Abstract
Introduction
Obsessive‐compulsive disorder (OCD) is among the most disabling chronic psychiatric disorders and has a significant negative impact on multiple domains of quality of life. Deep brain stimulation (DBS) is a treatment option for severe therapy‐resistant OCD.
Objective
To provide a detailed clinical description and treatment outcome analysis in a cohort of eight refractory OCD patients receiving ventral capsule/ventral striatum (VC/VS) stimulation with the intention to validate discriminating fiber bundles previously associated with clinical response.
Materials and Methods
The primary outcome measure (the Yale‐Brown Obsessive Compulsive Scale [Y‐BOCS]) and secondary outcomes depressive symptoms, anxiety, and quality of life were retrospectively analyzed. DBS leads were warped into standard stereotactic space. A normative connectome was used to identify the neural network associated with clinical outcome.
Results
With a median stimulation duration of 26 months, patients exhibited a mean Y‐BOCS reduction of 10.5 resulting in a response rate of 63%. Modulation of a fiber bundle traversing the anterior limb of the internal capsule (ALIC) was associated with Y‐BOCS reduction. This fiber bundle connected the frontal regions to the subthalamic nucleus (STN) and was functionally identified as the hyperdirect pathway of the basal ganglia circuitry.
Conclusion
Our findings show that in VC/VS stimulation, the neural network associated with clinical outcome shows overlap with that of previously described for other targets namely the anterior limb of the internal capsula, the nucleus accumbens, or the STN, which supports the evolvement from the concept of an optimal gray matter target to conceiving the target as part of a symptom modulating network.
Keywords: Connectivity analysis, deep brain stimulation, obsessive compulsive disorder, ventral capsule/ventral striatum
INTRODUCTION
Obsessive‐compulsive disorder (OCD) is characterized by the presence of time consuming unwanted and disturbing obsessions (thoughts, urges, or images) and/or repetitive behaviors or mental acts (compulsions) aimed at reducing or preventing anxiety or distress (1). In this heterogeneous condition, various kinds of obsessions and compulsions exist, pertaining to five main dimensions; safety, symmetry including repeating and counting compulsions, contamination, repugnant obsessions concerning sex, violence and religion, and hoarding (2, 3).
A range of interventions is effective in the management of OCD including cognitive behavioral therapy (CBT) and pharmacological therapy. A large body of evidence advocate on the use of selective serotonin reuptake inhibitors (SSRIs) and the tricyclic antidepressant clomipramine in the treatment of OCD, often used in combination with CBT (4, 5, 6). However, up to a 40–60% of the patients remain treatment‐refractory, commonly defined as a less than 25% reduction on the Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS), which urges the need for alternative treatment strategies, such as electrical stimulation of subcortical structures, for example, by way of deep brain stimulation (DBS) (7, 8, 9, 10). Based on both clinical and experimental studies, several targets for stimulation are defined including the ventral capsule/ventral striatum (VC/VS), nucleus accumbens (NAcc), the subthalamic nucleus (STN), the anterior limb of the internal capsule (ALIC), superolateral branch of the medial forebrain bundle, medial dorsal and ventral anterior nuclei of the thalamus (MD/vANT), the inferior thalamic peduncle (ITP), the bed nucleus of the stria terminalis (BNST), and the anterior cingulate cortex (ACC) (9, 11, 12, 13, 14, 15). Up to now, more than 200 patients have been treated with DBS for OCD (16). Regardless of the anatomical target, the treatment response seems to be highly variable with Y‐BOCS reductions ranging from 8 to 97%. The response rate, defined as a reduction in Y‐BOCS of 35% or more, is around 60% for the VC/VS target (16). Also improvements in general quality of life and OCD associated depression have been described (17). Only recently, Baldermann et al. showed that in patients receiving ALIC/NA stimulation modulating a frontothalamic fiber pathway was able to predict 40% of the variance in clinical outcome. This was later confirmed for the STN and the ALIC target in multiple cohorts (18, 19).
Here, we aim to constitute to the current OCD‐DBS paradigm shift away from stimulation of focal specific gray matter targets toward modulating specific brain networks. The present study aimed at testing whether the same fiber bundles previously associated with clinical response can be confirmed in a previously undescribed cohort of eight refractory OCD patients receiving VC/VS stimulation.
MATERIALS AND METHODS
Patients
In this retrospective cohort, eight patients were selected for VC/VS stimulation between the period of 2014–2019 according to the indication criteria based on the criteria proposed by Nuttin et al (20). These criteria included the diagnosis of severe OCD on the basis of DSM‐5 (1), with a Yale‐Brown Obsessive‐Compulsive Scale (Y‐BOCS) score of at least 30/40. This level of symptoms should have persisted for a minimum of five years, despite adequate trials of, or intolerance for, two SSRIs and clomipramine, augmentation strategies (i.e., antipsychotic medications), and CBT. The patient had to be at least 18 years of age and able to provide for informed consent. Exclusion criteria were substance abuse, current or past psychotic disorder, and comorbidities that made the patient ineligible for surgery. Referred cases were reviewed in a multidisciplinary DBS board. Patients were referred to an independent psychiatrist for a second opinion on whether all the criteria were met. See Table 1 for baseline characteristics.
Table 1.
Patient Characteristics.
| Patient | Sex | Age onset (years) | Education * | Disease duration (years) | Axis I comorbidity | Obsessions | Compulsions | Medication history | Psychotherapy | Previous DBS | Time to last follow‐up (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSRI | Antipsychotica | Clomipramine | |||||||||||
| 1 | Female | 15 | 4 | 33 | MDD | Perfectionism | Cleaning, ordering, Checking | Venlafaxine | Aripiprazol | CBT | STN | 74 | |
| 2 | Female | 20 | 6 | 20 | Fear of contamination | Washing, cleaning | Paroxetine, Cipramil, Sertralin | Olanzapine, Risperidon | CBT | 35 | |||
| 3 | Female | 22 | 6 | 31 | Fear of contamination, perfectionism | Cleaning, counting, checking | Sertraline, Citalopram, Duloxetine | Quetiapine | Yes | CBT | 28 | ||
| 4 | Male | 13 | 6 | 22 | MDD, ASD | Fear of harming others | Mental compulsions, washing | Fluoxetine | Quetiapine | Yes | CBT | 11 | |
| 5 | Female | 29 | 5 | 28 | MDD | Fear of harm, contamination | Mental compulsions, checking | Sertraline, Paroxetine, fluvoxamine | Yes | CBT | 20 | ||
| 6 | Male | 12 | 5 | 45 | MDD | Need for order, cleanliness | “Just‐Right” behavior | Paroxetine, Venlafaxine, Amitriptyline | Yes | CBT | 20 | ||
| 7 | Female | 6 | 4 | 22 | Fear of contamination, Fear of harming others | Cleaning, washing, checking, counting | Fluoxetine, Sertralin | CBT | 12 | ||||
| 8 | Female | 17 | 5 | 35 | ED: AN | Penance and reward | Cleaning, checking, exercise | Fluvoxamine, Paroxetine, Citalopram | Quetiapine | Yes | CBT | 10 | |
According to the Dutch Verhage classification of education.
AN, anorexia nervosa; ASD, autism spectrum disorder; ED, eating disorder; MDD, major depression disorder.
Procedure
For a detailed description of our stereotactic DBS procedures, see also previous publications (21, 22, 23). In short, all the surgical procedures were performed under general anesthesia with remifentanil and propofol. A Leksell stereotactic frame (Model G, Elekta Instrument Stockholm, Sweden) was mounted on the skull and a perioperative CT‐scan of the head with frame was acquired and fused with the preoperative MR images using Framelink software (Medtronic, Fridley, USA). The planned target was the VC/VS with the stereotactic coordinates: (−) 6 lateral of the middle of the bi‐commissural line (mid AC‐PC), 12 mm anterior of the mid AC‐PC and −3 mm under the bi‐commissural line. The target was adjusted based on the patient's individual anatomy. Typically, we planned a paraventricular trajectory, along which in the first three patients microelectrode recording was performed. As the VC/VS area showed no typical extracellular electrical activity, microelectrode recording was discontinued thereafter. All patients were finally implanted with bilateral quadripolar electrodes (Model 3387, Medtronic, Fridley, USA) along the central trajectory with variable contact points on target (Table 2) which were subsequently connected to an IPG (Activa PC, Medtronic, Fridley, USA).
Table 2.
Target Coordinates and Stimulation Parameters.
| Patient | Target coordinates * | Active contacts | Amplitude | Pulse width (msec) | Frequency (Hz) | ||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | ||||
| 1 | −7;12.8;−3 | 7;12.7;−3 | C+, 1− | C+, 9− | 7.5 mA | 90 | 110 |
| 2 | −6;14.5;−4 | 6;14.5;−4 | C+, 2− | C+, 10− | 5.5 V | 90 | 130 |
| 3 | −5.5;13;−3 | 6;12.5;−4 | C+, 2− | C+, 10− | 5.5 mA | 150 | 130 |
| 4 | −8;14;−1 | 7;13;0 | C+, 0− | C+, 8− | 3.5 mA | 90 | 130 |
| 5 | −7;12;−2 | 7;12;−2 | C+, 0− | C+, 8− | 7.0 mA | 60 | 130 |
| 6 | −6;14;−4 | 6;14;−4 | C+, 0− | C+, 8− | 5.0 mA | 90 | 130 |
| 7 | −6.5;13;−3 | 6;13;−3 | C+, 3− | C+, 11− | 8.2 V | 60 | 130 |
| 8 | −5.5;16;0.18 | 5.5;16;0.18 | C+, 0− | C+, 8− | 4.5 V | 90 | 110 |
Coordinates in native space; x, y, z from mid‐ACPC.
Imaging, Lead Localization, Estimation of the Volume of Tissue Activated and Connectivity Analyses
All subjects had a preoperative 3‐T magnetic resonance imaging (MRI; Philips, Eindhoven, The Netherlands) or 1.5‐T MRI in case of an implanted DBS system (STN, baseline characteristics). The sequence used was a 3D T1 (voxel size 1 × 1 × 1 mm) with gadolinium. Postoperatively, a CT (Voxel size 1 × 1 × 1 mm; Siemens, Erlangen, Germany) or a 1.5‐T T1 MRI was performed to localize the DBS leads.
DBS electrodes were localized using the Lead‐DBS pipeline (24). Postoperative CT and MR images were linearly coregistered to preoperative T1 images using Advanced Normalization Tools (ANT) (25). Subcortical refinement was applied (as a module in Lead‐DBS) to correct for brain shift that may have occurred during surgery. Images were then normalized into ICBM 2009b Nonlinear Asymmetric (montreal neurological institute [MNI]) template space using the SyN approach implemented in ANTs, with an additional subcortical refinement stage. Both coregistrations and normalizations were visually reviewed and refined, if needed. DBS electrodes were then localized using Lead‐DBS and warped into MNI space (19). As a relative measure for targeting precision and electrode registration, preoperative target AC‐PC coordinates were mapped into MNI space, where a distance of 2 mm was accepted as adequate (26). The Euclidean distance between the contact point and the closest in the shell of the target structure was calculated using MATLAB (R2020a, Mathworks, Natick, MA, USA). The volumes of tissue activation (VTAs) were estimated using a finite element method with patient specific stimulation parameters (Table 2). Gray matter was defined by the CIT168 Reinforcement Learning Atlas (27). Intersecting volumes of relevant gray matter structures within the CIT‐168 atlas with VTAs were calculated with the Lead‐DBS pipeline.
In order to validate discriminating fiber bundles previously associated with clinical response, we adapted the methodology of Irmen et al. (28). Accordingly, based on a normative connectome, individual fibers were assigned a “Fiber R‐score” by correlating the fiber tract's connectivity to E‐fields across patients with clinical outcome (29). In short, a fiber tract that passes close to an active contact of patients with Y‐BOCS improvement but far from active contacts in patients with Y‐BOCS worsening would receive a high Spearman's R‐value (and tracts exhibiting the inverse property received a highly negative R‐value) (29). R‐values were corrected for the stimulation amplitude. Validation of the tracts was sought by performing a k‐fold cross prediction (28, 30).
Stimulation, Data Collection, and Statistical Analyses
Typically, the monopolar stimulation of contact closest to target was turned‐on at low voltage several days after implantation. During regular follow‐up moments by the treating psychiatrist (AL) stimulation parameters were adapted (active electrode, pulse width, amplitude, and frequency) based on clinical response and stimulation related side effects. See Table 2 for the active electrode and stimulation parameters at time of last follow‐up. Patient characteristics, stimulation parameters, surgery or stimulation related complications, and psychiatric assessments were retrospectively collected at baseline and at the time of last follow‐up and included the Y‐BOCS, the Beck Depression Inventory–II (BDI‐II), 3‐level EQ‐5D, and the State‐Trait‐Anxiety‐Inventory (STAI) (31, 32, 33, 34, 35). EQ‐5D‐3L outcomes were presented as a single global health index with a weighted total value, according to the Dutch population (36). Responders were defined as patients with ≥35% Y‐BOCS reduction at the time of last follow‐up.
Clinical outcome variables, relative distances of active electrodes to atlas structures, VTA‐atlas intersection volumes between nonresponders and responders were compared using the Chi‐squared test, Student's t‐test, or Mann‐Whitney U where appropriate. The Kolmogorov‐Smirnov was used to test for normality. p values < 0.05 were considered statistically. All statistical analyses were performed using IBM SPSS Statistics, version 20 (IBM Corp., Armonk, NY, USA).
Ethical Statement
The work described was conducted in accordance with the Declaration of Helsinki. Approval by the institutional review board and patient consent were not required as the present study has no obligations to the Dutch Act of Scientific Research in Humans.
RESULTS
We included eight patients with a minimum duration of stimulation of ten months and a median of 26 months. Five patients were considered to be a responder while three remained nonresponsive, resulting in a response rate of 63%. The mean total Y‐BOCS reduction was 10.5, with an equal reduction in Y‐BOCS subscores for obsessions and compulsions (Table 3). Specified for responders, the mean total Y‐BOCS reduction was 16.6. There were no significant differences in age at surgery, age at onset, sex, disease duration, time of follow‐up, OCD severity, or the remainder outcome measures at baseline between responders versus nonresponders (see Supporting Information Table S1).
Table 3.
Clinical Outcome.
| Baseline [± SD] | Last follow‐up [± SD] | Mean difference [95% CI] | p value | |
|---|---|---|---|---|
| Y‐BOCS | ||||
| Total (8) | 33.12 [3.34] | 22.63 [7.91] | 10.5 [2.88;18.13] | 0.014 |
| Obsessions (7)* | 16 [1.63] | 11 [1.63] | 5 [0.44;9.57] | 0.036 |
| Compulsions (7)* | 16.57 [2.07] | 11.86 [3.98] | 4.71 [0.2‐;9.20 | 0.042 |
| BDI‐II (7) | 29.71 [9.05] | 21.43 [11.04] | 8.28 [‐5.39;21.96] | 0.189 |
| STAI (3) | ||||
| XI | 59.75 [15.05] | 42.33 [19.04] | 13.67 [1.41;25.92] | 0.041 |
| X2 | 69.14 [7.05] | 52.33 [22.03] | 10.67 [‐33.09:54.43] | 0.404 |
| EQ‐5D (5)* | ||||
| Index | 0.60 [0.14] | 0.65 [0.29] | 0.05 [‐0.37;0.49] | 0.686 |
| EQ‐VAS | 41.6 [7.73] | 63 [12.55] | ‐21.4[‐33.93;‐8.89] | 0.009 |
BDI‐II, Beck Depression Inventory–II; EQ‐VAS, EuroQol–Visual Analogue Scale; SD, standard deviation; STAI, State (X1)‐Trait (X2) Anxiety Inventory; Y‐BOCS, Yale‐Brown Obsessive Compulsive Scale.
The STAI (X1) score for anxiety symptoms improved significantly (from 59.75 ± 15.05 to 42.33 ± 19.04, p = 0.041). The EQ‐VAS as included in the EQ‐5D was significantly better postoperatively compared to baseline. When translated categorically, the mean BDI‐II scores clinically improved from clinically severe depression to moderate depression.
In a subsequent analysis, there were no significant differences in secondary outcome measures between responders versus nonresponders (Table 4). We refer to Supporting Information Table S2 for a detailed description of the observed complications within this cohort.
Table 4.
Clinical Outcome Responders Versus Nonresponders.
| Responders [±SD] | Nonresponders [±SD] | p value | |
|---|---|---|---|
| Y‐BOCS | 17.8 [5.41] | 30.67 [1.53] | 0.009 |
| BD‐II (7) | 18 [14.99] | 34 [9.45] | 0.25 |
| EQ‐5D (5) | |||
| Index | 0.67 [0.30] | 0.63 [0.30] | 0.76 |
| EQ‐VAS | 62.5 [12.58] | 65 [12.58] | 1 |
Electrodes were successfully registered in MNI space, with 95% of the contact points closest to target within an Euclidean distance ≤2.0 of the target coordinate in MNI space (26). Figure 1a shows the anatomical location of the active electrode (Table 2) during last follow‐up in MNI space with gray matter defined by the CIT168 Reinforcement Learning Atlas. Visually inspected, it seems that the active electrode of nonresponders show a more medial localization of the active contact points, especially on the left side (Fig. 1a). However, the X‐coordinate of the active electrode and the percentage of Y‐BOCS reduction were not significantly correlated (Pearson's r = −0.61; p = 0.10). Furthermore, no significant correlations were found between relative distances of active contact points with gray structures, as provided by the CIT168 Reinforcement Learning Atlas, and the percentage in Y‐BOCS reduction.
Figure 1.
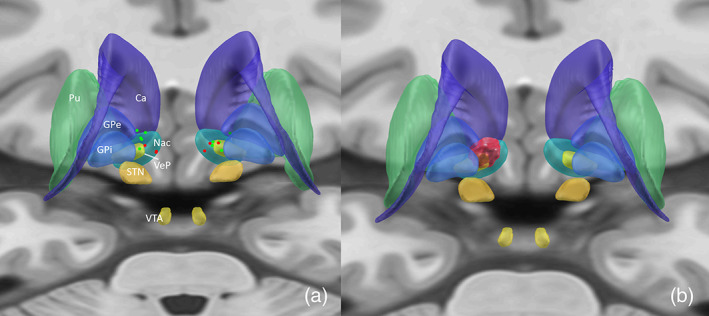
Note: P‐A visualization. Localization of the active electrodes mapped in ICBM 2009b Nonlinear Asymmetric MNI template (a) and the %Y‐BOCS reduction mapped on the Volume of Tissue activation for all patients, mirrored to the left side (b). Responders are shown in green, nonresponders in red (a). Ca, caudate nucleus; GPe, external globus pallidus; GPi, internal globus pallidus; NaC, nucleus accumbens; Pu, putamen; STN, subthalamic nucleus; VeP, ventral pallidum; VTA, ventral tegmental area. [Color figure can be viewed at wileyonlinelibrary.com]
There was no significant difference in the mean pooled VTAs between responders (424 mm3 [± 255]) and nonresponders (370 mm3 [± 158]) (p = 0.754). The intersecting volumes between the CIT‐168 gray matter structures and the VTAs were calculated. VTAs intersected with 9 out of the 16 structures in the atlas. None of the intersecting volumes significantly correlated with the percentage Y‐BOCS reduction (Supporting Information Table S3).
In an attempt to validate the results of Li et al. which identified a subtract of the ALIC, connecting the prefrontal cortex to the STN and the mediodorsal nucleus (MD) of the thalamus positively associated with Y‐BOCS reduction, we acquired a similar methodology as provided in Lead‐DBS. Fiber R‐values to E‐fields were assigned across patients with clinical outcome as performed in Irmen et al. (29) (Fig. 2).
Figure 2.
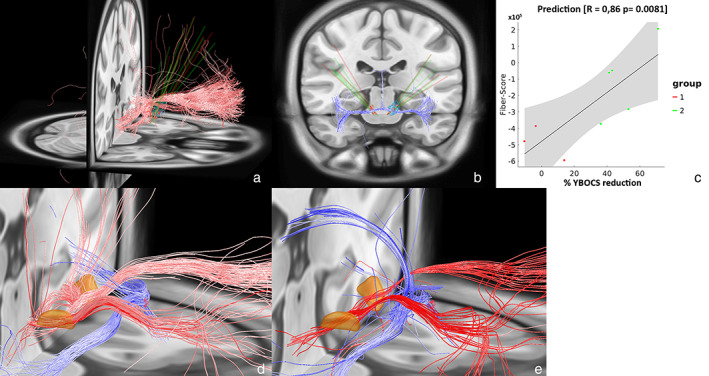
Left and middle: Positive (a) and negative (b) predicting fibers associated with clinical improvement are shown in red and blue. Right: Correlation between the degree of stimulated positive predictive tracts and percentage Y‐BOCS reduction (c). Gray shaded areas represent 95% confidence intervals. Group 1 and group 2 represent nonresponders and responders, respectively. This analysis is based on a normative connectome. d. The identified predicting fiber tracts as identified by Li et al. as available in Lead‐DBS. The STN is depicted in orange. e. Close‐up of figures (a) and (b) combined. [Color figure can be viewed at wileyonlinelibrary.com]
Unthresholded discriminating fiber tracts were identified which show a great overlap with the fiber tracts observed by Li et al. Using subsequent prediction analyses using k‐fold cross validation (K = 2), the degree of lead connectivity was strongly correlated with Y‐BOCS reduction (r = 0.76 at p = 0.011). However, also seemingly irrelevant tracts were identified, specifically tracts in the corpus callosum and in the temporal cortices (Supporting Information Fig. S1). Increasing the threshold of the tracts to be connecting if the E‐field magnitude >100.3 V/mm and >22% of the E‐fields, disregarded these irrelevant tracts but preserved the fiber tracts graphically similar to Li et al. (Fig. 2). The fiber bundles negatively associated with the percentage Y‐BOCS reduction are recognized as the posterior limb of the anterior commissure, connecting the bilateral temporal cortices and cingulate fiber bundles. The positive discriminating fibers connect the prefrontal cortex with the STN. In a subsequent prediction analyses using k‐fold cross validation (K = 2), the degree of lead connectivity was strongly correlated with clinical outcome (r = 0.86 at p = 0.008).
DISCUSSION
Our analysis supports that a subpart of the ALIC, that connects areas of the prefrontal cortex with the STN and medial (MD) nucleus of the thalamus, is associated with optimal clinical response in a cohort of eight patients receiving VC/VS stimulation for refractory OCD. With regard to the validity of clinical outcomes, the mean reduction in Y‐BOCS score of 31.7% is somewhat less favorable compared to the large cohort of Denys et al. (40%, SD = 9.4), lower than a large international prospective trial by Ménchon et al. (20%, SD = 9.5) (11, 37, 38). but within the confidence interval (CI) reported in the meta‐analyses by Alonso et al. (45.1%, 95% CI = 29.4–60.8%). Compared to Denys et al. and Alonso et al., we report a similar responder ratio (60% and 52%, respectively). As previously reported, a beneficial effect on state anxiety was observed (15, 39).
The mean EQ‐5D health index is within the confidence interval the EQ‐5D (0.67, 95% CI = 0.64–0.70) of large cohort of chronic and demographically comparable OCD patients (40). We did not observe an improvement in quality of life as observed in Ménchon et al. This may indicate that other factors than OCD severity contributed to the quality of life outcome, or reflect the lower sensitivity and precision of the EQ‐5D‐3L in our study compared to the EQ‐5D‐5L used by Menechon et al. (41) Mood improved in both responders and nonresponders, without significant between‐group differences, showing that effects of mood may be independent of effects on OCD symptoms.
Using the twofold cross‐validation method, we were able to validate the identified fiber tracts in our cohort. Of note, this correlation is somewhat circular and meant to describe the degree of how well discriminative tracts could explain the same sample of patients on which they were calculated. We were able to show these positive and negative fiber tracts with a relative low number of patients receiving a stimulation of a different target (VS/VS) compared to the four cohorts in which overlapping fiber bundles were originally identified, which either addresses pitfalls in methodology of using human scale diffusion weighted MRI images (DWI), a normative connectome, statistics and accuracies in lead localization which may result from the approach of warping electrodes into common space or cautiously validates the robustness of the findings. Without elaborating on the above standing technical issues, we would like to note that using a normative connectome, as provided by the well‐validated neuroimaging pipeline of Lead‐DBS, has abled further examination of stimulation effects, as patient‐specific DWI data was lacking in our cohort. However, in order to be clinical applicable, or to have an impact on stereotactic planning, these tracts have to be identified in native patient space. Individual anatomical variability of orbitofrontothalamic tracts has been observed, which may in turn partially explain for variation in treatment response (42).
The tract associated with good clinical outcome in the present study was identified as a subpart of the ALIC that connects areas of the prefrontal cortex with the STN and medial (MD) nucleus of the thalamus (19). Functionally, this tract is recognized as the hyperdirect pathway to the STN originating from the dorsal ACC (dACC) and the ventrolateral prefrontal cortex traversing within the ALIC, implicating the involvement for the limbic cortico‐basal ganglia‐thalamocortical circuit (19). The role of the hyperdirect pathway within this circuit may be explained by the hypothetical “pulley competition model.” In this model, it is suggested that the direct and indirect pathways compete throughout the basal ganglia, with the strength of each pathway acting as weights on opposing sides of a pulley. When activation of the direct pathway overpowers that of the indirect pathway, it results in facilitation or a concrete action if the difference exceeds a critical threshold (43). In this model, the role of the hyperdirect pathway is that of a brake that can cancel an action before the activation that leads to it reaches the critical threshold (43). Modulation of the hyperdirect pathway could thus result in a direct inhibition of the dACC's direct pathway. Hyperactivation of the dACC is observed in OCD, both at rest and during symptom provocation and may mediate the elevated fear and anxiety associated with OCD (44, 45, 46). Another role for the dACC in OCD may be recognized when introducing Hierarchical Reinforcement Learning (HRL), within its pathobiology. HRL is a machine learning paradigm that is increasingly used in behavioral sciences to explain normal and abnormal behavior. Within the HRL model, the ACC instigates a specific task appropriate to the environmental situation and subsequently instructs the actor module to perform this task. The dysfunctional behavior observed in OCD may emerge from a faulty task or option selection by the ACC, which ultimately is corrected by activation of the hyperdirect pathway by DBS (Bouwens van der Vlis et al., submitted).
Our clinical findings should be interpreted within the limits of this small‐sized retrospective open case study, lacking randomization and nonblinded assessment which may therefore be prone for systematic bias. Further, patients had continuous medication and psychotherapy during the follow‐up of the study. Therefore, a synergistic or confounding effect of cotreatment cannot be ruled out.
Taken together, the present study contributes to the available literature of VC/VS DBS as an effective and well‐tolerated treatment option for patients with refractory OCD and supports the finding that specifically modulating the limbic circuit is associated with treatment response. The latter fits the evolution from the search for a single, optimal gray matter target toward the conception of modulating networks that support particular symptom profiles. Expanding the connectomic analyses to targets which are not part of the classical cortico‐basal ganglia‐thalamocortical circuitry, that is, the ITP and the BNST, could reveal other differentiating brain networks. Finally, well‐controlled randomized studies in larger samples are needed to address clinical variability, including analyses of individual white matter tracts.
Authorship Statement
Drs. Bouwens van der Vlis, Vrij, Ackermans, Mulders, and Leentjens designed and conducted the study, including patient recruitment, data collection, and data analysis. Dr. Bouwens van der Vlis prepared the manuscript draft with important intellectual input from Drs. Ackermans, Leentjens, Schruers, Temel, Mulders, and Duits. All the authors approved the final manuscript. The Maastricht University Medical center provided funding for editorial support. Drs. Bouwens van der Vlis, Leentjens, and Ackermans had complete access to the study data.
Supporting information
Appendix S1: Supplementary Information
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: None.
Conflict of Interest: The authors reported no conflict of interest.
REFERENCES
- 1. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 2. Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive‐compulsive disorder in children and adults. Dev Psychopathol 2008;20:1251–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloch MH, Landeros‐Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta‐analysis of the symptom structure of obsessive‐compulsive disorder. Am J Psychiatry 2008;165:1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soomro GM, Altman D, Rajagopal S, Oakley‐Browne M. Selective serotonin re‐uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev 2008;1:CD001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skapinakis P, Caldwell DM, Hollingworth W et al. Pharmacological and psychotherapeutic interventions for management of obsessive‐compulsive disorder in adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2016;3:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gava I, Barbui C, Aguglia E et al. Psychological treatments versus treatment as usual for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev 2007;2:CD005333. [DOI] [PubMed] [Google Scholar]
- 7. Pallanti S, Quercioli L. Treatment‐refractory obsessive‐compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:400–412. [DOI] [PubMed] [Google Scholar]
- 8. Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med 2012;4:142rv148. [DOI] [PubMed] [Google Scholar]
- 9. Blomstedt P, Sjoberg RL, Hansson M, Bodlund O, Hariz MI. Deep brain stimulation in the treatment of obsessive‐compulsive disorder. World Neurosurg 2013;80:e245–e253. [DOI] [PubMed] [Google Scholar]
- 10. Görmezoğlu M, van der Vlis TB, Schruers K, Ackermans L, Polosan M, Leentjens AFG. Effectiveness, timing and procedural aspects of cognitive behavioral therapy after deep brain stimulation for therapy‐resistant obsessive compulsive disorder: a systematic review. J Clin Med 2020;9:2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alonso P, Cuadras D, Gabriels L et al. Deep brain stimulation for obsessive‐compulsive disorder: a meta‐analysis of treatment outcome and predictors of response. PLoS One 2015;10:e0133591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Koning PP, Figee M, van den Munckhof P, Schuurman PR, Denys D. Current status of deep brain stimulation for obsessive‐compulsive disorder: a clinical review of different targets. Curr Psychiatry Rep 2011;13:274–282. [DOI] [PubMed] [Google Scholar]
- 13. Nair G, Evans A, Bear RE, Velakoulis D, Bittar RG. The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder. J Clin Neurosci 2014;21:815–821. [DOI] [PubMed] [Google Scholar]
- 14. Coenen VA, Schlaepfer TE, Goll P et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive‐compulsive disorder. CNS Spectr 2017;22:282–289. [DOI] [PubMed] [Google Scholar]
- 15. Maarouf M, Neudorfer C, El Majdoub F, Lenartz D, Kuhn J, Sturm V. Deep brain stimulation of medial dorsal and ventral anterior nucleus of the thalamus in OCD: a retrospective case series. PLoS One 2016;11:e0160750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borders C, Hsu F, Sweidan AJ, Matei ES, Bota RG. Deep brain stimulation for obsessive compulsive disorder: a review of results by anatomical target. Ment Illn 2018;10:7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberg BD, Malone DA, Friehs GM et al. Three‐year outcomes in deep brain stimulation for highly resistant obsessive‐compulsive disorder. Neuropsychopharmacology 2006;31:2384–2393. [DOI] [PubMed] [Google Scholar]
- 18. Baldermann JC, Melzer C, Zapf A et al. Connectivity profile predictive of effective deep brain stimulation in obsessive‐compulsive disorder. Biol Psychiatry 2019;85:735–743. [DOI] [PubMed] [Google Scholar]
- 19. Li N, Baldermann JC, Kibleur A et al. A unified connectomic target for deep brain stimulation in obsessive‐compulsive disorder. Nat Commun 2020;11:3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nuttin BJ, Gabriëls LA, Cosyns PR et al. Long‐term electrical capsular stimulation in patients with obsessive‐compulsive disorder. Neurosurgery 2003;52:1263–1272. [DOI] [PubMed] [Google Scholar]
- 21. Schaper F, Zhao Y, Janssen MLF et al. Single‐cell recordings to target the anterior nucleus of the thalamus in deep brain stimulation for patients with refractory epilepsy. Int J Neural Syst 2019;29:1850012. [DOI] [PubMed] [Google Scholar]
- 22. Kocabicak E, Temel Y. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: surgical technique, tips, tricks and complications. Clin Neurol Neurosurg 2013;115:2318–2323. [DOI] [PubMed] [Google Scholar]
- 23. Ackermans L, Kuhn J, Neuner I, Temel Y, Visser‐Vandewalle V. Surgery for Tourette syndrome. World Neurosurg 2013;80:S29. [DOI] [PubMed] [Google Scholar]
- 24. Horn A, Li N, Dembek TA et al. Lead‐DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019;184:293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross‐correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis 2008;12:26–41. 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horn A, Kühn AA, Merkl A, Shih L, Alterman R, Fox M. Probabilistic conversion of neurosurgical DBS electrode coordinates into MNI space. Neuroimage 2017;150:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pauli WM, Nili AN, Tyszka JM. A high‐resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data 2018;5:180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irmen F, Horn A, Mosley P et al. Left prefrontal connectivity links subthalamic stimulation with depressive symptoms. Ann Neurol 2020;87:962–975. [DOI] [PubMed] [Google Scholar]
- 29. Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. The WU‐Minn human connectome project: an overview. Neuroimage 2013;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Treu S, Strange B, Oxenford S et al. Deep brain stimulation: imaging on a group level. Neuroimage 2020;219:117018. [DOI] [PubMed] [Google Scholar]
- 31. Abramowitz JS, Deacon BJ, Olatunji BO et al. Assessment of obsessive‐compulsive symptom dimensions: development and evaluation of the dimensional obsessive‐compulsive scale. Psychol Assess 2010;22:180–198. [DOI] [PubMed] [Google Scholar]
- 32. Goodman WK, Price LH, Rasmussen SA et al. The Yale‐Brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry 1989;46:1012–1016. [DOI] [PubMed] [Google Scholar]
- 33. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories ‐IA and ‐II in psychiatric outpatients. J Pers Assess 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 34. EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 35. Spielberger CD. State‐trait anxiety inventory. In: Weiner I, Craighead W, editors. The Corsini encyclopedia of psychology. Hoboken: Wiley, 2010; p. 1698–1699. [Google Scholar]
- 36. Lamers LM, McDonnell J, Stalmeier PF, Krabbe PF, Busschbach JJ. The Dutch tariff: results and arguments for an effective design for national EQ‐5D valuation studies. Health Econ 2006;15:1121–1132. [DOI] [PubMed] [Google Scholar]
- 37. Menchón JM, Real E, Alonso P et al. A prospective international multi‐center study on safety and efficacy of deep brain stimulation for resistant obsessive‐compulsive disorder. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Denys D, Graat I, Mocking R et al. Efficacy of deep brain stimulation of the ventral anterior limb of the internal capsule for refractory obsessive‐compulsive disorder: a clinical cohort of 70 patients. Am J Psychiatry 2020;177:265–271. [DOI] [PubMed] [Google Scholar]
- 39. Huff W, Lenartz D, Schormann M et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment‐resistant obsessive‐compulsive disorder: outcomes after one year. Clin Neurol Neurosurg 2010;112:137–143. [DOI] [PubMed] [Google Scholar]
- 40. Remmerswaal KCP, Batelaan NM, Hoogendoorn AW, van der Wee NJA, van Oppen P, van Balkom A. Four‐year course of quality of life and obsessive‐compulsive disorder. Soc Psychiatry Psychiatr Epidemiol 2020;55:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Janssen MF, Bonsel GJ, Luo N. Is EQ‐5D‐5L better than EQ‐5D‐3L? A head‐to‐head comparison of descriptive systems and value sets from seven countries. Pharmacoeconomics 2018;36:675–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Makris N, Rathi Y, Mouradian P et al. Variability and anatomical specificity of the orbitofrontothalamic fibers of passage in the ventral capsule/ventral striatum (VC/VS): precision care for patient‐specific tractography‐guided targeting of deep brain stimulation (DBS) in obsessive compulsive disorder (OCD). Brain Imaging Behav 2016;10:1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dunovan K, Verstynen T. Believer‐skeptic meets actor‐critic: rethinking the role of basal ganglia pathways during decision‐making and reinforcement learning. Front Neurosci 2016;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Milad MR, Rauch SL. Obsessive‐compulsive disorder: beyond segregated cortico‐striatal pathways. Trends Cogn Sci 2012;16:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holroyd CB, Umemoto A. The research domain criteria framework: the case for anterior cingulate cortex. Neurosci Biobehav Rev 2016;71:418–443. [DOI] [PubMed] [Google Scholar]
- 46. Breiter HC, Rauch SL, Kwong KK et al. Functional magnetic resonance imaging of symptom provocation in obsessive‐compulsive disorder. Arch Gen Psychiatry 1996;53:595–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


