Abstract
Background
Dogs play an important role as reservoirs of many zoonotic vector-borne pathogens worldwide, yet reports of canine vector-borne diseases (CVBDs) in Egypt are scarce.
Methods
Serum samples were collected from pet dogs (n = 500) of the three most common breeds (German Shepherd, Rottweiler and Pit Bull) in five Governates of Cairo (n = 230), Giza (n = 110), Al-Qalyubia (n = 60), Al-Gharbia (n = 60) and Kafr El-Sheikh (n = 40) with a hot desert climate. The presence of antibodies to Anaplasma spp. (A. phagocytophilum, A. platys), Ehrlichia spp. (E. canis, E. chaffeensis, E. ewingii), Borrelia burgdorferi (s.l.) and Dirofilaria immitis were assessed using IDEXX SNAP® 4Dx® ELISA tests. For each pathogen, risk factors (i.e. geographical area, keeping condition, sex, age, breed, tick infestation, weekly sanitation of dog enclosures and application of ectoparasiticides) were evaluated by logistic regression approach.
Results
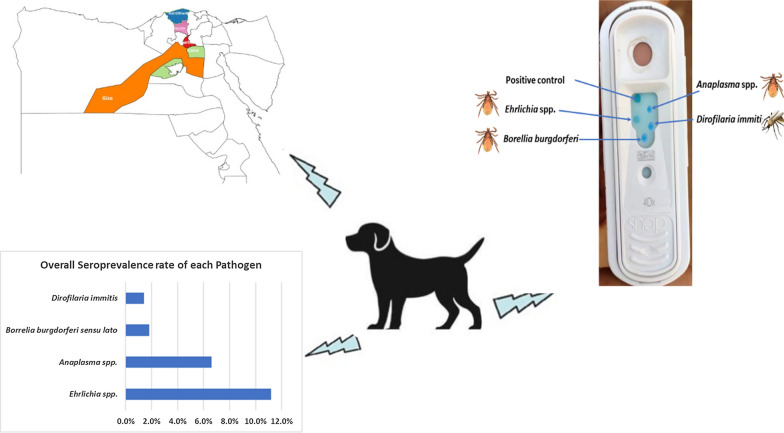
In total, 18.2% (n = 91, 95% CI 15.1–21.8) of dogs scored seropositive for at least one pathogen, the most frequent being Ehrlichia spp. (n = 56; 11.2%; 95% CI 8.7–14.3) followed by Anaplasma spp. (n = 33; 6.6%, 95% CI 4.7–9.1), Borrelia burgdorferi (s.l.) (n = 9; 1.8%, 95% CI 0.9–3.4) and D. immitis (n = 7; 1.4%, 95% CI 0.9–2.9). In the tested population, 15.4% (95% CI 12.5–18.8) of dogs were exposed to a single pathogen while 2.4 (95% CI 1.4–4.2) and 0.4% (95% CI 0.1–1.4) were simultaneously exposed to two or three pathogens, respectively. Major risk factors associated with VBDs were living outdoors (Anaplasma spp., P = 0.0001; Ehrlichia spp., P = 0.0001), female sex (Ehrlichia spp., P = 0.005), German Shepherd breed (Anaplasma spp., P = 0.04; Ehrlichia spp., P = 0.03), tick infestation (Anaplasma spp., P = 0.0001; Ehrlichia spp., P = 0.0001; B. burgdorferi (s.l.), P = 0.003; D. immitis, P = 0.02), irregular sanitation (Anaplasma spp., P = 0.0001; Ehrlichia spp., P = 0.0001; B. burgdorferi (s.l.), P = 0.002; D. immitis, P = 0.01) and not using ectoparasiticides (Anaplasma spp., P = 0.0001; Ehrlichia spp., P = 0.0001; B. burgdorferi (s.l.), P = 0.007).
Conclusion
To our knowledge, this is the first large-scale seroepidemiological study of CVBDs in Egypt. Considering that all of the detected pathogens are potentially zoonotic, effective ectoparasite control strategies, regular examination of pet dogs and successful chemoprophylaxis are advocated.
Keywords: Anaplasma, Borrelia, Canine vector-borne pathogens, Dirofilaria, Egypt, Ehrlichia, One-health, Zoonosis
Background
Vector-borne diseases (VBDs) are of global importance especially in the case of zoonotic infections which pose a direct threat to animal and human health [1–3]. These pathogens are circulated in animal and human communities by arthropod vectors including ticks, mosquitoes, fleas and phlebotominae sand flies [4, 5]. Canine vector-borne diseases (CVBDs) of viral, bacterial and protozoal origin are often widespread in tropical and subtropical regions [6], including in the Middle East and North Africa (MENA), because of the favourable climatic conditions for the perpetuation of arthropod vectors and development of canine vector-borne pathogens (CVBPs) [7]. Among CVBPs, tick-borne Ehrlichia spp., Anaplasma spp., Borrelia spp. and mosquito-borne Dirofilaria spp. are of great importance for dogs [8].
Dogs are the main reservoir hosts for zoonotic gram-negative intracellular bacteria Ehrlichia canis, E. ewingii and E. chaffeensis [9, 10]. Ehrlichia canis, the causative agent of canine monocytic ehrlichiosis (CME), is transmitted by Rhipicephalus sanguineus (s.l.) and is prevalent in dog populations worldwide [11, 12]. The clinical outcome of ehrlichiosis varies from mild symptoms to fatal illness in the chronic phase depending on the strain, individual immune response and presence of concomitant infections [4]. Although dogs may show nonspecific signs (e.g., fever, depression, weakness, lethargy, anorexia, weight loss and a mucopurulent nasal discharge), asymptomatic Ehrlichia infection may also occur [13–15].
Human and animal infections with Anaplasma species are increasingly recognized as important, occasionally emerging and potentially fatal tick-transmitted diseases of humans and animals [16, 17]. Among six recognized species in the genus Anaplasma, A. phagocytophilum, the causative agent of granulocytic anaplasmosis, is diagnosed in a wide range of warm-blooded hosts including dogs, cats, horses, sheep, goats, cattle, camels and humans [18, 19]. Anaplasma platys is a common VBP of dogs in MENA [20, 21] and has occasionally been detected in humans [22–24]. Dogs usually are silent carriers of the infection [25], with clinical signs (e.g. fever, lethargy, anorexia and thrombocytopenia) sometimes described [26].
Among bacteria of the genus Borrelia, which affect different animal species including humans, Borrelia burgdorferi (s.l.) species complex causes Lyme disease, which is considered a major zoonosis for which many animals species (e.g. reptiles, rodents, wild ruminants) are reservoirs and Ixodes spp. tick the primary vector [27, 28]. In dogs, B. burgdorferi (s.l.) most often causes nonspecific signs (e.g., fever, apathy, lethargy, renal damage and lymphadenopathy) but also severe arthritis and neurological disorders [29]. However, in the endemic areas the majority of seropositive dogs do not present any clinical signs of the infection although they often remain persistently infected for approximately 1 year [30].
Dirofilaria immintis (Spirurida, Onchocercidae) is the causative agent of canine heartworm disease, which is transmitted through the bite of several mosquito species worldwide [31]. Dirofilariosis may also affect other mammals including humans, leading to the formation of pulmonary nodules, which may be often confounded with pulmonary carcinoma [32]. Although most dogs infected by D. immitis—specially in endemic areas—are asymptomatic microfilaremic reservoirs, clinical signs depend on several factors, such as adult worm burden and localization [33]. The distribution of canine dirofilariosis in the MENA region, especially in North Africa, is not well known because of the paucity of epidemiological studies [31].
In Egypt, infection of dogs with E. canis [13, 34], D. immitis [35] and B. burgdorferi (s.l.) [36, 37] have been reported, in most cases based on small numbers of dogs and limited geographical areas. In this country Rh. sanguineus (s.l.) (brown dog tick), the competent vector of several tick-borne diseases [38, 39], has been prevalent in dog populations since ancient times [40–43]. DNA of E. canis and A. phagocytophilum has been detected in ticks attached to dogs [44, 45]. However, generally there are limited data on the occurrence of CVBDs in north African countries, e.g. Morocco [46], Algeria [47, 48] and Tunisia [49, 50].
The aim of the current study was to provide novel information on the seroprevalence and distribution of causative agents of monocytic ehrlichiosis, granulocytic anaplasmosis, Lyme disease and heartworm disease in dogs from five Governorates of Egypt and assess the risk factors associated with the infections.
Methods
Study area
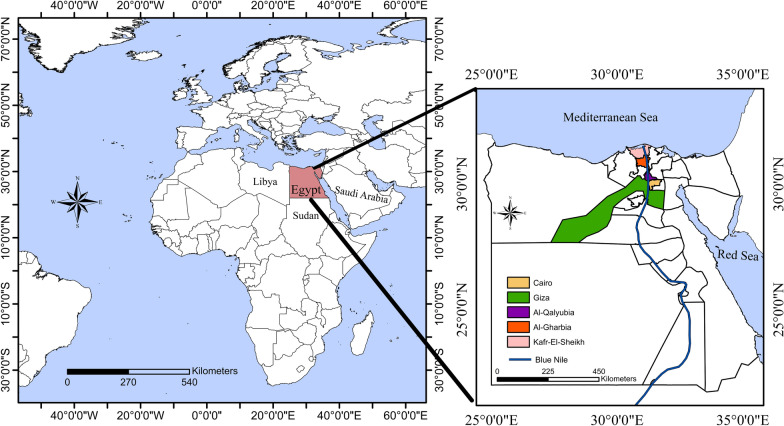
Egypt is a transcontinental country spanning the northeast corner of Africa and southwest corner of Asia. It is divided into 27 Governorates; the large regions of the Sahara desert, which constitute most of Egypt’s territory, are sparsely inhabited. The investigation was conducted in Cairo (30.0444°N, 31.2357°E), Giza (30.0131°N, 31.2089°E), Al-Qalyubia (30.3292°N, 31.2168°E), Al-Gharbia (30.8754°N, 31.0335°E) and Kafr El-Shaikh (31.1107°N, 30.9388°E) (Fig. 1). These Governorates essentially have a hot desert climate, which is classified as BWh by the Köppen-Geiger system.
Fig. 1.
Map indicating Governorates where animals included in the study were sampled
Sample collection
During 2018 and 2019, blood samples (ca. 2 ml) were collected from the cephalic or saphenous veins of 500 dogs of the three most common breeds raised in Egypt, i.e. German Shepherd, Rottweiler and Pit Bull, admitted to veterinary clinics of five cities in different Governorates, namely Naser City in Cairo (n = 230), 6th of October in Giza (n = 110), Benha in Al-Qalyubia (n = 60), Tanta in Al-Gharbia (n = 60) and Kafr El-Sheikh in the governate of the same name (n = 40). Since other breeds of dogs are rarely kept as pets in Egypt, they were excluded from this study. Dogs were grouped according to age into four groups: < 1 year (G1); between 1 and 3 (G2); between 3 and 5 (G3); > 5 years (G4). Animal data (i.e. age, sex, breed, tick infestation, weekly sanitation of the dog enclosures and tri-monthly application of ectoparasiticides) were recorded.
Serological examination
Sera were separated by centrifugation of blood (1500×g for 10 min) and preserved at − 20 °C until tested by IDEXX SNAP® 4Dx® (IDEXX Laboratories, Westbrook, ME, USA), which is a validated in-clinic ELISA test system. The kit simultaneously detects antibodies against immunodominant proteins of E. canis, E. chaffeensis, E. ewignii (peptides from p30 and p30-1 outer membrane proteins and p28 outer surface protein family), A. phagocytophilum, A. platys (peptide from the major surface protein p44/MSP2), B. burgdorferi (s.l.) (C6 peptide, derived from the IR6 region within the Borrelia membrane protein VlsE) [51] and D. immitis analyte derived from two antibodies (one for capture and the other for detection) specific to heartworm antigens [51–53]. The sensitivity and specificity of this kit are 93.2 and 99.2% for A. phagocytophilum, 89.2 and 99.2% for A. platys, 96.7 and 98.8% for B. burgdorferi (s.l.), 97.8 and 92.3% for E. canis and 98.9 and 99.3% for D. immitis [52–54].
Statistical analysis
Chi-square test and Fisher’s exact test were used to compare seropositivity to each pathogen, and the results were considered significant if P ≤ 0.05. In particular, P-values were calculated with Fisher’s exact test only for variables below five (keeping condition, sex, weekly sanitation of the dog enclosures, presence of ticks on the dog body and tri-monthly application of ectoparasiticides) for D. immitis and B. burgdorferi (s.l.); all other P-values were calculated with Chi-square test. Univariable logistic regression analysis was used to evaluate the association of prevalence of each pathogen and variables of location (five Governorates), keeping condition (indoors or outdoors), sex (male or female), age (three groups), breed (three breeds), weekly sanitation of the dog enclosures, tick infestation and tri-monthly application of ectoparasiticides. Variables with P ≤ 0.05 in the univariable analyses were conducted to the multivariable models. To determine the risk probability of CBVDs, the odds ratio (OR) and confidence interval (CI) of significant variables were calculated using the multivariant logistic regression model. Multiple linear regression analysis was used to determine the possible multiple collinearities of different variables included in this study. The Hosmer-Lemeshow test was calculated to assess the goodness of fit for each model. Statistical analyses were carried out using SPSS software (ver. 24.0, IBM, USA).
Results
Of 500 tested dogs, 91 (18.2%) scored seropositive for at least one pathogen, the most frequent being infection with Ehrlichia spp. (n = 56; 11.2%) followed by Anaplasma spp. (n = 33; 6.6%), B. burgdorferi (s.l.) (n = 9; 1.8%) and D. immitis (n = 7; 1.4%). In the tested population 15.4% of dogs were exposed to a single pathogen while 2.4% and 0.4% were simultaneously exposed to two or three pathogens, respectively (Table 1).
Table 1.
Number and percentage of dogs (n = 500) seropositive to Ehrlichia spp., Anapalsma spp., B. burgdorferi (s.l.) and D. immitis
| Pathogen | No. positive | Prevalence (%) | 95% CI |
|---|---|---|---|
| Exposure to one pathogen | |||
| Ehrlichia spp. | 44 | 8.8 | 6.5–11.7 |
| Anapalsma spp. | 24 | 4.8 | 3.1–7.1 |
| B. burgdorferi (s.l.) | 5 | 1 | 0.3–2.4 |
| D. immitis | 4 | 0.8 | 0.2–2.1 |
| Exposure to two pathogens | |||
| Ehrlichia spp. + Anapalsma spp. | 5 | 1 | 0.3–2.4 |
| Ehrlichia spp. + B. burgdorferi (s.l.) | 3 | 0.6 | 0.2–1.7 |
| Ehrlichia spp. + D. immitis | 2 | 0.4 | 0.1–1.4 |
| D. immitis + Anapalsma spp. | 2 | 0.4 | 0.1–1.4 |
| Exposure to three pathogens | |||
| Ehrlichia spp. + Anapalsma spp. + D. immitis | 1 | 0.2 | 0.04–1.1 |
| Ehrlichia spp. + Anapalsma spp. + B. burgdorferi (s.l.) | 1 | 0.2 | 0.04–1.1 |
The risk of exposure to pathogens was significantly associated with keeping condition, sex, breed, tick infestation, weekly sanitation of the dog enclosures and tri-monthly application of ectoparasiticides (Table 2). In particular, the risk of infection with Ehrlichia spp., Anaplasma spp. and B. burgdorferi (s.l.) was significantly associated with living outdoors, and CME was most prevalent in female dogs. Regarding breed of dogs, German Shepherds showed higher seroprevalence of Ehrlichia spp., Anaplasma spp. and B. burgdorferi (s.l.). Importantly, a significantly higher chance of seropositivity to all CVBDs was observed in dogs that lived in enclosures that were not sanitated, did not undergo ectoparasiticide application and were infested with ticks (Table 3). No statistical association was found between CVBDs and other variables. Multicollinearity analysis showed strong correlations between seropositivity to Anaplasma spp. and Ehrlichia spp. and tick infestation, not receiving adequate hygienic care and ectoparasiticides where the variance inflation factor (VIF) was 15.665 and 25.117, respectively. However, such correlations were observed for seropositivity to B. burgdorferi (s.l.) and D. immitis, i.e. VIF was 1 and 1.082, respectively.
Table 2.
Risk factors associated with seroprevalence rates of VBDs among dogs in Egypt with single and mixed infection (n = 91) according to different variables
| Variable | No. | Ehrlichia spp. | Anaplasma spp. | Borrelia burgdorferi (s.l.) | Dirofilaria immitis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) | 95% CIa | Chi square | Number (%) | 95% CI | Chi square | Number (%) | 95% CI | Chi square | Number (%) | 95% CI | Chi square | ||
| dfb | df | df | df | ||||||||||
| P-value | P-value | P-value | P-value | ||||||||||
| Governate | |||||||||||||
| Cairo | 230 | 24 (10.4) | 7.1–15 |
χ2 = 6.091 df = 4 P = 0.2 |
15 (6.5) | 3.9–10.5 |
χ2 = 5.694 df = 4 P = 0.2 |
5 (2.2) | 0.9–4.9 |
χ2 = 2.813 df = 4 P = 0.6 |
4 (1.7) | 0.6–4.3 |
χ2 = 1.782 df = 4 P = 0.7 |
| Giza | 110 | 19 (17.2) | 11.3–25.4 | 12 (10.9) | 6.3–18.1 | 2 (1.8) | 0.5–6.3 | 2 (1.8) | 0.5–6.3 | ||||
| Al-Qalyubia | 60 | 6 (10) | 4.6–20.1 | 2 (3.3) | 0.9–11.3 | 2 (3.3) | 0.9–11.3 | 1 (1.6) | 0.3–8.8 | ||||
| Al-Gharbia | 60 | 4 (6.6) | 2.6–15.9 | 3 (5) | 1.7–13.7 | 0 | 0–6 | 0 | 0–6 | ||||
| Kafr El-Sheikh | 40 | 3 (7.5) | 2.5–19.8 | 1 (2.5) | 0.4–12.8 | 0 | 0–8.7 | 0 | 0–8.7 | ||||
| Keeping condition | |||||||||||||
| Indoor | 310 | 12 (3.8) | 2.2–6.6 |
χ2 = 44.060 df = 1 P = 0.0001 |
9 (2.9) | 1.5–5.4 |
χ2 = 18.086 df = 1 P = 0.0001 |
2 (0.6) | 0.2–2.3 |
χ2 = 6.155 df = 1 P = 0.03 |
2 (0.6) | 0.1–2.3 |
χ2 = 2.071 df = 1 P = 0.1 |
| Outdoor | 190 | 44 (23.2) | 17.7–29.6 | 24 (12.6) | 8.6–18.1 | 7 (3.6) | 1.7–7.4 | 5 (2.6) | 1.1–6.1 | ||||
| Sex | |||||||||||||
| Male | 230 | 16 (6.9) | 4.1–11 |
χ2 = 7.712 df = 1 P = 0.005 |
11 (4.7) | 2.6–8.3 |
χ2 = 1.525 df = 1 P = 0.6 |
2 (0.8) | 0.2–3.1 |
χ2 = 2.086 df = 1 P = 0.2 |
2(0.8) | 0.2–3.1 |
χ2 = 0.868 df = 1 P = 0.3 |
| Female | 270 | 40 (14.8) | 11.1–19.5 | 22 (8.2) | 5.4–12 | 7 (2.5) | 1.2–5.2 | 5 (1.8) | 0.7–4.2 | ||||
| Age | |||||||||||||
| < 1 year | 30 | 3 (10) | 3.4–25.6 |
χ2 = 7.525 df = 3 P = 0.06 |
2 (6.6) | 1.8–21.3 |
χ2 = 2.1.525 df = 3 P = 0.6 |
1(3.3.) | 0.5–16.6 |
χ2 = 2.992 df = 3 P = 0.4 |
0 | 0–11.3 |
χ2 = 1.525 df = 3 P = 0.6 |
| 1–3 years | 210 | 16 (7.6) | 4.7–12 | 11 (5.2) | 2.9–9.1 | 2 (0.9) | 0.2–3.4 | 2 (0.9) | 0.2–3.4 | ||||
| 3–5 years | 180 | 22 (12.2) | 8.2–17.8 | 15 (8.3) | 5.1–13.3 | 3 (1.6) | 0.5–4.7 | 3 (1.6) | 0.5–4.7 | ||||
| > 5 years | 80 | 15 (18.7) | 11.7–28.6 | 5 (6.2) | 2.7–13.8 | 3 (3.7) | 1.2–10.4 | 2 (2.5) | 0.6–8.6 | ||||
| Breed | |||||||||||||
| German Shepherd | 260 | 38 (14.6) | 10.8–19.4 |
χ2 = 6.870 df = 2 P = 0.03 |
24 (9.2) | 6.2–13.3 |
χ2 = 6.290 df = 2 P = 0.04 |
6 (2.3) | 1.1–4.9 |
χ2 = 1.161 df = 2 P = 0.5 |
4 (1.5) | 0.6–3.8 |
χ2 = 0.550 df = 2 P = 0.7 |
| Rottweiler | 110 | 10 (9.1) | 5–15.9 | 5 (4.5) | 1.9–10.2 | 2 (1.8) | 0.5–6.3 | 2 (1.8) | 0.5–6.3 | ||||
| Pit Bull | 130 | 8 (6.2) | 3.1–11.6 | 4 (3.1) | 1.2–7.6 | 1 (0.7) | 0.1–4.2 | 1 (0.7) | 0.1–4.2 | ||||
| Season | |||||||||||||
| Spring | 190 | 26 (13.6) | 9.5–19.3 |
χ2 = 4.416 df = 3 P = 0.2 |
12 (6.3) | 3.6–10.7 |
χ2 = 3.938 df = 3 P = 0.3 |
4 (2.1) | 0.8–5.2 |
χ2 = 1.400 df = 3 P = 0.7 |
3 (2.1) | 0.8–5.2 |
χ2 = 2.955 df = 3 P = 0.4 |
| Summer | 175 | 21 (12) | 7.9–17.6 | 16 (9.1) | 5.7–14.3 | 4 (2.2) | 0.8–5.7 | 4 (2.2) | 0.8–5.7 | ||||
| Autumn | 90 | 7 (7.7) | 3.8–15.2 | 4 (4.4) | 1.7–10.8 | 1 (1.1) | 0.2–6 | 0 | 0.0–5.1 | ||||
| Winter | 45 | 2 (4.4) | 1.2–14.8 | 1 (2.2) | 0.3–11.5 | 0 | 0.0–9.8 | 0 | 0.0–9.8 | ||||
| Weekly sanitation of the dog enclosures | |||||||||||||
| Yes | 370 | 23 (6.2) | 4.2–9.2 |
χ2 = 35.346 df = 1 P = 0.0001 |
10 (2.7) | 1.4–4.9 |
χ2 = 35.064 df = 1 P = 0.0001 |
2 (0.5) | 0.1–1.9 |
χ2 = 12.771 df = 1 P = 0.002 |
2 (0.5) | 0.1–1.9 |
χ2 = 7.615 df = 1 P = 0.02 |
| No | 130 | 33(25.3) | 18.6–33.4 | 23 (18.5) | 12.7–26 | 7 (5.4) | 2.6–10.6 | 5 (3.8) | 1.6–8.6 | ||||
| Presence of tick on the dog body | |||||||||||||
| Yes | 140 | 48 (34.3) | 26.9–42.5 |
χ2 = 104.196 df = 1 P = 0.0001 |
22 (15.7) | 10.6–22.6 |
χ2 = 26.203 df = 1 P = 0.0001 |
7(5) | 2.4–9.9 |
χ2 = 11.264 df = 1 P = 0.003 |
5 (3.5) | 1.5–8.1 |
χ2 = 6.642 df = 1 P = 0.02 |
| No | 360 | 8 (2.2) | 1.1–4.3 | 11 (3.1) | 1.7–5.3 | 2 (0.5) | 0.12 | 2 (0.5) | 0.1–2 | ||||
| Tri-monthly application of ectoparasiticides | |||||||||||||
| Yes | 370 | 24 (6.4) | 4.4–9.4 |
χ2 = 31.790 df = 1 P = 0.0001 |
11 (2.9) | 1.6–5.2 |
χ2 = 30.370 df = 1 P = 0.0001 |
2 (0.5) | 0.1–1.9 |
χ2 = 8.604 df = 1 P = 0.007 |
2 (0.5) | 0.1–1.9 |
χ2 = 7.615 df = 1 P = 0.02 |
| No | 130 | 32 (24.6) | 18–32.6 | 22 (16.9) | 11.4–24.2 | 7 (5.4) | 2.6–10.6 | 5 (3.8) | 1.6–8.6 | ||||
| Total | 500 | 56 (11.2) | 8.7–14.3 | 33 (6.6) | 4.7–9.1 | 9 (1.8) | 0.9–3.3 | 7 (1.4) | 0.9–2.9 | ||||
P-values were calculated with Fisher’s exact test only for variables below five (keeping condition, sex, weekly sanitation of the dog enclosures, presence of tick on the dog body and tri-monthly application of ectoparasiticides) for D. immitis and B. burgdorferi (s.l.); all other P-values were calculated with Chi-square test
Significant variables (P-values ≤ 0.05) are marked in bold
aConfidence interval
bDegrees of freedom
Table 3.
Multivariant logistic regression analysis of risk factors associated with seroprevalence rates of VBDs in dogs in Egypt with single and mixed infection (n = 91) according to different variables
| Pathogen | Factor | ßa | SEb | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|---|---|---|
| Ehrlichia spp. | Keeping condition | |||||
| Outdoor | 2.013 | 0.314 | 7.5 | 3.8–14.6 | 0.0001* | |
| Sex | ||||||
| Female | 0.844 | 0.311 | 2.3 | 1.2–4.2 | 0.007⃰ | |
| Breed | ||||||
| Pit Bull (constant) | – | – | – | – | – | |
| German Shepherd | 0.959 | 0.405 | 2.6 | 1.2–5.7 | 0.02⃰ | |
| Rottweiler | 0.422 | 0.493 | 1.5 | 0.6–4 | 0.3 | |
| Weekly sanitation of the dog enclosures | ||||||
| No | 1.636 | 0.295 | 5.1 | 2.8–9.1 | 0.0001⃰ | |
| Presence of tick on the dog body | ||||||
| Yes | 3.143 | 0.399 | 22.9 | 10.4–50.2 | 0.0001⃰ | |
| Tri-monthly application of ectoparasiticides | ||||||
| No | 1.549 | 0.293 | 4.7 | 2.6–8.4 | 0.0001⃰ | |
| Anaplasma spp. | Keeping condition | |||||
| Outdoor | 1.576 | 0.403 | 4.8 | 2.1–10.6 | 0.001⃰ | |
| Breed | ||||||
| German Shepherd | 1.117 | 0.553 | 3.1 | 1.1–9.1 | 0.04⃰ | |
| Rottweiler | 0.405 | 0.684 | 1.5 | 0.4–5.7 | 0.5 | |
| Weekly sanitation of the dog enclosures | ||||||
| No | 2.046 | 0.394 | 7.7 | 3.5–16.7 | 0.0001⃰ | |
| Presence of tick on the dog body | ||||||
| Yes | 1.778 | 0.384 | 5.9 | 2.7–12.5 | 0.0001⃰ | |
| Tri-monthly application of ectoparasiticides | ||||||
| No | 1.894 | 0.385 | 6.6 | 3.1–14.1 | 0.0001⃰ | |
| B. burgdorferi (s.l.) | Keeping condition | |||||
| Outdoor | 1.773 | 0.807 | 5.8 | 1.2–28.6 | 0.02⃰ | |
| Weekly sanitation of the dog enclosures | ||||||
| No | 2.349 | 0.809 | 10.5 | 2.1–51.1 | 0.004⃰ | |
| Presence of tick on the dog body | ||||||
| Yes | 2.243 | 0.808 | 9.4 | 1.9–45.9 | 0.006⃰ | |
| Tri-monthly application of ectoparasiticides | ||||||
| No | 2.439 | 0.809 | 10.5 | 2.1–51.1 | 0.004⃰ | |
| D. immitis | Weekly sanitation of the dog enclosures | |||||
| No | 1.966 | 0.843 | 7.4 | 1.4–38.4 | 0.02⃰ | |
| Presence of tick on the dog body | ||||||
| Yes | 1.673 | 0.843 | 6.6 | 1.3–34.5 | 0.02⃰ | |
| Tri-monthly application of ectoparasiticides | ||||||
| No | 1.966 | 0.843 | 7.4 | 1.4–38.4 | 0.02⃰ | |
ß: Wald statistic; SE: standard error; 95% CI: 95% confidence interval; OR: odds ratio
*These parameters are statistically significant
Discussion
Data presented indicate that dog populations (i.e. 18.2%) in Egypt are exposed to CVBP, therefore posing threats to their own health and to people. This is largely due to the wide distribution of Rh. sanguineus (s.l.), the most common tick species infesting dogs in Egypt and a vector of canine ehrlichiosis and anaplasmosis [40–42]. Though little information is available about the prevalence of CVBDs in the MENA region, dogs often act as reservoirs of VBPs with prevalence of 18.8% in Qatar [55], 24.5% in Saudi Arabia [24], 38.1% in Iraq [21], 46.9% in Iran [20], 69.7% in Algeria [48], 73% in Israel [56] and 83.8% in Morocco [46]. In Egypt CVBDs were mostly observed in urban Governorates (i.e. Giza, Cairo and Al-Qalyubia) where keeping pet animals is more common, also indicating that dogs and people in these regions are at higher risk of acquiring VBDs of canine origin.
The most prevalent pathogen diagnosed in this study was Ehrlichia spp. An E. canis seroprevalence of 41% was reported in dogs from Cairo and Alexandria [34] where DNA of E. canis was also detected in Rh. sanguineus (s.l.) ticks collected on dogs [45]. In addition, the presence of E. canis is supported by the wide distribution in Egypt of the brown dog tick R. sanguineus (s.l.) [40], which is the recognized vector for this species [57]. Although the employed test could detect exposure to E. canis, E. chaffeensis and E. ewingii, in a previous study only E. canis, but not E. ewingii or E. chaffeensis, was molecularly diagnosed in blood of 39 seropositive dogs from Cairo, Giza and Al-Qalyubia [58]. Nonetheless, since E. ewingii and E. chaffeensis have been diagnosed in dogs, ticks and human patients from different countries in the African continent, e.g. Cameroon, Mali, Uganda and South Africa [59–61], further investigations should be carried out to characterize the species of Ehrlichia infecting dogs in Egypt.
To the best of our knowledge this is the first report of sero-reaction to A. phagocytophilum/A. platys in dogs from Egypt (6.6%). It seems that A. phagocytophilum infection of dogs is not prevalent in some regions of MENA, also considering that previous studies from Saudi Arabia, Qatar, Iraq and five regions of Iran failed to detect the infection [20, 21, 55, 62]. However, both A. phagocytophilum and A. platys were detected in Rh. sanguineus (s.l.) blood-feeding on dogs in Cairo and Giza [44, 45] and A. phagocytophilum in blood of five human patients in the Nile Delta [63].
The detection of nine dogs (1.8%) seropositive to B. burgdorferi (s.l.) suggests that the pathogen circulates in Egypt as indicated by previous studies where prevalence ranged from 23% [36] to 71.4% [37]. In addition, some tick species, e.g. Rh. sanguineus (s.l.), Rh. annulatus, Hyalomma excavatum, Hy. dromedarii, Amblyomma lepidum and Ornithodoros savignyi, scored molecularly positive for DNA of B. burgdorferi (s.l.) [36, 64]. The circulation of B. burgdorferi (s.l.) in Egypt has also been demonstrated [36, 63, 65, 66]. In addition, tick-borne relapsing fever (TBRF) caused by Borrelia persica, Borrelia microti, Borrelia latyschewii and Borrelia baltazardi is also endemic in MENA [67] including Egypt where relapsing fever Borrelia spp. were detected in Ornithodoros savignyi ticks and sera of camel, sheep, goat, cattle and buffalo [68, 69]. In particular, B. persica, which is transmitted by Ornithodoros tholozani, can infect dogs and has been reported from Egypt, Israel, Iran, Pakistan and former USSR Asian republics including Uzbekistan [70, 71]. Considering that yet available commercial point-of-care diagnostic kits such as SNAP® 4Dx® employed in this study do not detect TBRF in dogs, complementary tests for dogs living in the endemic areas are recommended.
The detection of seven dogs (1.4%) from Giza, Cairo and Al-Qalyubia seropositive to D. immitis corroborates an older report of microfilariae of D. immitis in blood smears from 8/19 imported German Shepherd dogs in Assiut Governorate, Upper Egypt [35]. In particular, antibodies to D. immitis were already detected from cats of Giza with prevalence of 3.4% [72]. While human cases with D. repens have been frequently reported in Egypt [73, 74], D. immitis was described once in a patient [75]. Data for canine dirofilariosis by D. immitis in North Africa are limited to few reports from Tunisia [49, 76], Algeria [77, 78] and Morocco [46]. It has been suspected that D. immitis is absent from some Middle Eastern countries such as Israel where D. repens is present [79]. In contrast, weighted prevalence of D. immitis infection in dog populations of Turkey and Iran was estimated to be 11.32% and 11.45%, respectively [80]. However, in a recent study in five geographical regions of Iran testing 354 dogs, no microfilaremic dog was found [20].
According to our findings, dogs that were living outdoors had a higher risk of being seropositive to Ehrlichia spp., Anaplasma spp. and B. burgdorferi (s.l.) probably as an effect of the higher chances of being exposed to bites of the brown dog tick Rh. sanguineus (s.l.) and other tick species that are competent vectors of these pathogens [5]. Furthermore, as expected, dogs that did not receive adequate hygienic care and antiparasitic treatments were more likely to be affected by CVBPs. Regular application of ectoparasiticides with potent anti-feeding and fast killing effects, repellents, insect growth regulators and juvenile hormone analogues combined with environmental treatment to reduce the number of adult and juvenile ticks are key control measures in managing CVBDs [81]. Ectoparasiticides, available in several formulations, such as pour-on, spot-on, shampoos, sprays and collars, have long-lasting effects [82] and are highly recommended to dog owners to prevent CVBDs.
In this study, seropositivity to Ehrlichia spp. was more frequent in female dogs. In contrast, some studies have found higher seropositivity to CVBDs in males due to behavioural characteristics that cause greater exposure to vectors than females [83, 84]. No sex-related correlation was recorded for other tested pathogens in this study.
German Shepherds showed higher seroprevalences of Ehrlichia spp. and Anaplasma spp. Although all breeds are prone to CVBDs, CME has been reported most frequently in the German Shepherds [85, 86]. German Shepherd dogs and Siberian Huskies are predisposed to developing more severe clinical signs of ehrlichiosis; therefore, these breeds have a worse prognosis [14]. An experimental study showed that the cell-mediated immune response to a challenge with E. canis was reduced in German Shepherds compared to Beagle dogs [87]. Examination of other dog breeds in Egypt though are very rare, and “baladi” stray dogs will shed light on the true occurrence of CVBDs in the country.
As a limitation of the employed commercial kit, PCR-positive/antibody-negative and antibody-positive/PCR-negative dogs have been reported [88]. The first case could represent early infections before the development of antibody responses; the second case may represent cases of past infections that may have been treated or spontaneously resolved. Furthermore, this commercial kit detects both IgM and IgG antibodies against Erhlichia spp., Anaplasma spp. and B. burgdorferi (s.l.). Although chronic long-term bacteremia is characteristic for some rickettsial agents, long-term persistence of IgG could occur, not reflecting the real “infection” status in some animals. Hence, seropositivity values must be interpreted as current infection with or previous exposure to the pathogens under assessment. Finally, the prevalence of D. immitis infection in Egypt should be carefully considered as an alarm bell for the introduction of a parasite not common in Egypt but which is expanding its range of distribution in southern regions of the Mediterranean Basin such as southern Italy [89].
Conclusion
The present study is the first comprehensive study for CVBD pathogens that has been conducted in Egypt to our knowledge and confirms the presence of Ehrlichia spp., Anaplasma spp., B. burgedorferi (s.l.) and D. immitis in dogs of different regions. The connection between these VBPs and their arthropod vectors in Egypt remains largely unknown and warrants further investigation. Considering that all of the detected pathogens are known zoonotic pathogens, effective ectoparasite control strategies, regular examination of pet dogs and successful chemoprophylaxis are advocated.
Acknowledgements
The authors thank the veterinarians for their support and help in providing data and sample collection throughout the study.
Abbreviations
- CVBPs
Canine vector-borne pathogens
- CVBDs
Canine vector-borne diseases
- OR
Odds ratio
- MENA
Middle East and North Africa
- DNA
Deoxyribonucleic acid
- CME
Canine monocytic ehrlichiosis
- TBRF
Tick-borne relapsing fever
- CI
Confidence interval
- ELISA
Enzyme-linked immunosorbent assay
- s.l.
Sensu lato
- VIP
Variance inflation factor
- IR6
Invariable region 6
- VlsE
Vmp-like sequence
- df
Degrees of freedom
Authors’ contributions
ASe conceived the study and performed field work. ASe, ADA and ASa performed laboratory work and analysed data. ASa and ASe wrote the first draft of the manuscript. DO reviewed the manuscript. All authors read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Declarations
Ethics approval and consent to participate
Blood samples for this study were approved by the Ethical Research Committee, Benha University, Egypt (Approval no.: BUFVTM045) and collected after owners’ consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdelfattah Selim, Email: abdelfattah.selim@fvtm.bu.edu.eg.
Abdullah D. Alanazi, Email: aalanazi@su.edu.sa
Alireza Sazmand, Email: alireza.sazmand@basu.ac.ir.
Domenico Otranto, Email: domenico.otranto@uniba.it.
References
- 1.Yuasa Y, Hsu T-H, Chou C-C, Huang C-C, Huang W-C, Chang C-C. The comparison of spatial variation and risk factors between mosquito-borne and tick-borne diseases: seroepidemiology of Ehrlichia canis, Anaplasma species, and Dirofilaria immitis in dogs. Comp Immunol Microbiol Infect Dis. 2012;35(6):599–606. doi: 10.1016/j.cimid.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.de la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Peña A, et al. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol. 2017;7:114. doi: 10.3389/fcimb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 2012;28(10):437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Otranto D, Dantas-Torres F, Breitschwerdt EB. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol. 2009;25(4):157–163. doi: 10.1016/j.pt.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Angelou A, Gelasakis AI, Verde N, Pantchev N, Schaper R, Chandrashekar R, et al. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasit Vectors. 2019;12(1):283. doi: 10.1186/s13071-019-3543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen V, Colella V, Greco G, Fang F, Nurcahyo W, Hadi U, et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasit Vectors. 2020;13:420. doi: 10.1186/s13071-020-04288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380(9857):1946–1955. doi: 10.1016/S0140-6736(12)61151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Günaydın E, Turut N, Akın B, Koç Ö, Ütük AE. A serosurvey on some canine vector-borne zoonoses (Anaplasma spp., Ehrlichia spp., Borrelia burgdorferi, Dirofilaria immitis and Leishmania spp.) in Osmaniye. Atatürk Üniv Vet Bilim Derg. 2019;14(2):151–158. [Google Scholar]
- 9.Breitschwerdt EB, Hegarty BC, Hancock SI. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36(9):2645–2651. doi: 10.1128/JCM.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30(1):261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso L, Mendão C, de Carvalho LM. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal-a national serological study. Parasit Vectors. 2012;5(1):62. doi: 10.1186/1756-3305-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Lee H, Park J-W, Yoon S-S, Seo H-J, Noh J, et al. Prevalence of antibodies against Anaplasma spp., Borrelia burgdorferi sensu lato, Babesia gibsoni, and Ehrlichia spp. in dogs in the Republic of Korea. Ticks Tick Borne Dis. 2020;11:101412. doi: 10.1016/j.ttbdis.2020.101412. [DOI] [PubMed] [Google Scholar]
- 13.Salem NY, Rakha GH, Baraka TA. Naturally occurring ehrlichiosis in Egyptian dogs. Iran J Vet Res. 2014;15(1):54–57. [Google Scholar]
- 14.Sainz Á, Roura X, Miró G, Estrada-Peña A, Kohn B, Harrus S, et al. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors. 2015;8(1):75. doi: 10.1186/s13071-015-0649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrus S, Aroch I, Lavy E, Bark H. Clinical manifestations of infectious canine cyclic thrombocytopenia. Vet Rec. 1997;141(10):247–250. doi: 10.1136/vr.141.10.247. [DOI] [PubMed] [Google Scholar]
- 16.Mendoza-Roldan J, Santhakumari Manoj R, Latrofa M, Iatta R, Annoscia G, Lovreglio P, et al. Role of reptiles and associated arthropods in the epidemiology of rickettsioses: a one health paradigm. PLOS Negl Trop Dis. 2021;15(2):e0009090. doi: 10.1371/journal.pntd.0009090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sazmand A, Harl J, Eigner B, Hodžić A, Beck R, Hekmatimoghaddam S, et al. Vector-borne bacteria in blood of camels in Iran: new data and literature review. Comp Immunol Microbiol Infect Dis. 2019;65:48–53. doi: 10.1016/j.cimid.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alanazi AD, Nguyen VL, Alyousif MS, Manoj RR, Alouffi AS, Ridolfi D, et al. Ticks and associated pathogens in camels (Camelus dromedarius) from Riyadh Province, Saudi Arabia. Parasit Vectors. 2020;13:110. doi: 10.1186/s13071-020-3973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013;3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iatta R, Sazmand A, Nguyen V-L, Nemati F, Bahiraei Z, Mazhar Ayaz M, et al. Vector-borne pathogens in dogs of different regions of Iran and Pakistan. Parasitol Res. 2021 doi: 10.1007/s00436-020-06992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otranto D, Iatta R, Baneth G, Cavalera MA, Bianco A, Parisi A, et al. High prevalence of vector-borne pathogens in domestic and wild carnivores in Iraq. Acta Trop. 2019;197:105058. doi: 10.1016/j.actatropica.2019.105058. [DOI] [PubMed] [Google Scholar]
- 22.Breitschwerdt EB, Hegarty BC, Qurollo BA, Saito TB, Maggi RG, Blanton LS, et al. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit Vectors. 2014;7(1):298. doi: 10.1186/1756-3305-7-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arraga-Alvarado CM, Qurollo BA, Parra OC, Berrueta MA, Hegarty BC, Breitschwerdt EB. Molecular evidence of Anaplasma platys infection in two women from Venezuela. Am J Trop Med Hyg. 2014;91(6):1161–1165. doi: 10.4269/ajtmh.14-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alanazi AD, Alouffi AS, Alyousif MS, Alshahrani MY, Abdullah HHAM, Abdel-Shafy S, et al. Molecular survey of vector-borne pathogens of dogs and cats in two regions of Saudi Arabia. Pathogens. 2021;10(1):25. doi: 10.3390/pathogens10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci. 2011;91(1):71–76. doi: 10.1016/j.rvsc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Granick JL, Armstrong PJ, Bender JB. Anaplasma phagocytophilum infection in dogs: 34 cases (2000–2007) J Am Vet Med Assoc. 2009;234(12):1559–1565. doi: 10.2460/javma.234.12.1559. [DOI] [PubMed] [Google Scholar]
- 27.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza-Roldan JA, Colella V, Lia RP, Nguyen VL, Barros-Battesti DM, Iatta R, et al. Borrelia burgdorferi (sensu lato) in ectoparasites and reptiles in southern Italy. Parasit Vectors. 2019;12(1):35. doi: 10.1186/s13071-019-3286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adaszek Ł, Gatellet M, Mazurek Ł, Dębiak P, Skrzypczak M, Winiarczyk S. Myocarditis secondary to Borrelia infection in a dog: a case report. Ann Parasitol. 2020;66(2):255–257. doi: 10.17420/ap6602.263. [DOI] [PubMed] [Google Scholar]
- 30.Goossens H, Van Den Bogaard A, Nohlmans M. Dogs as sentinels for human Lyme borreliosis in The Netherlands. J Clin Microbiol. 2001;39(3):844–848. doi: 10.1128/JCM.39.3.844-848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simón F, Siles-Lucas M, Morchón R, González-Miguel J, Mellado I, Carretón E, et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev. 2012;25(3):507–544. doi: 10.1128/CMR.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dantas-Torres F, Otranto D. Overview on Dirofilaria immitis in the Americas, with notes on other filarial worms infecting dogs. Vet Parasitol. 2020;282:109113. doi: 10.1016/j.vetpar.2020.109113. [DOI] [PubMed] [Google Scholar]
- 33.Panarese R, Iatta R, Latrofa MS, Zatelli A, Ćupina AI, Montarsi F, et al. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: an expanding threat in the Mediterranean region. Int J Parasitol. 2020;50(8):555–559. doi: 10.1016/j.ijpara.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Botros B, Elmolla M, Salib A, Calamaio C, Dasch G, Arthur R. Canine ehrlichiosis in Egypt: sero-epidemiological survey. Onderstepoort J Vet Res. 1995;62:41–43. [PubMed] [Google Scholar]
- 35.Abd El-Rahim I. Canine dirofilariasis among imported dogs in upper Egypt. Ass Vet Med J. 1998;40:121–132. [Google Scholar]
- 36.Elhelw RA, El-Enbaawy MI, Samir A. Lyme borreliosis: a neglected zoonosis in Egypt. Acta Trop. 2014;140:188–192. doi: 10.1016/j.actatropica.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Samir A, Fahmy A, Hatem EM, Orabi A. Occurrence of canine borreliosis in Egyptian dogs: a public health threat. Transl Biomed. 2015;6(29):1–4. [Google Scholar]
- 38.Latrofa MS, Dantas-Torres F, Giannelli A, Otranto D. Molecular detection of tick-borne pathogens in Rhipicephalus sanguineus group ticks. Ticks Tick borne Dis. 2014;5(6):943–946. doi: 10.1016/j.ttbdis.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806)(Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152(3–4):173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 40.Aziz ARA, Abou Laila MR, Bazh EK, Sultan K. Epidemiological study on brown dog tick Rhipicephalus sanguineus at Sadat District, Egypt. PSM Vet Res. 2017;2(1):1–5. [Google Scholar]
- 41.El-Gawady HM. Studies on ectoparasites of stray dogs in Ismailia city. Egypt Vet Med Soc Parasitol J. 2015;11(1):115–122. [Google Scholar]
- 42.Amin OM, Madbouly MH. Distribution and seasonal dynamics of a tick, a louse fly, and a louse infesting dogs in the Nile Valley and Delta of Egypt. J Med Entomol. 1973;10(3):295–298. doi: 10.1093/jmedent/10.3.295. [DOI] [PubMed] [Google Scholar]
- 43.Otranto D, Huchet J-B, Giannelli A, Callou C, Dantas-Torres F. The enigma of the dog mummy from Ancient Egypt and the origin of ‘Rhipicephalus sanguineus’. Parasit Vectors. 2014;7:2. doi: 10.1186/1756-3305-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghafar MW, Amer SA. Prevalence and first molecular characterization of Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis, in Rhipicephalus sanguineus ticks attached to dogs from Egypt. J Adv Res. 2012;3(2):189–194. doi: 10.1016/j.jare.2011.08.002. [DOI] [Google Scholar]
- 45.Nasr A, El Hariri M, Ghafar MW. Detection of Anaplasma platys and Ehrlichia canis in Rhipicephalus sanguineus ticks attached to dogs from Egypt; a public health concern. Vet Med J Giza. 2020;66:1–9. [Google Scholar]
- 46.Khatat SE, Khallaayoune K, Errafyk N, Van Gool F, Duchateau L, Daminet S, et al. Detection of Anaplasma spp. and Ehrlichia spp. anibodies, and Dirofilaria immitis antigens in dogs from seven locations of Morocco. Vet Parasitol. 2017;239:86–89. doi: 10.1016/j.vetpar.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Dahmani M, Loudahi A, Mediannikov O, Fenollar F, Raoult D, Davoust B. Molecular detection of Anaplasma platys and Ehrlichia canis in dogs from Kabylie, Algeria. Ticks Tick Borne Dis. 2015;6(2):198–203. doi: 10.1016/j.ttbdis.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Azzag N, Petit E, Gandoin C, Bouillin C, Ghalmi F, Haddad N, et al. Prevalence of select vector-borne pathogens in stray and client-owned dogs from Algiers. Comp Immunol Microbiol Infect Dis. 2015;38:1–7. doi: 10.1016/j.cimid.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Rjeibi M, Rouatbi M, Mabrouk M, Tabib I, Rekik M, Gharbi M. Molecular study of Dirofilaria immitis and Dirofilaria repens in dogs from Tunisia. Transbound Emerg Dis. 2017;64(5):1505–1509. doi: 10.1111/tbed.12541. [DOI] [PubMed] [Google Scholar]
- 50.M'ghirbi Y, Ghorbel A, Amouri M, Nebaoui A, Haddad S, Bouattour A. Clinical, serological, and molecular evidence of ehrlichiosis and anaplasmosis in dogs in Tunisia. Parasitol Res. 2009;104(4):767–774. doi: 10.1007/s00436-008-1253-4. [DOI] [PubMed] [Google Scholar]
- 51.Pantchev N, Schnyder M, Vrhovec MG, Schaper R, Tsachev I. Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol Res. 2015;114(1):117–130. doi: 10.1007/s00436-015-4518-8. [DOI] [PubMed] [Google Scholar]
- 52.Chandrashekar R, Mainville CA, Beall MJ, O’Connor T, Eberts MD, Alleman AR, et al. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc. 2010;71(12):1443–1450. doi: 10.2460/ajvr.71.12.1443. [DOI] [PubMed] [Google Scholar]
- 53.Stillman BA, Monn M, Liu J, Thatcher B, Foster P, Andrews B, et al. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc. 2014;245(1):80–86. doi: 10.2460/javma.245.1.80. [DOI] [PubMed] [Google Scholar]
- 54.Pantchev N, Pluta S, Huisinga E, Nather S, Scheufelen M, Vrhovec MG, et al. Tick-borne diseases (borreliosis, anaplasmosis, babesiosis) in German and Austrian dogs: status quo and review of distribution, transmission, clinical findings, diagnostics and prophylaxis. Parasitol Res. 2015;114(S1):S19–S54. doi: 10.1007/s00436-015-4513-0. [DOI] [PubMed] [Google Scholar]
- 55.Alho AM, Lima C, Latrofa MS, Colella V, Ravagnan S, Capelli G, et al. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Parasit Vectors. 2017;10:298. doi: 10.1186/s13071-017-2237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baneth G, Breitschwerdt EB, Hegarty BC, Pappalardo B, Ryan J. A survey of tick-borne bacteria and protozoa in naturally exposed dogs from Israel. Vet Parasitol. 1998;74(2–4):133–142. doi: 10.1016/S0304-4017(97)00149-0. [DOI] [PubMed] [Google Scholar]
- 57.Groves M, Dennis G, Amyx H, Huxsoll D. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus) Am J Vet Res. 1975;36(7):937–940. [PubMed] [Google Scholar]
- 58.Selim A, Abdelhady A, Alahadeb J. Prevalence and first molecular characterization of Ehrlichia canis in Egyptian dogs. Pak Vet J. 2020 doi: 10.29261/pakvetj/2020.061. [DOI] [Google Scholar]
- 59.Ndip LM, Ndip RN, Esemu SN, Walker DH, McBride JW. Predominance of Ehrlichia chaffeensis in Rhipicephalus sanguineus ticks from kennel-confined dogs in Limbe, Cameroon. Exp Appl Acarol. 2010;50(2):163. doi: 10.1007/s10493-009-9293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uhaa IJ, Maclean JD, Greene CR, Fishbein DB. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg. 1992;46(2):161–164. doi: 10.4269/ajtmh.1992.46.161. [DOI] [PubMed] [Google Scholar]
- 61.Proboste T, Kalema-Zikusoka G, Altet L, Solano-Gallego L, De Mera IGF, Chirife AD, et al. Infection and exposure to vector-borne pathogens in rural dogs and their ticks, Uganda. Parasit Vectors. 2015;8:306. doi: 10.1186/s13071-015-0919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandra S, Smith K, Alanazi AD, Alyousif MS, Emery D, Šlapeta J. Rhipicephalus sanguineus sensu lato from dogs and dromedary camels in Riyadh, Saudi Arabia: low prevalence of vector-borne pathogens in dogs detected using multiplexed tandem PCR panel. Fol Parasitol. 2019;66:007. doi: 10.14411/fp.2019.007. [DOI] [PubMed] [Google Scholar]
- 63.Ghafar MW, Eltablawy NA. Molecular survey of five tick-borne pathogens (Ehrlichia chaffeensis, Ehrlichia ewingii, Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Babesia microti) in Egyptian farmers. Glob Vet. 2011;7(3):249–255. [Google Scholar]
- 64.Adham FK, Abd-El-Samie E, Gabre RM, El Hussein H. Detection of tick blood parasites in Egypt using PCR assay II-Borrelia burgdorferi sensu lato. J Egypt Soc Parasitol. 2010;40(3):553–564. [PubMed] [Google Scholar]
- 65.Haberberger RL, Jr, Constantine NT, Schwan TG, Woody JN. Lyme disease agent in Egypt? Trans R Soc Trop Med Hyg. 1989;83(4):556. doi: 10.1016/0035-9203(89)90293-9. [DOI] [PubMed] [Google Scholar]
- 66.Hammouda NA, Hegazy IH, El-Sawy E. ELISA screening for Lyme disease in children with chronic arthritis. J Egypt Soc Parasitol. 1995;25(2):525–533. [PubMed] [Google Scholar]
- 67.Naddaf SR, Ghazinezhad B, Sedaghat MM, Asl HM, Cutler SJ. Tickborne relapsing fever in southern Iran, 2011–2013. Emerg Infect Dis. 2015;21(6):1078. doi: 10.3201/eid2106.141715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helmy N. Seasonal abundance of Ornithodoros (O.) savignyi and prevalence of infection with Borrelia spirochetes in Egypt. J Egypt Soc Parasitol. 2000;30(2):607–619. [PubMed] [Google Scholar]
- 69.Shanbaky NM, Helmy N. First record of natural infection with Borrelia in ornithodoros (Ornithodoros) savignyi. Reservoir potential and specificity of the tick to Borrelia. J Egypt Soc Parasitol. 2000;30(3):765–780. [PubMed] [Google Scholar]
- 70.Baneth G, Nachum-Biala Y, Halperin T, Hershko Y, Kleinerman G, Anug Y, et al. Borrelia persica infection in dogs and cats: clinical manifestations, clinicopathological findings and genetic characterization. Parasit Vectors. 2016;9(1):244. doi: 10.1186/s13071-016-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shirani D, Rakhshanpoor A, Cutler SJ, Ghazinezhad B, Naddaf SR. A case of canine borreliosis in Iran caused by Borrelia persica. Ticks Tick Borne Dis. 2016;7(3):424–426. doi: 10.1016/j.ttbdis.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 72.Al-Kappany Y, Lappin M, Kwok O, Abu-Elwafa S, Hilali M, Dubey J. Seroprevalence of Toxoplasma gondii and concurrent Bartonella spp., feline immunodeficiency virus, feline leukemia virus, and Dirofilaria immitis infections in Egyptian cats. J Parasitol. 2011;97(2):256–258. doi: 10.1645/GE-2654.1. [DOI] [PubMed] [Google Scholar]
- 73.Awadalla H, Bayoumi DM, Ibrahim IR. The first case report of suspected human dirofilariasis in the eyelid of a patient from Alexandria. J Egypt Soc Parasitol. 1998;28:941–943. [PubMed] [Google Scholar]
- 74.Abdel-Rahman S, Mahmoud A, Galal L, Gustinelli A, Pampiglione S. Three new cases of human infection with Dirofilaria repens, one pulmonary and two subcutaneous, in the Egyptian governorate of Assiut. Ann Trop Med Parasitol. 2008;102(6):499–507. doi: 10.1179/136485908X300904. [DOI] [PubMed] [Google Scholar]
- 75.Elnazer M. Human ectopic infection with Dirofilaria immitis (Leydi, 1865) representing first record from Egypt. Egypt Med J. 1994;11:435–438. [Google Scholar]
- 76.Chabchoub A, Landolsi F, Selmi C. Serological survey and clinical study of dirofilariosis with Dirofilaria immitis carried out in working dogs in Tunis region (Tunisia) Rev Méd Vét. 2003;154(11):673–678. [Google Scholar]
- 77.Meriem-Hind B-M, Mohamed M. Prevalence of canine Dirofilaria immitis infection in the city of Algiers, Algeria. Afr J Agric Res. 2009;4(10):1097–1100. [Google Scholar]
- 78.Tahir D, Damene H, Davoust B, Parola P. First molecular detection of Dirofilaria immitis (Spirurida: Onchocercidae) infection in dogs from Northern Algeria. Comp Immunol Microbiol Infect Dis. 2017;51:66–68. doi: 10.1016/j.cimid.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Capelli G, Genchi C, Baneth G, Bourdeau P, Brianti E, Cardoso L, et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit Vectors. 2018;11(1):663. doi: 10.1186/s13071-018-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anvari D, Narouei E, Daryani A, Sarvi S, Moosazadeh M, Hezarjaribi HZ, et al. The global status of Dirofilaria immitis in dogs: a systematic review and meta-analysis based on published articles. Res Vet Sci. 2020;131:104–116. doi: 10.1016/j.rvsc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Dantas-Torres F, Otranto D. Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors. 2014;7(1):22. doi: 10.1186/1756-3305-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Otranto D. Arthropod-borne pathogens of dogs and cats: from pathways and times of transmission to disease control. Vet Parasitol. 2018;251:68–77. doi: 10.1016/j.vetpar.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 83.Costa LM, Jr, Rembeck K, Ribeiro MFB, Beelitz P, Pfister K, Passos LMF. Sero-prevalence and risk indicators for canine ehrlichiosis in three rural areas of Brazil. Vet J. 2007;174(3):673–676. doi: 10.1016/j.tvjl.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Cortes S, Vaz Y, Neves R, Maia C, Cardoso L, Campino L. Risk factors for canine leishmaniasis in an endemic Mediterranean region. Vet Parasitol. 2012;189(2–4):189–196. doi: 10.1016/j.vetpar.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 85.Huxsoll DL, Hildebrandt PK, Nims RM, Amyx HL, Ferguson JA. Epizootiology of tropical canine pancytopenia. J Wildl Dis. 1970;6(4):220–225. doi: 10.7589/0090-3558-6.4.220. [DOI] [PubMed] [Google Scholar]
- 86.Jafari S, Gaur S, Hashemi A. Prevalence of Ehrlichia canis in dog population of Shiraz, Fars province of Iran. J Appl Anim Res. 1997;11(1):19–23. doi: 10.1080/09712119.1997.9706157. [DOI] [Google Scholar]
- 87.Nyindo M, Huxsoll D, Ristic M, Kakoma I, Brown J, Carson C, et al. Cell-mediated and humoral immune responses of German shepherd dogs and beagles to experimental infection with Ehrlichia canis. Am J Vet Res. 1980;41(2):250–254. [PubMed] [Google Scholar]
- 88.Wong SS, Teng JL, Poon RW, Choi GK, Chan K-H, Yeung ML, et al. Comparative evaluation of a point-of-care immunochromatographic test SNAP 4Dx with molecular detection tests for vector-borne canine pathogens in Hong Kong. Vector Borne Zoonotic Dis. 2011;11(9):1269–1277. doi: 10.1089/vbz.2010.0265. [DOI] [PubMed] [Google Scholar]
- 89.Mendoza-Roldan J, Benelli G, Panarese R, Iatta R, Furlanello T, Beugnet F, Zatelli A, Otranto D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009-2019: changing distribution patterns. Parasit Vectors. 2020;13(1):193. doi: 10.1186/s13071-020-04063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its additional files.