndrome related to hepatitis B virus (HBV) infection. Clin Nephrol 2006; 65: 208–210 [DOI] [PubMed] [Google Scholar]
- 19. Larsen CP, Messias NC, Silva FG. et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol 2013; 26: 709–715 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Z, Hever A, Bhasin N. et al. Secondary syphilis associated with membranous nephropathy and acute hepatitis in a patient with HIV: a case report. Perm J 2018; 22: 17–062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Numata A, Akimoto T, Toshima M. et al. Membranous nephropathy in an HIV-positive patient complicated with hepatitis B virus infection. Clin Exp Nephrol 2011; 15: 769–773 [DOI] [PubMed] [Google Scholar]
- 22. Fogo AB, Kashgarian M.. Glomerular diseases. In: Fogo AB, Kashgarian M (eds).Diagnostic Atlas of Renal Pathology. Third Edition). Philadelphia: Elsevier, 2017:19–294 [Google Scholar]
- 23. Dong HR, Wang YY, Cheng XH. et al. Retrospective study of phospholipase A2 receptor and IgG subclasses in glomerular deposits in Chinese patients with membranous nephropathy. PLoS One 2016; 11: e0156263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson LE, Widman D, Dikman SH. et al. Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin Arthritis Rheum 2002; 32: 163–173 [DOI] [PubMed] [Google Scholar]
- 25. Feng B, Eknoyan G, Guo Z-S. et al. Effect of interferon-alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27: 640–646 [DOI] [PubMed] [Google Scholar]
- 26. Haas M, Kaul S, Eustace JA.. HIV-associated immune complex glomerulonephritis with “lupus-like” features: a clinicopathologic study of 14 cases. Kidney Int 2005; 67: 1381–1390 [DOI] [PubMed] [Google Scholar]
- 27. Szczech LA, Gupta SK, Habash R. et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 2004; 66: 1145–1152 [DOI] [PubMed] [Google Scholar]