Abstract
Background
Previous studies reported reduced decision‐making abilities for patients with multiple sclerosis (MS) relative to healthy controls (HC). This study aimed to evaluate whether these problems arise when sampling information or when pondering about the evidence collected.
Methods
In a cross‐sectional, controlled study, 43 relapsing‐remitting MS patients (RRMS; Expanded Disability Status Scale 1.5, range 0–4) and 53 HC performed an information sampling task (‘beads task’), a health‐related framing task, and neuropsychological background tests.
Results
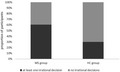
In the beads task, patients collected as much information as HC prior to a decision. However, there were twice as many patients as HC making irrational decisions, that is, decisions against the evidence collected (RRMS: 26/43, 60%; HC: 16/53, 30%; p = 0.003). Compared to HC, patients also showed a stronger framing effect, that is, they were more strongly biased by the way health‐related information was presented (p < 0.05, Cohen's d = 0.5). Overall, the framing effect predicted whether a participant would make irrational decisions (OR 2.12, 95% CI 1.29–3.49, p < 0.001).
Conclusions
Predecisional information sampling is intact in RRMS. However, compared to HC, patients are more likely to make irrational decisions and to be biased by the way health‐related information is framed. This warrants caution in communication, especially in the medical context, with patients.
Keywords: executive functions, logistic models, neuropsychological tests, relapsing‐remitting, risk taking
This study shows that patients with multiple sclerosis (MS) collect as much information as healthy controls (HC) do prior to making a decision. However, twice as many patients as HC make irrational decisions, that is, decisions against the evidence collected. Compared to HC, patients also show stronger framing effects, that is, they are more strongly biased by the way health‐related information is presented. These results warrant caution in communication, especially in the medical context, with patients.
INTRODUCTION
Several studies reported reduced decision‐making (DM) abilities for patients with multiple sclerosis (MS) relative to healthy controls (HC) [1]. Also, patients are more strongly biased than HC by the way information is presented, [2] known as the framing effect [3]. It is yet to be determined why DM problems arise in patients with MS. One might hypothesize that patients make less advantageous decisions than HC because they collect less evidence before making a decision. Alternatively, one might assume that patients collect as much information as HC but nonetheless make poorer decisions. This means that DM problems in MS might arise either when sampling information (insufficient) or when pondering about the evidence collected (misleading inference).
In this study, we compared the performance of relapsing‐remitting MS (RRMS) patients to that of HC on the ‘beads task’ [4]. This is an information sampling task that proved to be highly sensitive in detecting decisional impulsivity and frequent irrational decisions, for example, in patients with Parkinson's disease and in patients with restless legs syndrome under dopamine agonist treatment [5, 6]. The beads task allows assessment of not only the amount of the information collected before making a decision, but also whether individuals consider this information when making a decision. For example, after drawing three green beads, an individual may conclude that these beads were taken from a cup containing mainly green beads. This would be a rational decision. Alternatively, an individual may conclude that the beads were taken from a cup containing more blue beads than green beads. This would be an irrational decision. No study so far has investigated predecisional information sampling and these types of decision (rational, irrational) in MS patients.
In this study, we evaluated by means of the beads task how much evidence participants collect before making a decision and whether they take this information into consideration. Moreover, we investigated which clinical (for the patient group only), demographical, and neuropsychological measures correlate with and predict decision behaviour. We focused in particular on measures of executive functioning, number processing, and sensitivity to the information frame, since these have been previously found to correlate with DM in different neurological conditions as well as in the normal population [7]. Typically, higher executive functions, higher numerical abilities, and lower framing effects correlate with more advantageous DM [7].
METHODS
Participants
Between 2014 and 2017, 43 patients with a confirmed diagnosis of RRMS according to the 2010 McDonald criteria [8] and 53 HC agreed to participate in this study. HC did not report any health‐associated complaints. Exclusion criteria were: history of a major medical, psychiatric, or neurological disorder other than RRMS; substance abuse; or steroid treatment for MS relapse within 6 weeks prior to evaluation. We also excluded patients with current major depressive episodes (defined by an informal interview) or an Expanded Disability Status Scale (EDSS) [9] score higher than 4 to minimize the effects of mood and physical disability on performance in neuropsychological and decision measures. Table S1 (supplemental material only) reports current disease‐modifying therapy for the patient group. All participants had an estimated verbal IQ of at least 85. The study was approved by the ethics committee of the Medical University Innsbruck (ethical approval number: UN5044, 324/4.5) and conforms to the World Medical Association Declaration of Helsinki for studies involving human subjects. Written informed consent was obtained from all individuals before participation.
Procedure
All participants completed a neuropsychological background assessment, a health‐related framing task, and the beads task.
Neuropsychological background assessment
Tests assessed logical reasoning, response inhibition, verbal attention span, verbal working memory, mental complex calculation, ratio processing, and quantity discrimination. Participants also responded to a questionnaire on anxiety and depression symptoms (see supplemental material, online only).
Health‐related framing task
This task contains 20 statements about the success of fictive medications [10]. Participants are informed that medications are used for the treatment of mild diseases like a common cold. They are asked to evaluate them on a seven‐point color‐based scale from negative (left‐side, red colored) to positive (right‐side, green colored). Answers are recorded by the examiner by associating a number to each point (from 1, left‐most point to 7, right‐most point). Medications are described using either positive terms (eg, effective in 84% of cases) or negative terms (eg, ineffective in 16% of cases). In this study, we used as a measure of framing effect the difference between scores given to positively framed items (high percentage of effectiveness) and scores given to negatively framed items (low percentage of ineffectiveness) (for a detailed description, see supplemental material, online only). A score difference above zero indicates that positively framed items are evaluated more positively than negatively framed items.
Beads task
In this computerized task, [4] participants have to guess from which of two cups the computer is drawing colored beads. Before the task begins, participants are informed that the blue cup contains more blue beads than green beads, while the green cup contains more green beads than blue beads. They are also informed about the ratio of blue to green beads in each cup and whether they can lose points in case of a wrong decision (for a detailed description, see supplemental material, online only). In each trial (total N = 12), participants can either draw up to 10 beads before making a decision or immediately guess from which cup the bead has been drawn. Each additional draw costs a small fee. In this study, we analyzed the number of beads drawn before making a decision, the number of risky decisions (ie, decisions that are made when the same number of blue and green beads is shown), and the number of irrational decisions made against the evidence (eg, more blue beads shown, green cup chosen).
Statistics
We used SPSS software version 24.0). Groups were compared by Chi‐square test and Mann−Whitney U‐test where appropriate. A Bayesian independent‐sample t‐test was carried out for the number of beads drawn (this analysis was performed with JASP 0.12.2 software). We also computed Cohen's d effect‐ sizes for demographical and neuropsychological data (these are reported in Table 1). Effect sizes can be interpreted as small (d ≥ 2), medium (d ≥ 5), or large (d ≥ 8) according to Cohen's convention. A Spearman rank‐order correlation analysis on the whole sample explored the association between decision behavior with demographical and neuropsychological measures. An additional Spearman rank‐order correlation analysis was performed for the patient group between decision behavior, clinical characteristics, and fatigue scores. Finally, a multivariate hierarchical binary logistic regression analysis on the whole sample investigated which measures (group membership, neuropsychological scores) would best predict decision behavior (no irrational decisions versus at least one irrational decision). Significance was set at α=0.05. Results of the correlation analysis on the whole sample were interpreted as significant when p ≤ 0.002 (Bonferroni correction for multiple testing).
TABLE 1.
Characteristics and neuropsychological performance
| Parameter | Max. score | MS (n = 43) | HC (n = 53) | Zvalue | P value | Cohen’s d |
|---|---|---|---|---|---|---|
| Female a | 30 (69.8) | 29 (54.7) | n.a. | 0.132 b | n.a. | |
| Age (years) c | 40.0 (34.0–50.0) | 42.0 (30.0–55.0) | −0.11 | 0.915 d | 0.01 | |
| Education (years) c | 12.0 (11.0–13.0) | 12.0 (12.0–14.0) | −1.56 | 0.118 d | 0.18 | |
| EDSS c | 1.5 (1.0–2.0) | n.a. | ||||
| Disease duration (years) c , e | 8.15 (4.1–18.8) | n.a. | ||||
| FSS c | 3.7 (2.6–4.9) | n.a. | ||||
| Currently perceived fatigue c , f | 2.0 (1.0–3.0) | n.a. | ||||
| Logical reasoning (test score) c | 25 | 16.0 (12.0–18.0) | 18.0 (16.0–20.0) | −2.39 | 0.017 d | 0.54 |
| Response inhibition (time in ms) c | 412.0 (358.0–477.0) | 422.0 (375.0–451.0) | −0.08 | 0.935 d | 0.08 | |
| Response inhibition (errors) c | 1.0 (0–2.0) | 1.0 (0–1.0) | −1.40 | 0.161 d | 0.34 | |
| Verbal attention span (test score) c | 12 | 7.0 (6.0–8.0) | 8.0 (7.0–9.0) | −2.56 | 0.011 d | 0.56 |
| Verbal working memory (test score) c | 12 | 6.0 (5.0–7.0) | 6.0 (6.0–8.0) | −1.11 | 0.269 d | 0.20 |
| Mental complex calculation (correct answers) c | 16 | 15.0 (14.0–16.0) | 15.0 (14.0–16.0) | −0.37 | 0.708 d | 0.10 |
| Ratio processing (correct answers) c | 26 | 21.0 (12.0–24.0) | 22.0 (19.0–24.0) | −1.65 | 0.100 d | 0.45 |
| Quantity discrimination (Weber’s quotient) c | 0.18 (0.14–0.25) | 0.15 (0.14–0.19) | −1.49 | 0.137 d | 0.45 | |
| Framing effect c , g | 1.4 (0.4–2.6) | 0.8 (0.2–1.8) | −2.26 | 0.024 d | 0.49 | |
| Anxiety score c | 21 | 5.0 (2.0–7.0) | 4.0 (2.0–6.0) | −1.32 | 0.186 d | 0.32 |
| Depression score c | 21 | 3.0 (1.0–4.0) | 2.0 (1.0–4.0) | −0.78 | 0.436 d | 0.20 |
Bold values indicate statistical significance (p < 0.05).
Abbreviations: EDSS, Expanded Disability Status Scale; FSS, Fatigue Severity Scale; HC, healthy controls; MS, multiple sclerosis; n.a., not applicable.
Number (percentage).
Chi‐square test.
Median (interquartile range).
Mann−Whitney U‐test.
Information about disease duration was available for all but one patient.
From 0 – no fatigue to 10 – extreme fatigue.
Score difference between positively framed items (high percentage of effectiveness) and negatively framed items (low percentage of ineffectiveness).
RESULTS
Characteristics of the two groups are given in Table 1. Groups had comparable age, education, and gender distribution.
Neuropsychological background performance
Results are reported in Table 1. Median scores of both groups were in the average range of standardized norms in all tests. Patients scored lower than HC in measures of logical reasoning and verbal attention span. Cohen's d indicates a medium effect size for both these measures. Groups did not differ from each other in the remaining neuropsychological tests.
Health‐related framing task
The group difference was significant, with the Cohen's d indicating a small‐to‐medium effect size (Table 1). Compared to HC, patients obtained a larger score difference between evaluations given to positively framed items and evaluations given to negatively framed items, pointing to a stronger framing effect for them. This means patients were more strongly influenced than HC by the frame of health‐related statements.
Beads task
Groups did not differ in the number of beads drawn (BF01 = 3.51 ± 0.04%). Also, there was in both groups a comparable proportion of participants making risky decisions (number [percentage] MS: 6/43 [14.0%], HC: 13/53 [24.5%]; χ2 = 1.672, p = 0.196). By contrast, there were twice as many patients as HC making irrational decisions (MS: 26/43 [60.5%], HC: 16/53 [30.2%]; χ2 = 8.843, p = 0.003).
Factors associated with decision behavior
A higher number of irrational decisions correlated with a lower number of draws, a stronger framing effect, and lower scores in executive functions and ratio processing tests (Table 2). No significant correlation was found for the patient group between decision behavior, disease duration, EDSS, and fatigue scores (see supplemental material, online only).
TABLE 2.
Coefficients of a Spearman rank‐order correlation analysis for the whole sample
| (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | −0.319 (0.002) a | 0.186 (0.069) a | −0.277 (0.006) a | 0.461 (<0.001) a | −0.379 (<0.001) a | 0.077 (0.457) a | −0.363 (<0.001) a | −0.224 (0.028) a | −0.183 (0.074) a | −0.411 (<0.001) a | 0.211 (0.040) a | 0.176 (0.087) a | 0.098 (0.341) a |
| (2) | ‐ | −0.128 (0.213) a | 0.099 (0.337) a | −0.163 (0.112) a | 0.194 (0.058) a | 0.011 (0.918) a | 0.057 (0.578) a | 0.177 (0.085) a | 0.026 (0.805) a | 0.128 (0.213) a | −0.103 (0.320) a | 0.073 (0.483) a | 0.034 (0.745) a |
Bold values indicate statistical significance (p ≤ 0.002).
(1) Number of irrational decisions, (2) number of beads drawn, (3) age (years), (4) education (years), (5) framing effect (mean difference), (6) logical reasoning (correct answers), (7) response inhibition (response times in milliseconds), (8) verbal attention span (test score), (9) verbal working memory (test score), (10) mental complex calculation (correct answers), (11) ratio processing (correct answers), (12) quantity discrimination (Weber's quotient; higher quotients indicate lower quantity discrimination), (13) anxiety, and (14) depression.
rvalue (pvalue).
Factors predicting decision behavior
A multivariate hierarchical binary logistic regression analysis was performed for the whole group to predict decision behavior (irrational decisions: none vs. at least one). Group membership (HC vs. MS), executive functions (logical reasoning, verbal attention span), ratio processing, and the framing effect were entered subsequently as predictors of interest. For this analysis, we only used the cognitive variables that proved to be significantly correlated with decision behavior. The final model was significant (omnibus χ 2 = 39.02, df = 5, p < 0.001), accounting for between 33.4% and 44.8% of the variance in decision behavior. In Model 1, group membership emerged as significant predictor (p = 0.003). In Model 2, logical reasoning emerged as significant predictor (p = 0.007), while both group membership and verbal attention span failed to reach significance (both p > 0.05). In Model 3, none of the entered predictors reached significance (all p > 0.05). Finally, in Model 4, the framing effect predicted decision behavior above and beyond the variables entered in previous models (odds ratio 2.12, 95% CI 1.29–3.49; Table 3).
TABLE 3.
Multivariate hierarchical binomial logistic regression predicting decision behavior (no irrational decisions vs. at least one irrational decision)
| Model | Predictor | B | SE | Pvalue | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Model 1 | Group (reference category HC) | 1.263 | 0.432 | 0.003 | 3.537 | 1.516 | 8.251 |
| Constant | −0.838 | 0.299 | 0.005 | 0.432 | |||
| Model 2 | Group (reference category HC) | 0.827 | 0.481 | 0.085 | 2.287 | 0.891 | 5.869 |
| Logical reasoning | −0.197 | 0.073 | 0.007 | 0.821 | 0.712 | 0.947 | |
| Verbal attention span | −0.263 | 0.146 | 0.072 | 0.769 | 0.578 | 1.023 | |
| Constant | 4.622 | 1.542 | 0.003 | 101.677 | |||
| Model 3 | Group (reference category HC) | 0.815 | 0.490 | 0.096 | 2.259 | 0.865 | 5.898 |
| Logical reasoning | −0.114 | 0.087 | 0.189 | 0.893 | 0.753 | 1.058 | |
| Verbal attention span | −0.254 | 0.149 | 0.088 | 0.776 | 0.580 | 1.038 | |
| Ratio processing | −0.101 | 0.061 | 0.097 | 0.904 | 0.803 | 1.018 | |
| Constant | 5.164 | 1.625 | 0.001 | 174.868 | |||
| Model 4 | Group (reference category HC) | 0.746 | 0.524 | 0.154 | 2.109 | 0.756 | 5.886 |
| Logical reasoning | −0.035 | 0.096 | 0.717 | 0.966 | 0.801 | 1.165 | |
| Verbal attention span | −0.287 | 0.160 | 0.074 | 0.751 | 0.548 | 1.028 | |
| Ratio processing | −0.071 | 0.063 | 0.258 | 0.932 | 0.824 | 1.053 | |
| Framing effect | 0.752 | 0.254 | 0.003 | 2.122 | 1.290 | 3.491 | |
| Constant | 2.493 | 1.796 | 0.165 | 12.096 | |||
Bold values indicate statistical significance (p < 0.05).
Abbreviations: B, unstandardized regression coefficient; CI, confidence interval; Exp(B), odds ratio; HC, healthy controls; SE, standard error of B.
DISCUSSION
In this study, we demonstrated that predecisional information sampling is intact in RRMS. Indeed, patients collected as much evidence as HC before making a decision. However, there were twice as many patients as HC making irrational decisions, that is, decisions against the evidence collected. Patients were also more strongly influenced than HC by the frame of health‐related statements. Indeed, they obtained a larger score difference than HC between evaluations given to positively framed statements and evaluations given to negatively framed statements.
Patients’ decision behavior did not correlate with disease duration, physical disability, or perceived fatigue, which might be due to the fact that we recruited a quite homogeneous group of RRMS patients. A correlation analysis on the whole sample showed that a higher number of irrational decisions was associated with a lower number of draws, a stronger framing effect and lower scores in logical reasoning, verbal attention span, and ratio processing, that is, the ability to understand and process complex numerical information such as frequencies and percentages. This is in line with previous studies reporting that people with lower executive functions and lower numeracy are more likely to make disadvantageous decisions [11]. Moreover, and also in line with previous findings, [7] we found that the framing effect predicts whether a person would make irrational decisions.
Decision situations differ in the type and the amount of information they offer. In decision situations under risk, probabilities of the different outcomes, possible gains, and possible losses are explicitly given or can be inferred or computed. Differently, in decision situations under ambiguity, important information is missing or conflicting. In this case, individuals have to learn from experience and from feedback which options are advantageous [7, 12, 13, 14]. In recent years, a detailed assessment of DM behavior in MS has revealed a reduced performance for patients relative to HC both in situations of risk and in situations of ambiguity [1]. Patients differ from HC not only in quantitative measures such as the number of advantageous decisions, but also in qualitative measures such as performance speed and reaction to feedback [1]. The results of this study add importantly to the characterization of the effects of MS on DM. Indeed, we show that patients do not differ from HC in predecisional information processing as defined by the amount of information sampled prior to making a decision, but they do differ when pondering about this evidence. MS patients are more likely than HC to make irrational decisions. They also show a stronger framing effect.
Both irrational decisions and framing effects have been related to the use of an intuitive, less analytic processing approach [15, 16]. Following standard dual‐process cognitive models, people use two systems to interpret information and make decisions: a reflective and an intuitive processing system [17, 18, 19]. The reflective system involves reasoning, planning, cognitive control, and ratio processing. The intuitive system uses heuristics and assumptions developed on the base of intuitions, emotions, and experience. The intuitive system operates quickly and automatically, and provides an efficient and effective means for decision making in many contexts. However, it may also lead to cognitive biases or irrational ideas that promote excessive or risky behavior [15]. The reflective and intuitive systems may interact with each other [7]. Whether a person relies more on one or the other system depends – among other factors – on the situation type and the person's thinking style, [15] but also possibly on how good cognitive functions such as reasoning, cognitive control, or ratio processing are [7]. In this study, we found that MS patients scored lower than HC in logical reasoning and verbal attention span, cognitive measures that are negatively correlated with the number of irrational decisions and the framing effect. It may be possible that the intuitive approach helps people sparing cognitive resources and is therefore preferred by patients with cognitive alterations. This might lead to a higher number of irrational decisions and a stronger framing effect for the patients. Alternatively, it may also be hypothesized that the condition of having a chronic disease, whose origin and prognosis are quite uncertain, has an influence on how tasks are perceived and elaborated in the medical context. Possibly, this leads some patients towards a more “irrational” decision style and stronger framing effects. These speculations need to be verified in the future.
Future studies might also be interested in which structural or functional brain abnormalities are associated with frequent irrational decisions in MS. Previous studies have indicated an association of reduced performance on the Beads Task, in terms of poor information sampling and/or frequent irrational decisions, with changes in fronto‐subcortical circuits [4, 5, 6, 20]. A recent review has also suggested a relevant role of the dopaminergic system in the expression of different cognitive biases and distortions [21]. Whether this also applies for MS is yet to be determined.
One limitation of our study arises from the small sample size. Our sample is likely not fully representative of RRMS. As we excluded patients with an EDSS score higher than 4, we might have missed peculiarities related to disease severity. Moreover, the beads task does not explicitly explore medical decisions. Therefore, our results need to be interpreted cautiously. Finally, we should acknowledge that other methods can be adopted to assess information sampling. For example, the drift diffusion model pertains to the sequential sampling models in mathematical psychology and has been recently also used to study choice behavior in older people or in different clinical populations [22]. One of its advantages is to consider both response accuracy and response latency at the same time. Furthermore, it allows an assessment of the individual ability taking into account differences in the speed of peripheral processes such as encoding a stimulus or executing a response [22]. This is particularly relevant for the investigation of high‐level cognitive processes in clinical conditions like MS which are known to affect processing speed. Unfortunately, we did not collect response times with our paradigm and thus cannot apply an analysis following the drift diffusion model.
In recent decades, the variety of available disease‐modifying treatment options in MS has increased. As a consequence, treatment decisions have become much more complex. Also, there are therapies for MS that offer substantial benefits, but that are associated with potentially serious, even life‐threatening side effects [23]. Intact risk understanding and DM are therefore crucial to enable true shared decision making. In general, our findings strongly advocate for awareness of health professionals that their patients may tend to irrational decisions. Against this background, health professionals should communicate disease‐related information in an unambiguous way and discuss with patients their decisions.
ETHICAL STATEMENT
The study was approved by the ethics committee of the Medical University Innsbruck (ethical approval number: UN5044, 324/4.5) and conforms with the World Medical Association Declaration of Helsinki for studies involving human subjects. Written informed consent was obtained from all individuals before participation.
CONFLICT OF INTERESTS
None.
Funding information
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the Medical University Innsbruck (MUI‐START 2014‐05‐001).
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors thank all the patients and controls for their participation in the study.
The study was conducted at Medical University Innsbruck, Department of Neurology, Innsbruck, Austria.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Neuhaus M, Calabrese P, Annoni J‐M. Decision‐making in multiple sclerosis patients: a systematic review. Mult Scler Int. 2018;2018:7835952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zamarian L, Delazer M, Ehling R, et al. Improvement of medical judgments by numerical training in patients with multiple sclerosis. Eur J Neurol. 2019;26(1):106‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453‐458. [DOI] [PubMed] [Google Scholar]
- 4. Djamshidian A, O’Sullivan SS, Sanotsky Y, et al. Decision making, impulsivity, and addictions: do Parkinson’s disease patients jump to conclusions? Mov Disord Off J Mov Disord Soc. 2012;27(9):1137‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Djamshidian A, O’Sullivan SS, Foltynie T, et al. Dopamine agonists rather than deep brain stimulation cause reflection impulsivity in Parkinson’s disease. J Park Dis. 2013;3(2):139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heim B, Pertl M‐T, Stefani A, et al. Reflection impulsivity perceptual decision‐making in patients with restless legs syndrome. Ann Clin Transl Neurol. 2018;5(3):315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiebener J, Brand M. Decision making under objective risk conditions‐a review of cognitive and emotional correlates, strategies, feedback processing, and external influences. Neuropsychol Rev. 2015;25(2):171‐198. [DOI] [PubMed] [Google Scholar]
- 8. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. [zitiert 2017 Sep 20]. Available from: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444‐1452. [DOI] [PubMed] [Google Scholar]
- 10. Zamarian L, Benke T, Buchler M, Wenter J, Delazer M. Information about medications may cause misunderstanding in older adults with cognitive impairment. J Neurol Sci. 2010;298(1–2):46‐51. [DOI] [PubMed] [Google Scholar]
- 11. Brand M, Schiebener J, Pertl M‐T, Delazer M. Know the risk, take the win: how executive functions and probability processing influence advantageous decision making under risk conditions. J Clin Exp Neuropsychol. 2014;36(9):914‐929. [DOI] [PubMed] [Google Scholar]
- 12. Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision‐making in ambiguous and risky situations. Neural Netw Off J Int Neural Netw Soc. 2006;19(8):1266‐1276. [DOI] [PubMed] [Google Scholar]
- 13. Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision‐making. Science. 2005;310(5754):1680‐1683. [DOI] [PubMed] [Google Scholar]
- 14. Tobler PN, Weber EU. Valuation for risky and uncertain choices [Internet]. Neuroeconomics. Elsevier. 2014. [zitiert 2019 Apr 18]. Seite 149–172. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780124160088000097 [Google Scholar]
- 15. Armstrong T, Rockloff M, Browne M. Gamble with your head and not your heart: a conceptual model for how thinking‐style promotes irrational gambling beliefs. J Gambl Stud. 2020;36(1):183‐206. [DOI] [PubMed] [Google Scholar]
- 16. McElroy T, Seta JJ. Framing effects: an analytic–holistic perspective. J Exp Soc Psychol. 2003;39(6):610‐617. [Google Scholar]
- 17. Evans JSBT. In two minds: dual‐process accounts of reasoning. Trends Cogn Sci. 2003;7(10):454‐459. [DOI] [PubMed] [Google Scholar]
- 18. Reyna VF. How people make decisions that involve risk: a dual‐processes approach. Curr Dir Psychol Sci. 2004;13(2):60‐66. [Google Scholar]
- 19. Tversky A, Kahneman D. Extensional versus intuitive reasoning: the conjunction fallacy in probability judgment. Psychol Rev. 1983;90(4):293‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banca P, Lange I, Worbe Y, et al. Reflection impulsivity in binge drinking: behavioural and volumetric correlates: Impulsivity in binge drinking. Addict Biol. 2016;21(2):504‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cocker PJ, Winstanley CA. Irrational beliefs, biases and gambling: exploring the role of animal models in elucidating vulnerabilities for the development of pathological gambling. Behav Brain Res. 2015;279:259‐273. [DOI] [PubMed] [Google Scholar]
- 22. Forstmann BU, Ratcliff R, Wagenmakers E‐J. Sequential sampling models in cognitive neuroscience: advantages, applications, and extensions. Annu Rev Psychol. 2016;67:641‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta S, Weinstock‐Guttman B. Natalizumab for multiple sclerosis: appraising risk versus benefit, a seemingly demanding tango. Expert Opin Biol Ther. 2014;14(1):115‐126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data available on request from the authors.


