Abstract
Objective
To report the 2‐year efficacy and safety of tocilizumab (TCZ) in patients with polyarticular‐course juvenile idiopathic arthritis (JIA).
Methods
Patients ages 2–17 years with active polyarticular‐course JIA, in whom treatment with methotrexate was unsuccessful, received 16 weeks of open‐label intravenous TCZ in part 1 (once every 4 weeks: 8 mg/kg or 10 mg/kg for body weight [BW] <30 kg; 8 mg/kg for BW ≥30 kg). Assessments were based on the JIA–American College of Rheumatology (ACR) response (defined as percentage of improvement in ≥3 of the 6 JIA core response variables [CRVs]). Patients with at least a JIA‐ACR30 response (defined as ≥30% improvement in ≥3 of the 6 JIA CRVs without worsening in >1 of the remaining JIA CRVs by >30%) at week 16 were randomly assigned (1:1) to receive TCZ or placebo in part 2. Patients remained in part 2 until either week 40 or the occurrence of JIA flare. Upon starting part 3, all patients received open‐label TCZ. At week 104 of the study, efficacy was assessed using JIA‐ACR50/70/90 response rates (defined as 50%, 70%, or 90% improvement, respectively), achievement of inactive disease, and the Juvenile Arthritis Disease Activity Score in 71 joints (JADAS‐71). Safety was assessed in the all‐exposure population per 100 patient‐years of exposure.
Results
Overall, 188 patients entered part 1, 166 patients entered part 2, and 160 patients entered part 3. By week 104, among the 188 patients in the modified intent‐to‐treat group who received TCZ, JIA‐ACR50/70/90 response rates were 80.3%/77.1%/59.6%, respectively, the median JADAS‐71 score decreased from 3.6 at week 40 to 0.7 at week 104, 51.1% of patients had achieved inactive disease, and 31 of 66 patients who had been receiving glucocorticoids discontinued them. Adverse event (AE) and serious AE rates were 406.5 per 100 patient‐years and 11.1 per 100 patient‐years, respectively. The infection rate was 151.4 per 100 patient‐years, and the serious infection rate was 5.2 per 100 patient‐years.
Conclusion
Patients treated with TCZ for polyarticular‐course JIA showed high‐level disease control for up to 2 years. The TCZ safety profile was consistent with that previously reported.
INTRODUCTION
Juvenile idiopathic arthritis (JIA) describes a group of chronic arthritides of unknown etiology that begin before the age of 16 years and persist for longer than 6 weeks (1). Children with rheumatoid factor (RF)–positive polyarticular JIA, RF‐negative polyarticular JIA, or extended oligoarticular JIA can be referred to as having polyarticular‐course JIA (2); these patients are at risk of progressive joint damage, functional disability, and growth impairment (3, 4). Indeed, up to 30% of patients with polyarticular‐course JIA continue to experience active arthritis despite the use of disease‐modifying antirheumatic drugs, such as methotrexate (MTX), and/or biologic therapies, such as anti–tumor necrosis factor agents and CTLA‐4 blockade (5).
Serum levels of the proinflammatory cytokine interleukin‐6 (IL‐6) are often elevated in polyarticular‐course JIA and are associated with the extent and severity of active arthritis and with markers of inflammation, such as C‐reactive protein and erythrocyte sedimentation rate (ESR) (6). A randomized, double‐blind, placebo‐controlled, phase III withdrawal trial of the IL‐6 receptor inhibitor tocilizumab (TCZ) in patients with polyarticular‐course JIA (CHERISH) demonstrated improvement in JIA–American College of Rheumatology (ACR) responses, and significantly more placebo‐treated patients than TCZ‐treated patients experienced JIA flare during the 24‐week, double‐blind withdrawal period. This demonstrates that treatment with TCZ for up to 40 weeks was effective for improving the signs and symptoms of polyarticular‐course JIA in children with inadequate response to MTX (7). The observed safety profile of TCZ in this trial was consistent with that of adult patients with rheumatoid arthritis (RA) (7). Based on the results of this trial, TCZ was approved for the treatment of polyarticular‐course JIA in patients ≥2 years of age as an intravenous (IV) infusion once every 4 weeks at a dose of 10 mg/kg in patients with a body weight (BW) of <30 kg and 8 mg/kg in patients with a BW of ≥30 kg. TCZ as a subcutaneous injection was subsequently approved at a dose of 162 mg once every 3 weeks in patients with a BW of <30 kg and once every 2 weeks in patients with a BW of ≥30 kg, based on data from a phase Ib study with extrapolation to the efficacy and safety established in the CHERISH trial.
Here, we report the 2‐year safety and efficacy of TCZ in patients with polyarticular‐course JIA who participated in the CHERISH trial.
PATIENTS AND METHODS
Study design
CHERISH (ClinicalTrials.gov identifier: NCT00988221) (7) was a 3‐part, 104‐week, phase III study designed to investigate the efficacy and safety of IV TCZ in patients with polyarticular‐course JIA treated at centers that are part of the Paediatric Rheumatology International Trials Organisation (PRINTO) network (8) or the Pediatric Rheumatology Collaborative Study Group (PRCSG) (9) network (see Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract). In part 1, patients received IV TCZ according to BW every 4 weeks for 16 weeks; patients weighing ≥30 kg received TCZ 8 mg/kg, and patients weighing <30 kg were randomly assigned 1:1 to receive TCZ at either 8 mg/kg or 10 mg/kg. At week 16, patients with at least a JIA–American College of Rheumatology 30 (ACR30) response (defined as ≥30% improvement in ≥3 of the 6 JIA core response variables (CRVs) without worsening in >1 of the remaining JIA CRVs by >30%, compared with baseline [10–13]) entered the double‐blind withdrawal period (part 2). In part 2, patients were randomly assigned 1:1 to receive placebo or to continue TCZ, as in part 1, until week 40. Patients who experienced JIA flare (defined as ≥30% worsening in 3 of the 6 JIA CRVs, without improvement in >1 of the remaining JIA CRVs by >30%, compared with week 16 [7]) were allowed to enter part 3 early and received open‐label TCZ again until week 104 of the study (see Supplementary Methods for additional details, http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract). This study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and local laws and regulations.
Figure 1.
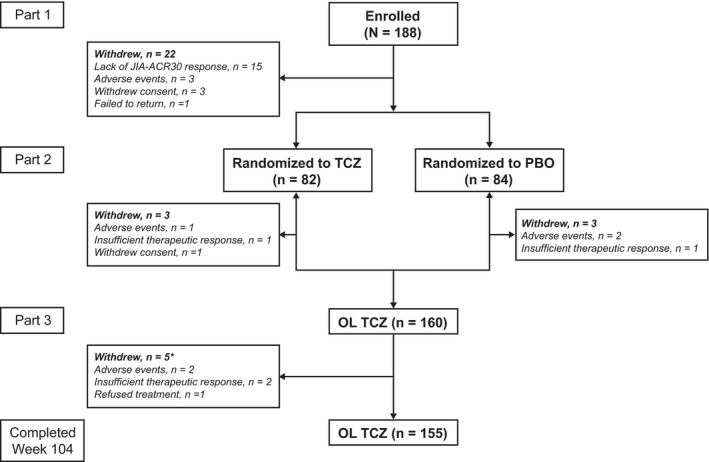
Summary of juvenile idiopathic arthritis (JIA) patient disposition in the modified intent‐to‐treat (mITT) population. The mITT population for reporting long‐term efficacy was defined as all enrolled patients who received ≥1 dose of tocilizumab (TCZ; n = 188). One patient randomly assigned to receive placebo (PBO) in part 2 withdrew while receiving TCZ 8 mg/kg in part 1; this patient was included among the patients randomly assigned to placebo who withdrew in part 2. * During part 3, 2 patients who had been randomly assigned to TCZ in part 2 withdrew due to adverse events, and 3 patients who had been randomly assigned to placebo in part 2 withdrew due to insufficient therapeutic response (n = 2) or refusal of treatment (n = 1). JIA‐ACR30 = ≥30% improvement in ≥3 of the 6 JIA core response variables defined by the American College of Rheumatology criteria; OL = open‐label.
Patients
Key patient eligibility criteria for the study have been published (7). Briefly, patients ages 2–17 years with polyarticular‐course JIA who had ≥5 joints with active arthritis, a disease duration of ≥6 months, and unsuccessful MTX treatment were eligible for participation, whereas patients with systemic JIA, persistent oligoarticular JIA, undifferentiated JIA, enthesitis‐related JIA, or psoriatic JIA were excluded. Stable concomitant doses of nonsteroidal antiinflammatory drugs, oral glucocorticoids (maximum 0.2 mg/kg/day or maximum daily dose of 10 mg, whichever was lower), and MTX (10–20 mg/m2 body surface area/week) were permitted. In part 3 of the study, patients could be considered for glucocorticoid dose reduction according to the protocol if they maintained at least a JIA‐ACR50 response (defined as ≥50% improvement in ≥3 of the 6 JIA CRVs without worsening of >1 of the remaining JIA CRVs by >30%) compared with baseline (10). Patients with inactive disease who had not received glucocorticoids for ≥6 months could be considered for MTX tapering/discontinuation per protocol. Informed consent was provided by a parent or guardian, and assent was obtained from the child in accordance with local regulations.
Assessments
Evaluation of long‐term efficacy was based on the modified intent‐to‐treat (mITT) population, defined as all patients enrolled in part 1 who received ≥1 dose of TCZ. Some long‐term efficacy end points were assessed in the long‐term extension (LTE) population, defined as all patients treated in part 1 who received blinded or open‐label TCZ during part 2 or part 3. Additional analyses were performed in patients who were randomly assigned to receive TCZ in part 2 and hence received TCZ from baseline through week 104 (TCZ continuous group). To investigate the effects of interrupting TCZ treatment, efficacy was evaluated separately in patients who were randomly assigned to the placebo group in part 2; these patients reinitiated treatment with open‐label TCZ upon receiving escape therapy in part 2 or upon entering part 3 of the study (TCZ restart group). The TCZ restart group included patients from the time they restarted TCZ treatment, either as escape therapy from the second visit in part 2 or as open‐label therapy in part 3; therefore, 84 weeks was the maximum length of TCZ treatment that could have been received at the end of the study in the TCZ restart group.
Efficacy assessment was based on JIA‐ACR response, defined as percentage improvement in ≥3 of the 6 JIA CRVs without worsening in >1 of the remaining JIA CRVs by >30%. Physician global assessment of disease activity (visual analog scale [VAS] range 0–100 mm), parent/patient global assessment of overall well‐being (VAS range 0–100 mm), number of joints with active arthritis, number of joints with limitation of movement, physical function based on Childhood Health Assessment Questionnaire Disability Index (CHAQ DI; range 0–3), and a laboratory measure of acute inflammation (ESR) were used in this trial (11). Efficacy was also assessed at weeks 40 and 104 of treatment, with consideration given to previous biologic use at baseline, concomitant MTX use, disease duration, and RF positivity. Inactive disease and remission were defined in accordance with ACR provisional criteria (12), except for allowing a physician global assessment of disease activity VAS score of ≤10 mm and an ESR of <20 mm/hour to be considered in inactive disease (13). Evaluations of JIA flare, JIA‐ACR responses, and clinically inactive disease status were performed in real time by independent masked evaluators at the coordinating centers of PRINTO and PRCSG, according to validated criteria (2, 11, 12, 13, 14). Disease activity was also assessed using the Juvenile Arthritis Disease Activity Score in 71 joints (JADAS‐71) with the ESR as a measure of inflammation, where a JADAS‐71 score of <1 reflects inactive disease, a JADAS‐71 score of <3.8 reflects low disease activity, and a JADAS‐71 score of 3.9–10.5 represents moderate disease activity (15, 16, 17).
Safety was evaluated in the all‐exposure/all‐TCZ population, which included all patients who received ≥1 dose of TCZ and had ≥1 postbaseline safety assessment or event. Rates of adverse events (AEs) and serious AEs (SAEs) were analyzed per 100 patient‐years of exposure to TCZ. AE system organ class and preferred terms were classified according to terminology for AEs found in the Medical Dictionary for Regulatory Activities, version 14.0 or 15.0. Laboratory measurements were assessed according to Common Terminology Criteria for Adverse Events, version 3.0 (18). Patients with elevations in liver enzymes were evaluated for hepatic injury (i.e., according to Hy’s law, with elevations of alanine aminotransferase [ALT] or aspartate aminotransferase [AST] >3× the upper limit of normal [ULN] and total bilirubin >2× ULN, potentially indicating serious hepatotoxicity) (19). Analysis of anti‐TCZ antibodies was performed using enzyme‐linked immunosorbent assay in serum samples collected at baseline and at selected postbaseline visits. Samples that tested positive in an anti‐TCZ antibody screening assay were further analyzed by a confirmation assay and neutralization assay (20).
Statistical analysis
For JIA‐ACR responses at the JIA‐ACR50/70/90 levels and inactive disease/clinical remission, patients who withdrew because of non–safety‐related reasons were classified as nonresponders; patients who withdrew because of safety‐related reasons had their last available response before withdrawal carried forward (last observation carried forward [LOCF]). LOCF was applied to missing core components at visits. For JADAS‐71, observed data were used, and patients who withdrew were not included in the analysis after the time of withdrawal. Changes in glucocorticoid dose were assessed in the all‐exposure/all‐TCZ population, and the numbers and proportions of patients who received glucocorticoid dose reduction or discontinued glucocorticoids were assessed as a proxy measure of efficacy in patients who were receiving oral glucocorticoids at baseline and had valid assessments at week 104. JIA‐ACR responses were also investigated in the mITT population, according to differences in previous use of biologics, concomitant use of MTX, duration of disease (duration of <2 years was considered early polyarticular‐course JIA), and RF positivity at baseline. Formal comparison of these subgroups was not planned, and therefore statistical comparisons were not performed. Change in patient‐reported outcomes from baseline included the CHAQ DI score, parent/patient global assessment of overall well‐being, and patient pain (each measured on a 100‐mm VAS).
RESULTS
Patient demographic data and disposition
Of the 188 patients enrolled in the study who received ≥1 dose of TCZ in part 1 (mITT population), 160 entered part 3, and 155 completed the study through week 104 (Figure 1). Thirty‐three patients (17.6%) withdrew from the study, 30 patients while receiving TCZ in parts 1, 2, or 3, and 3 patients while receiving placebo in part 2. Reasons for withdrawal during the entire 104‐week study period included the occurrence of AEs in 8 patients (4.3%; 6 TCZ, 2 placebo), insufficient therapeutic response in 19 patients (10.1%; 18 TCZ, 1 placebo), refusal of treatment or withdrawn consent in 5 patients (2.7%; all TCZ), and loss to follow‐up in 1 patient (0.5%; TCZ). The LTE population, which was used to assess certain efficacy end points, included only 163 patients, because 25 patients who did not receive TCZ during part 2 or 3 had no data after week 40 and were not included in this population. Three patients receiving placebo withdrew before part 3 and were excluded from the TCZ restart group; among the patients who withdrew from part 3 of the study, 2 patients were from the TCZ continuous group (both withdrew because of AEs) and 3 were from the TCZ restart group (2 withdrew because of insufficient therapeutic response and 1 refused treatment). Therefore, there were 82 patients in the TCZ continuous group and 81 patients in the TCZ restart group.
At baseline, patients had a mean disease duration of 4.2 years, high disease activity (mean JADAS‐71 score 33.5), >20 active joints, and moderately reduced physical function (Table 1). As previously reported (7), differences in baseline demographics between the BW‐based treatment groups (<30 kg TCZ 8 mg/kg, <30 kg TCZ 10 mg/kg, and ≥30 kg TCZ 8 mg/kg) were as expected. Patients with a BW of ≥30 kg were older, on average, and had longer disease duration than patients with a BW of <30 kg. At baseline, there were no meaningful differences between the treatment groups in the number of JIA‐ACR CRVs (7).
Table 1.
Baseline demographic and disease characteristics*
|
All TCZ (n = 188)† |
|
|---|---|
| Age, years | 11.0 ± 4.0 |
| Female sex, no. (%) | 144 (77) |
| White ethnicity, no. (%) | 150 (80) |
| Weight, kg | 39.6 ± 17.3 |
| Disease duration, years | 4.2 ± 3.7 |
| RF‐positive, no. (%) | 54 (29) |
| Previous DMARD use, no. (%)‡ | 134 (71) |
| Previous biologic use, no. (%) | 61 (32) |
| Active joints (range 0–71) | 20.3 ± 14.3 |
| Joints with LOM (range 0–67) | 17.6 ± 14.4 |
| PhGA VAS (range 0–100 mm) | 61.4 ± 20.7 |
| PGA VAS (range 0–100 mm) | 52.9 ± 25.0 |
| CHAQ DI score (range 0–3) | 1.4 ± 0.7 |
| ESR, mm/hour (ULN <20 mm/hour) | 34.8 ± 25.5 |
| JADAS‐71 | 33.5 ± 16.7 |
| Concomitant MTX, no. (%) | 148 (79) |
| MTX dosage, mg/m2/week | 13.0 ± 5.8 |
| Concomitant oral GC use, no. (%) | 86 (46) |
| GC dosage, mg/kg/day | 0.13 ± 0.05 |
Except where indicated otherwise, values are the mean ± SD. Median (range) values are 17 (4–68) for active joints and 13 (1–67) for joints with limitation of movement (LOM). RF = rheumatoid factor; PhGA = physician global assessment of disease activity; VAS = visual analog scale; PGA = parent/patient global assessment of overall well‐being; CHAQ DI = Childhood Health Assessment Questionnaire disability index; ESR = erythrocyte sedimentation rate; ULN = upper limit of normal; JADAS‐71 = Juvenile Arthritis Disease Activity Score in 71 joints.
Received ≥1 dose of tocilizumab (TCZ).
Includes all data on disease‐modifying antirheumatic drug (DMARD) use as collected by investigators on case report forms: sulfasalazine, gold, chloroquine, hydroxychloroquine, D‐penicillamine, azathioprine, cyclosporine, leflunomide, minocycline, cyclophosphamide, thalidomide, and gamma globulin. Data on concurrent methotrexate (MTX) use were collected separately.
Efficacy at week 104
JIA‐ACR responses and JADAS‐71 over time
JIA‐ACR responses were maintained from weeks 40 through 104 in the mITT population, with 151 patients (80.3%), 145 patients (77.1%), and 112 patients (59.6%) achieving JIA‐ACR50, JIA‐ACR70, and JIA‐ACR90 responses, respectively, at week 104 (Figure 2A). The TCZ continuous group (n = 82) demonstrated progressive improvement in JIA‐ACR responses through week 104, with 58 patients (70.7%) achieving a JIA‐ACR90 response (Supplementary Figure 2A, http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract). After TCZ treatment was restarted, 75.0% of patients in the TCZ restart group achieved a JIA‐ACR70 response, and 43.8% achieved a JIA‐ACR90 response by week 104 (Supplementary Figure 2B), which was comparable to the improvement from baseline observed in the TCZ continuous group (Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract).
Figure 2.
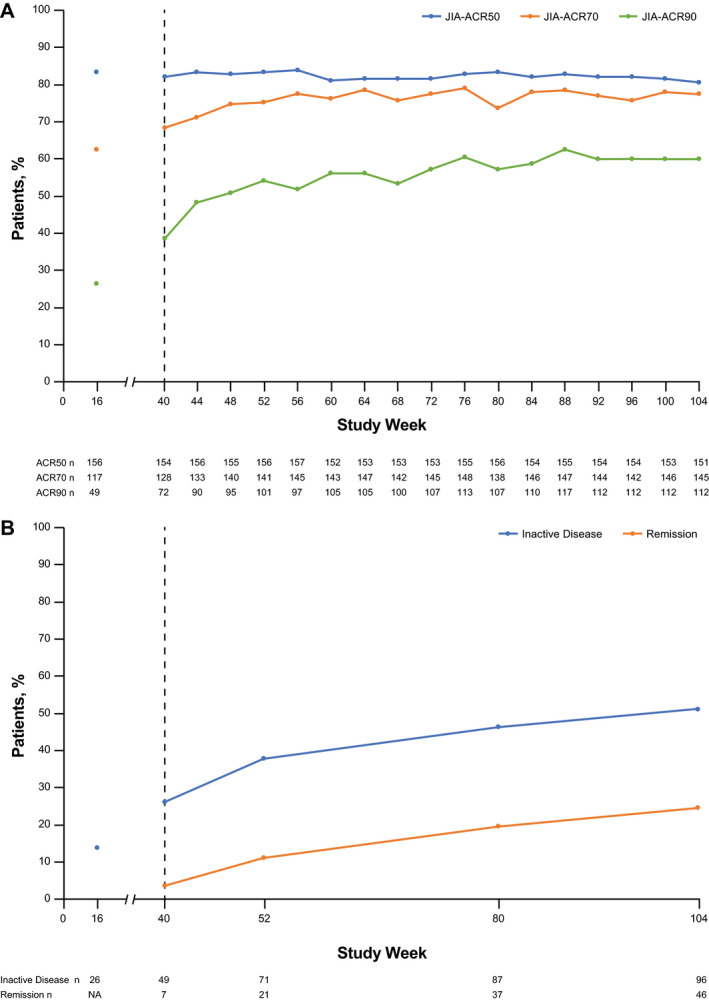
Proportions of patients in the mITT population (n = 188) achieving JIA‐ACR50/70/90 responses (A) and inactive disease and clinical remission (B) through week 104. Responders achieved JIA‐ACR50/70/90 responses or inactive disease/remission relative to baseline. After week 40, patients who were not randomly assigned in part 2 because they did not achieve at least a JIA‐ACR30 response were included and considered nonresponders. Patients who withdrew due to non–safety‐related reasons were included as nonresponders. For patients who withdrew due to safety reasons, last observation carried forward (LOCF) was used. LOCF was applied to missing ACR core components at each visit. Inactive disease was defined as the absence of active joints, no fever or physical examination features (including active uveitis) attributable to polyarticular‐course JIA, a physician global visual analog scale score of ≤10 mm, and a normal erythrocyte sedimentation rate (<20 mm/hour). Clinical remission was defined as meeting the criteria for inactive disease at all visits in the 6 months before and including the assessment day. See Figure 1 for other definitions.
In the mITT population, the number of patients who achieved inactive disease status according to ACR provisional criteria improved from 49 patients (26.1%) at week 40 to 96 patients (51.1%) at week 104; at the same time points, there were 7 patients (3.7%) and 46 patients (24.5%), respectively, whose disease was in clinical remission and were receiving medication (Figure 2B). In the TCZ continuous population, the number of patients who achieved JIA‐ACR inactive disease status improved from 33 patients (40.2%) at week 40 to 52 patients (63.4%) at week 104; at the same time points, there were 5 patients (6.1%) and 31 patients (37.8%), respectively, whose disease was in clinical remission and who were receiving medication (Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract).
The median JADAS‐71 score improved from 3.6 at week 40 to 0.7 at week 104 in the LTE population (Figure 3). Improvements in the median JADAS‐71 score from week 40 through week 104 were also observed in the TCZ continuous group (from 2.7 to 0.5) and the TCZ restart group (from 4.5 to 1.5) (Supplementary Figures 2C and D and Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract).
Figure 3.
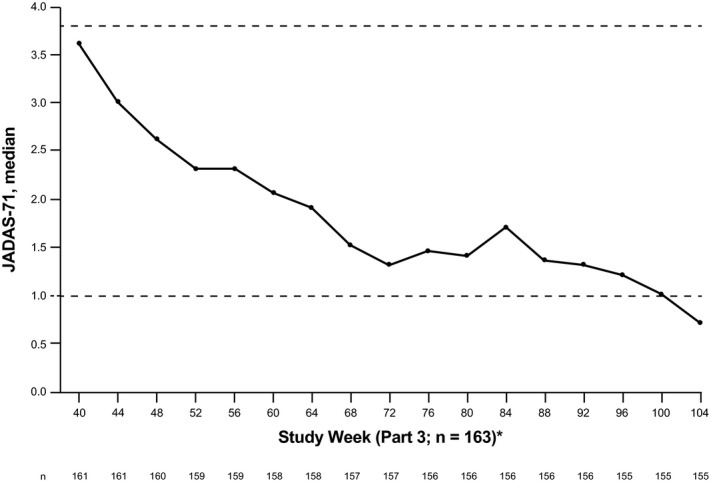
Median Juvenile Arthritis Disease Activity Score in 71 joints (JADAS‐71) in the long‐term extension (LTE) population (n = 163), according to study visit. All patients with a nonmissing assessment at each time point were included; there was no imputation for missing data. Last observation carried forward was used for missing core components. Dashed horizontal lines show inactive disease (JADAS‐71 <1) and low disease activity (JADAS‐71 <3.8).* The LTE population included all patients randomly assigned in part 2 who received ≥1 dose of tocilizumab (TCZ) either double‐blind or open‐label during parts 2 and 3 (25 patients who did not receive TCZ during parts 2 or 3 had no data from week 40 and were not included).
Reductions in glucocorticoid and MTX doses
Among patients who were receiving oral glucocorticoids at baseline (n = 86) (Table 1) and who had data available at week 104 (n = 66), 31 patients (47%) had discontinued oral glucocorticoids and 23 patients (35%) were receiving a reduced oral glucocorticoid dose by week 104. The mean daily oral glucocorticoid dose in the all‐exposure population (n = 188) was 0.06 mg/kg/day (median 0.00 [range 0.00–0.24]) at baseline and 0.02 mg/kg/day (median 0.00 [range 0.00–0.40]) at week 104 (n = 155 [LOCF; patients who withdrew were excluded]).
Among 127 of the 148 patients who were receiving MTX at baseline and had week 104 data available, 13 patients (10%) were receiving a reduced MTX dose by week 104, and 12 patients (9%) had discontinued MTX entirely. The mean ± SD MTX dose in the all‐exposure population decreased from 10.3 ± 7.4 mg/m2/week at baseline (n = 187) to 7.9 ± 6.4 mg/m2/week at week 104 (n = 155).
Patient‐reported outcomes
Mean CHAQ DI scores decreased from 0.48 at week 40 to 0.28 at week 104 in the LTE population (Supplementary Figure 3, http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract). Pain markedly decreased from baseline to week 104 in the TCZ continuous population (decrease in the mean ± SEM VAS score from 45.0 ± 3.1 to 10.3 ± 2.3) and in the TCZ restart population (Supplementary Figure 4, http://onlinelibrary.wiley.com/doi/10.1002/art.41528/abstract). Improvement was also seen in the patient/parent global assessment VAS score (mean ± SD change from baseline –36.6 ± 26.70 mm).
Figure 4.
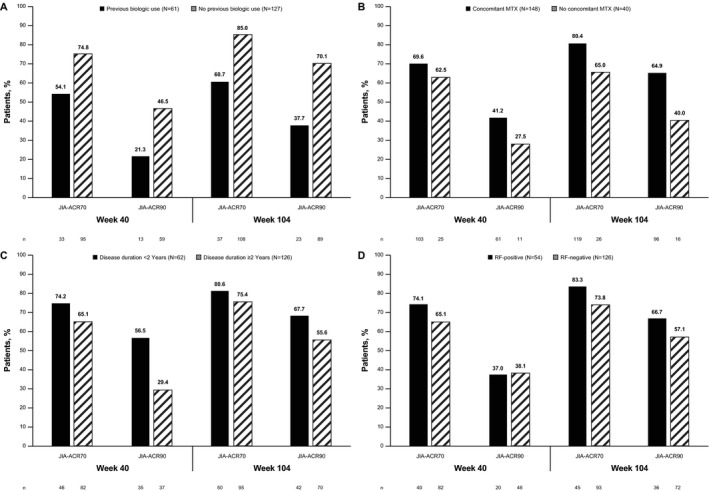
Proportion of patients in the mITT population (n = 188) with a JIA‐ACR70/90 response at weeks 40 and 104 based on previous biologic use (A), concomitant methotrexate (MTX) use (B), duration of disease (C), and rheumatoid factor (RF) status at baseline (D). Eight patients whose RF status at baseline was unknown were not included in the analsyis of RF status. See Figure 1 for other definitions.
Efficacy in subpopulations
Among subpopulations of the mITT population, numerically higher proportions of biologic‐naive patients (n = 127) achieved JIA‐ACR70/90 responses by week 104 compared with patients previously exposed to a biologic (n = 61) (85.0%/70.1% versus 60.7%/37.7%; Figure 4A). Numerically higher proportions of patients receiving concurrent MTX (n = 148) achieved JIA‐ACR70/90 responses compared with patients not receiving concomitant MTX (n = 40) (Figure 4B). The same was true for JIA‐ACR70/90 responses among patients with a disease duration of <2 years (n = 62) compared with those with a disease duration of ≥2 years (n = 126) (Figure 4C), though group differences were less pronounced. Except for the JIA‐ACR90 response at week 40, numerically higher proportions of patients who were RF‐positive at baseline (n = 54) achieved responses compared with those who were RF‐negative at baseline (n = 126) (Figure 4D).
Safety
The safety population (n = 188) provided information on 307.04 years of study duration. Overall rates of AEs and SAEs were comparable between weeks 40 and 104 (Table 2). Most SAEs were experienced by only 1 patient except for pneumonia (4 patients), bronchitis (2 patients), cellulitis (2 patients), uveitis (2 patients), and varicella (2 patients; both primary occurrences in patients who had not been vaccinated). A total of 1,248 AEs were reported in 169 patients (89.9%). The most frequently reported AEs were in the infections and infestations system organ class. Infection and infestation AEs occurred at rates of 163.7 per 100 patient‐years (95% confidence interval [95% CI] 145.8–183.3) at week 40 and 151.4 per 100 patient‐years (95% CI 138.0–165.9) at week 104.
Table 2.
Safety outcomes and abnormal laboratory parameters at week 40 and week 104 (all‐exposure/all‐tocilizumab population; n = 188)*
| Week 40† | Week 104 | |
|---|---|---|
| Safety outcome | ||
| Total patients with ≥1 AE, no. (%)‡ | 159 (84.6) | 169 (89.9) |
| Tocilizumab exposure, patient‐years | 184.44 | 307.04 |
| Total AEs, no. (rate per 100 patient‐years [95% CI])§ | 885 (479.8 [448.7–512.5]) | 1,248 (406.5 [384.2–429.7]) |
| Infections and infestations¶ | 302 (163.7) | 465 (151.4) |
| Musculoskeletal and connective tissue disorders# | 98 (53.1) | 121 (39.4) |
| JIA flares | 59 (32.0) | 65 (21.2) |
| Gastrointestinal disorders | 131 (71.0) | 159 (51.8) |
| Patients with ≥1 SAE, no. (%)‡ | 17 (9.0) | 26 (13.8) |
| Total SAEs (rate per 100 patient‐years [95% CI])§ | 23 (12.5 [7.9–18.7]) | 34 (11.1 [7.7–15.5]) |
| Infection SAEs, no. (rate per 100 patient‐years)§ | 9 (4.9) | 16 (5.2) |
| Pneumonia | 4 (2.2) | 4 (1.3) |
| Bronchitis | 2 (1.1) | 2 (0.7) |
| Cellulitis | 2 (1.1) | 2 (0.7) |
| Varicella | 1 (0.5) | 2 (0.7) |
| Other | – | 6 (1.9) |
| Patients with abnormal laboratory parameters, no. (%)‡ | ||
| ALT elevation | ||
| CTC grade 1 (>ULN–2.5× ULN) | 52 (28) | 65 (35) |
| CTC grade 2 (>2.5–5× ULN) | 8 (4) | 11 (6) |
| CTC grade 3 (>5–20× ULN) | 1 (0.5) | 4 (2) |
| AST elevation | ||
| CTC grade 1 (>ULN–2.5× ULN) | 32 (17) | 48 (26) |
| CTC grade 2 (>2.5–5× ULN) | 2 (1) | 3 (2) |
| CTC grade 3 (>5–20× ULN) | 1 (0.5) | 4 (2) |
| Total bilirubin elevation | ||
| CTC grade 1 (>ULN–1.5× ULN) | 14 (8) | 18 (10) |
| CTC grade 2 (>1.5–3× ULN) | 13 (7) | 14 (7) |
| CTC grade 3 (>3–10× ULN) | — | 2 (1)** |
| Neutrophil worst CTC grade | ||
| CTC grade 1 (<LLN–1.5 × 109/liter) | 14 (7) | 15 (8) |
| CTC grade 2 (<1.5 × 109/liter–1 × 109/liter) | 38 (20) | 44 (23) |
| CTC grade 3 (<1 × 109/liter–0.5 × 109/liter) | 6 (3) | 11 (6) |
| CTC grade 4 (<0.5 × 109/liter) | 1 (0.5)†† | – |
| Platelet worst CTC grade | ||
| CTC grade 1 (<LLN–75 × 109/liter) | 13 (7) | 17 (9) |
| CTC grade 2 (<75 × 109/liter–50 × 109/liter) | 1 (0.5) | 1 (0.5) |
| CTC grade 3 (<50 × 109/liter–25 × 109/liter) | 1 (0.5) | 1 (0.5) |
| CTC grade 4 (<25 × 109/liter) | 1 (0.5) | 1 (0.5) |
| LDL cholesterol ≥130 mg/dl | 8 (4)‡‡ | 10 (5) |
| Total cholesterol ≥200 mg/dl | 17 (9)‡‡ | 22 (12) |
Duration in study (years) = ([date of last assessment – date of first tocilizumab dose + 1]/365.25) – exposure to placebo treatment (years). Data on placebo treatment received in the part 2 withdrawal phase were excluded. 95% CI = 95% confidence interval; JIA = juvenile idiopathic arthritis; ALT = alanine aminotransferase; CTC = Common Toxicity Criteria; ULN = upper limit of normal; AST = aspartate aminotransferase; LLN = lower limit of normal; LDL = low‐density lipoprotein.
Includes all data collected until the last patient in the study completed week 40.
Multiple occurrences of the same adverse effect (AE) in a patient were counted only once.
Multiple occurrences of the same AE or serious AE (SAE) in a patient were counted at each occurrence.
Infestations reported in the infections and infestations system organ class were acarodermatitis (n = 3), enterobiasis (n = 3), parasitic infection (n = 2), lice infestation (n = 2), ascariasis (n = 1), and giardiasis (n = 1); the rest of the events were infections.
Sixty‐five events were JIA flares.
One patient was classified as having grade 4 bilirubin elevation due to a data error.
No grade 4 neutrophil count; unit conversion error (corrected to grade 2 neutrophil count).
Lipid data obtained from the data cutoff on May 3, 2012.
Infections and infestations were the most common system organ class (≥15% of patients) for AEs reported during 104 weeks of the study (71.3%), followed by musculoskeletal and connective tissue disorders (38.8%), gastrointestinal disorders (35.6%), respiratory, thoracic, and mediastinal disorders (28.7%), skin and subcutaneous tissue disorders (26.1%), and nervous system disorders (23.4%). The most common system organ class for SAEs was also infections and infestations (7.4%). Of those SAEs, 16 were reported as infections or infestations in 14 patients during the 104‐week study, for a rate of 5.2 per 100 patient‐years (95% CI 3.0–8.5); none were infestations. A total of 381 nonserious AEs were reported in 142 patients (75.5%); infections were the most common nonserious AEs (54.8%). No cases of tuberculosis, fungal infections, or other opportunistic infections developed, and there were no cases of gastrointestinal perforation.
Nine patients withdrew from study treatment because of AEs, 8 while receiving TCZ (scleroderma diagnosed by skin biopsy, abnormal bilirubin level, serum sickness–like reaction, JIA flare [1 patient withdrew from treatment because of this AE, but the reason for study withdrawal was insufficient therapeutic response], pneumonia, elevated transaminase levels [1 patient was randomly assigned to the placebo group but withdrew while receiving TCZ in part 1], benign intracranial hypertension, and pregnancy). One patient withdrew because of gastroenteritis while receiving placebo in part 2. No deaths were reported in the study, and there were no cases of macrophage activation syndrome.
Most changes in key laboratory parameters, including low‐density lipoprotein (LDL) cholesterol, total cholesterol, neutrophil count, and platelet abnormalities, were reported by week 40, with fewer occurrences reported from week 40 through week 104. Among 187 patients with assessments, ALT and AST levels remained within the normal range throughout study treatment in 107 patients (57.2%) and 132 patients (70.6%), respectively. Five patients had grade 3 elevations in ALT, AST, or both (>5–20× ULN) by week 104 (Table 2): 2 patients had no change in study drug because these elevations occurred at a single time point only; 2 patients had elevations associated with AEs of Epstein‐Barr virus, and the study drug was modified or interrupted because of them; and 1 patient had elevations recorded as an AE of hypertransaminasemia, which led to study drug discontinuation. Total bilirubin levels remained within the normal range throughout the study in 153 patients (81.8%). No patient experienced elevations in liver enzymes or hepatic events that met the criteria for Hy’s law (19); 1 patient experienced concomitant elevations in ALT (362 units/liter), AST (410 units/liter), and bilirubin (52 µmoles/liter) at week 80, which was suggestive of meeting the criteria for Hy’s law, but these elevations were attributed to an underlying Epstein‐Barr virus infection.
Postbaseline elevations of total cholesterol to ≥200 mg/dl and LDL cholesterol to ≥130 mg/dl were seen in 12% and 5% of patients, respectively (Table 2). Decreases in neutrophil count occurred in 70 patients (37.2%) between baseline and week 104, whereas neutrophil counts for 118 patients (62.8%) remained within the normal range. Eleven patients (5.9%) experienced grade 3 low neutrophil counts (<1.0–0.5 × 109/liter), none experienced grade 4 low neutrophil counts (<0.5 × 109/liter), and no events of neutrophil count decrease resulted in TCZ dose modification. No infections were reported during periods of neutropenia (within 30 days of a grade 3 low neutrophil count). Platelet count decreases occurred in 20 patients (10.6%) between baseline and week 104 but were generally mild (Table 2) and occurred once in different patients at individual time points. None of the patients with a decrease in platelet count of grade 2 or higher had associated concurrent bleeding events; therefore, these patients remained in the study.
One hundred eighty‐seven patients underwent immunogenicity screening assay at any time. Twenty patients (10.7%) had a positive anti‐TCZ assay result at baseline before exposure to TCZ; 4 patients (2.1%) who had a negative result at baseline had a positive postbaseline screening assay result, but only 1 of these patients (0.5%) had a positive confirmation and neutralizing assay result. This patient, treated with 10 mg/kg TCZ (BW of <30 kg), did not have hypersensitivity events but withdrew from the study due to insufficient therapeutic effect at week 16.
DISCUSSION
The efficacy of TCZ in patients with polyarticular‐course JIA previously demonstrated at week 40 (7) was maintained, if not further improved, through week 104. Notably, clinical remission was reached in ~25% of patients treated with TCZ for 104 weeks. Indeed, many patients successfully decreased or even discontinued glucocorticoids and/or MTX. The analysis of patients who received placebo during part 2 and restarted TCZ following flare or after week 40 of the study (TCZ restart population) showed efficacy comparable to that of patients treated with TCZ continuously to week 104, suggesting that efficacy can be recaptured after TCZ interruption.
Consistent with findings from other studies (21, 22), we found that polyarticular‐course JIA patients in whom previous biologic therapies were unsuccessful or who had longer disease durations experienced less pronounced improvement of JIA with TCZ treatment. Nevertheless, 60.7% of patients in our study who previously received biologics achieved a JIA‐ACR70 response by week 104. In contrast to adult patients with RA (23), combination therapy with TCZ and MTX seemed somewhat more effective than TCZ monotherapy in our study of patients with polyarticular‐course JIA. However, these results should be interpreted with caution because the study was not powered for comparison of TCZ monotherapy with TCZ in combination with MTX. Children whose duration of polyarticular‐course JIA exceeded 2 years before they received TCZ or those who were RF negative achieved JIA‐ACR70/90 responses less frequently than children with shorter disease duration or those who were RF positive. These observations are consistent with previous studies in JIA patients who were treated with biologics (24, 25). Long‐term use of glucocorticoids in children is highly undesirable because of impairment in growth (26), known long‐term cardiovascular risks, and other toxicities (27). In this study, glucocorticoid treatment was prescribed to 46% of polyarticular‐course JIA patients at baseline. TCZ therapy allowed for the discontinuation of steroids in 47% of these patients (31 of 66 patients) by week 104. As reported elsewhere (28), this reduction in glucocorticoid exposure, together with the degree of disease control achieved, is considered clinically relevant based on improved growth profiles.
On average, mild‐to‐moderate disability (29) at baseline improved with TCZ treatment over a 2‐year period. Pain is an important aspect of health‐related quality of life in patients with polyarticular‐course JIA (30). Changes in pain VAS scores >10 mm are considered clinically relevant for children (31). Patients who received TCZ continuously maintained improved pain control through week 104, and patients who restarted TCZ experienced improvement in pain upon the reinitiation of TCZ and then comparable pain control by 6 months after reinitiation, supporting the notion that major and clinically relevant improvement in patient pain can be recaptured with TCZ treatment.
The safety of TCZ in this polyarticular‐course JIA population was consistent with the known safety profile (7, 32, 33), and no new or unexpected safety concerns were reported. Infections, both serious and nonserious, were the most frequently reported AEs. As expected, the overall incidence of AEs and SAEs increased between weeks 40 and 104. However, standardized rates of AEs and SAEs at week 104 remained stable over time and were similar to those reported at week 40. Notably, the rates of serious infections remained stable between weeks 40 and 104. Patterns of laboratory abnormalities were consistent with those previously observed (7), and lipid parameters remained within normal ranges (34). Only 1 patient (0.5%) developed neutralizing anti‐TCZ antibodies; this low incidence of immunogenicity is consistent with that in RA patients treated with TCZ, for whom an incidence of 0.9% has been reported (35).
There are limitations to the interpretation of these long‐term data because there was no comparator after week 40, given the open‐label nature of part 3. Comparison of patients who received TCZ continuously throughout the study with those who received placebo treatment during part 2 enabled limited estimation of the effect of stopping and restarting TCZ treatment. Low patient numbers in the subgroup analyses should be noted in the interpretation of responses according to previous or concurrent medications and baseline disease characteristics. The number of polyarticular‐course JIA patients receiving TCZ monotherapy was relatively low, and analyses were not corrected for other patient characteristics such as disease duration and/or failure of other biologic treatments. Therefore, additional research is needed to evaluate the added benefits of MTX background therapy in polyarticular‐course JIA patients treated with TCZ. Longer‐term follow‐up and the availability of comparator groups from patients who have not received TCZ or who are treated with other biologic medications is needed for full understanding of the long‐term efficacy and safety of TCZ when used to treat polyarticular‐course JIA. An observational polyarticular‐course JIA registry study is being conducted to assess the longer‐term safety and effectiveness of up to 5 years of treatment with TCZ versus a comparator biologic.
In conclusion, this study has demonstrated that improvements in the signs and symptoms of polyarticular‐course JIA in children treated with TCZ were maintained or improved during longer‐term treatment up to 2 years and that the safety profile of TCZ remained consistent with that established in a much larger number of adults with RA, with no evidence of increasing toxicities over this period of time.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Brunner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Brunner, Ruperto, Zuber, Keltsev, Zavaler, Lovell, Martini, De Benedetti.
Acquisition of data
Brunner, Ruperto, Zuber, Cuttica, Keltsev, Xavier, Burgos‐Vargas, Penades, Silverman, Espada, Zavaler, Kimura, Duarte, Job‐Deslandre, Joos, Wimalasundera, Wells, Lovell, De Benedetti.
Analysis and interpretation of data
Brunner, Ruperto, Zuber, Keltsev, Penades, Zavaler, Douglass, Wimalasundera, Bharucha, Wells, Lovell, Martini, De Benedetti.
ROLE OF THE STUDY SPONSOR
F. Hoffmann‐La Roche Ltd was involved in the study design; collection, analysis, and interpretation of data; writing of the manuscript; and decision to submit the manuscript for publication. Writing assistance was provided by Sara Duggan, PhD, of ApotheCom and was funded by F. Hoffmann‐La Roche Ltd. Publication of this article was not contingent upon approval by F. Hoffmann‐La Roche Ltd.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the following collaborators: Ekaterina Alekseeva, Roger Allen, Eileen Baildam, David Cabral, Armando Calvo, Jeffrey Chaitow, Vyacheslav Chasnyk, Jose Chavez, Elisabetta Cortis, Maria Luz Gámir, Abraham Gedalia, Valeria Gerloni, Steven Goodman, Gerd Horneff, Kristin Houghton, Hans‐Iko Huppertz, Christian Jorgensen, Sheila Knupp, Katarzyna Kobusinska, Isabelle Kone‐Paut, Alan Martin, Kirsten Minden, Evgeny L. Nasonov, Karen Onel, Violetta Opoka‐Winiarska, Pierre Quartier, Athimalaipet Ramanan, Christoph Rietschel, Johannes Roth, Nadina Rubio, Alexey Sarychev, Kenneth Schikler, Heinrike Schmeling, Rayfel Schneider, Clovis Silva, Daniel Siri, Elzbieta Smolewska, Eunice Solis Vallejo, Alberto Spindler, Flavio Sztajnbok, Ewa Tuszkiewicz‐Misztal, Patricia Woo, Carine Wouters, Elena Zholobova, and Francesco Zulian.
References
- 1. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 2. Ruperto N, Giannini EH, Pistorio A, Brunner HI, Martini A, Lovell DJ. Is it time to move to active comparator trials in juvenile idiopathic arthritis? A review of current study designs [review]. Arthritis Rheum 2010;62:3131–9. [DOI] [PubMed] [Google Scholar]
- 3. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 4. Zak M, Muller J, Karup PF. Final height, armspan, subischial leg length and body proportions in juvenile chronic arthritis: a long‐term follow‐up study. Horm Res 1999;52:80–5. [DOI] [PubMed] [Google Scholar]
- 5. Lovell DJ. Update on treatment of arthritis in children: new treatments, new goals [review]. Bull NYU Hosp Jt Dis 2006;64:72–6. [PubMed] [Google Scholar]
- 6. De Benedetti F, Robbioni P, Massa M, Viola S, Albani S, Martini A. Serum interleukin‐6 levels and joint involvement in polyarticular and pauciarticular juvenile chronic arthritis. Clin Exp Rheumatol 1992;10:493–8. [PubMed] [Google Scholar]
- 7. Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular‐course juvenile idiopathic arthritis: results from a phase 3, randomised, double‐blind withdrawal trial. Ann Rheum Dis 2015;74:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruperto N, Martini A. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 9. Brunner HI, Rider LG, Kingsbury DJ, Co D, Schneider R, Goldmuntz E, et al. Pediatric Rheumatology Collaborative Study Group: over four decades of pivotal clinical drug research in pediatric rheumatology [review]. Pediatr Rheumatol Online J 2018;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ.et al, on behalf of the Pediatric Rheumatology Collaborative Study Group . Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med 2000;342:763–9. [DOI] [PubMed] [Google Scholar]
- 11. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 12. Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N, for the Childhood Arthritis and Rheumatology Research Alliance (CARRA), the Pediatric Rheumatology Collaborative Study Group (PRCSG), and the Paediatric Rheumatology International Trials Organization (PRINTO) . American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–36. [DOI] [PubMed] [Google Scholar]
- 13. Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004;31:2290–4. [PubMed] [Google Scholar]
- 14. Brunner HI, Lovell DJ, Finck BK, Giannini EH. Preliminary definition of disease flare in juvenile rheumatoid arthritis. J Rheumatol 2002;29:1058–64. [PubMed] [Google Scholar]
- 15. Consolaro A, Bracciolini G, Ruperto N, Pistorio A, Magni‐Manzoni S, Malattia C, et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum 2012;64:2366–74. [DOI] [PubMed] [Google Scholar]
- 16. Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni‐Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. [DOI] [PubMed] [Google Scholar]
- 17. Consolaro A, Ruperto N, Bracciolini G, Frisina A, Gallo MC, Pistorio A, et al. Defining criteria for high disease activity in juvenile idiopathic arthritis based on the juvenile arthritis disease activity score. Ann Rheum Dis 2014;73:1380–3. [DOI] [PubMed] [Google Scholar]
- 18. National Cancer Institute . Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 2006. URL: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 19. Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf 2006;15:241–3. [DOI] [PubMed] [Google Scholar]
- 20. Stubenrauch K, Wessels U, Birnboeck H, Ramirez F, Jahreis A, Schleypen J. Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: evaluation of an antidrug antibody ELISA using clinical adverse event‐driven immunogenicity testing. Clin Ther 2010;32:1597–609. [DOI] [PubMed] [Google Scholar]
- 21. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio‐Perez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double‐blind, placebo‐controlled withdrawal trial. Lancet 2008;372:383–91. [DOI] [PubMed] [Google Scholar]
- 22. Geikowski T, Becker I, Horneff G, German BIKER Registry Collaborative Study Group. Predictors of response to etanercept in polyarticular‐course juvenile idiopathic arthritis. Rheumatology (Oxford) 2014;53:1245–9. [DOI] [PubMed] [Google Scholar]
- 23. Gabay C, Riek M, Hetland ML, Hauge EM, Pavelka K, Tomsic M, et al. Effectiveness of tocilizumab with and without synthetic disease‐modifying antirheumatic drugs in rheumatoid arthritis: results from a European collaborative study. Ann Rheum Dis 2016;75:1336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horneff G, de Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis 2009;68:519–25. [DOI] [PubMed] [Google Scholar]
- 25. Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh‐Out cohort. Ann Rheum Dis 2015;74:1854–60. [DOI] [PubMed] [Google Scholar]
- 26. Wang SJ, Yang YH, Lin YT, Yang CM, Chiang BL. Attained adult height in juvenile rheumatoid arthritis with or without corticosteroid treatment. Clin Rheumatol 2002;21:363–8. [DOI] [PubMed] [Google Scholar]
- 27. Davis JM III, Kremers HM, Crowson CS, Nicola PJ, Ballman KV, Therneau TM, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population‐based cohort study. Arthritis Rheum 2007;56:820–30. [DOI] [PubMed] [Google Scholar]
- 28. Bharucha KN, Brunner HI, Penades IC, Nikishina I, Rubio‐Perez N, Oliveira S, et al. Growth during tocilizumab therapy for polyarticular‐course juvenile idiopathic arthritis: 2‐year data from a phase III clinical trial. J Rheumatol 2018;45:1173–9. [DOI] [PubMed] [Google Scholar]
- 29. Dempster H, Porepa M, Young N, Feldman BM. The clinical meaning of functional outcome scores in children with juvenile arthritis. Arthritis Rheum 2001;44:1768–74. [DOI] [PubMed] [Google Scholar]
- 30. Sawyer MG, Whitham JN, Roberton DM, Taplin JE, Varni JW, Baghurst PA. The relationship between health‐related quality of life, pain and coping strategies in juvenile idiopathic arthritis. Rheumatology (Oxford) 2004;43:325–30. [DOI] [PubMed] [Google Scholar]
- 31. Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med 2001;37:28–31. [DOI] [PubMed] [Google Scholar]
- 32. Genovese MC, Rubbert‐Roth A, Smolen JS, Kremer J, Khraishi M, Gomez‐Reino J, et al. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J Rheumatol 2013;40:768–80. [DOI] [PubMed] [Google Scholar]
- 33. Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther 2011;13:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Heart Lung and Blood Institute . National Institutes of Health. May 2001. ATP III at‐a‐glance quick desk reference. URL: https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf.
- 35. Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:1078–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


