Abstract
Compulsive alcohol use, or drinking that persists despite negative or aversive consequences, is a defining characteristic of alcohol use disorder. Here, chemogenetic technology (i.e. Designer Receptors Exclusively Activated by Designer Drugs; DREADDs) was used to inhibit or excite the NAc core or selectively inhibit D1- or D2 receptor-expressing neurons in the NAc core to understand the role of the NAc core and how these subpopulations of neurons may influence compulsive-like ethanol (EtOH) drinking using C57BL/6J, Drd1-cre, and Drd2-cre male and female mice. Compulsive-like EtOH drinking was modeled with a two-bottle choice, drinking in the dark paradigm. The major finding of this study was that mice decreased compulsive-like EtOH intake when the NAc core was inhibited and there was no change of EtOH + quinine intake when the NAc core was excited. Interestingly, inhibition of D1- or D2 receptor-expressing neurons did not alter compulsive-like EtOH intake. Control experiments showed that NAc core excitation and selective inhibition of D1- or D2- receptor-expressing neurons had no effect on baseline EtOH drinking, intake of water, or intake of quinine-adulterated water. CNO reduced amphetamine-induced locomotion in the D1-CRE+ (but not the D2CRE+) group in a control experiment. Finally, pharmacological antagonism of D1 and D2 receptors together, but not separately, reduced quinine-resistant EtOH drinking. These results suggest that the NAc core is a critical region involved in compulsive-like EtOH consumption, and that both D1- and D2 receptor-expressing medium spiny neurons participate in controlling this behavior.
Keywords: nucleus accumbens, drinking in the dark, dopamine, alcohol, aversion, reward
Graphical abstract
1. Introduction
Alcohol use disorder (AUD) affects an estimated 16 million individuals (NIAAA, 2015) and is the third leading cause of preventable death in the United States (Mokdad et al., 2005). AUD is characterized by uncontrollable, compulsive alcohol drinking, defined here as drinking that persists despite negative or aversive consequences (Association and Others, 2013). Due to the societal impact of AUD, it is imperative to understand the neural mechanisms that drive compulsive drinking in order to improve treatment and prevention initiatives.
In rodents, the neural mechanisms of compulsive-like drinking have been investigated using models that pair an aversive stimulus with ethanol (EtOH) delivery. In the present study, a two-bottle, limited access “drinking in the dark” (DID) procedure was used to model binge-like EtOH consumption and the bitter tastant quinine was added to the EtOH bottle to punish consumption. Numerous studies have demonstrated that rodents develop “aversion-resistant” drinking patterns, characterized by continued consumption of quinine-adulterated EtOH at concentrations of quinine that are normally avoided (Lesscher et al., 2010; Seif et al., 2013; Sneddon et al., 2019). A benefit of this model is that the effects of experimental manipulations can be tested on baseline EtOH drinking and quinine-resistant drinking, allowing identification of mechanisms specific to compulsive-like drinking.
Aversion-resistant drinking models have identified cortical and striatal circuits as key contributors to compulsive-like EtOH consumption across species (Chen and Lasek, 2019; Radke et al., 2017; Seif et al., 2015). The mPFC and insula have both been found to control aversion-resistant drinking via projections to subcortical structures (Halladay et al., 2020; Seif et al., 2013; Siciliano et al., 2019). In rodents, punished-suppression of EtOH-responding is associated with plasticity at mPFC inputs to nucleus accumbens (NAc) medium spiny neurons (MSNs) (Halladay et al., 2020; Seif et al., 2013). An fMRI study in humans also demonstrated that the NAc was active in heavy vs. light alcohol drinkers responding for alcohol under the threat of punishment with an aversive electric shock (Grodin et al., 2018).
NAc core MSNs generally express one of two types of dopamine receptors, excitatory D1-like receptors or inhibitory D2-like receptors (Gerfen and Surmeier, 2011). In mice, D1-receptor expressing “direct” pathway neurons (D1-neurons) project to the midbrain as well as to the ventral pallidum while D2-receptor expressing “indirect” pathway neurons (D2-neurons) project to the ventral pallidum and send collaterals to the D1-neurons (Dobbs et al., 2017; Kupchik et al., 2015; Robertson and Jian, 1995; Smith et al., 2013). An influential theory of MSN function posits that D1-neuron activity primarily mediates approach behaviors while D2-neurons are involved in aversion and punishment (Hikida et al., 2016; Lobo and Nestler, 2011). In line with this idea, plasticity in accumbal D1-neurons facilitates addictive behaviors (Bahi and Dreyer, 2012; Chandra et al., 2013) while increased D2-neuron activity is protective in this regard (Bock et al., 2013; Dobbs et al., 2017). Not all findings conform to this general model, however, and several studies have documented a role for D2-neurons in positive reinforcement (Natsubori et al., 2017; Soares-Cunha et al., 2019, 2016).
Considering the important role of NAc plasticity in promoting compulsive-like alcohol drinking, we investigated the role of NAc core D1- and D2-neurons in mediating resistance to quinine punishment in mice drinking a 15% EtOH solution. Using a chemogenetic approach, we first demonstrated that non-selective inhibition, but not excitation, of NAc core neurons reduced quinine-resistant EtOH drinking. Selective targeting and chemogenetic inhibition of D1- and D2-neurons, however, had no effect on drinking behaviors. Further, selective antagonism of D1 and D2 receptors (D1R and D2R) was ineffective in reducing quinine-resistant drinking while blockade of both receptors together reduced intake. These results suggest that both MSN subtypes in the NAc core are sufficient for expression of compulsive-like alcohol drinking.
2. Materials & Methods
2.1. Subjects
Experiments 1 (NAc-hM4Di), 2 (NAc-hM3Dq), and 5 (D1/D2R antagonism) used 60 adult male and female C57BL/6J generated from breeding pairs purchased from Jackson Labs (Bar Harbor, ME). Experiment 3 (D1-hM4Di) used 34 male and female B6;129-Tg (Drd1-cre)120Mxu/Mmjax mice generated from breeding pairs purchased from Jackson Labs (Stock no: 037156-JAX). Experiment 4 (D2-hM4Di) used 35 male and female B6.FVB(Cg)-Tg(Drd2-cre)ER44Gsat/Mmucd mice generated from breeding pairs purchased from the Mutant Mouse Research & Resource Centers (MMRRC) (Stock no: 032108-UCD; Davis, CA), a NIH funded strain repository, and were donated by the MMRRC at UC Davis. The original mouse strain was donated by the NINDS funded GENSAT BAC transgenic project. Mutant lines were bred on a C57BL/6J background and heterozygous males were bred with C57BL/6J females (Jackson Labs) to produce heterozygous Cre+ (CRE+) and wildtype (WT) Cre− offspring. Mice were matched for age (PND 60+) and sex before being assigned to experimental groups.
Mice were socially housed pre-surgery in standard shoe box udel polysufone rectangular mouse cages (18.4 cm x 29.2 cm x 12.7 cm) with high profile udel polysulfone mouse filtered tops (19.7 cm x 30.5 cm x 9.5 cm) on a 12:12 light/dark cycle. Mice were given standard care and had access to reverse-osmosis (RO) filtered water ad libitum and were fed LabDiet 5001 standard feed ad libitum. Following surgery, mice were transferred to individual cages and kept on a 12:12 dark/light cycle (i.e., lights off from 7:00 AM – 7:00 PM). All animals were cared for in accordance with the guidelines set by the National Institute of Health and all procedures were performed in accordance with Miami University’s animal care committee’s regulations.
2.2. Surgery
Mice were anesthetized with isoflurane and placed into a stereotaxic instrument (model SAS75, Kopf Instruments, Inc, Tujunga, CA). The nose bar was set at 0 mm then adjusted accordingly to ensure the head was flat. A midline incision was made over the skull then bilateral holes were drilled into the skull using coordinates: AP: +1.2 mm, ML: ± 1.0 mm, and DV: −4.5 mm, in respect to Bregma. These coordinates were identified using the Franklin & Paxinos mouse brain atlas (Franklin & Paxinos, 2008). Mice were injected with either the viral vector AAV8-hSyn-hm4D(Gi)-mCherry (ID: 50475-AAV8, Addgene) (Experiment 1; NAc-hM4Di), AAV8-hSyn-hm3D(Gq)-mCherry (ID: 50474-AAV8, Addgene) (Experiment 2; NAc-hM3Dq), or AAV8-hSyn-DIO-hM4D(Gi)-mCherry (ID: 44362-AAV8, Addgene) (Experiments 3 and 4; D1-hM4Di and D2-hM4Di) using a 32-gauge Hamilton syringe (0.3μl – 0.35μl/side over a period of 7 minutes) (Fig. 1A). The needle was removed from the surgical site approximately 3 minutes after the injection and sanitized using a sterile EtOH wipe and was allowed to dry before its next use. After the last injection, the surgical site was closed using VetBond tissue adhesive (Santa Cruz Biotechnology, Inc, Dallas, TX). Mice were then weighed and given a saline injection (10 mL/kg) before being returned to their home cage. The home cage was placed on a heating pad for at least 30 minutes before it was returned back to the colony room. Mice were given standard post-operative care for three days following surgery with 50-80 mg/kg/day of ibuprofen available in the drinking water. Mice were given at least a week of recovery before behavioral testing and at least three weeks between surgery and CNO injection.
Figure 1. Experimental timeline of drinking in the dark task.
A) NAc core neurons were targeted for chemogenetic manipulation. In Experiment 1 all neurons were inhibited. In Experiment 2 all neurons were excited. In Experiments 3 and 4 D1CRE+ or D2CRE+ neurons were selectively inhibited. B) For all experiments, mice were presented with bottles of 15% EtOH and water in the home cage and consumption was measured over a 2-h period for 12 sessions. Quinine (100 μM) was added to the EtOH bottle starting on session 13. VEH and CNO injections were administered 10 min before the start of sessions 13 and 15. A subset of animals in Experiments 1 and 2 and all animals in Experiments 3 and 4 also received a CNO injection on session 11, to assess the effects of chemogenetic manipulations on baseline EtOH drinking.
2.3. Drugs
Fifteen percent EtOH was prepared volume/volume in RO water. Quinine hemisulfate (Q1250-10G, Millipore-Sigma, St. Louis, MO) solutions were prepared weight/volume (0 μM, 100 μM) in 15% EtOH or RO water. Solutions were made fresh daily. Clozapine-n-oxide (CNO) (1.0 mg/kg; Tocris, Minneapolis, MN; Batch no: 7A/195565) was dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO) and diluted in 0.9% sterile saline to a final concentration of 0.1 mg/ml. Vehicle (VEH) solution was made by dissolving 0.13% DMSO in sterile saline as volume/volume. A water-soluble version of clozapine-n-oxide dihydrochloride (Tocris, Batch no: 2A/218143) dissolved in 0.9% sterile saline was used in some experiments. In these instances, 0.9% sterile saline served as the VEH solution. CNO was stored in 1.5 mL centrifuge tubes in −20°C in a dark container then thawed approximately one hour before use. D-amphetamine hemisulfate salt (2.5 mg/kg; Sigma, Batch no: SLCB1164) was diluted in 0.9% saline to a final concentration of 0.25 mg/ml. The D1 receptor antagonist, SCH 39166 hydrobromide (0.01, 0.03, and 0.1 mg/kg; Tocris; Batch no: 5A/230073, 5A/244259) and D2 receptor antagonist, L-741, 626 (0.1, 0.3, and 1.0 mg/kg; Tocris; Batch no: 5B/231438, 6A/247735) were dissolved in 20% DMSO and 0.9% saline (v/v). On days where subjects received an injection of both D1R (0.1 mg/kg) and D2R (1.0 mg/kg) antagonists, a cocktail was made and the drugs were administered from one syringe. All drugs were delivered in an intraperitoneal (i.p) injection volume of 10 ml/kg.
2.4. EtOH drinking in the dark
Experiments 1-4 tested chemogenetic manipulation of NAc MSNs during quinine-resistant drinking. Mice self-administered EtOH in a two-bottle limited access protocol for two hours a day, five days a week (Monday-Friday) for three weeks (Fig. 1B). One bottle contained 15% EtOH and the other contained RO water. Bottles were alternated at each session to equate side biases. Bottles were weighed before and after the two-hour drinking session. Water and EtOH bottles were also placed on two “dummy” cages to account for spillage and evaporation. To habituate subjects to injections, saline injections were given to all subjects one week prior to the DID procedure and after sessions 2, 4, 7, and 9. An injection of CNO (1.0 mg/kg) was given on session 11 to assess the effect of inhibition or excitation on baseline levels of EtOH consumption. In Experiment 1 (NAc-hM4Di) and Experiment 2 (NAc-hM3Dq), only a subset (n = 4, 10, respectively) of animals received this treatment. Quinine hemisulfate (100 μM) was added to the 15% EtOH solution on drinking sessions 13, 14, 15, and 16. CNO (1.0 mg/kg) or VEH injections were given on drinking sessions 13 and 15 ten minutes before bottles were placed on the cages. Mice were randomly assigned to receive VEH and CNO on sessions 13 and 15 using a crossover design (i.e., mice receiving VEH on session 13 received CNO on session 15 and vice versa). In Experiment 1 (NAc-hM4Di, n = 11) and Experiment 2 (NAc-hM3Dq, n = 8), drinking ended after session 15 for some mice.
Experiments 5 and 6 examined the effects of pharmacological antagonism of dopamine receptors on quinine-resistant drinking. Mice self-administered 15% EtOH in the drinking in the dark paradigm for 10 drinking sessions. Quinine was added to the EtOH solution from session 11 onward. Experiment 5 examined dose-dependent effects of the selected D1R and D2R antagonists on quinine-resistant drinking. Mice received systemic injections of VEH, SCH 39166 (0.01, 0.03, and 0.1 mg/kg), or L-741, 626 (0.1, 0.3, and 1.0 mg/kg) over seven drinking sessions (sessions 11, 13, 15, 17, 19, 21, and 23) using a Latin Square design. In Experiment 6, the effects of administering a cocktail of the two antagonists were assessed in a separate cohort of mice. This D1/D2R antagonist cocktail was first administered on session 10 to assess effects on baseline, quinine-free drinking. Next, using a crossover design, the D1/D2R antagonist cocktail or VEH was administered during quinine-resistant drinking on sessions 12 and 14.
2.5. Water drinking in the dark
In Experiments 2-4, the effects of DREADD manipulations on water consumption and quinine sensitivity were assessed. Two weeks following EtOH drinking, mice were presented with two bottles of RO water for two hours a day. Throughout testing, mice had access to water and food ad libitum and consumption was only measured within the two-hour window to mirror the EtOH DID. During the first week, mice received injections of CNO or VEH on two sessions using a crossover design (i.e., mice receiving VEH first received CNO next and vice versa.) The following week, 100 μM quinine hemisulfate was added to one of the bottles of RO water and mice received injections of CNO or VEH over two, non-consecutive sessions.
2.6. Open field test of locomotion
For Experiment 1 (NAc-hM4Di), at least two weeks following DID, locomotor behavior was tested in an open field. Mice (n = 11, CNO = 5, VEH = 6)) were placed in a 45 x 45 cm Plexiglass open field for a thirty-minute session. Mice were randomly assigned to receive either a VEH or CNO injection ten minutes prior to the session. ANY-Maze software (Stoelting, Wood Dale, IL) scored each session.
2.7. Amphetamine locomotion stimulation test
A subset of 23 mice from Experiments 3 (D1-hM4Di) and 4 (D2-hM4Di), were habituated to 14” x 14” x 8” locomotion chambers (Omnitech Electronics, Inc, Columbus, OH) for one hour for an amphetamine locomotion stimulation test. The following day, testing occurred in three phases. First, mice were placed into the locomotion chambers for one hour for baseline testing. For phase two, mice were injected with CNO (1.0 mg/kg) and were placed back into the chamber for fifteen minutes. Phase three consisted of an amphetamine injection (2.5 mg/kg) and testing in the chamber for one hour. Fusion 4.0 software (Omnitech Electronics, Inc, Columbus, OH) was used to measure locomotion. All testing was conducted during the dark phase of the light cycle and mice were transported to the testing room with a dark cover over their cages. Between testing, the chambers were cleaned with 1.4% hydrogen peroxide Clorox wipes.
2.8. Histology
At the end of the experiments, the mice were injected with a ketamine-xylazine cocktail solution (100 mg/kg ketamine + 10 mg/kg xylazine). When anesthetized, mice underwent an intracardiac perfusion with 0.9% sterile saline, followed by 30% sucrose formalin solution (weight/volume). The brains were kept in 30% sucrose formalin solution for at least 48 hours in 4°C before being sectioned on a cryostat (40 μm sections) at −20°C. Slides were cover slipped with VECTASHIELD mounting medium with DAPI counterstain (Vector Labs, Burlingame, CA). Images were taken using an Olympus AX-70 Wide-field Multi-mode microscope at 4X magnification. Subjects were removed from analyses if there was no expression of the virus or if a brain region outside of the NAc core was expressing virus. Adobe Photoshop, GNU Image Manipulation Program (GIMP) 2.10, BioRender, and GraphPad Prism software were used to create images.
2.9. Statistical analysis
Data from bottle weights for drinking were expressed as grams of EtOH or ml of water consumed per kilogram of body weight for each individual mouse and then averaged across groups. Consumption was calculated as (Initial Bottle Weight – Post Bottle Weight) – Average of Dummy Bottles. Preference was calculated as (Volume of EtOH/(Volume of EtOH + Water Consumption))*100. Consumption of water was calculated as the sum of the two water bottles for all subjects then averaged. All data were first analyzed with sex as a factor but were later collapsed when there were no significant effects or trends suggesting a sex difference. Results of all statistical tests meeting the threshold for significance (α = 0.05 unless otherwise specified) are reported in the text. Using data from Sneddon et al., 2019, an a priori power analysis was conducted to determine that the minimum number of subjects required per group to detect differences in quinine-resistant drinking was 14.
Experiments 1 and 2 used a completely within-subjects design to assess the effects of NAc core inhibition and excitation on EtOH drinking. Thus, effects of CNO on baseline drinking were analyzed with One-Way ANOVA and total consumption of quinine-adulterated EtOH was analyzed using a paired t-test. For the open field test, a Two-Way RM ANOVA was used. For Experiments 3 and 4, a mixed design was used and all data were analyzed using a Two-Way RM ANOVA. In cases where the assumption of sphericity was violated (ε < 0.75), the Greenhouse-Geisser correction was applied. Post hoc tests corrected for multiple comparisons (Holm-Sidak or Dunnett’s tests) were performed for follow-up comparisons. In Experiment 5, the effects of D1R and D2R antagonism on quinine-resistant drinking were assessed with a Mixed-Effects ANOVA. For Experiment 6, the effects of combined D1R and D2R antagonism on quinine-free baseline drinking (sessions 9 vs. 10) and quinine-resistant drinking (VEH vs. D1/D2R antagonists on sessions 12 and 14) were analyzed using paired t-tests. Because baseline drinking differed between males and females in this experiment, multiple t-tests were required and a Bonferroni correction was applied (α = 0.025). All analyses were completed using GraphPad Prism 8.0.1 (La Jolla, CA, USA).
Data from bottles spilling or measurement error were excluded from all analyses. In instances where data points were missing, data from drinking sessions 1-12 was analyzed by fitting a Mixed Effects ANOVA. In the absence of missing values, this method gives the same results as a repeated measures (RM) ANOVA. In the presence of random missing values, the data can be interpreted like RM ANOVA. An a priori decision was also made to exclude mice that failed to show escalation of drinking (< 1g/kg EtOH); one mouse met this criterion and was excluded from all analyses.
3. Results
3.1. Experiment 1: Inhibition of the NAc core with hM4Di decreased aversion-resistant intake of quinine-adulterated EtOH.
The contribution of the NAc core to quinine-resistant EtOH drinking was first tested with chemogenetic inhibition targeting all NAc core neurons. Robust mCherry expression was observed in cell bodies in the NAc core. The average range of expression along the anterior-posterior axis was 1.4 mm (Fig. 2A). Across drinking sessions 1-12, all mice (n = 15, male = 9, female = 6) escalated consumption of 15% EtOH. A One-Way Mixed Effects ANOVA examining EtOH consumption on sessions 1-12 revealed a main effect of session (F(5.451,75.82) = 7.089, p < 0.001) (Fig. 2B). A post hoc Dunnett’s multiple comparisons test comparing consumption on sessions 2-12 to session 1 demonstrated a significant increase in drinking on sessions 6 (p = 0.034), 7 (p = 0.016), 8 (p = 0.009), 9 (p = 0.008), 10 (p = 0.023), 11 (p < 0.0001), and 12 (p < 0.0001). The effect of CNO on baseline EtOH drinking was assessed in a subset of animals (n = 4). A One-Way RM ANOVA analyzing consumption on sessions 10 (2.958 ± 0.381 g/kg), 11 (3.409 ± 0.422 g/kg), and 12 (3.060 ± 0.3533 g/kg) in this subset of animals found no differences [all data mean ± SEM].
Figure 2. Inhibition of the NAc core decreased aversion-resistant intake of quinine-adulterated EtOH.
A) Visual representation of hM4Di DREADD expression of neurons in the NAc core. B) Mice (n = 15) escalated their consumption of 15% EtOH and C) preferred 15% EtOH more than water. * p < 0.05, ** p < 0.01, vs. session 1 (Dunnett’s). D) Inhibition of the NAc core reduced intake of 15% EtOH + quinine. * p < 0.05 vs. VEH (Paired t-test).
All mice preferred 15% EtOH to water across drinking sessions. A One-Way Mixed Effects ANOVA examining preference on sessions 1-12 showed an increase in preference for EtOH vs. water with a main effect of session (F(5.287,73.54) = 3.581, p < 0.001) (Fig. 2C). A post hoc Dunnett’s test revealed a difference between drinking session 1 and sessions 8 (p = 0.005), 10 (p = 0.035), and 12 (p = 0.036).
A paired t-test demonstrated a significant difference in consumption of quinine-adulterated EtOH on CNO vs. VEH days, suggesting that NAc core inhibition reduced aversion-resistant drinking (t(14) = 2.591, p = 0.021) (Fig. 2D). A paired t-test showed no difference in preference for quinine-adulterated EtOH vs. water on CNO vs. VEH days. The order in which CNO or VEH injections were given also did not influence consumption or preference. Two-Way RM ANOVAs analyzing consumption and preference on sessions 13 and 15 revealed no effects of treatment or treatment order.
To assess if inhibition of the NAc core altered locomotor behavior, a subset of mice (n = 11, CNO = 5, VEH = 6) were injected with CNO or VEH and then underwent a thirty-minute open field test. A Two-Way RM ANOVA identified no significant differences in locomotor behavior between CNO (total distance traveled: 84.415 ± 6.097 cm) and VEH (total distanced traveled: 73.407 ± 4.459 cm) treatment groups (main effect of time: F(5.148, 51.48) = 4.985, p < 0.001 and no effect of treatment: F(1,9) =2.22, p = 0.170).
3.2. Experiment 2 Excitation of the NAc core with hM3Dq did not influence aversion-resistant intake of quinine-adulterated EtOH
The ability of increases in neuronal activity in the NAc core to affect EtOH drinking and resistance to quinine was tested next with chemogenetic excitation. Robust mCherry expression was observed in cell bodies in the NAc core. The average range of expression along the anterior-posterior axis was 1.2 mm (Fig. 3A). All mice (n = 18, male = 9, female = 9) consistently consumed 15% EtOH across drinking sessions 1-12. A Two-Way Mixed Effects ANOVA on escalation of EtOH consumption revealed a main effect of sex (F(1,16) = 24.765, p < 0.001) and no interaction (Fig. 3B). A post hoc Holm-Sidak multiple comparisons test identified a significant difference between males and females on drinking sessions 1 (p = 0.026) and 3 (p = 0.006). The effect of CNO on baseline EtOH drinking was assessed in a subset of animals (n = 10). There were no sex differences in this subset of animals on these drinking sessions, so data were collapsed across sex for analysis. A One-Way RM ANOVA analyzing consumption on sessions 10 (2.480 ± 0.235 g/kg), 11 (1.739 ± 0.426 g/kg), and 12 (1.615 ± 0.238 g/kg) in this subset of animals showed no effect of CNO on baseline EtOH drinking (F(1.359, 12.230) = 3.495, p = 0.076).
Figure 3. Excitation of the NAc core did not influence aversion-resistant intake of quinine-adulterated EtOH.
A) Visual representation of hM3Dq DREADD expression of neurons in the NAc core. B) Mice (n = 26) consistently consumed 15% EtOH and C) preferred 15% EtOH more than water. # p < 0.05, ## p < 0.01 comparing males vs. females (Holm-Sidak). D) Excitation of the NAc core did not alter intake of 15% EtOH + quinine. E) NAc core excitation did not alter consumption of water or F) water + quinine.
Mice increased their preference for 15% EtOH vs. water across drinking sessions. A Mixed Effects ANOVA identified an escalation of preference with a main effect of session (F(11,175) = 4.106, p < 0.001) and an interaction of session X sex (F(11,175) = 0.022, p = 0.009). (Fig. 3C). Post hoc tests found no significant differences in preference between the sexes on any session. Further, comparisons of preference in each sex to session 1 were not statistically significant.
Excitation of the NAc core did not significantly alter consumption of quinine-adulterated 15% EtOH. A paired t-test revealed no significant differences in consumption (Fig. 3D) or preference between CNO and VEH treatment. The order in which CNO or VEH injections were given also did not influence consumption or preference. Two-Way RM ANOVAs analyzing consumption and preference on sessions 13 and 15 revealed no effects of treatment or treatment order.
For a subset of mice (n = 10), the effects of excitation of the NAc core on intake of water and sensitivity to quinine in water were assessed. Paired t-tests revealed no differences in consumption of water (Figure 3E) or quinine-adulterated water (Figure 3F) within subjects following treatment with CNO or VEH.
3.3. Experiment 3: Inhibition of D1 receptor-expressing neurons with hM4Di did not influence baseline drinking or aversion-resistant intake of quinine-adulterated EtOH
To determine whether the effects of NAc core inhibition are mediated by D1-neurons, targeted chemogenetic inhibition of this subpopulation of MSNs was performed next. Robust mCherry expression was observed in cell bodies in the NAc core. The average range of expression along the anterior-posterior axis was 0.98 mm (Fig. 4A). All mice (n = 26, D1CRE+ = 17, D1WT = 9) escalated their drinking of 15% EtOH across drinking sessions 1-12. A Mixed Effects ANOVA on consumption revealed a main effect of session (F(6.533,146.7) = 2.489, p = 0.022) and no effect of genotype or interaction (Fig. 4B). Because consumption did not differ between the genotypes, data were collapsed and escalation of drinking was assessed with post hoc Dunnett’s multiple comparisons test vs. session 1. Consumption was significantly different on sessions 4 (p = 0.005), 9 (p = < 0.001), and 11 (p = 0.040). A Two-Way RM ANOVA of consumption on sessions 10, 11, and 12 showed no effect of D1 receptor-expressing neuron inhibition on baseline EtOH consumption (F(1.850,44.41) = 0.232, p = 0.777).
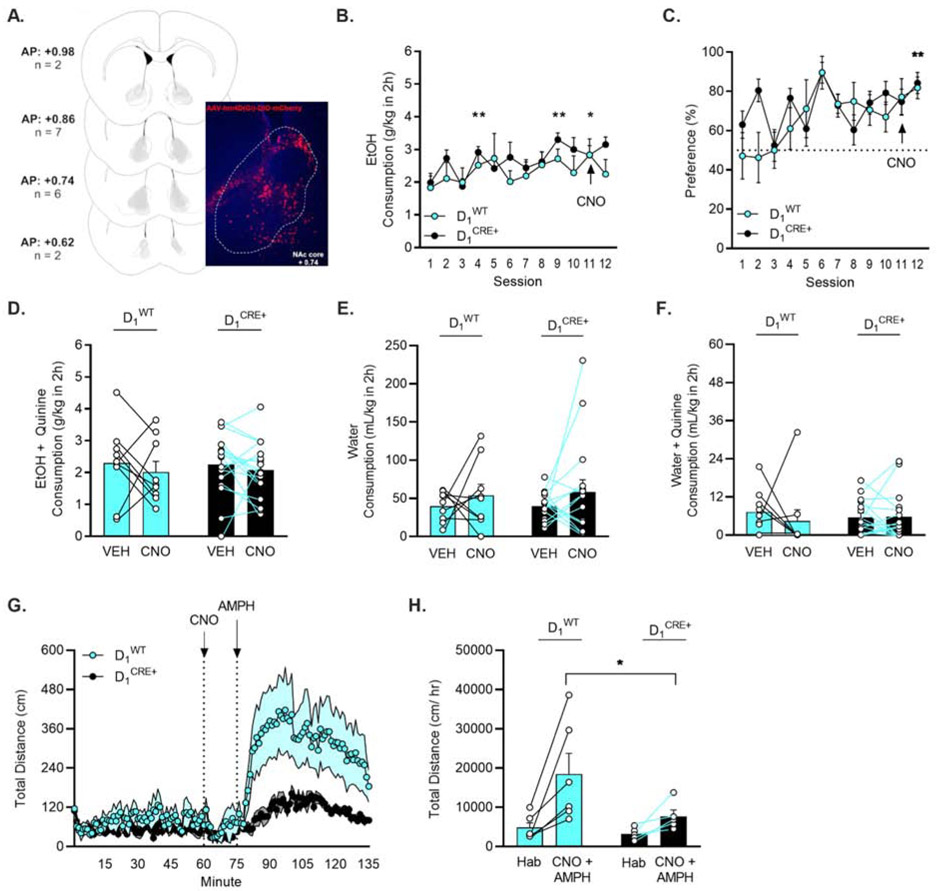
Figure 4. Inhibition of D1 receptor-expressing neurons did not influence escalation of drinking or aversion-resistant intake of quinine-adulterated EtOH.
A) Visual representation of hM4Di-DIO DREADD expression in D1 receptor-expressing neurons in the NAc core. B) D1CRE+ (n = 19) and D1WT (n = 9) mice escalated their consumption of 15% EtOH and C) preferred 15% EtOH more than water. * p < 0.05, ** p < 0.01 vs. session 1 (Dunnett’s). D) Inhibition of D1 receptor expressing neurons in the NAc core did not alter intake of 15% EtOH + quinine. E) D1-neuron inhibition did not alter consumption of water or F) water + quinine but G-H) reduced amphetamine-induced locomotion (significant time X genotype interaction, p < 0.001). *p < 0.05 (Holm-Sidak).
Preference for 15% EtOH vs. water also escalated over sessions. A Mixed Effects ANOVA demonstrated an escalation of preference with a main effect of session (F(6.271,139.7) = 3.497, p = 0.003) (Fig. 4C). When data were collapsed across genotype, a post hoc Dunnett’s multiple comparisons test showed a difference in preference between drinking sessions 1 vs. 12 (p = 0.003).
D1-neuron inhibition did not influence quinine-resistant EtOH drinking. Two-Way RM ANOVAs found no effect of genotype or CNO treatment on consumption (Fig. 4D) or preference for quinine-adulterated EtOH in D1WT and D1CRE+ subjects. The order in which CNO or VEH injections were given also did not influence consumption or preference. Two-Way RM ANOVAs in each genotype analyzing consumption and preference on sessions 13 and 15 revealed no effects of treatment or treatment order.
The effects of inhibition of D1-neurons in the NAc core on water consumption and sensitivity to quinine were tested following the EtOH drinking paradigm. Two-Way RM ANOVAs demonstrated no effect of CNO injection on consumption of water or quinine-adulterated water in D1WT and D1CRE+ subjects (Figure 4E-F).
In a subset of mice (D1WT = 6; D1CRE+ = 5), inhibition of D1-neurons decreased amphetamine-induced locomotor stimulation, as revealed by a main effect of time (F(1.700, 15.30) = 9.353; p = 0.003) and an interaction of time X genotype (F(134,1206) = 3.455; p < 0.001) (Fig. 4G). This effect persisted when data were collapsed to compare the habituation vs. CNO + amphetamine phases of the experiment, a post hoc Holm-Sidak multiple comparisons test identified a significant difference between the D1WT and D1CRE+ during the CNO + amphetamine phase (p = 0.046) (Fig. 4H). Further, a post hoc Holm-Sidak multiple comparisons test revealed the D1WT group significantly increased their locomotion during the CNO+ amphetamine timepoint (p = 0.006) while D1CRE+ mice did not (p = 0.264).
3.4. Experiment 4: Inhibition of D2 receptor-expressing neurons with hM4Di did not influence baseline drinking or aversion-resistant intake of quinine-adulterated EtOH
The contribution of D2-neurons to the effects of NAc core inhibition on quinine-resistant EtOH drinking was also assessed with targeted chemogenetic inhibition. Robust mCherry expression was observed in cell bodies in the NAc core. The average range of expression along the anterior-posterior axis was 0.82 mm (Fig. 5A). All mice (n = 25, D2CRE+ = 14, D2WT = 11) consistently consumed 15% EtOH across drinking sessions 1-12. A Mixed Effects ANOVA on consumption demonstrated a main effect of session (F(5.758,123.5) = 3.248, p = 0.006) and an interaction of session X genotype (F(11,236) = 2.288, p = 0.011) (Fig. 5B). A post hoc Holm-Sidak multiple comparisons test showed a difference in consumption between the D2CRE+ and D2WT groups on session 12 (p = 0.037). A Two-Way RM ANOVA of consumption on sessions 10, 11, and 12 found an interaction of session X genotype (F(2,46) = 3.325, p = 0.045). A post hoc Dunnett’s multiple comparisons test comparing sessions 10 and 12 to session 11 revealed that the D2WT mice drank significantly less 15% EtOH on session 12 compared to session 11 (p = 0.022) (Fig. 5B).
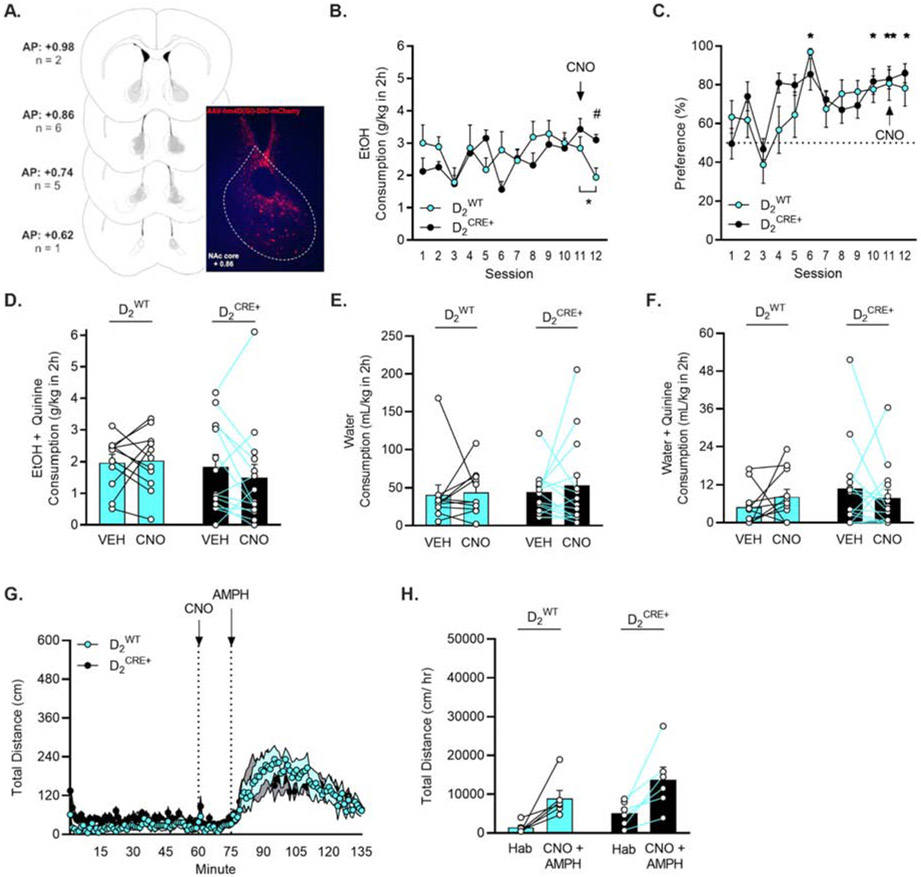
Figure 5. Inhibition of D2receptor-expressing neurons did not influence escalation of drinking or aversion-resistant intake of quinine-adulterated EtOH.
A) Visual representation of hM4Di-DIO DREADD expression of D2 receptor-expressing neurons in the NAc core. B) D2CRE+ (n = 16) and D2WT (n = 11) mice consistently consumed 15% EtOH. Consumption on session 12 was reduced in D2WT mice. *p < 0.05 vs. session 11 (paired t-test), # p < 0.05 comparing D2CRE+ vs. D2WT (Holm-Sidak). C) Mice preferred 15% EtOH more than water. * p < 0.05, ** p < 0.01 vs. session 1 (Dunnett’s). D) Inhibition of D2 receptor expressing neurons in the NAc core did not alter intake of 15% EtOH + quinine. E) D1-neuron inhibition did not alter consumption of water or F) water + quinine. G-H) Inhibition of D2 receptor expressing neurons did not influence amphetamine-induced locomotion.
All mice preferred 15% EtOH vs. water. A Mixed Effects ANOVA showed an escalation of preference for EtOH vs. water with a main effect of session (F(6.382,136.9) = 7.362, p < 0.001) and no interaction with genotype (Fig. 5C). When data were collapsed across genotypes, a post hoc Dunnett’s test revealed a difference in preference between drinking session 1 and session 6 (p = 0.014), 10 (p = 0.021), 11 (p = 0.001), and 12 (p = 0.016).
D2-neuron inhibition did not influence quinine-resistant EtOH drinking. Two-Way RM ANOVAs revealed no effect of genotype or CNO treatment on consumption or preference for quinine-adulterated EtOH in D2WT and D2CRE+ subjects (Fig. 5D). The order in which CNO or VEH injections were given also did not influence consumption or preference. Two-Way RM ANOVAs in each genotype analyzing consumption and preference on sessions 13 and 15 revealed no effects of treatment or treatment order.
The effects of inhibition of D2-neurons in the NAc core on water consumption and sensitivity to quinine were tested following the EtOH drinking paradigm. Two-Way RM ANOVAs demonstrated no effect of CNO injection on consumption of water or quinine-adulterated water in D2WT or D2CRE+ subjects (Figure 5E-F).
In a subset of mice (D2WT = 6; D2CRE+ = 6), inhibition of D2-neurons did not alter amphetamine-induced locomotor stimulation. A Two-Way RM ANOVA demonstrated a main effect of time (F(2.127, 21.27) = 14.20; p < 0.001) and no interaction with genotype (Fig. 5G). When data were collapsed to compare the habituation vs. CNO + amphetamine phases of the experiment, a RM Two-Way ANOVA showed a main effect of phase (F(1,10) = 21.42, p < 0.001) but not genotype (F(1,10) = 3.310, p = 0.099). A post hoc Holm-Sidak multiple comparisons test revealed that locomotion during the CNO + amphetamine phase was increased in both genotypes, D2WT (p = 0.013) and D2CRE+ (p = 0.011) (Fig. 5H).
3.5. Experiments 5 and 6: Combined, but not selective, antagonism of D1 and D2 receptors decreased aversion-resistant intake of quinine-adulterated EtOH.
To assess the contribution of both D1 and D2 receptors, pharmacological antagonism was used. The effects of selective antagonism of either D1R or D2R on quinine-resistant drinking were first established. All mice (n = 13, male = 7, female = 6) consistently consumed 15% EtOH across drinking sessions 1-10. A Two-Way RM ANOVA revealed a main effect of session (F(9,99) = 6.63, p < 0.001) and no sex differences. When data were collapsed by sex, a post hoc Dunnett’s multiple comparisons test showed that mice escalated their drinking on session 9 compared to session 1 (p = 0.027) (Fig. 6A). All mice preferred 15% EtOH vs. water. A Mixed Effects ANOVA demonstrated an escalation of preference for EtOH vs. water with a main effect of session (F(3.526, 38.40) = 5.63, p = 0.002) and a post hoc Dunnett’s test found that preference on session 7 was significantly higher vs. session 1 (p = 0.047) (Fig. 6B).
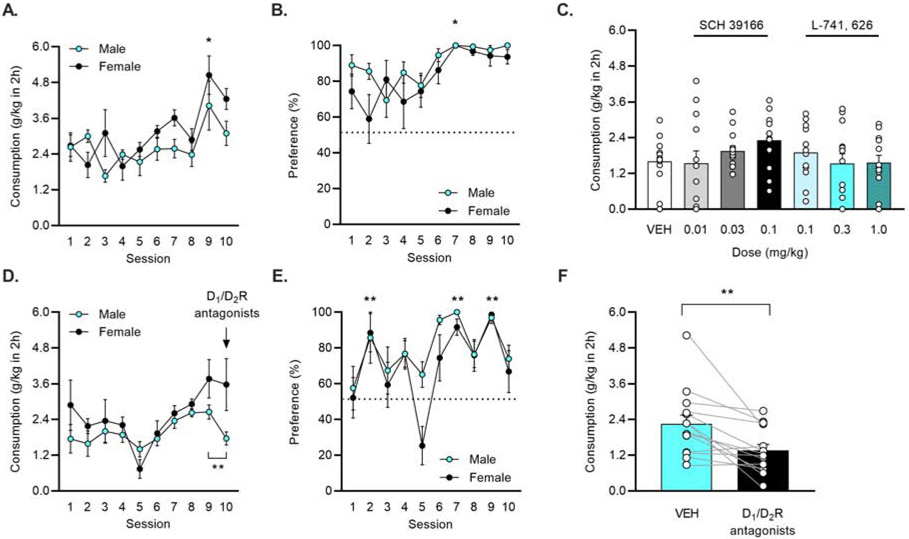
Figure 6: Combined antagonism of D1 and D2 receptors decreased aversion-resistant intake of quinine-adulterated EtOH.
A) Male (n = 7) and female (n = 6) mice consistently consumed 15% EtOH and B) preferred 15% EtOH more than water. * p < 0.05, vs. session 1 (Dunnett’s). C) When compared to VEH sessions, neither the D1R antagonist SCH 39166 nor the D2R antagonist L-741, 626 reduced consumption of quinine-adulterated 15% EtOH at any dose (data collapsed by sex as no sex differences were observed). D) A new cohort of male (n = 7) and female (n = 7) mice consistently consumed 15% EtOH. Injection of the D1/D2R antagonist cocktail reduced drinking in male, but not female, mice. **p < 0.01 comparing sessions 9 and 10 (paired t-test). E) Mice preferred 15% EtOH more than water. ** p < 0.01 vs. session 1 (Dunnett’s). F) Antagonism of D1 and D2 receptors reduced consumption of 15% EtOH + quinine. **p < 0.001 vs VEH (paired t-test).
Antagonism of D1 or D2 receptors across a range of doses did not alter consumption of quinine-adulterated EtOH compared to the VEH. Mixed Effects ANOVA comparing the seven treatments (VEH, three doses of SCH 39166, and three doses of L-741, 626), found no differences, regardless of whether sex was included as a factor (Fig. 6C).
Next, a new cohort of mice was used to assess the effect of combined D1 and D2 receptor blockade on quinine-resistant intake. All mice (n = 14, male = 7, female = 7) consistently consumed 15% EtOH across drinking sessions 1-10. A Two-Way RM ANOVA identified main effects of session (F(3.89,46.68) = 3.646, p = 0.012) and sex (F(1,12) = 8.948, p = 0.011), with females consuming more 15% EtOH compared to males. Follow-up comparisons between males and females were not significant. On session 10, the D1/D2R cocktail was administered to all mice to assess the effects of the drugs on quinine-free, baseline drinking. A paired t-test between sessions 9 and 10 revealed that males significantly decreased their intake (p = 0.003) while females did not (p = 0.854). (Fig. 6D). All mice preferred 15% EtOH vs. water. A Two-Way ANOVA demonstrated an escalation of preference for EtOH vs. water with a main effect of session (F(9,108) = 7.533, p < 0.001). A post hoc Dunnett’s multiple comparisons test identified difference in preference vs. session 1 on sessions 2, 7, and 9 (all p < 0.01) (Fig. 6E).
Antagonism of both D1 and D2 receptors reduced consumption of quinine-adulterated EtOH. A paired t-test identified a significant difference between VEH and treatment with the D1 and D2 antagonists (p = 0.003) (Fig. 6F).
4. Discussion
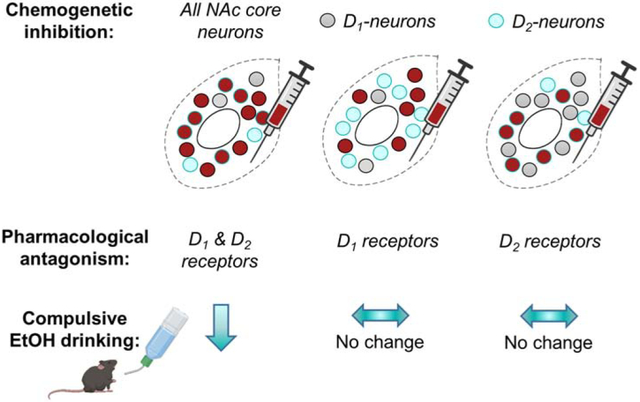
A major finding of this study is that chemogenetic inhibition of the NAc core decreased consumption of quinine-adulterated EtOH. This finding is consistent with previous reports demonstrating a role for the NAc core in compulsive-like EtOH drinking (Grodin et al., 2018; Seif et al., 2015, 2013). Chemogenetic excitation of the NAc core did not influence quinine-resistant drinking in this study and neither inhibition nor excitation influenced baseline levels of EtOH consumption. Further, selective inhibition of D1-neurons or D2-neurons was ineffective in altering baseline EtOH consumption and quinine-resistant drinking. Pharmacological antagonism of dopamine receptors demonstrated that quinine-resistant drinking was reduced only by combined D1R and D2R blockade. Together, these results suggest that the NAc core is an important regulator of compulsive-like drinking and that either subpopulation of NAc core MSNs can drive this behavior.
While previous studies have demonstrated that cortical afferents to the NAc core (Seif et al., 2015, 2013) and shell (Halladay et al., 2020) participate in punished EtOH drinking, the current data confirm that chemogenetic inhibition of neurons in the NAc core influences this behavior in a similar manner. The absence of an effect of this treatment on baseline (i.e., quinine-free) EtOH drinking suggests that chemogenetic inhibition of the NAc core selectively influences quinine-resistant drinking. These latter findings add to an already conflicting literature regarding the effects of chemogenetic manipulations on baseline EtOH drinking in the drinking in the dark paradigm, which includes reports suggesting that inhibition with hM4Di decreases (Cassataro et al., 2014), increases (Purohit et al., 2018), or has no effect on EtOH drinking (Pozhidayeva et al., 2020). Similarly, while hM3Dq did not influence EtOH consumption or preference in the current study, previous reports suggest chemogenetic excitation with hM3Dq decreases drinking (Pozhidayeva et al., 2020; Purohit et al., 2018) or has no effect (Cassataro et al., 2014). One difference between these prior studies and the work presented here is that chemogenetic manipulations were carried out over multiple drinking sessions in the former whereas CNO was only injected before one drinking session in the current study. In addition, the current study used a two-bottle choice procedure while mice in the studies cited were presented with EtOH only. It must also be noted that only a subset of mice in Experiment 1 received CNO prior to the quinine drinking sessions, and thus any conclusions about the effects of neuronal inhibition on baseline EtOH drinking are preliminary. Another interesting finding during the baseline drinking sessions is that D1/D2R antagonism reduced consumption in male, but not female, mice suggesting that dopamine receptors may regulate EtOH drinking in a sex-dependent fashion.
In Experiment 2, chemogenetic excitation had no effect on quinine-resistant drinking. However, these results are admittedly difficult to interpret given that mice were already demonstrating resistance to quinine and increases in this construct would not necessarily translate into increases in consumption or preference. Considering that drinking in the dark is a model of binge-like EtOH consumption, it is also possible that consumption was at a ceiling and could not be increased any further. Notably, mice in this experiment consistently consumed the EtOH solution and did not seem to show an apparent escalation. To clarify these findings, future studies could assess whether aversion-resistant drinking can be induced in mice who have not yet developed compulsive behavior or examine the effects of NAc excitation on consumption of EtOH adulterated with higher concentrations of quinine known to suppress drinking (Sneddon et al., 2019).
Inhibition of neither D1- nor D2 receptor-expressing MSNs altered baseline EtOH drinking or compulsive-like intake of quinine-adulterated EtOH. Control experiments further suggest that D1- and D2- neuron inhibition does not affect baseline EtOH drinking, water intake, or sensitivity of mice to quinine in water. Additional control experiments designed to test the efficacy of the chemogenetic approach suggest that these manipulations were effective as inhibition of D1-neurons decreased amphetamine-induced locomotion (Ferguson et al., 2011). Importantly, this effect was not replicated in mice expressing hM4Di in D2-neurons. While locomotion was increased in both D1WT and D2WT mice following amphetamine challenge, this effect appeared smaller in the D2 line. A less robust response to amphetamine may have obscured any effects of chemogenetic inhibition on locomotor behavior. We also cannot rule out that differences in expression efficiency between viral vectors or strain differences between the Drd1-cre and Drd2-cre lines influenced the observed pattern of results, although robust expression of hM4Di was confirmed in all experiments.
We predicted that inhibition of D1-neurons alone would be sufficient to reduce quinine-resistant EtOH drinking, which would be consistent with the proposed role of D1-neurons in motivated behavior and work by Halladay et al. (2020) demonstrating that punishment-resistant EtOH responding is associated with increased AMPA/NMDA receptor ratios at glutamatergic inputs to D1-neurons, but not D2-neurons. Instead, our results demonstrate that reduced activity in D1-neurons alone is not sufficient to restore quinine’s ability to suppress EtOH drinking. Combined with the results of Experiment 1, in which all NAc core neurons were targeted and inhibited, the chemogenetic results suggest that D1- and D2- neurons work together to drive aversion-resistant drinking. This conclusion is further supported by the results of our pharmacological experiments demonstrating that combined D1R and D2R antagonism, but not blockade of either receptor alone, reduced quinine-resistant drinking. Some studies do suggest a combined and/or complementary influence of NAc D1 and D2 pathways on motivated behavior (Natsubori et al., 2017; Radke and Gewirtz, 2012; Soares-Cunha et al., 2016; Steinberg et al., 2014; Volkow and Morales, 2015). For example, photostimulation of either D1- or D2-neurons is sufficient to increase motivation for a food reward (Soares-Cunha et al., 2016). Further, dopamine-mediated reinforcement, as measured with intracranial self-stimulation of VTA dopamine neurons, requires activation of both D1 and D2 receptors (Steinberg et al., 2014). Based on this type of evidence, one possibility is that aversion-resistant EtOH drinking relies on adaptations in both subpopulations of NAc MSNs. At least one study in the dorsal striatum found that chronic, intermittent alcohol drinking potentiates glutamatergic NMDA receptor activity in D1-neurons and GABAergic activity in D2-neurons (Cheng et al., 2017). While there are still many unanswered questions about how plasticity in D1- vs. D2-neurons contributes to aversion-resistant drinking, the current results highlight the fact that this behavior likely requires a shift in the balance of activity between D1- and D2-neuron subpopulations in the NAc.
Considering evidence demonstrating that D1-neuron activity is increased during reward-seeking while D2-neuron activity is decreased (Calipari et al., 2016), it is somewhat surprising that combined chemogenetic inhibition of the NAc reduced quinine-resistance while inhibition of D1-neurons alone was ineffective. One possible explanation is that chemogenetic inhibition throughout the two-hour drinking session reduced the relatively high baseline firing rate that has been observed in D2-neurons (Calipari et al., 2016), impairing the ability of these neurons to regulate motivated behavior through reductions in neuronal firing. Another possibility is that, although D1 and D2 receptor-expressing MSNs in the NAc core account for 90-95% of all neurons in this region (Lobo and Nestler, 2011), they are not the only neurons in this region that could be contributing to aversion-resistant behavior. Specifically, the role of cholinergic interneurons, which are important regulators of striatal dopamine release (Cragg, 2006; Threlfell et al., 2012), in compulsive-like reward seeking has yet to be investigated.
Sex differences in aversion-resistant alcohol drinking or the effects of chemogenetic and pharmacological manipulations were not observed in this study. This result may be specific to the paradigm used, as we have previously reported that quinine-resistance is similar between male and female mice in the drinking in the dark paradigm (Sneddon et al., 2019). Other studies, including our own, have reported a female vulnerability to aversion-resistant drinking using other EtOH drinking paradigms (Fulenwider et al., 2019; Radke et al., 2019; Sneddon et al., 2020; Xie et al., 2019). It will be important for future studies to determine whether any of the mechanisms investigated here contribute to such sex differences in aversion-resistant drinking. Additionally, while the current findings support prior work in rats and humans (Seif et al., 2013; Grodin et al., 2018), it will be important to continue exploring the role of NAc circuits in compulsive-like EtOH drinking across species and drinking models. Because C57BL/6J mice develop aversion-resistance very quickly, mice in this study did not have extended EtOH exposure. As such, additional neuroadaptations in the NAc that promote quinine-resistance might be uncovered following chronic, intermittent EtOH exposure (Radke et al., 2017).
5. Conclusions
Together, these studies validate that the NAc core is an important region contributing to addictive pathology, including aversion-resistant EtOH drinking. Manipulation of D1- and D2 neurons together, but not alone, decreased quinine-resistant intake. Future studies are necessary to determine if both D1- and D2 receptor-expressing neurons contribute to aversion-resistant EtOH drinking or if other neuronal populations are mediating this behavior.
Highlights.
Compulsive-like drinking was assessed as intake of 15% EtOH mixed with quinine.
Chemogenetic inhibition of NAc core neurons reduced quinine-resistant drinking.
Targeted inhibition of D1 and D2 medium spiny neuron subtypes was ineffective.
Selective antagonism of D1 and D2 receptors was ineffective.
Combined antagonism of D1 and D2 receptors reduced quinine-resistant drinking.
Acknowledgements
The authors would like to thank Brandon Arnold, Sachi Bhati, Bridget Dames, Jenelle DeMedio, Kaila Fennel, Marissa Muench, Austin Nader, Olivia Ramsey, Joshua Setters, Natalie Shand, and Annemarie Thomas for assistance with behavioral experiments.
Funding: This work was supported by the National Institutes of Health (AA027915 to AKR and NS118727 to EAS) and awards from the College of Arts and Sciences (AKR), the Office of Research for Undergraduates (JWF and KMS), and the Graduate School (EAS) at Miami University.
Abbreviations:
- AUD
Alcohol use disorder
- EtOH
ethanol
- NAc core
nucleus accumbens core
- DREADDs
Designer Receptors Exclusively Activated by Designer Drugs
- CNO
clozapine-N-oxide
- VEH
vehicle
- D1R
dopamine type-1 receptors
- D2R
dopamine type-2 receptors
- MSN
medium spiny neuron
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Association, A.P., Others, 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Bahi A, Dreyer J-L, 2012. Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology 222, 141–153. 10.1007/s00213-011-2630-8 [DOI] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA, 2013. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat. Neurosci 16, 632–638. 10.1038/nn.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ, 2016. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc. Natl. Acad. Sci. U. S. A 113, 2726–2731. 10.1073/pnas.1521238113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassataro D, Bergfeldt D, Malekian C, Van Snellenberg JX, Thanos PK, Fishell G, Sjulson L, 2014. Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology 39, 283–290. 10.1038/npp.2013.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R, Lenz JD, Gancarz AM, Chaudhury D, Schroeder GL, Han M-H, Cheer JF, Dietz DM, Lobo MK, 2013. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front. Mol. Neurosci 6, 13. 10.3389/fnmol.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, Wang J, 2017. Distinct Synaptic Strengthening of the Striatal Direct and Indirect Pathways Drives Alcohol Consumption. Biol. Psychiatry 81, 918–929. 10.1016/j.biopsych.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lasek AW, 2019. Perineuronal nets in the insula regulate aversion-resistant alcohol drinking. Addict. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, 2006. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 29, 125–131. 10.1016/j.tins.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Lemos JC, Alvarez VA, 2017. Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders. Genes Brain Behav. 16, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PEM, Dong Y, Roth BL, Neumaier JF, 2011. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat. Neurosci 14, 22–24. 10.1038/nn.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Price ME, Hafeez H, Schank JR, 2019. Sex Differences in Aversion-Resistant Ethanol Intake in Mice. Alcohol Alcohol 54, 345–352. 10.1093/alcalc/agz022 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ, 2011. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–466. 10.1146/annurev-neuro-061010-113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, Momenan R, 2018. Neural Correlates of Compulsive Alcohol Seeking in Heavy Drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 3, 1022–1031. 10.1016/j.bpsc.2018.06.009 [DOI] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, Piantadosi PT, Authement ME, Lieberman AG, Spitz NA, Coden K, Glover LR, Costa VD, Alvarez VA, Holmes A, 2020. Prefrontal Regulation of Punished Ethanol Self-administration. Biol. Psychiatry 87, 967–978. 10.1016/j.biopsych.2019.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Morita M, Macpherson T, 2016. Neural mechanisms of the nucleus accumbens circuit in reward and aversive learning. Neurosci. Res 108, 1–5. 10.1016/j.neures.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW, 2015. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci 18, 1230–1232. 10.1038/nn.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HMB, van Kerkhof LWM, Vanderschuren LJMJ, 2010. Inflexible and indifferent alcohol drinking in male mice. Alcohol. Clin. Exp. Res 34, 1219–1225. 10.1111/j.1530-0277.2010.01199.x [DOI] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ, 2011. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat 5, 41. 10.3389/fnana.2011.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL, 2005. Correction: actual causes of death in the United States, 2000. JAMA 293, 293–294. 10.1001/jama.293.3.293 [DOI] [PubMed] [Google Scholar]
- Natsubori A, Tsutsui-Kimura I, Nishida H, Bouchekioua Y, Sekiya H, Uchigashima M, Watanabe M, de Kerchove d’Exaerde A, Mimura M, Takata N, Tanaka KF, 2017. Ventrolateral Striatal Medium Spiny Neurons Positively Regulate Food-Incentive, Goal-Directed Behavior Independently of D1 and D2 Selectivity. J. Neurosci 37, 2723–2733. 10.1523/JNEUROSCI.3377-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozhidayeva DY, Farris SP, Goeke CM, Firsick EJ, Townsley KG, Guizzetti M, Ozburn AR, 2020. Chronic Chemogenetic Stimulation of the Nucleus Accumbens Produces Lasting Reductions in Binge Drinking and Ameliorates Alcohol-Related Morphological and Transcriptional Changes. Brain sciences 10, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit K, Parekh PK, Kern J, Logan RW, Liu Z, Huang Y, McClung CA, Crabbe JC, Ozburn AR, 2018. Pharmacogenetic manipulation of the nucleus accumbens alters binge-like alcohol drinking in mice. Alcohol. Clin. Exp. Res 42, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Gewirtz JC, 2012. Increased dopamine receptor activity in the nucleus accumbens shell ameliorates anxiety during drug withdrawal. Neuropsychopharmacology 37, 2405–2415. 10.1038/npp.2012.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Held IT, Sneddon EA, Riddle CA, Quinn JJ, 2019. Additive influences of acute early life stress and sex on vulnerability for aversion-resistant alcohol drinking. Addict. Biol e12829. 10.1111/adb.12829 [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A, 2017. Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict. Biol 22, 423–434. 10.1111/adb.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Jian M, 1995. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience 64, 1019–1034. 10.1016/0306-4522(94)00426-6 [DOI] [PubMed] [Google Scholar]
- Seif T, Chang S-J, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci 16, 1094–1100. 10.1038/nn.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW, 2015. D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharmacology 40, 2357–2367. 10.1038/npp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Noamany H, Chang C-J, Brown AR, Chen X, Leible D, Lee JJ, Wang J, Vernon AN, Vander Weele CM, Kimchi EY, Heiman M, Tye KM, 2019. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012. 10.1126/science.aay1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW, 2013. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr. Opin. Neurobiol 23, 546–552. 10.1016/j.conb.2013.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon EA, Ramsey OR, Thomas A, Radke AK, 2020. Increased Responding for Alcohol and Resistance to Aversion in Female Mice. Alcohol. Clin. Exp. Res 10.1111/acer.14384 [DOI] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK, 2019. Sex differences in binge-like and aversion-resistant alcohol drinking in C57BL/6J mice. Alcohol. Clin. Exp. Res 43, 243–249. 10.1111/acer.13923 [DOI] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, Sousa N, Rodrigues AJ, 2016. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun 7, 11829. 10.1038/ncomms11829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, Gaspar R, Sotiropoulos I, Sousa N, Rodrigues AJ, 2019. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol. Psychiatry 10.1038/s41380-019-0484-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH, 2014. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One 9, e94771. 10.1371/journal.pone.0094771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ, 2012. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64. 10.1016/j.neuron.2012.04.038 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M, 2015. The Brain on Drugs: From Reward to Addiction. Cell 162, 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Xie Q, Buck LA, Bryant KG, Barker JM, 2019. Sex Differences in Ethanol Reward Seeking Under Conflict in Mice. Alcohol. Clin. Exp. Res 43, 1556–1566. 10.1111/acer.14070 [DOI] [PMC free article] [PubMed] [Google Scholar]