Abstract
Nonclassical monocytes maintain vascular homeostasis by patrolling the vascular endothelium, responding to inflammatory signals, and scavenging cellular debris. Nonclassical monocytes also prevent metastatic tumor cells from seeding new tissues, but whether the patrolling function of nonclassical monocytes is required for this process is unknown. To answer this question, we utilized an inducible-knockout mouse that exhibits loss of the integrin-adaptor protein Kindlin-3 specifically in nonclassical monocytes. We show that Kindlin-3-deficient nonclassical monocytes are unable to patrol the vascular endothelium in either the lungs or periphery. We also find that Kindlin-3-deficient nonclassical monocytes cannot firmly adhere to, and instead ‘slip’ along, the vascular endothelium. Loss of patrolling activity by nonclassical monocytes was phenocopied by ablation of LFA-1, an integrin-binding partner of Kindlin-3. When B16F10 murine melanoma tumor cells were introduced into Kindlin-3-deficient mice, nonclassical monocytes showed defective patrolling towards tumor cells and failure to ingest tumor particles in vivo. Consequently, we observed a significant, 4-fold increase in lung tumor metastases in mice possessing Kindlin-3-deficient nonclassical monocytes. Thus, we conclude that the patrolling function of nonclassical monocytes is mediated by Kindlin-3 and essential for these cells to maintain vascular endothelial homeostasis and prevent tumor metastasis to the lung.
Graphical Abstract
INTRODUCTION
Cancer is a leading cause of death in the United States and metastasis of primary cancer cells to distant organs increases morbidity in cancer patients [1]. For example, the lungs are frequently targeted for colonization by several tumor types [2, 3]. Invasion of circulating tumor cells into lung tissue is met by waves of neutrophils, dendritic cells, and monocytes, which control the expansion or repulsion of metastases [4–7]. Nonclassical monocytes (CD16+CD14low in humans and CX3CR1highLy6C− in mice) are predominantly located in the blood and highly vascularized tissues such as the lungs [8, 9]. These cells patrol along the vascular endothelium, where they play important roles in mediating homeostasis and repair [10], and detecting and clearing pathogens [11]. Additionally, our laboratory has shown that nonclassical monocytes scavenge invading tumor cells and thus prevent tumor colonization in the lungs [7].
Integrin activation and clustering are essential for the motility of monocytes as well as the adherence of these cells to the endothelium [12, 13]. LFA-1 (composed of the αL and β2 subunits) is the dominant integrin responsible for mediated nonclassical monocyte patrolling [8]. Kindlin-3, a leukocyte-specific adapter protein that binds to the cytoplasmic tails of β1 and β2 integrins, promotes high-affinity conformation and adhesion of leukocytes to the endothelium [14, 15]. A functional role for Kindlin-3 in nonclassical monocytes has not yet been reported. Moreover, whether patrolling action by nonclassical monocytes mediates anti-tumoral immunity and/or modulation of the tumor immune microenvironment remains to be investigated.
In this study, we show that in the absence of Kindlin-3, nonclassical monocytes cannot firmly adhere to the vascular endothelium and instead ‘slip’ along the endothelium and thus fail to patrol. This observation was phenocopied in LFA-1-deficient nonclassical monocytes. Importantly, loss of functional patrolling results in perturbed endothelial homeostasis and prevents nonclassical monocytes from sensing, migrating towards, and ingesting metastasizing tumor cells, leading to increased tumor burden.
RESULTS
Kindlin-3 is necessary for patrolling behavior of nonclassical monocytes in the periphery.
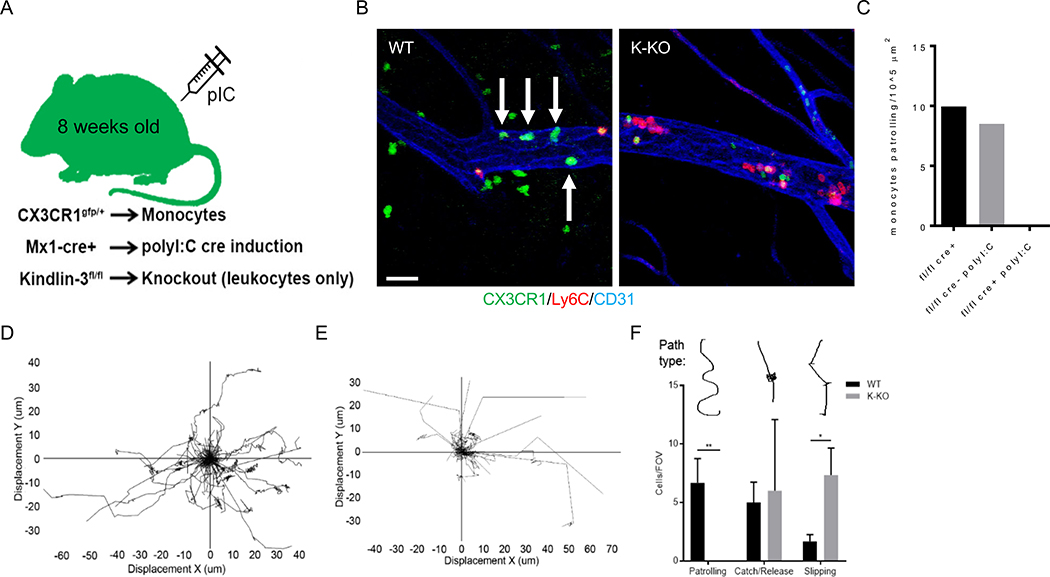
To determine the role of nonclassical monocyte patrolling in preventing tumor cell metastasis, we generated an inducible Fermt3 (Kindlin-3) knockout (KO) mouse where nonclassical monocytes are present in normal numbers but unable to patrol the vasculature. Interferon-inducible Mx1-Cre mice express Cre recombinase under the Mx1 promoter following stimulation with poly(I:C) [16]. Therefore, we crossed Fermt3fllox/flox mice with Mx1-Cre+/− mice to generate animals with inducible loss of Kindlin-3 (Fermt3fllox/flox;Mx1-Cre+/−, K-KO mice) in hematopoietic cells [17]. To image nonclassical monocytes selectively via live intravital microscopy (IVM), we crossed Fermt3fllox/flox;Mx1-Cre+/− mice with Cx3cr1gfp/+ mice to generate mice with GFP+ labeled nonclassical monocytes (Fermt3fllox/flox;Mx1-Cre+/−;Cx3cr1gfp/+ mice) (Fig. 1A). Murine nonclassical monocytes exhibit high levels of CX3CR1 and Cx3cr1gfp/+ mice constitutively express GFP under the Cx3cr1 promoter [18], making this reporter line well-suited for labeling nonclassical monocytes. Furthermore, in contrast to Nr4a1 reporter mice, which have been previously used in monocyte studies and in which Nr4a1 is expressed by multiple immune cell types, Cx3cr1 is enriched in monocytes and lowly expressed in certain T cell subsets [19]. At approximately 8 weeks of age, K-KO and control mice were injected with poly(I:C). We then performed quantitative RT-PCR (qPCR) 2 weeks later on blood monocytes from these animals and confirmed loss of Fermt3 expression in K-KO nonclassical monocytes (Sup Fig. 1). After in vivo injections of tumor necrosis factor (TNF)-α to stimulate an inflammatory response, nonclassical monocytes patrolled the femoral vasculature normally in control mice (fl/fl Cre+ or fl/fl cre- polyI:C; Fig. 1B–C and Video S1). However, in the K-KO mouse that received poly(I:C) (fl/fl cre+ poly I:C), all nonclassical monocytes within the field of view (FOV) failed to patrol the vasculature (Fig. 1B–C; Video S2).
Figure 1. Kindlin-3 is necessary for patrolling behavior of nonclassical monocytes in the periphery.
A) Schematic for Kindlin-3 mouse model. B) Snapshots of CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre−/− (WT) and CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre+/− (K-KO) mouse femoral vasculature. Time-lapse images were acquired using intravital confocal microscopy (IVM) on the femoral vasculature of 12-week old female cohort mice. Accompanying videos are in Supporting Information section. Mice were injected intraperitoneal (i.p.) with 500 ng recombinant mouse TNF-α in PBS 1–2 hours before imaging. Patrolling monocytes (green cells, no red) are highlighted by white arrows. Scale bar = 30 μM. C) Number of patrolling nonclassical monocytes (GFP+) per 105 μm surface area of blood vessel. Graph is representative of 3 biological replicates. Mice designated fl/fl cre+ without poly(I:C) and fl/fl cre- with poly(I:C) are experimental controls representing no Kindlin-3 excision, while fl/fl cre+ with poly(I:C) represents a mouse with the Kindlin-3 gene excised. Bars within the graph are the averages (means) of 5 fields of view (FOV), 18 minutes per time-lapse image, per mouse. Spider plots of WT D) and K-KO E) nonclassical monocyte tracks in mouse lung during homeostasis (GFP+, Ly6C− or Gr-1−). F) Nonclassical monocyte tracks were binned into 3 groups: 1-patrolling, 2-adherence/release (arresting for more than 2 frames on the endothelium without crawling), or 3-slipping (briefly stopping along endothelium repeatedly for less than 2 frames). 1 frame = approximately 7 seconds. *p<0.05, **p<0.01.
Kindlin-3 mediates high-affinity conformation and clustering of β-integrins, which can increase leukocyte firm adhesion and patrolling along the endothelium [20–22]. Nonclassical monocytes from control mice exhibited characteristically slow, long-distance, and omni-directional patrolling tracks both in the vasculature and in situ (Fig. 1D), whereas K-KO nonclassical monocytes exhibited straight tracks with few contact points on the endothelium (Fig. 1E). Moreover, in control animals, nonclassical monocytes patrolled (as defined by nonlinear crawling for at least 1 minute), while K-KO nonclassical monocytes could not crawl through lung blood vessels or parenchyma for long periods of time or distances. Instead, K-KO nonclassical monocytes exhibited transient adherence without crawling (adherence/release, > 2 frames or approximately >14 seconds) or brief adherence to the endothelium via a ‘slipping’ mechanism (slipping, < 2 frames adhered; Fig. 1F). However, the numbers of nonclassical monocytes capable of adhering to the endothelium for more than 2 frames (> 14+ seconds) were not significantly different between WT and K-KO mice, suggesting that transitioning from adherence to patrolling involves a multi-step process that Kindlin-3 mediates, which is a distinct process from rolling or other types of adherence to the endothelium. Overall, our findings indicate that Kindlin-3 is required for the normal patrolling function of nonclassical monocytes. They also highlight the generation of a new mouse model with which to study the roles of nonclassical monocytes in vascular homeostasis and disease.
Kindlin-3 is required for nonclassical monocyte recruitment to sites of invading melanoma metastases in the lung and for cancer particle uptake.
We next asked whether Kindlin-3 is necessary for nonclassical monocyte migration towards metastasizing tumor cells within the lungs. WT and K-KO mice were injected intravenously with 3 × 105 B16F10-RFP tumor cells and the lungs of live mice were imaged using intravital microscopy (Fig. 2A, Video S3). At 30 minutes post-injection, we observed a significant decrease in the number of GFP+ cells patrolling and accumulating within 30 μm of RFP+ tumor cells (circle overlay) in lungs of K-KO mice compared to WT mice (Fig. 2A/B, Video S4), indicating defective migration of K-KO nonclassical monocytes towards invading tumor cells. Additionally, we confirmed the absence of functionally patrolling nonclassical monocytes within the FOV of the lung vasculature in K-KO mice (Fig. 2C). Importantly, the frequencies of CX3CR1-GFP+ CD11b+ cells in the lung after polyI:C injection, but prior to cancer injection, were similar between WT and K-KO mice (Sup Fig. 2A). Likewise, we did not observe differences in the total numbers of CX3CR1-GFP+ cells within the entire FOV (Sup Fig. 2B). Thus, the numbers of patrolling monocytes were similar, but their ability to patrol towards metastasizing tumor cells was greatly impaired, demonstrating that Kindlin-3 action is necessary for nonclassical monocytes to sense and migrate towards newly seeded cancer cells during early metastasis.
Figure 2. Nonclassical monocytes lacking Kindlin-3 fail to enter lung tissue and do not surround invading metastases.
A) Snapshots of lung intravital imaging (Leica SP8, 25× 0.8 NA objective) in CX3CR1gfp/+Kindlin-3fl/flMx1-cre- (WT) or cre+ (K-KO) mice 30 minutes after i.v. injection of 3×105 B16F10-RFP cells. Scale bar = 50 uM. B) Analysis of intravital microscopy imaging from A), using Imaris software to detect the average number of GFP+ monocytes per for every frame of the recording within 30 μM of B16F10-RFP cells. n = 3 mice per group, independent experiments. C) Number of patrolling nonclassical monocytes per FOV from A). D) Flow cytometry gating for selection of vascular (CD45v) or interstitial (CD45i) leukocytes, followed by monocyte gating using CD115 and CD11b markers. Lungs were mechanically dissociated so as not to lose CD115 expression. E) Ly6C− monocyte frequencies within interstitial lung tissues out of total CD45+ live cells. n = 6–7 per group. F) Ly6C− monocyte frequencies within the vascular niches of the lung tissue out of total CD45+ live cells. n=6–7 mice per group. G) 3×105 B16F10 cells were labeled with CellTrace Violet and injected i.v. into DsRed:K-KO BMT mice. 16 hours later, classical (Ly6C+) and nonclassical (Ly6C−) monocytes from WT (DsRed) donors or K-KO donors within the same mice were assessed for uptake of B16F10-violet particles per 105 CD45+ cells in the lung. n = 8 mice per group. Kolmogorov-Smirnov nonparametric test for single comparisons, Kruskal-Wallis nonparametric test for multiple comparisons. ns = not significant, *p<0.05, **p<0.01, ****p<0.0001.
Due to the highly vascularized nature of the lungs, we wondered if K-KO nonclassical monocytes become trapped in the microvasculature of the lung. To differentiate between vascular- and tissue-resident immune cells in the lungs, CD45-BV650 was i.v. injected 1 minute prior to harvesting the lungs of CX3CR1gfp/+;Fermt3fllox/flox;Mx1-Cre+/− mice to label cells residing in the vasculature (CD45v cells). We then stained for total CD45+ cells in the interstitium with CD45-APC by flow cytometry (CD45i cells; Fig. 2D). Afterwards, we gated CD45i and CD45v cells for monocytes and found that K-KO Ly6C− nonclassical monocyte numbers were significantly decreased in the interstitium compared to controls (Fig. 2E). In contrast, there was no difference in Ly6C− nonclassical monocyte numbers within the lung vasculature (Fig. 2F), while the frequency of Ly6C− nonclassical monocytes residing in the lung vasculature was more than 50-fold higher than those residing in the interstitium. These data indicate that patrolling monocytes preferentially occupy the vasculature and that their response to metastatic cancer cells makes them ideal sensors for early cancer invasion into the lung.
Moreover, Ly6C− nonclassical monocytes that are able to migrate out of the vasculature and into the lung tissue in the K-KO mice showed a significant reduction in their ability to ingest tumor cells/particles in a DsRed:K-KO bone marrow transfer (BMT) mouse model (Fig. 2G). However, uptake of labeled tumor particles by Ly6C+ classical monocytes was not significantly affected by the Kindlin-3 deletion, indicating the importance of the patrolling function mediated by Kindlin-3 in nonclassical monocytes. Thus, Kindlin-3-mediated patrolling by nonclassical monocytes is essential for the anti-metastatic functions of nonclassical monocytes.
Kindlin-3 is required for LFA-1 utilization by nonclassical monocytes for firm adhesion and cell-cell interactions with invading metastases.
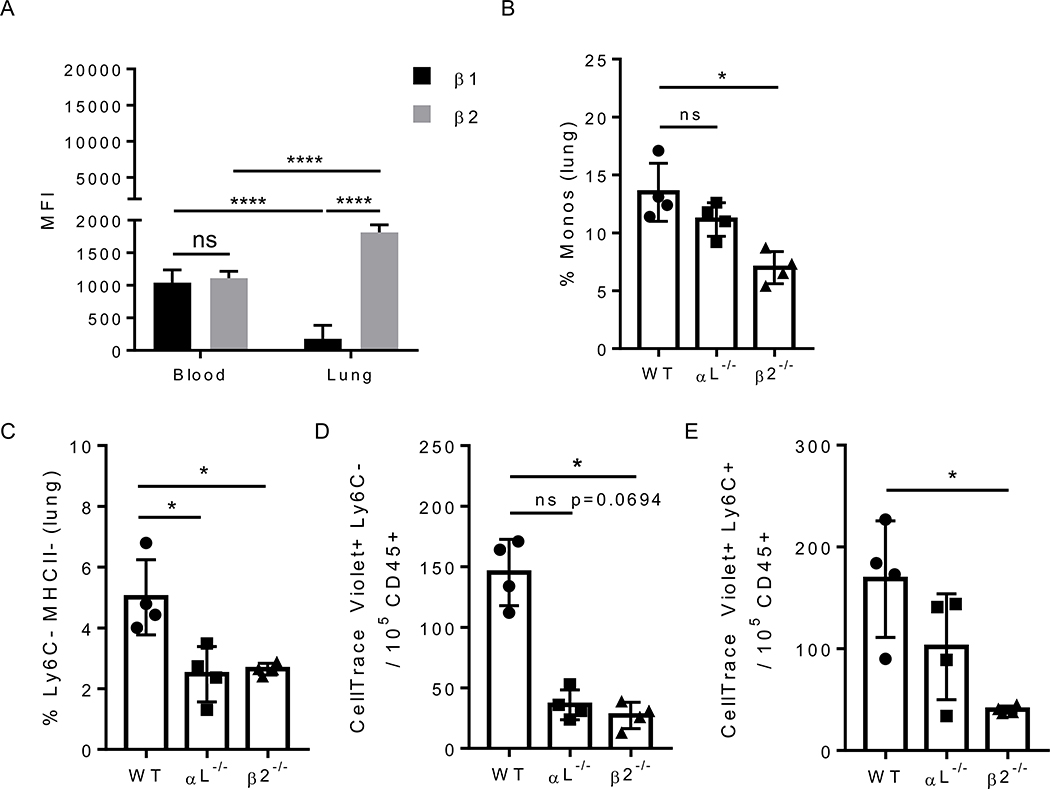
Kindlin-3 binds the cytoplasmic tails of both β1 and β2 integrins in leukocytes [14]. Nonclassical monocytes use the integrin LFA-1 (composed of subunits αL and β2) to patrol the vasculature [8] and preferentially express β2 integrins within the lung microenvironment (Fig. 3A). Therefore, we asked whether or not the loss of either of the two LFA-1 subunits phenocopies the loss of patrolling seen in Fermt3−/− nonclassical monocytes during metastasis. First, we found reduced frequencies of monocytes in the lungs of mice transplanted with either αL−/− or β2−/− bone marrow compared to mice transplanted with WT marrow (Fig. 3B). Specifically, Ly6C− monocytes deficient in either αL or β2 were reduced 2-fold within the lungs compared to WT controls (Fig. 3C). Second, we found a significant and dramatic reduction in tumor cell uptake by lung nonclassical monocytes that were lacking either integrin subunit of LFA-1 (Fig. 3D). In comparison, classical monocytes displayed a significant decrease in cancer uptake only when β2 was deficient (Fig. 3E), confirming that LFA-1 signaling is crucial for nonclassical monocytes to patrol towards, and interact with, invading tumor cells in the lung vasculature.
Figure 3. Patrolling monocytes increase their expression of LFA-1 on their surface in the lung environment and require integrins for sensing tumor cells.
A) MFI of beta integrins ß1 and ß2) present on Ly6C- monocytes from blood and lung in healthy WT mice by flow cytometry. n = 4 mice per group. B) Frequency of CD115+ monocytes in the lungs of E2:integrin KO BMT and C) the specific frequency of Ly6C− monocytes in the lungs of E2:integrin KO BMT mice. n = 4 mice per group. Numbers of Ly6C− D) or Ly6C+ E) monocytes that are positive for B16F10 cell particles (CellTrace Violet-labeled) 16 hours after i.v. injection in E2:integrin KO BMT mice, n = 4 mice per group. Multiple t tests with Holm-Sidak method for grouped analysis, Kruskal-Wallis nonparametric test for multiple comparisons. ns = not significant, *p<0.05.
Mice lacking patrolling monocytes show endothelial cell dysfunction in the lungs.
We next wished to examine the impact of lack of Ly6C− nonclassical monocyte patrolling on vascular endothelial homeostasis. We hypothesized that when nonclassical monocytes fail to patrol, endothelial homeostasis and responses to metastatic cancer cell seeding would be disturbed. To accomplish this, we used our E2-enhancer deficient (E2) mouse, which specifically depletes the nonclassical monocyte population through deletion of a regulatory 1500 bp enhancer region within the Nr4a1 gene that controls nonclassical monocyte development [23]. Since this mouse model maintains all immune cells except for nonclassical monocytes, we filled this void by mixing bone marrow from K-KO (Fermt3flox/flox;Mx1-Cre+/−) or WT (Fermt3flox/flox;Mx1-Cre−/−) donors. Thus, half of the mixed chimera bone marrow donated by the E2-deficient mouse would possess all normal immune cells except for nonclassical monocytes, while the other half would fill the niche with either Kindlin-3-deficient nonclassical monocytes or WT nonclassical monocytes (Fig. 4A). Recipient mice are wild-type C57BL/6J with normal vascular endothelium. Such a mouse model allows us to selectively examine the impacts of Kindlin-3 deletion in nonclassical monocytes on endothelial homeostasis.
Figure 4. Dysregulation of endothelial cell mRNA transcripts when nonclassical monocytes fail to patrol.
A) Scheme of bone marrow transplantation for integrin knockout chimeras with E2 bone marrow donors at a 50:50 ratio. B-M) Endothelial cells were digested out of lungs harvested from E2:WT and E2:K-KO BMT mice, with and without i.v. injection of 5×105 B16F10 cells, and endothelial cells were then sorted and purified for mRNA. B-M) qPCR for inflammatory cytokines, integrin ligands, and other signaling cytokines were run in duplicate with Hprt used as a normalization control. n = 3–4 mice per group. N) Heat map of transcript signatures for each group of mixed BMT mice with and without B16F10 cancer cells injected. Each gene expression value was rescaled to reflect the range of expression for each individual gene. Blue = Homeostasis, Pink = Metastasis. ns = not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
After allowing these mice to fully reconstitute, we FAC-sorted CD45−CD31+ endothelial cells (EC) from lungs of recipient mice possessing either E2:WT bone marrow (control) or E2:K-KO bone marrow (Kindlin-3-deficient) (Sup Fig. 3). We measured mRNA transcript levels of cytokines and adhesion molecules present in the endothelial cells by qPCR during both homeostasis and metastasis of i.v. injected B16F10 cancer cells (Fig. 4B–G; Blue and Pink panels, respectively). Interestingly, IL-1β, IL-6, CX3CL1, ICAM-1, ICAM-2, and VCAM mRNA levels were all significantly increased in E2:K-KO EC compared to E2:WT EC at homeostatic conditions (Fig. 4B–G). Upregulation of adhesion molecules and inflammatory cytokines are hallmarks of EC activation and inflammation [24]. Thus, in E2:K-KO mice, nonclassical monocytes were unable to patrol the endothelium, and consequently, these mice presented with inflamed lung endothelium at homeostasis.
ECs express transcripts involved in immune cell activation, proliferation, and recruitment [25–27]. We therefore hypothesized that ECs in mice with defective nonclassical monocytes would exhibit dysfunctional responses to tumor cell metastasis. To address this question, we first measured the relative abundance of several cytokine and receptor transcripts involved in immune cell recruitment and inflammation by qPCR (Fig. 4H–M). M-CSF, GM-CSF, and IL-15 were significantly decreased in E2:K-KO mouse lung ECs in metastasis, compared to E2:WT ECs, while CXCL1 was significantly increased. IL-6 and VCAM were also down-regulated in E2:K-KO ECs in response to tumor cell accumulation. TNF-α and TGFβR were upregulated in mice treated with B16F10 cells, regardless of the patrolling ability of nonclassical monocytes.
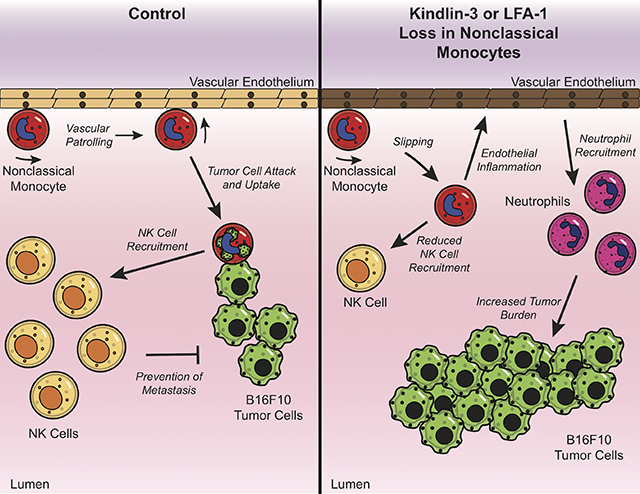
Overall, these data indicate that nonclassical monocytes prime the lungs to produce robust anti-tumoral responses, based on their ability to make cell-to-cell contacts with ECs through Kindlin-3-mediated integrin adhesion (Fig. 4N).
Increased metastasis in absence of patrolling activity of nonclassical monocytes.
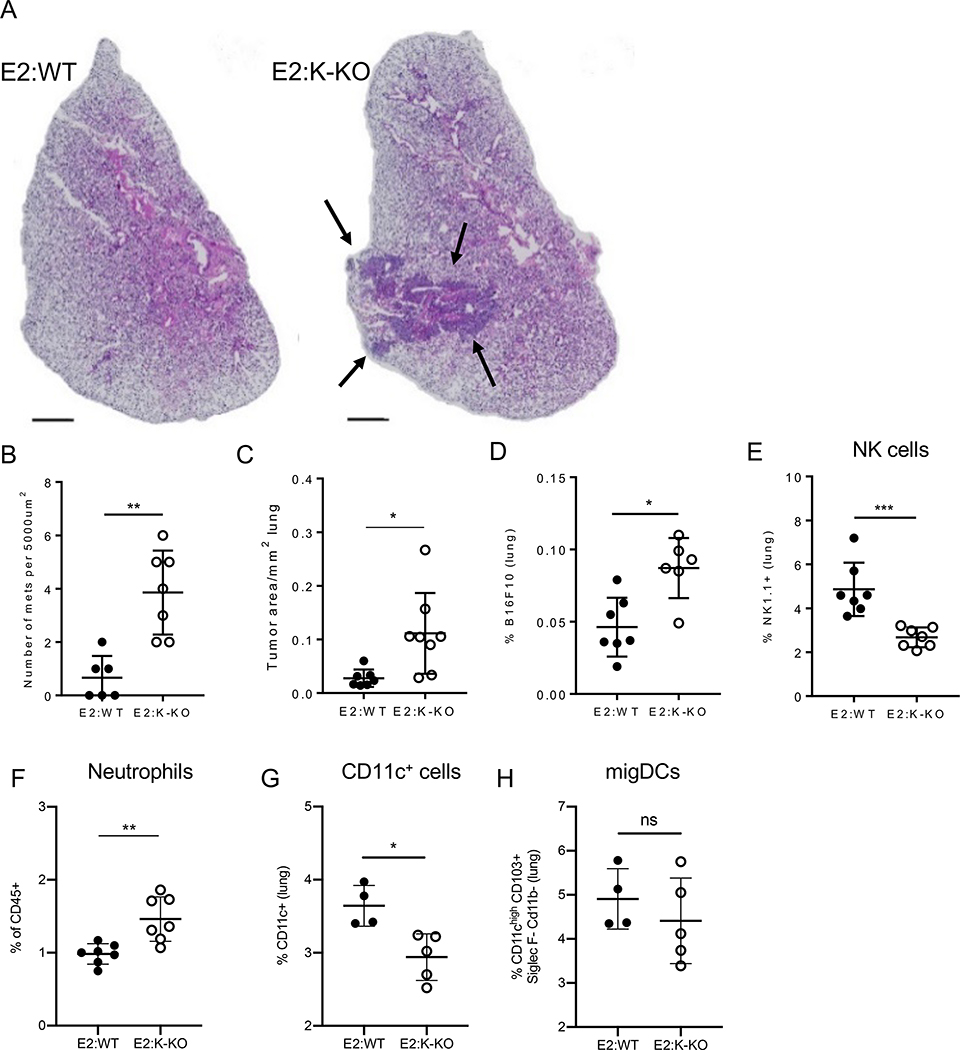
In Nr4a1- and E2-deficient mice, both of which lack nonclassical monocytes, lung metastases grow faster, with greater numbers of metastatic sites [7, 23]. Thus, nonclassical monocytes play key roles in the clearance of newly seeded cancer cells in the lung microvasculature. However, whether the patrolling activity of nonclassical monocytes is indispensable for controlling tumor metastasis is unclear. To investigate the consequences of having nonclassical monocytes present in normal numbers but functionally unable to patrol in vivo, we returned to the E2:Kindlin-3 haploinsufficient BMT model (introduced above in Fig. 2). We injected 5 × 105 B16F10 cells to mice and quantified the number of resulting metastatic sites by hematoxylin and eosin staining 14 days later (Fig. 5A). Mice with WT nonclassical monocytes developed fewer numbers of metastases, compared to mice reconstituted with K-KO nonclassical monocytes. The area of metastases per mm2 of lung was 4–5-fold higher in E2:K-KO mice (Fig. 5B–C). The frequency of B16F10-RFP cells was significantly increased in E2:K-KO compared to E2:WT mouse lungs as early as 2 days post injection (Fig. 5D).
Figure 5. Metastatic cancer is better controlled when nonclassical monocytes are able to patrol.
A) Hematoxylin and eosin (H&E) stained lungs were prepared from E2:WT and E2:K-KO mice i.v. injected with 5×105 B16F10 cells 2 weeks before harvesting. Images represent one biological triplicate. Scale bar 1000 μM. Error bars = 95 % CI. B) The area of total tumor mass over total lung area for each H&E slide analyzed. C) Number of B16F10 metastases quantified per section of H&E slide analyzed. Each point is an individual section from a total of 3 biological replicates. D) E2:WT and E2:K-KO BMT mice were sacrificed 16 hours after B16F10-RFP injection and the frequency of B16F10-RFP cells out of all lung cells was measured between E2:WT and E2:K-KO BMT mice. n = 6–7 mice per group. E) frequency of NK1.1+ cells in lungs of E2:WT compared to E2:K-KO mice. n = 6–7 mice per group. F) frequency of Ly6G+ neutrophils in the vasculature of lungs of E2:WT compared to E2:K-KO mice. n = 6–7 mice per group. G) Frequency of CD11c+ cells in lungs of E2:WT compared to E2:K-KO mice. n = 6–7 mice per group. H) Frequency of migratory dendritic cells (DCs) in lungs of E2:WT versus E2:K-KO mice. Kolmogorov-Smirnov nonparametric test for single comparisons. ns = not significant, *p<0.05, **p<0.01, ***p<0.001.
We have previously reported that nonclassical monocytes recruit NK cells to sites of early lung metastasis [7, 28]. We thus examined the abundance of NK cells recruited to the lungs during metastasis in mice that had impaired nonclassical monocyte patrolling. We found that NK cells were significantly decreased in E2:K-KO mouse lungs (Fig. 5E), as earlier predicted by the decrease of IL-15 transcripts in E2:K-KO endothelial cells (Fig. 4K). Neutrophil frequencies increased (Fig. 5F) in the absence of functional nonclassical monocyte patrolling, which correlate with the increased CXCL1/IL-8 mRNA expression in E2:K-KO endothelial cells (Fig. 4J). These cellular compositional changes suggest the presence of a more pro-metastatic environment in the lung in the absence of functionally patrolling nonclassical monocytes. CD11c+ cell frequency in the lung significantly decreased in the haploinsufficient BMT, suggesting that patrolling monocytes are required either for their recruitment or proliferation during metastasis (Fig. 5G). This change in frequency may be due to lower levels of M-CSF and GM-CSF transcripts generated by the endothelium in the absence of interaction with patrolling monocytes (Fig. 4H and I, respectively). The frequency of migratory dendritic cells in the lungs during metastasis did not change in the presence of Kindlin-3 sufficient or haploinsufficient nonclassical monocytes (Fig. 5H). Overall, our data show that nonclassical monocytes affect the lung microenvironment, both during homeostasis and metastasis, by influencing endothelial cell responses and innate cell recruitment and proliferation.
DISCUSSION
We previously reported that nonclassical monocytes play important roles in the recognition of metastasizing cancer cells in the vasculature. Here we find that Kindlin-3 is essential for the patrolling and anti-metastatic functions of nonclassical monocytes in the lung vasculature. As there is currently no mouse model for examining gene knockouts specifically in nonclassical monocytes, we utilized a mixed chimeric BMT mouse model to determine the consequences of Kindlin-3 loss selectively in nonclassical monocytes. Using this model, we show that lung-resident nonclassical monocytes with loss of Kindlin-3-mediated patrolling ingested fewer cancer particles than their WT counterparts. Moreover, the lack of functionally patrolling nonclassical monocytes was associated with impairment of lung endothelial homeostasis, reduced anti-tumoral NK cell recruitment, and increased numbers of neutrophils in tumor-bearing lungs. As such, mice with Kindlin-3-deficient monocytes developed an increased number of tumor metastases. These findings indicate that nonclassical monocyte patrolling has a direct, critical impact on the generation of anti-tumoral responses in the lungs.
Geissmann and colleagues have previously reported that nonclassical monocytes employ LFA-1 to patrol [8], but the requirement for firm adhesion to mediate patrolling was not identified. Thus, we show that firm adhesion by nonclassical monocytes via Kindlin-3 and LFA-1 is required to initiate their patrolling, and we designate the inability of these cells to patrol due to a lack of firm adhesion as “slipping.” We uncovered pronounced changes in endothelial cell (EC)-based transcription of cytokines and chemokines that directly affected the recruitment and differentiation of myeloid and lymphoid cells in the lungs. We also determined that patrolling by nonclassical monocytes maintains vascular endothelial homeostasis. Indeed, our observation of endothelial dysfunction, characterized by increased cytokine production by ECs, in the absence of nonclassical monocyte patrolling reinforces the notion that nonclassical monocytes communicate with the endothelium via cell-to-cell contact.
We observed reduced M-CSF and GM-CSF production by endothelial cells during tumor metastasis. However, the overall abundance of nonclassical monocytes in the lungs remained comparable between homeostatic and metastatic conditions. As nonclassical monocytes quickly respond to invading tumor cells [6, 7], we anticipate that altered expression of these colony-stimulating factors is likely a downstream consequence of direct interactions between nonclassical monocytes and endothelial cells.
VCAM, a ligand for VLA-4 integrin, has been shown to play several distinct roles in cancer progression. For example, while VCAM is implicated as a mode of entry for circulating tumor cells into metastatic sites [29], VCAM also functions as an adhesion molecule on endothelial cells that allows anti-tumoral effector CD8 T cells to enter tumors [30]. Therefore, regulation of VCAM is an important factor in determining metastatic outcomes. Why VCAM-1 expression is lower in E2:K-KO endothelial cells during metastasis is unclear, but may relate to dysregulated endothelial cell homeostasis, as suggested by the data herein.
Despite CXCL1 being elevated 2-fold in E2:K-KO mice compared to wild-type mice during metastasis, we did not observe a corresponding increase in neutrophil frequencies or numbers. However, neutrophil recruitment typically occurs a few hours after B16F10 cell injection [5, 6], whereas our study took place 16 hours post-injection. Additionally, whether patrolling-deficient nonclassical monocytes can drive anti-tumoral immune cell recruitment via chemokine secretion remains to be determined. Future work is needed to understand how interactions between patrolling monocytes, tumor cells, and other immune cells within the metastatic niche, drive an anti-tumoral response during early-stage metastasis.
In summary, our work shows that cooperation between Kindlin-3 and LFA-1 is required for patrolling by nonclassical monocytes. We also find that nonclassical monocyte patrolling is essential for the anti-metastatic functions of these cells as well as their involvement in vascular homeostasis. Thus, nonclassical monocytes and their patrolling functions may represent new targets for anti-tumor therapies directed at tumor metastasis.
METHODS
Mice and Reagents
Fermt3fllox/flox mice were kindly gifted to us by the laboratory of Dr. Klaus Ley at La Jolla Institute for Immunology (LJI). C57Bl/6J, E2-deficient, CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre+/− and CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre−/− mice were bred in-house. CD45.1 (002014) and DsRed*MST (006051) mice were purchased from the Jackson Laboratory. B16F10-RFP (AntiCancer Incorporated) and B16F10 cells (ATCC CRL-6475) were cultured according to ATCC protocols. Poly(I:C) (Sigma P1530) was used to induce Cre expression in Mx1-Cre+/− mice by injecting 200 uL i.p. every other day for 5 days. B16F10 cells were labeled with CellTrace Violet (Thermo Fisher C34571) according to the manufacturer’s instructions. B16F10 injections were performed retro-orbitally for overnight (16 hour) experiments, or by tail-vein for longer experiments. All injections were 0.4 μM filtered and resuspended in 100 μL sterile PBS.
Bone marrow transplants
Bone marrow donors were sacrificed and bones were separated from marrow by sterile centrifugation (8000 x g 30 seconds). Cells were resuspended in sterile PBS, washed and filtered twice, and counted with a BD Coulter Counter. CD45.1 recipients were irradiated with 900 rads and rehoused in sterile cages with sterile food, a gel cup and water (supplemented with Equisol 0.5mL/8oz). Mice were anesthetized with isoflurane immediately after irradiation and retro-orbitally injected with 107 bone marrow cells from mixed bone marrow donors (50:50 ratio).
Flow Cytometry
Blood was obtained either by retro-orbital bleeds or by cardiac puncture. Retro-orbital bleeds were carried out under anesthesia using micro-hematocrit capillary tubes (Fisherbrand 22–362-566) and 2 mM EDTA (final concentration) to prevent clotting. Cardiac punctures were performed post-mortem using 25 G needles loaded with 2 mM EDTA. Blood samples were first lysed of red blood cells with 1x lysis buffer (0.15 M NH4Cl, 10 mM NaHCO3, 1.1mM EDTA-disodium in sterile H20) for 10 minutes at 4°C on a rotator. Lungs were first weighed (total lungs and left lobe), then either mechanically dissociated (left lobe) with a 70 μM cell strainer and syringe plunger, or diced and enzymatically digested (remainder of lung lobes) in 1 mL of Liberase TM and DNaseI in HBSS at 37°C shaker for 30 minutes in a microtube shaker (all Thermo Fisher). Bone marrow was centrifuged out of tibias (1 per mouse) and RBC lysed for 5 minutes at room temperature. All samples were resuspended in FACS buffer (1x PBS, 1 % BSA, 2 mM EDTA) with 1:200 Fc blocker (Biolegend; 93) and stained with antibody cocktails for 10 minutes on ice. CXCR4 (Biolegend; L276F12) staining was done at 37°C for 15 minutes before adding the remaining antibodies and incubating on ice. For labeling of vascular CD45+ cells, 5uL of anti-mouse CD45 BV650 (Biolegend, clone 30-F11) was diluted into 50uL sterile PBS (room temp) and injected into each mouse 1 minute before sacrificing the animal. Lungs were removed without perfusion and immediately put on ice in cold PBS for further processing. For these samples, an additional stain of anti-mouse CD45 APC (Biolegend, clone 30-F11) was used to label all CD45+ cells, including interstitial immune cells that would not have been exposed to the vascular stain. The following antibodies were used for flow cytometry: CD45 (30-F11), CD115 (AFS98), CD11b (M1/70), Ly6G (1A8), Ly6C (HK1.4), CD3 (17A2), CD19 (6D5), B220 (RA3–6B2), NK1.1 (PK136), MHCII (M5/114.15.2), CD11c (3.9), F4/80 (BM8), CD54 (YN1/1.7.4), CD11a (I21/7), CD18 (1B4/CD18), LFA-1 (H155–78), CD62L (MEL-14), CD117 (2B8), all from Biolegend; Live/Dead (Tonbo; 13–0870). FACS experiments were performed on a LSRII (BD Biosciences) and analyzed with FlowJo software (TreeStar version 10.7).
FACS Sorting
Sorting was performed by the LJI Flow Cytometry Core Facility on a FACSAria (BD Biosciences). Fc block was added before all staining and primary conjugated antibodies were incubated with cells in FACS buffer (PBS, 1 % BSA, 2 mM EDTA, 0.05 % NaN3) for 15 minutes covered at 4°C.
Intravital Imaging
All intravital imaging was performed on a Leica SP8 confocal microscope with a 25× 0.95 NA objective using LAF X software. Mice were surgically prepared as previously described for imaging of the femoral vasculature [31]. Antibody labeling of Ly6C (HK1.4; Biolegend) and CD31 (390; Biolegend) was done by injecting 2–5 μg of each antibody 5 minutes prior to imaging the animal. For stimulation of monocyte patrolling, 500 ng of recombinant mouse TNF-α in PBS was injected i.p. 1 hour before imaging the animal. Lung imaging was prepared as described previously [6, 7, 32]. Antibodies to label CD31 (Biolegend; 390), Gr-1 (Biolegend; RB68C5) or Ly6C (Biolegend; HK1.4) were injected as described above. B16F10-RFP cells were injected retro-orbitally. Mice were imaged for approximately 1 hour after surgery. Timelapse images were analyzed using Imaris (Bitplane) 9.1 software. Images were drift-corrected and GFP+Gr-1− (or Ly6C−) monocytes were tracked using spot selection. For binning path types: monocytes that moved at least 5 μm (track displacement) in the lung tissue or endothelium for more than 60 seconds (track duration) were counted as patrolling; monocytes that stopped momentarily (1–2 frames at a time) were counted as slipping; monocytes that stopped on the endothelium for more than 2 frames, regardless of whether they remained or entered the circulation again were counted as arrested.
Hematoxylin and eosin staining of lung sections
Histology services were provided by the LJI Histology Core Facility. 5 μm sections were deparaffinized with ProPar and rehydrated in a series of graded alcohols, stained with hematoxylin and eosin using a regressive staining protocol, dehydrated, and embedded with #1.5 coverglass (Fisherbrand). Sections were imaged on an AxioScan Z1 automated slide scanner (Zeiss) with 20× 0.8 NA objective.
Quantitative RT-PCR (qPCR)
E2:Kindlin-3 BMT mice were injected with 3×105 B16F10-RFP cells approximately 16 hours prior to harvesting. Lungs were perfused with 10 mL of PBS with 2 mM EDTA. Lungs were diced and enzymatically digested as described in Flow Cytometry. Cells were stained with Live/Dead (Tonbo; Ghost Dye Violet 510), CD31 (Biolegend; 390), and CD45 (Biolegend; 30-F11). Endothelial cells were identified as Live/Dead−CD45−CD31+ and sorted into PBS with 2% FBS. Cells were resuspended in Trizol (Thermo Fisher 15596026) to obtain purified RNA using Zymo Research Direct-zol kit (R2052). cDNA was made using iScript Reverse Transcription Supermix (Bio-Rad 1708840) from equal amounts of RNA input and 20 μL reaction volumes were run on a Roche 480 Lightcycler for 50 cycles. Samples from 3–4 mice per group were run in duplicate. The following Taqman probes from Thermo Fisher were used to quantify gene expression: HPRT (Mm03024075_m1), Il-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), CXCL1 (Mm04207460_m1), IL-15 (Mm00434210_m1), CX3CL1 (Mm00436454_m1), TNF-α (Mm00443258_m1), VEGFR (Mm00438980_m1), ICAM-1 (Mm00516023_m1), ICAM-2 (Mm00494862_m1), VCAM (Mm01320970_m1), M-CSF (Mm00432686_m1), GM-CSF (Mm01290062_m1). Ct values were averaged per sample and gene expression was normalized to HPRT expression using delta delta Ct calculations.
Statistics
Monocyte frequencies and numbers were compared between different conditions using a Kolmogorov-Smirnov test without assuming consistent SD. Grouped analysis (monocyte subsets and path types) were done by using multiple t-tests with Holm-Sidak method for multiple comparisons. B16F10 uptake by monocyte subsets was analyzed with a Kruskal-Wallis test. qRT-PCR experiments were analyzed by two-way ANOVA (WT, WT+B16, K-KO, K-KO+B16) or Kolmogorov-Smirnov tests without assuming consistent SD.
Supplementary Material
Supplemental Figure 1. Fermt3 is excised after polyI:C injection. Cohort mice (same age/gender/parents) were intraperitoneally (i.p.) injected with 200 ug polyI:C three times (every other day for 5 days). A) After 2 weeks mice were sacrificed and blood was harvested and magnetically sorted for monocytes. qPCR for Fermt3 expression was performed using Taqman probes.
Supplemental Figure 2. CX3CR1 GFP expressing cells do not change significantly within lung tissue or field of view during imaging. Lungs from CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre−/− (WT) and CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre+/− (K-KO) mice were digested for flow cytometry analyses at 16 hours after B16F10 melanoma cell injection. A) FACS plot representative of n = 5 mice. B) All GFP expressing cells with the entire FOV from each time lapse video was counted and plotted per video across all mice. n = 3 mice per group. Kolmogorov-Smirnov nonparametric test. ns = not significant.
Supplemental Figure 3. Sorted endothelial cells from digested lungs. A) Lungs were digested from E2:WT and E2:K-KO mice and sorted based on live CD31+ endothelial cells. n = 3–4 mice per group.
ACKNOWLEDGEMENTS
We thank Angela Denn at the LJI Histology Core Facility for expert help with the H&E sectioning and staining. All flow cytometry cell sorting was done by the LJI Flow Cytometry Core. Special thanks to Denise, Robin, Chris and Lara. Help with statistics and heat maps was provided by Alex Buckley. For helpful discussions regarding microscopy, we thank Zbigniew Mikulski in the LJI Imaging Core. We thank Deborah Yoakum for help with mouse breeding. This study was supported by NIH F31 HL132538-03 (to P.M.M.) and NIH P01HL136275, R01 HL134236 and R01 CA202987 (all to C.C.H.). The FACS Aria III Cell Sorter was acquired through the NIH Shared Instrumentation Grant Program S10 RR027366-01A1 (to LJI).
Footnotes
CONFLICTS OF INTEREST DISCLOSURE
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Steeg PS (2016) Targeting metastasis. Nature reviews 16, 201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J (2009) Metastasis: from dissemination to organ-specific colonization. Nature reviews 9, 274–84. [DOI] [PubMed] [Google Scholar]
- 3.Weidle UH, Birzele F, Kollmorgen G, Ruger R (2016) Molecular Basis of Lung Tropism of Metastasis. Cancer Genomics Proteomics 13, 129–39. [PubMed] [Google Scholar]
- 4.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, Hovav AH, Henke E, Fridlender ZG, Granot Z (2015) Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 10, 562–73. [DOI] [PubMed] [Google Scholar]
- 5.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, Krummel MF (2016) Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 531, 513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC (2015) Patrolling monocytes control tumor metastasis to the lung. Science 350, 985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–70. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 10.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F (2013) Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33, 375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakkert BC, Kuijpers TW, Leeuwenberg JF, van Mourik JA, Roos D (1991) Neutrophil and monocyte adherence to and migration across monolayers of cytokine-activated endothelial cells: the contribution of CD18, ELAM-1, and VLA-4. Blood 78, 2721–6. [PubMed] [Google Scholar]
- 13.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH (2010) LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol 185, 7057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, Wang HV, Sperandio M, Fassler R (2009) Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med 15, 300–5. [DOI] [PubMed] [Google Scholar]
- 15.Mory A, Feigelson SW, Yarali N, Kilic SS, Bayhan GI, Gershoni-Baruch R, Etzioni A, Alon R (2008) Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Blood 112, 2591. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn R, Schwenk F, Aguet M, Rajewsky K (1995) Inducible gene targeting in mice. Science 269, 1427–9. [DOI] [PubMed] [Google Scholar]
- 17.Malinin NL, Plow EF, Byzova TV (2010) Kindlins in FERM adhesion. Blood 115, 4011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20, 4106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA (2011) T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208, 1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, Moser M, Metin A, Fried M, Tomlinson I, Hogg N (2009) Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med 15, 306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretti FA, Moser M, Lyck R, Abadier M, Ruppert R, Engelhardt B, Fassler R (2013) Kindlin-3 regulates integrin activation and adhesion reinforcement of effector T cells. Proc Natl Acad Sci U S A 110, 17005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manevich-Mendelson E, Feigelson SW, Pasvolsky R, Aker M, Grabovsky V, Shulman Z, Kilic SS, Rosenthal-Allieri MA, Ben-Dor S, Mory A, Bernard A, Moser M, Etzioni A, Alon R (2009) Loss of Kindlin-3 in LAD-III eliminates LFA-1 but not VLA-4 adhesiveness developed under shear flow conditions. Blood 114, 2344–53. [DOI] [PubMed] [Google Scholar]
- 23.Thomas GD, Hanna RN, Vasudevan NT, Hamers AA, Romanoski CE, McArdle S, Ross KD, Blatchley A, Yoakum D, Hamilton BA, Mikulski Z, Jain MK, Glass CK, Hedrick CC (2016) Deleting an Nr4a1 Super-Enhancer Subdomain Ablates Ly6C(low) Monocytes while Preserving Macrophage Gene Function. Immunity 45, 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S (2003) New markers of inflammation and endothelial cell activation: Part I. Circulation 108, 1917–23. [DOI] [PubMed] [Google Scholar]
- 25.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA (1994) Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 180, 1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berard M, Brandt K, Bulfone-Paus S, Tough DF (2003) IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol 170, 5018–26. [DOI] [PubMed] [Google Scholar]
- 27.Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, Huttenlocher A (2017) Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Rep 19, 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narasimhan PB, Eggert T, Zhu YP, Marcovecchio P, Meyer MA, Wu R, Hedrick CC (2020) Patrolling Monocytes Control NK Cell Expression of Activating and Stimulatory Receptors to Curtail Lung Metastases. J Immunol 204, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng VWT, Soto MS, Khrapitchev AA, Perez-Balderas F, Zakaria R, Jenkinson MD, Middleton MR, Sibson NR (2019) VCAM-1-targeted MRI Enables Detection of Brain Micrometastases from Different Primary Tumors. Clin Cancer Res 25, 533–543. [DOI] [PubMed] [Google Scholar]
- 30.Woods AN, Wilson AL, Srivinisan N, Zeng J, Dutta AB, Peske JD, Tewalt EF, Gregg RK, Ferguson AR, Engelhard VH (2017) Differential Expression of Homing Receptor Ligands on Tumor-Associated Vasculature that Control CD8 Effector T-cell Entry. Cancer Immunol Res 5, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcovecchio PM, Thomas GD, Mikulski Z, Ehinger E, Mueller KAL, Blatchley A, Wu R, Miller YI, Nguyen AT, Taylor AM, McNamara CA, Ley K, Hedrick CC (2017) Scavenger Receptor CD36 Directs Nonclassical Monocyte Patrolling Along the Endothelium During Early Atherogenesis. Arterioscler Thromb Vasc Biol 37, 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF (2011) Stabilized imaging of immune surveillance in the mouse lung. Nat Methods 8, 91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Fermt3 is excised after polyI:C injection. Cohort mice (same age/gender/parents) were intraperitoneally (i.p.) injected with 200 ug polyI:C three times (every other day for 5 days). A) After 2 weeks mice were sacrificed and blood was harvested and magnetically sorted for monocytes. qPCR for Fermt3 expression was performed using Taqman probes.
Supplemental Figure 2. CX3CR1 GFP expressing cells do not change significantly within lung tissue or field of view during imaging. Lungs from CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre−/− (WT) and CX3CR1gfp/+Fermt3fllox/flox;Mx1-Cre+/− (K-KO) mice were digested for flow cytometry analyses at 16 hours after B16F10 melanoma cell injection. A) FACS plot representative of n = 5 mice. B) All GFP expressing cells with the entire FOV from each time lapse video was counted and plotted per video across all mice. n = 3 mice per group. Kolmogorov-Smirnov nonparametric test. ns = not significant.
Supplemental Figure 3. Sorted endothelial cells from digested lungs. A) Lungs were digested from E2:WT and E2:K-KO mice and sorted based on live CD31+ endothelial cells. n = 3–4 mice per group.