Abstract
Objective
To examine the association between social isolation and mortality and incident diseases in middle-aged adults in urban and rural communities from high-income, middle-income and low-income countries.
Design
Population-based prospective observational study.
Setting
Urban and rural communities in 20 high income, middle income and low income.
Participants
119 894 community-dwelling middle-aged adults.
Main outcome measures
Associations of social isolation with mortality, cardiovascular death, non-cardiovascular death and incident diseases.
Results
Social isolation was more common in middle-income and high-income countries compared with low-income countries, in urban areas than rural areas, in older individuals and among women, those with less education and the unemployed. It was more frequent among smokers and those with a poorer diet. Social isolation was associated with greater risk of mortality (HR of 1.26, 95% CI: 1.17 to 1.36), incident stroke (HR: 1.23, 95% CI: 1.07 to 1.40), cardiovascular disease (HR: 1.15, 95% CI: 1.05 to 1.25) and pneumonia (HR: 1.22, 95% CI: 1.09 to 1.37), but not cancer. The associations between social isolation and mortality were observed in populations in high-income, middle-income and low-income countries (HR (95% CI): 1.69 (1.32 to 2.17), 1.27 (1.15 to 1.40) and 1.47 (1.25 to 1.73), respectively, interaction p=0.02). The HR associated with social isolation was greater in men than women and in younger than older individuals. Mediation analyses for the association between social isolation and mortality showed that unhealthy behaviours and comorbidities may account for about one-fifth of the association.
Conclusion
Social isolation is associated with increased risk of mortality in countries at different economic levels. The increasing share of older people in populations in many countries argues for targeted strategies to mitigate its adverse effects.
Keywords: public health
Key questions.
What is already known?
With ageing populations, urbanisation and fewer extended families, social isolation is becoming more common.
Social isolation is associated with negative health consequences.
Prior studies on social isolation are mainly from high-income countries, primarily from urban populations.
What are the new findings?
For the first time, we investigate associations between social isolation and health outcomes in middle-aged community-dwelling adults from urban and rural sites in high-income, middle-income and low-income countries.
The mortality risk of social isolation is consistently observed among diverse populations regardless of residence area (rural or urban) and country income level.
The mortality risk was partly explained by unhealthy behaviours and baseline comorbidities.
Social isolation is associated with increased risk of incident stroke, cardiovascular disease and pneumonia.
What do the new findings imply?
Our study shows that the risk of mortality associated with social isolation is observed consistently among diverse populations regardless of residence area (rural or urban) and country income level. Healthcare workers and policy-makers should consider social isolation as an added risk factor for premature death.
Introduction
Social isolation is characterised as the absence of social relationships1 in the forms of social contacts, social resources and participation in social or religious activities.2 3 Ageing populations, urbanisation and fewer extended families are increasing levels of social isolation in many countries. In meta-analyses, social isolation was associated with a 29% increase in the risk of death4 and a 29% increase in the risk of coronary heart disease and 32% increase in the risk of stroke.5 However, most these studies are from high-income countries (HICs), primarily from urban populations, and with a focus on older people6–9 with few studying the general adult population. None examined whether there are differences in observed associations in countries at different levels of economic development. This last point is important because while social networks in poor countries may be stronger, social services provided by governments or other organisations may be weaker.10 Furthermore, family and social structures in rural communities may differ from that in urban communities. Here, we examine the relationship between social isolation and health outcomes in middle-aged community-dwelling adults from urban and rural sites in several HIC, middle-income country (MIC) and low-income country (LIC).
Methods
The objective of this study was to examine the association between social isolation and both mortality and certain incident diseases in middle-aged adults in urban and rural communities from HIC, MIC and LIC. We hypothesised that social isolation is associated with increased risk of mortality in populations everywhere but the magnitude of associations between social isolation and outcomes may vary by the economic development of a country, by urban or rural residence, between men and women and by age group.
This is a secondary analysis of the Prospective Urban Rural Epidemiology (PURE) study, which is a prospective, population-based cohort study that has recruited community-dwelling adults aged 35–70 years old from both urban and rural areas.11 Details of the sampling methods, response rates, documentation of events and their adjudication have been published previously and are summarised in the online supplemental appendix 1. Countries selected were classified according to the World Bank scheme as HIC, MIC and LIC at the beginning of the study in 2006. The HIC include Canada, Saudi Arabia, Sweden and the United Arab Emirates. The MIC include Argentina, Brazil, Chile, China, Colombia, Iran, Malaysia, Palestine, Philippines, Poland, South Africa and Turkey. The LIC include Bangladesh, India, Pakistan, Tanzania and Zimbabwe. Although some of the countries have subsequently been reclassified as their economic status has changed, for consistency with previous publications from PURE, we have retained the countries within their original economic categories assigned at the beginning of the study. Information on lifestyles, health-related risk factors, the presence of chronic disease and outcomes were collected using standardised methods (online supplemental appendix 2). Sampling aimed to achieve a broadly representative sample of adults living in each community. Although different sites used varying methods of approaching households depending on what was practical in each setting, all enrol individuals irrespective of the presence of pre-existing conditions. At least three attempts to contact individuals were made in all approaches. If a household was eligible (at least one member was between the ages of 35 and 70 years), then all consenting and eligible individuals were enrolled. Once recruited, all participants were invited to a clinic to complete a standardised set of questionnaires and measurements. Participants or other family members were contacted in person or by telephone at least every 3 years to document deaths and key non-fatal events. Information on medically certified death was accessed through administrative registries, where available. Otherwise, event documentation was obtained from household interviews, medical records, death certificates, verbal autopsies12 and other sources. Participants from China (n=47 927) are not included in this study since local ethics committees did not allow some of the key questions on social isolation.
bmjgh-2020-004124supp001.pdf (109.2KB, pdf)
Patient and public involvement
The study participants and the public were not involved in the design and conduct of this study.
Assessment of social isolation
A single measure of social isolation has not yet been agreed. Berkman and Syme constructed their social network index (The Social Network Index; SNI) to assess social relations in 1979.2 Subsequent studies have used the SNI or variations of it as measures of social isolation. The SNI comprises four domains; information on partnership, contact with family members or friends, engagement in religious activities and membership in organisations or clubs. In this analysis, we measured social isolation using an adaptation of the SNI. The social isolation scale was constructed using five items from the PURE baseline questionnaire relevant to the SNI:
Marital status (scored as 1 for any of the following: never married, widowed, separated, or divorced and 0 otherwise).
‘Can you count on your family members in a difficult situation?’ (possible responses include none, little, moderate/average and a great deal)—scored as 1 for ‘none’ or ‘little’ and 0 for ‘moderate’ or ‘a great deal’
‘Can you count on any organization in a difficult situation?’ (possible responses include none, little, moderate/average and a great deal—scored as 1 for ‘none’ or ‘little’ and 0 for ‘moderate’ or ‘a great deal’.
‘Are you a member of any religious group?’ (yes=0, no=1).
‘Are you a member of any social group?’ (yes=0, no=1).
The social isolation scale ranges from 0 to 5. Individuals who score 0 are defined as having the most social support and those who score 5 are defined as having maximum social isolation. In preparatory work, we explored using the scale as a continuous or binary predictor and confirmed the relationship with mortality was non-linear and it was more appropriate to treat social isolation as a binary variable. We therefore considered individuals with a score of 4 or 5 as being socially isolated.
Outcome
The outcomes of interest for this analyses were all-cause mortality, cardiovascular mortality, non-cardiovascular mortality and incidence of selected diseases (myocardial infarction, stroke, heart failure, cardiovascular disease (CVD), cancer, pneumonia, chronic obstructive pulmonary disease (COPD) and injury). CVD included fatal or non-fatal myocardial infarction, stroke, heart failure and other fatal CVD events.
Statistical analysis
The characteristics of participants in each of the two groups (social isolation vs no social isolation) were compared using χ2 tests for categorical variables and student t-test or the Mann-Whitney U test for continuous variables. Multivariable logistic regression analyses with social isolation as a dependent variable were conducted to evaluate factors associated with social isolation. We used Cox proportional-hazard regression models to evaluate the relationship between social isolation and mortality. To account for the clustered nature of the data, we used shared frailty models in which the community to which each individual belongs served as the clustering variable. In the models, those with a social isolation score of 0–3, served as the reference group. The adjusted model included following baseline variables: age, sex, education attainment (presecondary, secondary or postsecondary education), residence area (rural or urban), country income (LIC, MIC or HIC), smoking, alcohol use, physical inactivity, diet score, hypertension, diabetes, coronary artery disease, depression and disabilities. We also performed Cox regression analyses using the adjusted model to evaluate the relationship between social isolation and incident disease (myocardial infarction, stroke, heart failure, CVD, cancer, pneumonia, COPD and injury). The incidence rates of each outcome were expressed in person-years (per thousand). The CIs are calculated using the quadratic approximation to the Poisson log likelihood for the log-rate parameter. Definitions and values of baseline participant characteristics are reported in online supplemental appendix 3. To quantify the contribution of risk factors to mortality, population attributable fractions were calculated13 from a Cox proportional regression model, in which social isolation, education attainment, smoking, alcohol, physical inactivity, diet quality, hypertension, diabetes and depression were included. Mediation analyses were performed to examine what factors mediate any relationship between social isolation and mortality. Factors chosen as the candidate potential mediators were behavioural factors (current smoking, current alcohol, physical inactivity and low diet quality) and comorbidities (hypertension, diabetes, abdominal obesity, coronary artery disease, stroke, cancer, disabilities and depression). The analytical methods are provided in online supplemental appendix 4. To minimise the potential for reverse causality, we performed sensitivity analyses in which participants with diseases at baseline or those who developed clinical outcomes within the first 2 years of follow-up were excluded. STATA V.15.1 was used for statistical analyses and graphs.
Results
Characteristics of participants with and without social isolation
A flow chart describing the selection of the study population is provided in online supplemental figure 1. A total of 119 894 individuals were enrolled between 6 July 2005 and 2 June 2016, of whom 118 764 with the social isolation scale recorded were included in this study. The proportions of participants from LIC, MIC and HIC were 31.9% (n=37 863), 52.9% (n=62 855) and 15.2% (n=18 046), respectively. The prevalence of social isolation (social isolation scale of 4 or 5) was 10.9% (n=12 992). Socially isolated participants were older and more likely to be women (table 1). They had higher prevalence of baseline comorbid conditions including hypertension, diabetes mellitus, coronary artery disease, stroke, cancer, COPD, as well as depression. Table 2 shows participants’ characteristics associated with social isolation. Older age, being female, with a low level of education and unemployed were associated with increased odds of being socially isolated. Social isolation was more common in urban than rural areas; and in MICs and HICs compared with LICs. Current smoking, poor diet and disabilities were associated with social isolation.
Table 1.
Baseline characteristics of study participants with and without social isolation
| Characteristic | No social isolation (n=1 05 772) |
Social isolation (n=12 992) |
P value Univariate analysis |
| Age, year | 50.1±9.8 | 52.2±10.2 | <0.0001 |
| Women (%) | 59 567 (56.3) | 9402 (72.4) | <0.0001 |
| Hypertension (%) | 22 682 (21.5) | 3372 (26.0) | <0.0001 |
| Diabetes mellitus (%) | 10 066 (9.5) | 1357 (10.5) | 0.001 |
| Coronary artery disease (%) | 3115 (3.0) | 443 (3.4) | 0.003 |
| Stroke (%) | 1396 (1.3) | 271 (2.1) | <0.0001 |
| Cancer (%) | 1646 (1.6) | 255 (2.0) | 0.001 |
| COPD (%) | 845 (0.9) | 159 (1.2) | 0.001 |
| Depression (%) | 15 570 (14.8) | 2670 (20.6) | <0.0001 |
COPD, chronic obstructive pulmonary disease.
Table 2.
Factors associated with social isolation using multivariable logistic regression analyses
| Adjusted OR (95% CI) | |
| Age, 10-year increase | 1.06 (1.03 to 1.09) |
| Women (vs men) | 2.17 (2.06 to 2.29) |
| Education attainment level Presecondary (vs secondary or postsecondary) |
1.37 (1.29 to 1.45) |
| Unemployed vs employed | 1.16 (1.10 to 1.22) |
| Residence in urban (vs rural) |
1.64 (1.55 to 1.73) |
| Country income level (low as reference) | |
| Middle vs low | 2.41 (2.25 to 2.57) |
| High vs low | 2.03 (1.85 to 2.22) |
| Current smoking (vs former or never smoking) | 1.33 (1.25 to 1.41) |
| Low diet score (lowest tertile of diet score) (vs the other two tertiles) | 1.12 (1.07 to 1.18) |
| Current alcohol use (vs former or never drinking) | 1.04 (0.98 to 1.10) |
| Physical inactivity (vs WHO recommended physical activity) | 1.04 (0.97 to 1.11) |
| Number of comorbidities≥2 (vs one or no comorbidities) | 1.06 (0.97 to 1.15) |
| Number of disabilities≥2 (vs one or no disabilities) | 1.27 (1.20 to 1.35) |
ORs were adjusted for age, sex, education attainment, employment status, residence area, country income level, smoking, alcohol, presence of physical inactivity, diet score, presence of comorbidities and presence of disabilities.
bmjgh-2020-004124supp002.pdf (85.7KB, pdf)
bmjgh-2020-004124supp003.pdf (103.3KB, pdf)
Social isolation by country income
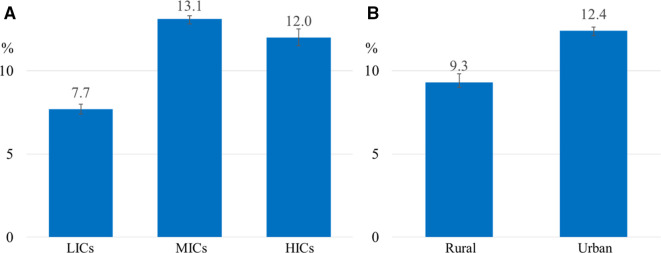
The age-sex adjusted prevalence of social isolation in LIC, MIC and HIC were 7.7%, 13.1% and 12.0%, respectively (figure 1A). Table 3 shows the participant characteristics of participants associated with social isolation. Women were more likely to be socially isolated, consistently across countries at all income levels. The directions of the association between other factors and social isolation were inconsistent across different country income levels. For example, low education was associated with social isolation in LICs and MICs, while no association was observed in HICs. Unemployment was strongly associated with social isolation in LICs, while similar associations were not observed in MICs or HICs. Higher age was associated with social isolation in LICs and MICs but not in HICs.
Figure 1.
Age-sex adjusted prevalence of social isolation by country income levels (A) and by residence areas (B). The prevalence of social isolation is the lowest in the low-income countries (A). The prevalence of social isolation is higher in the urban areas (B). HICs, high-income countries; LICs, low-income countries; MICs, middle-income countries.
Table 3.
Variations in the association of factors that are associated with social isolation by income level of countries using multivariable logistic regression analyses
| Variables | Adjusted OR (95% CI) | P for interaction | ||
| Country income level | ||||
| Low (n=37 863) |
Middle (n=62 855) |
High (n=18 046) |
||
| Age, 10-year increase | 1.08 (1.02 to 1.15) | 1.18 (1.14 to 1.22) | 0.92 (0.86 to 0.98) | <0.0001 |
| Women (vs men) | 1.42 (1.22 to 1.64) | 2.65 (2.47 to 2.84) | 1.46 (1.30 to 1.64) | <0.0001 |
| Education attainment level Presecondary (vs secondary or postsecondary) |
2.64 (2.30 to 3.02) | 1.16 (1.08 to 1.24) | 1.16 (0.97 to 1.38) | <0.0001 |
| Unemployed vs employed | 3.52 (3.05 to 4.05) | 0.93 (0.87 to 0.99) | 0.99 (0.87 to 1.14) | <0.0001 |
| Residence area urban (vs rural) |
1.12 (0.99 to 1.26) | 1.67 (1.56 to 1.79) | 1.72 (1.49 to 1.98) | <0.0001 |
| Current smoking (vs former or never smoking) | 0.73 (0.62 to 0.87) | 1.37 (1.27 to 1.48) | 1.91 (1.66 to 2.19) | <0.0001 |
| Low diet score (lowest tertile of AHEI) (vs the other two tertiles) | 0.44 (0.37 to 0.52) | 1.35 (1.27 to 1.44) | 1.04 (0.92 to 1.17) | <0.0001 |
| Current alcohol use (vs former or never drinking) | 1.54 (1.27 to 1.87) | 1.34 (1.26 to 1.44) | 0.48 (0.43 to 0.55) | <0.0001 |
| Physical inactivity (vs WHO recommended physical activity) | 0.78 (0.67 to 0.90) | 0.84 (0.77 to 0.92) | 1.84 (1.60 to 2.12) | <0.0001 |
| Number of comorbidities≥2 (vs one or no comorbidities) | 0.86 (0.66 to 1.13) | 1.03 (0.93 to 1.15) | 1.24 (1.03 to 1.49) | 0.01 |
| Number of disabilities≥2 (vs one or no disabilities) | 1.03 (0.90 to 1.19) | 1.21 (1.13 to 1.31) | 1.26 (1.06 to 1.48) | 0.2 |
ORs were adjusted for age, sex, education attainment, employment status, residence area, smoking, alcohol, presence of physical inactivity, diet score, presence of comorbidities and presence of disabilities.
AHEI, alternative healthy eating index.
Social isolation in urban and rural populations
The age-sex adjusted prevalence of social isolation in rural areas was 9.3% compared with 12.4% in urban areas (figure 1B). The participant characteristics stratified urban and rural residence associated with social isolation are shown in online supplemental appendix 5. The patterns of all variables except for employment status and current smoking were similar irrespective of rural or urban area of residence. Older age, women, low education, poor diet, current alcohol use and disabilities were associated with increased odds of being socially isolated. Unemployment was associated with social isolation in rural but not urban residence. Current smoking was associated with social isolation in urban but not in rural residence.
Association between social isolation and mortality
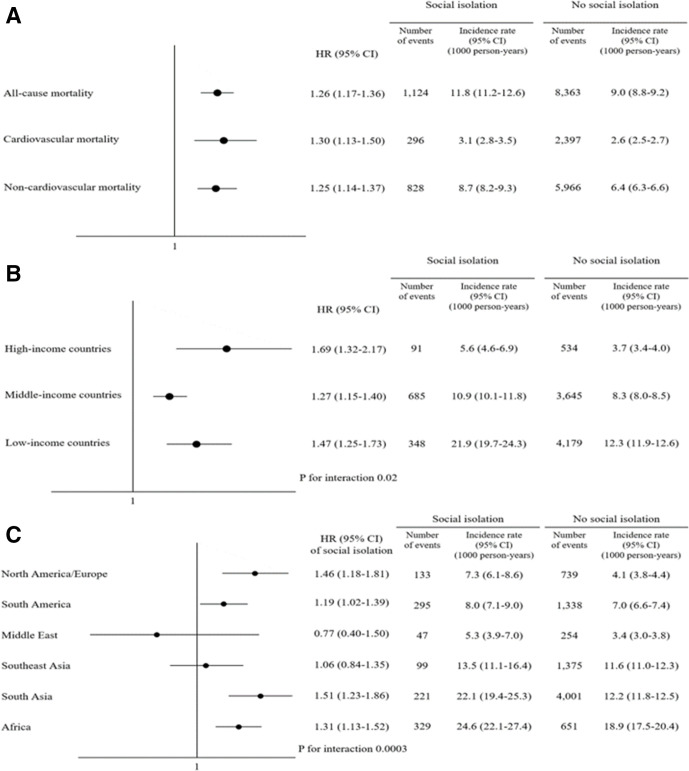
Survival analyses were conducted in 115 816 (97.5%) individuals whose vital status was available. During the mean follow-up of 9.0 years, we observed 9487 (8.2%) deaths (2693 cardiovascular and 6794 non-cardiovascular). The adjusted HR of mortality for social isolation was 1.26 (95% CI: 1.17 to 1.36) (figure 2A). The adjusted HR for cardiovascular and non-cardiovascular mortality were 1.30 (95% CI: 1.13 to 1.50), and 1.25 (95% CI: 1.14 to 1.37), respectively. The magnitude of mortality risks associated with social isolation was greatest in HICs (figure 2B). While social isolation was consistently associated with increased risk of all-cause mortality regardless of age, sex, area of residence and country income level, the magnitude of the association was greater in younger adults and men.
Figure 2.
The mortality risk of social isolation. Social isolation is associated with increase in the risk of all-cause, cardiovascular and non-cardiovascular mortality (A). The mortality risk associated with social isolation is greatest in HICs (B). The incidence rates of death were higher among the socially isolated and the mortality risk of social isolation was observed across regions with some random variations (C). HICs, high-income countries.
Population attributable fractions were examined to quantify the contribution of social isolation to all-cause mortality. These are compared with similar data for other risk factors. The population attributable fraction of social isolation was 2.4%, which is modest compared with the other risk factors (figure 3). Regional variations in mortality risks associated with social isolation are shown in figure 2C. Overall, mortality rates were higher among the socially isolated and although with some variations across regions. The magnitude of the association between social isolation and mortality was greatest in South Asia, North America/Europe followed by Africa and South America. The association was not significant in Middle East and Southeast Asia.
Figure 3.
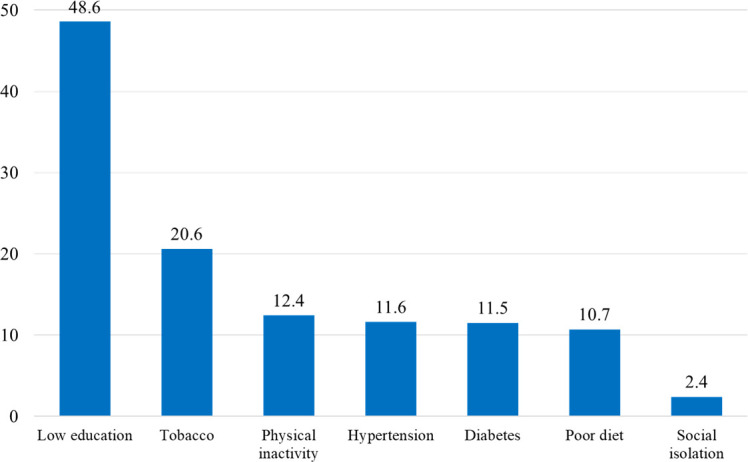
The population attributable fraction of mortality for risk factors in the overall population. Social isolation is a modest but significant contributor to mortality in the whole study participants. Education and smoking substantially contributed to mortality.
Mediation analyses for the association between social isolation and mortality showed that unhealthy behaviours accounted for 18% of the association, while comorbidities explained 3% of the association. A model adjusted for both behavioural factors and comorbidities showed that these variables accounted for 21% of the association.
Association between social isolation and incident disease
During follow-up, a new myocardial infarction occurred in 3417 (3.0%), a new stroke in 2129 (1.8%), new onset heart failure in 827 (0.7%), a new cancer in 4377 (3.8%), pneumonia in 2578 (2.2%), a new diagnosis of COPD in 1423 (1.2%) and hospitalisation for injury in 13 608 (11.7%). Figure 4 shows that after adjustment social isolation was associated with an increased risk of stroke (HR: 1.23, 95% CI: 1.07 to 1.40) and CVD (HR: 1.15, 95% CI: 1.05 to 1.25). For non-cardiovascular events, a significant association was observed only for pneumonia (HR: 1.22, 95% CI: 1.09 to 1.37). The associations stratified by country income levels are shown in online supplemental figure 2.
Figure 4.
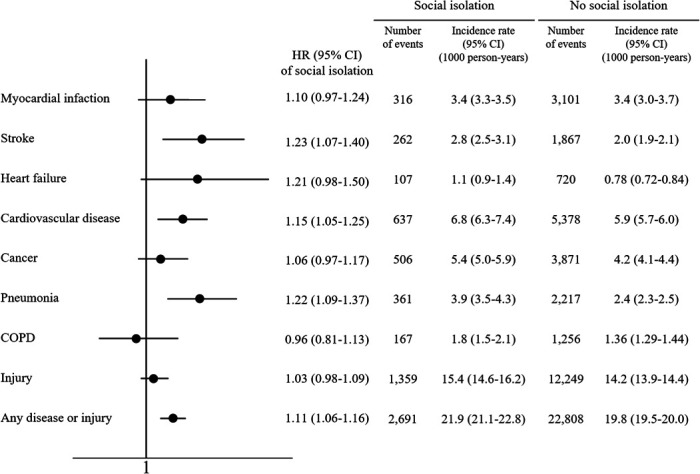
Multivariable Cox regression analyses for the association between social isolation and incident diseases. Social isolation is significantly associated with increased risk of stroke, cardiovascular disease and injury while no associations are observed in relation to other incident diseases. HRs are adjusted for age, sex, education, residence area, country income level, smoking, alcohol, hypertension, diabetes, coronary artery disease and stroke. COPD, chronic obstructive pulmonary disease.
bmjgh-2020-004124supp004.pdf (100.3KB, pdf)
To further examine the impact of social isolation on mortality, case fatality rates within 90 days from the occurrence of new illnesses were assessed after a clinical event (myocardial infarction, stroke, heart failure, CVD, cancer, pneumonia, COPD and injury). Only for stroke was the case fatality rates higher in the socially isolated (online supplemental figure 3). The higher risk and case fatality rates of strokes could partly explain their increased risk of cardiovascular mortality.
bmjgh-2020-004124supp005.pdf (1MB, pdf)
Discussion
Principal findings
There are four main findings from our study: (1) social isolation is more common among women, older individuals, those with low levels of education or unemployed, living in urban areas and in richer countries; (2) social isolation is independently associated with increased risk of mortality after adjusting for conventional risk factors for CVD and this is observed in HIC, MIC and LIC. The mortality risk was partly explained by unhealthy behaviours and baseline comorbidities; (3) social isolation is associated with increased risk of incident stroke, CVD and pneumonia and (4) the population attributable fraction of social isolation on mortality is 2.4%.
Comparison with other studies
Factors associated with higher prevalence of social isolation
Previous studies have shown that older adults are at risk of being socially isolated due to limited mobility caused by chronic illnesses and disabilities or reduced social ties through life events such as retirement or loss of their spouses, family members or friends.4 14 15 Our findings are consistent with this. Previous studies have reported contradictory findings on gender difference in the prevalence of social isolation.16–18 In our study, social isolation was more common among women. This may be due to our finding that women had lower rates of education and lower employment outside the home. In addition, lack of social supports might limit interactions with other individuals or groups, particularly in LICs and MICs. However, the higher rates of social isolation among women were observed in countries at all economic levels although most prominent in LICs.
Current smoking was more common in socially isolated people in our study, consistent with prior reports that those who are socially isolated are more likely to engage in unhealthy behaviours.16 19 20 In a French cohort,21 socially isolated men were more likely to be smokers and to indulge in heavy episodic drinking. Smokers might be forced to move to the periphery of social networks under pressure to avoid interactions with non-smokers due to concerns about the health risks of secondhand smoke.19 22 Socially isolated individuals were less likely to quit smoking as compared with those with social ties.23 Poor diet quality was also more common in those who were socially isolated. Social connection could increase the likelihood of engaging in health-promoting behaviours, presumably because people with social connections may receive advice or support from other people, while social isolation might reduce people’s sense of obligation to stay healthy, which results in engaging in unhealthy behaviours. On the other hand, social networks could be limited by the presence of disabilities and chronic diseases as they form barriers to social interactions.24 25
Variations in social isolation in HIC, MIC and LIC
The major differences between HIC, MIC and LIC with respect to patterns of social isolation that we observed are as follows. (1) Older age was not associated with social isolation in HICs but was in LICs and MICs. We hypothesise that better social networks and community services for older people in HICs may account for this pattern. (2) The association between social isolation and current smoking was strong in HICs, while no association was observed in LICs. It is possible that smoking might encourage social isolation in HICs where smoking is less socially acceptable and public smoking is more strictly restricted. (3) In LICs, low education and unemployment were strongly associated with social isolation, suggesting that social connectedness is more affected by economic opportunities in LICs. (4) Urban residence was strongly associated with social isolation particularly in MICs and HICs. Urban communities might have weaker social connectedness through having fewer opportunities for social contacts, especially in highly developed countries.24
Variation in social isolation in urban versus rural settings
We found that social isolation was more common in urban areas than in rural areas. This might reflect a lack of interest in remaining connected or lack of interpersonal relationships in urban areas perhaps shaped by different values that focus on personal success.24 Furthermore, women in urban communities were more likely to be socially isolated than their counterparts in rural settings.
Despite the similarities in factors associated with social isolation, the magnitude of certain associations was different between rural and urban areas. For example, a stronger association was observed in women in urban areas, which may related to smaller social networks than their rural peers possibly due to greater economic independence or barriers of personal relationships with individuals in their communities which may hinder building social relationships. We also found that disability was more closely related to social isolation in rural areas as compared with urban areas. We speculate that larger interpersonal distances in rural settings may have a disproportionately large isolating effect on those with disabilities.26
Adverse health consequences associated with social isolation
In line with previous research, our study shows that social isolation is associated with a 26% increased risk of mortality with similar results for cardiovascular and non-cardiovascular mortality. The increased mortality risk was observed in different age groups, in men and women, in those living in rural or urban areas or in countries at different income levels. There has been a paucity of data regarding underlying mechanisms through which social isolation has influence on mortality. A study in the UK of people with a mean age of 57 years and mean follow-up of 6.5 years found that lifestyle behaviours, socioeconomic factors and mental health could explain 64% of the mortality risk associated with social isolation.27 In our study, mediation analyses of the association between social isolation and mortality attributed 21 of the association to unhealthy behaviours and baseline comorbidities.
Previous studies have produced conflicting results on the association between social isolation and incident CVD.5 16 28 A meta-analysis reported an increased risk of coronary heart disease and stroke in individuals reporting social isolation,5 but more recent studies did not report support this conclusion.28 29 These inconsistent results could be explained by differences in study populations or definitions of social isolation. Our study found that social isolation was associated with increased risk of CVD and particularly stroke. The higher case fatality rates from stroke, along with their higher incidence of strokes, could partly explain the increased risk of cardiovascular mortality.
Evidence of an association between social isolation and non-CVD is scarce. We only found a significant association for pneumonia despite non-cardiovascular mortality being higher in people with social isolation. The increased risk of non-cardiovascular mortality might be explained by self-harm, substance abuse or suicide associated with social isolation though detailed information on these events were not available in this study. Previous studies showed that socially isolated individuals are at high risk of cancer, pulmonary disease as well as infection.30 31 Fewer social ties were associated with a higher risk of developing respiratory disease,32 33 which were consistent to our finding. The link between social isolation and non-CVD could be explained by older age, unhealthy lifestyle behaviours and pre-existing chronic illnesses,17 which may make them vulnerable to death. Also, social networks might play a role in resisting infection through regulation of the immune system.34
Strengths and weaknesses of the study
Our study is the first study to examine the associations of social isolation with health outcomes in 20 countries from five continents, including LIC, MIC and HIC and from urban and rural communities. The large size of our study and the diversity of the populations has allowed examination of the consistency or heterogeneity of associations in different settings and in different subgroups of the population.
Our study has some potential limitations. First, it is not possible to exclude unmeasured confounding factors such as feelings of loneliness, history of substance abuse and criminal records or victimhood in this observational study, although a wide range of potential explanatory factors were studied. Second, reverse causality could be a concern. We conducted sensitivity analyses to address this concern. In those analyses, individuals with disease at baseline or those who developed clinical outcomes within the first 2 years of follow-up were excluded, which did not alter our results. Third, covariates adjusted for the analyses were assessed only at baseline, but demographic data including country income levels and other socioeconomic factors could have changed over the study period. However, since the association between social isolation and clinical outcomes is similar in HIC, MIC and LIC, a shift in the categorisation of countries from one economic group to another would not be expected to materially alter our results. Regarding our mediation analyses, potential mediators were not independent of each other since some factors (ie, smoking, alcohol) may mediate not only mortality but also some comorbidities (ie, CVD, cancer). Thus, the estimates for the mediation effects may be affected by other factors. Besides that, since potential mediators were only assessed at baseline, causal relationship among social isolation, potential mediators and outcomes cannot be reliably derived using our current study design. In our analyses, only high alcohol intake defined as >14 drinks/week for women or 21 drinks/week for men was associated with increased risk of mortality in a multivariable Cox regression analysis (HR: 1.48, 95% CI: 1.31 to 1.67), pointing to the particular importance of socially isolated people taking care about excessive alcohol intake. Finally, our social isolation scale did not include information on living alone, subjective social isolation (ie, loneliness), or social network size that may provide a more nuanced reflection of social isolation. Future studies should include such information as well as new concepts which emerge in this field of scholarship.
Implications for clinicians and policy-makers
Our findings support for strategies to address several factors (lower socioeconomic status and unhealthy lifestyles) and consequences of social isolation. Healthcare workers and policy-makers may wish to consider social isolation as an added factor in identifying individuals at higher risk who may benefit from specific measures that go beyond the usual preventive and treatment strategies, to mitigate their higher risk.
Conclusions
Our study is the first to demonstrate the associations between social isolation and health outcomes in middle-aged community-dwelling adults from urban and rural sites in HIC, MIC and LIC. Social isolation is associated with increased risk of death and morbidity among diverse populations across the world. It should be considered as an added risk factor to that conferred by conventional risk factors. The best ways of addressing this issue, whether intensive use of proven therapies and lifestyle modification or measures to improve social support remain unclear and may be context dependent.
Acknowledgments
PURE Project Office Staff, National Coordinators, Investigators, and Key Staff:
Project office (Population Health Research Institute, Hamilton Health Sciences and McMaster University, Hamilton, Canada): S Yusuf* (Principal Investigator).
S Rangarajan (Program Manager); K K Teo, S S Anand, C K Chow, M O’Donnell, A Mente, D Leong, A Smyth, P Joseph, M Duong, R D’Souza, M Walli-Attaei, S Islam (Statistician), W Hu (Statistician), C Ramasundarahettige (Statistician), P Sheridan (Statistician), S Bangdiwala, L Dyal, B Liu (Biometric Programmer), C Tang (Biometric Programmer), X Yang (Biometric Programmer), R Zhao (Biometric Programmer), L Farago (ICT), M Zarate (ICT), J Godreault (ICT), M Haskins (ICT), M Jethva (ICT), G Rigitano (ICT), A Vaghela (ICT), M Dehghan (Nutrition Epidemiologist), A Aliberti, A Reyes, A Zaki, B Connolly, B Zhang, D Agapay, D Krol, E McNeice, E Ramezani, F Shifaly, G McAlpine, I Kay, J Rimac, J Swallow, M Di Marino, M Jakymyshyn, M(a) Mushtaha, M(o) Mushtaha, M Trottier, N Aoucheva, N Kandy, P Mackie, R Buthool, R Patel, R Solano, S Gopal, S Ramacham, S Trottier
Core Laboratories: G Pare, M McQueen, S Lamers, J Keys (Hamilton), X Wang (Beijing, China), A Devanath (Bangalore, India).
Argentina: R Diaz*, A Orlandini, P Lamelas, M L Diaz, A Pascual, M Salvador, C Chacon; Bangladesh: O Rahman*, R Yusuf*, S A K S. Ahmed, T Choudhury, M Sintaha, A Khan, O Alam, N, Nayeem, S N Mitra, S Islam, F Pasha; Brazil: A Avezum*, C S Marcilio, A C Mattos, G B Oliveira; Canada: K Teo*, S Yusuf*, Sumathy Rangarajan, A Arshad, B Bideri, I Kay, J Rimac, R Buthool, S Trottier, G Dagenais, P Poirier, G Turbide, AS Bourlaud, A LeBlanc De Bluts, M Cayer, I Tardif, M Pettigrew, S Lear, V de Jong, A N Saidy, V Kandola, E Corber, I Vukmirovich, D Gasevic, A Wielgosz, A Pipe, A Lefebvre, A Pepe, A Auclair, A Prémont, A S Bourlaud; Chile: F Lanas*, P Serón, M J Oliveros, F Cazor, Y Palacios; China: Liu Lisheng*, Li Wei*, Chen Chunming#, Zhao Wenhua. Hu Bo, Yin Lu, Zhu Jun, Liang Yan, Sun Yi, Wang Yang, Deng Qing, Jia Xuan, He Xinye, Zhang Hongye, Bo Jian, Wang Xingyu, Liu Xu, Gao Nan, Bai Xiulin, Yao Chenrui, Cheng Xiaoru, Wang Chuangshi, Li Sidong, Liu Weida, Lang Xinyue, Liu Xiaoyun, Zhu Yibing, Xie Liya, Liu Zhiguang, Ren Yingjuan, Dai Xi, Gao Liuning, Wang Liping, Su yuxuan, Han Guoliang, Song Rui, Cao Zhuangni, Sun Yaya, Li Xiangrong, Wang Jing, Wang Li, Peng Ya, Li Xiaoqing, Li Ling, Wang Jia, Zou Jianmei, Gao Fan, Tian Shaofang, Liu Lifu, Li Yongmei, Bi Yanhui, Li Xin, Zhang Anran, Wu Dandan, Cheng ying, Xiao Yize, Lu Fanghong, Li Yindong, Hou Yan, Zhang Liangqing, Guo Baoxia, Liao Xiaoyang, Chen Di, Zhang Peng, Li Ning, Ma Xiaolan, Lei Rensheng, Fu Minfan, Liu Yu, Xing Xiaojie, Yang Youzhu, Zhao Shenghu, Xiang Quanyong, Tang Jinhua, Liu Zhengrong, Qiang Deren, Li Xiaoxia, Xu Zhengting, Aideeraili. Ayoupu, Zhao Qian; Colombia: P Lopez-Jaramillo*, P A Camacho-Lopez, M Perez, J Otero-Wandurraga, D I Molina, C Cure-Cure, JL Accini, E Hernandez, E Arcos, C Narvaez, A Sotomayor, F Manzur, H Garcia, G Sanchez, F Cotes, A Rico, M Duran, C Torres; India: Bangalore - P Mony *, M Vaz*, S Swaminathan, AV Bharathi, K Shankar, A V Kurpad, K G Jayachitra, H A L Hospital, AR Raju, S Niramala, V Hemalatha, K Murali, C Balaji, A Janaki, K Amaranadh, P Vijayalakshmi, Chennai - V Mohan*, R M Anjana, M Deepa, K Parthiban, L Dhanasekaran, SK Sundaram, M Rajalakshmi, P Rajaneesh, K Munusamy, M Anitha, S Hemavathy, T Rahulashankiruthiyayan, D Anitha, R. Dhanasekar, S. Sureshkumar, D Anitha, K Sridevi, Jaipur - R Gupta, R B Panwar, I Mohan, P Rastogi, S Rastogi, R Bhargava, M Sharma, D Sharma, Trivandrum - V Raman Kutty, K Vijayakumar, S Nair, Kamala R, Manu MS, Arunlal AR, Veena A, Sandeep P Kumar, Leena Kumari, Tessi R, Jith S, K Ajayan, G Rajasree, AR Renjini, A Deepu, B Sandhya, S Asha, H S Soumya, Chandigarh- R Kumar, M Kaur, P V M Lakshmi, V Sagar J S Thakur, B Patro, R Mahajan, A Josh, G Singh, K Sharma, P Chaudary, Iran: R Kelishadi*, A Bahonar, N Mohammadifard, H Heidari, Kazakhstan: K Davletov*, B Assembekov, B Amirov; Kyrgyzstan: E Mirrakhimov*, S Abilova, U Zakirov, U Toktomamatov; Malaysia: UiTM - K Yusoff*, T S Ismail, K Ng, A Devi, N Mat-Nasir, AS Ramli, MNK Nor-Ashikin, R Dasiman, MY Mazapuspavina, F Ariffin, M Miskan, H Abdul-Hamid, S Abdul-Razak, N Baharudin, NMN Mohd-Nasir, SF Badlishah-Sham, MS Mohamed-Yassin, M Kaur, M Koshy, F A Majid, N A Bakar, N Zainon, R Salleh, SR Norlizan, NM Ghazali, M Baharom, H Zulkifli, R Razali, S Ali, CWJCW Hafar, F Basir; UKM - Noorhassim Ismail, M J Hasni, M T Azmi, M I Zaleha, R Ismail, K Y Hazdi, N Saian, A Jusoh, N Nasir, A Ayub, N Mohamed, A Jamaludin, Z Rahim; Occupied Palestinian Territory: R Khatib*, U Khammash, R Giacaman; Pakistan: R Iqbal*, R Khawaja, I Azam, K Kazmi; Peru: J Miranda*, A Bernabe Ortiz, W Checkley, R H Gilman, L Smeeth, R M Carrillo, M de los Angeles, C Tarazona Meza; Philippines: A Dans*, H U Co, J T Sanchez, L Pudol, C Zamora-Pudol, L A M Palileo-Villanueva, M R Aquino, C Abaquin, SL Pudol, K Manguiat, S Malayang; Poland: W Zatonski*, A Szuba, K Zatonska, R Ilow#, M Ferus, B Regulska-Ilow, D Różańska, M Wolyniec; Saudi Arabia: KF AlHabib*, M Alshamiri, HB Altaradi, O Alnobani, N Alkamel, M Ali, M Abdulrahman, R Nouri; South Africa: L Kruger*, A Kruger#, P Bestra, H Voster, A E Schutte, E Wentzel-Viljoen, FC Eloff, H de Ridder, H Moss, J Potgieter, A Roux, M Watson, G de Wet, A Olckers, J C Jerling, M Pieters, T Hoekstra, T Puoane, R Swart*, E Igumbor, L Tsolekile, K Ndayi, D Sanders, P Naidoo, N Steyn, N Peer, B Mayosi#, B Rayner, V Lambert, N Levitt, T Kolbe-Alexander, L Ntyintyane, G Hughes, J Fourie, M Muzigaba, S Xapa, N Gobile, K Ndayi, B Jwili, K Ndibaza, B Egbujie; Sweden A Rosengren*, K Bengtsson Boström, A Rawshani, A Gustavsson, M Andreasson, L Wirdemann; Tanzania: K Yeates*, M Oresto, N West Turkey: A Oguz*, N Imeryuz, Y Altuntas, S Gulec, A Temizhan, K Karsidag, K B T Calik, A K Akalin, O T Caklili, M V Keskinler, K Yildiz; United Arab Emirates: A H Yusufali, F Hussain, M H S Abdelmotagali, D F Youssef, O Z S Ahmad, F H M Hashem, T M Mamdouh, F M AbdRabbou, S H Ahmed, M A AlOmairi, H M Swidan, M Omran, N A Monsef; Zimbabwe: J Chifamba*, T Ncube, B Ncube, C Chimhete, G K Neya, T Manenji, L Gwaunza, V Mapara, G Terera, C Mahachi, P Murambiwa, R Mapanga, A Chinhara
*National Coordinator
#Deceased
PURE Country Institution Names:
Institution
South Africa
Faculty of Health Science
North-West University
Potchefstroom Campus
University of the Western Cape
Department of Dietetics and Nutrition
Private Bag X17, 7535
Bellville, South Africa
Zimbabwe
University of Zimbabwe
College of Health Sciences
Physiology Department
Harare, Zimbabwe
Tanzania
Pamoja Tunaweza Health Research Centre, Moshi, Tanzania
Division of Nephrology, Department of Medicine
Queen's University
China
National Centre for Cardiovascular Diseases
Cardiovascular Institute & Fuwai Hospital
Chinese Academy of Medical Sciences
167, Bei Li Shi Lu, Beijing, China
Fuwai Hospital
167 Beilishi Rd. Xicheng District
Beijing. 100037 China
Philippines
University of Philippines, Section of Adult Medicine & Medical Research Unit, Manila, Philippines
Pakistan
Department of Community Health Sciences and Medicine
Aga Khan University
Stadium Road, P.O Box 3500
Karachi Pakistan
India, Bangalore
St John's Medical College and Research Institute Bangalore 560034, India
India, Chennai
Madras Diabetes Research Foundation &
Dr. Mohan’s Diabetes Specialities Centre, Chennai
India Jaipur
Eternal Heart Care Centre and Research Institute, Jaipur
India, Trivandrum
Health Action by People,
Thiruvananthapuram, Kerala, 695011 INDIA
India, Chandigarh
School of Public Health, Post Graduate Institute of Medical Education & Research, Chandigarh (India)
Bangladesh
Independent University, Bangladesh
Bashundhara, Dhaka
Bangladesh
Malaysia
Universiti Teknologi MARA, Sungai Buloh, Selangor, Malaysia AND UCSI University, Cheras, Selangor, Malaysia
Department of Community Health. Faculty of Medicine. University Kebangsaan Malaysia. Kuala Lumpur. Malaysia
Poland
Wroclaw Medical University Department of Internal Medicine; Department of Social Medicine Borowska 213 street; 50- 556 Wroclaw, Poland
Department of Epidemiology,
The Maria Skłodowska-Curie Memorial Cancer Center and Institute of Oncology
02-034 Warsaw, 15B Wawelska str.
Poland
Turkey
Istanbul Medeniyet University
Istanbul, Turkey
Sweden
Sahlgrenska Academy
University of Gothenburg
Sweden
Iran
Isfahan Cardiovascular Research Center, Isfahan Research Institute
Isfahan University of Medical Sciences, Isfahan, Iran
UAE
Dubai Medical University, Hatta Hospital, Dubai Health Authority, Dubai, United Arab Emirates
Saudi Arabia
Department of Cardiac Sciences, King Fahad Cardiac Center
College of Medicine
King Saud University
Riyadh, Saudi Arabia
Palestine
Institute of Community and Public Health, Birzeit University, Ramallah, occupied Palestinian territory
Canada
Université Laval Institut universitaire de cardiologie et de pneumologie de Québec, Quebec
Canada G1V 4G5
Simon Fraser University,
Dept. of Biomedical Physiology & Kinesiology, BC, Canada
Department of Medicine,
University of Ottawa,
Ottawa, Canada
Population Health Research Institute, McMaster University, Hamilton Health Sciences, Hamilton, Ontario, Canada
Argentina
Estudios Clinicos Latinoamerica ECLA
Rosario, Santa Fe
Argentina
Department of Chronic Diseases
South American Center of Excellence for Cardiovascular Health (CESCAS)
Institute for Clinical Effectiveness and Health Policy (IECS)
Brazil
Dante Pazzanese Institute of Cardiology;
Hospital Alemao Oswaldo Cruz
Sao Paulo, SP Brazil
Colombia
Facultad de Ciencias de la Salud, Universidad de Santander (UDES), Bucaramanga, Santander,
Fundacion Oftalmologica de Santander (FOSCAL)
Floridablanca-Santander, Colombia
Chile
Universidad de La Frontera
Temuco, Chile
Ecuador
DECANO
Facultad de Ciencias de la Salud Eugenio Espejo
Universidad Tecnológica Equinoccial
Dirección: Av. Mariscal Sucre s/n y Av. Mariana de Jesús, Quito Ecuador
Peru
CRONICAS Centro de Excelencia en Enfermedades Crónicas | www.cronicas-upch.pe
Universidad Peruana Cayetano Heredia | www.upch.edu.pe
Av. Armendáriz 497, Miraflores, Lima
Russia
Research Institute for Complex Issues of Cardiovascular Diseases, Kemerovo, Russia
Institute For Medical Education, Yaroslav-the-Wise Novgorod State University Ministry of Education and Science of the Russian Federation
Russia, Saint-Petersburg, 197022,
Karpovka river emb., Bld.13, office 28
Kazakhstan
Research Institute of Cardiology & Internal Diseases, Almaty, Kazakhstan
Kyrgyzstan
Kyrgyz Society of Cardiology, National Center of Cardiology and Internal Disease, Bishkek, Kyrgyzstan
Footnotes
Handling editor: Sanni Yaya
Twitter: @DarrylLeong, @rajeevgg
Contributors: RN, DL and SY contributed to the study concept and design. SIB, SI and SR were responsible for the acquisition of data. RN, DL, MMK and SY drafted the manuscript. SVS, AJA, KEY, SAL, RG, AY, ALD, AS, KFA, MK, OR, PS, RD, TP, WL, YZ, YS, PL-J, JC, IR, KK, RK, AR, RK, LIAKR, SI and KT contributed to the critical revision of the manuscript. SY is guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: SY is supported by the Marion W Burke endowed chair of the Heart and Stroke Foundation of Ontario. The PURE study is an investigator-initiated study that is funded by the Population Health Research Institute, Hamilton Health Sciences Research Institute (HHSRI), the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, Support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research, through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care and through unrestricted grants from several pharmaceutical companies [with major contributions from AstraZeneca (Canada), Sanofi-Aventis (France and Canada), Boehringer Ingelheim (Germany and Canada), Servier, and GlaxoSmithKline], and additional contributions from Novartis and King Pharma and from various national or local organisations in participating countries. These include: Argentina: Fundacion ECLA (Estudios Clínicos Latino America); Bangladesh: Independent University, Bangladesh and Mitra and Associates; Brazil: Unilever Health Institute, Brazil; Canada: This study was supported by an unrestricted grant from Dairy Farmers of Canada and the National Dairy Council (U.S.), Public Health Agency of Canada and Champlain Cardiovascular Disease Prevention Network; Chile: Universidad de La Frontera [DI13-PE11]; China: National Center for Cardiovascular Diseases and ThinkTank Research Center for Health Development; Colombia: Colciencias (grant 6566-04-18062 and grant 6517-777-58228); India: Indian Council of Medical Research; Malaysia: Ministry of Science, Technology and Innovation of Malaysia (grant number: 100-IRDC/BIOTEK 16/6/21 [13/2007], and 07-05-IFN-BPH 010), Ministry of Higher Education of Malaysia (grant number: 600-RMI/LRGS/5/3 [2/2011]), Universiti Teknologi MARA, Universiti Kebangsaan Malaysia (UKM-Hejim-Komuniti-15-2010); occupied Palestinian territory: the United Nations Relief and Works Agency for Palestine Refugees in the Near East, occupied Palestinian territory; International Development Research Centre, Canada; Philippines: Philippine Council for Health Research and Development; Poland: Polish Ministry of Science and Higher Education (grant number: 290/W-PURE/2008/0), Wroclaw Medical University; Saudi Arabia: Saudi Heart Association, Dr.Mohammad Alfagih Hospital, The Deanship of Scientific Research at King Saud University (Research group number: RG -1436-013), Riyadh; Saleh Hamza Serafi Chair for Research of Coronary Heart Disease, Umm AlQura University, Makkah, Saudi Arabia; South Africa: The North-West University, SA and Netherlands Programme for Alternative Development, National Research Foundation, Medical Research Council of South Africa, The South Africa Sugar Association, Faculty of Community and Health Sciences; Sweden: Grants from the Swedish state under the Agreement concerning research and education of doctors; the Swedish Heart and Lung Foundation; the Swedish Research Council; the Swedish Council for Health, Working Life and Welfare, King Gustaf V:s and Queen Victoria Freemason’s Foundation, AFA Insurance; Turkey: Metabolic Syndrome Society, AstraZeneca, Sanofi Aventis; United Arab Emirates: Sheikh Hamdan Bin Rashid Al Maktoum Award For Medical Sciences and Dubai Health Authority, Dubai.
Disclaimer: The external funders and sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The PURE protocol was approved by the ethics committees in the participating centres. All participants provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request. An exclusive licence: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to (i) publish, reproduce, distribute, display and store the Contribution, (ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution and convert or allow conversion into any format including without limitation audio, (iii) create any other derivative work(s) based in whole or part on the on the Contribution, (iv) to exploit all subsidiary rights to exploit all subsidiary rights that currently exist or as may exist in the future in the Contribution, (v) the inclusion of electronic links from the Contribution to third party material where-ever it may be located and (vi) licence any third party to do any or all of the above. All research articles will be made available on an open access basis.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Umberson D, Karas Montez J, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav 2010;51:S54–66. 10.1177/0022146510383501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkman LF, Syme SL, networks S. Social networks, host resistance, and mortality: a nine-year follow-up study of ALAMEDA County residents. Am J Epidemiol 1979;109:186–204. 10.1093/oxfordjournals.aje.a112674 [DOI] [PubMed] [Google Scholar]
- 3.Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot 2000;14:362–70. 10.4278/0890-1171-14.6.362 [DOI] [PubMed] [Google Scholar]
- 4.Holt-Lunstad J, Smith TB, Baker M. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci J Assoc Psychol Sci 2015;10:227–37. [DOI] [PubMed] [Google Scholar]
- 5.Valtorta NK, Kanaan M, Gilbody S, et al. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart 2016;102:1009–16. 10.1136/heartjnl-2015-308790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller BK, Magnuson TM, Cernin PA, et al. The significance of social network in a geriatric assessment population. Aging Clin Exp Res 2003;15:512–7. 10.1007/BF03327375 [DOI] [PubMed] [Google Scholar]
- 7.Kreibig SD, Whooley MA, Gross JJ. Social integration and mortality in patients with coronary heart disease: findings from the heart and soul study. Psychosom Med 2014;76:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarma EA, Kawachi I, Poole EM, et al. Social integration and survival after diagnosis of colorectal cancer. Cancer 2018;124:833–40. 10.1002/cncr.31117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai R, Yasunaga M, Nishi M, et al. Co-Existence of social isolation and homebound status increase the risk of all-cause mortality. Int. Psychogeriatr. 2019;31:703–11. 10.1017/S1041610218001047 [DOI] [PubMed] [Google Scholar]
- 10.Pantoja T, Opiyo N, Lewin S. Implementation strategies for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev 2017;9:CD011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818–27. 10.1056/NEJMoa1311890 [DOI] [PubMed] [Google Scholar]
- 12.Gajalakshmi V, Peto R, Kanaka S, et al. Verbal autopsy of 48 000 adult deaths attributable to medical causes in Chennai (formerly Madras), India. BMC Public Health 2002;2:7. 10.1186/1471-2458-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eide GE, Heuch I. Average attributable fractions: a coherent theory for apportioning excess risk to individual risk factors and subpopulations. Biom. J. 2006;48:820–37. 10.1002/bimj.200510228 [DOI] [PubMed] [Google Scholar]
- 14.Iliffe S, Kharicha K, Harari D. Health risk appraisal in older people 2: the implications for clinicians and commissioners of social isolation risk in older people. Br J Gen Pract J R Coll Gen Pract 2007;57:277–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Pantell M, Rehkopf D, Jutte D, et al. Social isolation: a predictor of mortality comparable to traditional clinical risk factors. Am J Public Health 2013;103:2056–62. 10.2105/AJPH.2013.301261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar A, McMunn A, Banks J, et al. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychology 2011;30:377–85. 10.1037/a0022826 [DOI] [PubMed] [Google Scholar]
- 17.Steptoe A, Shankar A, Demakakos P, et al. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A 2013;110:5797–801. 10.1073/pnas.1219686110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandervoort D. Social isolation and gender. Curr Psychol 2000;19:229–36. 10.1007/s12144-000-1017-5 [DOI] [Google Scholar]
- 19.Kobayashi LC, Steptoe A, Isolation S. Social isolation, loneliness, and health behaviors at older ages: longitudinal cohort study. Annals of Behavioral Medicine 2018;52:582–93. 10.1093/abm/kax033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YC, McClintock MK, Kozloski M. Social isolation and adult mortality: the role of chronic inflammation and sex differences. J Health Soc Behav 2013;54:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkman LF, et al. Social integration and mortality: a prospective study of French employees of electricity of France-Gas of France: the GAZEL cohort. Am J Epidemiol 2004;159:167–74. 10.1093/aje/kwh020 [DOI] [PubMed] [Google Scholar]
- 22.Lauder W, Mummery K, Jones M, et al. A comparison of health behaviours in Lonely and non-lonely populations. Psychol Health Med 2006;11:233–45. 10.1080/13548500500266607 [DOI] [PubMed] [Google Scholar]
- 23.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med 2008;358:2249–58. 10.1056/NEJMsa0706154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havens B, Hall M, Sylvestre G, et al. Social isolation and loneliness: differences between older rural and urban Manitobans. Can. J. Aging 2004;23:129–40. 10.1353/cja.2004.0022 [DOI] [PubMed] [Google Scholar]
- 25.Locher JL, Ritchie CS, Roth DL, et al. Social isolation, support, and capital and nutritional risk in an older sample: ethnic and gender differences. Soc Sci Med 2005;60:747–61. 10.1016/j.socscimed.2004.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menec VH, Newall NE, Mackenzie CS, et al. Examining individual and geographic factors associated with social isolation and loneliness using Canadian longitudinal study on aging (CLSA) data. PLoS One 2019;14:e0211143. 10.1371/journal.pone.0211143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elovainio M, Hakulinen C, Pulkki-Råback L, et al. Contribution of risk factors to excess mortality in isolated and Lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Health 2017;2:e260–6. 10.1016/S2468-2667(17)30075-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valtorta NK, Moore DC, Barron L, et al. Older Adults’ Social Relationships and Health Care Utilization: A Systematic Review. Am J Public Health 2018;108:e1–10. 10.2105/AJPH.2017.304256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart 2018;104:1536–42. 10.1136/heartjnl-2017-312663 [DOI] [PubMed] [Google Scholar]
- 30.Ikeda A, Kawachi I, Iso H, et al. Social support and cancer incidence and mortality: the JPHC study cohort II. Cancer Causes Control 2013;24:847–60. 10.1007/s10552-013-0147-7 [DOI] [PubMed] [Google Scholar]
- 31.McAllister DA, Morling JR, Fischbacher CM, et al. Socioeconomic deprivation increases the effect of winter on admissions to hospital with COPD: retrospective analysis of 10 years of national hospitalisation data. Primary Care Respiratory Journal 2013;22:296–9. 10.4104/pcrj.2013.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, et al. Social ties and susceptibility to the common cold. JAMA 1997;277:1940–4. 10.1001/jama.1997.03540480040036 [DOI] [PubMed] [Google Scholar]
- 33.Jordan RE, Hawker JI, Ayres JG, et al. Effect of social factors on winter hospital admission for respiratory disease: a case–control study of older people in the UK. Br J Gen Pract 2008;58:e1–9. 10.3399/bjgp08X302682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull 1996;119:488–531. 10.1037/0033-2909.119.3.488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-004124supp001.pdf (109.2KB, pdf)
bmjgh-2020-004124supp002.pdf (85.7KB, pdf)
bmjgh-2020-004124supp003.pdf (103.3KB, pdf)
bmjgh-2020-004124supp004.pdf (100.3KB, pdf)
bmjgh-2020-004124supp005.pdf (1MB, pdf)