Abstract
Background
Previous studies in patients on haemodialysis (HD) have shown an association of fibroblast growth factor 23 (FGF23) with all-cause mortality. As of yet, the result of FGF23 lowering on mortality is unknown in this population.
Methods
FGF23 was measured in a subset of 404 patients from the Dutch CONvective TRansport STudy (CONTRAST study) [a randomized trial in prevalent dialysis patients comparing HD and haemodiafiltration (HDF) with clinical outcome] at baseline and Months 6 and 12. A substantial decline of FGF23 change over time was anticipated in patients randomized to HDF since HDF induces higher dialytic clearance of FGF23. The associations of both baseline FGF23 and 6-months change in FGF23 with all-cause mortality were analysed. In addition, the difference in FGF23 change between HD and HDF was explored. Furthermore, the role of dialysis modality in the association between FGF23 change and outcome was analysed.
Results
No association was observed between quartiles of baseline FGF23 and all-cause mortality. Over 6 months, FGF23 declined in patients on HDF, whereas FGF23 remained stable in patients on HD. A decrease in FGF23 was not associated with improved survival compared with a stable FGF23 concentration. However, increasing FGF23 was associated with a significantly higher mortality risk, both in crude and fully adjusted models [hazard ratio 2.01 (95% confidence interval 1.30–3.09)].
Conclusion
Whereas no association between a single value of FGF23 and all-cause mortality was found, increasing FGF23 concentrations did identify patients at risk for mortality. Since lowering FGF23 did not improve outcome, this study found no argument for therapeutically lowering FGF23.
Keywords: chronic haemodialysis, CKD-MBD, clinical trial, ESRD, FGF-23, haemodiafiltration, haemodialysis, survival analysis
INTRODUCTION
Mortality rates in patients treated with haemodialysis (HD) remain unacceptably high despite contemporary medication and modern dialysis equipment. Besides classical risk factors for cardiovascular disease (CVD), such as smoking, diabetes and hypertension, non-classical risk factors such as fluid overload and chronic kidney disease–associated mineral bone disease (CKD-MBD) contribute to this high mortality risk [1–3]. However, intervention studies targeting these CKD-MBD components generally failed to show a beneficial effect on CVD morbidity and mortality [4–8]. More recently, fibroblast growth factor 23 (FGF23) has emerged as a key player in MBD pathophysiology. FGF23 is a bone-derived hormone maintaining phosphate balance by enhancing its renal excretion [9–11]. During the course of CKD, FGF23 values rise, reaching the highest levels in end-stage renal disease (ESRD) patients on dialysis [12]. Although this physiological adaptation is crucial for maintaining phosphate balance in early CKD, prolonged exposure in advanced CKD may have deleterious effects [13–15]. Several observational studies have shown an independent association between increased FGF23 levels and mortality through all stages of CKD, including dialysis [16–19]. However, it is debated whether interventions that lower FGF23 levels lower mortality risk. Previous studies in patients on HD have been performed in incident dialysis patients and lack longitudinal FGF23 measurements or have observed the natural history of FGF23 evolution over time [20, 21]. In the current study we explored the association between the change in FGF23 over time and all-cause mortality in a cohort of patients treated with either low-flux HD or haemodiafiltration (HDF) [22]. This is of interest, as convective transport has been shown to effectively lower FGF23 [23–25].
MATERIALS AND METHODS
Study design and patient population
For the present study, data from the CONTRAST study (ClinicalTrials.gov NCT00205556) were used. CONTRAST was a randomized controlled clinical trial evaluating the effect of online post-dilution HDF versus low-flux HD on mortality and cardiovascular outcomes. The methods of the CONTRAST study have been described elsewhere [22, 26]. In short, data were collected in 29 dialysis centres in The Netherlands, Canada and Norway between 2004 and 2010. Adult patients with ESRD on HD two or three times weekly for at least 2 months were eligible for inclusion. Exclusion criteria were treatment with HDF or high-flux HD in the 6 months prior to randomization, an expected lifespan >3 months, severe non-compliance to dialysis treatment or participation in another clinical intervention trial. CONTRAST was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by central and all local medical ethics review boards. Before enrolment, written informed consent was obtained from all participants [26].
Of the original 714 patients enrolled in CONTRAST, stored plasma samples from baseline were available in 404 patients for measurement of FGF23. Those samples originated from 17 of the 29 participating centres in the CONTRAST study, of which it was logistically feasible to collect and store extra plasma samples at −80°C [27]. Of these, plasma samples were available at Month 6 in 341 patients and at 12 months in 291 patients.
Data collection
At baseline, data on demographic characteristics, medical history, medication and duration of dialysis were recorded. Every 3 months laboratory values, medication, treatment characteristics and information on clinical events were obtained. Blood samples were drawn at the beginning of dialysis. The mean delivered convection volume (substitution volume plus net ultrafiltration) in HDF was estimated with the following formula: mean delivered convection volume = (HDF treatments/total number of treatments) × mean convection volumes of the three treatments preceding the quarterly visit [22].
Laboratory measurements
All routine laboratory measurements were analysed locally by the participating hospitals using standard laboratory techniques. From the stored samples at −80°C the C-terminal FGF23 was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) (Immutopics, San Clemente, CA, USA). The intra- and interassay coefficient of variation of this assay are <5% and <16%, respectively [28]. If values were >16 000 RU/mL, then additional dilution was performed in order to optimize the validity of the test results.
Statistical analysis
Baseline characteristics were presented as proportions, means with their corresponding standard deviations or as medians with interquartile ranges (IQRs) when data had a skewed distribution.
For all mortality analyses, an intention-to-treat approach was applied. Follow-up for survival was complete, as patients who discontinued the randomized treatment (e.g. due to renal transplantation, a switch to peritoneal dialysis or moving to a non-participating centre) were still followed for cause-specific mortality until the end of the study. Since transplantation during follow-up is a competing treatment affecting mortality, all models were adjusted for transplantation by adding transplantation as a time-varying covariate, as recently suggested [29]. Adjusted models were corrected for age, diabetes, history of CVD, dialysis vintage, residual kidney function and serum phosphate concentration.
First, the relation between quartiles of baseline FGF23 concentration and all-cause mortality was analysed using Cox proportional hazard models.
Second, we explored the possible difference in the rate of change of FGF23 in HDF compared with HD using generalized linear mixed models with an interaction term between treatment modality and time. When appropriate, stratified models were fitted. For these analyses, models with a random slope, random intercept or both were used depending on the lowest Akaike information criterion. Change in FGF23 concentrations in 6-months time were calculated as absolute and relative change.
In a supplemental analysis, we investigated whether the relative change in FGF23 over 6 months was different for different tertiles of the mean reached convention volume. For this, a P-value for trend was calculated. Convective volume for the HD group was set to zero, assuming differences in net ultrafiltration between study arms did not differ.
Third, the association between the change in FGF23 concentrations over 6 months and all-cause mortality was analysed. An interaction term was used to evaluate if the 6-months change in FGF23 differed between HD and HDF in a Cox regression model. Since no interaction was found, we continued with the pooled cohort. Patients were categorized into tertiles with either declining, relatively stable or increasing FGF23 concentrations as determined by the absolute difference between FGF23 concentration from baseline to Month 6. Stable FGF23 concentrations were set as the reference and defined by the mid-tertile range. Both crude and adjusted models were fitted with identical confounders as described above. In addition, baseline FGF23 was added in a model. Lastly, treatment modality was added to the model in a final Cox regression model.
Hazard ratios (HRs) are reported with 95% confidence intervals (CIs). All P-values are two-sided and considered statistically significant if <0.05.
Sensitivity analysis
In a sensitivity analysis, crude and adjusted Cox regression models were fitted for baseline FGF23 quartiles using an ‘on treatment’ approach. In addition, a Cox regression model with time-varying covariate analysis was performed at baseline and at 12 months with the quartiles of baseline FGF23 concentrations.
RESULTS
Baseline characteristics
Baseline characteristics for the entire CONTRAST cohort and the subgroup of 404 patients with FGF23 measurements are shown in Table 1. In the FGF23 cohort, the mean age was 63.5 ± 13.9 years, 38.1% were female and 43.1% had a history of CVD. No calcimimetics were used. The median FGF23 was 4384 RU/mL (IQR 1829–12 210). No remarkable differences were observed between patients randomized to HD or HDF at baseline, including baseline FGF23 (P for difference 0.54).
Table 1.
Baseline characteristics
| Baseline characteristics | Contrast population | FGF23 cohort | FGF23 cohort-HD | FGF23 cohort-HDF |
|---|---|---|---|---|
| n | 714 | 404 | 202 | 202 |
| Demographic characteristics | ||||
| Age (years) | 64.1 ± 13.7 | 63.5 ± 13.9 | 63.1 ± 13.6 | 63.9 ± 14.1 |
| Gender (female), % | 37.7 | 38.1 | 35.1 | 41.1 |
| Race (Caucasian), % | 84 | 82 | 81 | 84 |
| Body mass index (kg/m²) | 25.4 ± 4.8 | 25.0 ± 4.8 | 25.2 ± 4.7 | 24.7 ± 4.86 |
| Medical history | ||||
| Diabetes, % | 25 | 21 | 20 | 23. |
| Current smoking, % | 19 | 20 | 20 | 10 |
| Prior CVD, % | 56 | 43 | 43 | 43 |
| Prior kidney transplantation, % | 11 | 10 | 15 | 6 |
| Dialysis vintagea (years), median (IQR) | 2.0 (1.0–4.0) | 1.8 (0.9–3.3) | 2 (1.0–3.7) | 1.7 (0.9–3.0) |
| Dialysis characteristics | ||||
| Kt/V | 1.40 ± 0.22 | 1.38 ± 0.21 | 1.36 ± 0.17 | 1.40 ± 0.24 |
| Average dialysis time (hours) | 3.77 ± 0.38 | 3.78 ± 0.39 | 3.78 ± 0.38 | 3.78 ± 0.40 |
| Residual kidney function,b % | 52.7 | 56.4 | 54 | 58.9 |
| If yes, 24-h urine (mL/24 h), median (IQR) | 700 (350–1150) | 700 (324–1110) | 625 (352–1100) | 750 (275–1150) |
| If yes, eGFRc (mL/min/1.73 m²), median (IQR) | 1 (0–3) | 1 (0–3) | 0 (0–3) | 1 (0–4) |
| Laboratory values | ||||
| Haemoglobin (mmol/L) | 7.3 ± 0.8 | 7.4 ± 0.8 | 7.3 ± 0.7 | 7.4 ± 0.8 |
| Creatinine (µmol/L) | 860.8 ± 255.5 | 866.5 ± 251.9 | 897.5 ± 257.7 | 835.4 ± 242.6 |
| Serum albumin (g/L) | 40.4 ± 3.8 | 40.0 ± 4.1 | 40.1 ± 4.26 | 40.0 ± 6.9 |
| Calcium (mmol/L) | 2.31 ± 0.18 | 2.31 ± 0.17 | 2.31 ± 0.17 | 2.32 ± 0.18 |
| Phosphate (mmol/L) | 1.64 ± 0.49 | 1.66 ± 0.52 | 1.64 ± 0.47 | 1.69 ± 0.56 |
| PTH (pmol/L), median (IQR) | 20.5 (10.5–35.2) | 21.0 (11.0–36.0) | 21.5 (11–37.9) | 20.4 (10.6–35.0) |
| FGF23 (RU/mL), median (IQR) | 4384 (1829–12 210) | 4384 (1829–12 210) | 4983 (1815–12 265) | 3691 (1826–12 293) |
| Medication prescription, % | ||||
| Active vitamin D | 66.9 | 70.0 | 74.1 | 65.8 |
| Calcium-containing phosphate binder | 41.4 | 49.6 | 50.2 | 49.6 |
| Non-calcium-containing phosphate binder | 66.8 | 69.8 | 72.8 | 66.8 |
| RAS inhibitors | 49.4 | 53.5 | 53.5 | 53.5 |
| β-blockers | 53.7 | 56.2 | 59.4 | 53 |
| Statins | 52.0 | 51.2 | 47.5 | 55 |
Data are presented as mean ± SD unless stated otherwise.
Dialysis vintage is the sum of time on HD and/or PD.
Residual kidney function defined as diuresis >100 mL/24 h.
Estimated glomerular filtration rate as the mean of urea and creatinine clearance in 24-h urine collection. RAS, renin–angiotensin system; PTH, parathyroid hormone; PD, peritoneal dialysis.
Baseline FGF23 and risk for all-cause mortality
The median follow-up of 404 patients in the FGF23 cohort was 3.0 years (IQR 1.5–4.4). In total, 156 patients died during follow-up. The results of the survival analyses are shown in Table 2. The HR for all-cause mortality did not differ between quartiles of baseline FGF23, both in the crude and the adjusted models. The sensitivity analyses (on-treatment analysis and time-varying covariate analysis) yielded similar results (Supplementary data, Tables S1 and S2).
Table 2.
HRs of baseline quartiles of FGF23 for all-cause mortality
| Quartile | FGF23 values | Crude HR (95% CI) | Model 1 HR (95% CI)a | Model 2 HR (95% CI)b |
|---|---|---|---|---|
| 1 | Lowest to 1829 | Ref | Ref | Ref |
| 2 | 1829–4383 | 1.20 (0.79–1.83) | 1.16 (0.76–1.76) | 1.08 (0.69–1.70) |
| 3 | 4384–12 209 | 0.94 (0.60–1.45) | 1.10 (0.71–1.71) | 1.12 (0.68–1.83) |
| 4 | 12 210 to highest | 0.72 (0.45–1.16) | 1.05 (0.65–1.70) | 1.08 (0.62–1.88) |
Cox regression, intention to treat analysis. Results are shown as HRs with 95% CIs. All models are adjusted for transplantation by adding transplantation as a time-varying covariate.
Model 1 = adjusted for age.
Model 2 = Model 1 plus adjustment for sex, diabetes, history of CVD, dialysis vintage, residual kidney function and phosphate.
FGF23 over time: impact of HDF
The rate of change of FGF23 was statistically significantly different between HD and HDF (P for interaction <0.001) (Figure 1). In patients on HDF, FGF23 decline was 432 RU/mL/month (95% CI –663 to −201; P < 0.001), while FGF23 concentrations in patients on HD did not change [103 RU/mL/month (95% CI −83–290); P = 0.276]. Additional analysis on convection volume showed a positive relation between the decline in FGF23 and convection volume (P for trend 0.02, as shown in Supplementary data, Figure S1).
FIGURE 1.
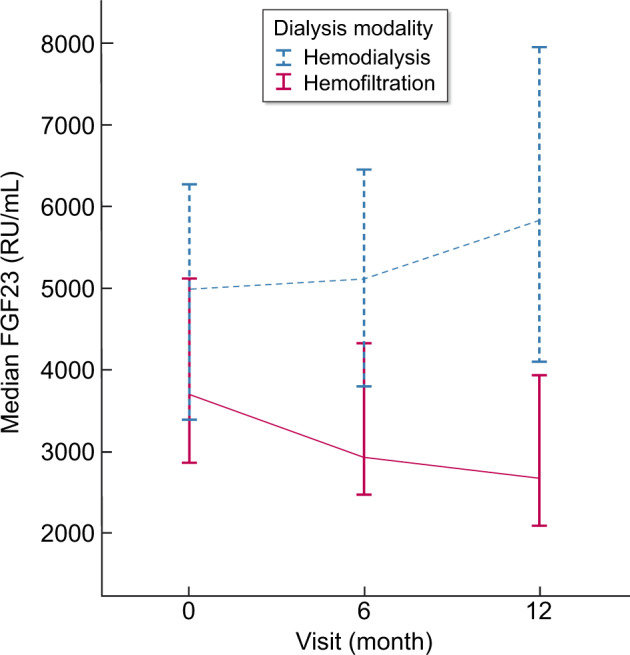
Course of FGF23 concentrations over time in patients on hemodialysis and hemodiafiltration. FGF23 concentrations are depicted as medians and corresponding 95% CIs at baseline, 6 and 12 months.
Change in FGF23 concentrations and risk of all-cause mortality
The 341 patients for whom measurements of both baseline and Month 6 were available were divided into tertiles of change in FGF23. Table 3 demonstrates HRs for all-cause mortality for the tertiles of FGF23 change. Figure 2 shows the survival curve of tertiles of FGF23 change in 6-months time. Decreasing FGF23 was not associated with all-cause mortality [HR 0.96 (95% CI 0.61–1.50)], whereas increasing FGF23 was associated with an increased mortality risk [HR 1.56 (95% CI 1.03–2.35); P = 0.03]. Adjusted analyses yielded similar results [HR 2.01 (95% CI 1.32–3.07); P = 0.001].
Table 3.
HRs for all-cause mortality of tertiles of FGF23 change during 6 months
| Tertiles | Stable FGF23 (n = 115) | Decreasing FGF23 (n = 114) | Increasing FGF23 (n = 114) |
|---|---|---|---|
| Median change of FGF23a | −275 (−825–33) | −4972 (−14 418 to −2582) | 3339 (1226–7310) |
| Ref. | HR (95% CI) | HR (95% CI) | |
| Crude | Ref. | 0.96 (0.61–1.50) | 1.56 (1.03 – 2.35*) |
| Model 1b | Ref. | 1.26 (0.79–2.01) | 2.01 (1.32 – 3.07*) |
| Model 2c | Ref. | 1.25 (0.78–2.02) | 2.01 (1.32 – 3.07*) |
| Model 3d | Ref. | 1.25 (0.78–2.02) | 2.00 (1.30 – 3.09*) |
Results are shown as HRs with 95% CIs. All models are adjusted for transplantation by adding transplantation as a time-varying covariate.
Depicted are median change of FGF23 concentrations (RU/mL) in 6 months with IQR.
Model 1 = adjusted for age, sex, diabetes, history of CVD, dialysis vintage, residual kidney function and phosphate.
Model 2 = Model 1 plus adjustment for baseline FGF23 concentration.
Model 3 = Model 2 plus adjustment for dialysis modality.
Asterisks indicate a statistically significant difference in hazard at the level of P ≤ 0.05.
FIGURE 2.
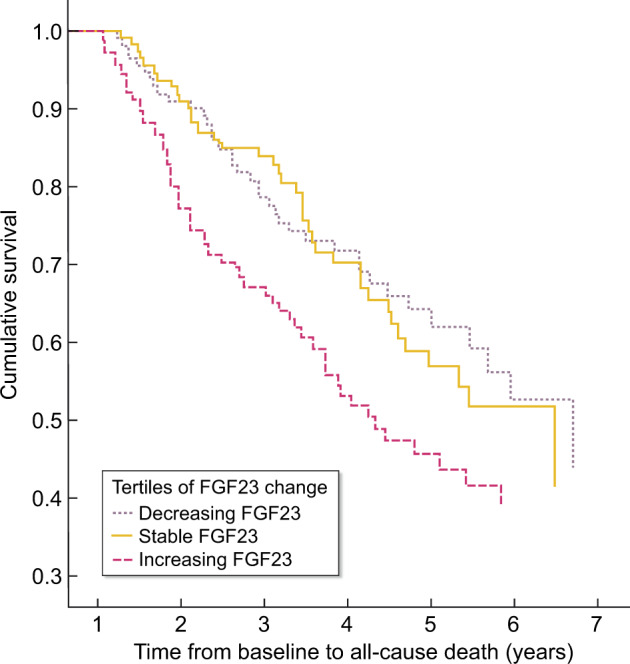
Survival curve of tertiles of FGF23 change in 6-months time. Kaplan–Meier: log rank P = 0.015 (indicates a difference between three groups)
HDF induced reduction of FGF23 and its association with all-cause mortality
Adding dialysis modality to the model (Table 3, Model 3) to explore the potential treatment-related reduction of mortality risk did not change the HRs for all-cause mortality [HR 2.01 (95% CI 1.30–3.09); P = 0.002]. Furthermore, no significant interaction was found between treatment modality and FGF23 change (P = 0.11), indicating that the relation between FGF23 decrease or increase is not different between HD and HDF.
DISCUSSION
In this study we found no association between baseline FGF23 concentration and all-cause mortality among prevalent dialysis patients. Next, we confirmed that HDF is capable of substantially reducing plasma FGF23 concentrations. Most remarkable, we found that only increasing FGF23 concentrations over 6 months were associated with an increased all-cause mortality risk, while decreasing FGF23 concentrations did not associate with mortality, both compared with rather stable FGF23 concentrations. Treatment modality did not modify these risks.
The absence of an association between a single FGF23 measurement and mortality risk is in contrast with some previous findings that showed FGF23 in patients on HD is independently associated with all-cause mortality [18, 30]. However, those studies were performed among incident HD patients or patients initiating dialysis, compared with the prevalent HD patients in our study. In turn, the results of a recent analysis of the large Japanese Dialysis Outcomes and Practice Patterns Study cohort were in line with our findings. This study found no association between FGF23 and all-cause mortality in the adjusted model of patients with a dialysis vintage >3.5 years [31]. In our study, the median dialysis vintage was 1.8 (IQR 0.9–3.3) years. In addition, two other studies in prevalent HD patients found no association of baseline FGF23 with all-cause mortality [32, 33]. Moreover, this absence of an association between FGF23 and all-cause mortality was recently confirmed in patients treated by either HD or peritoneal dialysis [34]. Collectively, these data, together with our current analysis, suggest that the association between a single value of FGF23 and long-term outcome may disappear with dialysis duration. These observations may be explained by survival bias; those patients surviving on dialysis may be less susceptible to detrimental effects of FGF23 (assuming direct causality) or the processes causing FGF23 to rise. However, two other studies in prevalent HD patients demonstrated an association between FGF23 and all-cause mortality [19, 35]. In the study by Nowak et al. [35], among 239 patients, the average dialysis vintage was 59 months and the mean FGF23 concentration was 883 RU/mL, which is exceptionally low, thereby possibly limiting the external validity of that finding. In comparison, in our current study, median baseline FGF23 was 4384 RU/mL. In the study by Jean et al. [19], a relatively small single-centre study, phosphate control was exceptionally good (in part due to 5- to 8-h dialysis sessions). These findings complicate the interpretation of the divergent effects of FGF23.
In general, FGF23 declined in patients treated with HDF while FGF23 in most patients on HD remained stable. Although this difference between HD and HDF was not adjusted for change in the use of phosphate binders, it was previously demonstrated in this cohort that patients on HDF used slightly less phosphate binders. Thus, if any, the absence of data on phosphate binders in the present analysis induces a bias towards zero [36]. FGF23 reduction in HDF is most likely explained by its middle-molecule size (32 kDa), which can be cleared by HDF and not by low-flux HD [37]. Previous studies showed a reduction ratio of serum FGF23 within a single HDF session of around 50% (±25%), with FGF23 present in dialysate samples [23–25]. Our study is the first study to show that FGF23 in patients on HDF decline over time and that this reduction increases with higher convection volume.
The primary finding from our study was the association of increasing FGF23 concentration in 6 months with all-cause mortality as compared with stable FGF23, while a decline and stable FGF23 did not differ in terms of mortality risk. These findings were unaltered after adjustment for all relevant confounders. The increased mortality risk of increasing FGF23 is in line with a recent report from the Chronic Renal Insufficiency Cohort, a large population of nearly 4000 patients with CKD Stages 2–4 [20], and extends these findings to patients with CKD 5D.
In turn, we did not find that a decline of FGF23 was associated with improved survival. This finding seems to contradict a post hoc analysis of the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events trial. Here, a >30% reduction of FGF23 in 20 weeks among participants allocated to cinacalcet was associated with a reduced risk on the composite outcome of cardiovascular mortality, sudden cardiac death and heart failure [38]. However, in the placebo-treated group, there were also (yet fewer) patients with >30% FGF23 reduction in whom no association with cardiovascular events was found. This suggests that not the decline of FGF23 itself, but the way it was achieved, determined the incidence of the composite endpoint. In addition, another study examining trajectories of FGF23 in HD patients found no benefit for patients with decreasing levels compared with the group with high stable levels of FGF23 [21].
Since our study showed that FGF23 concentrations can be lowered with HDF, while increasing FGF23 concentrations associated with increased mortality, it is tempting to speculate that HDF may prevent this from happening. However, a decline of FGF23 did not confer improved mortality risk, as outlined above, and the addition of dialysis modality to the model did not alter the elevated mortality risk associated with increasing FGF23 concentrations. In addition, the change of FGF23 in relation to all-cause mortality was not different between HD and HDF. The latter finding suggests that the effect of FGF23 change on mortality is not affected by dialysis modality. This finding resembles previous observations of β2-microglobulin during HDF, a protein of comparable molecular weight to FGF23 and of similar kinetics. Despite its association with cardiovascular outcome, the superior reduction of β2-microglobulin by HDF as compared with low-flux HD did not associate with a survival benefit [39, 40].
So despite the substantial variability of FGF23 in our study, a dose–response association between the change in FGF23 and mortality was absent. Only patients with an increase of FGF23 were at risk, suggesting that FGF23 is more likely a tell-tale sign of a high-risk phenotype instead of a direct uraemic toxin, which is in line with the conclusion of a recent meta-analysis of studies that addressed the association of FGF23 and mortality and other endpoints [41].
The present study has several limitations: our analysis of FGF23 change in 6 months was necessarily limited to patients who had survived these six additional months. We evaluated the course of FGF23 concentrations in 6 months because we anticipated the largest effect of dialysis modality on FGF23 levels in this short time window, with limited additional diverging effects after this time point. However, a longer time window might have induced an even more substantial decline of FGF23. In addition, the high FGF23 concentrations in our study may simply be too high to induce a beneficial treatment effect on mortality, even with moderate lowering. A possible beneficial effect of lowering of FGF23 might also be masked by exposure to stronger competing risks. This would explain the discrepancy with the study by Isakova et al. [20] in CKD Stages 2–4, in which the median FGF23 was much lower when compared with our study. Another limitation is that this study was performed in countries with a mainly Caucasian population, which may reduce the applicability of our conclusions to other populations. However, no important interaction between FGF23 and race has been demonstrated in previous studies. Lastly, since this is a post hoc analysis, we cannot rule out the possibility that titrating treatments on change in FGF23 concentration may yield different results, and residual confounding cannot be excluded.
An important strength of the present study is the use of serial measurements for FGF23 in the survival analyses. In addition, we performed separate sample handling for the higher FGF23 concentrations, since ELISA loses precision in the extreme values. Furthermore, we performed several sensitivity analyses to increase the validity of our findings and competing risk by renal transplantation was taken into account by a time-varying analysis. Another merit of the study is the strongly diverging FGF23 concentrations in this population. HDF has been shown to be an efficacious manner to lower FGF23 in patients on dialysis, which created the unique opportunity to study the impact of a substantial decline of FGF23 on outcome. Furthermore, this study was performed in a well-defined cohort of patients on HD and HDF, in which the data were collected meticulously and follow-up for mortality was complete.
In conclusion, in prevalent dialysis-dependent patients, thenbaseline FGF23 value is not associated with all-cause mortality, suggesting that the association between FGF23 and long-term outcome may disappear with dialysis duration. Furthermore, decreasing FGF23 concentrations over time are not associated with a lower mortality risk. This observation argues against a benefit of interventions that lower FGF23 in prevalent patients on HD. Finally, since increasing FGF23 does associate with increased risk for all-cause mortality, serial FGF23 measurements could identify dialysis-dependent patients at highest risk.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
Executive Committee CONTRAST study: University Medical Center Utrecht: P.J. Blankestijn; Amsterdam UMC, location VU University Medical Center, Amsterdam: M.P.C. Grooteman, M.J. Nubé, P.M. ter Wee; Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht: M.L. Bots; and Maasstad Hospital, Rotterdam: M.A. van den Dorpel.
FUNDING
CONTRAST was financially supported by a grant from the Dutch Kidney Foundation (Nierstichting Nederland, grant C02.2019) and unrestricted grants from Fresenius Medical Care (The Netherlands) and Gambro Lundia AB (Sweden). Additional support was received from the Dr E.E. Twiss Fund, Roche Netherlands, the International Society of Nephrology/Baxter Extramural Grant Program and the Dutch Organization for Health Research and Development (ZonMW, grant 17088.2802).
CONFLICT OF INTEREST STATEMENT
None declared. We hereby declare that the results presented in this article have not been published previously in whole or part, except in abstract format.
Contributor Information
the CONTRAST Study Group:
P J Blankestijn, M P C Grooteman, M J Nubé, P M ter Wee, M L Bots, and M A van den Dorpel
REFERENCES
- 1. Kestenbaum B, Sampson JN, Rudser KD. et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005; 16: 520–528 [DOI] [PubMed] [Google Scholar]
- 2. Covic A, Kothawala P, Bernal M. et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24: 1506–1523 [DOI] [PubMed] [Google Scholar]
- 3. Hecking M, Moissl U, Genser B. et al. Greater fluid overload and lower interdialytic weight gain are independently associated with mortality in a large international hemodialysis population. Nephrol Dial Transplant 2018; 33: 1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Block GA, Wheeler DC, Persky MS. et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012; 23: 1407–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raggi P, Chertow GM, Torres PU. et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011; 26: 1327–1339 [DOI] [PubMed] [Google Scholar]
- 6. Chue CD, Townend JN, Moody WE. et al. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 2013; 24: 842–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thadhani R, Appelbaum E, Pritchett Y. et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012; 307: 674–684 [DOI] [PubMed] [Google Scholar]
- 8.EVOLVE Trial Investigators. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012; 367: 2482–2494 [DOI] [PubMed] [Google Scholar]
- 9. Ix JH, Shlipak MG, Wassel CL. et al. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant 2010; 25: 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimada T, Hasegawa H, Yamazaki Y. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004; 19: 429–435 [DOI] [PubMed] [Google Scholar]
- 11. Gattineni J, Bates C, Twombley K. et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 2009; 297: F282–F291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isakova T, Wahl P, Vargas GS. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011; 79: 1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mirza MA, Larsson A, Lind L. et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009; 205: 385–390 [DOI] [PubMed] [Google Scholar]
- 15. Yilmaz MI, Sonmez A, Saglam M. et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int 2010; 78: 679–685 [DOI] [PubMed] [Google Scholar]
- 16. Scialla JJ, Xie H, Rahman M. et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 2014; 25: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bouma-de KA, Bots ML, Vervloet MG. et al. Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant 2014; 29: 88–97 [DOI] [PubMed] [Google Scholar]
- 18. Gutierrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jean G, Terrat JC, Vanel T. et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 2009; 24: 2792–2796 [DOI] [PubMed] [Google Scholar]
- 20. Isakova T, Cai X, Lee J. et al. Longitudinal FGF23 trajectories and mortality in patients with CKD. J Am Soc Nephrol 2018; 29: 579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jovanovich A, You Z, Isakova T. et al. Fibroblast growth factor 23 trajectories in chronic hemodialysis patients: lessons from the HEMO study. Am J Nephrol 2019; 49: 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grooteman MP, van den Dorpel MA, Bots ML. et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 2012; 23: 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patrier L, Dupuy AM, Granger Vallee A. et al. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J Nephrol 2013; 26: 342–349 [DOI] [PubMed] [Google Scholar]
- 24. Cernaro V, Lucisano S, Canale V. et al. Acetate-free biofiltration to remove fibroblast growth factor 23 in hemodialysis patients: a pilot study. J Nephrol 2018; 31: 429–433 [DOI] [PubMed] [Google Scholar]
- 25. Choo SZ, Polkinghorne KR, Kerr PG.. Biochemical comparison of 8 h haemodialysis and 4 h haemodiafiltration, and two dialysis membranes, in a randomized cross‐over trial. Nephrology 2019; 24: 542–549. [DOI] [PubMed] [Google Scholar]
- 26. Penne EL, Blankestijn PJ, Bots ML. et al. Effect of increased convective clearance by on-line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients—the Dutch CONvective TRAnsport STudy (CONTRAST): rationale and design of a randomised controlled trial [ISRCTN38365125]. Curr Control Trials Cardiovasc Med 2005; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. den Hoedt CH, Bots ML, Grooteman MP. et al. Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int 2014; 86: 423–432 [DOI] [PubMed] [Google Scholar]
- 28. Heijboer AC, Levitus M, Vervloet MG. et al. Determination of fibroblast growth factor 23. Ann Clin Biochem 2009; 46: 338–340 [DOI] [PubMed] [Google Scholar]
- 29. Hazelbag CM, Peters SAE, Blankestijn PJ. et al. The importance of considering competing treatment affecting prognosis in the evaluation of therapy in trials: the example of renal transplantation in hemodialysis trials. Nephrol Dial Transplant 2017; 32: ii31–ii39 [DOI] [PubMed] [Google Scholar]
- 30. Scialla JJ, Parekh RS, Eustace JA. et al. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol 2015; 42: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Komaba H, Fuller DS, Taniguchi M. et al. FGF23 and mortality in a large cohort of prevalent hemodialysis patients: results from the J-DOPPS. ASN Kidney Week, Abstract No. TH-PO787, ASN Abstract book 2017
- 32. Hsu HJ, Wu MS.. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci 2009; 337: 116–122 [DOI] [PubMed] [Google Scholar]
- 33. Sugimoto H, Ogawa T, Iwabuchi Y. et al. Relationship between serum fibroblast growth factor-23 level and mortality in chronic hemodialysis patients. Int Urol Nephrol 2014; 46: 99–106 [DOI] [PubMed] [Google Scholar]
- 34. Olauson H, Qureshi AR, Miyamoto T. et al. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant 2010; 25: 3033–3038 [DOI] [PubMed] [Google Scholar]
- 35. Nowak A, Friedrich B, Artunc F. et al. Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PLoS One 2014; 9: e100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Penne EL, van der Weerd NC, van den Dorpel MA. et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis 2010; 55: 77–87 [DOI] [PubMed] [Google Scholar]
- 37. Humalda JK, Riphagen IJ, Assa S. et al. Fibroblast growth factor 23 correlates with volume status in haemodialysis patients and is not reduced by haemodialysis. Nephrol Dial Transplant 2016; 31: 1494–1501 [DOI] [PubMed] [Google Scholar]
- 38. Moe SM, Chertow GM, Parfrey PS. et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Circulation 2015; 132: 27–39 [DOI] [PubMed] [Google Scholar]
- 39. Liabeuf S, Lenglet A, Desjardins L. et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int 2012; 82: 1297–1303 [DOI] [PubMed] [Google Scholar]
- 40. Cheung AK, Rocco MV, Yan G. et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol 2006; 17: 546–555 [DOI] [PubMed] [Google Scholar]
- 41. Marthi A, Donovan K, Haynes R. et al. Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: a meta-analysis. J Am Soc Nephrol 2018; 29: 2015–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


