INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is the classic example of fibrotic interstitial lung disease (ILD) with prevalence of 14.0 to 42.7 per 100,000 persons [1] and estimated median survival of 3.8 years [2]. Computed tomography (CT) is the current standard imaging modality for assessment of ILD [3,4] and specific CT findings can obviate the need for biopsy [5] for defining usual interstitial pneumonia (UIP) patterns [4,5] establishing the diagnosis. CT is widely available and offers excellent spatial resolution, but drawbacks include exposure to ionizing radiation, limited tissue characterization, and limited ability to assess dynamic contrast enhancement or response to antifibrotic therapies.
Magnetic resonance imaging (MRI) has been investigated as a potential tool for evaluation of interstitial lung diseases and fibrosis [6-9] because of its superior ability of characterizing tissue properties, lack of ionizing radiation and dynamic image acquisition capabilities. However, lung MRI is limited by long acquisition time, poor spatial resolution, low signal-to-noise ratio, susceptibility and motion artifacts [10]. The long scan time and breath-hold requirements can be particularly challenging in patients with ILD who often have limited breath-holding capabilities. Therefore, to be able to image ILD effectively, an MR sequence should overcome MR- and patient-related issues, provide reasonable resolution to visualize small pulmonary structures, and be reproducible. 3D Dynamic contrast-enhanced free-breathing imaging has the potential to overcome these challenges [11]. prototype 4D Stack of Stars GRE (StarVIBE) (Siemens, Erlangen, Germany) is a fast and highly motion-resistant 3D sequence which can image the entire chest in free breathing during dynamic contrast administration. At least one study has assessed StarVIBE for imaging the lungs as part of in radiation therapy planning for lung cancer [12].
The objective of this study is to evaluate StarVIBE MR sequence in assessment of dynamic contrast enhancement of lung parenchyma in IPF and healthy controls, comparing anatomic findings with CT and with a more established MRI sequence, steady-state free precession (SSFP).
MATERIALS AND METHODS
This prospective study was approved by the Institutional Review Board and informed consent was obtained from participants.
Subjects (Table 1)
Table 1.
Demographics (n = 25 subjects).
| Subject | Age | Sex | Diagnosis | Number of MRI studies |
CT correlation |
Interval between CT and MR (days) |
|---|---|---|---|---|---|---|
| HC1 | 53 | M | Healthy | 1 | NA | NA |
| HC2 | 58 | F | Healthy | 1 | NA | NA |
| HC3 | 54 | M | Healthy | 1 | NA | NA |
| HC4 | 51 | M | Healthy | 1 | NA | NA |
| HC5 | 59 | M | Healthy | 1 | NA | NA |
| HC6 | 52 | M | Healthy | 1 | NA | NA |
| HC7 | 54 | F | Healthy | 1 | NA | NA |
| HC8 | 60 | M | Healthy | 1 | NA | NA |
| HC9 | 50 | M | Healthy | 1 | NA | NA |
| HC10 | 60 | M | Healthy | 1 | NA | NA |
| HC11 | 58 | M | Healthy | 1 | NA | NA |
| HC12 | 54 | F | Healthy | 1 | NA | NA |
| IPF1 | 64 | F | Familial IPF | 2 | yes | 48 |
| IPF2 | 72 | M | IPF | 1 | yes | 27 |
| IPF3 | 61 | F | IPF | 1 | yes | 111 |
| IPF4 | 51 | M | IPF | 2 | yes | 70 |
| IPF5 | 73 | F | IPF | 1 | yes | 66 |
| IPF6 | 62 | F | Familial IPF | 1 | yes | 303 |
| IPF7 | 60 | M | Familial IPF | 1 | yes | 62 |
| IPF8 | 61 | M | IPF | 2 | yes | 105 |
| IPF9 | 78 | M | IPF | 1 | yes | 7 |
| IPF10 | 61 | M | IPF | 1 | yes | 373 |
| IPF11 | 71 | M | Familial IPF | 1 | yes | 725 |
| IPF12 | 79 | M | IPF | 1 | yes | 7 |
| IPF13 | 68 | F | IPF | 1 | yes | 1112 |
MRI: Magnetic resonance Imaging; CT: Computed tomography; HC: Healthy control; IPF: Idiopathic pulmonary fibrosis; M: Male; F: Female; NA: Not applicable.
The study included 13 subjects [5F:8M; average age 66±8.1 years] with IPF (including 4 with familial IPF). The diagnosis was established based on clinical features and CT findings consistent with UIP or probable UIP [4,5]. 12 healthy controls [3F:9M; average age 55±3.6 years] were recruited through a hospital-wide research volunteer program. Exclusion criteria were contraindications to MR scanning due to implanted devices, allergy to gadolinium, eGFR<60l/min/1.73m2 and respiratory infection within 6 months.
Imaging methods: MRI and CT
All subjects underwent MRI of the chest on a 3-T Prisma Fit scanner (Siemens Healthcare, Erlangen, Germany) with an 18-channel body array and 32-channel spine array, using only a maximum of 12 channels at a time. The 3-plane localizer was followed by single breath-hold axial imaging with SSFP (Siemens’ TrueFISP – True Fast Imaging with Steady-state Precession), and by StarVIBE during free-breathing. This sequence employs in-plane radial sampling on a T1-weighted spoiled-gradient recalled echo acquisition; radial sampling is performed with a 3D “stack-of-stars” approach, which acquires the XY-plane along radial spokes and the Z-direction with Cartesian sampling [13]. Axial SSFP images were acquired with 5 mm slice thickness, repetition time (TR) of 434.35-582.62 ms, echo time (TE) of 1.19 ms, 256 x 256 matrix, flip angle (FA) of 41 degrees. StarVIBE was performed with a FA of 12 degrees, TE of 1 ms, TR of 3 ms, approximately 40 K radials, three-dimensional FOV of 400 mm and pixel resolution of (1.5-2.0) x (1.5-2.0) x 3 mm3. Imaging was performed before intravenous administration of gadoterate meglumine (Dotarem, Guerbet, USA) to confirm coverage and quality, and then repeated multiple times during and after contrast injection up to 160-360 seconds. For the purposes of this study, only the images acquired up to 160 seconds were reviewed as these acquisitions were consistently available for all subjects. The interval between each post-contrast acquisition varied from 2.0 to 7.6 seconds. The IV contrast was administered at a dose of 0.1 mmol/kg at the rate of 2 cc per second. Additional MR sequences were also obtained in some patients as part of the development of the research protocol but not assessed in this study. Healthy controls underwent a single MRI, three subjects with pulmonary fibrosis underwent two MRIs (intervals of 81, 91 and 112 days between scans) and the other ten pulmonary fibrosis subjects underwent a single MRI.
IPF subjects also underwent high-resolution CT imaging for clinical indications on different multidetector scanners, with standard high-resolution protocol with supine inspiration and expiration images and prone imaging (kV of 120, automatic exposure control, and slice thickness of 0.8–1.5 mm). The median interval between CT and MRI was 70 days, with CT preceding MRI in all but two subjects.
Image evaluation
All imaging studies were evaluated in consensus by two thoracic radiologists with 8 and 15 years of experience, unaware of clinical data. The StarVIBE sequences were evaluated on the coronal acquisition and in reformatted axial and sagittal planes and SSFP images were assessed only in the acquired axial plane.
Assessment of image quality and resolution
SSFP and StarVIBE images were assessed subjectively for quality, signal-to-noise ratio (SNR), and artifacts. The subjective quality and noise were graded on a 5-point scale with 5 being best, and 1 being the worst. The grading of artifacts was on a 4-point scale with 0 for no artifacts and 3 for nondiagnostic study. Spatial resolution was assessed based on the visibility of bronchi, pulmonary vessels, pleural fissures, and lymph nodes. The bronchi and pulmonary vessels were assessed on a 5-point scale based on the smallest airway and vessel that was clearly visible (1 being the trachea or main pulmonary artery, 5 being subsegmental branches). The visibility of small mediastinal lymph nodes and of pleural fissures was each graded on a 2-point scale (yes/no). The objective assessment of noise was performed by measuring the signal intensity and standard deviation of axial images in SSFP and axial images in StarVIBE. The ROI was placed in identical locations in axial images that were free of artifacts. The standard deviation is considered the measure of the noise in an image ( https://www.acr.org/-/media/ACR/Files/Radiology-Safety/MR-Safety/MRGlossary.pdf). The ratio of signal intensity and standard deviation(noise) was generated to develop objective ratio, that is independent of effect of IV gadolinium on signal intensity. The cross-sectional diameter of ascending and descending aorta was measured at identical locations in axial images of SSFP and StarVibe to determine the effect of motion on dimensions of the aorta.
Assessment of parenchymal abnormalities
The lung parenchyma on MRI was evaluated for reticulation and ground-glass opacities, traction bronchiectasis, cystic changes, and consolidation; readers were blinded to CT findings for this assessment. Reticulation and ground-glass opacities were grouped in one category based on authors’ prior experience and frequent co-existence of these findings in patients with fibrosis and were defined as areas of increased signal intensity compared to the normal lung parenchyma. Traction bronchiectasis were defined as dilated, tubular and branching structures of decreased signal intensity, contiguous to the central airways; cystic changes as circumscribed areas of reduced signal intensity and represented honeycombing or traction bronchiolectasis; and consolidation as increased signal intensity that obscured vascular structures. In the StarVIBE images, the consistency of the location of the findings over the multiple acquisitions that lasted up to 160 seconds was noted and graded on a 3-point scale as poor, reasonable and excellent.
Comparison of MRI and CT
After the evaluation of the MRI, the CT images were assessed for reticulation, ground-glass opacities, traction bronchiectasis, honeycombing, and consolidation, according to the Fleischner society guidelines [14]. StarVIBE images were then compared side-by-side with CT to identify all abnormal findings. The concordance was ranked 1-3, 3 being a perfect match in the sense that for every abnormal area on CT (the standard of reference), similar findings were noted on MRI.
Statistics
The imaging characteristics were summarized descriptively. Continuous data were described as mean ± standard deviation or median (range) and categorical data were described as frequency with percentage. The Wilcoxon rank-sum test was performed to compare continuous characteristics between groups, Wilcoxon signed-rank test was applied to test numeric features between paired groups, and McNemar’s test was applied to test binary features between paired groups. All tests were two-sided, and p<0.05 was considered significant. The paired sample 2 tailed student t test was performed to compare the image noise and effect of motion on caliber of ascending and descending aorta. Analysis was performed with R software (v3.3.1; R Foundation for Statistical Computing, Vienna, Austria). Only the results of the first MRI of subjects who underwent more than one exam were included in the statistical analysis. The accuracy of MRI (CT being the gold standard) was calculated as: (true positive + true negative) / (total number of scans).
RESULTS
All subjects completed the MRI scans successfully. There were 28 MRI scans, 12 in healthy controls and 16 in IPF subjects. No contrast adverse reactions were noted.
Image quality and resolution (Table 2 and 3)
Table 2.
Comparative image assessment between steady-state free precession imaging and dynamic 3D contrast-enhanced StarVIBE (n = 25 subjects).
| Characteristics | SSFP | StarVIBE | P-value |
|---|---|---|---|
| Median (range) |
Median (range) | Wilcoxon signed-rank test |
|
| Quality | 4 (3-4) | 4 (3-4) | >0.99 |
| Noise | 4 (3-4) | 3 (3-4) | 0.39 |
| Artifacts | 1 (0-2) | 0 (0-2) | <0.01 |
| Smallest visualized airway* | 3 (3-4) | 4 (3-5) | <0.01 |
| Smallest visualized pulmonary artery branch** | 4 (4-5) | 5 (4-5) | <0.01 |
| N (%) | McNemar's test | ||
| Fissures | 1 (4%) | 16 (64%) | <0.01 |
| Lymph nodes | 25 (100%) | 25 (100%) | >0.99 |
SSFP: Steady-state free precession; Smallest airway
1-5, 1 being the trachea and 5 being subsegmental bronchi; Smallest pulmonary artery branch
1-5, 1 being the main pulmonary artery and 5 being subsegmental branches.
Table 3.
Comparative analysis of noise and aortic dimensions between SSFP and StarVibe images.
| SSFP (Mean ± SD) | StarVIBE (Mean ± SD) | P-value | |
|---|---|---|---|
| Signal Intensity | 22.07 ± 7.44 | 43.30 ± 21.76 | >0.99 |
| Noise | 8.73 ± 5.84 | 12.67 ± 8.16 | 0.39 |
| Signal intensity/Noise | 2.97 ± 0.84 | 4.27 ± 2.49 | p = .006 |
| Ascending aorta (mm) | 33.07 ± 4.15 | 33.39 ± 3.86 | p = .52 |
| Ascending aorta (mm) | 24.64 ± 3.24 | 24.75 ± 3.76 | p = .83 |
The SSFP images were obtained without IV gadolinium in breath-hold and StarVIBE images were obtained after IV gadolinium during free-breathing.
The subjective scores of StarVIBE and SSFP for quality (median 4 for both sequences) were similar (Table 2). The median score for SNR was lower for StarVIBE, 3 compared to 4 for SSFP images, but the difference was not significant. The score for artifacts was significantly lower for StarVIBE (median 0) than SSFP images (median 1) (p<0.01). The artifacts on SSFP were mostly in the paramediastinal region, related to phase-encoded motion artifacts from cardiac and vascular pulsation, whereas the artifacts on StarVIBE images were due to minimal blurring from respiratory motion. The resolution for bronchi, vessels, and fissures was significantly higher in StarVIBE (p<0.01 for all). Both SSFP and StarVIBE were able to depict mediastinal lymph nodes in all subjects. The objective measurements of signal intensity, noise and ratio of signal intensity to the noise and measurements of ascending and descending thoracic aorta are summarized in Table 3. The noise was significantly less in StarVibe images (p=0.006). There was no significant no difference measurements of ascending and descending aorta (p> 0.5 and >0.8).
Detection of parenchymal abnormalities (Table 4, Figures 1 and 2)
Table 4.
Comparative analysis of findings between CT and MRI in patients with pulmonary fibrosis (n = 13 subjects).
| Finding | MRI sequence | Present on CT (N) | |||
|---|---|---|---|---|---|
| No | Yes | Accuracy | |||
| Reticulation | SSFP | No | 0 | 4 | 69.00% |
| Yes | 0 | 9 | |||
| StarVIBE | No | 0 | 1 | 92.00% | |
| Yes | 0 | 12 | |||
| Cysts | SSFP | No | 9 | 4 | 69.00% |
| Yes | 0 | 0 | |||
| StarVIBE | No | 9 | 3 | 77.00% | |
| Yes | 0 | 1 | |||
| Traction bronchiectasis | SSFP | No | 2 | 4 | 69.00% |
| Yes | 0 | 7 | |||
| StarVIBE | No | 1 | 1 | 85.00% | |
| Yes | 1 | 10 | |||
| Consolidation | SSFP | No | 13 | 0 | 100.00% |
| Yes | 0 | 0 | |||
| StarVIBE | No | 13 | 0 | 100.00% | |
| Yes | 0 | 0 | |||
SSFP: Steady-state free precession; Accuracy: (true positive + true negative) / (total number of scans).
Figure 1.
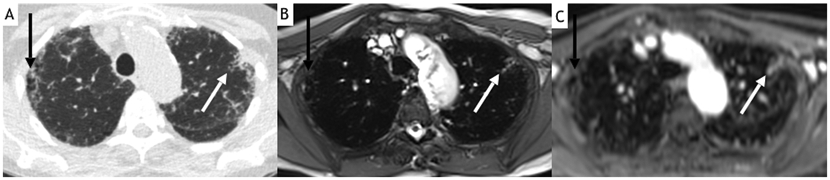
73-year-old female with idiopathic pulmonary fibrosis. Axial noncontrast CT (A) demonstrates bilateral subpleural ground-glass opacities and reticulation, right upper lobe honeycombing (black arrow) and left upper lobe traction bronchiolectasis (white arrow). Both axial steady-state free precession imaging (SSFP) (B) and 160-s post-contrast StarVIBE (C) images from MRI performed 2 months after the CT demonstrate similar findings. Note also how findings are much more evident on StarVIBE than on SSFP images, and improved conspicuity of the left major fissure (which is only barely visible on SSFP). Also note the pulsation artifacts along the aortic arch in the SSFP image.
Figure 2.
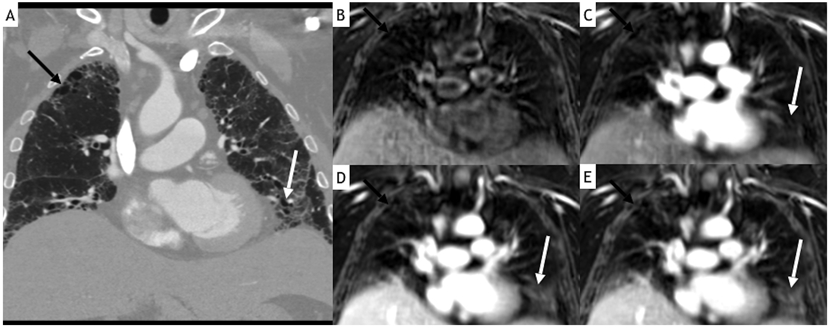
60-year-old female with familial idiopathic pulmonary fibrosis. Coronal reformat contrast-enhanced CT image (A) demonstrates bilateral left greater than right subpleural reticulation and ground-glass opacity, right apical honeycombing (black arrow), and left basilar traction bronchiectasis (white arrow). Coronal StarVIBE images before (B) and at 53 s (C), 105 s (D) and 160 s (E) after contrast administration demonstrate increased conspicuity of findings in delayed images, particularly of traction bronchiectasis (most of which cannot be confidently appreciated before contrast administration).
Reticulation/ground-glass opacities were noted in 13 subjects on CT, in 12 on StarVIBE (accuracy 92%) and 9 on SSFP (accuracy 69%). CT identified traction bronchiectasis in 11 subjects, StarVIBE in 11 (accuracy 85%, as there was one false-positive case) and SSFP in 7 (accuracy 69%). Honeycombing or traction bronchiolectasis were present in 4 subjects on CT, in 1 subject on StarVIBE (accuracy 77%) and 0 subjects on SSFP (accuracy 69%). There was no consolidation. There was excellent consistency in the location and distribution of the abnormalities across all StarVIBE images acquired for up to 160 seconds during free-breathing. For the three subjects that underwent repeat MRI on a different date, parenchymal abnormalities were also consistent between examinations.
Side to side comparison of MRI and CT
The correlation between CT and StarVIBE image findings was ranked as 3, i.e, for every abnormal area on CT, corresponding findings were noted on MRI. However, the areas of abnormality on CT were more extensive than could be appreciated on MRI, and many findings on MRI could only be identified in retrospect after using the CT as a reference.
Scan duration
The scan time, measured between the localizer images and the end of 160-second images of the StarVIBE series, averaged 29±6 minutes. This variability was due to additional sequences performed in many patients for research purposes but not evaluated as part of this study. During the 160 seconds of dynamic imaging an average of 34.2±13.0 acquisitions were obtained.
DISCUSSION
Our study showed that free-breathing StarVIBE MRI has good spatial and temporal resolution in patients with IPF and healthy controls for image interpretation without significant breathing-related artifacts. StarVIBE was superior to SSFP sequence, the latter of which is often used to assess the lungs due to its very fast acquisition and good spatial resolution [15,16]. The StarVIBE images had good quality and few artifacts throughout the dynamic acquisition.
Recent research comparing StarVIBE with breath-hold contrast-enhanced gradient recalled echo sequences for radiation therapy planning in lung cancer [12] demonstrated superior image quality with StarVIBE particularly on patients with poor breath-holding capabilities. In our study, the CT-detected lung parenchymal abnormalities were identified independently in both studied sequences, but contrast-enhanced StarVIBE had greater accuracy than breath-hold noncontrast SSFP imaging. The parenchymal abnormalities such as reticulation and ground-glass opacity were easier to see in dynamic contrast-enhanced images compared to pre-contrast StarVIBE images due to increased signal provided by gadolinium enhancement. However, the overall extent of parenchymal abnormalities was better appreciated on CT, and MR had slightly lower sensitivity to detect honeycombing and traction bronchiolectasis. While the lower SNR and spatial resolution are likely responsible for this difference, it is also worth noting that there is lack of experience even among dedicated thoracic radiologists in interpreting ILD on MRI. This limited experience is also the likely reason for a single false-positive finding of traction bronchiectasis on StarVIBE. The spatial resolution of StarVIBE, while certainly not as high as that of CT, was found to be more than acceptable, particularly in contrast-enhanced images, supported by the consistent visualization of small pulmonary vessels, mediastinal nodes and pleural fissures and objective lower image noise.
Recent research has shown the importance of assessing flow dynamics in patients with ILD and correlation with development of subsequent fibrosis. Mirsadraee et al. [6] evaluated healthy controls and IPF subjects with a breath-hold sequence and demonstrated that areas of fibrosis showed increased uptake of gadolinium compared to the lung in healthy controls and suggested contrast-enhanced MRI could identify areas of early fibrosis before these were evident on CT. Yi et al. [9] evaluated patients with idiopathic interstitial pneumonia and noted that T2-hyperintensity and early post-contrast enhancement with rapid wash-out correlated with active inflammation on biopsy, while delayed enhancement was associated with fibrosis. Weatherley et al. [17,18] assessed the use of dynamic contrast-enhanced MRI in subjects with IPF and demonstrated a correlation between lung fibrosis and increased transit time on perfusion analysis. Montesi et al. [11] showed that capillary permeability is diffusely increased throughout the lungs in patients with ILD, not just in the subpleural lung more involved by fibrosis. Most of these studies mostly used MR sequences that required breath holding, a task that is inherently difficult in patients with ILD. Furthermore, to assess the flow dynamics, multiple rapid image acquisitions are required of the entire lungs. Our study demonstrate that the StarVIBE sequence is an excellent tool to image the lungs with minimal artifacts and consistent image quality throughout the whole 160-second acquisition. The consistency was noted in different subjects and also during repeat MR imaging in the same subjects. Although we assessed images up to only 160 seconds after contrast administration for this study, the scanning can potentially be continued for much longer as there is no need for breath-holding. The total duration of the MRIs would depend mostly on the performance of additional sequences and intended duration of dynamic contrast imaging; for example, in our study, an MRI could be performed in 4 minutes (less than 1 minute for localizer images and 160 seconds for the StarVIBE images). The volumetric coverage of the entire lungs allows for near-isotropic reformats and review of images in axial or any other planes that are deemed optimal for evaluation of ILD. The high temporal resolution might allow characterization of tissue perfusion kinetics in the lung and help investigate the pathophysiology associated with ILD. Analysis of lung tissue kinetics was outside the scope of this analysis which focused on the assessment of the diagnostic quality of the images of the MRI sequences compared to CT.
Dynamic contrast-enhanced MRI is extensively used in other parts of the body including prostate and breast [19,20] using imaging sequences such as VIBE, but these are sensitive to motion, requiring breath-holds for lung imaging [10]. The motion-resistance provided by the radial-sampling of the k-space in the StarVIBE makes this an ideal sequence for imaging moving structures [12, 21,22].
Our study has several limitations. First, ground-glass opacities and reticulation were grouped in the assessment of parenchymal abnormalities, and therefore the accuracy for identifying each cannot be assessed separately. However, the authors feel that these findings frequently co-exist. Second, there was no histopathology proof of fibrosis, only CT correlation. Third, CT and MR were not performed concurrently. As such there could be interval changes in the lung parenchyma during the time interval between the two scans, although given the high correlation noted, it is unlikely that substantial changes were present. Lastly, subjective assessment of noise was performed by standards routinely used for assessment of CT images but has not been validated for MR image assessment.
In summary, we demonstrate that free-breathing contrast-enhanced MRI can obtain diagnostic quality images in patients with IPF with adequate spatial resolution and can allow assessment of perfusion dynamics. MRI is still less available and costlier than CT, and while authors do not believe that the former should replace the latter for routine clinical care, MRI might help further elucidate the physiopathology of ILD by enabling finer tissue characterization and assessment of dynamic intravenous contrast kinetics. Dynamic contrast enhancement evaluation might provide insight into hemodynamic processes not apparent on CT imaging, as well as add to other potential future developments in MRI, such as with agents that target collagen [23].
Abbreviations:
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- CT
computed tomography
- MRI
magnetic resonance imaging
- SSFP
steady-state free precession
- SNR
signal-to-noise ratio
- ROI
region of interest
Footnotes
Conflicts: SRD: Provides independent image analysis for hospital contracted clinical research trials programs for Merck, Pfizer, Bristol Mayer Squibb, Novartis, Roche, Polaris, Cascadian, Abbvie, Gradalis, Clinical Bay, Zai laboratories. Received honorarium from: Siemens, not related to work.
SBM: SBM is supported by research awards from the Francis Family Foundation and the Scleroderma Foundation. SBM has received research funding through her institution from United Therapeutics outside the scope of this work.
REFERENCES
- [1].Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G, Incidence and prevalence of idiopathic pulmonary fibrosis, Am. J. Respir. Crit. Care Med 174 (2006) 810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- [2].Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR, Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11., Lancet. Respir. Med 2 (2014) 566–72. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- [3].Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ, An Official ATS/ERS/JRS/ALAT Statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management, Am. J. Respir. Crit. Care Med 183 (2011) 788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, Goldin JG, Hansell DM, Inoue Y, Johkoh T, Nicholson AG, Knight SL, Raoof S, Richeldi L, Ryerson CJ, Ryu JH, Wells AU, Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper, Lancet Respir. Med 6 (2018) 138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- [5].Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, Flaherty KR, Wells A, Martinez FJ, Azuma A, Bice TJ, Bouros D, Brown KK, Collard HR, Duggal A, Galvin L, Inoue Y, Jenkins RG, Johkoh T, Kazerooni EA, Kitaichi M, Knight SL, Mansour G, Nicholson AG, Pipavath SNJ, Buendía-Roldán I, Selman M, Travis WD, Walsh SLF, Wilson KC, Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline, Am. J. Respir. Crit. Care Med 198 (2018) e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- [6].Mirsadraee S, Tse M, Kershaw L, Semple S, Schembri N, Chin C, Murchison JT, Hirani N, Van Beek EJR, T1 characteristics of interstitial pulmonary fibrosis on 3T MRI — a predictor of early interstitial change ?, Quant Imaging Med Surg. 6 (2016) 42–49. doi: 10.3978/j.issn.2223-4292.2016.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lutterbey G, Gieseke J, von Falkenhausen M, Morakkabati N, Schild H, Lung MRI at 3.0 T: A comparison of helical CT and high-field MRI in the detection of diffuse lung disease, Eur. Radiol 15 (2005) 324–328. doi: 10.1007/s00330-004-2548-1. [DOI] [PubMed] [Google Scholar]
- [8].Biederer J, Mirsadraee S, Beer M, Molinari F, Hintze C, Bauman G, Both M, van Beek EJR, Wild J, Puderbach M, MRI of the lung (3/3)-current applications and future perspectives, Insights Imaging. 3 (2012) 373–386. doi: 10.1007/s13244-011-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yi CA, Lee KS, Han J, Chung MP, Chung MJ, Shin KM, 3-T MRI for differentiating inflammation- and fibrosis-predominant lesions of usual and nonspecific interstitial pneumonia: comparison study with pathologic correlation., AJR. Am. J. Roentgenol 190 (2008) 878–85. doi: 10.2214/AJR.07.2833. [DOI] [PubMed] [Google Scholar]
- [10].Wild JM, Marshall H, Bock M, Schad LR, Jakob PM, Puderbach M, Molinari F, van Beek EJR, Biederer J, MRI of the lung (1/3): Methods, Insights Imaging. 3 (2012) 345–353. doi: 10.1007/s13244-012-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Montesi SB, Rao R, Liang LL, Goulart HE, Sharma A, Digumarthy SR, Shea BS, Seethamraju RT, Caravan P, Tager AM, Gadofosveset-enhanced lung magnetic resonance imaging to detect ongoing vascular leak in pulmonary fibrosis, Eur. Respir. J 51 (2018) 1800171. doi: 10.1183/13993003.00171-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kumar S, Rai R, Stemmer A, Josan S, Holloway L, Vinod S, Moses D, Liney G, Feasibility of free breathing Lung MRI for Radiotherapy using non-Cartesian k-space acquisition schemes., Br. J. Radiol 90 (2017) 20170037. doi: 10.1259/bjr.20170037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kean M, Moon A, Radial 3D VIBE in the Pediatric Abdomen. A Report on Our Initial Experience, MAGNETOM Flash. (2014) 6. https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@imaging/@mri/documents/download/mday/mjq2/~edisp/improving_robustness_using_radial_vibe-01176267.pdf https://static.healthcare.siemens.com/si (accessed February 15, 2019). [Google Scholar]
- [14].Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J, Fleischner Society: glossary of terms for thoracic imaging., Radiology. 246 (2008) 697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- [15].Failo R, Wielopolski PA, Tiddens HAWM, Hop WCJ, Mucelli RP, Lequin MH, Lung morphology assessment using MRI: A robust ultra-short TR/TE 2D steady state free precession sequence used in cystic fibrosis patients, Magn. Reson. Med 61 (2009) 299–306. doi: 10.1002/mrm.21841. [DOI] [PubMed] [Google Scholar]
- [16].Biederer J, Beer M, Hirsch W, Wild J, Fabel M, Puderbach M, van Beek EJR, MRI of the lung (2/3). Why… when … how?, Insights Imaging. 3 (2012) 355–371. doi: 10.1007/s13244-011-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weatherley N, Marshall H, Hughes P, Austin M, Smith L, Renshaw S, Bianchi S, Wild J, Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the Evaluation of Perfusion Heterogeneity in Idiopathic Pulmonary Fibrosis., in: Proc. from Am. Thorac. Soc. 2018 Int. Conf. May 18-23; San Diego, CA, 2018: pp. A5925–A5925). [Google Scholar]
- [18].Weatherley ND, Stewart NJ, Chan HF, Hughes PJC, Marshall H, Austin M, Smith L, Collier G, Rao M, Norquay G, Renshaw SA, Bianchi SM, Wild JM, S73 Probing diffusion and perfusion in idiopathic pulmonary fibrosis with hyperpolarised Xenon and dynamic contrast-enhanced magnetic resonance imaging, Thorax. 73 (2018) A44 LP–A45. doi: 10.1136/thorax-2018-212555.79. [DOI] [Google Scholar]
- [19].Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton M, Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study, Lancet. 389 (2017) 815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- [20].Zhang M, Horvat JV, Bernard-Davila B, Marino MA, Leithner D, Ochoa-Albiztegui RE, Helbich TH, Morris EA, Thakur S, Pinker K, Multiparametric MRI model with dynamic contrast-enhanced and diffusion-weighted imaging enables breast cancer diagnosis with high accuracy, J. Magn. Reson. Imaging (2018) 1–11. doi: 10.1002/jmri.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Block KT, Chandarana H, Milla S, Bruno M, Mulholland T, Fatterpekar G, Hagiwara M, Grimm R, Geppert C, Kiefer B, Sodickson DK. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J Korean Soc Magn Reson Med. 2014. June;18(2):87–106. [Google Scholar]
- [22].Li HH, Zhu H, Yue L, Fu Y, Grimm R, Stemmer A, Fu CX, Peng WJ, Feasibility of free-breathing dynamic contrast-enhanced MRI of gastric cancer using a golden-angle radial stack-of-stars VIBE sequence: comparison with the conventional contrast-enhanced breath-hold 3D VIBE sequence., Eur. Radiol 28 (2018) 1891–1899. doi: 10.1007/s00330-017-5193-1. [DOI] [PubMed] [Google Scholar]
- [23].Caravan P, Yang Y, Zachariah R, Schmitt A, Mino-Kenudson M, Chen HH, Sosnovik DE, Dai G, Fuchs BC, Lanuti M, Molecular magnetic resonance imaging of pulmonary fibrosis in mice, Am. J. Respir. Cell Mol. Biol 49 (2013) 1120–1126. doi: 10.1165/rcmb.2013-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


