Abstract
Objective
To describe the clinical characteristics and outcomes of hospitalized coronavirus disease 2019 (COVID-19) patients in a middle east respiratory syndrome coronavirus (MERS-CoV) referral hospital during the peak months of the pandemic.
Design
A single-center case series of hospitalized individuals with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in King Saud University Medical City (KSUMC), an academic tertiary care hospital in Riyadh, Saudi Arabia.
Clinical and biochemical markers were documented. Risks for ventilatory support, intensive care unit (ICU) admission and death are presented.
Results
Out of 12,688 individuals tested for SARS-CoV-2 by real time reverse transcriptase polymerase reaction (RT-PCR) from June 1 to August 31, 2020, 2,683 (21%) were positive for COVID-19. Of the latter, 605 (22%) patients required hospitalization with a median age of 55, 368 (61%) were male. The most common comorbidities were hypertension (43%) and diabetes (42%). Most patients presented with fever (66%), dyspnea (65%), cough (61%), elevated IL-6 (93.5%), D-dimer (90.1%), CRP (86.1%), and lymphopenia (41.7%). No MERS-CoV co-infection was detected. Overall, 91 patients (15%) died; risk factors associated with mortality were an age of 65 years or older OR 2.29 [95%CI 1.43–3.67], presence of two or more comorbidities OR 3.17 [95%CI 2.00–5.02], symptoms duration of seven days or less OR 3.189 [95%CI (1.64 – 6.19]) lymphopenia OR 3.388 [95%CI 2.10–5.44], high CRP OR 2.85 [95%CI 1.1–7.32], high AST OR 2.95 [95%CI 1.77–4.90], high creatinine OR 3.71 [95%CI 2.30–5.99], and high troponin-I OR 2.84 [95%CI 1.33–6.05].
Conclusion
There is a significant increase in severe cases of COVID-19. Mortality was associated with older age, shorter symptom duration, high CRP, low lymphocyte count, and end-organ damage.
Keywords: COVID-19, SARS-CoV-2, MERS-CoV, Mortality
Introduction
Since the emergence of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Wuhan, China, in December 2019 and the declaration of the coronavirus 2019 (COVID-19) as a pandemic by the World Health Organization (WHO) on March 11, 2020, it has infected more than 88 million individuals around the world with almost two million deaths as of January 7, 2020 (World Health Organization, n.d.). In the Kingdom of Saudi Arabia (KSA), 363,582 individuals have been infected with 6,282 deaths, with a national case fatality ratio (CFR) of 1.7% (Saudi Arabia Ministry of Health, n.d.a, n.d.b), which is less than that reported by the United States (CFR of 2.7%), Italy (CFR of 9.5%), and China (CFR 5.2%) (World Health Organization, n.d.). Many studies have been published describing patient demographics, clinical course, and outcomes from those countries (Argenzian et al., 2020, Docherty et al., 2020, Lechien et al., 2020, Richardson et al., 2020).
The first case of COVID-19 in KSA was reported on March 2, 2020 (Barry et al., 2020b), and the peak of the pandemic was reported in June and July 2020, with 4,919 individuals being the highest number of cases recorded in a single day on June 18, 2020 (Saudi Arabia Ministry of Health, n.d.a, n.d.b; World Health Organization, n.d.).
The Middle East Respiratory Syndrome Corona Virus (MERS-CoV) originated in KSA (Zaki et al., 2012) and is the only country in the world where it is still endemic, causing multiple nosocomial outbreaks (Alenazi et al., 2017, Amer et al., 2018, Assiri et al., 2013, Balkhy et al., 2016, Barry et al., 2020c, Drosten et al., 2015, Fagbo et al., 2015, Oboho et al., 2015). At the time of writing, the most recent reported case of MERS-CoV was on May 31, 2020 (Saudi Arabia Ministry of Health, n.d.a, n.d.b). Per the latest situation update from the WHO, a total of 2,519 laboratory-confirmed MERS-CoV infections, including 866 associated deaths (CFR 34.3%), were reported globally. Most of these cases were in KSA (2,121 cases), including 788 related deaths (CFR 37.1%) between June 2012 and January 2020 (WHO, n.d.).
We describe the clinical characteristics and outcomes of hospitalized patients with COVID-19 in an academic hospital, which serves as a MERS-CoV referral center, during the peak months of the pandemic in KSA. This is a follow-up to our previously published data from the same hospital during the pandemic’s early months (Barry et al., 2020a).
Materials and methods
Study design
This is a single-center case series conducted at King Saud University Medical City (KSUMC) of hospitalized COVID-19 patients during the peak of the COVID-19 pandemic, between June 1 to August 31, 2020. We included all individuals aged 14 years or older who were hospitalized for at least 24 h, with real-time reverse transcriptase-polymerase chain reaction (RT-PCR) confirmed COVID-19 during the study period. Patients who were still hospitalized by the end of the study period were followed until discharge or in-hospital death, with the last follow-up event occurring on October 15, 2020.
Disease severity was classified based on the Saudi Ministry of Health (MOH) severity definitions (Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19, n.d.) into asymptomatic, upper respiratory tract illness (URTI), mild to moderate pneumonia, severe pneumonia (in which non-invasive ventilation or mechanical ventilation is required), and critical cases, in which acute respiratory distress syndrome (ARDS), overt sepsis, or multiorgan dysfunction were documented. MERS-CoV RT-PCR was done for all patients who met the case definition as per the MOH (Ministry of Health Command and Control Center, 2018).
All patients were admitted to single rooms under droplet and contact precautions, as per hospital policy, or in airborne infection isolation rooms with 6–12 air-changes per hour, or, when those options were not available, in a single room with a high-efficiency particulate air filter, if aerosol-generating medical procedures were required with the use of fit-tested N95 masks.
This analysis received ethical approval from the KSUMC Institutional Review Board bearing project number E20-4979. Oral informed consent was obtained for data use from all study participants.
Laboratory testing
All hospitalized patients underwent a nasopharyngeal and/or tracheal aspirate upon admission, once obtained it was sent in viral transport medium UTM® (Copan, Brescia, Italy). We defined confirmed COVID-19 as a positive result for both SARS-CoV-2 E and S genes using the RealStar® SARS-CoV-2 real-time Reverse Transcriptase PCR (RT-PCR) kit (Altona®-Diagnostics, Hamburg, Germany) and Rotor-gene Q system (Qiagen®, Santa Clarita, CA, USA) in our institute’s molecular laboratory. We confirmed a sample with a cycle threshold (Ct) value ≤29 for both SARS-CoV-2 E and S genes as a positive case, whereas a sample with a single gene detection or Ct value ≥29 was confirmed by repeating the test on an Xpert® Xpress SARS-CoV-2 kit and GeneXpert XVI system (Cepheid®, Sunnyvale, CA, USA), which detects SARS-CoV-2 E and N genes. When indicated, we used the same samples to test for MERS-CoV RNA in which, after extraction, the RNA was reverse transcribed to cDNA. This was then amplified and screened to detect MERS-CoV upE and ORF1a genes using the specific primers and probes of the RealStar® MERS-CoV RT-PCR kit (Altona® Diagnostics) on the Rotorgene Q instrument (Qiagen®).
Data collection
Patient data was maintained in individual electronic health care records of all those with confirmed COVID-19. The information recorded in health care records included demographic data, medical history, epidemiological exposure, underlying comorbidities, symptoms, physical signs, laboratory results, MERS-CoV PCR, bacterial cultures results, chest X-rays and computed tomographic (CT) scans, treatment measures (i.e., anti-viral therapy, corticosteroids, monoclonal antibody treatment, supportive care), in-hospital complications, admission to the intensive care unit (ICU), and clinical outcomes. The sequential organ failure assessment (SOFA) was determined on the day of ICU admission.
Statistical analysis
Continuous measurements are presented as mean (SD) if they are normally distributed or median [interquartile range (IQR)] if they are not, and categorical variables are counted in percentages. For laboratory results, we assessed whether the measurements were outside the normal range. We used SPSS software (version 25, IBM Corp., Armonk, NY, USA) for statistical analysis, including a univariate analysis to obtain odds ratio (OR) and 95% confidence interval (CI) on the association of the study variables with severity, ICU admission, and mortality. The Haldane–Anscombe correction was used for odds ratio calculation when one of the cells had a value of zero. Pearson’s chi-squared test was performed to calculate the p-value in the analysis’s categorical variables, and a p-value of <0.05 was considered significant. P-values generated by Fisher’s exact test (instead of those generated by Pearson’s chi-squared) were used when >20% of the cells had an expected count of less than five.
Results
Demographics
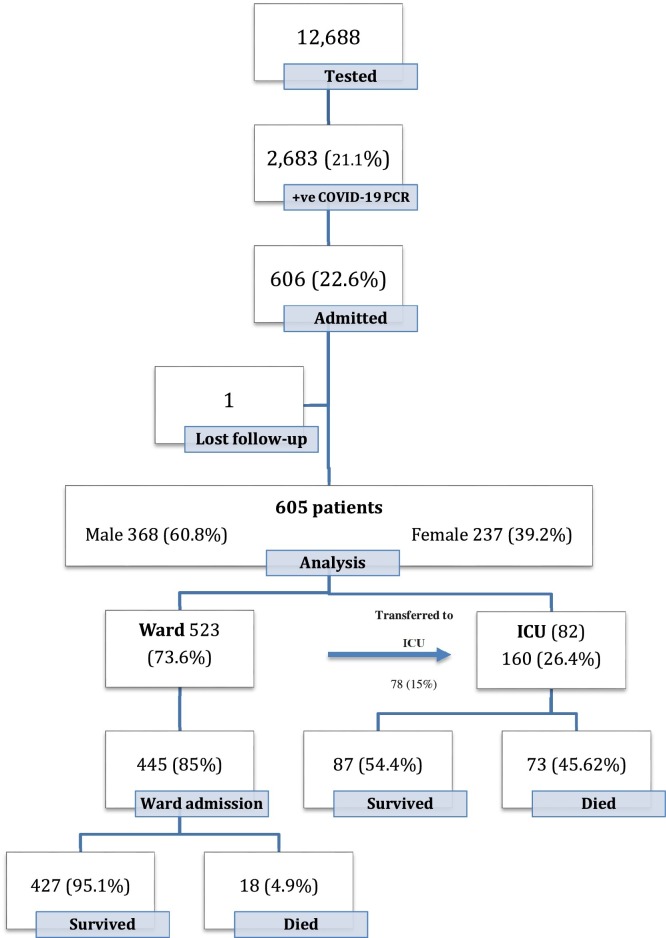
During the peak of the COVID-19 pandemic in KSA from June 1 to August 31, 2020, a total of 12,688 patients aged 14 years and above were tested for COVID-19 by RT-PCR at KSUMC, Riyadh. Of those, 2683 patients (21.1%) tested positive, and of those tested positive, 606 patients (22.6%) required hospitalization. Only one patient was lost to follow-up due to transfer to another hospital; the remaining 605 patients met the inclusion criteria (Figure 1 ). Demographic characteristics are shown in Table 1 .
Figure 1.
Patients flow.
Table 1.
Demographic data of 605 hospitalized patients with SARS-CoV-2 infection at King Saud University Medical City.
| (n) | (%) | ||
|---|---|---|---|
| Gender | Female | 237 | 39 |
| Male | 368 | 61 | |
| Age | 14–64 | 455 | 75 |
| 65 or older | 150 | 25 | |
| Occupation | HCWs | 26 | 4 |
| Non-HCWs | 579 | 96 | |
| Nationality | Saudi | 353 | 58 |
| Non-Saudi | 252 | 42 | |
| BMI | Obese | 261 | 43 |
| Overweight | 177 | 29 | |
| Normal | 112 | 19 | |
| Underweight | 10 | 2 | |
| Not documented | 45 | 7 | |
| Comorbid Conditions | HTN | 262 | 43 |
| DM | 259 | 43 | |
| DLP | 92 | 15 | |
| CAD | 64 | 11 | |
| HF | 42 | 7 | |
| CKD | 37 | 6 | |
| CVA | 20 | 3 | |
| Active cancer | 16 | 3 | |
| Autoimmune diseases | 12 | 2 | |
| COPD | 9 | 2 | |
| Pregnancy | Yes | 34 | 14 |
| No | 203 | 86 | |
HCWs: health care workers, DM: diabetes mellitus, HTN: hypertension, DLP: dyslipidemia, CAD: coronary artery disease, HF: heart failure, CKD: chronic kidney disease, CVA: cerebrovascular accident, COPD: chronic obstructive pulmonary disease.
Among the 605 patients, 368 (60.8%) were male, and 237 (39.2%) were female. The overall age ranged from 16 to 101 years old with a median age of 55 years. A total of 353 patients (58%) were Saudi nationals. The majority of patients had a high body mass index (BMI), of which 261 were obese (43%). The most frequent comorbidities were hypertension in 262 patients (43%) and diabetes mellitus in 259 patients (43%). Thirty-four patients were pregnant, representing 14% of the sample. Health care workers represented only 4%, one of whom had a history of PCR confirmed MERS-CoV in 2015; none of the other patients had a past history of the disease.
Patients’ disposition
Of 445 (73.6%) admitted to the ward, 78 (15%) required transfer to the ICU, while 82 (13.5%) were admitted directly to the ICU, for a total of 160 (26.4%) patients requiring ICU admission. Overall, 514 (85%) were discharged from the hospital, while 91 patients (15%) died during their hospital stay. The median time to discharge or mortality was seven and 13 days, respectively. Reasons for ICU admission included respiratory distress (79.4%), transfer from another hospital on mechanical ventilation (6.3%), hypotension (5.6%), decreased level of consciousness (3.1%), cardiac/respiratory arrest (1.9%), and others (Table 2 ). Out of those who required ICU admission, 74 patients (46.3%) had two or more comorbid illnesses, while 86 patients (53.7%) had only one or no comorbid illness. Of those who required ICU admission, 32.5% had a temperature of ≥38 °C, and 69.4% had a respiratory rate >24/min in the first 24 h of admission. In the ICU, 38.1%, 46.9%, and 45.6% of patients needed vasopressors, mechanical ventilation, and non-invasive ventilation, respectively. One patient in the ICU required extracorporeal membrane oxygenation (ECMO) but did not survive.
Table 2.
Clinical characteristics of 160 hospitalized COVID-19 patients who required admission to the ICU at King Saud University Medical City.
| Variables |
Count | Death in ICU | |
|---|---|---|---|
| n = 160 (%) | n = 73 (45.6%) | ||
| Initial admission | Ward then ICU | 78 (48.8) | 31 (42.5) |
| Direct to ICU | 82 (51.2) | 42 (57.5) | |
| Reason for ICU admission | Cardiac/respiratory arrest | 3 (1.9) | 1 (1.4) |
| Respiratory distress | 127 (79.4) | 56 (76.7) | |
| Hypotension | 9 (5.6) | 4 (5.5) | |
| Decreased level of consciousness | 5 (3.1) | 3 (4.1) | |
| Transferred from another hospital | 10 (6.3) | 7 (9.6) | |
| Others (Post-operative, DKA, severe electrolyte disturbances) | 6 (3.6) | 2 (2.7) | |
| Number of comorbid conditions. | Two or more comorbid conditions | 74 (46.3) | 42 (57.5) |
| One or no comorbid condition | 86 (53.8) | 31 (42.5) | |
| Temperature in the first 24 hours of hospital admission. | ≥38 °C | 52 (32.5) | 25 (34.2) |
| <38 °C | 108 (67.5) | 48 (65.8) | |
| Respiratory rate in the first 24 hours of hospital admission. | >24 bpm | 111 (69.4) | 46 (63) |
| ≤24 bpm | 49 (30.6) | 27 (37) | |
| Need for vasopressors | Yes | 61 (38.1) | 52 (71.2) |
| No | 99 (61.9) | 21 (28.8) | |
| Respiratory support | None | 6 (3.8) | 0 (0) |
| Facemask | 6 (3.8) | 0 (0) | |
| Non-invasive ventilation : HFNC, BIPAP, CPAP | 73 (45.6) | 9 (12.3) | |
| Invasive mechanical ventilation | 75 (46.9) | 64 (87.7) | |
| Median ventilator days (IQR) | 7 days (15) | ||
| Need for ECMO | Need for ECMO | 1 (0.6) | 1 (1.4) |
| Median ICU stay (IQR): 8 days (11) | |||
| Median hospital stay (IQR): 17 days (18) | |||
IQR: interquartile range, DKA: diabetic ketoacidosis, HFNC: high-flow nasal cannula, BIPAP: bilevel positive airway pressure, CPAP: continuous positive airway pressure, ECMO: extracorporeal membrane oxygenation.
Clinical presentation
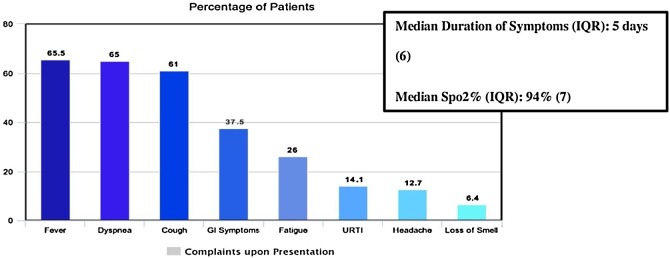
The most common presenting symptoms were fever (65.5%) dyspnea (65%), cough (61%), and gastrointestinal symptoms (37.5%) which included any of the following: nausea, vomiting, diarrhea, (Figure 2 ). The median duration of symptoms (IQR) was five days.
Figure 2.
Presenting complaints of hospitalized COVID-19 Patients, King Saud University Medical City, Saudi Arabia.
In the first 24 h of presentation, median oxygen saturation (IQR) was 94%, median temperature (range) was 37.3 °C (36°–40.2 °C), median respiratory rate (IQR) was 23 bpm, and median systolic blood pressure (IQR) was 118 mmHg.
In the first 48 h of admission, laboratory investigations revealed that 9.8% of patients had a WBC count of less than 4.0 × 109/L, while 13.6% of patients had a platelet count less than 150 × 109/L, and 583 (41.7%) had a lymphocyte count less than 1.0 × 109/L. Most patients showed raised Interleukin-6 (IL-6), D-dimer, aspartate aminotransferase (AST), C-reactive protein (CRP), and ferritin levels, in addition to a prolonged QT interval on baseline electrocardiogram (ECG) (Table 3 ). During admission, chest X-rays (CXR) and CT scans were obtained from 545 and 45 patients, respectively. One hundred and fifty-nine (29.2%) patients had normal CXR, while the other 69.8% had evidence of lung infiltrates. Of those CXR showing infiltrates, 88.5% were bilateral, and 11.5% were unilateral. Of the 45 chest CT scans done, 11.1% were normal, 35.6% showed ground-glass opacification (GGO), 11.1% showed consolidation, 35.5% showed both GGO and consolidation, and 6.7% showed pulmonary embolism. From all COVID-19 confirmed cases, 194 patients (32%) met the case definition for MERS-CoV and were tested for it by RT-PCR. No MERS-CoV co-infection was detected among any of those patients. Additional microbiological testing revealed no influenza-A (184 tested) or influenza-B (185 tested) co-infections. Blood cultures were obtained from 332 patients, and only 20 (6%) were positive, while sputum cultures were obtained from 129 patients, and 18 samples grew various bacteria, while one sample grew Aspergillus niger.
Table 3.
Laboratory investigations and radiological findings in hospitalized COVID-19 patients, King Saud University Medical City, Saudi Arabia.
| Study variables | Number of patients | Median, (IQR) | Abnormal | |
|---|---|---|---|---|
| cutoff | n (%) | |||
| Basic investigations | ||||
| White blood cells (x10^9/L) | 605 | 7 (4.3) | <4 | 59 (9.8) |
| Lymphocytes (x10^9/L) | 583 | 1.1 (0.8) | <1 | 243 (41.7) |
| Platelets count (x10^9/L) | 605 | 233 (12) | <150 | 82 (13.6) |
| D-dimer (mcg/mL) | 534 | 1.1 (1.34) | >0.45 | 481 (90.1) |
| ALT (units/L) | 592 | 38 (36) | >61 | 146 (24.7) |
| AST (units/L) | 541 | 42 (39) | >37 | 291 (53.8) |
| Serum creatinine (mcmol/L) | 604 | 81 (45) | >115 | 121 (20) |
| Inflammatory markers | ||||
| Lactate (mmol/L) | 345 | 1.6 (0.8) | >2 | 84 (24.3) |
| Ferritin (mcg/L) | 529 | 575 (986) | >400 | 321 (60.7) |
| C-reactive protein (CRP) (mg/L) | 503 | 89 (105) | >20 | 433 (86.1) |
| Procalcitonin (PCT) (ng/mL) | 405 | 0.12 (0.375) | >0.5 | 107 (26.4) |
| Interleukin-6 (IL.6) (pg/mL) | 262 | 57.09 (113.3) | >7 | 245 (93.5) |
| Troponin (ng/L) | 433 | 7.4 (21) | >100 | 33 (7.6) |
| Creatinine kinase (CK) (units/L) | 328 | 138 (257.5) | >308 | 87 (26.5) |
| ECG: QT interval (ms) | 122 | 449.5 (39) | >440 | 79 (64.8) |
| Microbiology | ||||
| MERS CoV RT-PCR | 194 | Positive | 0 (0) | |
| HIV Ag/Ab | 372 | Positive | 0 (0) | |
| Hepatitis BsAg | 407 | Positive | 10 (2.5) | |
| TB QuantiFERON Gold Plus | 316 | Positive | 12 (3.8) | |
| Sputum culture | 129 | Positive | 18 (14) | |
| Blood culture | 332 | Positive | 20 (6) | |
| Imaging | ||||
| Chest X-ray | 545 | Normal | 159 (29.2) | |
| Infiltration. | 381 (69.9) | |||
| Extent | ||||
| Unilateral | 44 (11.5) | |||
| Bilateral | 337 (88.5) | |||
| Isolated Pleural Effusion | 5 (0.9) | |||
| CT chest | 45 | Normal | 5 (11.1) | |
| GGO | 16 (35.6) | |||
| Consolidation | 5 (11.1) | |||
| Both (GGO and Consolidation) | 16 (35.6) | |||
| PE | 3 (6.7) |
CT: computed tomography, GGO: ground-glass opacity, ECG: electrocardiogram, HIV: human immunodeficiency virus, TB: tuberculosis, IQR: interquartile range, MERS-CoV: Middle East respiratory syndrome coronavirus, RT-PCR: reverse transcription-polymerase chain reaction, ALT: alanine aminotransferase, AST: aspartate aminotransferase, PE: pulmonary embolism.
Treatment
Treatment of COVID-19 patients varied according to severity and complications, including corticosteroids, interferon beta, anticoagulants, tocilizumab, anti-viral, anti-malaria, and antibacterial agents (Table 4 ). The most common treatment regimen included hydroxychloroquine, lopinavir/ritonavir, favipiravir, remdesivir, coricosteroids, and tocilizumab (Table 4).
Table 4.
Diagnosis and treatment among hospitalized COVID-19 patients, King Saud University Medical City, Saudi Arabia.
| Study variable | n (%) | Died n = 91 (%) |
Survived n = 514 (%) |
|---|---|---|---|
| Diagnosis stratified based on experimental therapy | |||
| Asymptomatic | 93 (15.4) | 2 (2.2) | 91 (97.8) |
| - Acyclovir | 1 | 0 (0) | 1 (100) |
| - coricosteroids | 6 | 0 (0) | 6 (100) |
| Upper respiratory tract infection | 30 (5) | 0 (0) | 30 (100) |
| - Hydroxychloroquine | 1 | 0 (0) | 1 (100) |
| - Acyclovir | 1 | 0 (0) | 1 (100) |
| - Favipiravir | 1 | 0 (0) | 1 (100) |
| - Coricosteroids | 6 | 0 (0) | 6 (100) |
| Mild\moderate pneumonia | 269 (44.5) | 5 (1.9) | 264 (98.1) |
| - Hydroxychloroquine | 4 | 0 (0) | 4 (100) |
| - Lopinavir/Ritonavir. | 1 | 0 (0) | 1 (100) |
| - Favipiravir | 2 | 0 (0) | 2 (100) |
| - Remdesivir | 1 | 0 (0) | 1 (100) |
| - coricosteroids | 145 | 2 (1.4) | 143 (98.6) |
| - Tocilizumab | 1 | 0 (0) | 1 (100) |
| Severe pneumonia | 107 (17.7) | 12 (11.2) | 95 (88.8) |
| - Hydroxychloroquine | 1 | 0 (0) | 1 (100) |
| - Lopinavir/ritonavir + ribavirin | 1 | 0 (0) | 1 (100) |
| - Favipiravir | 1 | 0 (0) | 1 (100) |
| - Remdesivir | 2 | 0 (0) | 2 (100) |
| - coricosteroids | 97 | 10 (10.3) | 87 (89.7) |
| - Tocilizumab | 8 | 0 (0) | 8 (100) |
| Critical pneumonia | 106 (17.5) | 72 (67.9) | 34 (32.1) |
| - Lopinavir/ritonavir + ribavirin | 5 | 3 (60) | 2 (40) |
| - Favipiravir | 2 | 2 (100) | 0 (0) |
| - Interferon-beta 1 | 1 | 1 (100) | 0 (0) |
| - coricosteroids | 97 | 63 (64.9) | 34 (35.1) |
| - Tocilizumab | 28 | 18 (64.3) | 10 (35.7) |
| Therapy duration | |||
| Steroids, median = 9 days (IQR = 5) | |||
| Favipiravir, median = 9.5 (Range 7–10) | |||
| Remdesivir, duration = 10 days | |||
| Lopinavir/ritonavir + ribavirin, median = 11.5 (IQR = 11) | |||
| Other treatment | |||
| Antibacterial: | |||
| None | 113 (18.7) | 1 (0.9) | 112 (99.1) |
| One antibacterial | 82 (13.6) | 8 (9.8) | 74 (90.2) |
| Two or more antibacterial agents | 410 (67.8) | 82 (20) | 328 (80) |
| Anticoagulant: | 558 (92.2) | ||
| - In-hospital DVT prophylactic anticoagulation | 432 (77.4) | 46 (10.6) | 386 (89.4) |
| - In-hospital therapeutic anticoagulation for suspected PE, ACS, severe disease with high D-dimer | 112 (20.1) | 41 (36.6) | 71 (63.4) |
| - Already on anticoagulation before COVID-19 diagnosis | 14 (2.5) | 1 (7.1) | 13 (92.9) |
IQR: interquartile range, ACS: acute coronary syndrome, PE: pulmonary embolism, DVT: deep venous thrombosis.
Outcome
Older patients (age group ≥65 years) had high mortality (p < 0.001) with an OR = 2.297 [95%CI 1.437–3.671]; however, there was no statistically significant association between age groups and severity of infection or ICU admission. Male patients had more severe disease (p = 0.004), but there was no significant association between gender and ICU admission or mortality. BMI showed no significant association with severity of infection, ICU admission, or mortality; mortality was recorded to be higher in patients with BMI ≥ 30 as compared to overweight, normal weight, and under-weight patients but did not show statistical significance. Non-health care workers (nHCWs) had more severe infections (p = 0.003, OR = 6.880 [95%CI 1.610–29.402]) and were more likely to be admitted to the ICU (p = 0.035) compared to HCWs. There were no deaths among the 26 (4%) hospitalized HCWs.
None of the 34 pregnant women admitted with COVID-19 had a severe disease or required ICU admission. Twenty-five women (73.53%) were asymptomatic, three (8.82%) had URTI, and six (17.65%) had mild/moderate pneumonia. Twenty-nine (85.29%) were in their third trimester, four (11.76%) were in the second trimester, and only one was in her first trimester and was presenting with ectopic pregnancy. There was no association observed between gestational age and disease severity. Out of the nine symptomatic patients, six received ceftriaxone, and only one was given dexamethasone due to hypoxia. None were given hydroxychloroquine or anti-viral agents. Most of the patients were admitted for delivery or other obstetric indications, and only one patient was admitted due to symptomatic COVID-19 infection with moderate pneumonia and hypoxia. Regarding obstetrics complications, 15 (44.16%) had an uneventful course, while the rest had different complications, including decelerations in cardiotocography (42.11%), per-vaginal bleeding (21.05%), decreased fetal movement (15.78%), pre-eclampsia (15.78%), and pre-term labor (10.53%). Eighteen (66.67%) pregnant women had a spontaneous vaginal delivery, while nine (33.34%) required emergency cesarean section. The COVID-19 status of the newborns was not well documented; 15 out of 27 (55.56%) newborns tested negative for COVID-19, and the rest were of unknown status due to lack of documentation. All 34 women were discharged with a median hospital stay of seven days.
Nationality had a significant association with severity of infection and ICU admission, with non-Saudis exhibiting more severe disease (p < 0.001) and more likely to be admitted to the ICU (p = 0.004), although there was no significant impact on mortality (p = 0.982). Mortality was significantly higher among patients who were admitted to the ICU directly, compared to those who were admitted initially to the ward (p < 0.001; OR = 10.157 [95%CI 6.018–17.144]). Mortality was also significantly higher among those who reported symptom duration of seven days or fewer in comparison to those who had more than seven days of symptoms prior to presentation (p < 0.001; OR = 3.189 [95%CI 1.643–6.190]). A low saturation of oxygen (SpO2) of less than 94% in the first 24 h, or a ferritin level of more than 400 mcg/L in the first 48 h, had a significant association with more severe disease and ICU admission; however, these showed no statistically significant association with mortality. High CRP levels (more than 20 mg/L) in the first 48 h had a statistically significant association with more severe infection (p = 0.002) and mortality (p = 0.023; OR = 2.856 [95%CI 1.113–7.327]) but no statistically significant association with ICU admission. Severe infection led to higher mortality (p < 0.001; OR = 35.81 [95%CI 16.15–79.42]) (Table 4).
Other factors, namely having two or more comorbidities, RR of more than 24/min in the first 24 h, low lymphocyte count (<1 × 10^9/L), high PCT (>0.5 ng/mL), markers of end-organ damage: high aspartate transaminase (AST) levels (>37 units/L), high creatinine levels (>115 mcmol/L), high troponin I (>100 ng/L), and CXR infiltrates on arrival were all significantly associated with more severe disease, ICU admission, and mortality (Table 5 ).
Table 5.
Factors associated with ICU admission and outcome among hospitalized COVID-19 patients, King Saud University Medical City, using univariate analysis.
| Factors | Severe infection | ICU admission | Death | |||
|---|---|---|---|---|---|---|
| OR (95% CI) * | P value | OR (95% CI)* | P value | OR (95% CI)* | P value | |
| Demographic data | ||||||
| Age ≥ 65 (vs. <65) | – | 0.156 | – | 0.255 | 2.3 (1.4–3.7) | <0.001 |
| Males (vs. Females) | 1.7 (1.2–2.4) | 0.004 | – | 0.100 | – | 0.700 |
| BMI | – | 0.502 | – | 0.342 | – | 0.365 |
| Non-HCWs (vs. HCWs) | 6.89 (1.6–29.4) | 0.003 | 20.3 (1.2–334.7)** | 0.035 | – | 0.109 |
| Non-Saudi (vs. Saudi) | 1.9 (1.3–2.6) | <0.001 | 1.7 (1.2–2.5) | 0.004 | – | 0.982 |
| Clinical data upon Presentation | ||||||
| Presence of >1 comorbidity (vs. ≤1) | 1.6 (1.2–2.3) | 0.005 | 1.7 (1.2–2.5) | 0.003 | 3.2 (2.0–5.0) | <0.001 |
| Admitted initially to ICU (vs. Ward) | – | – | – | – | 10.2 (6.0–17.1) | <0.001 |
| Symptoms for ≤7 days (vs. >7 days) | – | – | – | 0.418 | 3.2 (1.6–6.2) | <0.001 |
| RR >24 breaths/min (vs. ≤ 24) | 5.3 (3.7–7.7) | <0.001 | 4.6 (3.1–6.9) | <0.001 | 2.5 (1.6–4.0) | <0.001 |
| SpO2 < 94 % (vs. ≥ 94%) | 3.2 (2.2–4.5) | <0.001 | 2.4 (1.6–3.5) | <0.001 | 1.5 (1.0–2.350) | 0.083 |
| Laboratory findings and chest X-ray in the first 48 h of admission | ||||||
| Lymphocyte count < 1 (vs. ≥ 1) | 4.3 (3.0–6.2) | <0.001 | 3.9 (2.7–5.8) | <0.001 | 3.4 (2.1–5.5) | <0.001 |
| D-dimer > 0.45 (vs. ≤ 0.45) | 1.9 (1.0–3.6) | 0.043 | – | 0.231 | – | 0.154 |
| AST > 37 (vs. ≤ 37) | 3.2 (2.2–4.6) | <0.001 | 2.4 (1.7–3.6) | <0.001 | 3.0 (1.8–4.9) | <0.001 |
| Creatinine > 115 (vs. ≤ 115) | 2.2 (1.5–3.3) | <0.001 | 2.5 (1.6–3.8) | <0.001 | 3.7 (2.3–6.0) | <0.001 |
| Ferritin > 400 (vs. ≤ 400) | 1.9 (1.3–2.7) | 0.001 | 1.9 (1.3–2.9) | 0.002 | – | 0.312 |
| CRP > 20 (vs. ≤ 20) | 2.5 (1.4–4.5) | 0.002 | 1.7 (1.0–3.1) | 0.084 | 2.9 (1.1–7.3) | 0.023 |
| PCT ≥ 0.5 (vs. < 0.5) | 3.0 (1.9–4.9) | <0.001 | 3.5 (2.0–6.2) | <0.001 | 4.7 (2.0–11.2) | <0.001 |
| IL-6 > 7 (vs. ≤ 7) | – | 0.637 | – | 0.441 | 0.8 (0.7–0.8) | 0.013 |
| Trop I > 100 (vs. ≤ 100) | 2.7 (1.3–5.8) | 0.007 | 3.2 (1.5–6.6) | 0.001 | 2.8 (1.3–6.1) | 0.005 |
| Infiltrate on initial CXR (vs. No infiltrate) | 4.0 (2.6–4.8) | <0.001 | 2.4 (1.5–3.8) | <0.001 | 2.2 (1.2–3.9) | 0.006 |
OR: odds ratio, CI: confidence interval, Ref: reference value, BMI: body mass index, HCW: health care worker, ICU: intensive care unit, RR: respiratory rate, SpO2: oxygen saturation, AST: aspartate transaminase, CRP: c-reactive protein, PCT: procalcitonin, IL-6: interleukin-6, Trop-I: troponin I, CXR: chest x-ray.
OR calculated for severity, ICU admission, and death, respectively
Haldane-Anscombe corrected odds ratio.
Discussion
This detailed COVID-19 case series from a country endemic with MERS-CoV describes the clinical characteristics and risk factors in hospitalized patients aged 14 years and older with laboratory-confirmed SARS-CoV-2 infection during the peak months of the pandemic. This study is a follow-up to our first detailed case series, which emerged from KSA during the early months of the pandemic (Barry et al., 2020a). It reveals that a fifth of patients tested for COVID-19 were positive, and 23% required hospitalization. Of those, 26% required ICU care, which is a significant rise from the earlier months, in which only a tenth of all patients tested positive, 16% required hospitalization, and 22% required ICU care. In this analysis, the COVID-19 case fatality rate was 3.4% of all those who tested positive in our hospital, which is also a significant rise from 0.2% in the preceding months. Among all hospitalized patients, 15% died, and for the subset of patients requiring ICU admission, 46% died, compared to 12% and 50%, respectively, in the early months. As in the earlier cohort, from 194 (32%) patients who were also tested for MERS-CoV as per case definition, none had any evidence of co-infection.
In-hospital mortality was associated with advanced age (≥65 years), presence of comorbidities, severity of infection upon presentation, and presence of end-organ damage. In a study conducted in Georgia in the United States, the CFR was 17.1% for non-ICU cases and 30.6% for ICU cases (Gold et al., 2020). Another study from Detroit presented a similar CFR of 5.1% and 39% for general practice and ICU cases, respectively (Suleyman et al., 2020). In China, a meta-analysis of COVID-19 clinical characteristics revealed an overall CFR of 3.6% (Fu et al., 2020), while in the UK, a study of 20,133 patients showed a CFR of 26%, with the median age of victims being 80 years old (Docherty et al., 2020).
Hospital stay was notably longer among patients hospitalized during the peak (median 17 days) in comparison to those hospitalized at the beginning of the pandemic in KSUMC (median five days) (Barry et al., 2020a), which indicates worsening disease severity and an increase in severe/critical cases. These findings are consistent with findings from Wuhan, China, describing the clinical characteristics and outcomes of severe or critical COVID-19, which showed a mean hospital stay of 15 days (Li et al., 2020). Another study from China showed a mean hospital stay of eleven days in general and 21 days for severe/critical cases (Zhou et al., 2020). In the US, it was five days for non-severe cases and 15 days for severe cases requiring ICU admission (Suleyman et al., 2020).
The predominant symptoms were fever, cough, and dyspnea. Our cohort showed a higher percentage of dyspnea (65%) compared to other reports from KSA (27%) (Sarfraz et al., 2020), Turkey (27.8%) (Altunok et al., 2020), and China (6.9%) (Tian et al., 2020). Regarding the 160 patients admitted to the ICU, the fatality rate (CFR) was 47.6%. In comparison, a case series from Kuwait showed a similar CFR of 43.7% (Ayed et al., 2020), but was much less than that reported from Spain (72.9%) (Jiménez et al., 2020). There was no statistically significant difference in age group or gender for ICU admission, which is similar to other studies (Ayed et al., 2020, Jiménez et al., 2020). This could be attributed to a younger population with only 150 patients aged ≥65 years, while other cohorts had higher numbers (Richardson et al., 2020).
Upon admission, infiltrate on CXR was an independent risk factor for ICU admission and death with OR 4.042 [95%CI 2.593–4.848] and 2.192 [95%CI 1.233–3.898], respectively. This was not shown in other studies (Ayed et al., 2020), although they had a significant infiltrate rate upon presentation. Low lymphocyte count, high AST, acute kidney injury (AKI), and raised inflammatory markers (ferritin, CRP, and PCT) were all observed as factors associated with severe disease, ICU admission, and death, which is consistent with other studies (Lechien et al., 2020, Richardson et al., 2020).
The majority of pregnant women included in our study were asymptomatic, with no associations identified between gestational age and disease severity, which is consistent with the results of a large meta-analysis including published data from different countries and populations (Juan et al., 2020). COVID-19 status could not be retrieved for all neonates; however, no neonatal or maternal deaths were recorded.
Multiple drugs were used to manage hospitalized patients' conditions, and coticosteroids were commonly used among different disease spectra. Survival rates were compared between the five groups (asymptomatic, URTI, mild and severe pneumonia, and critically ill), and corticosteroids were found to be successful in the asymptomatic and those with pneumonia in addition to the severe group, compared with the critically ill patients, where the survival rate was poor, which is most likely secondary to hospital stay complications, including pulmonary embolism, cardiopulmonary arrest, and anoxic brain damage. The RECOVERY randomized control trial conducted among COVID-19 patients, where dexamethasone was given to 2104 patients and compared to 4321 patients managed by the standard of care, found that 28-day mortality was lower in the group who received dexamethasone while on oxygen therapy, either supplemental or invasive (The RECOVERY Collaborative Group, 2020). Another prospective meta-analysis in 1703 critically ill patients that compared systemic glucocorticoids with standard care showed that corticosteroids lowered 28-day mortality (Sterne et al., 2020).
Several drugs were used to manage COVID-19, including remdesivir (Sanders et al., 2020). In our center, remdesivir was given as part of the WHO Solidarity trial (WHO Solidarity Trial Consortium, 2020), although the number was quite low, no mortality was observed. A double-blind, randomized, placebo-controlled trial was conducted by Beigel et al. (2020) among 1062 patients who underwent randomization, with 541 assigned to remdesivir and 521 to placebo, it was found that remdesivir predicted a shorter recovery time in those with lower respiratory tract infection compared to the placebo group.
Triple therapy that includes protease inhibitors, interferon, and ribavirin was used for a small number of patients, also as part of the Solidarity trial (WHO Solidarity Trial Consortium, 2020). Of five patients in critical condition who received it, only two survived. A multicenter, prospective, open-label, randomized phase 2 trial in Hong Kong showed that early triple therapy was superior to lopinavir-ritonavir alone regarding shortening symptoms duration and hospital stay (Hung et al., 2020).
Tocilizumab is a monoclonal antibody against interleukin-6 (IL-6) (Luo et al., 2020). It was given to 28 critically ill patients with 18 mortalities. A cohort studying the association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19 was conducted by Gupta et al., and the primary outcome of early treatment with tocilizumab showed lower in-hospital mortality rates (Gupta et al., 2020). Additionally, Salvarani et al. conducted a randomized clinical trial of hospitalized adult patients with COVID-19 pneumonia and did not show any disease progression benefit compared to standard of care (Salvarani et al., 2020). Hydroxychloroquine was also used initially for a few patients as part of the Solidarity trial but was soon discontinued when its use was revoked (US Food and Drug Administration, 2020). None of the six patients who received it died; however, only one had severe disease.
Study limitations
Our study has several limitations, including data emerging from a single-center, lack of data on viral load, and epidemiological data. Only a third of patients were tested for MERS-CoV infections.
Conclusion
In conclusion, this follow-up case series found a significant increase in the number of severe cases of COVID-19, predominantly in male patients younger than 65 years old. Mortality was associated with older age, multiple comorbidities, shorter symptom duration, tachypnea, abnormal CXR on admission, direct admission to ICU, low lymphocyte count, high CRP, and evidence of end-organ damage. No MERS-CoV co-infections were detected.
Availability of data and materials
All the data for this study will be made available upon reasonable request to the corresponding author.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
Acknowledgments
None.
References
- Alenazi T.H., Al Arbash H., El-Saed A., Alshamrani M.M., Baffoe-Bonnie H., Arabi Y.M. Identified transmission dynamics of Middle East respiratory syndrome coronavirus infection during an outbreak: implications of an overcrowded emergency department. Clin Infect Dis. 2017;65:675–679. doi: 10.1093/cid/cix352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altunok E.S., Alkan M., Kamat S., Demirok B., Satici C., Demirkol M.A. Clinical characteristics of adult patients hospitalized with laboratory-confirmed COVID-19 pneumonia. J Infect Chemother. 2021;27(2):306–311. doi: 10.1016/j.jiac.2020.10.020. Epub 2020 Oct 23. PMID: 33191111; PMCID: PMC7584418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer H., Alqahtani A.S., Alzoman H., Aljerian N., Memish Z.A. Unusual presentation of Middle East respiratory syndrome coronavirus leading to a large outbreak in Riyadh during 2017. Am J Infect Control. 2018;46:1022–1025. doi: 10.1016/j.ajic.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenzian M.G., Bruc S.L., Slate C.L., Tia J.R., Baldwi M.R., Barr R.G. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369 doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayed M., Borahmah A.A., Yazdani A., Sultan A., Mossad A., Rawdhan H. Assessment of clinical characteristics and mortality-associated factors in COVID-19 critical cases in Kuwait. Med Princ Pract. 2020;369:407–416. doi: 10.1159/000513047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhy H.H., Alenazi T.H., Alshamrani M.M., Baffoe-Bonnie H., Arabi Y., Hijazi R. Description of a hospital outbreak of Middle East respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2016;37:1147–1155. doi: 10.1017/ice.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry M., Almohaya A., Alhijji A., Akkielah L., Alrajhi A. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic Area. J Epidemiol Glob Health. 2020;10:214–221. doi: 10.2991/jegh.k.200806.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry M., Ghonem L., Alsharidi A., Alanazi A., Alotaibi N., Al-Shahrani F. Coronavirus disease-2019 pandemic in the Kingdom of Saudi Arabia: mitigation measures and hospital preparedness. J Nat Sci Med. 2020;3:155–158. doi: 10.4103/JNSM.JNSM_29_20. [DOI] [Google Scholar]
- Barry M., Phan M.V., Akkielah L., Al-Majed F., Alhetheel A., Somily A. Nosocomial outbreak of the Middle East Respiratory Syndrome coronavirus: a phylogenetic, epidemiological, clinical and infection control analysis. Travel Med Infect Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:1–12. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Muth D., Corman V.M., Hussain R., Al Masri M., HajOmar W. An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60:369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbo S.F., Skakni L., Chu D.K.W., Garbati M.A., Joseph M., Peiris M. Molecular epidemiology of hospital outbreak of Middle East respiratory syndrome, Riyadh, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21:1981–1988. doi: 10.3201/eid2111.150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.A.W., Wong K.K., Szablewski C.M., Patel P.R., Rossow J., da Silva J. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19—Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:545–550. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51. doi: 10.1001/jamainternmed.2020.6252. PMID: 33080002; PMCID: PMC7577201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chu T.W.-H., Chu M.-Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E., Fontán-Vela M., Valencia J., Fernandez-Jimenez I., Álvaro-Alonso E.A., Izquierdo-García E. Characteristics, complications and outcomes among 1549 patients hospitalised with COVID-19 in a secondary hospital in Madrid, Spain: a retrospective case series study. BMJ Open. 2020;10:1–10. doi: 10.1136/bmjopen-2020-042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu G., Yu H., Peng X., Luo Y., Cao C. Clinical characteristics and outcomes of 74 patients with severe or critical COVID-19. Am J Med Sci. 2020;360:229–235. doi: 10.1016/j.amjms.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID‐19: a single-center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Command and Control Center . 2018. Middle East respiratory syndrome coronavirus; guidelines for healthcare professionals. Version 5.1 May 21. [Google Scholar]
- Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S. 2014 MERS-CoV outbreak in Jeddah — a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs. standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. PMID: 33080005; PMCID: PMC7577199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sarfraz S., Mohammed A., Reem A., Fahad A., Faisal A., Rayan A. Clinical characteristics of patients with COVID-19 in Saudi Arabia—a single center experience. Res Rev Infect Dis. 2020;3:68–74. doi: 10.36959/719/568. [DOI] [Google Scholar]
- Saudi Arabia Ministry of Health. COVID 19 Dashboard: Saudi Arabia. n.d. https://covid19.moh.gov.sa/.
- Saudi Arabia Ministry of Health. MOH health events: Epi-week 23, 2020, n.d. https://moh.gov.sa/EN/CCC/EVENTS/NATIONAL/PAGES/2020.ASPX. (Accessed 17 October 2020).
- Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19. (Version 21) July 31, 2020, n.d. https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf.
- Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. JAMA. 2020;324:1330. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration . 2020. Coronavirus (COVID-19) Update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. [Google Scholar]
- WHO. MERS situation update January 2020. Eastern Mediterr Reg Off. n.d. http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html. (Accessed 23 October 2020).
- WHO Solidarity Trial Consortium, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V. Repurposed antiviral drugs for Covid-19 — interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. Epub 2020 Dec 2. PMID: 33264556; PMCID: PMC7727327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO coronavirus disease (COVID-19) Dashboard. n.d. https://covid19.who.int/. (Accessed 10 October 2020).
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data for this study will be made available upon reasonable request to the corresponding author.