Abstract
Hepatitis B surface antigen (HBsAg) seroclearance is regarded as the ideal endpoint for antiviral treatment. However, reports on the durability of and outcomes after HBsAg seroclearance are few, which has become a focus in clinical practice. This meta‐analysis was performed to evaluate the durability and hepatocellular carcinoma (HCC) incidence after HBsAg seroclearance after treatment cessation. We searched PubMed, Embase, Medline and Web of Science for studies that reported the durability and HCC incidence after HBsAg seroclearance published between 1 January 2000 and 31 January 2020. Data were analysed by a random‐effects model. Thirty‐eight studies and 43,924 patients were finally included. The results showed that HBsAg seroclearance was durable, with a pooled recurrence rate of 6.19% (95% CI: 4.10%–8.68%). There was no significant difference in recurrence rates after different seroclearance methods or among recurrence types and different regions. Anti‐HBs seroconversion resulted in a significantly reduced recurrence rate (RR = 0.25, p < .001). Patients who experienced HBsAg seroclearance had significantly lower HCC incidence than HBsAg‐positive (RR = 0.41, p < .001). The pooled HCC incidence after HBsAg seroclearance was 1.88%; this rate was reduced to 0.76% among patients without baseline cirrhosis. In conclusion, the analysis during an average follow‐up of 4.74 years suggested that in patients who experienced sustained HBsAg seroclearance and anti‐HBs seroconversion, this was associated with low HCC incidence. Patients without baseline cirrhosis benefited even more. We emphasize the importance of gaining HBsAg seroclearance while highlighting the benefits of achieving this as early as possible.
Keywords: HBsAg, HBV, HCC, meta‐analysis, recurrence rate
1. INTRODUCTION
Chronic hepatitis B virus (HBV) infection is currently a leading cause of cirrhosis and hepatocellular carcinoma (HCC). 1 With the development of antiviral treatment, it is believed that relying on viral suppression (sustained undetectable HBV DNA levels) and seroconversion/loss of hepatitis B e antigen (HBeAg) alone cannot lead to maximum clinical benefits. Hepatitis B surface antigen (HBsAg) seroclearance, which refers to the loss of detectability of serum HBsAg with or without anti‐HBs, is regarded as the ideal endpoint for antiviral treatment. HBsAg seroclearance substantially reduces the risk of HCC and disease progression and is known as a "functional cure". 2 , 3
Despite the significance of obtaining HBsAg seroclearance, few studies have investigated the durability, the recurrence rate and related risk factors after HBsAg seroclearance. HBsAg loss is rare; therefore, there are few large or even sufficient sample cohorts available for analysis. With the long‐term use of antiviral therapy, especially nucleos(t)ide analogues (NAs), the adverse outcomes of liver inflammation and necrosis caused by HBV (such as acute and subacute liver failure) can be controlled, whereas the incidence of chronic proliferative diseases such as cirrhosis and HCC caused by HBV is not significantly reduced. Is the ideal endpoint for antiviral therapy associated with significantly favourable clinical outcomes? This has become the focus in both research and the clinic. Therefore, we performed a systematic review and meta‐analysis of published literature to evaluate the durability and risk of HCC development after HBsAg seroclearance after treatment discontinuation.
2. METHOD
The review was registered in the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/PROSPERO): CRD42020172902.
2.1. Data sources and search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines 4 and conducted a search for studies that reported the durability of HBsAg seroclearance and the recurrence rate and incidence of HCC after HBsAg seroclearance in HBV patients. We searched PubMed, Embase, Medline and Web of Science for studies published between 1 January 2000 and 31 January 2020. The search strategy included Medical Subject Heading terms and a range of relevant keywords, including “chronic hepatitis B”, “hepatitis B virus”, “hepatitis B surface antigen or HBsAg”, “seroclearance or loss”, “seroconversion”, “clearance”, “undetectable”, “durability”, “seroreversion”, “persistent”, “liver cancer”, “hepatocellular carcinoma or HCC”, “long‐term outcome”, “advanced liver disease” and “clinical outcome”. In addition, references of the included articles and relevant systematic reviews were also searched manually to identify additional articles. Study authors were contacted directly if necessary for more details. Randomized trials, prospective and retrospective cohort studies and clinical cohort studies were eligible for inclusion. Case reports, reviews, cross‐sectional studies and letters or comments were excluded. There were no geographic restrictions. The search was limited to journal articles written in English.
2.2. Study selection and data extraction
We firstly screened the titles, abstracts and keywords to identify relevant articles for inclusion. Then, the abstracts of the retained studies were read in detail. Finally, the full texts of the retained studies were reviewed, and duplicate references were excluded. Two reviewers independently screened the articles and then assessed the studies for relevance and methodological quality. We planned to resolve any discrepancies through discussion and, if necessary, we would involve a neutral third investigator.
The inclusion criteria for the identification of eligible studies were as follows: (a) the studies had cohorts of confirmed chronic hepatitis B (CHB) patients; (b) more than 10 patients who experienced HBsAg seroclearance were reported, whether spontaneously or after antiviral therapy; (c) sufficient data on the primary outcomes were available, including durability of seroclearance, the recurrence rate, or the incidence of HCC in patients after HBsAg seroclearance after the cessation of treatment, and an average of more than 1 year of follow‐up was performed. In contrast, studies with insufficient data, those lacking available full texts, and those with patients who had undergone liver transplantation or had decompensated liver disease/HCC before HBsAg seroclearance were excluded. All the included studies must addressed patients with CHB rather than acute HBV infection. Furthermore, we excluded patients coinfected with HIV, HCV and/or HDV to reduce the effects of heterogeneity and other factors on the analysis.
Data were extracted from the articles by two reviewers using standardized forms as follows: study variables, including the article author, publication year, country or region, study design and total sample size; patient variables, including age and sex; outcome measures, including the number of patients with HBsAg seroclearance (spontaneous or post‐treatment), the number of cases of recurrence after HBsAg seroclearance (The recurrence type was defined as the reappearance of HBsAg, HBV DNA, or both during follow‐up after treatment cessation), the proportion of patients who developed HCC after HBsAg seroclearance (including those with liver cirrhosis at the time of HBsAg seroclearance) and the duration of follow‐up (mean or median). Additionally, we extracted the number of patients positive for hepatitis B surface antibodies (antiHBs) after HBsAg seroclearance and the proportion of patients who developed HCC who remained HBsAg‐positive.
2.3. Assessment of evidence quality
Two reviewers used the Newcastle‐Ottawa assessment scale (NOS) to assess the methodological quality and risk of bias in the nonrandomized studies. 5 This scale judges three general areas comprising 8 items: the selection of study groups, comparability of groups and ascertainment of outcomes. The maximum total score is 9; the studies with total scores ≥7 were regarded as good quality, those with scores from 4 to 6 were deemed fair quality, and those with scores <4 were considered poor quality (Table S1).
2.4. Statistical analysis
We calculated the pooled event rate by using a Freeman‐Tukey double arcsine transformation to stabilize the variances and to allow for studies with zero rates in the pooled analysis. In addition, 95% confidence intervals (CIs) were generated by the Wilson method rather than the asymptotic method. 6 The resulting values were then back‐transformed and are presented in the figures. For heterogeneous data, a random‐effects model was used. Statistical heterogeneity was assessed by the I 2 statistic and Cochran's Q statistic. Publication bias was evaluated with a funnel plot and Egger's test. 7
Subgroup analyses were performed based to examine whether the durability of HBsAg seroclearance was modified by other variables, including the HBsAg seroclearance methods, types of recurrence, different regions and anti‐HBs positive conversion. In addition, regarding HCC incidence, we compared the HCC incidence in patients with and without baseline cirrhosis at the time of HBsAg seroclearance to assess the impact of HBsAg seroclearance on the prognosis of HBV patients. We further analysed the difference in the HCC incidence of post‐treatment versus spontaneous HBsAg seroclearance patients. All analyses were performed with STATA, version 12.0. Results with p < .05 were considered statistically significant, and p values were two tailed.
3. RESULT
A total of 4510 studies were identified for potential inclusion (Figure 1). After the removal of duplicates, 1805 studies were retained for further evaluation. Next, 146 studies were retained after the initial screening of the abstracts and texts. Then, after reading the full texts and evaluating them according to the inclusion and exclusion criteria, 108 studies were excluded; 9 studies lacked full‐text articles, 3 had small sample sizes, 25 reported irrelevant outcomes, 41 provided inadequate data on our outcomes, and 30 failed to exclude patients coinfected with HIV, HCV and/or HDV. Finally, 38 full‐text articles were included in this analysis. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45
FIGURE 1.
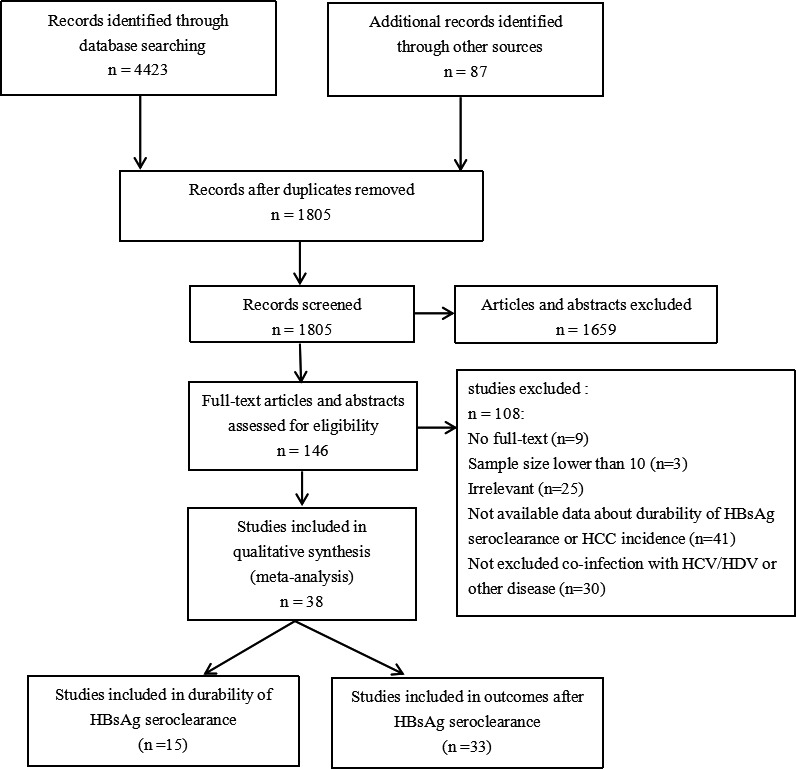
Study selection process
3.1. Characteristics of studies
The characteristics of the included studies are shown in Table 1. Twenty‐eight studies came from the Asia‐Pacific regions, 8 , 11 , 28 , 29 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 40 , 41 , 42 accounting for the majority of the included studies. Five were from Europe, 9 , 13 , 18 , 36 , 39 4 were from the Americas, 10 , 27 , 30 , 45 and 1 was a multicentre cohort study. 14 Fifteen studies reported recurrence‐related outcomes after HBsAg seroclearance, 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 14 of which included patients with HBsAg seroclearance after NA treatment and/or common interferon and Peg‐interferon (IFN; Peg‐IFN) treatment. Only 1 study had patients who all spontaneously achieved HBsAg clearance. Definitions of HBsAg seroclearance were provided. There were 4 studies not mentioned, 3 studies defined HBsAg negativity as HBsAg levels <0.05 IU/ml (or lower limit of detection of 0.05 IU/ml), 1 defined as loss of HBsAg detectability at least once in the serum after treatment, 2 defined as persistent absence of HBsAg at least 1 year (or 6 months) and until the time of analysis, and 5 defined as the absence of HBsAg on two consecutive determinations at least 6 months apart. Moreover, 33 studies reported HCC incidence‐related outcomes, 23 of which provided information on the patients with and without baseline cirrhosis, and 16 studies reported the incidence of HCC in patients with sustained HBsAg positivity, which was compared with the incidence in patients who achieved HBsAg seroclearance.
TABLE 1.
Characteristics of studies
| Study | Year | Region | Design | Total number | HBsAg seroclearance number | HBsAg seroreversions number | Male (%) | Age (mean or median) | Follow‐up years after HBsAg seroclearance (mean or median) | Liver cirrhosis | HCC incidences | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Post‐NUC/IFN | Total | HBsAg(+) | DNA(+) | ||||||||||
| Wu | 2019 | China | Retrospective | 1276 | 238 | 238 | 16 | 8 | 2 | 63.9 | 36 ± 11 | 3.08 (1.3–4.7) | 0 | 0/238 |
| Suárez | 2019 | Spain | Retrospective | 69 | 69 | 69 | 1/64 | 0 | 1 | 91.3 | 51.7 ± 11.9 | 3.15 (2.0–4.6) | 10 | 1/69 |
| Alawad | 2019 | USA | Retrospective | 787 | 65 | 46 | 3 | 2 | 0 | 80 | 49 ± 14 | 9.58 (1.1–29.4) | 7 | 0/65 |
| Li | 2019 | China | Prospective | 172 | 172 | 172 | 23 | 19 | 1 | 78.41 | 42.60 ± 10 | 1 | 14 | 1/172 |
| Yip | 2018 | Hongkong, China | Retrospective | 4080 | 2211 | 365 | 89 | 89 | 0 | 63.1 | 56.8 ± 14 | 1.95 (0.8–3.5) | NA | 24/2122 |
| Stelma | 2017 | The Netherlands | Prospective | 92 | 16 | 16 | 0 | 0 | 0 | 73.9 a | 39.5 (19–69) a | 5 | 14 a | 1/92 a |
| Chi | 2017 | The Netherlands, Canada, China | Retrospective | 5872 | 54 | 54 | 6 | 1 | 5 | 87 | 48 ± 12 | 1.6 (0.5–2.7) | 8 | 0/54 |
| Wong | 2017 | Asian | Retrospective | 1072 | 49 | 49 | 6 | 6 | 0 | 64.6 a | NA | 2.27 | 88 a | 44/1072 a |
| Seto | 2016 | Hongkong, China | Retrospective | 51 | 51 | 51 | 2 | 1 | 0 | 82.4 | 48.7 (39.3–58.6) | 4.3 (2.0–6.9) | 5 | NA |
| Li | 2016 | China | Retrospective | 28 | 28 | 28 | 0 | 0 | 0 | 92.9 | 47.1 (27–67) | 2.3 (0.8–7.5) | NA | NA |
| Lauret | 2015 | Spain | Prospective | 612 | 78 | 22 | 2 | 2 | 0 | 71.79 | 49.5 (16–74) | 5.13 (0.7–17.1) | 12 | 1/78 |
| Kim, Lim | 2014 | Korea | Retrospective | 5409 | 110 | 110 | 7 | 1 | 6 | 76.36 | 42 ± 10 | 2.1 (287 patient‐years) | 34 | 1/110 |
| Chu | 2012 | Taiwan, China | Retrospective | 118 | 118 | 0 | 21 | 0 | 21 | 85 | 51.1 ± 9.4 | 10 | 10 | NA |
| Yuen | 2008 | Hongkong, China | Retrospective | 298 | 298 | 13 | 6/99 | 0 | 6 | 70.81 | 49.6 (4.1–84.7) | 3.03 (0.5–18.5) | NA | 7/298 |
| Arase | 2006 | Japan | Retrospective | 231 | 231 | 75 | 4 | 0 | 4 | 80.52 | 51 (23–66) | 6.5 (1–23.6) | 67 | 2/231 |
| Yip | 2019 | Hongkong, China | Retrospective | 17,499 | 376 | 376 | NA | NA | NA | 74.2 | 47.8 ± 14.2 | 5.9 (3.8–7.7) | 23 | 2/376 |
| Song | 2018 | China | Prospective | 3635 | 652 | 0 | NA | NA | NA | NA | NA | 9 a | NA | 8/652 |
| Yip | 2017 | Hongkong, China | Retrospective | 4568 | 4568 | 853 | NA | NA | NA | 62.9 | 56.7 ± 13.8 | 3.4 (1.5–5.0) | 839 | 54/4568 |
| Chen | 2016 | Taiwan, China | Retrospective | 422 | 422 | 110 | NA | NA | NA | 76.3 | 50.4 ± 11.2 | 8.93 ± 5.62 | 44 | 5/422 |
| Gounder | 2016 | Alaska | Retrospective | 1346 | 238 | 0 | NA | NA | NA | 64 | 28.8 (15.9–42.2) | 11.7 (6.5–18.3) | NA | 3/226 |
| Park | 2016 | Korea | Retrospective | 1919 | 90 | 0 | NA | NA | NA | 65.56 | NA | NA | 24 | 4/83 |
| Tseng | 2015 | Taiwan, China | Retrospective | 2121 | 338 | 0 | NA | NA | NA | 71.6 | 28‐75 a | 3 a | 0 | 5/338 |
| Ferreira | 2014 | Brazil | Retrospective | 548 | 40 | 0 | NA | NA | NA | 55 | 37.7 ± 13.3 | 15.8 a | 0 | 0/40 |
| Cho | 2014 | Korea | Retrospective | 2392 | 166 | 17 | NA | NA | NA | 63.3 a | 49.3 ± 0.6 a | 3.53 | NA | 10/166 |
| Kim, Lee | 2014 | Korea | Retrospective | 829 | 829 | 105 | NA | NA | NA | 69.36 | 52.3 ± 9.3 | 3.2 (1.8–6.1) | 98 | 19/829 |
| Orito | 2014 | Japan | Retrospective | 602 | 13 | 13 | NA | NA | NA | 63.3 a | 52 (21–79) a | 7.5 a | 0 | 0/13 |
| Liu | 2014 | Taiwan, China | Prospective | 2946 | 529 | 0 | NA | NA | NA | 75.43 | 30–60 | 48,149.1 p/y a | 0 | 8/529 |
| Tseng | 2012 | Taiwan, China | Prospective | 668 | 130 | NA | NA | NA | NA | 54.5 a | ≥28 | 9.4 ± 5.7 | 1 | 1/130 |
| Idilman | 2012 | Turkey | Retrospective | 183 | 10 | 10 | NA | NA | NA | 66.6 | 45.5 ± 11 | 2.87 ± 1.87 | 0 | 0/10 |
| Kim Ji Hoon | 2011 | Korea | Retrospective | 96 | 96 | 5 | NA | NA | NA | 80.21 | 46.4 ± 9.9 | 4.7 (0.6–19.8) | 24 | 6/96 |
| Fwu | 2009 | Taiwan, China | Retrospective | 1,782,401 | 31,088 | NA | NA | NA | NA | 0 | 28.72 ± 4.23 a | 8.07 a | 0 | 8/31,088 |
| Moucari | 2009 | France | Retrospective | 97 | 28 | 28 | NA | NA | NA | 82.14 | 43 (23–73) a | 14 a | 10 | 0/28 |
| Kim Jeong | 2008 | Korea | Retrospective | 215 | 11 | 0 | NA | NA | NA | 54.55 | 52 (40–67) | 2.5 | 0 | 0/11 |
| Nam | 2007 | Korea | Retrospective | 4061 | 47 | 0 | NA | NA | NA | 64.98 a | 46.2 ± 15.7 | 7.3 a | 7 | 9/47 |
| Ahn | 2005 | Korea | Retrospective | 49 | 49 | 0 | NA | NA | NA | 73.47 | 50 (27–72) | 1.6 (0.4–3.2) | 17 | 5/49 |
| Yuen | 2004 | Hongkong, China | Retrospective | 184 | 92 | 6 | NA | NA | NA | 70.65 | 48.8 ± 13.81 | 4.3 | NA | 5/92 |
| Chen | 2002 | Taiwan, China | Retrospective | 218 | 218 | 0 | NA | NA | NA | 78.9 | 44.8 ± 11.1 | 5.1 (1–14.9) | 29 | 3/218 |
| McMahon | 2001 | USA | Retrospective | 1536 | 106 | NA | NA | NA | NA | 59.1 | NA | 12.3 a | NA | 2/106 |
Abbreviation: NA, Not available.
Data from the whole cohort.
3.2. Durability of HBsAg seroclearance
In 15 studies that reported the durability of HBsAg seroclearance, the longest average follow‐up period was 9.58 (1.1–29.4) years, and the shortest was 1 year. The median follow‐up for all studies was 4.74 (1.45–12.76) years. A total of 3584 patients experienced HBsAg seroclearance among 20,167 CHB patients, and 186 patients had experienced recurrence by the end of follow‐up. The pooled recurrence rate after HBsAg seroclearance was 6.19% (95% CI: 4.10–8.68, I 2 = 78.2%, random‐effects model) (Figure 2). We found no significant publication bias, as expected, based on the funnel plot (Figure S1) and results of Egger's test (p = .246). In two studies with relatively fewer HBsAg seroclearance patients, the recurrence rates were as low as zero after an average follow‐up of 3 years. 13 , 17 The recurrence type included the positive of HBsAg, HBV DNA, or both during follow‐up after treatment cessation. In some studies that reported the reappearance of HBV DNA, the definition of recurrence was different due to the difference in the lower limit of detection of HBV DNA during different periods; one of them defined recurrence as HBV DNA >4 IU/ml, one defined it as >60 IU/ml, one defined it as >400 copies/ml, and 7 studies defined it as >20 IU/ml. Then, we noticed that several studies reported a higher recurrence rate within one year after HBsAg seroclearance. In a further analysis, one year after follow‐up, 8 studies reported that a total of 73 patients had experienced recurrence from among 1046 HBsAg seroclearance patients, and the pooled rate was 6.49% (95% CI: 3.29–10.65, Figure S2). There was no significant publication bias among these studies according to Egger's test (p = .945).
FIGURE 2.
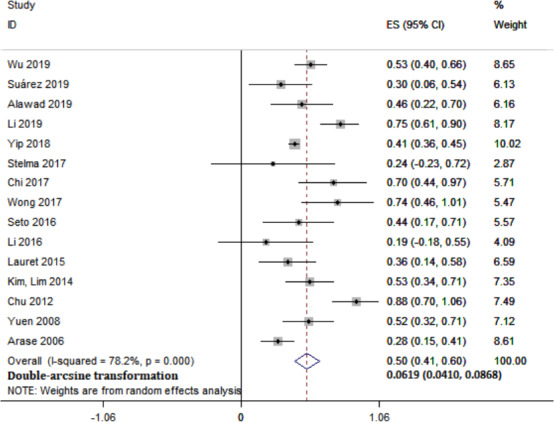
Meta‐analysis of overall pooled recurrence rate in CHB patients after HBsAg seroclearance
3.3. Subgroup meta‐analyses and HBsAg reversion risk factors
To evaluate whether the durability of HBsAg seroclearance is influenced by other variables and reduce the heterogeneity among studies, we further performed a subgroup analysis. First, the analysis was stratified by the methods of HBsAg seroclearance, namely spontaneous, after treatment with NAs and after IFN/peg‐IFN treatment. A total of 2680 cases were spontaneous, 747 occurred after NA treatment, and 561 occurred after IFN/peg‐IFN treatment (including NAs+IFN), and the recurrence rates were 4.08% (95% CI: 3.26–5.00), 5.79% (95% CI: 3.42–8.71) and 6.12% (95% CI: 2.26–11.69), respectively (Figure S3). There was no significant difference among these groups (p = .64). Second, the type of recurrence after HBsAg seroclearance, namely positivity for HBsAg, positivity for HBV DNA and positivity for both, was used to stratify the data. The recurrence rates were 4.47% (95% CI: 2.75–6.58), 4.53% (95% CI: 1.69–8.65) and 2.41% (95% CI: 1.28–3.91), respectively (Figure S4). No significant difference was found (p = .44). Furthermore, since these studies come from different regions, we separately pooled the recurrence rate in Asia‐Pacific regions and non‐Asia‐Pacific regions to see if there is any difference. In 15 studies that reported the durability of HBsAg seroclearance, 10 from the Asia‐Pacific regions and 4 from non‐Asia‐Pacific regions, the last one was a multicentre cohort study. The recurrence rates were 6.91% (95% CI: 4.20–10.23) in Asia‐Pacific regions and 3.26% (95% CI: 1.35–5.95) in non‐Asia‐Pacific regions (Figure S5). No significant difference was found (p = .14). Finally, previous studies have reported that anti‐HBs positivity or high anti‐HBs levels are a predictor of a sustained functional cure. 8 , 11 , 19 Ten studies reported the status of anti‐HBs. The results showed that the recurrence rate of patients with positive anti‐HBs seroconversion was significantly lower than that of patients with negative anti‐HBs seroconversion (RR = 0.25, 95% CI: 0.09–0.42, p < .001, I 2 = 0%) (Figure 3).
FIGURE 3.

Meta‐analysis of recurrence rate after HBsAg seroclearance among anti‐HBs‐positive or anti‐HBs‐negative patients
3.4. Incidence of HCC after HBsAg seroclearance
Thirty‐three studies reported the incidence of HCC, and a total of 194 patients among 43,573 CHB patients developed HCC after HBsAg seroclearance. Sixteen studies among 33 provided the incidence of HCC among patients who remained HBsAg‐positive. We found that patients who experienced HBsAg seroclearance had a significantly lower HCC incidence than HBsAg‐positive patients (RR = 0.41, 95% CI: 0.22–0.76, p < .001, I 2 = 58%) (Figure 4). In 8 studies, there were no HCC incidence after HBsAg seroclearance. Overall, the pooled incidence rate of HCC after HBsAg seroclearance was 1.88% (95% CI: 1.16–2.76, I 2 = 93.2%, random‐effects model) (Figure 5). The results suggested that there is a certain risk of HCC occurrence in patients with HBsAg seroclearance, although they were considered to have significantly more favourable clinical outcomes than those who remain positive. In all 33 studies, twenty‐three studies reported the cirrhosis status of patients at the time of HBsAg seroclearance. HCC occurred in 49 of 6967 non‐cirrhotic patients, and the pooled incidence rate of HCC was 0.76% (95% CI: 0.56–0.97, I 2 = 0%) (Figure S6). This rate in non‐cirrhotic patients was markedly lower than that in all HBsAg seroclearance patients (1.88%). There was no significant heterogeneity among those studies. Then, thirteen studies further reported the incidence of HCC stratified by the presence of cirrhosis before seroclearance. Patients without baseline cirrhosis were associated with a significantly lower HCC incidence after HBsAg seroclearance (RR = 0.17, 95% CI: 0.12–0.25, p < .001) (Figure S7). Finally, we tried to assess whether post‐treatment versus spontaneous HBsAg seroclearance would have any difference in HCC incidence. Nine studies among 33 included patients with HBsAg seroclearance after NA treatment and/or IFN treatment, while 8 studies had patients who all spontaneously achieved HBsAg clearance. The pooled incidence rates of HCC were 0.83% (95% CI: 0.35–1.52) and 3.53% (95% CI: 1.58–6.19), respectively (Figure S8). There was significant difference between them (p = .003). This result showed that the incidence of HCC in patients after treatment was significantly lower than that in patients with spontaneous clearance. Our analysis suggested that cirrhosis at the time of HBsAg seroclearance was a crucial risk factor for HCC occurrence, and the early acquisition of HBsAg seroclearance by treatment leads to favourable clinical outcomes.
FIGURE 4.
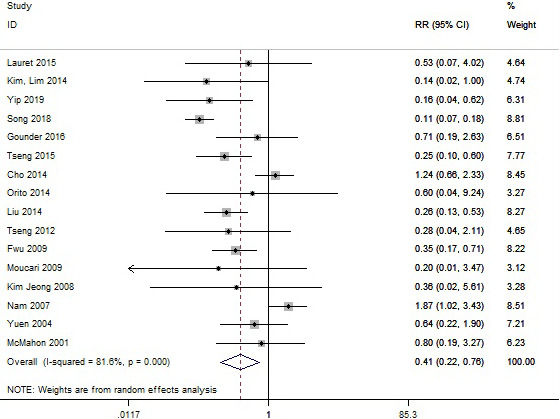
Meta‐analysis of HCC incidence among CHB patients with HBsAg seroclearance male or HBsAg‐positive
FIGURE 5.
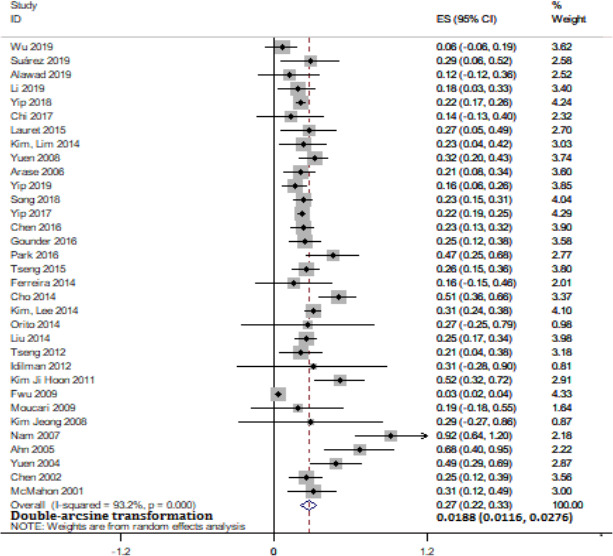
Meta‐analysis of overall pooled HCC incidence in CHB patients after HBsAg seroclearance
4. DISCUSSION
This systematic review and meta‐analysis included 38 studies and data from 43,924 patients with HBsAg seroclearance. To the best of our knowledge, this is the first systematic study with a large sample size to analyse the durability of and recurrence rate after HBsAg seroclearance. HBsAg seroclearance or seroconversion has been considered the ideal treatment endpoint for CHB patients, 2 , 46 which can be achieved spontaneously or induced by NA and/or IFN treatment. It should be noted that there is a phenomenon of “S‐escape” mutations in the HBsAg gene, this may result in a status of undetectable serum HBsAg with detectable serum and/or intrahepatic HBV DNA which named occult hepatitis B infection (OBI). 47 OBI may result from patients with chronic hepatitis B who achieved HBsAg seroclearance. However, a functional cure does not mean that HBV has been eradicated from a virological perspective. Thus, this has become the focus in the clinical setting, and it is necessary to evaluate the durability of HBsAg seroclearance and whether it is associated with significantly favourable clinical outcomes.
In terms of durability, this analysis of 15 studies, which included 3584 patients with HBsAg seroclearance among 20,167 CHB patients, resulted in a pooled recurrence rate of 6.19% during an average follow‐up of 4.74 years and suggested that HBsAg seroclearance is durable. A few studies reported significant differences in the recurrence rates after HBsAg seroclearance, ranging from zero 13 , 17 to as high as 13%, 11 which may be related to differences in study regions, subjects and sample size. Five studies reported a higher recurrence rate within one year after HBsAg seroclearance, 8 , 14 , 19 , 20 , 21 and the rate increased slowly or decreased after one year. The recurrence rate was 6.49% at 1 year of follow‐up, which was slightly higher than the total recurrence rate. The follow‐up time for the determination of the durability of HBsAg seroclearance may be set at 1 year. Furthermore, anti‐HBs positivity was significantly associated with a lower recurrence rate (RR = 0.25). However, some studies have reported results inconsistent with this. HBV DNA can reappear when HBsAg is negative and anti‐HBs is positive. The presence of variations in the S region could exist. Patients with mutations could develop the intracellular retention of HBsAg proteins and disturbances of viral protein secretion, leading to the dissociation of HBsAg production and viral replication. 48 , 49 Several studies provided explanations for the cases of recurrence, including patients who received immunosuppressive therapy or hormone therapy, as well as drug resistance sites detected during NA treatment before HBsAg seroclearance. 8 , 16 , 18 These conditions increase the risk of recurrence in patients with HBsAg seroclearance and require surveillance. In addition, the results showed that there was no significant difference in the recurrence rate based on the HBsAg seroclearance method, recurrence type, or different regions, suggesting that regardless of which method was used to obtain HBsAg seroclearance, it will be durable. Besides, there was a trend towards lower recurrence in the western studies, the reason for this observation may be related to the number of studies from the West was relatively small, as well as most of western patients developed anti‐HBs after HBsAg seroclearance, and the presence of the antibody was sustained.
In terms of the clinical outcome, the analysis of 33 studies including 45,531 patients with HBsAg seroclearance suggested that patients who experienced HBsAg seroclearance had a significantly decreased HCC incidence compared with those who remained HBsAg‐positive (RR = 0.41). However, approximately 1.88% of patients may still develop HCC after HBsAg seroclearance. In the data from the 23 studies, we excluded patients with cirrhosis before HBsAg seroclearance, and the pooled rate of HCC occurrence fell to 0.76%. Further analysis of 13 studies involving patients with and without cirrhosis at baseline suggested that the absence of cirrhosis was associated with a significantly lower HCC incidence after HBsAg seroclearance compared with the presence of cirrhosis (RR = 0.17). This is consistent with previous reports that cirrhosis at the time of HBsAg seroclearance might contribute to HCC development. 50 , 51 Our analysis and related studies suggest that patients who experience HBsAg seroclearance have favourable clinical outcomes, and achieving a functional cure early in the absence of cirrhosis can result in a relatively better prognosis. Furthermore, patients after treatment was associated with lower risk of HCC occurrence compared to those with spontaneous clearance. Finally, other risk factors related to HCC, such as HBV genotype, age and gender, were not analysed in this study due to limited data.
Several limitations of this meta‐analysis should be addressed. First, the limited available data about the durability of HBsAg seroclearance, as well as the short follow‐up periods among the included studies, limited the possibility of calculating the recurrence rate over a longer time and investigating more factors in subgroup meta‐analyses. Second, some patients with reactivation of HBV had S region mutations, immunosuppressive therapy, or other relevant therapy, which may have affected the analysis of recurrence rates. Furthermore, the annual HCC incidence after HBsAg seroclearance could not be calculated in this study, mainly due to the various follow‐up times of the studies, and the follow‐up durations in several studies were relatively short for the observation of HCC occurrence. Finally, there was some heterogeneity in our analysis, which might be because of the different regions and characteristics of the patient populations, as well as the various sample sizes and follow‐up times. In addition, such inherent heterogeneity might be caused by the methodology involved in a meta‐analysis of pooled proportions to some extent, which is difficult to avoid, although we eliminated heterogeneity via our study design and subgroup analyses as much as possible.
HBsAg seroclearance has been recommended as an ideal endpoint of antiviral treatment by the HBV management guidelines, and the durability of and clinical outcomes after HBsAg seroclearance have become the focus in the clinical setting. Many clinicians recognize the importance of these issues, but there is a lack of evidence‐based medical knowledge. Our results showed that HBsAg seroclearance was durable and that anti‐HBs seroconversion was a protective factor against recurrence. Patients who experienced HBsAg seroclearance had a low HCC incidence and favourable clinical outcomes, and those without baseline cirrhosis had a significantly lower HCC incidence compared with those with cirrhosis. We emphasize the importance of obtaining early HBsAg seroclearance.
CONFLICT OF INTEREST
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTION
A. S., X.W. and X.C. conceived and designed the protocol and study. J.L. and Y.J., identified studies to be screened. L.M., Y.Z. and C.S. identified studies for eligibility, extracted data and assessed the methodologic quality of included studies. A.S. performed the analysis with assistance from Z.H., C.S. and X.C. All authors read and approved the final manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Thirteenth Five‐Year Major Science and Technology Projects (2017ZX10202201; 2017ZX10201021‐001‐008; 2017ZX10302201‐004‐003; 2017ZX10202203‐006). Capital Health Research and Development Projects (2020‐1‐2181). The Key R & D and transformation plan in Qinghai Province (No.2017‐SF‐159). High‐level and Innovative One Thousand Talent Program in Qinghai Province (2016).
Aixin Song and Xiaoxiao Wang contributed equally to this work.
Contributor Information
Chengli Shen, Email: chengli.shen@osumc.edu.
Xinyue Chen, Email: chenxydoc@ccmu.edu.cn.
DATA AVAILABILITY STATEMENT
All the data used to support the findings of this study are included within the article and Supplementary Information file.
REFERENCES
- 1. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373(9663):582‐592. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu ; European Association for the Study of the Liver . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370‐398. [DOI] [PubMed] [Google Scholar]
- 3. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 2, 2018.
- 6. Freeman M, Tukey J. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607‐611. [Google Scholar]
- 7. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Y, Liu Y, Lu J, et al. Durability of interferon‐induced hepatitis B surface antigen seroclearance. Clin Gastroenterol Hepatol. 2020;18:514‐516.e2. [DOI] [PubMed] [Google Scholar]
- 9. Suarez E, Buti M, Rodriguez M, et al. Hepatitis B surface antigen loss after discontinuing nucleos(t)ide analogue for treatment of chronic hepatitis B patients is persistent in White patients. Eur J Gastro Hepatol. 2019;31(2):267‐271. [DOI] [PubMed] [Google Scholar]
- 10. Alawad AS, Auh S, Suarez D, Ghany MG. Durability of spontaneous and treatment‐related loss of hepatitis B s antigen. Clin Gastroenterol Hepatol. 2020;18:700‐709.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li MH, Yi W, Zhang L, et al. Predictors of sustained functional cure in hepatitis B envelope antigen‐negative patients achieving hepatitis B surface antigen seroclearance with interferon‐alpha‐based therapy. J Viral Hepat. 2019;26(Suppl 1):32‐41. [DOI] [PubMed] [Google Scholar]
- 12. Yip TC, Wong GL, Wong VW, et al. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue‐treated patients. J Hepatol. 2018;68:63‐72. [DOI] [PubMed] [Google Scholar]
- 13. Stelma F, van der Ree MH. HBsAg loss after peginterferon‐nucleotide combination treatment in chronic hepatitis B patients: 5 years of follow‐up. J Viral Hepat. 2017;24(12):1107‐1113. [DOI] [PubMed] [Google Scholar]
- 14. Chi H, Wong D, Peng J, et al. Durability of response after hepatitis B surface antigen seroclearance during nucleos(t)ide analogue treatment in a multiethnic cohort of chronic hepatitis B patients: results after treatment cessation. Clin Infect Dis. 2017;65(4):680‐683. [DOI] [PubMed] [Google Scholar]
- 15. Wong RJ, Nguyen MT, Trinh HN, et al. Hepatitis B surface antigen loss and sustained viral suppression in Asian chronic hepatitis B patients: a community‐based real‐world study. J Viral Hepat. 2017;24(12):1089‐1097. [DOI] [PubMed] [Google Scholar]
- 16. Seto WK, Cheung KS, Wong DK, et al. Hepatitis B surface antigen seroclearance during nucleoside analogue therapy: surface antigen kinetics, outcomes, and durability. J Gastroenterol. 2016;51(5):487‐495. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, You X, Zhu C. The effect after cessation of nucleos(t)ide analog(ue)s for chronic hepatitis B virus infection patients who had serum HBsAg negative is durable. Hepatology. 2016;64:937A. [Google Scholar]
- 18. Lauret E, Gonzalez‐Dieguez ML, Rodriguez M, et al. Long‐term outcome in Caucasian patients with chronic hepatitis B virus infection after HBsAg seroclearance. Liver Int. 2015;35(1):140‐147. [DOI] [PubMed] [Google Scholar]
- 19. Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63(8):1325‐1332. [DOI] [PubMed] [Google Scholar]
- 20. Chu CM, Liaw YF. Prevalence of and risk factors for hepatitis B viremia after spontaneous hepatitis B surface antigen seroclearance in hepatitis B carriers. Clin Infect Dis. 2012;54(1):88‐90. [DOI] [PubMed] [Google Scholar]
- 21. Yuen MF, Wong DK, Fung J, et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135(4):1192‐1199. [DOI] [PubMed] [Google Scholar]
- 22. Arase Y, Ikeda K, Suzuki F, et al. Long‐term outcome after hepatitis B surface antigen seroclearance in patients with chronic hepatitis B. Am J Med. 2006;119(1):71.e9‐71.e16. [DOI] [PubMed] [Google Scholar]
- 23. Yip TC, Wong GL, Chan HL, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol. 2019;70(3):361‐370. [DOI] [PubMed] [Google Scholar]
- 24. Song C, Zhu J, Ge Z, et al. Spontaneous seroclearance of hepatitis B surface antigen and risk of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(6):1204‐1206. [DOI] [PubMed] [Google Scholar]
- 25. Yip TC, Chan HL, Wong VW, Tse YK, Lam KL, Wong GL. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol. 2017;67(5):902‐908. [DOI] [PubMed] [Google Scholar]
- 26. Chen YC, Jeng WJ, Chien RN, Chu CM, Liaw YF. Clinical outcomes after spontaneous and nucleos(t)ide analogue‐treated HBsAg seroclearance in chronic HBV infection. Aliment Pharmacol Ther. 2016;43(12):1311‐1318. [DOI] [PubMed] [Google Scholar]
- 27. Gounder PP, Bulkow LR, Snowball M, et al. Nested case‐control study: hepatocellular carcinoma risk after hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther. 2016;43(11):1197‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park YM, Lee SG. Clinical features of HBsAg seroclearance in hepatitis B virus carriers in South Korea: a retrospective longitudinal study. World J Gastroenterol. 2016;22(44):9836‐9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tseng TC, Liu CJ, Chen CL, et al. Higher lifetime chance of spontaneous surface antigen loss in hepatitis B carriers with genotype C infection. Aliment Pharmacol Ther. 2015;41(10):949‐960. [DOI] [PubMed] [Google Scholar]
- 30. Ferreira SC, Chacha SG, Souza FF, et al. Factors associated with spontaneous HBsAg clearance in chronic hepatitis B patients followed at a university hospital. Ann Hepatol. 2014;13(6):762‐770. [PubMed] [Google Scholar]
- 31. Cho JY, Paik YH, Sohn W, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63(12):1943‐1950. [DOI] [PubMed] [Google Scholar]
- 32. Kim GA, Lee HC, Kim MJ, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol. 2015;62(5):1092‐1099. [DOI] [PubMed] [Google Scholar]
- 33. Orito E, Hasebe C, Kurosaki M, et al. Risk of hepatocellular carcinoma in cirrhotic hepatitis B virus patients during nucleoside/nucleotide analog therapy. Hepatol Res. 2015;45(8):872‐879. [DOI] [PubMed] [Google Scholar]
- 34. Liu J, Yang HI, Lee MH, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut. 2014;63(10):1648‐1657. [DOI] [PubMed] [Google Scholar]
- 35. Tseng TC, Liu CJ, Yang HC, et al. Determinants of spontaneous surface antigen loss in hepatitis B e antigen‐negative patients with a low viral load. Hepatology. 2012;55(1):68‐76. [DOI] [PubMed] [Google Scholar]
- 36. Idilman R, Cinar K, Seven G, et al. Hepatitis B surface antigen seroconversion is associated with favourable long‐term clinical outcomes during lamivudine treatment in HBeAg‐negative chronic hepatitis B patients. J Viral Hepat. 2012;19(3):220‐226. [DOI] [PubMed] [Google Scholar]
- 37. Kim JH, Lee YS, Lee HJ, et al. HBsAg seroclearance in chronic hepatitis B: implications for hepatocellular carcinoma. J Clin Gastroenterol. 2011;45(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 38. Fwu CW, Chien YC, Kirk GD, et al. Hepatitis B virus infection and hepatocellular carcinoma among parous Taiwanese women: nationwide cohort study. J Natl Cancer Inst. 2009;101(14):1019‐1027. [DOI] [PubMed] [Google Scholar]
- 39. Moucari R, Korevaar A, Lada O, et al. High rates of HBsAg seroconversion in HBeAg‐positive chronic hepatitis B patients responding to interferon: a long‐term follow‐up study. J Hepatol. 2009;50(6):1084‐1092. [DOI] [PubMed] [Google Scholar]
- 40. Kim JH, Lee JH, Park SJ, et al. Factors associated with natural seroclearance of hepatitis B surface antigen and prognosis after seroclearance: a prospective follow‐up study. Hepatogastroenterology. 2008;55(82–83):578‐581. [PubMed] [Google Scholar]
- 41. Nam SW, Jung JJ, Bae SH, et al. Clinical outcomes of delayed clearance of serum HBsAG in patients with chronic HBV infection. Korean J Intern Med. 2007;22(2):73‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahn SH, Park YN, Park JY, et al. Long‐term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol. 2005;42(2):188‐194. [DOI] [PubMed] [Google Scholar]
- 43. Yuen M‐F, Wong D‐H, Sablon E, et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology. 2004;39(6):1694‐1701. [DOI] [PubMed] [Google Scholar]
- 44. Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123(4):1084‐1089. [DOI] [PubMed] [Google Scholar]
- 45. McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135(9):759‐768. [DOI] [PubMed] [Google Scholar]
- 46. Block TM, Gish R, Guo H, et al. Chronic hepatitis B: what should be the goal for new therapies? Antiviral Res. 2013;98(1):27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yip TC, Wong GL. Current knowledge of occult hepatitis B infection and clinical implications. Semin Liver Dis. 2019;39(2):249‐260. [DOI] [PubMed] [Google Scholar]
- 48. Kalinina T, Riu A, Fischer L, Santantonio T, Will H, Sterneck M. Selection of a secretion‐incompetent mutant in the serum of a patient with severe hepatitis B. Gastroenterology. 2003;125(4):1077‐1084. [DOI] [PubMed] [Google Scholar]
- 49. Pollicino T, Amaddeo G, Restuccia A, et al. Impact of hepatitis B virus (HBV) preS/S genomic variability on HBV surface antigen and HBV DNA serum levels. Hepatology. 2012;56(2):434‐443. [DOI] [PubMed] [Google Scholar]
- 50. Liu F, Wang XW, Chen L, Hu P, Ren H, Hu HD. Systematic review with meta‐analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther. 2016;43(12):1253‐1261. [DOI] [PubMed] [Google Scholar]
- 51. Kuang X‐J, Jia R‐R, Huo R‐R, et al. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Viral Hepat. 2018;25(9):1026‐1037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All the data used to support the findings of this study are included within the article and Supplementary Information file.


