Abstract
Background
Over the last six decades (earliest included publication from 1959), clinical trials of migraine preventive treatments have led to the regulatory approval of many medications and devices. Despite similar clinical goals, the outcomes and endpoints used in these trials are broad and not well standardized.
Objective
To describe results from a systematic literature review focused on outcomes and endpoints used in preventive migraine clinical trials.
Method
A systematic literature review, following a pre‐specified (unregistered) protocol developed to adhere to recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses, was conducted to characterize the endpoints and outcomes used in preventive migraine clinical trials. Predetermined terms were searched in PubMed on October 28, 2019. Data related to trial design, subject characteristics, outcomes, and endpoints reported in each publication were extracted. Descriptive summaries of these features were tabulated for the recent subset of publications, published during or after 1988, that were randomized, blinded, and focused on pharmacological or device therapies for the preventive treatment of migraine.
Results
The initial literature search identified 1506 publications, of which 757 publications were eligible for data extraction. Of specific clinical interest were the recent subset of 268 articles (268/757, 35.4%) fulfilling the targeted criteria. Results showed that the outcomes used to define endpoints varied substantially across publications. For example, in the recent subset of publications, 68.7% (184/268) of the publications examined ≥1 migraine‐specific outcome, 39.6% (106/268) examined ≥1 headache‐specific outcome, 50.7% (136/268) examined ≥1 acute/rescue medication use outcome, 40.3% (108/268) examined ≥1 headache‐related patient‐reported outcome measure (PROM), and 22.0% (59/268) examined ≥1 non‐headache‐specific PROM. Furthermore, the definition of the endpoints used (e.g., change from baseline, fixed timepoint comparisons, categorization of “responders” to treatment based on wide variety of “responder definitions”) also differed across publications.
Conclusion
Publications from clinical trials of preventive migraine pharmacologic and device treatments differed in terms of study design, endpoint definitions, and how endpoints and outcomes were measured. Although there were common outcomes and endpoints used across publications, no clear “standardized” set of endpoints and outcomes emerged. The inconsistencies in endpoints and outcomes within this literature suggest that the development of a uniform set of outcomes and endpoints could improve the clinical meaningfulness of clinical trial results, facilitate cross‐trial comparisons and better inform patient care. This standard set of outcomes and endpoints should be statistically robust and informed by the priorities of various stakeholders, most importantly, the needs and preferences of people living with migraine.
Keywords: clinical outcome assessment, clinical trial design, endpoints, outcomes, patient reported outcome measures, preventive migraine
INTRODUCTION
Migraine is a prevalent neurologic disease that has been linked to a broad range of negative outcomes including disability and impairment, reduced health‐related quality of life, high direct and indirect costs, a range of comorbidities, and adverse family, professional, and social consequences. 1 , 2 , 3 , 4 , 5 , 6 , 7 In 2016, it was estimated that the total costs of migraine in the United States alone was $36 billion. 7 Given the clear health and financial burden of migraine on patients, their families, and society as a whole, there have been substantial efforts to develop effective and tolerable migraine therapies for more than half a century. 8
Organizations and agencies such as the International Headache Society (IHS), the American Headache Society (AHS), the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the US National Institute for Neurologic Diseases (NINDS) Common Data Elements have provided guidance to help improve the quality of migraine clinical trials and to inform clinical practice. 9 , 10 , 11 , 12 , 13 , 14 Published clinical trial guidelines address several important topics including patient selection, trial design, outcomes, endpoints, evaluation of results, and statistical analyses. Although each of these topics is critical to advancing the area of migraine therapeutics, the current work focuses specifically on characterizing the endpoints and corresponding outcomes used in preventive migraine clinical trials. The term outcome usually refers to the construct or variable being measured (e.g., number of migraine days) while the endpoint is a parameter analyzed in the trial (e.g., change in migraine days per 4 weeks from the 4 week baseline run‐in phase to 9‐ to 12‐week post‐treatment).
Our systematic review of the literature on preventive migraine clinical trials in adults (18+ years old) integrates information regarding the clinical endpoints and outcomes used to evaluate preventive migraine treatment efficacy and other outcomes. This work is the first step of a broader, federally funded project aimed at formulating a recommended set of outcomes and endpoints for preventive and acute treatment trials in migraine. We have used the range of outcomes and endpoints identified by this systematic review to inform qualitative work with patients on the burden of migraine, the benefits they seek from treatment, and the strengths and limitations of available therapies. As we move forward with our project, this systematic review will support future development of endpoints and outcome measures for preventive migraine clinical trials.
METHODS
A systematic literature review, consistent with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (followed a pre‐specified, but unregistered protocol), was conducted to evaluate the range of outcome measures and endpoints used in clinical trials of migraine preventive treatments. 15 Briefly, PRISMA provides a checklist related to consensus recommendations for the development and execution of high‐quality systematic literature reviews that includes pre‐specification of eligibility criteria for located publications, the database to be used for the search as well as draft search terms, the standardized process used to review located publications including record tracking/data management systems to be used, the data planned to be extracted from each publication meeting inclusion criteria, and the plan for summarizing the extracted information.
Search strategy
PubMed (https://pubmed.ncbi.nlm.nih.gov/) was used as the primary database queried to identify initial articles for review. PubMed filters were used to limit results to human clinical trials and to articles published in English. No period restrictions were imposed on the results and the search was conducted on October 28, 2019.
The PubMed search term used to identify the initial articles was as follows:
(((((((preventive) OR prophylaxis) OR prophylactic) AND migraine [Mesh]) AND Clinical Trial[ptyp]) AND Humans[Mesh]) AND English[lang]).
The title and abstract of each publication returned from the search were screened by two methodologists (CH and JM), using the Covidence online systematic review tool (https://www.covidence.org/), for relevance to the stated goals. The inclusion and exclusion criteria are provided in Table 1.
TABLE 1.
Inclusion and exclusion criteria used in publication screening process
| Study characteristics | Inclusion | Exclusion |
|---|---|---|
| Patient population | Interventional, adult preventive migraine trial | Trials using only pediatric patients (<18 years old) (mixed adult and pediatric trials were included) |
| All migraine types, including subtypes (e.g., menstrual migraine; medication overuse if sample is specified as migraine patients) were included | Trials with ONLY healthy volunteers given a preventive intervention (mixed healthy/migraine samples were included) | |
| Interventions | Interventions can be pharmacological (e.g., pills, injections), devices, physical (acupuncture, massage, exercise, etc.), dietary, or other novel treatments intended to prevent or lessen the frequency of migraine attacks/days | Acute migraine trials were excluded (mixed trials with preventive and acute outcomes included) |
| Comparators | Any | |
| Outcomes | Any | |
| Study design/publication characteristics | Open‐label studies and Phase 4 trials | Observational studies, surveys (not Post‐Marketing Phase 4), epidemiological studies, etc. |
| Pilot studies with migraine patients | Letters to the editor (including those describing trials), abstracts/papers from conference proceedings, case reports/studies | |
| Language | English | Non‐English |
Once the initial list of screen‐pass publications was compiled, a review of the reference section in each publication was undertaken to locate any potentially relevant publications. Newly located articles (n = 251 non‐duplicate additional articles) were added to the “initial” list and titles and abstracts were submitted to the screening review (as detailed above) for inclusion/exclusion in the final version of the initial list.
Study selection
With the candidate reference list finalized, a brief review of each full publication was undertaken by two of four methodologists (DB, CH, JM, and LS) to confirm the relevance of the article to the current goals. With an agreed‐upon positive assessment from the brief review, the publication was included in the final references list. All agreed upon negative reviews resulted in the exclusion of the publication from this list. Disagreements on the status of an article were reviewed by a doctoral‐level study team member (DB), blind to previous votes, and a discussion among the reviewers determined the final status of an article regarding inclusion/exclusion in the final list of publications slated for extraction.
Data extraction
For the final list of publications, pre‐identified salient key features of each preventive migraine clinical trial were extracted. This included extracting all available information related to year of publication, journal name, ClinicalTrials.gov identifier(s), trial name, phase of trial (I–IV), 16 general description of the trial design, sample size, patient descriptives (age, gender/sex, race, ethnicity), salient migraine characteristics (migraine with or without aura only, menstrual migraine or menstrually related migraine only, episodic vs. chronic migraine, etc.), and type of treatment investigated (pharmacologic, neurostimulation, behavioral, complimentary and integrative treatments, etc.). Older terms for migraine were reclassified to current terminology per the expert guidance of RL and DB: classic migraine to migraine with aura, common migraine to migraine without aura, and transformed migraine to chronic migraine. Additionally, data extraction from the articles included the concepts (i.e., the domain being measured such as disability or pain) examined, the endpoints used, and any specific PROMs used. Endpoint designations such as primary, secondary, and tertiary were not captured because they were not consistently reported.
Data related to the descriptive trial information were extracted by trained research assistants (AP, LO, SH, JW, and TN). A second research assistant independently extracted the same data for approximately 5% of candidate publications and rater/extractor agreement kappas were calculated. Data related to the concepts, outcomes, and endpoints examined were extracted by one of four doctoral‐level methodologists (DB, CH, JM, and AP) into a standardized, structured Microsoft Excel worksheet.
Quality assessment of included studies
Given the purpose of this systematic review, an assessment of study quality was not applicable.
Analyses
Tables were planned to present information from the articles in a digestible fashion, including summary tables focused on the study design characteristics, demographics for the samples used in preventive migraine trials, and outcomes (migraine/headache days, attacks, hours, patient‐reported outcomes measures [PROMs]) and endpoints (change from baseline, fixed timepoint comparisons, responder definitions) used.
Outcomes were classified within five broad categories:
Migraine‐Focused Outcomes—Days, Attacks, Total Hours, Index (various combinations of attack duration and pain intensity/severity), Pain/Intensity/Severity, and Duration (per attack)
Headache‐Focused Outcomes—Days, Attacks, Total Hours, Index (various combinations of attack duration and pain intensity/severity), Pain/Intensity/Severity, and Duration (per attack)
Acute/Rescue Medication Outcomes—Days of use, Doses/Uses of medication
Headache/Migraine‐related PROMs—such as the Migraine Disability Assessment Test (MIDAS), 17 the 6‐item Headache Impact Test short form (HIT‐6), 18 Migraine‐specific Quality of Life (MSQ) 19 , 20 and other measures of migraine‐specific quality of life, cognitive constructs, disability and impairment due to migraine and/or headache. Disability/Impairment is a general category (not a single item/scale) that consists of a range of disability, impact, and impairment outcomes. These measures are placed in the Headache/Migraine‐related PROMs category for this review.
Non‐headache‐specific PROMs—includes such measures as the Short Form Health Survey (SF‐36), 21 various depression and anxiety measures, patient global impression of change (PGIC), patient global impression of severity (PGIS), and treatment efficacy/satisfaction/preference items. The measurement of patient global impression, treatment efficacy, satisfaction, and preference varied across manuscripts (e.g., different response scales, varied verbal labels) but were grouped into general categories for tabled results.
These five broad categories were developed by the first author (JM) after reviewing an initial set of five publications and studying the types of information commonly reported and the level of detail available. Review of these broad categories was undertaken by two expert migraine researchers (RL and DB) independently to evaluate their face validity and make suggestions for modification. The outcomes listed in categories 1, 2, and 3 above are exhaustive of the outcomes included in those categories.
In addition, each endpoint can be classified into one of three broad categories, based on how the outcome variable was used to classify the endpoint:
Change from baseline
Fixed timepoint
Responder definitions (≥50% reduction, ≥75% reduction, 100% reduction, Other definitions)
Kappa statistics were calculated to measure inter‐rater reliability for data extraction. Descriptive statistics were calculated for the categorical (N, %) and continuous (mean, standard deviation [SD], minimum [Min], 25th percentile [Q1], median, 75th percentile [Q3], maximum [Max]) variables of interest. Analyses were conducted in SAS 9.4.
Given the substantive goals of the current study, a recent randomized and blinded subset of publications was selected from the broader article list to better understand endpoints in outcomes used in more modern trials. This subset of articles consisted of articles published in 1988 or later (following the International Classification of Headache Disorders (ICHD)‐1 publication) 22 that used randomized and blinded designs and focused on pharmacological treatments or medical devices, excluding behavioral, lifestyle, and complimentary and integrative medicine studies. We refer to this subset as the “recent randomized and blinded” publications.
RESULTS
Study selection and extraction
Of the 1506 publications found through the initial search and reference section reviews, 757 publications were included for data extraction (Figure 1). The online supplemental materials provide a complete list of all publications located from the PubMed search and their ultimate status regarding inclusion/exclusion in the final selection of articles. Based on the 757 publications, inter‐rater agreement kappas for the descriptive variables extracted (age, sex, study design characteristics, etc.) had an average kappa estimate of 0.87, which was well above the recommended lower bound of 0.6. 23
FIGURE 1.
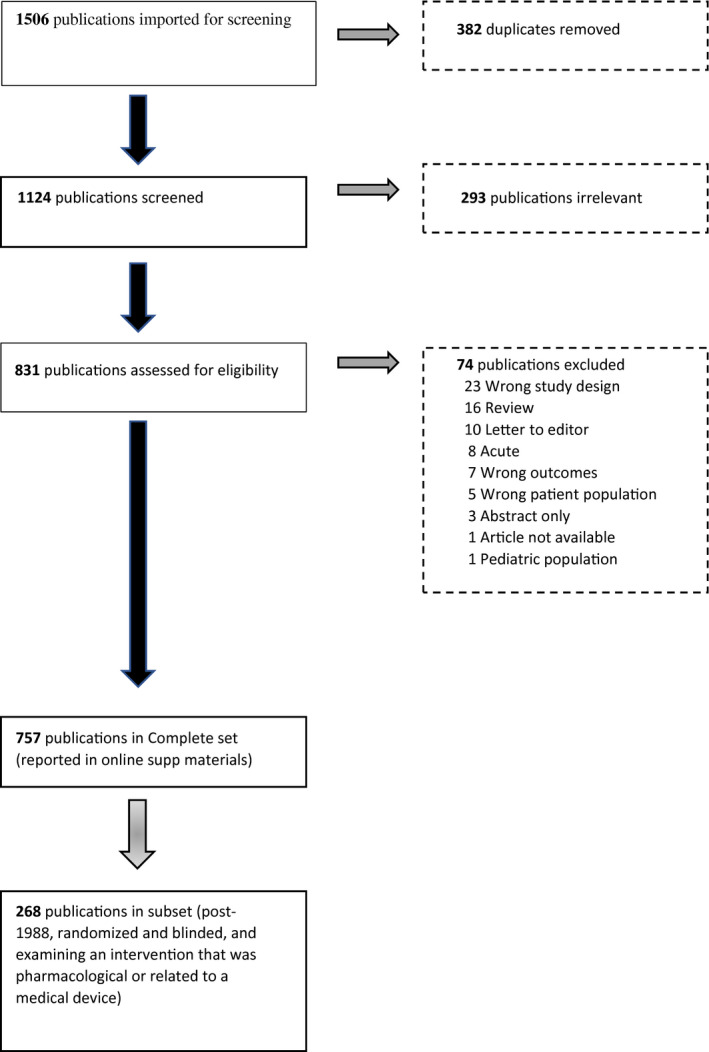
PRISMA diagram of article flow through the systematic literature review of preventive migraine trials
Primary publication set study characteristics
Table 2 shows the study characteristics for the full set of 757 publications. Over half of the publications were placebo/sham controlled (441/757, 58.3%), almost two‐thirds were blinded and randomized (476/757, 62.9%), and about two‐thirds used one of the iterations of the ICHD criteria for migraine (497/757, 65.7%). Most publications examined ≥1 efficacy outcome (747/757, 98.7%) or a safety outcome (631/757, 83.4%). Nearly three‐quarters of publications investigated pharmacological/medication treatments (555/757, 73.3%), 11.0% (83/757) examined complementary and alternative treatments (acupuncture, herbal remedies, osteopathic manipulation, etc.), 4.0% (30/757) examined medical devices (e.g., neurostimulation devices, dental devices), and 2.8% (21/757) examined behavioral interventions.
TABLE 2.
General publication characteristics (n = 757 publications)
| Study characteristic | Percent | N |
|---|---|---|
| Study purpose(s) | ||
| Efficacy assessed | 98.7 | 747 |
| Safety assessed | 83.4 | 631 |
| Pharmacokinetic study | 1.2 | 9 |
| Study/design features | ||
| Study 1988 or later | 77.1 | 584 |
| Randomized | 74.2 | 562 |
| Blinded | 65.8 | 498 |
| Randomized and blinded | 62.9 | 476 |
| ICHD migraine criteria used | 65.7 | 497 |
| Placebo/Sham controlled | 58.3 | 441 |
| Crossover design | 19.0 | 144 |
| Open‐label study | 22.5 | 170 |
| Intervention studied | ||
| Pharmacological/medication | 73.3 | 555 |
| Complimentary and integrative (acupuncture, osteopathic manipulation, herbal treatment, etc.) | 11.0 | 83 |
| Other/multiple categories | 5.0 | 38 |
| Medical device (electrical stimulation, dental plate) | 4.0 | 30 |
| Biobehavioral/psychological (e.g., biofeedback, cognitive behavioral therapy) | 2.8 | 21 |
| Lifestyle (e.g., diet or exercise) | 2.2 | 17 |
| Surgical e.g., (patent foramen ovale closure, other) | 1.7 | 13 |
The denominator for all percentages is 757.
Recent randomized and blinded subset
Given the clinical utility of describing endpoints and outcomes used in more current preventive migraine clinical trials, a subset of 268 studies was selected from the broader publication list (268/757, 35.4% of articles). Most of the publications in this recent randomized and blinded subset used an ICHD criteria for migraine (226/268, 84.3%). All subsequent results are reported for this subset of studies. Results from the full sample of publications are available in the supplemental online materials.
Sample characteristics
Pooling over the available demographic information from the recent randomized and blinded publication subset, the median total sample size was 92.5 (25th percentile: 52; 75th percentile: 355) (268/268, 100% of reported sample size). Of publications that reported age, sex/gender, race, and/or ethnicity descriptive statistics, the average age was found to be 39.4 (SD = 4.1) (239/268, 89.2% of studies reported age), with 82.2% of patients identifying as female (253/268, 94.4% of studies reported gender), and 86.9% of patients reported as White/Caucasian (86/268, 32.1% reported race). Almost 76% (203/268, 75.7%) of the publications examined migraine (unspecified/multiple types) and 17.2% (46/268) of publications examined chronic/transformed migraine exclusively.
Outcomes and endpoints
Within the recent randomized and blinded subset of 268 studies, over two‐thirds of the publications examined ≥1 migraine‐specific outcomes (184/268, 68.7%), 39.6% (106/268) examined ≥1 headache‐specific outcomes, 50.7% (136/268) examined ≥1 acute/rescue medication use outcomes (acute and/or rescue medication use was not reliably differentiated in publications and are amalgamated here as well), 40.3% (108/268) examined ≥1 headache‐related PROMs, and 22% (59/268) examined ≥1 non‐headache‐specific PROMs (Table 3). There were various combinations of outcomes used across publications (Table 4). The most common strategy used only migraine‐focused outcomes (47/268, 17.5%), although the publication could include multiple migraine‐related outcomes (e.g., attack frequency, days with migraine headache, pain intensity, duration of pain). The next most common combination was migraine‐focused outcomes paired with ≥1 acute/rescue medication use outcomes (40/268, 14.9%), followed by migraine‐focused outcomes with acute/rescue medication outcomes and ≥1 PROMs (37/268, 13.8%).
TABLE 3.
Outcomes assessed across recent randomized and blinded subset of publications (n = 268 publications)
| Outcome type | Percent | N |
|---|---|---|
| Migraine‐focused outcome | 68.7 | 184 |
| Headache‐focused outcome | 39.6 | 106 |
| Acute/rescue medication use | 50.7 | 136 |
| Migraine‐related PROMs | 40.3 | 108 |
| Non‐headache‐specific PROMs | 22.0 | 59 |
The denominator for all percentages is 268.
TABLE 4.
Combinations assessed across the recent randomized and blinded subset of publications (n = 268 publications)
| Migraine‐focused outcomes | Headache‐focused outcomes | Acute/rescue medication use | PROMs (both headache‐related and non‐headache‐specific) | Percent | N |
|---|---|---|---|---|---|
| Yes | No | No | No | 17.5 | 47 |
| Yes | No | Yes | No | 14.9 | 40 |
| Yes | No | Yes | Yes | 13.8 | 37 |
| No | Yes | No | Yes | 8.2 | 22 |
| Yes | Yes | Yes | Yes | 8.2 | 22 |
| Yes | No | No | Yes | 7.5 | 20 |
| No | Yes | No | No | 6.7 | 18 |
| No | Yes | Yes | No | 4.9 | 13 |
| No | Yes | Yes | Yes | 4.9 | 13 |
| Yes | Yes | Yes | No | 3.4 | 9 |
| No | No | No | No | 3.0 | 8 |
| No | No | No | Yes | 3.0 | 8 |
| Yes | Yes | No | Yes | 1.9 | 5 |
| Other | 2.2 | 6 | |||
The denominator for all percentages is 268.
Migraine‐focused outcomes and endpoints
Of the 184 publications evaluating ≥1 migraine‐focused outcomes, 69.0% (127/184) examined migraine attacks, 51.1% (94/184) evaluated migraine days, and 48.4% (89/184) evaluated migraine pain (left side of Table 5). Migraine pain was most often assessed as “pain severity” or “pain intensity” on a variety of metrics, including a visual analog scale (VAS) of varied lengths or through ordinal categories—often with 4 response categories, but sometimes with as few as 3 or as many as 11 response options. An “index” was used in 9.2% (17/184) of the publications, but within this grouping the actual factors included in the calculation of the index value varied among publications. Often, publications used index = pain intensity*frequency, but other index definitions were used (e.g., some incorporated duration). With respect to timing of endpoints, the majority of the publications (160/184, 87.0%) evaluated change from baseline and a somewhat limited number of publications (55/184, 29.9%) examined between‐group differences within a pre‐specified, fixed time interval.
TABLE 5.
Migraine‐focused and headache‐focused outcomes, endpoints, and responder definitions in the recent randomized and blinded subset of publications (n = 268)
| Migraine‐focused (n = 184) | Headache‐focused (n = 106) | |||
|---|---|---|---|---|
| Percent | N | Percent | N | |
| Outcomes | ||||
| Attacks | 69.0 | 127 | 28.3 | 30 |
| Days | 51.1 | 94 | 74.5 | 79 |
| Pain intensity | 48.4 | 89 | 38.7 | 41 |
| Duration (e.g., average length of attack) | 32.6 | 60 | 15.1 | 16 |
| Hours (e.g., total headache hours per 4 weeks) | 10.9 | 20 | 17.9 | 19 |
| Index | 9.2 | 17 | 18.9 | 20 |
| Endpoint timing | ||||
| Change from baseline | 87.0 | 160 | 87.7 | 93 |
| Fixed timepoint | 29.9 | 55 | 28.3 | 30 |
| Responder definition | 55.4 | 102 | 40.6 | 43 |
| Responder definitions | ||||
| >50% reduction | 94.1 | 96 | 90.7 | 39 |
| >75% reduction | 18.6 | 19 | 7.0 | 3 |
| 100% reduction | 17.6 | 18 | 7.0 | 3 |
| Other responder definition | 10.8 | 11 | 18.6 | 8 |
Index definitions varied across publications. In general, indexes were defined as a combination of combination of Frequency (days, attacks, or hours), Pain/Intensity/Severity, or Duration (per attack). For example, index = pain intensity*frequency was a commonly used index approach. The denominators for Migraine‐Focused and Headache‐Focused percentages under Outcomes and Endpoint timing are 184 and 106. The denominators for Migraine‐Focused and Headache‐Focused percentages under Responder definitions are 102 and 43. Columns N’s can exceed the total number of Migraine‐Focused or Headache‐Focused articles due to publications evaluating multiple outcomes.
About 55% of publications (102/184, 55.4%) using migraine‐focused outcomes examined differences between groups created by ≥1 within‐person meaningful change threshold values (also known as responder definitions) applied to the target variable (e.g., ≥50% reduction in monthly migraine days, ≥75% reduction in migraine attacks). Of the 102 publications that used responder definitions, the vast majority examined 50% thresholds (96/102, 94.1%).
Headache‐focused outcomes and endpoints
Table 5 shows that 74.5% (79/106) of the 106 publications that utilized ≥1 headache‐focused outcomes examined headache days, 28.3% (30/106) examined headache attacks, and 38.7% (41/106) evaluated headache pain. Almost 90% of the 106 publications examined change from baseline in ≥1 headache‐focused outcomes (93/106, 87.7%) while 28.3% (30/106) examined group differences at fixed times. Just under 41% (43/106, 40.6%) compared treatments by the proportion of patients achieving a set responder definition. Of the 43 publications that used responder definitions, the vast majority (39/43, 90.7%) examined the 50% threshold.
Acute/rescue medication use
Within the recent randomized and blinded subset, 136 publications (136/268, 50.7%) assessed the use of acute or rescue medication as outcomes. About one‐third (49/136, 36.0%) of these acute/rescue medication use publications examined days of acute medication use and two‐thirds (91/136, 66.9%) examined number of doses used (4/136, 2.9% of articles examined both number of days and doses). With respect to the timing of acute/rescue medication use endpoints, 82.4% (112/136) of the 136 publications examined change from baseline and 25.7% (35/136) compared treatment groups at fixed timepoints. Very few publications systematically examined responder definitions for acute/rescue medication use in preventive trials (8/136, 5.9%). For example, it was uncommon for publications to evaluate endpoints such as the proportion of subjects showing a ≥50% reduction in rescue medication doses from baseline.
Headache‐related PROMs
Of the 108 publications within the recent randomized and blinded subset that used headache‐related PROMs (108/268, 40.3%), the MIDAS (53/108, 49.1%), MSQ (34/108, 31.5%), and the HIT‐6 (33/108, 30.6%) were the most frequently encountered measures used as outcomes (Table 6). More than 90% of the 108 publications that included ≥1 headache‐related PROMs examined change from baseline (99/108, 91.7%) and 23.1% (25/108) utilized a fixed time comparison, typically comparing groups cross‐sectionally at several different timepoints, including the end of the blinded trial phase. Few publications examined headache‐related PROMs in conjunction with one or more responder definitions (13/108, 12.0%). There were other headache‐related PROMs used less frequently in studies (under five publications) that are not displayed in Table 6. Readers can see the full list of “named” headache‐related PROMs encountered in all 757 publications in the online supplemental materials.
TABLE 6.
Headache‐related PROMs and endpoints (n = 108 publications)
| Percent | N | |
|---|---|---|
| Headache‐related PROMs Used | ||
| MIDAS | 49.1 | 53 |
| MSQ (all versions) | 31.5 | 34 |
| HIT‐6 | 30.6 | 33 |
| Disability/impairment | 22.2 | 24 |
| Endpoint definitions for PROMs | ||
| Change from baseline | 91.7 | 99 |
| Fixed timepoint | 23.1 | 25 |
| Responder definition | 12.0 | 13 |
Only PROMs seen in five or more publications are displayed. The denominator for all percentages is 108.
Abbreviations: HIT‐6, 6‐item Headache Impact Test short form; MIDAS, Migraine Disability Assessment; MSQ, Migraine‐specific Quality of Life Questionnaire.
Non‐headache‐specific PROMs
Of the 59 publications in the recent randomized and blinded 268 publication subset that used ≥1 non‐headache‐specific PROM (59/268, 22%), a Patient Global Impression of Change item (PGIC; 15/59, 25.4%), the SF‐36 (14/59, 23.7%), Beck Depression Inventory (BDI; 10/59, 16.9%), and a treatment satisfaction item (8/59, 13.6%) were the most frequently used general outcomes (Table 7). However, the PGIC and treatment satisfaction/efficacy items were varied with respect to wording (if given), as well as the number of response options, and the verbal labels applied to those response options.
TABLE 7.
Non‐headache‐specific PROMs seen in five or more publications and endpoints (n = 59 publications)
| Percent | N | |
|---|---|---|
| Non‐headache‐specific PROMs used | ||
| PGIC (item) | 25.4 | 15 |
| SF‐36 | 23.7 | 14 |
| BDI | 16.9 | 10 |
| Treatment satisfaction (item) | 13.6 | 8 |
| Treatment efficacy (item) | 10.2 | 6 |
| Endpoint definition | ||
| Change from baseline | 71.2 | 42 |
| Fixed timepoint | 49.2 | 29 |
| Responder definition | 3.4 | 2 |
Only PROMs seen in five or more publications are displayed. The denominator for all percentages is 59.
Abbreviations: BDI, Beck Depression Inventory; PGIC, patient global impression of change; SF‐36, Short Form Health Questionnaire (36 items).
There was a range of other outcomes used in migraine trials occurring less frequently (fewer than five publications). In addition to established measures of psychiatric comorbidities (such as the Hospital Anxiety and Depression Scale (HADS) 24 or the Patient Health Questionnaire (PHQ‐9) 25 ), there were items or scales unique to specific authors or author‐groups. For the interested reader, a breakdown of all “named” non‐headache‐specific PROMs seen in the examined publications (for the full N = 757 publication sample) is provided in the online supplemental materials.
Almost three‐quarters of the 59 publications that used ≥1 non‐migraine/headache‐specific PROM examined change from baseline (42/59, 71.2%) while 49.2% (29/59) examined differences between groups at fixed timepoints. Only 3.4% (2/59) of the examined 59 publications using a non‐headache‐specific PROM investigated groups created by applying a responder definition to the PROM scores (Table 7).
DISCUSSION
This systematic review of the preventive migraine clinical trial literature showed that publications differed substantially with regard to selection of outcomes and endpoint definitions. While the initial extraction process produced 757 articles, given the clinical and research goals, a subset of 268 studies that were published in 1988 or later and adhered to certain design characteristics (e.g., blinded, randomized) were of primary focus. Findings demonstrated that although some outcomes (e.g., migraine attacks, migraine headache days, rescue/acute medication use) and endpoints (e.g., change from baseline, responder definitions) appeared fairly regularly, there was not a clear “standardized” set of endpoints and outcomes being used despite the recommendations from organizations and agencies such as the AHS, IHS, FDA, NINDS, and EMA. 9 , 10 , 11 , 12 , 13 , 14
With regard to general outcomes used in the 268 article subset, 68.7% of the publications examined ≥1 migraine‐specific outcome, 39.6% examined ≥1 headache‐specific outcome, 50.7% examined ≥1 acute/rescue medication use outcome, 40.3% examined ≥1 headache‐related PROM, and 22.0% examined ≥1 non‐headache‐specific PROM. In general, across the outcomes, endpoints were frequently defined as change from baseline and, for headache‐related outcomes, ≥50% responder definitions were often used.
There were substantial differences among publications in the PROMs that were used. For instance, of the 108 publications within the noted subset that used headache‐related PROMs, the MIDAS (49.1%), MSQ (31.5%), and the HIT‐6 (30.6%) were most often selected. However, a variety of other PROMs were also used less consistently and captured areas such as disability/impairment, self‐efficacy, and other measures of quality of life. The broad area of non‐headache‐specific PROMs (e.g., SF‐36, PGIC, treatment efficacy) was even more variable.
The current work is not without limitations. Given the article selection criteria, there is a possibility of publication bias for positive clinical studies and selective reporting of supportive results. It is likely that we also did not identify every preventive migraine trial publication; however, our sample of 757 fully reviewed and extracted publications is likely large enough to be representative of the field. Additionally, the terms “publication” or “article” are used throughout because in some cases, ≥2 publications came from a single clinical study, which leaves within‐study dependencies unaccounted for when tabulating frequencies of outcomes and endpoints. In addition, there are many limitations posed by the scientific literature itself. This a large body of work encompassing several decades, over which many things changed including criteria, definitions, and terminology of headache types and criteria for the conduct of research and research customs. As a result, there were elements that were not able to be consistently assessed such as endpoint designations (e.g., primary, secondary, and tertiary). Clinical trial identifiers, registry information, and study names (which would facilitate easier grouping of manuscripts form the same parent study) of clinical trials were not systematically reported or collected.
CONCLUSION
This systematic review demonstrated that there were substantial cross‐publication differences in the outcomes and endpoints used in preventive migraine trials. While there were common elements across trials, for example, assessing change from baseline in migraine days or headache days, there was tremendous variability in other areas, including the selection and application of PROMs. In addition, endpoints were calculated in three broad ways: change from baseline, fixed timepoint assessments, and responder definitions. Empirical work is required to determine if these approaches or other approaches optimize power and sensitivity to change. Novel approaches could include analysis of trajectories of change, 26 time to event analysis, or regression adjustment for baseline differences. Qualitative work is required to identify aspects of the migraine experience central to patients. This could include assessing cognitive effects, patient designated most bothersome symptoms or other migraine features (neck pain, osmophobia). Taken together, a standardization process that is designed to integrate the voices of patients with rigorous methodological techniques could improve evaluations of preventive migraine treatments, capture outcomes most meaningful to patients, facilitate cross‐trial comparisons, and better inform clinical decision‐making.
CONFLICT OF INTEREST
CRH, TKN, and RJW are full‐time employees of Vector Psychometric Group, LLC. JSM is a full‐time employee of Vector Psychometric Group, LLC and has received honoraria/payment/reimbursement from the journal Cephalalgia (biostatistics editor). JSM has also received research grants/support from Amgen, Inc. and the National Headache Foundation. DCB is a part‐time employee of Vector Psychometric Group, LLC which has received a grant from the Food and Drug Administration for the MICOAS research project and has received grant support from the National Headache Foundation and grant support and honoraria from Allergan, Amgen, Biohaven, Lilly, Novartis and Promius/Dr. Reddys. She serves on the editorial board of Current Pain and Headache Reports. RBL has received grant support from the National Institutes of Health, the Food and Drug Administration, the National Headache Foundation, and the Migraine Research Fund. He serves as consultant, serves as an advisory board member, has received honoraria from or conducted studies funded by Alder, Abbvie/Allergan, American Headache Society, Biohaven, Eli Lilly, eNeura Therapeutics, Lundbeck, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff's Headache, 8th Edition (Oxford University Press, 2009). He holds stock options in eNeura Therapeutics and Biohaven. PJG reports, over the last 36 months, grants and personal fees from Amgen and Eli‐Lilly and Company, grant from Celgene, and personal fees from Alder Biopharmaceuticals, Aeon Biopharma, Allergan, Biohaven Pharmaceuticals Inc., Cerecin, Clexio, Electrocore LLC, eNeura, Epalex, GlaxoSmithKline, Impel Neuropharma, Lundbeck, MundiPharma, Novartis, Pfizer, Sanofi, Santara Therapeutics, Satsuma, Teva Pharmaceuticals, Trigemina Inc., WL Gore, and personal fees from MedicoLegal work, Massachusetts Medical Society, Up‐to‐Date, Oxford University Press, and Wolters Kluwer. DWD reports consulting: Amgen, Allergan, Abbvie, Eli Lilly, Novartis, Lundbeck, AEON, Clexio, Cerecin, Biohaven, Linpharma, Promius, eNeura, Impel, Theranica, WL Gore, Nocira, XoC, Zosano, Upjohn (Division of Pfizer), Pieris, Revance, Equinox. Honoraria: CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Majallin LLC, Medlogix Communications, Miller Medical Communications, Southern Headache Society (MAHEC), WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Research Support: Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Patient Centered Outcomes Research Institute (PCORI). Stock Options/Shareholder/Patents/Board of Directors: Aural analytics (options), ExSano (options), Palion (options), Healint (Options), Theranica (Options), Second Opinion/Mobile Health (Options), Epien (Options/Board), Nocira (options), Ontologics (Options/Board), King‐Devick Technologies (Options/Board), Precon Health (Options/Board). Patent 17189376.1‐1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis.
AUTHOR CONTRIBUTIONS
Study concept and design: James S. McGinley, Carrie R. Houts, Richard B. Lipton, R. J. Wirth, and Dawn C. Buse. Acquisition of data: James S. McGinley, Carrie R. Houts, Tracy K. Nishida, and Dawn C. Buse. Analysis and interpretation of data: James S. McGinley, Carrie R. Houts, Tracy K. Nishida, Dawn C. Buse, Peter J. Goadsby, David W. Dodick, Richard B. Lipton, and R. J. Wirth. Drafting of the manuscript: James S. McGinley and Carrie R. Houts. Revising it for intellectual content: James S. McGinley, Carrie R. Houts, Tracy K. Nishida, Dawn C. Buse, Peter J. Goadsby, David W. Dodick, Richard B. Lipton, and R. J. Wirth. Final approval of the completed manuscript: James S. McGinley, Carrie R. Houts, Tracy K. Nishida, Dawn C. Buse, Peter J. Goadsby, David W. Dodick, Richard B. Lipton, and R. J. Wirth.
Supporting information
File S1‐S2
ACKNOWLEDGMENTS
We would like to thank Sabrina Howland, BA, Lindsay Olix, BA, Ashley Polk, MA, Leah Sutton, MA, and Julia Warren, MA, for assisting in the literature review process.
Views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Funding Information
This publication was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award (UG3FD006795) totaling $1,286,743 with 100 percent funded by FDA/HHS. The contents are those of the authors and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
REFERENCES
- 1. Silberstein SD, Lee L, Gandhi K, Fitzgerald T, Bell J, Cohen JM. Health care resource utilization and migraine disability along the migraine continuum among patients treated for migraine. Headache. 2018;58:1579‐1592. [DOI] [PubMed] [Google Scholar]
- 2. Lipton RB, Hamelsky SW, Kolodner KB, Steiner TJ, Stewart WF. Migraine, quality of life, and depression: a population‐based case–control study. Neurology. 2000;55:629‐635. [DOI] [PubMed] [Google Scholar]
- 3. Terwindt GM, Ferrari MD, Tijhuis M, Groenen SM, Picavet HS, Launer LJ. The impact of migraine on quality of life in the general population: the GEM study. Neurology. 2000;55:624‐629. [DOI] [PubMed] [Google Scholar]
- 4. Tietjen GE, Herial NA, Hardgrove J, Utley C, White L. Migraine comorbidity constellations. Headache. 2007;47:857‐865. [DOI] [PubMed] [Google Scholar]
- 5. Radat F, Swendsen J. Psychiatric comorbidity in migraine: a review. Cephalalgia. 2005;25:165‐178. [DOI] [PubMed] [Google Scholar]
- 6. Buse DC, Scher AI, Dodick DW, et al. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the CaMEO study. Mayo Clin Proc. 2016;91:596‐611. [DOI] [PubMed] [Google Scholar]
- 7. Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58:700‐714. [DOI] [PubMed] [Google Scholar]
- 8. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 10. Diener HC, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. 2019;39:687‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tassorelli C, Diener HC, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815‐832. [DOI] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration . Migraine: Developing Drugs for Acute Treatment Guidance for Industry; 2018. https://www.regulations.gov/document?D=FDA-2014-D-1540-0008. Accessed September 9, 2020. [Google Scholar]
- 13. National Institute of Neurological Disorders and Stroke . Headache: NINDS Common Data Elements. https://www.commondataelements.ninds.nih.gov/headache. Accessed September 9, 2020. [Google Scholar]
- 14. European Medicines Agency . Clinical Investigation of Medicinal Products for Treatment of Migraine. https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-migraine. Accessed September 9, 2020. [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Clin Epidemiol. 2009;62:1006‐1012. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Food and Drug Administration . What are the Different Types of Clinical Research?. https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research. Accessed September 9, 2020. [Google Scholar]
- 17. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache‐related disability. Neurology. 2001;56:S20‐S28. [DOI] [PubMed] [Google Scholar]
- 18. Kosinski M, Bayliss MS, Bjorner JB, et al. A six‐item short‐form survey for measuring headache impact: The HIT‐6™. Qual Life Res. 2003;12:963‐974. [DOI] [PubMed] [Google Scholar]
- 19. Jhingran P, Osterhaus JT, Miller DW, Lee JT, Kirchdoerfer L. Development and validation of the migraine‐specific quality of life questionnaire. Headache. 1998;38:295‐302. [DOI] [PubMed] [Google Scholar]
- 20. Martin BC, Pathak DS, Sharfman MI, et al. Validity and reliability of the migraine‐specific quality of life questionnaire (MSQ Version 2.1). Headache. 2000;40:204‐216. [DOI] [PubMed] [Google Scholar]
- 21. Ware JE Jr, Kosinski M, Bjorner JB, Turner‐Bowker DM, Gandek B, Maruish ME. User’s Manual for the SF‐36v2® Health Survey. 2nd ed. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 22. Headache Classification Committee of the International Headache Society . Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8:1‐96. [PubMed] [Google Scholar]
- 23. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 24. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 25. Kroenke K, Spitzer RL, Williams JB. Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGinley JS, Wirth RJ, Houts CR. Growth curves for headache research: a multilevel modeling perspective. Headache. 2019;59:1063‐1073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1‐S2


